Abstract
This study demonstrated the successful recovery of zinc, lead, and copper collective concentrates from historical metal-bearing mine tailings (sulfide–polymetallic ore with a composition of 7.38% Zn, 1.45% Pb, and 0.49% Cu) using froth flotation techniques, which were originally developed during uranium ore mining. Froth flotation techniques were used to justify suitability for recovering metals. The effects of a dosage of the foaming agent Polyethylene glycol (PEG 600) at 50 and 100 g t−1, collector types Aerophine 3418A (AERO), Danafloat 067 (DF), and potassium ethyl xanthate (KEX) at 50 and 80 g t−1, and a suspension density of 300 and 500 g L−1 on froth flotation collective concentrates were investigated. The final collective concentrate achieved recoveries exceeding 91% for lead (Pb), 88% for copper (Cu), and 87% for zinc (Zn). The obtained concentrates were analyzed using Atomic Absorption Spectroscopy (AAS) and X-ray Fluorescence Spectrometry (XRF), while selected samples were further examined via Scanning Electron Microscopy (SEM) with Energy Dispersive X-ray Spectroscopy (EDS). The resulting sulfide concentrates can subsequently be treated using suitable hydrometallurgical techniques. The application of these concentrates in metal production would help reduce the environmental burden of mining activities.
1. Introduction
The global mining industry faces a significant challenge with the depletion of high-grade ore deposits, which has increased the reliance on low-grade ore stockpiles to meet the rising demand for valuable metals. These stockpiles, often considered waste in the past due to their low metal content, are now becoming crucial sources of metals such as zinc, lead, copper, and other strategic minerals [1,2].
Historically, base metals such as zinc, lead, and copper have been efficiently recovered from sulfide ores using froth flotation. However, the rising global demand for these metals, combined with the depletion of high-grade ores, has driven the mining industry toward processing lower-grade and more complex ores, including oxidized and partially oxidized deposits [3,4]. These ores pose significant challenges due to their altered mineralogy and surface chemistry, which reduce flotation efficiency. Conventional flotation techniques often result in poor recoveries when applied to oxidized base metal ores. This is largely due to the reduced hydrophobicity of oxidized minerals and the limited effectiveness of standard reagents. As a result, research has increasingly focused on advanced flotation strategies such as sulfidization and novel collectors to improve metal recoveries from these more problematic resources [5,6,7,8].
Zinc, lead, and copper are critical non-ferrous metals extensively used across various industries (including automotive, construction, batteries, electronics, and coatings) due to their distinct physical and chemical properties. Zinc is primarily extracted from sphalerite (ZnS); lead is obtained mainly from galena (PbS); and copper typically occurs in minerals such as chalcopyrite (CuFeS2), which is often found alongside lead–zinc ores in polymetallic deposits [9,10,11,12,13,14,15].
Flotation is a fundamental and extensively utilized separation technique in the field of mineral processing, playing a vital role in the beneficiation of a wide range of ore types. As a physicochemical method, flotation enables the selective separation and concentration of valuable minerals from gangue, thus producing high-grade concentrates that serve as essential feedstock for downstream metallurgical operations such as smelting, hydrometallurgy, and electrorefining [13,16,17]. This process is especially important for the pre-treatment of ores containing non-ferrous metals, precious metals, and industrial minerals, making it a foundation of modern mineral processing operations. The effectiveness of flotation, however, is influenced by a range of mineralogical and chemical factors, including oxidation. Oxidation processes (natural or induced) can substantially modify key physicochemical properties of minerals. These include changes in surface chemistry, crystal structure, zeta potential, and elemental composition, all of which can affect reagent adsorption and, consequently, flotation selectivity and recovery efficiency. Understanding and controlling these oxidation effects is crucial for optimizing flotation performance, particularly in the processing of complex or low-grade ores [18,19,20].
In conclusion, the strategic processing of historical stockpiles and mine waste materials containing low concentrations of valuable base metals such as zinc, lead, and copper represents a growing area of interest in sustainable mineral resource management. Sustainable management of mineral resources and their waste streams is crucial for addressing environmental challenges, reducing the ecological footprint, and supporting the transition to low-carbon economies by recovering valuable materials from mine tailings. The recovery of metals from mining waste can also help minimize environmental risks and achieve a circular economy model. Hydrometallurgical processing yielded more than 87% zinc in sulfuric acid [21,22,23]. The central objective of this study is to evaluate and optimize mineral processing techniques capable of economically and efficiently recovering these metals from secondary sources, which have traditionally been underutilized due to their complex mineralogy and lower grades. Froth flotation emerges as the most suitable and adaptable method, owing to its ability to selectively separate sulfide minerals from gangue under variable conditions of reagent dosage and slurry density.
The application of flotation to low-grade polymetallic tailings not only enables the recovery of residual metals that would otherwise remain unrecovered but also contributes to the reduction of environmental liabilities associated with tailing storage facilities.
The novelty of this research lies in its systematic investigation of froth flotation process optimization tailored specifically for low-grade, polymetallic tailings, which are typically characterized by complex mineral associations. Unlike previous studies that often focus on either reagent selection or process parameters in isolation, this study uniquely integrates the optimization of collector type, reagent dosage, slurry density, and frother concentration to maximize recovery and concentrate grade across multiple metals, such as zinc, lead, and copper. Special emphasis is placed on the comparative performance of specific collectors, under varying conditions, providing new insights into their selectivity and effectiveness in multi-metal systems. This comprehensive approach provides a novel framework for enhancing flotation efficiency under suboptimal ore conditions, offering practical guidelines for the economic reprocessing of stockpile tailings, a crucial step forward as high-grade ore reserves diminish and the demand for critical metals intensifies globally.
