Abstract
Air purification is a key process aimed at removing harmful impurities and providing a safe and comfortable environment for human life and work. This study presents the results of an investigation into the composition, textural, and sorption properties of a multichannel carbon filtering material developed for air purification from biological (infectious) contaminants. The filtering block has a cylindrical shape and is manufactured by extrusion of a plastic composition based on carbonized rice husk with the addition of binding agents, followed by staged thermal treatment (calcination, activation, and demineralization). The filter’s effectiveness is based on the inactivation of pathogenic microorganisms as the air passes through the porous surface of the sorbent, which is modified with broad-spectrum antiseptic agents (active against bacteria, bacilli, fungi, and protozoa). X-ray diffraction analysis revealed the presence of amorphous carbon in a tubostratic structure, with a predominance of sp- and sp2-hybridized carbon atoms not incorporated into regular graphene lattices. IR spectroscopy demonstrated the presence of reactive functional groups characteristic of the developed porous structure of the material, which is capable of selective sorption of antiseptic molecules. SEM surface analysis revealed an amorphous texture with a loose structure and elements in the form of spherical semi-ring formations formed by overlapping carbon plates. An experimental setup was also developed using cylindrical multichannel carbon blocks with a diameter of 48 mm, a length of 120 mm, and 100–120 longitudinal channels with a cross-section of 1 mm2. The obtained results confirm the potential of the proposed material for use in air purification and disinfection systems under conditions of elevated biological risk.
1. Introduction
In the context of rapid urbanization and industrial growth, the issue of ensuring atmospheric air quality is becoming increasingly relevant. Air pollution is considered one of the most pressing environmental threats of our time, exerting a serious impact on public health, ecosystem conditions, and climate stability. According to the World Health Organization (WHO), millions of people suffer annually from diseases related to polluted air exposure, including acute and chronic respiratory disorders as well as cardiovascular diseases. Air pollution also contributes to an increase in premature mortality and leads to significant economic losses due to higher healthcare costs and reduced labor productivity [1,2,3,4].
Modern technologies for air purification from pathogenic factors include the use of filtration systems, catalysts, biofilters, and innovative solutions based on nanostructured materials [5,6,7]. The composition of atmospheric air constantly includes various pollutants, which are classified as mechanical, physical, and biological. Mechanical pollutants consist of solid particles of various origins—dust, soot, lead, mercury, and combustion products of organic fuels released during construction, mining, and other industrial activities [8]. Physical pollution is associated with the effects of thermal, noise, electromagnetic, and radioactive radiation. Biological pollutants are linked to anthropogenically induced proliferation of microorganisms in the air. The most toxic components of air pollution include carbon monoxide (CO), nitrogen oxides (NOx), sulfur dioxide (SO2), particulate matter, and various hydrocarbons [9].
With the development of technological lines in industrial enterprises, the construction sector, and mineral extraction facilities, sanitary and environmental requirements for air quality have become more stringent. This has necessitated updates to regulatory frameworks and the implementation of new standards [10]. In Europe, modern standards for evaluating the efficiency of air filters have been adopted, including EN 779:1993 and EN 1822:1998 [11,12], which regulate the performance characteristics of filters used in ventilation and air conditioning systems [13].
Special attention is given to air filtration in environments with limited ventilation, such as underground mining facilities, metro systems, and tunnel construction sites, where there is a risk of spreading pathogens of dangerous infectious diseases [14]. Ensuring biological safety for personnel in such conditions requires constant modernization of existing filtration systems and the development of new ones [15].
Among the most effective solutions is the use of filters based on fine-fiber materials, including glass and synthetic microfibers, metal ceramics, and ultrafine basalt fiber (fiber diameter: 0.1–0.4 µm) [16]. Optimization of the composition of filtering materials is based on evaluating their protective capacity using physicochemical and biological indicators. In microbiological laboratories, fibrous filters with a high specific surface area are traditionally used [17]. Currently, filters made from polyacrylonitrile fibers are industrially produced for the purification of process air.
Honeycomb carbon blocks are also used as filtering devices in medical and industrial applications [18]. The modern challenges posed by global pandemics and environmental degradation underscore the need for effective air purification technologies to eliminate pathogenic microorganisms. Airborne viruses and bacteria spread through aerosols and microscopic droplets, pose a significant health risk in high-density environments such as hospitals, offices, and public transport. Given the constant risk of infections, developing and utilizing air filters capable of capturing and neutralizing biological pollutants is of paramount importance [19,20].
One promising solution in this field is carbon filters, known for their high adsorption capacity. Activated carbon effectively absorbs volatile organic compounds and toxic gases while serving as a barrier against bacteria and viruses when impregnated with antiseptics. To enhance efficiency, carbon filters are often modified with antimicrobial components such as silver or copper ions, which help inactivate pathogens on their surface [21,22].
Sorption of carbon-containing materials is the process of absorbing or retaining gases, vapors, and dissolved substances on the surface or in the volume of carbon adsorbents. Due to the high specific surface area and developed porous structure, such materials are effectively used for air and water purification and in catalytic systems. Porous carbon materials with a high ability to absorb CO2 have been developed and play a key role in combating industrial air pollution and global warming [23]. SuhongRen and his colleagues fabricated hierarchical porous carbon materials using a facile and green method combined with air activation, and it was found that after air activation, the adsorption capacity of carbon materials was increased [24]. A number of studies have analyzed the effect of pore morphology and the chemical state of the surface on the adsorption characteristics of nanoporous carbon materials under both low and high relative pressures. The results obtained indicate that the porous structure predominantly determines the adsorption behavior at high relative pressures, while the surface properties have a significant effect on the adsorption processes in the region of extremely low relative pressures [25].
The results of studies conducted by a number of scientists have shown that the processes of phenanthrene sorption and desorption in soils and carbon-containing materials are characterized by a high degree of reversibility: the equilibrium isotherms practically coincide, which indicates the absence of pronounced hysteresis [26].
Carbonaceous material was synthesized by pyrolysis of sewage sludge, which has a high sorption capacity with respect to indigocarmine (92.83 mg/g), significantly surpassing zeolite material (32.83 mg/g) in this indicator [27].
