Abstract
The equipment has been developed and the process of granulation of liquid selenium into water has been implemented. The process of selenium granulation is implemented at a temperature of 225–250 °C through unheated sieves with a diameter of holes of 1–2 mm at a density of perforation from 279 to 417 holes/dm2. The specific productivity reaches 180 kg/(dm2 × h), which corresponds to industrial-scale production. Electron microscopy studies revealed the localization of impurity elements in both the raw and granulated selenium. The composition and concentration of elements in localized areas were found to be random. For example, one area contained (by mass %) C—7.64; S—6.04; As—4.85; and Pb—40.72. Another, located nearby, contained C—4.68; Te—45.42; and Pb—12.67. In granulated selenium there was a noted increase in the number of spherical or close to spherical in shape voids, on the boundaries of which impurity elements were concentrated. The change in chemical composition of granules in comparison with the initial selenium was not established. The use of a granulated element is accompanied by simplification of manipulations with dispersed material and improvement of dosing conditions in technological processes using selenium.
1. Introduction
The problem of granulation of materials has received a great deal of attention from scientists engaged in the development of new materials with increased requirements for strength, durability, a special composition and complex internal structure, as well as other properties. The process of granulation is due to the convenience of dosing, for example, in the alloying of steels with ferroalloys and logistics in large-scale production and slags of different composition.
Granulation is subjected both to high-temperature melts: slags, matte, ferroalloys refractory metals and their mixtures, and materials with low melting point—polymers and sulfur.
The technical design of granulation processes of liquid slags [1,2,3] and metals is devoted to a large number of developments, including [4,5,6,7,8,9,10]. Melt jet crushing and granulation were performed by water, air, mechanical means or a combination of methods. In one paper [11], the results of metal melting with feeding through holes of minimum diameter with the disintegration of a metal droplet by a high-speed gas jet before the metal droplet enters water are presented.
One paper [12] considers air granulation of the main furnace slag to improve immobilization by granules of heavy non-ferrous metals Al–Zn–Mg–Cu by centrifugation with ultra-high cooling rates of granules. A technique to increase the crystallization rate by continuously venting the vapor layer between the granule and water is proposed. This leads to an increase in the strength characteristics of granulated aluminum alloys, Al–Zn–Mg–Cu.
The author of [13] presented the development and testing of a new granulation machine to obtain uniform in size and shape granules of zinc, aluminum and magnesium cooled at high speed to the state of amorphous or close to amorphous materials. The process is organized as a controlled film flow and a method of controlled jet disintegration into granules.
A considerable amount of technical development and research has been devoted to the granulation of ferroalloys and refractory materials [14,15,16,17,18].
In one paper [14], GRANSHOT granulation process and granulated ferroalloy is found to be a highly productive process that replaces the traditional casting, crushing, and screening operations. Granulated ferroalloy is demanded by steel producers. Authors of [15] developed and mastered the process of ferroalloy granulation in industry to reduce operating costs and improve transportation and dosage conditions in steel-making processes.
Authors of [16] showed the possibility of obtaining high-quality spherical granules with different densities from various alloys of tantalum, molybdenum, nickel and steel. The relationship between the granulometric composition and physical properties of alloys and the parameters of the melting process has been established. As a result of granulation, the strength characteristics increase. Analysis of the microstructure of granules of various alloys indicates a fine dendritic structure and the absence of large second-phase separations.
In [17] the process of obtaining spherical-shaped granules of metal composite material (MCM) based on molybdenum with the initial fraction size of 40 ÷ 63 µm was investigated. The principal possibility of spheroidization of high-temperature MCMs based on refractory metals reinforced with particles of ceramic compounds is shown. The necessity of a deeper study and selection of spheroidization modes during processing in the plasma of electric arc discharge has been revealed.
In [18], three methods of gold bead production were investigated: pouring method, heating method and crucible method. The granule formation, surface morphology and microstructure were analyzed. On the basis of experiments of granule production, probable methods of ancient gold ball production were determined.
