One-Pot Improvement of Stretchable PEDOT/PSS Alginate Conductivity for Soft Sensing Biomedical Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
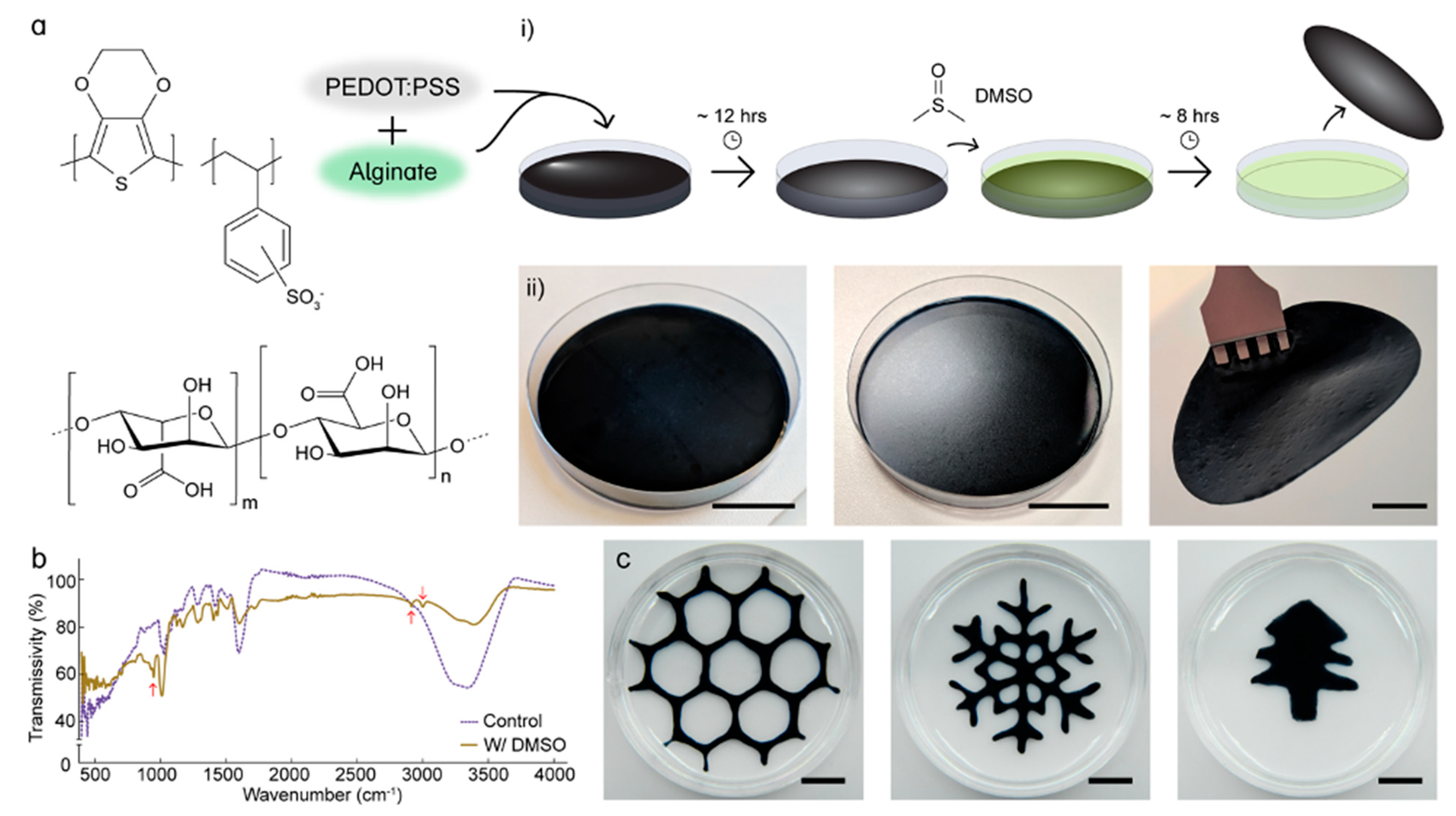
2.2. PEDOT/ALG Hydrogel Film Preparation
2.3. DMSO Post-Treatment
2.4. Resistance Measurements
2.5. Dynamic Mechanical Analysis (DMA)
3. Results
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. PEDOT/ALG Film Patterning
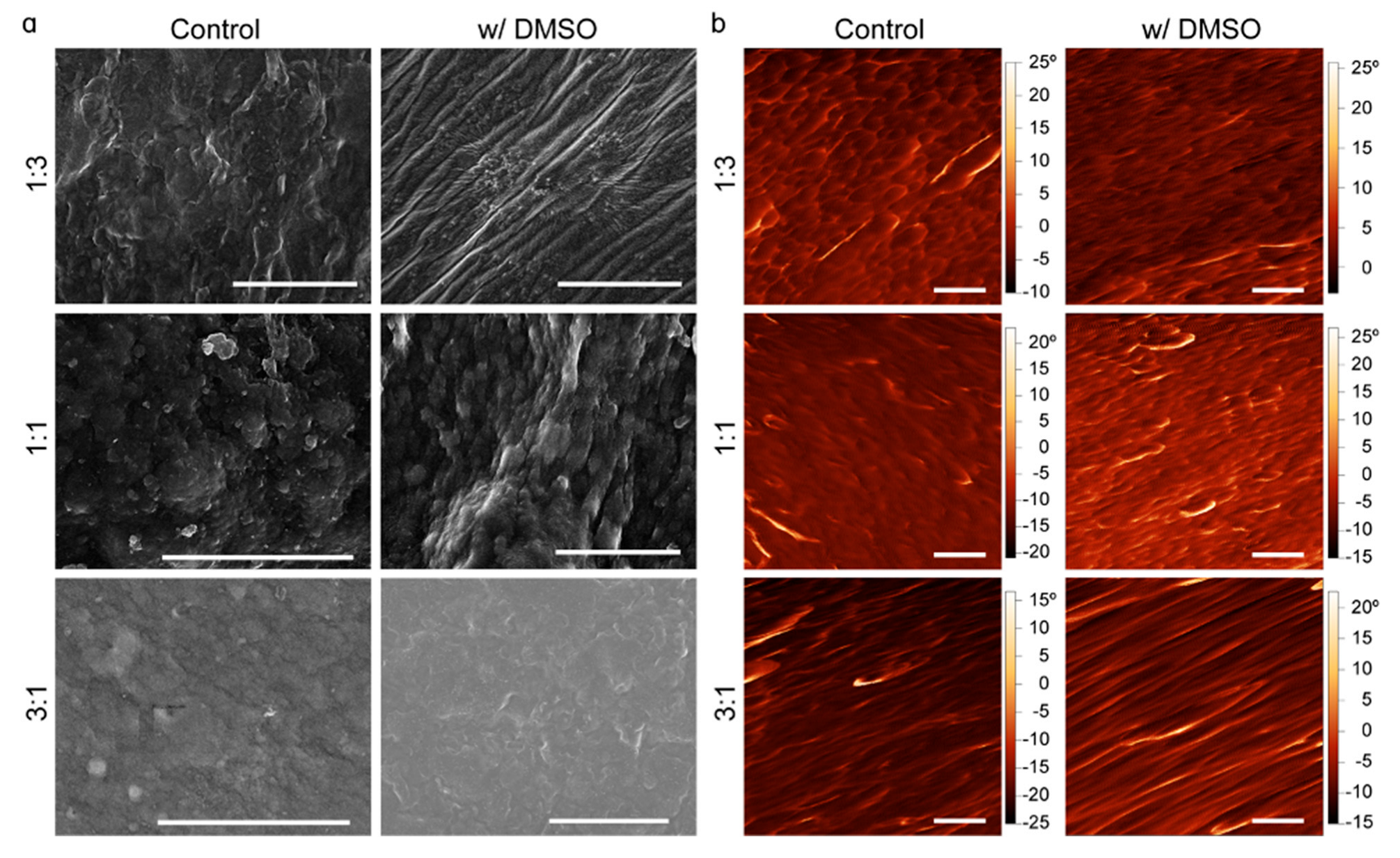
3.3. Scanning Electron Microscopy (SEM)
3.4. Atomic Force Microscopy (AFM)
3.5. Resistance Measurements
3.6. Dynamic Mechanical Analysis (DMA)
3.7. Electromyography (EMG)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Libanori, A.; Chen, G.; Zhao, X.; Zhou, Y.; Chen, J. Smart textiles for personalized healthcare. Nat. Electron. 2022, 5, 142–156. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Liu, Y. Wearable electronics based on stretchable organic semiconductors. Small 2023, 19, 2206309. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Cheng, W. One-dimensional nanomaterials for soft electronics. Adv. Electron. Mater. 2017, 3, 1600314. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Huang, Z.; Xu, S. Materials and structures toward soft electronics. Adv. Mater. 2018, 30, 1801368. [Google Scholar] [CrossRef]
- Jang, K.-I.; Li, K.; Chung, H.U.; Xu, S.; Jung, H.N.; Yang, Y.; Kwak, J.W.; Jung, H.H.; Song, J.; Yang, C.; et al. Self-assembled three-dimensional network designs for soft electronics. Nat. Commun. 2017, 8, 15894. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.-Y.; Chiang Foo, C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, transparent, ionic conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef]
- Byun, S.-H.; Sim, J.Y.; Zhou, Z.; Lee, J.; Qazi, R.; Walicki, M.C.; Parker, K.E.; Haney, M.P.; Choi, S.H.; Shon, A.; et al. Mechanically transformative electronics, sensors, and implantable devices. Sci. Adv. 2019, 5, eaay0418. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Zhu, X.X. Rational design of thermoresponsive polymers in aqueous solutions: A thermodynamics map. Prog. Polym. Sci. 2019, 90, 269–291. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2019, 4, 159–171. [Google Scholar] [CrossRef]