2. Materials and Methods
2.1. The Examined Sample
The raw material utilized in this study was sourced from polymetallic mine waste stockpiles located in the Příbram mining district of the Czech Republic. These stockpiles are remnants of historical mining operations, which were primarily focused on uranium and base metal extraction [24,25]. Over decades of mining activity, a substantial volume of material accumulated, forming extensive surface deposits that now represent a potential secondary resource for valuable metals.
The total mass of these stockpiles is estimated at approximately 39 million tons, of which around 380,000 tons were classified as sulfide polymetallic ore based on historical and recent geochemical assessments. The sample investigated was sulfide polymetallic ore (polymetallic tailings), which was obtained during the mining of uranium ore. This material contains notable concentrations of lead (Pb) and zinc (Zn), alongside economically significant quantities of critical raw materials (CRMs), including copper (Cu), niobium (Nb), tantalum (Ta), and cobalt (Co).
2.2. Sample Preparations
The polymetallic tailings from uranium mining were stockpiled. A random sampling procedure was then used to obtain a gross sample, which was then adjusted to a representative sample, delivered to our laboratories.
Due to the fine dissemination and intimate association of valuable minerals with gangue, it is essential to liberate the minerals prior to separation. The raw material used in this study was received in a particle size range of 0–8 mm. Initial comminution was carried out in two stages. Primary crushing was completed using a jaw crusher BB500 (Retsch GmbH, Haan, North Rhine-Westphalia, Germany). Grinding was performed using a laboratory ball mill TM 500 (Retsch GmbH, Haan, North Rhine-Westphalia, Germany). The material originated from a stockpile (not from flotation tailings); it represented run-of-mine ore that had not undergone prior beneficiation. Consequently, the larger particle sizes present in the stockpile were less susceptible to oxidation, primarily due to their lower specific surface area, which reduces exposure to atmospheric conditions over time.
2.3. Particle Size Measurment
The particle size distribution of the sample was determined using a Malvern Mastersizer 3000 laser diffraction particle size analyzer (Malvern Instruments Ltd., Malvern, Worcestershire, UK). For the analysis, a representative subsample was prepared by dispersing the material in demineralized water to achieve a pulp concentration of approximately 30 wt.%, maintained at room temperature. The suspension was agitated at 690 rpm for 10 min to ensure complete dispersion of the mineral particles. Each sample was measured in triplicate, and the results from a total of 15 measurements were averaged to determine the final particle size distribution.
The particle size distribution (PSD) of the ore, obtained through Mastersizer analysis, is represented in Figure 1. According to the laser scattering data, the particle size distribution of the sample ranged from 0.1 to 1000 μm, with a P80 of 128 μm and a P50 less than 41 μm.
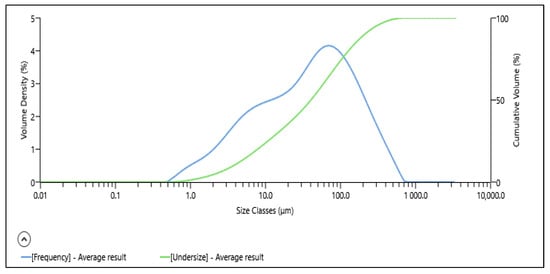
Figure 1.
Particle size distribution of the ore.
2.4. Chemical Analysis
Samples were pulverized to achieve a grain size with 85% passing below 0.075 mm. The resulting mineral concentrates were analyzed using accredited analytical protocols specific to each concentrate type. Quantitative elemental analysis was performed using a combination of X-ray fluorescence spectrometry (XRF) (SPECTRO Analytical Instruments GmbH, Kleve, Germany) and atomic absorption spectroscopy AAS (iCE 3300 AA-ThermoFisher Scientific, Grand Island, NY, USA), depending on the elemental composition and detection requirements.
The chemical analysis of raw ore is presented in Table 1 and indicates that the ore is a valuable secondary raw material, particularly due to its content of zinc, lead, and copper. When compared with primary raw materials, it is worth noting that copper ores generally contain between 0.5% and 2% Cu; commercially significant lead ores typically have a lead content ranging from 3% to 10%, with an average of 3.4% Pb; zinc ores usually contain 5% to 15% Zn; and lead–zinc ores typically comprise 1% to 5% Pb and 1% to 10% Zn [26,27,28].

Table 1.
Chemical analysis of the sample ore.
2.5. SEM–EDX Analysis
Selected samples and feed were characterized by the scanning electron microscope (SEM) (MIRA 3 FE-SEM microscope TESCAN, Brno, Czech Republic) equipped with a high-resolution cathode (Schottky field emitter) and with a three-lens Wide Field OpticsTM design and Energy-dispersive X-ray detector (EDX) (Oxford Instruments, Abingdon, UK).
The morphology of the particles of the ore made by the SEM method is documented in Figure 2a and the result of EDX analysis in Figure 2b. The results of the surface EDX analysis show similar values to the ore composition determined by the AAS method. The presence of copper, lead, and iron was below the detection limit of determination. The above implies the presence of metals in the form of sulfides.