In [19,20] the process of aqueous granulation of copper sulfide was studied; an increase in temperature up to 1350 °C promotes the formation of particles of size −10.0 + 5.0 mm, and a decrease to 1250 °C promotes smaller classes: −1.6 + 1.0; −1.0 + 0.63; and −0.63. In the considered temperature range, the cooling time of particles of all classes exceeds the time of spheroidization, which promotes the formation of spherical particles. Melt temperature does not affect the chemical composition of copper sulfide granules. The main constituent phases of the granules were chalcosine (Cu2S) with monoclinic lattice in the amount of more than 80%, the same with hexagonal lattice 8 ÷ 9% and digenite (Cu1.78S)—4 ÷ 12%. Dispersed inclusions of metallic copper are present. During implementation of the project of the complex for continuous conversion of copper nickel-containing matte of MMC Norilsk Nickel PJSC, studies were performed to study the influence of the composition of granulated matte on the change in pH of water and a noticeable acidification of water was established.
Zharov M.V. studied the granulation processes of aluminum-based alloys [21,22,23]. It was found that the greatest influence on the cooling rate and crystallization rate of metal and alloy granules is exerted by the vapor shell formed around the melt drop, which arises due to the transformation of boundary layers of water into a vapor state. In order to intensify heat removal and accelerate the crystallization process of the granules, a method has been proposed for vapor shell elimination. The method is based on increasing the initial velocity of the molten droplet upon contact with the cooling medium. The obtained pressed semifinished products formed from granules obtained in this way are characterized by an increase in the strength characteristics of the material by 15–18% compared to materials obtained by traditional granulation methods.
In [23], several methods to produce nickel aluminide granules were examined, and it was established that the Plasma Rotating Electrode Process (PREP) provides the optimal technology to obtain high-quality NiAl granules.
The team of authors of [24] proposed a device to granulate the aluminum alloys, consisting of a casting container connected to a vibrator with openings at its bottom and a vessel filled with a cooling liquid. Due to certain parameters of perforation and the distance from the upper level of the coolant to the outer end surface of the bottom of the casting vessel, an increase in the quality of granules is achieved.
In [25] the questions of structure and physicochemical properties of liquid metals and alloys are covered, the mechanisms of destruction of metal melts jet by high-energy gas are considered, and the results of experimental studies on the influence of technological parameters of melt jet on forming, structure and dispersibility of powder particles are generalized. Technological schemes, equipment and optimal variants of processes to obtain powders of metals and multi-component alloys are described.
The authors of [26] have developed technologies and devices for deep purification of metals to obtain especially pure metals, zinc and cadmium with a content of the basic element of 99.99995 wt. % and lead—99.99996 wt. %; methods of their granulation into water and passivation by chemical method in anhydrous organic solvents on the basis of dimethyl formamide are developed.
There is a known method of obtaining granulated sulfur [27], in which the sulfur melt with a temperature 5 °C above the crystallization temperature is fed into the granulation zone upwards by means of a sprayer into a layer of sulfur nucleates with a temperature of 30–65 °C, which are maintained in a fluidized bed, and subjected to contact with gas. The melt at the outlet of the atomizer is in contact with a powerful gas stream with a velocity of 150–200 m/s and melt temperature. The resulting sulfur granules are continuously removed from the furnace, dispersed, large ones are crushed and, together with small granules, are returned to granulation.
The author of [28] made an attempt to model the granulation process by self-decay of a metal melt jet. The model allows determining and predicting technological (jet height, melt temperature and drop diameter) and design parameters of the unit. The model was initially tested on beryllium fluoride melt. However, the author does not provide data on the ratio of calculated and experimental data.
Thus, despite a very large number of studies, papers and constructive solutions on the technical design of granulation of melts, metals and elements with different physical properties and melting point, no information on granulation of molten selenium has been found. At the same time, the use of granulated selenium is accompanied by a reduction in costs, simplification of manipulations with dispersed material and improvement of dosing conditions in technological processes using selenium. In this regard, we have performed work on the granulation of liquid selenium and the structural design of the process.
2. Methodology
The elemental composition of the initial selenium was determined on an X-ray fluorescence wave-dispersive spectrometer Axios 1 kW (PANalytical, Almelo, The Netherlands).
Electron microscopic studies were performed on an electron probe microanalyzer JSM-8230 (JEOL, Tokyo, Japan). The electron probe performed under the following conditions: accelerating voltage—20 kV; current—5 nA.
Selenium pip before melting and granules of fraction +2.0–2.5 mm obtained through sieve holes of 1.5 mm were used as the object of study.