- Meng, K.; Zhao, S.; Zhou, Y.; Wu, Y.; Zhang, S.; He, Q.; Wang, X.; Zhou, Z.; Fan, W.; Tan, X.; et al. A wireless textile-based sensor system for self-powered personalized health care. Matter 2020, 2, 896–907. [Google Scholar] [CrossRef]
- Choi, C.; Choi, M.K.; Hyeon, T.; Kim, D.-H. Nanomaterial-based soft electronics for healthcare applications. ChemNanoMat 2016, 2, 1006–1017. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and soft electronics using liquid metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Bae, S.; Oh, I.; Jeong, J.; Kang, B.; Hwang, S.; Lee, S.; Son, H.; Moon, B.; Ko, M.; et al. 3D printer-based encapsulated origami electronics for extreme system stretchability and high areal coverage. ACS Nano 2017, 11, 2714–2721. [Google Scholar] [CrossRef]
- Morikawa, Y.; Yamagiwa, S.; Sawahata, H.; Numano, R.; Koida, K.; Ishida, M.; Kawano, K. Ultrastretchable kirigami bioprobes. Adv. Healthc. Mater. 2018, 7, 1800130. [Google Scholar] [CrossRef]
- Kang, D.; Pikhitsa, P.V.; Choi, Y.W.; Lee, C.; Shin, S.S.; Piao, L.; Park, B.; Suh, K.-Y.; Kim, T.-i.; Choi, M. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature 2014, 516, 222–226. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly stretchable, elastic, and ionic conductive hydrogel for artificial soft electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Stoyanov, H.; Kollosche, M.; Risse, S.; Waché, R.; Kofod, G. Soft conductive elastomer materials for stretchable electronics and voltage controlled artificial muscles. Adv. Mater. 2012, 5, 578–583. [Google Scholar] [CrossRef]
- Jang, K.-I.; Chung, H.U.; Xu, S.; Lee, C.-H.; Luan, H.; Jeong, J.; Cheng, H.; Kim, G.-T.; Han, S.-Y.; Lee, J.-W.; et al. Soft network composite materials with deterministic and bio-inspired designs. Nat. Commun. 2015, 6, 6566. [Google Scholar] [CrossRef]