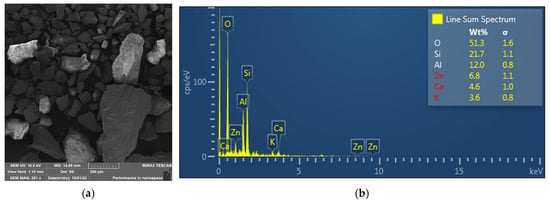
Figure 2.
Results of SEM analysis of the ore sample (a); EDX analysis of the ore (b).
2.6. XRD Analysis
Powder X-ray diffraction (XRD) analysis was performed using a Panalytical X’Pert PRO MPD diffractometer equipped with a Co anode (λKα = 0.17902 nm), an RTMS detector (X’Celerator), and fixed divergence slits in conventional reflection geometry. The measurement parameters included a step size of 0.033° 2θ, time per step of 160 s, and a scanning range of 5–100° 2θ, resulting in a total acquisition time of 3563 s per scan.
The diffraction data was processed using Malvern Panalytical HighScore 5.2 Plus and Bruker AXS DIFFRAC Plus Topas 4 software. (Semi) quantitative phase analysis was conducted using the Rietveld refinement method, with quantification performed for crystalline phases only.
Qualitative phase analysis of the ore sample prior to froth flotation was conducted using X-ray diffraction (XRD), with the resulting diffraction patterns presented in Figure 3. The mineralogical composition revealed that the ore primarily consists of sulfide minerals, which are known to be amenable to froth flotation techniques.
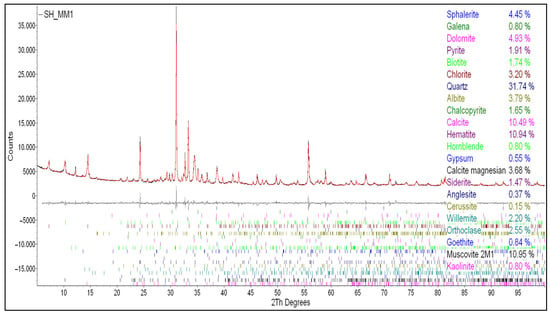
Figure 3.
XRD spectrum of the ore sample.
Specifically, zinc was identified in the form of sphalerite (ZnS), lead as galena (PbS), and copper as chalcopyrite (CuFeS2). These sulfide minerals are commonly processed using froth flotation due to their surface chemistry. The presence of these sulfide phases confirms that the ore is well-suited for flotation-based beneficiation. It is also known that the origin of selenide mineralization in the Příbram uranium district occurred between the calcite–uraninite and carbonate–sulphidic stages [25].
In addition to the economically valuable sulfides, several gangue minerals were detected. These included albite (NaAlSi3O8), representing aluminum-bearing phases, quartz (SiO2) as the primary silicate component, pyrite (FeS2) as an iron sulfide frequently associated with base metal ores, and calcite (CaCO3) as a carbonate phase. While these gangue minerals were not targeted for recovery, their identification is important for understanding the mineralogical complexity of the ore and for optimizing flotation selectivity and reagent schemes.
2.7. Processes of Froth Flotation
Flotation experiments were conducted using an automated laboratory froth flotation machine equipped with a 2 L Denver-type flotation cell (Outotec GTK LabCell™ Flotation Machine, Metso, Espoo, Finland).
The experiments were performed at a constant temperature of 25 °C, with the slurry containing varying solids concentrations depending on test requirements. After transferring a pre-weighed amount of sample into the flotation cell, agitation was initiated at a speed of 1100 rpm. Reagents were subsequently added, and the slurry was conditioned for a duration ranging from 5 to 25 min, based on the type and number of reagents used in each individual test. Following the conditioning period, air was introduced into the cell at a flow rate of 5 L·min−1, electronically controlled to ensure consistency across all experiments. After flotation, the concentrates were collected, dried, and assayed for Zn, Pb, and Cu using atomic absorption spectroscopy (AAS), and metal recoveries ε in the concentrates were calculated using Equation (1).
where C and F represent the dried weights of the flotation concentrate and feed, respectively, whereas c and f are the metal grades in the concentrate and feed, respectively.
ε = Cc/Ff × 100 (%)
The following reagents were used in all flotation experiments: polyethylene glycol (PEG 600) as the frother and potassium ethylxanthate (KEX), Aerophine 3418A (Aero, Sodium diisobutyldithiophosphinate), and Danafloat 067 (DF) (a water-soluble salt of aryl dithiophosphoric acid) as collectors. Detailed information about the reagents used is provided in Table 2.

Table 2.
Reagents used for froth flotation fests.
Given that the flotation process was aimed at producing a collective concentrate containing multiple valuable sulfide minerals, the use of copper sulfate (commonly employed as an activator for sphalerite) was omitted. This approach was chosen to avoid selective activation and instead promote the simultaneous recovery of zinc, lead, and copper into a single concentrate.
The design of experiments (DoE) for flotation tests using the ore is presented in Table 3; with the yield of each test, the yield of concentrate refers to the mass percentage of the final concentrate obtained from the total mass of the feed material.

Table 3.
Design of experiments + yield of concentrate and waste (wt.%).
The basic technological scheme of the flotation process is shown in Figure 4.