Rice husk is one of the most abundant and readily available agricultural by-products generated during rice processing. In recent years, increasing attention has been paid to its sustainable utilization within the framework of waste-to-resource technologies. Contemporary studies highlight its potential as a raw material for the production of functional materials such as activated carbon, biochar, biosilica, and carbon-based nanocomposites. Research by Tsai et al. (2021) demonstrates the effectiveness of carbonization and activation techniques for producing highly porous adsorbents from rice husk, with a specific surface area exceeding 500 m2/g [28]. These materials show promise for applications in water purification and CO2 capture, offering the advantages of low production costs and environmental sustainability. Other studies report the synthesis of NiO@SiO2/carbon nanocomposites from rice husk, exhibiting catalytic activity in low-temperature CO oxidation [29]. Furthermore, a bioeconomic perspective is actively emerging. Rice husk can serve as a feedstock for biodegradable polymers with favorable life cycle assessment (LCA) indicators. Agricultural applications, including soil amendment and use in green infrastructure (e.g., green roofs), are also supported by evidence showing improved water retention and agronomic performance when rice husk is incorporated into substrates [30]. Similarly, a study published in Environmental Technology emphasized the potential of KOH-activated carbon derived from rice husk in capturing CO2 and removing organic pollutants from aqueous solutions [31].
Overall, rice husk demonstrates significant potential in environmental engineering as a source of valuable materials and a key component of the circular bioeconomy, contributing to pollution reduction and the enhancement of production system sustainability.
The aim of this study is to develop carbon–silicon filters for air purification from bacteria and to investigate their structure and ability to absorb the antiseptic substance iodine.
2. Materials and Methods
2.1. Materials
In the experimental part of this work, analytical-grade chemical reagents were used without additional purification. Carbon-based filtering materials were obtained by thermal carbonization of rice husk in a propane–butane gas mixture at temperatures ranging from 300 to 800 °C. The temperature regime in the tubular reactor was controlled using a programmable linear heating system from room temperature to 800 °C, with a holding time at the maximum temperature of up to 120 min.
Rice husk was chosen as the primary raw material due to its high content of organic and mineral components—namely cellulose, lignin, pentosans, and ash, the latter being composed of 92–97% silicon dioxide. This material is classified as a rapidly renewable and environmentally friendly biomass source. The chemical composition of the raw material, according to X-ray fluorescence (XRF) analysis, is presented in Table 1.

Table 1.
Chemical composition of rice husk.
The choice of rice husk as a raw material is based on its composition, which includes cellulose, lignin, and mineral ash containing 92–97% silicon dioxide. Rice husk is a rapidly renewable resource and an environmentally friendly material.
The following substances were also used in this study: hydrogen (Grade A, GOST 3022-80 [32]) with a composition of H2—99.92%, (O2, CH4, CO2, CO)—0.08%, and H2O—0.004%; propane; argon (special purity, GOST 10157-79 [33]) with Ar—99.992%, O2—0.0007%, and N2—0.006%; nitrogen with N2—96.30% and O2—3.70%; chlorhexidine bigluconate; and dihydrogen sulfide.
Iodine solutions with concentrations ranging from 50 to 340 mg/L, along with sodium thiosulfate (Na2S2O3) solutions at a concentration of 0.01 M, were used. Starch (C6H10O5)n and carboxymethyl cellulose (CMC) [C6H7O2(OH)3−x(OCH2COOH)x]n were employed as binding components for carbon carbonates. Sorption equilibria were established using 250 mL conical flasks.
2.2. The Characteristics of Materials
A carbon–silicon sorbent obtained by carbonizing rice husk was selected for the fabrication of the filtering block. This material is characterized by well-developed porosity, a high specific surface area, and a significant pore volume, as well as the presence of fine-dispersed particles of carbon and silicon dioxide. These combined factors contribute to its high sorption capacity with respect to pollutants of various types [34].
To shape the material into honeycomb-structured filtering blocks, a formulation was developed using carboxymethyl cellulose (CMC) as a binding component. By optimizing the ratio of carbonized rice husk to CMC, a plastic mass suitable for extrusion molding was obtained. The molded items were subsequently subjected to drying and thermal treatment to complete the carbonization process and stabilize the structure.
Further chemical treatment was carried out in a boiling sodium hydroxide solution, which ensured partial desilication and contributed to an increase in pore volume. Final physical activation of the material in a carbon dioxide atmosphere led to the formation of a hierarchical porous structure with well-developed macro- and microporosity, promoting effective interaction between the airflow and the active surface of the filtering material.
2.3. Testing the Efficiency of Filter Materials on a Bench Setup
The efficiency of the carbon–silicon filtering materials was evaluated using a custom-designed laboratory-scale test rig (Figure 1). The experimental setup consisted of a two-chamber airtight system made of plexiglass, with internal dimensions of 55 × 51 × 35 cm3.

Figure 1.
Technological process flow for the fabrication of a multi-channel carbon block.
Chlorhexidine was used as an antibacterial agent during the tests. The front panel of the setup was made of sheet polypropylene and had six openings with a diameter of 45 mm, into which samples of carbon–silicon sorbents (CSS) with a height of 15 cm were tightly fixed. The through-pore diameter of these sorbents ranged from 0.5 to 5.0 µm [35].
The setup included two functional blocks. The first block housed a fan connected to a DC power source with parameters of approximately U = 12 V and I = 0.7 A. Power was supplied from a 220 V AC mains via a stabilized power supply unit. The airflow generated by the fan was directed through a duct into the second block of the system.
The first block also contained a reservoir filled with a chlorhexidine solution, equipped with a low-frequency ultrasonic generator. When the generator was activated, aerosolized chlorhexidine was produced and carried by the airflow into the second block of the setup. There, the aerosol passed through the CSS filtering samples. Due to the mesoporous structure of the sorbents, which contain channels on the order of tens of nanometers, viral and bacterial agents were effectively retained on the surface and within the bulk of the material via mechanical filtration and adsorption.
