Abstract
The processing of metallurgical slags is an urgent task, as they contain residual amounts of precious and non-ferrous metals such as gold, silver, copper and zinc. The efficiency of extraction of these metals directly depends on the granulometric composition of the processed material, which determines the need for its detailed analysis. The purpose of this study is to analyze the effect of the granulometric composition of slags on the efficiency of extraction of non-ferrous metals using the flotation method. For this purpose, studies were carried out, including granulometric analysis, chemical composition analysis and flotation tests using Na2S, KAX and 3418A reagents. The analysis showed that the main part of the slag consisted of particles less than 3.36 mm, while the content of copper was 0.60%, zinc was 2.37%, gold was 0.1 g/t and silver was 7.2 g/t. Flotation experiments confirmed that the use of Na2S and 3418A increased the recoverability of copper and zinc, and reducing the particle size to d80 <10 microns increased the efficiency of copper extraction by 7%. Thus, the optimization of flotation processes and the control of granulometric composition make it possible to increase the efficiency of metallurgical waste processing, reduce losses of valuable metals and reduce the environmental burden.
1. Introduction
In modern conditions of mining and processing of ore materials, special attention is paid to the integrated use of raw materials and the maximum extraction of valuable components. One of the promising areas is the processing of metallurgical slags, which are man-made mineral resources containing residual amounts of non-ferrous and precious metals. Their effective extraction contributes not only to increasing the profitability of production but also to reducing the anthropogenic impact on the environment [1,2,3,4,5,6]. In particular, in slags of pyrometallurgical origin, copper and zinc are often preserved in sulfide or oxidized form, as well as in association with noble metals, which makes them a promising source of secondary raw materials.
One of the promising recycling facilities is a man-made slag deposit formed as a result of long-term operation of the former Shymkent Lead Metallurgical Enterprise in the territory of the Republic of Kazakhstan. It is estimated that the total amount of accumulated waste is about 1.9 million tons. These slags are characterized by a residual content of non-ferrous and precious metals, including copper, zinc, silver and gold, which determines their industrial value as a source of secondary raw materials. The significant scale of the deposit and the availability of valuable components actualize the need to develop and optimize effective technological solutions for their processing in order to re-engage in the production cycle and minimize environmental risks [7,8,9].
The granulometric composition of the processed material plays a key role in the efficiency of precious metal extraction processes. The particle size determines the surface area available for interaction with reagents and also affects the processes of flotation, leaching and gravity enrichment. Studies show that different fractions of the material can exhibit different degrees of metal recoverability, which makes granulometric analysis an important stage in the development and optimization of slag processing technologies [10,11,12,13,14,15,16].
Among modern slag processing methods, flotation extraction technology occupies a special place, which is widely used due to its high selectivity and efficiency [17,18]. Thus, it has been shown that flotation of copper sulfides from slags using various reservoir combinations allows for achieving a high degree of recoverability under optimal pH conditions and fine grinding [19]. Preliminary sulfidation (for example, using Na2S) additionally increases the efficiency of collecting oxidized forms of copper [20]. In addition, modern research emphasizes that the successful application of flotation requires precise control over particle size and phase distribution, which is especially important in the processing of complex man-made waste [21].
The relevance of the study is due to the need to increase the efficiency of metallurgical waste processing and to search for new methods for their enrichment aimed at extracting both non-ferrous (copper, zinc) and precious metals (gold, silver). Various approaches to slag processing are used in global practice, including X-ray fluorescence sorting, magnetic separation and hydrometallurgical methods. For example, studies have shown that X-ray fluorescence sorting makes it possible to effectively isolate non-ferrous metals from industrial waste, depending on the properties of the particle surface [22,23,24,25]. In addition, it was found that the characteristics of the granulometric composition have a significant effect on the processes of metal reduction in the peripheral zone of the blast furnace [26,27].
The present work is devoted to the study of the processing of metallurgical slags for the purpose of secondary extraction of valuable metals. The main focus is on the study of the effect of the granulometric composition on the efficiency of flotation extraction of non-ferrous metals. In the course of the study, laboratory experiments were conducted to determine the granulometric composition of slags and analyze the effect of fractional composition on extraction efficiency, and a comparative analysis of various combinations of flotation reagents was also conducted. The results obtained will make it possible to develop recommendations for optimizing the processing of metallurgical waste, increasing the recoverability of valuable metals and minimizing losses during their processing. The work is aimed at improving the efficiency of the secondary use of slags as an alternative raw material, which is of both economic and environmental importance.