The object of study was water after granulation. The ratio of the mass of cooled granules and water was 2:1. The comparison was carried out with distilled water.
The spectrum was obtained on an IR Fourier spectrometer “FT/IR-6X” (FT/IR-6X High-end model JASCO, Tokyo, Japan) in the spectral range of 4000–400 cm−1 on an ATR PRO ONE X, ATR Crystal Diamond attachment, Spectra Manager Ver. 2.5 software. The spectrum analysis was carried out using specialized literature and IR spectral databases for the KnowItAll Ver. 23.1 software.
3. Description of Experiments and Their Discussion
Selenium obtained after vacuum distillation at the industrial prototype of vacuum distillation unit of rough selenium produced by Kazakhmys Smelting LLP was used as the material to be granulated. The content of the main component in refined selenium amounted to 99.51 wt. %, including impurities, wt. %: 0.057 Mg; 0.009 Na; 0.001 Al; 0.001 Si; 0.015 S; 0.005 Cl; 0.008 Ca; 0.001 Pb; 0.001 Cr; 0.004 Fe; 0.001 Ni; 0.001 Cu; and 0.386 Te.
At the first stage the option of combining the distillation purification process with selenium conversion into vapor phase and granulation of liquid selenium condensate by jet self-decomposition into distilled water was considered.
The schematic diagram of the installation is given in Figure 1. The unit is a retort equipped with a spigot for condensation and drainage of liquid selenium, made of quartz glass. The retort is installed in the resistance furnace on a heat-insulating insert. The retort is provided with a lid separated from the selenium by a heat-insulating insert. A container with distilled water is installed under the condensate spigot.
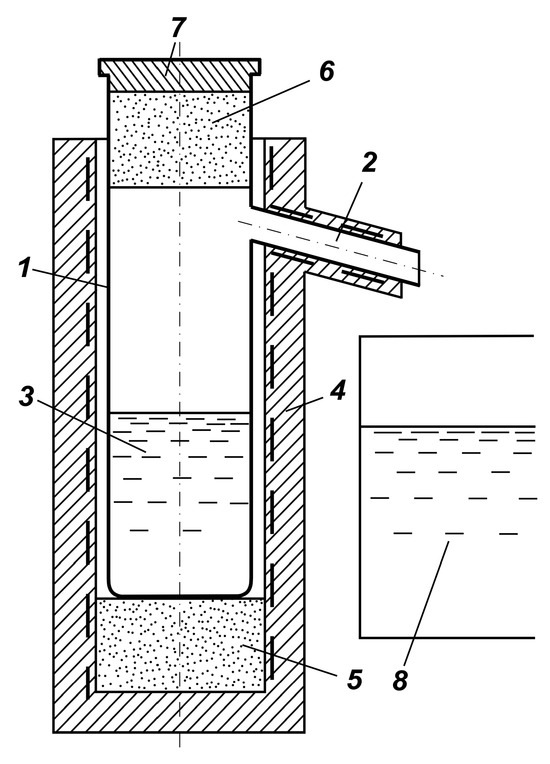
Figure 1.
Scheme of the installation for granulation of liquid selenium condensate: (1) retort, (2) pipe for condensate, (3) selenium, (4) resistance furnace, (5), (6) heat insulating inserts, (7) lid, (8) water tank.
The process is performed as follows. In the retort in the cold state, 1.5 kg of initial selenium is loaded, the heat-insulating insert is set and the lid closed. The retort is placed in the resistance furnace on the heat-insulating insert, so that the temperature in the retort during heating is constant throughout the volume. Electric power is supplied to the resistance furnace and the condensate pipe heater. The loaded selenium is heated to boiling point at atmospheric pressure, 672 °C, and converted to vapor state. The vapor is condensed in the spigot at a temperature of 225–240 °C. The liquid condensate of selenium drains off, crushed into droplets, which fall into water. Capacity is determined by the amount of electrical power supplied and the area of vaporization of liquid selenium. The process is stable and does not require precise maintenance of parameters.
As a result, disk-shaped granules were obtained (Figure 2) with an average size of 72 measurements on the diameter of 4 mm at the impact of the drop with water. This is due to the fact that the temperature of liquid condensate of selenium was slightly higher than 225–240 °C (we did not measure it) and, when falling from a height to water of 100–150 mm and quenching, it took the specified shape.

