The Adsorptive Removal of Paracetamol as a Model Pollutant from an Aqueous Environment Using Activated Carbons Made from Selected Nutshells as Agricultural Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Analysis of Biomass Composition
2.3. Preparation of Activated Carbons
2.4. Adsorbents Characterization
2.5. Batch Adsorption Studies
3. Results and Discussion
3.1. Chemical Analysis of Nutshells
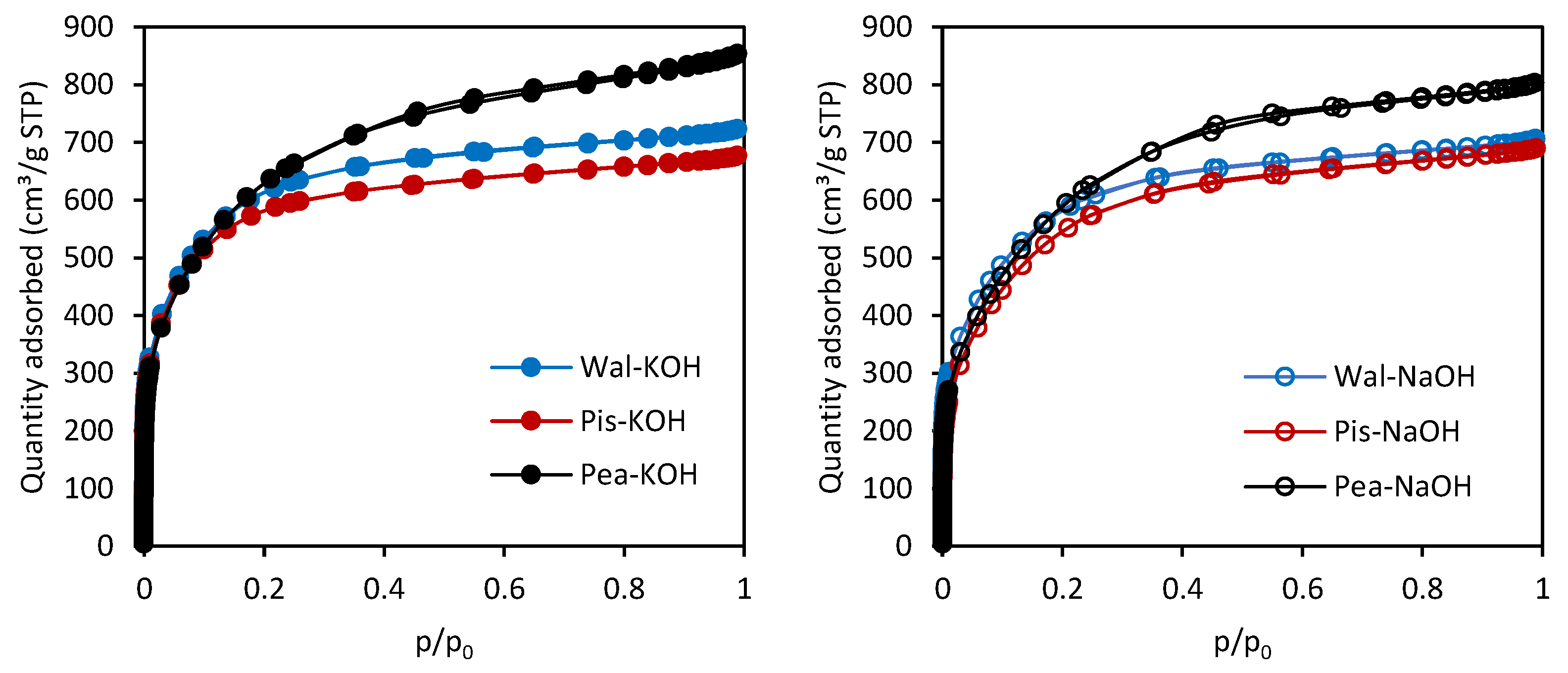
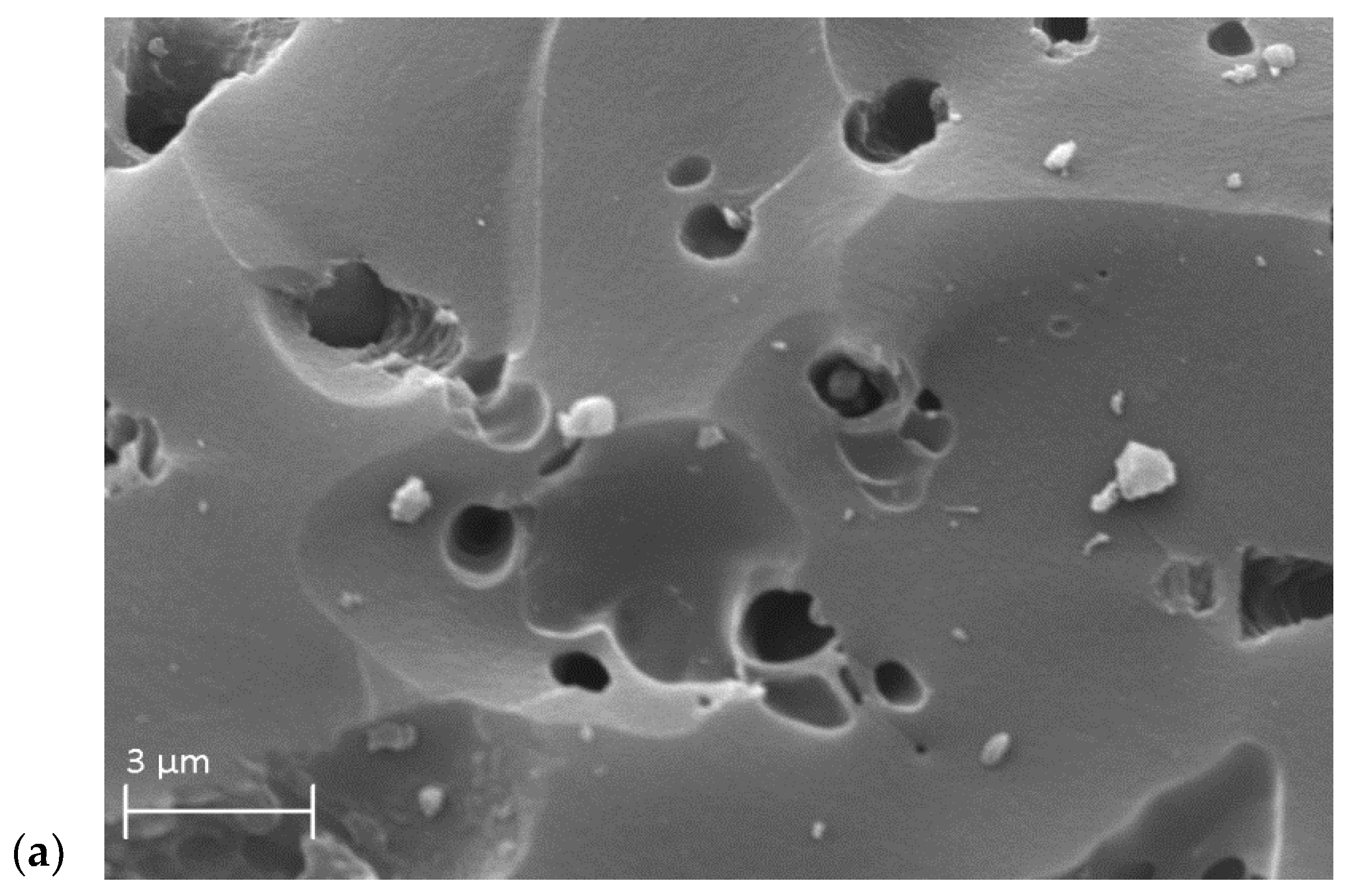
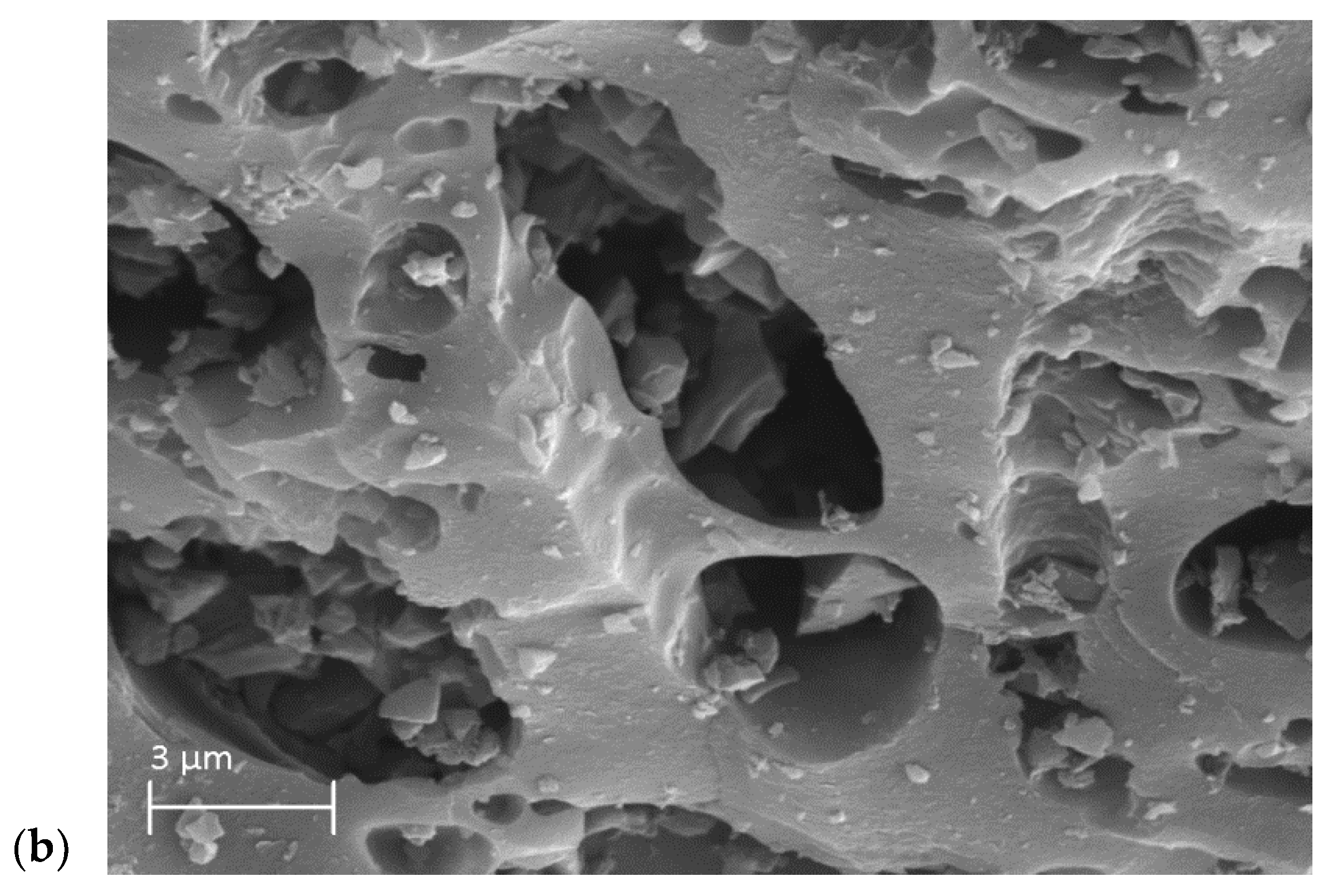
3.2. The Characterization of the Activated Carbons
3.3. Adsorption Study
3.3.1. Effect of Solution pH
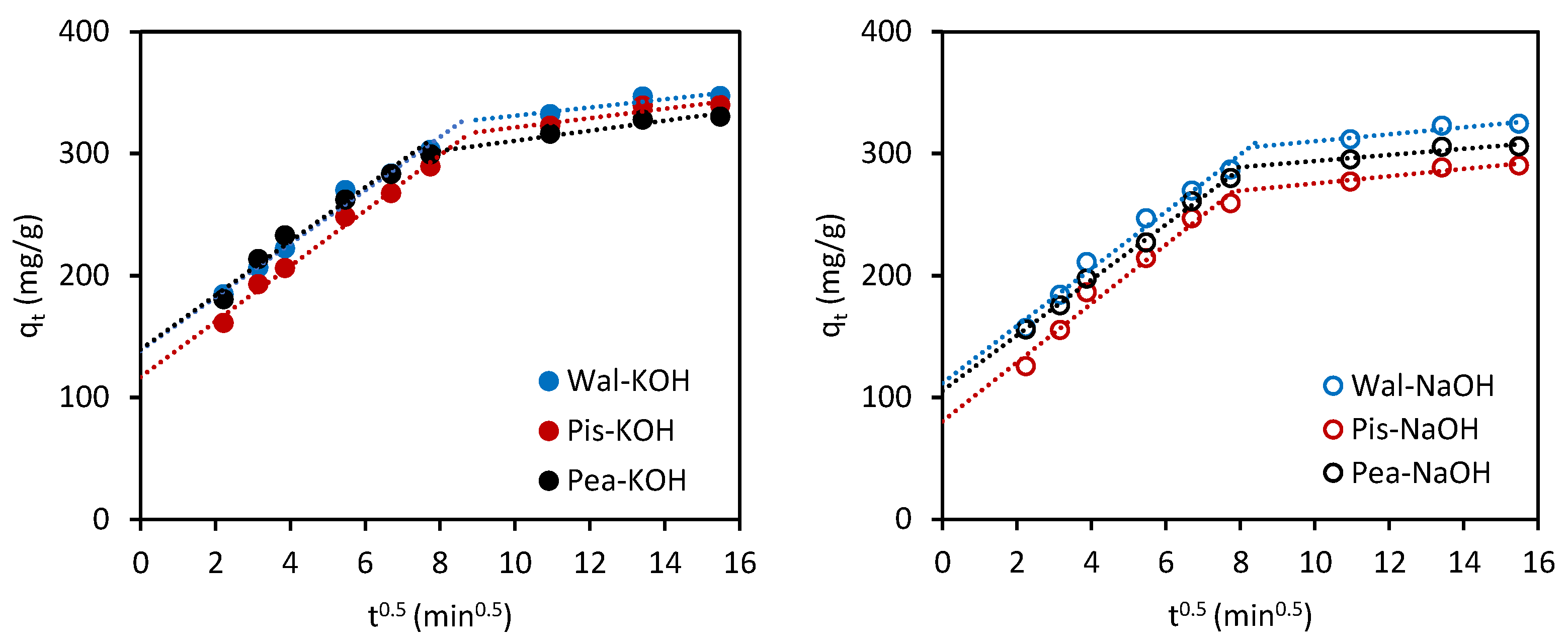
3.3.2. Adsorption Kinetics
- -
- The adsorption rate is controlled by only one step (qt = f(t0.5) is a straight line);
- -
- The adsorption is complex; both stages, film diffusion and intraparticle diffusion, control the adsorption rate (qt = f(t0.5) is not linear over the whole range, and a broken line is observed);
- -
- Intra-particle diffusion is the primary rate-limiting step controlling the whole adsorption process (qt = f(t0.5) passes through the origin; intercept = 0);
- -
- Film diffusion is the primary rate-limiting step that determines the overall adsorption process (qt = f(t0.5) does not pass through the origin; intercept ≠ 0);
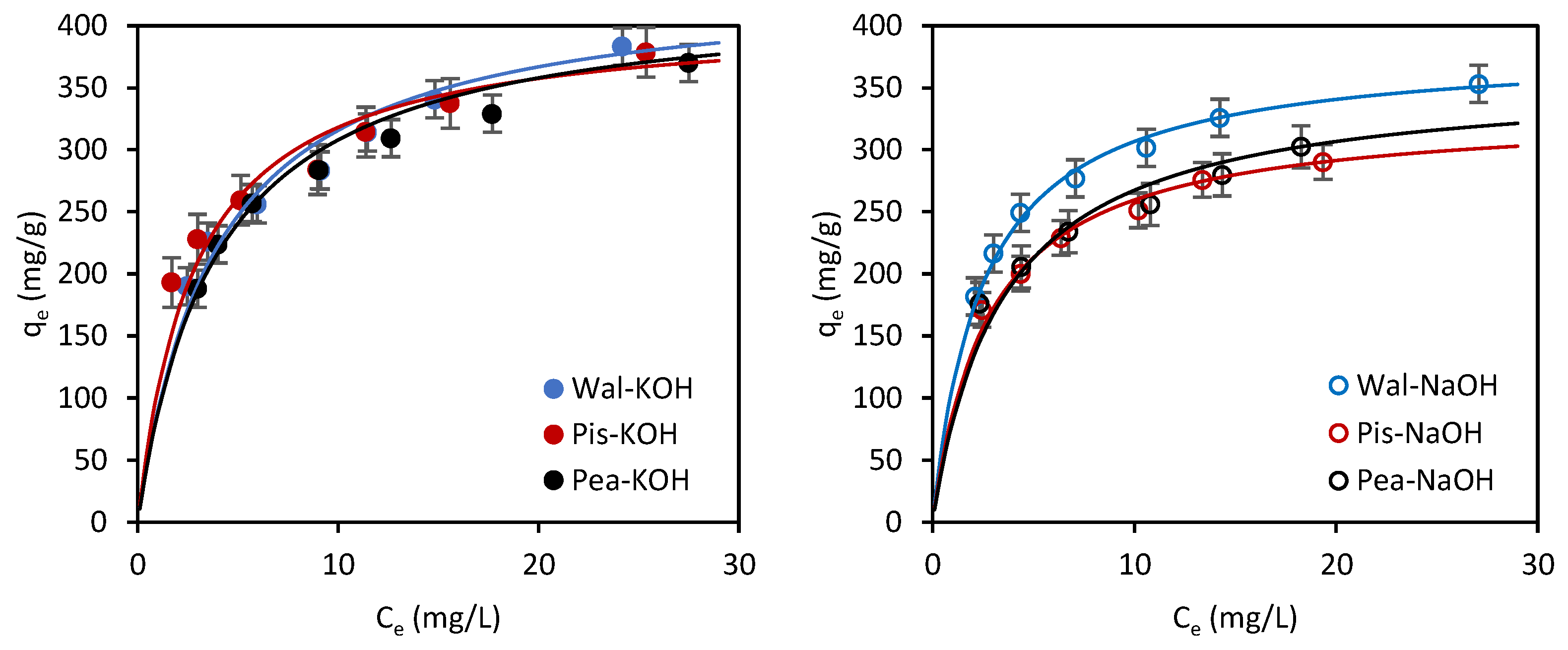
3.3.3. Adsorption Isotherms
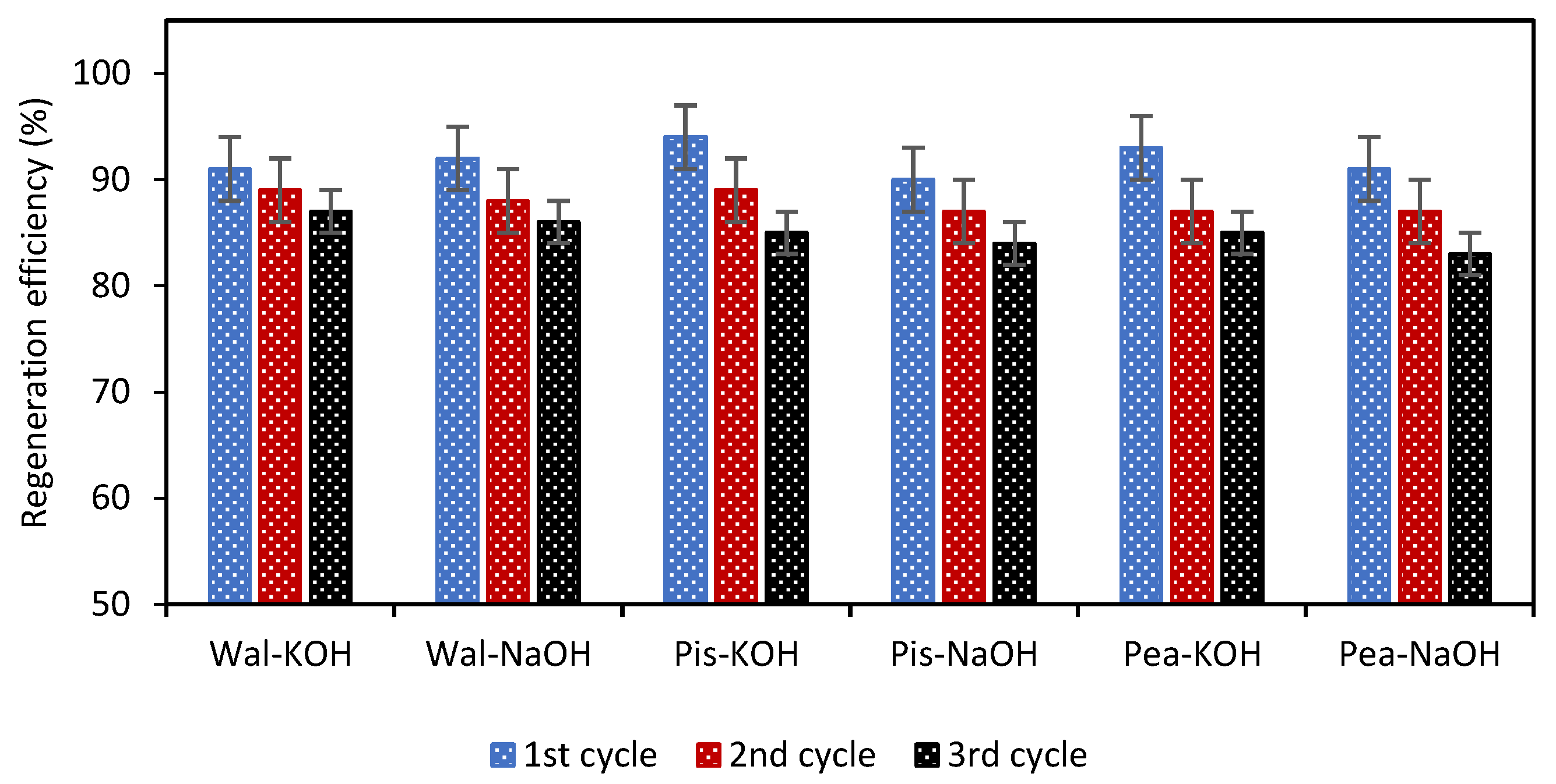
3.4. The Regeneration and Reuse of the Activated Carbons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A.H.A.; Barros, R. Pharmaceuticals in Water: Risks to Aquatic Life and Remediation Strategies. Hydrobiology 2023, 2, 395–409. [Google Scholar] [CrossRef]
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef] [PubMed]
- Dolatimehr, A.; Mahyar, A.; Barough, S.P.H.; Mahmoodi, M. Insights into the efficiencies of different biological treatment systems for pharmaceuticals removal: A review. Water Environ. Res. 2024, 96, e11153. [Google Scholar] [CrossRef] [PubMed]
- Boroń, M.; Pawlas, K. Farmaceutyki w środowisku wodnym–przegląd literatury Pharmaceuticals in aquatic environment–literature review. Probl. Hig. Epidemiol. 2015, 96, 357–363. [Google Scholar]