2. Materials and Methods
The lead production slag dump analyzed in this study is located opposite the lead plant on the left bank of the Badam River and is a truncated cone-shaped mountain of loose granular slag of a black color, mainly with a grain size from 0.5 to 15 mm. However, there are pieces of slag with a size of up to 60–70 mm. The volume of slag in the slag dump is 988,924 m3, with a total weight of about 1.9 million tons (Figure 1). The study included sampling of slag from the slag dump, grinding using a mill and then conducting a granulometric analysis using the sieve method and chemical analysis to determine the content of the slag, as well as flotation experiments [28,29].

Figure 1.
Slag dump of lead production.
The studied slag is a man-made formation of pyrometallurgical origin, obtained as a result of processing lead concentrates. According to chemical and phase analysis data, the slag contains copper in the form of sulfides, oxides (CuO, Cu2O), as well as residual inclusions of metallic copper. Zinc is also present in an oxidized form and as part of a silicate matrix. The structure of the material is heterogeneous, with the presence of a glassy phase, which requires preliminary fine grinding to effectively release valuable phases before flotation.
For a more detailed study of metallurgical waste samples taken from the facilities of the former Shymkent Lead Plant, a complete elemental analysis was performed for the content of more than 40 chemical elements using mass spectrometry and inductively coupled plasma optical emission spectrometry. During the study, samples related to various stages of production (slag and concentrate obtained from this slag) were analyzed in order to determine the content of macro-, micro- and rare earth elements, as well as precious metals. The average data on the content of the elements, taking into account the extended measurement uncertainty, are presented in Table 1.

Table 1.
Elemental composition of metallurgical waste samples, mg/kg.
The results of the analysis are presented in Table 1 and demonstrate a high content of macronutrients such as Fe, Ca, Al, Mg, K and Na. In terms of non-ferrous metals, the content of copper was recorded at 5100 mg/kg and lead at 860 mg/kg. As for precious metals, the content of Au, Ag and Pt in the initial slag was below the detection limit (<0.002 mg/kg), with the exception of palladium Pd, which was detected at a concentration of 2.4 mg/kg. This indicates an extremely low total content of precious metals in the initial sample, which requires additional enrichment to extract them. Nevertheless, significantly higher concentrations of the following elements were recorded in the concentrate obtained as a result of flotation enrichment: Cu up to 140,000 mg/kg, Zn up to 41,000 mg/kg, Au up to 5.3 mg/kg and Ag up to 190 mg/kg, indicating their selective accumulation and possible presence in the slag in a bound or dispersed state. This underlines the importance of determining the optimal degree of grinding in order to release finely dispersed inclusions of Cu, Ag and Au, facilitating their further extraction during flotation.
A 178 × 356 mm “Unal” rod mill operating at 45 rpm was used for the study. The sample, pre–ground to a size of 1 mm, weighing about 1000 g, was processed at a ratio of solid–liquid = 1:1 for different times given below. The particle size distribution was recorded after each stage of using wet sieving. The results are shown in Table 2.

Table 2.
Values of grinding time and size distribution.
3. Experimental Part
Flotation studies were aimed at studying the effect of the type of reagents, their order of administration and the degree of grinding on the efficiency of extracting the studied elements from slags. All experiments were based on a single technological scheme, including the stages of crushing, flotation, purification and control processing, as shown in Figure 2.
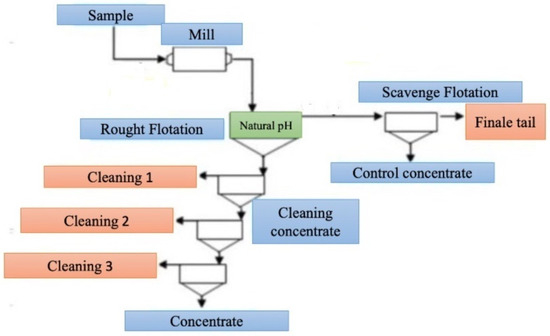
Figure 2.
Combined technological flowchart of flotation experiments.
- Flotation experiments with Na2S-3418A
At the first stage, sulfidization with various amounts of Na2S was carried out before flotation. Reagent 3418A was used as a collector. Flotation tests were performed at the natural pH of the slag pulp (pH ≈ 9.50 and above). To assess the effect of acidity, a control experiment was conducted with the pH reduced to 7.60. The coarse concentrate was subjected to up to six stages of purification. The residues after purification were additionally examined in order to determine the residual content of valuable metals. Since the number of cleaning steps differed, after cleaning, they are expressed as “residue after cleaning”.