- Byun, J.; Oh, E.; Lee, B.; Kim, S.; Lee, S.; Hong, Y. A single droplet-printed double-side universal soft electronic platform for highly integrated stretchable hybrid electronics. Adv. Funct. Mater. 2017, 27, 1701912. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Zhang, H.; Xie, M.; Duana, X. PEDOT:PSS: From conductive polymers to sensors. Nanotechnol. Precis. Eng. 2021, 4, 045004. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Sekine, T.; Takeda, Y.; Yokosawa, K.; Matsui, H.; Kumaki, D.; Shiba, T.; Nishikawa, T.; Tokito, S. Fully printed PEDOT:PSS-based temperature sensor with high humidity stability for wireless healthcare monitoring. Sci. Rep. 2020, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Chen, H.; Lv, D.; Li, W.; Liu, X.; Zhang, Q.; Yu, X.; Han, Y. Highly Conductive Inkjet-Printed PEDOT:PSS Film under Cyclic Stretching. ACS Appl. Mater. Interfaces 2023, 15, 28503–28515. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, L.; Yue, S.; Yu, S.; Wei, J.; Ouyang, J. Enhancement in the mechanical stretchability of PEDOT:PSS films by compounds of multiple hydroxyl groups for their application as transparent stretchable conductors. Macromolecules 2013, 54, 1234–1242. [Google Scholar] [CrossRef]
- Yu, Y.; Peng, S.; Blanloeuil, P.; Wu, S.; Wang, C.H. Wearable temperature sensors with enhanced sensitivity by engineering microcrack morphology in PEDOT:PSS–PDMS sensors. ACS Appl. Mater. Interfaces 2020, 12, 36578–36588. [Google Scholar] [CrossRef]
- Stříteský, S.; Marková, A.; Víteček, J.; Šafaříková, E.; Hrabal, M.; Kubáč, L.; Kubala, L.; Weiter, M.; Vala, M. Printing inks of electroactive polymer PEDOT:PSS: The study of biocompatibility, stability, and electrical properties. J. Biomed. Mater. Res. Part A 2018, 106, 1121–1128. [Google Scholar] [CrossRef]