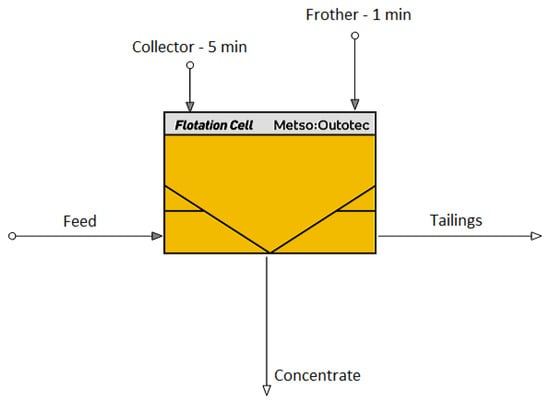
Figure 4.
The basic technological scheme of flotation experiments, made by HSC Chemistry 10 software (10.5.4 vision), Modul HSC Sim [29].
3. Results and Discussion
3.1. Forth Flotation Tests
Froth flotation experiments were conducted using automated flotation, as was described in the methodology section. All adjustable parameters on the flotation unit were standardized and kept constant across all experiments to ensure comparability. The primary variables evaluated for their influence on flotation performance were slurry density and reagent dosage. The corresponding results are summarized in Table 4. All the flotation tests were done in duplicate to ensure reproducibility. The results presented in Table 3 represent the average values from duplicate runs.

Table 4.
Results of experiments.
3.1.1. Effect of Reagents and Flotation Parameters on Zinc Recovery in Collective Concentrate
For all flotation experiments executed with the ore, metal recoveries were calculated individually for each target metal. Following flotation, the mass of froth (concentrate) products was collected, and the product yield was determined accordingly. Based on the analytical results, grade–recovery curves were constructed to illustrate the metallurgical performance under varying flotation conditions (Figure 5a,b).
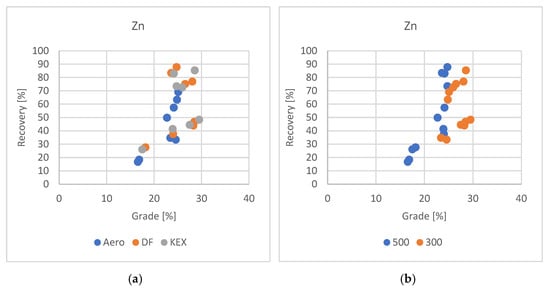
Figure 5.
Grade/recovery diagram of zinc results with respect to collector used (a) and grade/recovery diagram of zinc results with respect to slurry density (b).
The effects of frother dosage, type of collector, dosage of collector, and slurry density on froth flotation concentrates were examined.
The experimental results indicate that DF and KEX are the most effective collectors for zinc flotation, exhibiting comparable recovery efficiencies, as reported in previous studies. The mixture of auxiliary collectors of Aero 3477, Aerophine 3418, and Florrea 2255 with Potassium Amyl Xanthate (PAX) improved Zn recovery in comparison with the test in which PAX was added alone in the Zn rougher flotation stage [30,31]. The zinc concentrate grades produced with these reagents were also closely aligned. Moreover, slurry density was observed to significantly influence flotation outcomes: lower densities generally improved concentrate grade, although this was accompanied by a reduction in overall zinc recovery.
Flotation tests conducted with a collector DF dosage at 50 g·t−1, combined with a high frother concentration and increased slurry density, resulted in a zinc recovery of 87.76% and a concentrate grade of 24.76% Zn. These conditions suggest that the cooperation between collector and frother dosages, along with a denser pulp environment, enhances bubble–particle interactions and promotes more efficient zinc attachment and transport to the froth phase. The relatively high zinc grade achieved under these conditions also indicates improved selectivity, with reduced entrainment of gangue minerals.
3.1.2. Effect of Reagents and Flotation Parameters on Lead Recovery in Collective Concentrate
The flotation results for lead were more pronounced compared to those for zinc, exhibiting clearer trends and greater consistency. Among the tested collectors, Aero and DF demonstrated the highest effectiveness, consistently achieving the highest concentrate grades along with substantial recovery rates across multiple tests. Additionally, the influence of slurry density on lead flotation performance was evident, with higher slurry densities proving more favorable for maximizing lead recovery (Figure 6a,b).
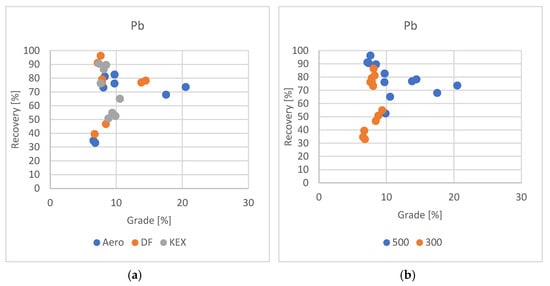
Figure 6.
Grade/recovery diagram of lead results with respect to collector used (a) and grade/recovery diagram of lead results with respect to slurry density (b).