- Flotation experiments with KAX-Na2S-3418 A
In the second variant, KAX was added directly to the mill during grinding. At the control flotation stage, Na2S was additionally introduced, with an exposure time of 5 min, after which the collector 3418A was used. This approach made it possible to evaluate the effect of preliminary modification of the surface of KAX particles and subsequent sulfidization. The flotation efficiency was also studied for various particle fractions.
- Flotation experiments with the sample after grinding of coarse residues
The third option involved re-crushing the residue after rough flotation. In this case, Na2S was not used. Only the KAX collector was added to the pulp at the grinding stage, and at subsequent stages, 3418A was added. This scheme was aimed at testing the possibility of efficient copper extraction without prior sulfidization while maintaining the natural pH of the medium.
4. Results and Discussion
To increase the recoverability of the studied element from metallurgical slag, various flotation schemes using Na2S, 3418A and KAX reagents were considered. Both the nature of the reagent and the sequence of its administration had a significant impact on the efficiency of the process. In particular, when using Na2S in combination with the collector 3418A, after the sulfidization stage, a noticeable improvement in the recoverability of copper was achieved, especially in fine fractions, due to the activation of oxidized forms of copper. At the same time, the use of KAX alone, without prior sulfidization, proved to be less effective for such forms, which emphasizes the importance of choosing a flotation scheme depending on the composition and phase state of the slags. These results confirm the need for individual selection of flotation reagents and processing conditions depending on the mineral composition of the slag.
4.1. Time of Grinding and Size Distribution of Coarse Sludge Residues
Table 2 shows the results in terms of the dependence of the cumulative particle size distribution on the grinding time, where the proportion of sifted particles corresponds to different time intervals.
Table 2 shows an accelerated decrease in the proportion of large particles and a gradual increase in the content of small fractions over the grinding time. The most intensive grinding takes place between 17 and 25 min, after which the process slows down significantly. For large particles (for example, 150 microns), the cumulative yield reaches 100% by 35 min of grinding. At the same time, for smaller particles (for example, 20 microns), the cumulative increase is noticeable even after 45 min, which indicates the continued crushing of the material.
The results show the efficiency of rod mills for grinding slag: after 35 min, most of the material reaches a size of less than 75 microns. When grinding for up to 60 min, almost all the material passes through a sieve with a mesh size of 20 microns. This significantly affects the processing steps such as flotation or hydrometallurgy. This is especially important for the stages of flotation and hydrometallurgical processing, where the particle size has a decisive influence on metal recovery. Similar grinding patterns have been described in other studies. Thus, Liu et al. (2019) found that an increase in time during the grinding of dry-granulated blast furnace slag makes it possible to reach an average size of about 16.6 microns, which affects the technological properties of the material, including reactivity [27]. At the same time, Li et al. (2024) noted that excessive grinding can lead to particle agglomeration and a decrease in process efficiency [29]. These observations confirm the need to optimize the grinding time and control the dispersion of the material during the processing of man-made waste.
As part of the control flotation, to study the effect of the particle size (38 microns), coarse flotation residues were subjected to repeated grinding. Experiments on control flotation were carried out with particle sizes in the following fractions: 24 microns, 20 microns and <10 microns (Table 3).

Table 3.
Results of particle size distribution analysis during the grinding of coarse residues.
Table 3 shows the results of the particle size distribution after grinding the coarse residues in various time intervals. When analyzing the particle distribution after grinding coarse particles, the following changes were observed. For synchronization of 38 microns, the weight fraction drops from 8.37% (30 min) to 5.28% (60 min), while the cumulative value remains at 100% for the lower time intervals, which indicates the complete removal of large particles. At the same time, the mass fraction of particles with particle sizes of 20 microns varies slightly: it decreases from 17.6 at 30 min to 16.15% at 60 min; however, a cumulative increase in the proportion from 91.6 to 94.72% indicates a gradual decrease in large particles. The most noticeable increase occurs within 20 microns, where the weight fraction of fine particles increases from 74.0 (30 min) to 78.57% (60 min), and the cumulative value for this group reaches 93.72%. These results indicate the effectiveness of long-term grinding to prepare the material for further processing.