- Umar, M.; Azis, H.A. Photocatalytic Degradation of Organic Pollutants in Water. In Organic Pollutants-Monitoring, Risk and Treatment; Rashed, M.N., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z.A. Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Finn, M.; Giampietro, G.; Mazyck, D.; Rodriguez, R. Activated Carbon for Pharmaceutical Removal at Point-of-Entry. Processes 2021, 9, 1091. [Google Scholar] [CrossRef]
- Brigante, M.; Avena, M. Biotemplated synthesis of mesoporous silica for doxycycline removal. Effect of pH, temperature, ionic strength and Ca2+ concentration on the adsorption behavior. Microporous Mesoporous Mater. 2016, 225, 534–542. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Y.; Xiang, Y.; Meng, S.; Liu, X.; Yu, J.; Yang, J.; Zhang, J.; Qin, P.; Luo, L. Single and simultaneous adsorption of pefloxacin and Cu (II) ions from aqueous solutions by oxidized multiwalled carbon nanotube. Sci. Total Environ. 2019, 646, 29–36. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, J.; Li, Y.; Xia, S.; Zhao, J.; Minale, T.M.; Gu, Z. Coadsorption of tetracycline and copper (II) onto struvite loaded zeolite—An environmentally friendly product recovered from swine biogas slurry. Chem. Eng. J. 2019, 371, 366–377. [Google Scholar] [CrossRef]
- Ghemit, R.; Makhloufi, A.; Djebri, N.; Flilissa, A.; Zerroual, L.; Boutahala, M. Adsorptive removal of diclofenac and ibuprofen from aqueous solution by organobentonites: Study in single and binary systems. Groundw. Sustain. Dev. 2019, 8, 520–529. [Google Scholar] [CrossRef]
- Yao, N.; Zhang, X.T.; Yang, Z.; Yang, W.B.; Tian, Z.Q.; Zhang, L.M. Norfloxacin and Bisphenol-A removal using temperature-switchable graphene oxide. ACS Appl. Mater. Interfaces 2018, 10, 29083–29091. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, C.; Yang, Z.; Tian, Z.Q.; Lu, L.S.; Yang, W.B.; Wang, Y.P.; Zhang, L.M.; Li, A.M.; Chen, J.Q. Enhanced adsorption of pharmaceuticals onto core-brush shaped aromatic rings-functionalized chitosan magnetic composite particles: Effects of structural characteristics of both pharmaceuticals and brushes. J. Cleaner Prod. 2018, 172, 1025–1034. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Singh, B.; Bishnu, A. A comprehensive review on activated carbon from pyrolysis of lignocellulosic biomass: An application for energy and the environment. Carbon Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2024, 11, 6204. [Google Scholar] [CrossRef]