Figure 2.
Selenium granules after distillation and granulation of condensate.
When converted to spherical shape by the expression, , where: D—granule diameter, cm; Q—granule mass, g; and d—selenium density, g/cm3, the drop-granule diameter was 0.31 cm = 3.1 mm.
Large power consumption due to the transfer of the entire mass of selenium in the vapor state and insignificant purification from low volatile impurities, lead chromium, iron, nickel, and copper in the absence of such for tellurium, prompted the search for another technical design of the process.
Another solution of technical design of the granulation process was the flow of liquid selenium at a temperature of 225–240 °C through calibrated holes with self-decomposition of the jet on granules into water.
The scheme of the installation for implementation of the mentioned process is shown in Figure 3.
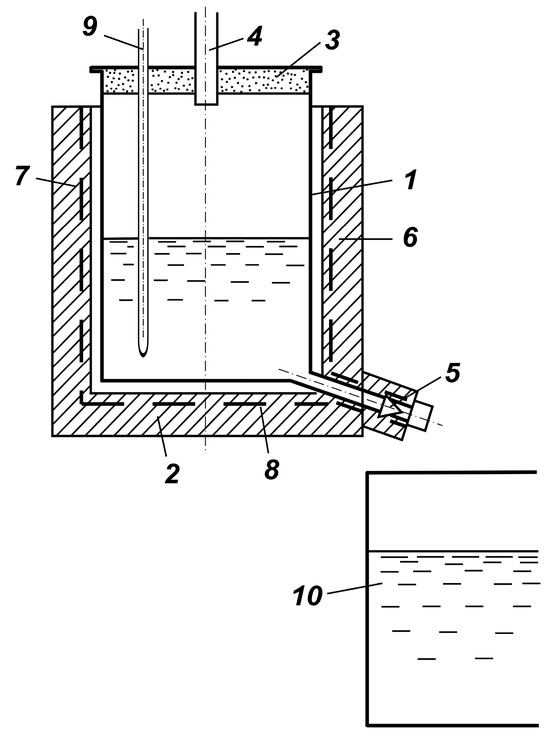
Figure 3.
Scheme of the selenium granulator by flow through the hole: (1) body, (2) bottom base, (3) cover, (4) gas duct, (5) needle tap, (6) heat insulation, (7), (8) heaters, (9) thermocouple cover, (10) water tank.
The device contains a housing 1, equipped in the lower part with a spigot with a replaceable tip with a different diameter of holes. A needle tap 5 is mounted on the spigot. The housing has a bottom part 2. Resistance heaters 7 and 8 and heat-insulating layer 6 are mounted on the housing and bottom part. The housing is covered with cover 3, in which gas duct 4 and thermocouple cover 9 are installed. The pipe with calibrated orifice is placed above the container with distilled water. The distance from the calibrated hole to the water cutoff in the initial period is 100 mm. The gas duct pipe is connected to the exhaust ventilation.
The granulation process is performed as follows. The initial selenium subject to granulation is loaded into the housing. The temperature is raised by turning on the heaters to 240–250 °C. The needle tap organizes the flow of selenium through the spigot and the hole. Due to self-decomposition of the stream selenium, drops fall into a container with water. In the process of experiments, it was found that changing the diameter of the calibrated orifice from 3 to 6 mm has no effect on the size of the obtained granules. As a result, granules of almost the same size of 2.9–3.0 mm are obtained (Figure 4).

Figure 4.
Selenium granulated by jet expulsion.
The process runs stably at a constant temperature of molten selenium. If necessary, an additional amount of initial selenium can be loaded into the housing.
Such a process design significantly reduces energy costs compared to the previous variant of granule production. However, the process is characterized by relatively low productivity of the equipment.
A significant increase in specific productivity was achieved by technical design of the granulation process by passing the flow of liquid selenium through sieves with different diameter of holes and density of perforation (Figure 5).