Figure 3 shows that with particle sizes of about 20 microns, the cumulative distribution increases over the grinding time, reaching about 80% after 20 min, slightly more than 85% after 30 min and about 93% after 60 min. For smaller particles (<20 microns), the largest increase in cumulative mass is observed during the transition from 30 to 60 min of grinding. At the same time, for large particles (>24 microns), the cumulative value remains close to 100% regardless of the grinding time, which indicates the almost complete removal of large fractions. Thus, an increase in the grinding time leads to a more significant reduction in particle size and a significant increase in the proportion of small fractions.
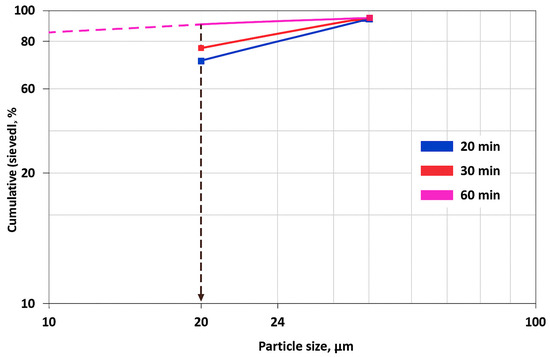
Figure 3.
The effect of grinding time on the particle size distribution of coarse residues. Note: The dash lines in the figure indicate the cumulative mass distributions of particles of various size fractions depending on the grinding time.
In the work of Marwa (2023), it was found that small waste fractions (<10 mm) exhibit high values of acidity and intensive metal leaching, emphasizing the importance of fineness control to reduce environmental risks [24]. The article by Lyalyuk et al. (2013) emphasizes the importance of coke crushing to improve the gas permeability of the blast furnace charge, where reducing large fractions (>80 mm) to 1–2% increases furnace productivity and reduces coke consumption [26]. In addition, data from Jani et al. (2018) indicate that even for landfill waste, fine fractions (<10 mm) are of key importance for the mobility of heavy metals (Zn, Cu, Cr), which is important to consider during processing and reclamation [16]. Pfandl et al. (2019) noted that the efficiency of X-ray fluorescence sorting of non-ferrous metals from slags strongly depends on the particle size range (optimally 10–32 mm), and smaller fractions require separate processing [13]. Thus, our own results confirm the importance of choosing the optimal grinding time in order to maximize the release of valuable components, minimize large fractions and improve technological performance during subsequent processing stages. This is consistent with the literature data on the need to control the particle distribution at different stages of raw material preparation.
4.2. The Effect of Particle Size Distribution on the Extraction of the Studied Components from Slags
Particle size plays a key role in the efficiency of the flotation process, as it directly affects the interaction of reagents with metallurgical waste containing valuable elements. Table 4 shows the values of the results of flotation experiments using various reagents (Na2S-3418A, KAX-Na2S-3418A) with different particle fractions.

Table 4.
Estimated values of flotation experiments.
The results of the total flotation and residue presented in Table 4 reflect the average values of the content of the studied elements obtained from a series of experiments. However, during flotation with six purification stages, in some experiments, the copper content in the concentrate reached 20.15%. The table data also show that with optimal granulometric composition (especially for the fraction at 38 microns), copper extraction reaches maximum values of about 58–59%, with a copper content in the concentrate of up to 1.91–1.94%, depending on the reagent scheme used. Under these conditions, the zinc content in the concentrate increases to 3.28–4.02% when extracted to 28.45%. At the same time, the content of copper and zinc in the residue after flotation decreases to 0.36–0.40% and 2.83–3.53%, respectively, which indicates the efficiency of the process. Thus, the total flotation ensures the extraction of up to half of the copper and a significant proportion of the zinc, while significantly reducing the metal content in the unselected residue. It is shown that with coarse flotation, it is possible to extract almost 50% of the copper, and the maximum Cu content in the concentrate can reach 20.15% after six-stage purification.
The results of the total flotation and residue shown in Table 4 indicate that with an optimal granulometric composition (especially with a fraction of 38 microns), the copper recovery reaches maximum values of about 58–59%, with a copper content in the concentrate of up to 1.91–1.94%, depending on the reagent application scheme. The zinc content in this case also increases to 3.28–4.02% with an extraction up to 28.45%. At the same time, the proportion of Cu and Zn in the residue after flotation decreases to 0.36–0.40% and 2.83–3.53%, respectively, which indicates the efficiency of the extraction process. Thus, the total flotation allows for extracting up to half of the copper and a significant part of the zinc, leaving a smaller proportion of metals in the remainder.