- Gonzalez-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sust. Energ. Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Antal, M.J. Biomass Pyrolysis: A Review of the Literature Part 1. Carbohydrate Pyrolysis; Boer, K.W., Duffie, J.A., Eds.; Advances in Solar Energy; American Solar Energy Society: Boulder, CO, USA, 1982; pp. 61–111. [Google Scholar]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresource Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef]
- Vinod, A.; Pulikkalparambil, H.; Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Recent advancements in lignocellulose biomass-based carbon fiber: Synthesis, properties, and applications. Heliyon 2023, 9, e13614. [Google Scholar] [CrossRef]
- Seifert, K. Zur Frage der Cellulose-Schnellbestimmung nach der Acetylaceton-Methode. Das Papier 1960, 14, 104–106. [Google Scholar]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Dąbek, L.; Świątkowski, A. The use of modified multi-walled carbon nanotubes for the removal of selected pharmaceuticals from the aqueous environment. Desalin. Water Treat. 2023, 288, 60–71. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Nasser, S.M.; Abbas, M.; Trari, M. Understanding the rate-limiting step adsorption kinetics onto biomaterials for mechanism adsorption control. Prog. React. Kinet. Mec. 2024, 49, 1–26. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.X. The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Dąbek, L.; Świątkowski, A. The influence of the shape and grain size of commercial activated carbons on their sorption efficiency towards organic water pollutants. Desalin. Water Treat. 2025, 321, 100996. [Google Scholar] [CrossRef]
- Antal, M.J.; Allen, S.G.; Dai, X.; Shimizu, B.; Tam, M.S.; Grønli, M. Attainment of the Theoretical Yield of Carbon from Biomass. Ind. Eng. Chem. Res. 2000, 39, 4024–4031. [Google Scholar] [CrossRef]