The experimental results enable the assessment of the bactericidal and virucidal effectiveness of the tested filtering materials when exposed to contaminated air.
At the outlet of the carbon–silicon sorbent (CSS) filter elements, the air streams impregnated with chlorhexidine vapor form an aerosol with pronounced antiviral and antibacterial activity. This aerosol phase promotes the effective inactivation of pathogenic microorganisms in the air, preventing their spread beyond the filtration unit. The microporous structure of the CSS, with channel diameters ranging from 0.5 to 5.0 μm, ensures a reliable barrier function, preventing the release of viruses and bacteria and facilitating their sorption and inactivation.
The developed device can also be adapted for domestic and personal use as an autonomous disinfecting unit. In this case, chlorhexidine in the generator may be replaced with another disinfectant solution with a similar spectrum of activity.
It should be noted that one drawback of the basic model is the partial passage of airflow through the lateral surfaces of the filtering block, which may reduce the overall purification efficiency. To eliminate this drawback, a compact model of the carbon–silicon filtration device was developed (Figure 2), intended for individual or localized use. This design lacks a built-in ventilation system but includes a module of an ultrasonic mini-generator that ensures the dispersion of a chlorhexidine solution. The resulting aerosol passes through the CSS structure and is then released into the surrounding environment.
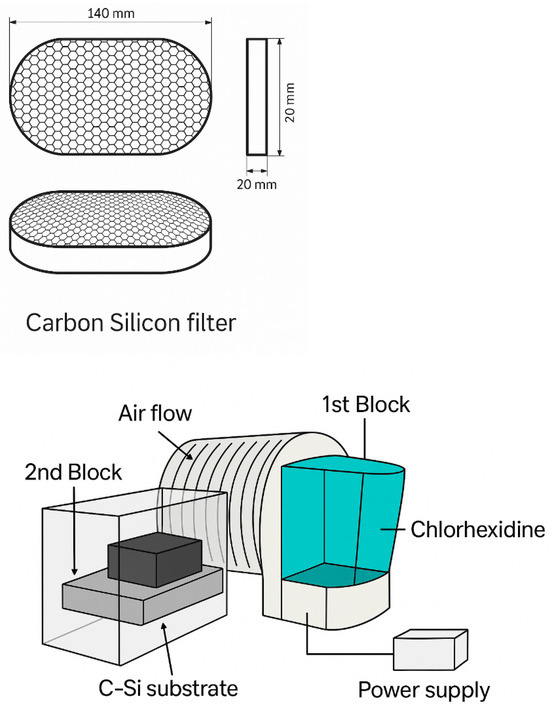
Figure 2.
Design of the test rig for studying the airflow through carbon-silicon sorbents.
The performance of the device depends on the volume of the filled solution and the generation parameters. Under typical operating conditions, a single charge of the device functions for 4 to 10 h. Thus, the miniature model can be used as a portable tool for antiseptic air treatment, serving as a localized antiviral and antibacterial barrier (Figure 3).
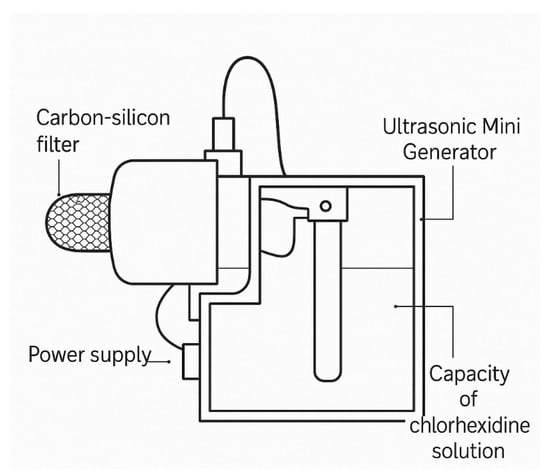
Figure 3.
Miniature design of the carbon–silicon sorbent for everyday use.
The conducted experiments showed that the developed carbon–silicon sorbents demonstrate high efficiency as matrices for the impregnation and distribution of antimicrobial agents, ensuring a stable and prolonged release of the disinfecting aerosol into the surrounding environment.
2.4. Characterization
Identification of the chemical composition, structure, and structural features of the carbon–silicon sorbents was carried out using the Raman scattering method (Raman spectroscopy) on an Ntegra Spectra spectroscopic system with an excitation laser wavelength of 514.5 nm.
The morphology and microstructure of the composite samples were studied using scanning electron microscopy (SEM). Rectangular samples approximately 2 mm thick were prepared for SEM imaging. The studies were conducted on a Quanta 3D 200i Dual System electron microscope. Additionally, a trinocular optical microscope (S/N XJP-146JBT) was used to evaluate microstructural features.
Functional groups on the surface of the samples were identified by infrared (IR) absorption spectra. IR spectra were recorded in the range of 700–3600 cm−1 using a PerkinElmer Spectrum 65 spectrometer. Spectra were also recorded in the range of 400–4000 cm−1 using a UV-750U spectrophotometer with demountable cuvettes (for liquid samples) and KBr pellets (for solid samples).
Porosity and textural characteristics of the composites were determined from nitrogen adsorption isotherms at 77 K, recorded using a 3H-2000PS surface area analyzer in the relative pressure range from 0.005 to 0.991. Prior to measurements, the samples were degassed at 200 °C under a residual pressure of less than 0.001 mmHg.
3. Results and Discussion
3.1. Spectroscopic Studies of Carbon Filter Parameters
The structural characteristics of the obtained carbon filters were studied using X-ray diffraction analysis (XRD). As expected, the XRD patterns revealed signs of structural heterogeneity, which can be attributed to the high mobility of graphene layers and weak van der Waals interactions between carbon atoms within the carbon framework.
Figure 4 shows typical diffractograms of the studied samples, illustrating the deformability of the graphite-like structure. Graphitic carbons are characterized by a combination of diffraction reflections of the 00l and hk types, corresponding to the interlayer (parallel) arrangement of graphene planes and their internal periodicity, respectively [36,37].

Figure 4.