The results show that nearly half of the Cu can be recovered during rougher flotation, and the Cu grade in the concentrate can reach up to 20.15% after six stages of cleaning. The use of Na2S during coarse flotation had a positive effect, but no effect was observed during control flotation, which is consistent with the conclusions (Sajjad & Otsuki, 2022), which emphasize that the effectiveness of reagents and their interaction with minerals depend on the stages of the process and the characteristics of the suspension [30]. Also, reducing the pH level in the control flotation proved to be insufficiently effective. An increase in flotation efficiency was achieved by regulating the particle size distribution, which fully corresponds to the conclusions Kazemi et al. [31], where the optimal particle fraction of +40–60 microns gave the greatest Cu extraction in laboratory conditions (Table 4) [31,32,33].
The conducted studies on lead-produced slags revealed important patterns: the particle fraction with an upper limit of 38 microns, corresponding to the threshold value of one of the sieve classifications, showed the best efficiency in flotation tests. Although 38 microns is not an average size or an actual modal value, it was this fraction that was used as the boundary value in determining the technologically optimal distribution for the initial stage of flotation, ensuring sufficient contact area with the reagents and minimizing copper losses.
This is also confirmed by studies Chi Wang et al. [34], which show that fine particles can improve interaction with air bubbles and reagents, but their excess can negatively affect the process due to aggregation and changes in the rheological properties of the pulp [34,35].
Reducing the particle size to <10 microns demonstrated a positive effect on copper extraction: Cu extraction increased by about 7%, and the overall flotation efficiency reached about 60%.
Despite the fact that, according to the classical theory of flotation, the efficiency of extracting particles with a size of less than 20 microns decreases due to a decrease in the probability of their collision with air bubbles, an inverse relationship is observed in the experiments: with a decrease in particle size to <10 microns, the degree of copper extraction increases. This effect is due to a number of factors. Firstly, a decrease in particle size is accompanied by an increase in specific surface area, which increases the contact of minerals with reagents and promotes more efficient sulfidization, especially in the presence of Na2S and collectors of type 3418A. Secondly, under experimental conditions, the flotation modes were optimized—the pH level and the amount and sequence of reagent injection—which minimized the negative impact of aggregation and instability of the finely dispersed phase.
It should be noted that the nature of the slag phase composition may contribute to this behavior, since the studied slag contains both sulfide and oxidized forms of copper (cuprite Cu2O, tenorite CuO), as well as finely distributed metal inclusions in a silicate and vitreous matrix. By reducing the particle size to the submicron level, it becomes possible to more completely release these forms of copper previously encapsulated in the matrix, which increases their availability for interaction with reagents and inclusion in the flotation process. This explanation is consistent with the literature data, emphasizing the importance of the degree of separation and specific surface area of minerals in the processing of man-made raw materials.
In addition, it is important to take into account the mineral composition of the slags: copper can be associated with finely dispersed phases of silver and gold, which increases the efficiency of their extraction together in the concentrate. Finally, the use of modern approaches, such as the use of nanobubbles and stabilized flotation systems, has made it possible to compensate for the limitations of traditional theory and ensure effective capture of particles with a size of less than 10 microns. Thus, an increase in the degree of Cu extraction with a decrease in granulometry is associated not only with the physico-chemical characteristics of the material but also with the correct adaptation of the flotation scheme to the dispersed composition of the suspension.
This effect is associated with an increase in the available surface of minerals for interaction with reagents and air bubbles, which improves the formation of partially bubbled aggregates and promotes the better separation of valuable components from waste rock. Similar approaches aimed at improving the efficiency of flotation of fine particles using nanobubbles and nanoparticles are discussed in detail in [36] (Sigauke et al., 2025), which emphasizes their role in increasing hydrophobicity, stabilizing foam and improving separation [36,37,38].
However, it is important to take into account that excessive grinding, resulting in a particle size of <10 microns, is not always the optimal solution. If the grinding is too fine, a number of negative consequences are observed, including a deterioration in pulp filtration, an increase in the viscosity of the slurry, the formation of aggregates of fine particles and a significant increase in energy consumption for grinding. These problems reduce the efficiency of the subsequent stages of the process, require a longer time for the dehydration of concentrates and can lead to the loss of some valuable components due to non-selective capture of particles in tailings. This is confirmed in the work in [39] (Zeng et al., 2023), where the authors emphasize the importance of finding a balance between the degree of grinding and the efficiency of flotation, noting that excessive grinding contributes to the formation of aggregates (“card houses”), increased viscosity and deterioration of filtration, which is especially critical in the design and modernization of processing plants [39,40,41].