- Yang, W.; Shimizu, I.; Ono, T.; Kimura, Y. Preparation of biodegradable foam from walnut shells treated by subcritical water. J. Chem. Technol. Biotechnol. 2014, 90, 44–49. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Hao, J.; Wang, W. Study of Almond Shell Characteristics. Materials 2018, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Jovičič, N.; Antonovič, A.; Matin, A.; Antolovič, S.; Kalambura, S.; Krička, T. Biomass Valorization of Walnut Shell for Liquefaction Efficiency. Energies 2022, 15, 495. [Google Scholar] [CrossRef]
- Cho, C.H.; Hatsu, M.; Takamizawa, K. The production of D-xylose by enzymatic hydrolysis of agricultural wastes. Water Sci. Technol. 2002, 45, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefin. 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Robles, E.; Izaguirre, N.; Martin, A.; Moschou, D.; Labidi, J. Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making. Molecules 2021, 26, 1371. [Google Scholar] [CrossRef]
- Jadhav, B.; Roy, R.; Rahman, M.S.; Raynie, D.E. Extraction and Depolymerization of Lignin from Pine Sawdust and Pistachio Shells. Biomass 2022, 2, 348–357. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Aminul Islam, M.; Nazal, M.K.; Sajid, M.; Suliman, M.A. Adsorptive removal of paracetamol from aqueous media: A review of adsorbent materials, adsorption mechanisms, advancements, and future perspectives. J. Mol. Liq. 2024, 396, 123976. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Tran, H.N.; Juang, R.-S.; Dat, N.D.; Tomul, F.; Ivanets, A.; Woo, S.H.; Hosseini-Bandegharaei, A.; Nguyen, V.P.; Chao, H.-P. Adsorption process and mechanism of acetaminophen onto commercial activated carbon. J. Environ. Chem. Eng. 2020, 8, 104408. [Google Scholar] [CrossRef]
- El Saied, M.; Shaban, S.A.; Mostafa, M.S.; El Naga, A.O.A. Efficient adsorption of acetaminophen from the aqueous phase using low-cost and renewable adsorbent derived from orange peels. Biomass Convers. Biorefin. 2024, 14, 2155–2172. [Google Scholar] [CrossRef]
- Gatrouni, M.; Asses, N.; Bedia, J.; Belver, C.; Molina, C.B.; Mzoughi, N. Acetaminophen Adsorption on Carbon Materials from Citrus Waste. C 2024, 10, 53. [Google Scholar] [CrossRef]