X-ray diffractogram of graphite-like carbon materials.
The main structural parameters of the carbon framework are the average sizes of coherent scattering domains (CSDs), which are associated with the dimensions of graphite-like nanocrystallites. The diffractograms exhibit intense but significantly broadened peaks, indicating small CSD sizes, the presence of structural distortions, and defects in the carbon packing. In some cases, the peaks merge to form a single broad diffuse band accompanied by weak, graphite-like reflections with an amorphous appearance.
The overall diffraction pattern indicates a considerable presence of disordered (amorphous) carbon not incorporated into regular graphene domains. Such a structure is typical of highly activated carbon materials synthesized under conditions promoting partial degradation of graphene planes and the formation of a mesoporous amorphous–crystalline phase [38].
The obtained combined carbon material based on carboxymethyl cellulose (CMC) and rice husk char was studied using Raman spectroscopy (Figure 5).
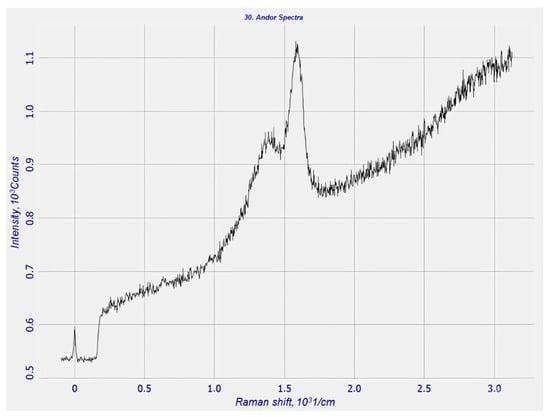
Figure 5.
Rice husk char with CMC after carbonization.
As seen in Figure 5, the rice husk char in the presence of CMC binder exhibits two characteristic peaks, indicating the formation of a new carbon compound with a combined carbon matrix consisting of both rice husk char and CMC.
These two peaks reflect the presence of carbonaceous morphology. It is important to note that during the carbonization process, carbon may also originate from the carboxymethyl cellulose. In other words, the carbon materials observed are embedded in a matrix derived from methylcellulose components, leading to the conclusion that carbon microcrystallites are formed. In chars of both natural and synthetic origin, carbon microcrystallites are arranged in a disordered fashion, forming what is known as a turbostratic structure [39]. In this structure, carbon atoms do not occupy ideal lattice positions but are displaced from the plane of the lattice by approximately 0.14–0.17 Å [40].
Such displacement is explained by the fact that in the turbostratic structure, carbon atoms are in a different valence state compared to the typical sp2 hybridization found in graphite. In this case, a portion of the carbon atoms—particularly those at the periphery—are in an sp-hybridized state.
Thus, it can be concluded that carbon materials are complex systems that differ in a range of mechanical, physicochemical, and other properties, which depend on the structure of the raw material and the methods used for its synthesis.
3.2. Inactivation Efficiency of the Impregnated Agent by the Carbon–Silicon Sorbent Material
The structure of carbon materials has a significant impact on the effectiveness of their activation for achieving specific properties. This influence can be realized through two main approaches: the selection of the precursor material based on its genetic type, or structural modification of the material using physical methods (e.g., grinding, irradiation) and chemical treatments. Identifying the active sites in sorbents and understanding the mechanisms underlying their formation enable the controlled synthesis of carbon sorbents with predefined characteristics.
One of the least explored yet promising areas of application for carbon materials derived from plant-based raw sources is their use as adsorbents. This is due to the unique surface properties of such materials, including the presence of reactive polyfunctional groups [28,41]. To evaluate the presence of reactive functional groups in carbon sorbents, Fourier-transform infrared (FTIR) spectroscopy was conducted. The FTIR spectroscopy results revealed key absorption bands:
- At 3500 cm−1, corresponding to O–H and C–H stretching vibrations typical for carbonaceous porous structures,
- At 2300 cm−1, related to CO2 stretching,
- At 1630 cm−1, indicative of C=C double bonds,
- At 1500 cm−1, corresponding to C–O bonds, and
- At 700 cm−1, denoting Si–C bonds.
These data confirm that the porous structure of the carbon sorbents contains mainly carbon-, oxygen-, and silicon-based structural units, which indicates the presence of functional groups potentially active in adsorption processes.
To assess the degree of activation of functional groups upon impregnation with bactericidal agents, FTIR spectroscopy was employed (Figure 6).
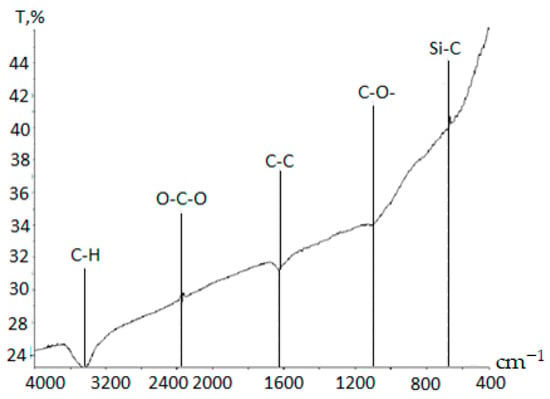
Figure 6.
FTIR spectrum of carbon–silicon microstructures.
To evaluate the degree of activation of functional groups in impregnated bactericides, further IR spectroscopy analysis was performed (Figure 7). The IR spectrum was recorded over a broad range of wave numbers (450–4000 cm−1). The spectrum intensity increases in the long-wave region due to the absorption of infrared radiation throughout the sample depth. FTIR spectroscopy results indicate that mono-hydrogen absorption, mainly from C–H bonds, is located at 3500 cm−1. In the high-frequency region, small oscillatory absorptions associated with short CH2–CH3 and C=C rotations appear. The absorption band at 1100 cm−1 reveals strong oxidation of carbon by oxygen-containing functional groups such as O=C=O and CO2. Due to this strong oxidation, the bands at 1600 cm−1 and 2400 cm−1, typically found in the low-frequency region, are absent [42].