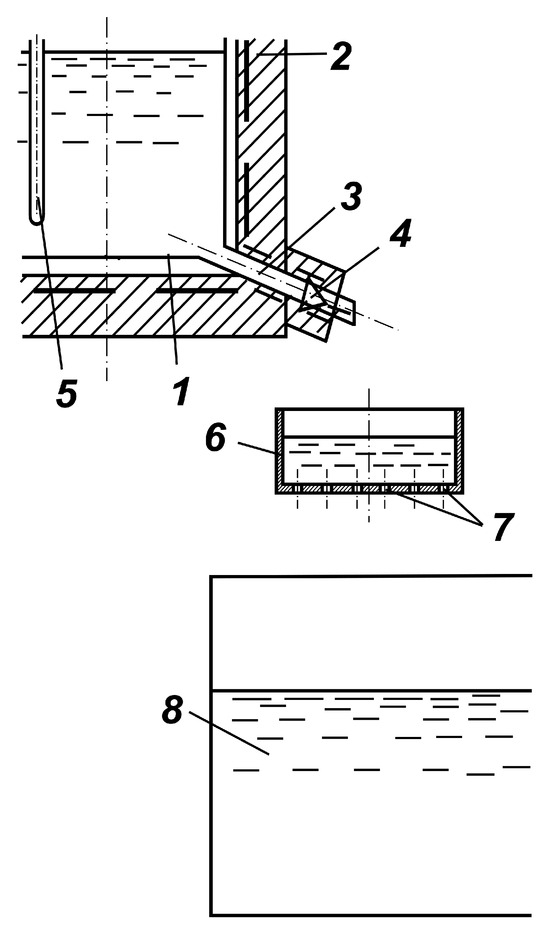
Figure 5.
Scheme of granulator by passing liquid selenium through sieves: (1) container with selenium, (2) resistance furnace, (3) drain pipe; (4) needle tap, (5) thermocouple cover, (6) sieve, (7) sieve holes, (8) water tank.
The equipment contains a container with selenium placed in a resistance furnace. The vessel is provided with a drain connection with a needle tap. A sieve with a diameter of 70 mm with holes is placed below the cut of the drainage pipe at a distance of 95 mm. A water tank is placed under the sieve. The distance from the bottom of the sieve to the water level is 140 mm. The distance from the drain pipe cut to the bottom of the sieve is 70 mm.
The process is performed as follows. The flow of molten selenium from the container at a certain temperature (225–250 °C) by opening the needle tap through the spigot is poured at a certain rate onto the sieve so that, on the sieve, a layer of liquid selenium of a thickness of 3–10 mm formed. Droplets falling into the water are formed when the selenium flows through the holes and with self-decomposition of the jet.
Conditions of selenium granulation are given in the Table 1.

Table 1.
Conditions of liquid selenium granulation.
In all experiments, a large specific productivity of obtaining a granulated element was observed, which can be recommended for industrial production if the equipment is compact. However, at high productivity, the formation of bars with the ratio of diameter to length of 1:10 ÷ 15 and filamentous formations were observed. A decrease in the process speed on lowering the temperature of the melt is expected so, when changing the temperature from 250 to 225 °C and the diameter of holes to 2.0 mm, the specific granulation rate changed from 181.3 to 31.1 kg/(dm2 × h). The latter seems to be due to a decrease in the viscosity of molten selenium. The granules were extracted from water and subjected to natural drying. The yield of granulation product fractions during sieving is presented in Table 2.

Table 2.
Fractional composition of granulation products.
It can be seen that, with decreasing the diameter of the sieve holes and temperature of 250 °C, the granules of one or two sizes prevail. Increasing the size of the sieve apertures puts the process in an unstable state with the predominance of formations in the form of bars of different shapes and lengths. However, if the granulation process has the purpose of transferring selenium ingots to the ground state used in practical application, the latter is acceptable. When granulating selenium at a temperature of 250 °C through a sieve with a hole diameter of 1.5 mm, the predominant amount of more than 95% of granules with a size of (−2.5 + 2.0) mm was obtained; Figure 6.

Figure 6.
Selenium granules obtained through a sieve with holes of 1.5 mm.
It can be seen that almost all granules have the same size and spherical shape.
When the water temperature increases during selenium granulation above 50–55 °C, the formation of conglomerates from selenium granules is possible (Figure 7) due to insufficient cooling rate, as it is known that, at 70 °C, the element softens.
Figure 7.
Conglomerate of selenium granules.