Under the same flotation conditions—utilizing a DF collector dosage of 80 g·t−1, high frother concentration, and increased slurry density—lead recovery reached 91% with a corresponding concentrate grade of 7.21% Pb, representing one of the most balanced outcomes in terms of both recovery and grade. When the DF dosage was further increased beyond 80 g·t−1, an even higher lead recovery of 96% was achieved, accompanied by a slightly improved grade of 7.65% Pb. These results suggest that lead flotation performance is highly responsive to collector dosage, with increased DF concentrations enhancing the hydrophobicity of lead-bearing particles and promoting more efficient attachment to air bubbles. Numerous studies have explored the concept that for large particles, collision efficiency is higher under immobile surfaces, while for small particles, it is lower compared to mobile surfaces [32,33].
Higher slurry density (500 g·L−1) significantly improved lead recovery in this study. The denser pulp enhanced particle–bubble collision frequency, which benefitted well-liberated galena (PbS). The authors [34] investigated the use of ultrasound and KEX to enhance PbS flotation, achieving 77.5% recovery at 30 W power and 2 min of conditioning, while improving hydrophobicity, as confirmed by more negative zeta potential and reduced bubble–particle attachment time.
While it was technically possible to obtain significantly higher concentrate grades—up to 20% Pb—this improvement came at the expense of substantially reduced recovery rates, reflecting the inherent compromise between achieving higher concentrate purity and maintaining high recovery rates. This is often observed in flotation systems where increased selectivity leads to the rejection of valuable particles that are less hydrophobic or more finely disseminated.
Notably, the concentrate grade can be further improved during the cleaning stage of the froth flotation process, where additional separation steps help remove entrained gangue material and enhance the purity of the product. Implementing multi-stage cleaning or regrinding of intermediate products could serve as an effective strategy to achieve higher lead grades while minimizing recovery losses. These findings underscore the importance of optimizing reagent dosages and flotation circuit design to achieve the desired balance between metal recovery and concentrate quality.
3.1.3. Effect of Reagents and Flotation Parameters on Copper Recovery in Collective Concentrate
Copper recoveries exhibited the most favorable performance when using the DF collector, with experimental results consistently indicating high efficiency under optimized flotation conditions, as shown in Figure 7a,b. In the best-performing test, copper recovery exceeded 95%, with multiple tests achieving recoveries above 90%, demonstrating the strong affinity of DF for copper-bearing minerals, particularly chalcopyrite (CuFeS2), which was identified as the primary copper phase in the ore.
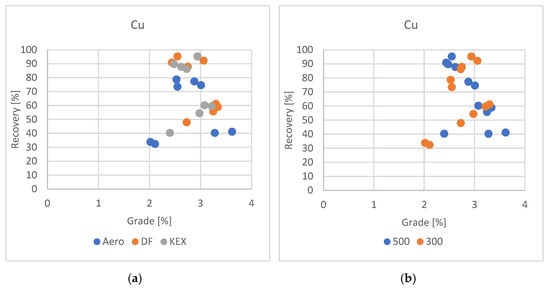
Figure 7.
Grade/recovery diagram of copper results with respect to collector used (a) and grade/recovery diagram of copper results with respect to slurry density (b).
Although the KEX collector also yielded recovery rates exceeding 90% in isolated instances, its overall performance was less consistent across varying test conditions and metals. In contrast, DF not only ensured excellent recovery of copper but also delivered superior results for zinc and lead flotation, making it a more versatile and effective collector for this specific polymetallic ore.
Copper recovery, particularly of chalcopyrite (CuFeS2), also improved with higher slurry density. Denser pulp conditions extended particle residence time and enhanced collector–particle interaction, supporting better flotation performance. Chalcopyrite benefits from stronger particle–bubble interactions under these conditions, especially when using selective collectors [35].
Given the complex mineralogical composition of ore containing a combination of sulfide minerals including sphalerite, galena, and chalcopyrite, the selection of a collector that performs efficiently across multiple target metals is essential. The comprehensive effectiveness of DF suggests that it is the most suitable reagent for maximizing the overall metallurgical performance in the froth flotation of this ore type, achieving both high recovery rates and favorable concentrate grades. DF collectors exhibit strong selectivity due to their molecular structure, which contains functional groups with high affinity for metal sulfide surfaces. These groups form stable chemisorbed complexes, particularly with Pb2⁺ and Zn2⁺ ions, enhancing selectivity for galena and sphalerite over gangue minerals. Compared to conventional xanthates, DF collectors offer better adsorption strength and specificity, improving flotation performance under complex ore conditions and reducing unwanted activation or entrainment of non-target phases [36,37].
Based on the comprehensive evaluation of the flotation test results, it can be concluded that the optimal conditions for concentrating the valuable metals such as copper, lead, and zinc from this polymetallic ore involve a specific combination of reagent dosages and pulp characteristics. The most effective configuration was achieved using the DF collector at a dosage of 50 g·t−1, complemented by a frother dosage of 100 g·t−1 and a slurry density of 500 g·L−1 (test no. 7).
Although numerous studies have investigated using XRF analysis of froth flotation concentrates, only a limited number have investigated the suitability of using superior identification methods, such as SEM-EDX and XRD analysis [38,39,40]. However, these studies confirm the suitability of these analytical methods for the analysis of ore-dressing products. The attached images display SEM and EDX micrographs of the most enriched flotation concentrates obtained during the experiments (Figure 8). These microanalytical techniques were employed to investigate the morphology and elemental composition of the concentrate particles. The SEM images reveal well-defined mineral surfaces and agglomerates, while the corresponding EDX spectra confirms the significant presence of zinc and lead. These findings provide direct evidence of successful mineral enrichment, demonstrating that both zinc and lead were effectively concentrated during the flotation process. The localization and distribution of these elements within the sample further support the efficiency of the applied collectors and flotation conditions.