The significant content of precious metals (Au, Ag) should also be noted, which showed that their magnitudes in concentrates at 38 microns reached 1.3 g/t of gold and 416 g/t of silver, although the content of these metals in the initial sample was quite low. This was due to the fact that gold and silver are often associated with finely dispersed minerals, which makes them more vulnerable to flotation extraction in fine fractions. Thus, the obtained results and literature data emphasize that achieving maximum efficiency of flotation of copper and precious metals requires an integrated approach: particle size control, optimization of grinding time, proper selection of flotation reagents, consideration of pulp rheology and, if necessary, the introduction of innovative methods—from flotation with carriers to the use of nanotechnology. All of this raises the relevance of further research on optimizing the processing of metallurgical waste and improving the economic and environmental efficiency of processes [42].
5. Conclusions
The conducted research has shown that the granulometric composition of slags significantly affects the efficiency of extraction of non-ferrous and precious metals. The optimal particle size of about 38 microns ensures the highest efficiency of copper flotation, and reducing the size to <10 microns additionally increases extraction but is accompanied by a deterioration in filtration and an increase in energy consumption. Noble metals (Au, Ag) are concentrated mainly in fine fractions, which makes their extraction effective at the stage of copper flotation. The data obtained make it possible to recommend the optimization of grinding modes and the selection of flotation reagents to increase the profitability of metallurgical waste processing and reduce the environmental burden.
Author Contributions
Conceptualization, A.S.; data curation, N.A.; formal analysis, B.B.; investigation, N.M.; methodology, B.R.; validation, Z.S. and B.G.; writing—original draft preparation, N.M.; supervision, A.S.; writing—review and editing, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant BR24993178 “Development of innovative technology for processing secondary resources—waste from metallurgical industries, by-products of oil refining and oil production”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Rakhadilov Bauyrzhan was employed by the company PlasmaScience LLP. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Maslak, O.I.; Hryshko, N.Y.; Hlazunova, O.O.; Maslak, M.V.; Yakovenko, Y.Y.; Savielova, A.D. Prospects of the recycling of metallurgical waste. IOP Conf. Ser. Earth Environ. Sci. 2024, 1415, 012128. [Google Scholar] [CrossRef]
- Bulaev, A.G.; Muravyov, M.I.; Pivovarova, T.A.; Fomchenko, N.V.; Kondrat’eva, T.F. Bioprocessing of mining and metallurgical wastes containing nonferrous and precious metals. Adv. Mater. Res. 2013, 825, 301–304. [Google Scholar] [CrossRef]
- Adesina, A. Performance and sustainability overview of alkali-activated self-compacting concrete. Waste Dispos. Sustain. Energy 2020, 2, 165–175. [Google Scholar] [CrossRef]
- Muravyov, M.I.; Bulaev, A.G.; Kondrat’eva, T.F. Complex treatment of mining and metallurgical wastes for recovery of base metals. Miner. Eng. 2014, 64, 63–66. [Google Scholar] [CrossRef]
- Ban, J.; Sun, K.; Yao, J.; Sunahara, G.; Hudson-Edwards, K.; Jordan, G.; Alakangas, L.; Ni, W.; Poon, C.S. Advances in the use of recycled non-ferrous slag as a resource for non-ferrous metal mine site remediation. Environ. Res. 2022, 213, 113533. [Google Scholar] [CrossRef]
- Yang, S.J.; Zhang, L.W.; Yu, D.H. Intensive development and comprehensive utilization of metallurgical slag. Appl. Mech. Mater. 2012, 174–177, 1424–1428. [Google Scholar] [CrossRef]