- Al-Rub, F.A.A.; Fares, M.M.; Al-Banna, L.N. Toward Cleaner Ecosystems; Elimination of Paracetamol Drug via Mesoporous Activated Carbon Date Pits. Chem. Sci. Int. J. 2024, 33, 1–21. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Rychlicki, G. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: The temperature dependence of adsorption at the neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2000, 163, 135–150. [Google Scholar] [CrossRef]
- Terzyk, A.P. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 23–45. [Google Scholar] [CrossRef]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of Solution pH on the Adsorption of Paracetamol on Chemically Modified Activated Carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Lladó, J.; Lao-Luque, C.; Ruiz, B.; Fuente, E.; Solé-Sardans, M.; Dorado, A.D. Role of activated carbon properties in atrazine and paracetamol adsorption equilibrium and kinetics. Process Saf. Environ. Prot. 2015, 95, 51–59. [Google Scholar] [CrossRef]
- Hamadneh, I.; Abu-Zurayk, R.A.; Aqel, A.; Al-Mobydeen, A.; Hamadneh, L.; Al-Dalahmeh, Y.; Hannoon, F.; Albuqain, R.; Alsotari, S.; Al-Dujaili, A.H. Impact of H3PO4-activated carbon from pine fruit shells for paracetamol adsorption from aqueous solution. Desalin. Water Treat. 2022, 264, 293–306. [Google Scholar] [CrossRef]
- Bursztyn Fuentes, A.L.; Benito, D.E.; Montes, M.L.; Scian, A.N.; Lombardi, M.B. Paracetamol and Ibuprofen Removal from Aqueous Phase Using a Ceramic-Derived Activated Carbon. Arab. J. Sci. Eng. 2023, 48, 525–537. [Google Scholar] [CrossRef]
- Mandal, S.; Stephen, D.; Janardhanan, S.K. Activated carbon with composite pore structures made from peanut shell and areca nut fibers as sustainable adsorbent material for the efficient removal of active pharmaceuticals from aqueous media. RSC Sustain. 2024, 2, 3022–3035. [Google Scholar] [CrossRef]
- Wong, S.; Lim, Y.; Ngadi, N.; Mat, R.; Hassan, O.; Inuwa, I.M.; Mohamed, N.B.; Low, J.H. Removal of acetaminophen by activated carbon synthesized from spent tea leaves: Equilibrium, kinetics and thermodynamics studies. Powder Technol. 2018, 338, 878–886. [Google Scholar] [CrossRef]
- Haro, N.K.; Dávila, I.V.J.; Nunes, K.G.P.; de Franco, M.A.E.; Marcilio, N.R.; Féris, L.A. Kinetic, equilibrium and thermodynamic studies of the adsorption of paracetamol in activated carbon in batch model and fixed-bed column. Appl. Water Sci. 2021, 11, 38. [Google Scholar] [CrossRef]