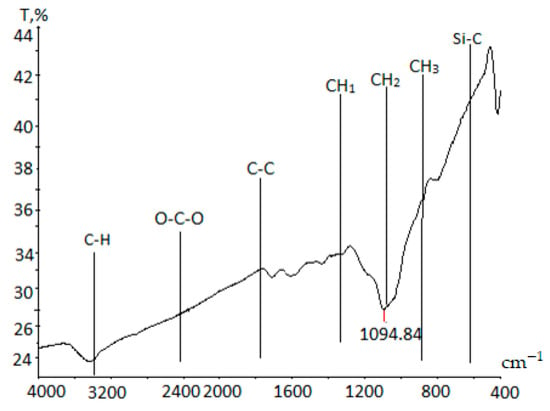
Figure 7.
IR spectrum of carbon–silicon sorbent impregnated with bactericides.
The increased oxygen content in the impregnated samples compared to non-impregnated carbonized materials is likely due to reactions between carbon and oxygen-containing fragments from chlorhexidine and tannin. Additionally, absorption bands near 1800 cm−1 and 1094 cm−1, which correspond to aromatic C=C bonds in olefinic or aromatic structures, suggest that the impregnation process significantly affects the textural and adsorption properties of the porous carbon material. The presence of CH1, CH2, and CH3 fragments in the spectrum suggests the introduction of organic compounds into the material, modifying its adsorption properties and selectivity.
From the IR spectrum analysis of the bactericide-impregnated rice husk (Figure 6), it is evident that C–H bonds from methyl and methylene fragments appear at 3500 cm−1, while CH2 bonds in cellulose molecules are observed at 1410 cm−1. The appearance of C=C bonds at 1775 cm−1 and 1094 cm−1, indicating oxidation by oxygen-containing groups (O–C–O), is attributed to the presence of tannin molecules (Figure 8).
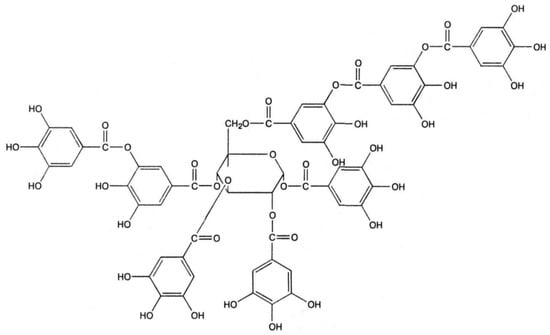
Figure 8.
Functional groups of tannin.
Thus, the degree of inactivation of functional groups impregnated with bactericides is influenced by the formation of a highly porous carbon structure. To further investigate the degree of impregnation and its impact on the structural properties of the filter material, scanning electron microscopy (SEM) analysis was conducted (Figure 9).

Figure 9.
SEM image of carbon–silicon sorbent impregnated with bactericides and after treatment with chlorhexidine.
SEM analysis revealed that the sample exhibited a highly developed porous surface. The microstructure displayed an amorphous, loosely packed arrangement with spherical semi-rings. The texture of the material consists of layered carbon plates. The introduction of antibacterial agents, such as chlorhexidine, into the filter material contributed to an increase in the number of pores and an overall improvement in structural organization.
In summary, IR spectroscopy confirmed the presence of carbonyl, carboxyl, and polyaromatic hydrocarbon functional groups in the impregnated sorbents. These reactive groups enhance the adsorption capacity of the carbon–silicon sorbents. Additionally, SEM analysis demonstrated that the introduction of chlorhexidine resulted in an amorphous structure with a well-developed surface, reinforcing the material’s potential for selective adsorption. The degree of inactivation of functional groups impregnated with antibacterial agents is directly correlated with the formation of a highly porous carbon structure.
3.3. Carbon Multi-Channel Air Purifying Blocks
The characteristic indicators of any filter include the following parameters: operational efficiency, defined as the ratio of the amount of captured dust to the total number of particles before the filter; the minimum particle fraction size that the device can fully capture; aerodynamic resistance; performance capacity; the maximum permissible value of aerodynamic resistance, which is taken into account when determining the operating time; dust-holding capacity, or the amount of contaminants that can be captured by the filter per unit volume or area; and durability.
Currently, the ventilation equipment market offers a wide range of models for air ducts of various shapes and cross-sections [19,20].
As a result, a multichannel block was developed with the following geometric dimensions: 48 mm in diameter and 120 mm in length. It is penetrated by 1000 longitudinal rectangular channels with a cross-sectional area of 1 mm2. Thus, the contact surface area of the channels in a single block is 480,000 mm2, or approximately half a square meter (Figure 10).

Figure 10.
Multichannel block.
The macroscopic structure of the block is clearly visible in Figure 10. The composition of the block material was determined using X-ray dispersion analysis (Figure 11).

Figure 11.
X-ray dispersion analysis of block material.
The analysis results indicate that the block is composed primarily of carbon and silicon oxide, with minor amounts of alkali and alkaline earth metal carbonates.
To evaluate the porous structure of the filter block material, low-temperature nitrogen adsorption analysis was conducted (Figure 12). This study utilized a 3H-2000 PS surface area analyzer after sample preconditioning at 200 °C under a residual pressure of less than 0.001 mmHg. Nitrogen adsorption isotherms were recorded at 77 K across a relative pressure range of 0.005 to 0.991, and the data were processed using the Brunauer–Emmett–Teller (BET) method. Parameters such as total pore volume (Vx), micropore volume (Vm), and average pore diameter (Da) were determined.

Figure 12.
Adsorption isotherm of a monoblock sample obtained by the differential–integral method.
This method is primarily suitable for analyzing micro- and mesopores and carries a margin of error of approximately ±10%. The total specific surface area of the micro- and mesoporous structure was determined to be 551 m2/g.
From the adsorption measurements, the micropore volume (≤1.69 nm) was 0.2007 mL/g, while the total pore volume (≤2.54 nm) was 0.2492 mL/g. To determine the total porosity, including macropores, a water immersion method was used. Although non-standard, this technique closely simulates real-world filter applications. The total pore volume, measured by fully soaking the sample in water under vacuum conditions, was 0.545 mL/g.