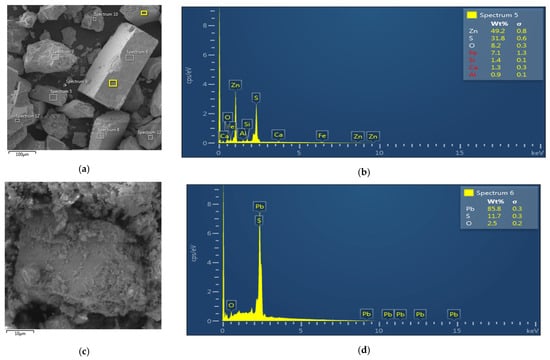
Figure 8.
Results of SEM analysis of concentrate (a,c); EDX analysis of the concentrate (b,d) (concentrate no. 7).
The micrographs show that the flotation product contains particles with high mineralogical purity. Specifically, spectrum 5 reveals a particle composed almost entirely of sphalerite, while spectrum 6 identifies a nearly pure galena particle. These observations confirm the selective nature of the flotation process and the successful isolation of individual sulfide phases within the concentrate.
The results obtained from XRD analyses of both the feed material and the final flotation concentrate (sample no. 7) provide clear evidence of the selective enrichment of sulfide minerals during the flotation process (Figure 9).
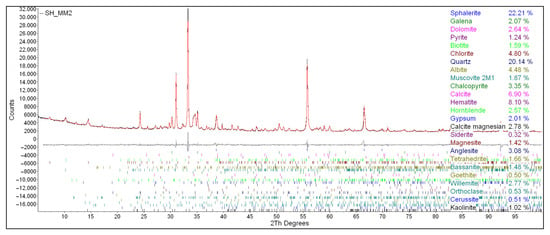
Figure 9.
XRD Spectrum of the concentrate from concentrate no. 7.
Notably, sphalerite (ZnS) exhibited the most significant increase in concentration, rising from 4.45 wt% in the feed to 22.21 wt% in the final concentrate, indicating highly effective recovery of zinc-bearing minerals.
Similarly, galena (PbS), the primary lead-bearing phase, increased from 0.80 wt% in the feed to 2.07 wt% in the concentrate, while chalcopyrite (CuFeS2), representing the copper phase, rose from 1.65 wt% to 3.35 wt%. These enrichment trends confirm that the applied flotation conditions were capable of effectively concentrating sulfide minerals even from relatively low-grade ores, demonstrating the process’s efficiency and selectivity.
The research on fine disseminated particle size, poor mineral liberation degree, and serious argillization can help select suitable methods in the separation of minerals [38].
The stockpile studied shares mineralogical and geochemical traits common to many polymetallic legacy tailings worldwide [41,42].
It should be acknowledged that the flotation tests conducted in this study are specific to the mineralogical composition of the investigated polymetallic stockpiles. Therefore, the optimized conditions reported here may not be directly transferable to other mine waste materials with differing mineralogies or geochemical characteristics. Thus, the optimized flotation methods developed here can be applied to similar mine wastes to enhance metal recovery and concentrate quality. This research offers practical insights for improving the sustainable reprocessing of tailings, supporting future guidelines for more efficient and environmentally responsible resource recovery in mining.
4. Conclusions
This study demonstrated effective recovery of zinc, lead, and copper collective concentrates from metal-bearing historical mining tailings (sulfide polymetallic ore), obtained during the mining of uranium ore by froth flotation techniques.
- Valuable metals including zinc, lead, and copper were successfully recovered from historical uranium and polymetallic tailings through the application of froth flotation techniques. This approach demonstrates the potential of reprocessing legacy mining waste to recover critical resources that would otherwise remain untapped and continue contributing to environmental liabilities.
- The tailings, containing 7.38% Zn, 1.45% Pb, and 0.49% Cu, yielded exceptionally high metal recovery rates under optimized flotation conditions. Specifically, recoveries exceeded 96% for lead, 87% for zinc, and 88% for copper, in the collective concentrate, confirming the technical feasibility and efficiency of the selected flotation process for extracting valuable metals from complex tailings material.
- The final flotation concentrates achieved metal grades of 7.65% Pb, 24.76% Zn, and 3.5% Cu, indicating effective enrichment of lead, zinc, and copper from the processed ore.
- Among the tested flotation reagents, DF 607 emerged as the most effective collector for all three metals—lead, zinc, and copper—delivering consistently high recovery rates and favorable concentrate grades. The most effective configuration was achieved using the DF collector at a dosage of 50 g·t−1, complemented by a frother dosage of 100 g·t−1 and a slurry density of 500 g·L−1.
- Slurry density was found to significantly influence flotation performance, with clear trends observed across different metals. Lower slurry densities enhanced the grade of zinc concentrates by improving selectivity but led to a reduction in overall zinc recovery. Conversely, higher slurry densities proved more beneficial for lead and copper recovery, likely due to improved collision frequency between particles and bubbles in denser pulp conditions.
- The high-grade concentrates obtained from this process are well-suited for further metallurgical treatment, such as hydrometallurgical extraction or smelting.