- Ramli, M.R.M.; Shoparwe, N.F.; Ahmad, M.A.; Yusop, M.F.M. Acetaminophen removal using porous activated carbon derived from corn cob: Optimization and mass transfer modelling. J. Chem. Technol. Biotechnol. 2024, 99, 2088–2106. [Google Scholar] [CrossRef]



| CAS No. | 103-90-2 |
|---|---|
| Empirical formula | C8H9NO2 |
| Structure |  |
| Molecular weight (g/mol) | 151.165 |
| Water solubility (g/L) | 14 |
| pKa | 9.53 |
| Nutshells | Content (%) | |||
|---|---|---|---|---|
| Cellulose | Holocellulose | Lignin | Hemicelluloses | |
| walnut | 27.21 | 56.16 | 39.23 | 28.95 |
| peanut | 44.84 | 66.57 | 31.44 | 21.73 |
| pistachio | 43.05 | 80.35 | 16.91 | 37.30 |
| Sample | SBET (m2/g) | VT (cm3/g) | Vmi (cm3/g) | Vme (cm3/g) | Vmi/VT (%) | dh (nm) |
|---|---|---|---|---|---|---|
| Wal-KOH | 2040 | 1.107 | 0.958 | 0.149 | 86.5 | 2.17 |
| Wal-NaOH | 1865 | 1.079 | 0.910 | 0.169 | 84.3 | 2.32 |
| Pis-KOH | 1980 | 1.036 | 0.908 | 0.128 | 87.6 | 2.10 |
| Pis-NaOH | 1710 | 1.054 | 0.853 | 0.201 | 80.9 | 2.47 |
| Pea-KOH | 1995 | 1.296 | 0.985 | 0.311 | 76.0 | 2.60 |
| Pea-NaOH | 1795 | 1.226 | 0.920 | 0.306 | 75.0 | 2.73 |
| AC Sample | Types of Acidic Groups [mmol/g] | Overall Acidity [mmol/g] | Overall Basicity [mmol/g] | Acidic/Basic Groups Ratio | pHPZC | ||
|---|---|---|---|---|---|---|---|
| Phenolic | Lactonic | Carboxylic | |||||
| Wal-KOH | 0.51 | 0.40 | 0.04 | 0.95 | 0.79 | 1.20 | 6.90 |
| Wal-NaOH | 0.63 | 0.20 | 0.03 | 0.86 | 0.99 | 0.87 | 7.20 |
| Pis-KOH | 0.78 | 0.39 | 0.02 | 1.19 | 0.78 | 1.52 | 6.70 |
| Pis-NaOH | 0.53 | 0.25 | 0.05 | 0.83 | 0.79 | 1.05 | 7.05 |
| Pea-KOH | 0.89 | 0.39 | 0.03 | 1.31 | 0.99 | 1.32 | 6.85 |
| Pea-NaOH | 0.63 | 0.17 | 0.02 | 0.82 | 0.89 | 0.92 | 7.15 |
| Sample | Mass Loss (%) in Temperature Range (°C) | |||
|---|---|---|---|---|
| 200–400 | 400–700 | 700–900 | Σ 200–900 | |
| Wal-KOH | 2.85 | 8.99 | 5.83 | 17.69 |
| Wal-NaOH | 2.64 | 8.03 | 5.51 | 16.18 |
| Pis-KOH | 2.92 | 8.13 | 5.78 | 16.83 |
| Pis-NaOH | 2.78 | 7.86 | 5.79 | 16.43 |
| Pea-KOH | 2.51 | 8.61 | 5.89 | 17.01 |
| Pea-NaOH | 2.27 | 7.08 | 5.27 | 14.62 |