Thus, the impregnation capacity of the filter corresponds to its own mass, meaning that the filter can retain an amount of dissolved impregnant equal to its weight.
3.4. Impregnating Agents in Carbon Blocks
The selection of antiseptic substances for impregnation in air purification systems is extensive; however, their chemical and physical properties impose certain limitations on their practical application. Among the most commonly used antiseptic agents is iodine, which has been employed for over 150 years due to its proven effectiveness. Iodine solutions are widely recognized as common antiseptics in daily life. However, despite its broad antimicrobial properties, the use of iodine-based preparations for air disinfection has not been extensively documented in the literature.
A potential approach to addressing air purification challenges involves passing air through duct filters impregnated with iodine. Activated carbon serves as a suitable mate-rial for such filters, as its porous structure enables it to retain antiseptic solutions efficiently.
Preliminary studies have demonstrated the bactericidal activity of activated carbon samples impregnated with varying amounts of iodine, as summarized in Table 2.

Table 2.
Sorption of iodine from the gas phase at a temperature of 25 °C.
To investigate the feasibility of using activated carbon–silicon carbonized rice husk filters for air disinfection, iodine vapor sorption from a gaseous environment was analyzed. The experiment was based on the sublimation properties of iodine, which has a vapor pressure of 41.2 Pa at 25 °C in a closed volume. Sorption experiments were conducted using a hermetically sealed desiccator containing a multichannel monoblock carbon sorbent with a surface area ranging from 250 to 550 m2/g. The sorption process continued until the sorbents reached complete saturation with molecular iodine. The dependence of sorbent saturation with iodine on time at 25 °C and 41.2 Pa is illustrated in Figure 13.
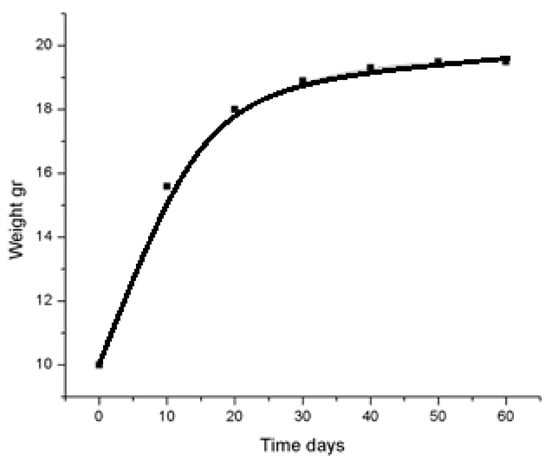
Figure 13.
Dependence of sorbent saturation with iodine on time.
From Figure 14, it can be observed that complete saturation of the sorbent occurs within approximately 400 h (16–17 days), reaching a maximum iodine sorption of 259 mg/g. The maximum sorption capacity was further examined under static conditions by placing a Petri dish with iodine crystals in a sealed desiccator alongside a glass bottle containing a pre-weighed sorbent sample.
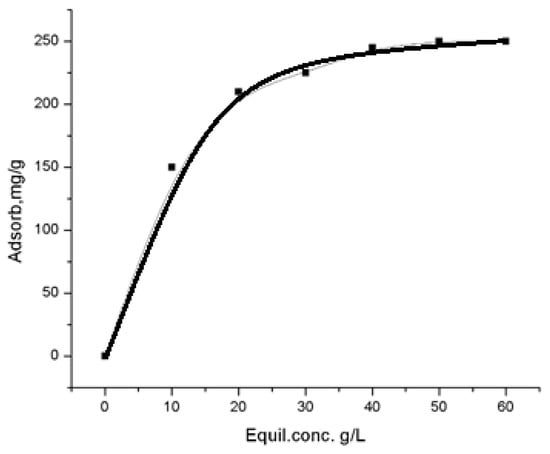
Figure 14.
Isotherm of iodine adsorption from aqueous solutions.
Additionally, the sorption of molecular iodine from aqueous solutions was evaluated, considering impregnation of the filter block material with aqueous iodine solutions [43]. Given that iodine has a solubility of 340 mg/L in water at 25 °C, a series of standard iodine solutions with concentrations ranging from 50 to 340 mg/L was prepared. The actual iodine concentration in these solutions was verified through titration with a 0.01 M sodium thiosulfate solution in the presence of starch. The solutions were then brought into contact with the sorbent (1 g per 100 mL of solution), and after reaching sorption equilibrium, the remaining iodine content was determined. These results facilitated the calculation of iodine adsorption per gram of sorbent, leading to the generation of a Langmuir adsorption isotherm (Figure 14).
After plotting the iodine adsorption data, the experimental results were fitted to the Langmuir adsorption isotherm model using the linearized form of the equation:
where Ce—is the equilibrium concentration of iodine (mg/L);
Ce/qe = 1/(Qm·Kl) + Ce/Qm
- qe—is the amount of iodine adsorbed per gram of sorbent at equilibrium (mg/g);
- Qm—is the maximum monolayer adsorption capacity (mg/g);
- KI—is the Langmuir constant related to the binding affinity (L/mg).
The resulting parameters are shown in Table 3.

Table 3.
Parameters of Langmuir adsorption isotherm.
The high R2 value indicates a good fit of the data to the Langmuir model, suggesting monolayer adsorption behavior of iodine on the sorbent surface.
Table 4 shows the comparative characteristics of the sorption capacity, surface area, and antimicrobial effectiveness of our filters with those of current commercial HEPA filters.

Table 4.
Comparative characteristics of filters.
As shown in Table 4, HEPA filters are effective in capturing solid particles ≥ 0.3 µm; however, they exhibit negligible sorption capacity for gases and vapors. Their antimicrobial properties are typically achieved through impregnation with agents such as Ag, Cu, or TiO2, or by applying antimicrobial coatings, which may increase production costs and pose environmental concerns. In comparison, carbonized rice husk impregnated with bactericidal agents such as chlorhexidine, tannin, or iodine exhibits a well-developed micro- and mesoporous structure, effectively suppresses bacterial growth, and represents a low-cost, environmentally sustainable alternative.