- In addition to generating economic value, the reprocessing of these tailings contributes to sustainable mining practices by recovering residual metals, minimizing waste, and significantly reducing the environmental footprint of past mining activities.
Author Contributions
Conceptualization, M.L. and M.M.; methodology, M.M. and M.S.; software, M.S. and D.M.B.; validation, M.L. and M.S.; formal analysis, M.M. and M.L.; investigation, M.M.; resources, M.S. and I.Ď.; data curation, M.M. and M.L.; writing—original draft preparation, M.M.; writing—review and editing, M.L.; visualization, M.L. and M.S.; supervision, M.L. and M.S.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the European Institute of Innovation and Technology (EIT), a body of the European Union, under Horizon Europe, the EU Framework Programme for Research and Innovation (Project DYNOSORT Dynamic ore sorting of polymetallic stockpiles, grant number: 21050). This work was supported by the Slovak Cultural and Educational Grant Agency under the grants’ project KEGA 020TUKE-4/2024 “Adaptability of education with a focus on strategic support of companies to ensure the sustainable quality of processes”.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nkuna, R.; Ijoma, G.N.; Matambo, T.S.; Chimwani, N. Accessing Metals from Low-Grade Ores and the Environmental Impact Considerations: A Review of the Perspectives of Conventional versus Bioleaching Strategies. Minerals 2022, 12, 506. [Google Scholar] [CrossRef]
- Hermanus, M. Mining redesigned—Innovation and technology needs for the future-A South African perspective. J. South. Afr. Inst. Min. Metall. 2017, 117, 811–818. [Google Scholar] [CrossRef]
- Parker, G.K.; Buckley, A.N.; Woods, R.; Hope, G.A. The interaction of the flotation reagent, n-octanohydroxamate, with sulfide minerals. Miner. Eng. 2012, 36–38, 81–90. [Google Scholar] [CrossRef]
- Nkosi, N.; Nheta, W. Pretreatment and recovery of base metals from oxidised ores by froth flotation technology—A review. Miner. Eng. 2024, 218, 109024. [Google Scholar] [CrossRef]
- Moon, K.S.; Fuerstenau, D.W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates. Int. J. Miner. Process. 2003, 72, 11–24. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Sun, W.; Hu, Y. The flotation and adsorption of mixed collectors on oxide and silicate minerals. Adv. Colloid Interface Sci. 2017, 250, 1–14. [Google Scholar] [CrossRef]
- Bilal, M.; Park, I.; Hornn, V.; Ito, M.; Hassan, F.U.; Jeon, S.; Hiroyoshi, N. The Challenges and Prospects of Recovering Fine Copper Sulfides from Tailings Using Different Flotation Techniques: A Review. Minerals 2022, 12, 586. [Google Scholar] [CrossRef]
- Qin, S.; Dou, S.; Ma, S.; Zhang, Z.; Hu, Y.; Li, Y.; Liu, P.; Lin, F.; Zhao, H. Enhanced recovery of low-grade copper ore and associated precious metals from iron tailings: A case study in China. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 699, 134656. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, T.; Shen, Z.; Ma, S.; Shi, S.; Deng, J.; Deng, J. The depression mechanism of magnesium on the flotation of sphalerite with different iron contents: DFT and experimental studies. Sep. Purif. Technol. 2025, 353, 128379. [Google Scholar] [CrossRef]
- Abkhoshk, E.; Jorjani, E.; Al-Harahsheh, M.S.; Rashchi, F.; Naazeri, M. Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy 2014, 149, 153–167. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Han, G.; Feng, Q. Effect of copper ions on surface properties of ZnSO4-depressed sphalerite and its response to flotation. Sep. Purif. Technol. 2019, 228, 115756. [Google Scholar] [CrossRef]
- Nayak, A.; Jena, M.S.; Mandre, N.R. Beneficiation of Lead-Zinc Ores–A Review. Miner. Process. Extr. Metall. Rev. 2022, 43, 564–583. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Han, Y. Efficient flotation separation of lead–zinc oxide ores using mineral sulfidation reconstruction technology: A review. Green Smart Min. Eng. 2024, 1, 175–189. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Zhang, Z.; Liu, M.; Zeng, G.; Liu, G. The selective flotation separation of galena from sphalerite with 5-heptyl-1,3,4-thiadiazole-2-thione: Emphasizing on adsorption mechanism. Appl. Surf. Sci. 2025, 702, 163204. [Google Scholar] [CrossRef]
- Prior, T.; Giurco, D.; Mudd, G.; Mason, L.; Behrisch, J. Resource depletion, peak minerals and the implications for sustainable resource management. Glob. Environ. Change 2012, 22, 577–587. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Ma, Z.; Peng, W.; Huang, Y.; Wang, W.; Cao, Y. Recent advances in oxidation pretreatment and flotation separation of metal sulfide ores. Green Smart Min. Eng. 2025, 2, 142–155. [Google Scholar] [CrossRef]
- Qi, M.; Peng, W.; Wang, W.; Cao, Y.; Zhang, L.; Huang, Y. A novel molybdenite depressant for efficient selective flotation separation of chalcopyrite and molybdenite. Int. J. Min. Sci. Technol. 2024, 34, 1179–1196. [Google Scholar] [CrossRef]
- Mesa, D.; Brito-Parada, P.R. Scale-up in froth flotation: A state-of-the-art review. Sep. Purif. Technol. 2019, 210, 950–962. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Sun, W.; Gao, Y.; Kowalczuk, P.B. Froth flotation of fluorite: A review. Adv. Colloid Interface Sci. 2021, 290, 102382. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Wen, S.; Wang, H.; Zhao, W.; Han, G. Flotation of copper oxide minerals: A review. Int. J. Min. Sci. Technol. 2022, 32, 1351–1364. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Méndez, A.; Rodríguez-Pacheco, R.; Paz-Ferreiro, J.; Gascó, G. Recovery of zinc and copper from mine tailings by acid leaching solutions combined with carbon-based materials. Appl. Sci. 2021, 11, 5166. [Google Scholar] [CrossRef]
- James, L.P.; Cooksey, W.H.; Park, M.E.; Sung, K.Y. Economic and Environmental Applications for Recent Innovations of Nonferrous Metallurgy in Some Industrial Nations. Korean J. Chem. Eng. 2001, 18, 948–954. [Google Scholar] [CrossRef]
- Kursunoglu, S. A Review on the Recovery of Critical Metals from Mine and Mineral Processing Tailings: Recent Advances. J. Sustain. Metall. 2025, 1–28. [Google Scholar] [CrossRef]
- Uranium Mine No. 16. Available online: https://www.mindat.org/loc-25641.html (accessed on 22 June 2025).