| Kinetic Model | Activated Carbon | |||||
|---|---|---|---|---|---|---|
| Wal-KOH | Wal-NaOH | Pis-KOH | Pis-NaOH | Pea-KOH | Pea-NaOH | |
| qe(EXP) (mg/g) | 347.3 | 324.3 | 339.9 | 290.3 | 330.2 | 306.1 |
| PFO | ||||||
| qe1(CAL) (mg/g) | 309.6 | 251.4 | 369.9 | 182.9 | 259.2 | 167.3 |
| k1 (1/min) | 0.0412 | 0.0263 | 0.0414 | 0.0261 | 0.0221 | 0.0235 |
| R2 | 0.942 | 0.976 | 0.938 | 0.962 | 0.969 | 0.960 |
| RMSE | 56.3 | 40.1 | 50.2 | 48.4 | 21.9 | 19.5 |
| PSO | ||||||
| qe2(CAL) (mg/g) | 357.1 | 333.3 | 357.1 | 303.0 | 344.8 | 322.6 |
| k2 (g/mg∙min) | 3.16·10−4 | 3.33·10−4 | 2.57·10−4 | 3.41·10−4 | 4.02·10−4 | 3.55·10−4 |
| R2 | 0.999 | 0.999 | 0.998 | 0.999 | 0.999 | 0.998 |
| RMSE | 10.5 | 13.8 | 20.1 | 9.08 | 1.24 | 1.3 |
| Isotherm Model | Activated Carbon | |||||
|---|---|---|---|---|---|---|
| Wal-KOH | Wal-NaOH | Pis-KOH | Pis-NaOH | Pea-KOH | Pea-NaOH | |
| Langmuir | ||||||
| qm (mg/g) | 437.8 | 383.1 | 408.1 | 332.2 | 411.5 | 359.7 |
| KL (L/mg) | 0.259 | 0.402 | 0.354 | 0.358 | 0.268 | 0.290 |
| R2 | 0.994 | 0.998 | 0.993 | 0.999 | 0.997 | 0.995 |
| RMSE | 5.55 | 4.22 | 6.31 | 3.22 | 5.92 | 5.29 |
| Freundlich | ||||||
| KF ((mg/g) (L/mg)1/n) | 172.0 | 188.4 | 160.5 | 147.9 | 162.6 | 149.9 |
| 1/n | 0.244 | 0.247 | 0.231 | 0.285 | 0.289 | 0.299 |
| R2 | 0.992 | 0.991 | 0.966 | 0.968 | 0.970 | 0.990 |
| RMSE | 7.14 | 12.8 | 20.9 | 22.1 | 15.6 | 8.21 |
| Temkin | ||||||
| AT (L/g) | 4.030 | 8.470 | 9.826 | 9.855 | 4.307 | 7.194 |
| bT (kJ/mol) | 30.07 | 35.23 | 37.04 | 40.86 | 32.02 | 37.77 |
| R2 | 0.987 | 0.985 | 0.984 | 0.983 | 0.990 | 0.991 |
| RMSE | 15.4 | 14.6 | 7.50 | 15.2 | 8.56 | 8.16 |
| ACs | ΔG° (kJ/mol) | Separation Factor (RL) | |
|---|---|---|---|
| min. | max. | ||
| Wal-KOH | −23.6 | 0.031 | 0.072 |
| Wal-NaOH | −24.7 | 0.020 | 0.047 |
| Pis-KOH | −24.3 | 0.023 | 0.053 |
| Pis-NaOH | −24.4 | 0.022 | 0.052 |
| Pea-KOH | −23.7 | 0.031 | 0.069 |
| Pea-NaOH | −23.8 | 0.028 | 0.064 |
| Adsorbent | Surface Area (m2/g) | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|
| Wal-KOH | 2041 | 437.8 | This study |
| Wal-NaOH | 1864 | 383.1 | This study |
| Pis-KOH | 1978 | 408.1 | This study |
| Pis-NaOH | 1710 | 332.2 | This study |
| Pea-KOH | 1995 | 411.5 | This study |