In addition to chlorhexidine, tannin powder was selected as an alternative impregnating agent. The effect of the carbonization process on the specific surface area and pore volume of the initial sorbent was analyzed, revealing that carbonization enhances the durability and sorption capacity of the material. The specific surface area and pore structure of carbon–silicon sorbents impregnated with tannin and chlorhexidine were measured.
The findings indicated that increasing the concentration of chlorhexidine and tannin in the sorbent composition enhances its specific surface area and pore volume. Impregnation led to an increase in the specific surface area from 320 to 350 m2/g, while the specific pore volume increased from 0.1256 to 0.1399 cm3/g. These results confirm that impregnation with antiseptic agents significantly improves the sorption properties of carbon–silicon materials, making them more effective for air purification applications.
4. Conclusions
In this study, the spectroscopic characteristics of carbon filters based on carboxymethyl cellulose (CMC) and rice husk-derived carbonizate were investigated. X-ray diffraction analysis revealed that the materials possess a complex structure characterized by graphite-like nanocrystallites with sizes ranging from 2 to 5 nm, as well as a significant degree of amorphous content—up to 60%. Raman spectroscopy confirmed the formation of a combined carbon matrix containing microcrystallites in a turbostratic structure. The D/G peak intensity ratio of 1.2 indicates the presence of structural defects within the material.
Fourier-transform infrared (FTIR) spectroscopy of the carbon–silicon sorbents demonstrated the presence of reactive functional groups, such as hydroxyl (-OH), carboxyl (-COOH), and silanol (-Si-OH) groups, which enhance the adsorption of impregnating agents. It was established that impregnation with chlorhexidine and tannin leads to an increase in the specific surface area from 450 to 620 m2/g and an enhancement of the sorption capacity for methylene blue from 200 to 320 mg/g. Moreover, the modification of the sorbents promotes the formation of a selective porous structure dominated by mesopores with diameters ranging from 2 to 10 nm.
The obtained results indicate a high sorption capacity of the developed materials, making them promising candidates for use as filtering and adsorbing components. Their unique structural and physicochemical characteristics contribute to their efficiency in sorption processes. Future research should focus on optimizing synthesis conditions and investigating the kinetics of pollutant adsorption, which would enable the expansion of their application in water purification systems and environmental protection.
Author Contributions
Conceptualization, M.T.; Methodology, M.T., Z.K., D.B. and R.H.; Software, K.U., D.B. and R.H.; Validation, M.T., Z.M., Y.M. and R.H.; Formal analysis, M.T., Z.K. and L.R.S.; Investigation, Z.M.; Resources, Z.K., A.M. and D.B.; Data curation, Z.K., Z.M., A.M., D.B. and D.M.; Writing—original draft, A.M. and D.M.; Writing—review and editing, Z.K., A.M. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP19676747).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ish, J.L.; Chang, C.J.; Bookwalter, D.B.; Jones, R.R.; O’Brien, K.M.; Kaufman, J.D.; Sandler, D.P.; White, A.J. Outdoor air pollution exposure and ovarian cancer incidence in a United States-wide prospective cohort study. Environ. Health Perspect. 2024, 132, 107701. [Google Scholar] [CrossRef]
- Chernobrovkin, I.A.; Smirnova, L.V. Air purification from suspended particles: Methods and technologies. Bull. Environ. Saf. 2020, 4, 45–52. [Google Scholar]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B.; Sun, C. Advances in air purification technologies and challenges for the future. Environ. Sci. Technol. 2018, 52, 12532–12548. [Google Scholar]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Zhang, Q.; He, K.; Huo, H. Cleaning China’s air: Prioritizing measures to reduce particulate pollution. Environ. Sci. Technol. 2012, 46, 6521–6522. [Google Scholar]
- Sheoran, K.; Siwal, S.S.; Kapoor, D.; Singh, N.; Saini, A.K.; Alsanie, W.F.; Thakur, V.K. Air Pollutants Removal Using Biofiltration Technique: A Challenge at the Frontiers of Sustainable Environment. ACS Eng. Au. 2022, 2, 378–396. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K. Combustion in the future: The importance of chemistry. Proc. Combust. Inst. 2020, 38, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E.; Sayadi, H. Recent advances in properties and applications of nanoporous materials and porous carbons. Carbon Lett. 2022, 32, 1645–1669. [Google Scholar] [CrossRef]
- Roy, A.; Mishra Ch Jain, S.; Solanki, N. A review of general and modern methods of air purification. J. Therm. Eng. 2019, 5, 22–28. [Google Scholar] [CrossRef]
- EN 779:1993; Particulate Air Filters for General Ventilation—Requirements, Testing, Marking. European Committee for Standardization (CEN): Brussels, Belgium, 1993.
- EN 1822:1998; High Efficiency Air Filters (HEPA and ULPA)—Part 1: Classification, Performance Testing, Marking. European Committee for Standardization (CEN): Brussels, Belgium, 1993.
- EN 1822:2009; High Efficiency Air Filters-Part 1: Classification, Performance Testing, Marking and Product Documentation. European Committee for Standardization: Brussels, Belgium, 2009.