- Lim, B.; Aylmore, M.; Alorro, R.D. Technospheric Mining of Critical and Strategic Metals from Non-Ferrous Slags. Metals. 2024, 14, 804. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Chen, Y.; Huang, J. A Novel Non-Ferrous Metals Price Forecast Model Based on LSTM and Multivariate Mode Decomposition. Axioms 2023, 12, 670. [Google Scholar] [CrossRef]
- Nowińska, K.; Grzesik, B.; Kokowska-Pawłowska, M.; Nowak, J. Use of Mineral Waste for the Production of Artificial Aggregates. Appl. Sci. 2024, 14, 11734. [Google Scholar] [CrossRef]
- Matinde, E.; Simate, G.S.; Ndlovu, S. Mining and metallurgical wastes: A review of recycling and re-use practices. J. S. Afr. Inst. Min. Met. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Fomchenko, N.; Muravyov, M. Sequential Bioleaching of Pyritic Tailings and Ferric Leaching of Nonferrous Slags as a Method for Metal Recovery from Mining and Metallurgical Wastes. Minerals 2020, 10, 1097. [Google Scholar] [CrossRef]
- Dong, K.; Wang, H.; Jiang, Z. Case Analysis of Blast Furnace Consumption Technology and Process. In Resource Utilization of Solid Waste by Thermometallurgy in Steel Processes; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Pfandl, K.; Küppers, B.; Scheiber, S.; Stockinger, G.; Holzer, J.; Pomberger, R.; Antrekowitsch, H.; Vollprecht, D. X-ray fluorescence sorting of non-ferrous metal fractions from municipal solid waste incineration bottom ash processing depending on particle surface properties. Waste Manag. Res. 2019, 38, 111–121. [Google Scholar] [CrossRef]
- Zhang, T.S.; Yu, Q.J.; Wei, J.X.; Zhang, P.P.; Li, S. Effect of size fraction of ground granulated blast furnace slag on its strength contribution and hydraulic activity. Adv. Sci. Lett. 2011, 4, 1286–1291. [Google Scholar] [CrossRef]
- Petlovanyi, M.; Malashkevych, D.; Sai, K.; Bulat, I.; Popovych, V. Granulometric composition research of mine rocks as a material for backfilling the mined-out area in coal mines. Min. Miner. Depos. 2021, 15, 122–129. [Google Scholar] [CrossRef]
- Jani, Y.; Pehme, K.; Bucinskas, A.; Kriipsalu, M.; Burlakovs, J.; Hogland, W. Speciation of Cu, Zn and Cr in Excavated Fine Fraction of Waste at two Landfills. Iran. J. Energy Environ. 2018, 9, 86–90. [Google Scholar] [CrossRef]
- Valderrama, L.; Tapia, J.; Pavez, O.; Santander, M.; Rivera, V.; Gonzalez, M. Recovery of Copper from Slags Through Flotation at the Hernán Videla Lira Smelter. Minerals 2024, 14, 1228. [Google Scholar] [CrossRef]
- Panayotova, M. Control of Non-Ferrous Metal-Sulfide Minerals’ Flotation via Pulp Potential. Minerals 2023, 13, 1512. [Google Scholar] [CrossRef]
- Roy, S.; Rehani, S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 143, 43–49. [Google Scholar] [CrossRef]
- Carranza, F.; Romero, R.; Mazuelos, A.; Iglesias, N.; Forcat, O. Biorecovery of copper from converter slags: Slags characterization and exploratory ferric leaching tests. Hydrometallurgy 2009, 97, 39–45. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour. Conserv. Recycl. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Karimov, K.; Turakhodjaev, N.; Akhmedov, A.; Tashbulatov, S. A mathematical model of the technology of extraction of copper from industrial slags. E3S Web Conf. 2021, 264, 04077. [Google Scholar] [CrossRef]
- Kölking, M.; Flamme, S.; Heinrichs, S.; Schmalbein, N.; Jacob, M. More resource efficient recycling of copper and copper alloys by using X-ray fluorescence sorting systems: An investigation on the metallic fraction of mixed foundry residues. Waste Manag. Res. 2024, 42, 814–822. [Google Scholar] [CrossRef]
- Marwa, A. Effect of Particle Size on Leachate Formation Characteristics from Gold Mine Waste Rocks: At Source Acid Mine Drainage Management in Tanzania. J. Appl. Sci. Environ. Manag. 2023, 27, 2387–2392. [Google Scholar] [CrossRef]
- Pereira, G.V.d.A.; Pereira, W.V.d.S.; Ramos, S.J.; Guimarães, J.T.F.; Covre, W.P.; Dias, Y.N.; Fernandes, A.R. Bioavailable and Bioaccessible Fractions of Potentially Toxic Elements in Copper Mining Wastes in the Southeastern Amazon. Minerals 2025, 15, 140. [Google Scholar] [CrossRef]