| Pea-NaOH | 1794 | 359.7 | This study |
| commercial WG12 | 980 | 277.3 | [30] |
| Filtrasorb-400 (F-400) | 1234 | 261.1 | [48] |
| AC from pine fruit shells | 1022 | 256.4 | [49] |
| GS50 AC (reduced) | 816 | 245.7 | [47] |
| commercial AC | 1248 | 221.0 | [41] |
| commercial L2S | 960 | 208.3 | [30] |
| commercial F300 | 995 | 250.1 | [30] |
| AC from date pits | 838 | 196.0 | [44] |
| commercial GS50 AC | 842 | 183.4 | [47] |
| Norit PK 1-3 (NPK) | 782 | 150.1 | [48] |
| commercial AC (Clarimex) | 1050 | 146.2 | [50] |
| AC from orange peels | 1069 | 118.0 | [42] |
| ceramic-derived AC | 895 | 109.8 | [50] |
| GS50 AC (oxidized) | 876 | 106.9 | [47] |
| AC from peanut shell (H3PO4) | 689 | 67.57 | [51] |
| AC from spent tea leaves | 1203 | 59.19 | [52] |
| sludge-based AC (SBC) | 260 | 53.75 | [48] |
| AC from citrus waste | 273 | 49.00 | [43] |
| commercial AC (Exodus) | 543 | 43.50 | [53] |
| carbon nanotubes | 179 | 29.85 | [25] |
| corn cob-based AC | 976 | 21.14 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doczekalska, B.; Kuśmierek, K.; Świątkowski, A. The Adsorptive Removal of Paracetamol as a Model Pollutant from an Aqueous Environment Using Activated Carbons Made from Selected Nutshells as Agricultural Waste. Processes 2025, 13, 2198. https://doi.org/10.3390/pr13072198
Doczekalska B, Kuśmierek K, Świątkowski A. The Adsorptive Removal of Paracetamol as a Model Pollutant from an Aqueous Environment Using Activated Carbons Made from Selected Nutshells as Agricultural Waste. Processes. 2025; 13(7):2198. https://doi.org/10.3390/pr13072198
Chicago/Turabian StyleDoczekalska, Beata, Krzysztof Kuśmierek, and Andrzej Świątkowski. 2025. "The Adsorptive Removal of Paracetamol as a Model Pollutant from an Aqueous Environment Using Activated Carbons Made from Selected Nutshells as Agricultural Waste" Processes 13, no. 7: 2198. https://doi.org/10.3390/pr13072198
APA StyleDoczekalska, B., Kuśmierek, K., & Świątkowski, A. (2025). The Adsorptive Removal of Paracetamol as a Model Pollutant from an Aqueous Environment Using Activated Carbons Made from Selected Nutshells as Agricultural Waste. Processes, 13(7), 2198. https://doi.org/10.3390/pr13072198