- Li, Y.; Cao, W.; Liu, Z.; Zhang, Y.; Chen, Z.; Zheng, X. A personalized electronic textile for ultrasensitive pressure sensing enabled by biocompatible MXene/PEDOT:PSS composite. Carbon Energy 2024, 6, e530. [Google Scholar] [CrossRef]
- Soni, M.; Bhattacharjee, M.; Ntagios, M.; Dahiya, R.S. Printed temperature sensor based on PEDOT:PSS-graphene oxide composite. IEEE Sens. J. 2020, 20, 7525–7531. [Google Scholar] [CrossRef]
- Sasaki, R.; Katsuhara, M.; Yoshifuji, K.; Komoriya, Y. Novel dry EEG electrode with composite filler of PEDOT:PSS and carbon particles. In Proceedings of the 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Lo, L.-W.; Zhao, J.; Wan, H.; Wang, Y.; Chakrabartty, S.; Wang, C. An inkjet-printed PEDOT:PSS-based stretchable conductor for wearable health monitoring device applications. ACS Appl. Mater. Interfaces 2021, 13, 5768–5776. [Google Scholar] [CrossRef]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Soft-matrices based on silk fibroin and alginate for tissue engineering. Int. J. Biol. Macromol. 2016, 89, 161–171. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanganeh, S.; Escobar, A.R.; Cao, H.; Tseng, P. One-Pot Improvement of Stretchable PEDOT/PSS Alginate Conductivity for Soft Sensing Biomedical Processes. Processes 2025, 13, 2173. https://doi.org/10.3390/pr13072173
Zanganeh S, Escobar AR, Cao H, Tseng P. One-Pot Improvement of Stretchable PEDOT/PSS Alginate Conductivity for Soft Sensing Biomedical Processes. Processes. 2025; 13(7):2173. https://doi.org/10.3390/pr13072173
Chicago/Turabian StyleZanganeh, Somayeh, Alberto Ranier Escobar, Hung Cao, and Peter Tseng. 2025. "One-Pot Improvement of Stretchable PEDOT/PSS Alginate Conductivity for Soft Sensing Biomedical Processes" Processes 13, no. 7: 2173. https://doi.org/10.3390/pr13072173
APA StyleZanganeh, S., Escobar, A. R., Cao, H., & Tseng, P. (2025). One-Pot Improvement of Stretchable PEDOT/PSS Alginate Conductivity for Soft Sensing Biomedical Processes. Processes, 13(7), 2173. https://doi.org/10.3390/pr13072173