- Škácha, P.; Sejkora, J.; Plášil, J. Selenide mineralization in the příbram uranium and base-metal district (Czech Republic). Minerals 2017, 7, 91. [Google Scholar] [CrossRef]
- Haldar, S.K. Mineral Exploration: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Calvo, G.; Mudd, G.; Valero, A.; Valero, A. Decreasing ore grades in global metallic mining: A theoretical issue or a global reality? Resources 2016, 5, 36. [Google Scholar] [CrossRef]
- Wang, G.C. The Utilization of Slag in Civil Infrastructure Construction; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Toolbars, T. Sim Module—Common Tools Table of Contents. Available online: https://www.metso.com/globalassets/portfolio/hsc-chemistry/40-sim-common-tools.pdf (accessed on 4 February 2025).
- Heydari, G.; Mehrabani, J.V.; Bagheri, B. Selective separation of galena and sphalerite from pyrite-rich lead-zinc ores: A case study of the Kooshk mine, Central Iran. Int. J. Min. Geo-Eng. 2019, 53, 43–50. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, S.; Mandre, N.R. Flotation behavior of a complex lead-zinc ore using individual collectors and its blends for lead sulfide. J. Dispers. Sci. Technol. 2023, 44, 1703–1710. [Google Scholar] [CrossRef]
- Zhuo, Q.; Liu, W.; Xu, H.; Sun, X.; Zhang, H.; Zheng, X.; Wei, H. Research progress of relative motion between particles and bubbles in froth flotation. Meitan Xuebao/J. China Coal Soc. 2019, 44. [Google Scholar] [CrossRef]
- Li, S.; Jue, K.; Sun, C. Effect of bubble surface properties on bubble–particle collision efficiency in froth flotation. Minerals 2020, 10, 367. [Google Scholar] [CrossRef]
- Gungoren, C.; Baktarhan, Y.; Demir, I.; Ozkan, S.G. Enhancement of galena-potassium ethyl xanthate flotation system by low power ultrasound. Trans. Nonferrous Met. Soc. China 2020, 30, 1102–1110. [Google Scholar] [CrossRef]
- Turysbekov, D.; Tussupbayev, N.; Narbekova, S.; Kaldybayeva, Z. Combined microflotation effects in polymetallic ores beneficiation. SN Appl. Sci. 2023, 5, 124. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Z.; Mulenga, H.; Sun, W.; Cao, J.; Gao, Z. Synthesis of a novel collector based on selective nitrogen coordination for improved separation of galena and sphalerite against pyrite. Chem. Eng. Sci. 2020, 226, 115860. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Flotation of mixed oxide sulphide copper-cobalt minerals using xanthate, dithiophosphate, thiocarbamate and blended collectors. Miner. Eng. 2019, 138, 246–256. [Google Scholar] [CrossRef]
- Xu, W.; Shi, B.; Tian, Y.; Chen, Y.; Li, S.; Cheng, Q.; Mei, G. Process mineralogy characteristics and flotation application of a refractory collophanite from Guizhou, China. Minerals 2021, 11, 1249. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Jia, X.; Cai, J.; Su, C.; Yu, X.; Zheng, Q.; Peng, R.; Shen, P.; Liu, D. Role of ammonium phosphate in improving the physical characteristics of malachite sulfidation flotation. Physicochem. Probl. Miner. Process. 2023, 59, 161510. [Google Scholar] [CrossRef]
- Mavhungu, E.M.S.; Nheta, W.; Rose, D. Characterization of Hydrocarbons Contaminated Platinum Group Metals Mine Sludge from the Bushveld Complex. In Proceedings of the World Congress on Mechanical, Chemical, and Material Engineering (MCM’22), Prague, Czech Republic, 31 July–2 August 2022. [Google Scholar]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A review of circular economy strategies for mine tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Forbes, E.; Verster, I.; Jokovic, V.; Awatey, B.; Parbhakar-Fox, A. Review on advances in mineral processing technologies suitable for critical metal recovery from mining and processing wastes. Clean. Eng. Technol. 2022, 7, 100451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).