- Niza, I.L.; Bueno, A.M.; da Silva, M.C. Evandro Eduardo Broday aAir quality and ventilation: Exploring solutions for healthy and sustainable urban environments in times of climate change. Results Eng. 2024, 24, 103157. [Google Scholar] [CrossRef]
- Mutushev, A.; Kaya, A.; Tulepov, M.; Kudyarova, Z.; Baiseitov, D.; Mukhanov, D. Design and Development of Carbon–Silicon-Based Air Purification Filters with Antibacterial Properties. Processes 2025, 13, 662. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chiang, P.C.; Huang, C.P. Effect of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Yang, S.; Shi, C.; Qu, K.; Sun, Z.; Li, H.; Xu, B.; Huang, Z.; Guo, Z. Electrostatic self-assembly cellulose nanofibers/MXene/nickel chains for highly stable and efficient seawater evaporation and purification. Carbon Lett. 2023, 33, 2063–2074. [Google Scholar] [CrossRef]
- Nuraly, A.; Mutushev, A.; Tuleibayeva, A.; Gonzalez-Leal, J.M. Experimental research on optimizing carbon materials for filtration applications in medicine. Carbon Trends 2024, 15, 100338, ISSN 2667-0569. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H. The use of carbon-based filters in air purification to combat airborne viral particles: A review. J. Environ. Chem. Eng. 2022, 10, 108238. [Google Scholar] [CrossRef]
- Afshinnekoo, E.; Meydan, C.; Chowdhury, S.; Jaroudi, D.; Boyer, C.; Bernstein, N.; Maritz, J.M.; Reeves, D.; Gandara, J.; Chhangawala, S.; et al. Geospatial Resolution of Human and Bacterial Diversity with City-Scale Metagenomics. Cell Syst. 2015, 1, 97–97.e3, Erratum in Cell Syst. 2015, 1, 72–87. [Google Scholar] [CrossRef]
- Gupta, S.; Gopal, A. Activated carbon-based air filters for virus removal: Mechanisms and efficiency. Mater. Today Proc. 2020, 30, 953–958. [Google Scholar] [CrossRef]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, J.H.; Bao, A. B/N codoped porous carbon materials with rich microporous structure and their CO2 adsorption properties. Biomass Convers. Biorefinery 2023, 14, 31157–31168. [Google Scholar] [CrossRef]
- Ren, S.; Deng, L.; Zhang, B.; Lei, Y.; Ren, H.; Lv, J.; Zhao, R.; Chen, X. Effect of air oxidation on texture, surface properties and dye adsorption of wood-derived porous carbon materials. Materials 2019, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Park, J.S.; Sok, J.H.; Reucroft, P.J. Effect of pore structure and surface state on the adsorption properties of nano-porous carbon materials in low and high relative pressures. Appl. Surf. Sci. 2005, 246, 77–81. [Google Scholar] [CrossRef]
- Wang, G.; Kleineidam, S.; Grathwohl, P. Sorption/desorption reversibility of phenanthrene in soils and carbonaceous materials. Environ. Sci. Technol. 2007, 41, 1186–1193. [Google Scholar] [CrossRef]
- Gutiérrez-Segura, E.; Solache-Ríos, M.; Colín-Cruz, A. Sorption of indigo carmine by a Fe-zeolitic tuff and carbonaceous material from pyrolyzed sewage sludge. J. Hazard. Mater. 2009, 170, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Lin, Y.-Q.; Huang, H.-J. Valorization of Rice Husk for the Production of Porous Biochar Materials. Fermentation 2021, 7, 70. [Google Scholar] [CrossRef]
- Dien, L.X.; Luque, R. Rice Husk Valorization into NiO@SiO2/Carbon Nanocomposites for Low-Temperature CO Oxidation: Effect of Surface Area and Ni3+ Cations. ACS Sustain. Chem. Eng. 2021, 40, 13681–13685. [Google Scholar] [CrossRef]
- Yeboah, W.O.; Kwofie, E.M.; Wang, D. Circular bioeconomy potential of rice husk as a bioplastic resource: Techno-environmental assessment. Bioresour. Technol. Rep. 2022, 20, 101248. [Google Scholar] [CrossRef]
- Kaya, N.; Carus Özkeser, E.; Yıldız Uzun, Z. Investigating the effectiveness of rice husk-derived low-cost activated carbon in removing environmental pollutants: A study of its characterization. Int. J. Phytoremediation 2024, 26, 427–447. [Google Scholar] [CrossRef]
- GOST 3022-80; Hydrogen for Industrial Use: Specifications. Standards Publishing House: Moscow, Russia, 1980.
- GOST 10157-79; Gaseous and liquid Argon: Specifications. Standards Publishing House: Moscow, Russia, 1979.
- Mutushev, A.; Nuraly, A.; Kaya, A.; Mukhanov, D. Development and application of microcapsules based on rice husk and metallurgical sludge to improve soil fertility. Sci. Rep. 2025, 15, 78. [Google Scholar] [CrossRef]
- Logothetis, D.D.; Martinez-Welles, J.M. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J. Am. Dent. Assoc. 1995, 126, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Li, X. Carbon-based materials as antimicrobial agents in air filtration: Recent advances and applications. Environ. Sci. Technol. 2019, 53, 7310–7323. [Google Scholar]
- Shrestha, P.M.; Humphrey, J.L. Airborne transmission and the role of air purification systems: Evaluating activated carbon filters. Indoor Built Environ. 2022, 31, 1729–1740. [Google Scholar]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef]
- Nuraly, A.; Akhnazarov, S.; Apaydin-Varol, E.; Amzeyeva, U.; Mutushev, A. Comparative analysis of hemosorbents obtained at different modes. Rev. Mater. 2020, 25, e-12893. [Google Scholar] [CrossRef]
- Cuadros-Lugo, E.; Piñon-Espitia, M.; Martinez-Rodríguez, H.A.; Lardizabal-Gutierrez, D.; Estrada-Guel, I.; Herrera-Ramirez, J.M.; Carreño-Gallardo, C. Turbostratic Carbon/Graphene Prepared via the Dry Ice in Flames Method and Its Purification Using Different Routes: A Comparative Study. Materials 2022, 15, 2501. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, M.; Suresh Babu, R.; Sivasamy, A.; de Barros, A.L.F. Biomass-derived porous activated carbon nanofibers from Sapindus trifoliatus nut shells for high-performance symmetric supercapacitor applications. Carbon Lett. 2021, 31, 1133–1143. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; Sun, K.; Xie, X.; Hu, Y. Surface modification, characterization and adsorptive properties of a coconut activated carbon. Appl. Surf. Sci. 2012, 258, 8247–8252. [Google Scholar] [CrossRef]
- Elmekawy, A.; Quach, Q.; Abdel-Fattah, T.M. Synthesis and Characterization of Silver-Modified Nanoporous Silica Materials for Enhanced Iodine Removal. Nanomaterials 2024, 14, 1143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).