- Lyalyuk, V.P.; Shmel’tser, E.O.; Lyakhova, I.A.; Kassim, D.A.; Tarakanov, A.K.; Otorvin, P.I. Changes in Granulometric Composition of Blast- Furnace Coke. Coke Chem. 2013, 56, 456–460. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Q.; Yu, Q. The effect of size distribution of slag particles obtained in dry granulation on blast furnace slag cement strength. Powder Technol. 2020, 362, 32–36. [Google Scholar] [CrossRef]
- Buchwald, V.F.; Wivel, H. Slag analysis as a method for the characterization and provenancing of ancient iron objects. Mater. Charact. 1998, 40, 73–96. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Liu, Y.; Chu, J.; Zhang, T.; Wang, W. Influence of the Steel Slag Particle Size on the Mechanical Properties and Microstructure of Concrete. Sustainability 2024, 16, 2083. [Google Scholar] [CrossRef]
- Sajjad, M.; Otsuki, A. Correlation between Flotation and Rheology of Fine Particle Suspensions. Metals 2022, 12, 270. [Google Scholar] [CrossRef]
- Kazemi, F.; Bahrami, A.; Ghorbani, Y.; Danesh, A.; Abdollahi, M.; Falah, H.; Salehi, M. The interaction and synergic effect of particle size on flotation efficiency: A comparison study of recovery by size, and by liberation between lab and industrial scale data. Rud.-Geol.-Naft. Zb. (Min.-Geol.-Pet. Eng. Bull.) 2023, 38, 622–699. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Safari, M.; Hoang, D.H.; Khoshdast, H.; Albijanic, B.; Kowalczuk, P.B. Technological assessments on recent developments in fine and coarse particle flotation systems. Miner. Eng. 2022, 180, 107509. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Safari, M.; Hoang, D.H. Fine, Coarse and Fine-Coarse Particle Flotation in Mineral Processing with A Particular Focus on the Technological Assessments. In Proceedings of the 2nd International Conference on Mineral Science, Online, 1–15 March 2021. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Mao, S.; Qin, S. Effects of Fine Minerals on Pulp Rheology and the Flotation of Diaspore and Pyrite Mixed Ores. Minerals 2020, 10, 60. [Google Scholar] [CrossRef]
- Wang, L.; Li, C. A Brief Review of Pulp and Froth Rheology in Mineral Flotation. J. Chem. 2020, 2020, 3894542. [Google Scholar] [CrossRef]
- Sigauke, T.; Johnson, O.T.; Ndeshimona, V.L.; Mashingaidze, M.M. Advancements in nanotechnology for the enhanced flotation of fine mineral particles: A review. Discov. Appl. Sci. 2025, 7, 317. [Google Scholar] [CrossRef]
- Abd El-Rahiem, F.H. Recent Trends in Flotation of Fine Particles. J. Min. World Express 2014, 3, 63. [Google Scholar] [CrossRef]
- Bilal, M.; Park, I.; Hornn, V.; Ito, M.; Hassan, F.U.; Jeon, S.; Hiroyoshi, N. The Challenges and Prospects of Recovering Fine CopperSulfides from Tailings Using Different Flotation Techniques: A Review. Minerals 2022, 12, 586. [Google Scholar] [CrossRef]
- Zeng, G.; Zhu, Y.; Chen, W. A Brief Review of Micro-Particle Slurry Rheological Behavior in Grinding and Flotation for Enhancing Fine Mineral Processing Efficiency. Minerals 2023, 13, 792. [Google Scholar] [CrossRef]
- Sokolovic, J.; Miskovic, S. The effect of particle size on coal flotation kinetics: A review. Physicochem. Probl. Miner. Process. 2018, 54, 1172. [Google Scholar] [CrossRef]
- Sygusch, J.; Wilhelm, T.; Furat, O.; Bachmann, K.; Schmidt, V.; Rudolph, M. Application of Multivariate Tromp Functions for Evaluating the Joint Impact of Particle Size, Shape and Wettability on the Separation of Ultrafine Particles via Flotation. Powders 2024, 3, 338–366. [Google Scholar] [CrossRef]
- Strube, F.; Guy, B.M.; Pereira, L.; Ebert, D.; Zgheib, A.; Fischer, M.; Möckel, R.; Schmidt, A.; Rudolph, M. Batch Flotation of Lithium-Bearing Slag—A Special Focus on the Phase Properties of Engineered Artificial Minerals for Enhancing the Recycling of End-of-Life Lithium-Ion Batteries. Minerals 2025, 15, 334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).