As a consequence, during selenium granulation, it is necessary to maintain the temperature of the water into which the granules fall not higher than 40–45 °C and, if possible, to remove the granules from the place of falling into the water.
Thus, as a result of technical and technological research, equipment was developed and the process of granulation of liquid selenium into water was implemented. When the process of granulation of selenium with a temperature of 220–250 °C through unheated sieves with a hole diameter of 1–2 mm was utilized, a specific productivity of up to 180 kg/(dm2 × h) was achieved. The latter corresponds to the productivity of industrial production.
4. Electron Microscopic Studies of Selenium Before and After Granulation
Due to the fact that, during granulation, the initial selenium hardening in water takes place, it is of interest to analyze electron microscopic studies of the element before and after granulation.
The electron microscopic image of a spherical granule with low magnification (×40) corresponds to Figure 8. The image contains spherical and other shaped voids. The composition of the surface outside the depressions formed after grinding the granules corresponds to 100% selenium.
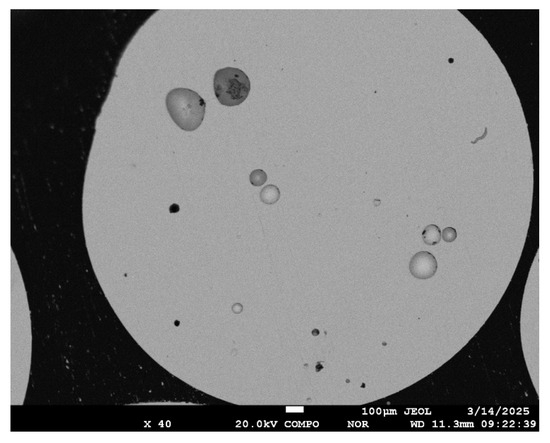
Figure 8.
Microstructure of the cross-section of a selenium granule.
The concentration of impurities inside the void formations is noted, the elemental composition of which is given below.
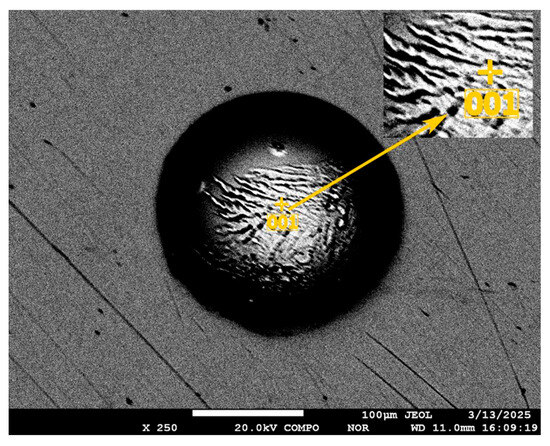
Figure 9.
A spherical void in the mass of initial selenium.

Table 3.
Composition and amount of elements at the bottom of the depression.
Carbon-dominated inclusions are observed in similar locations (Figure 10); composition is given in Table 4.
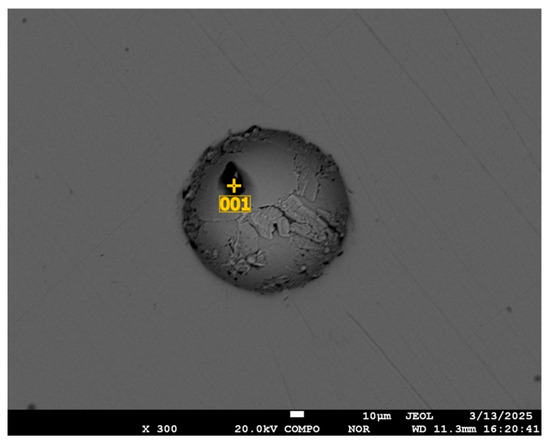
Figure 10.
Spherical void and impurity in the mass of initial selenium.

Table 4.
Composition and amount of inclusion elements in the depression.
Impurity elements are also localized outside the spherical formations in the selenium mass (Figure 11, Table 5).

Figure 11.
Impurity elements in the mass of initial selenium.

Table 5.
Composition and amount of localized impurity elements.
Similar studies were performed for granulated selenium. The presence of spherical or close to spherical in shape voids, but in greater quantity, as well as the concentration of impurity elements at the selenium boundary, was noted (Figure 12). The composition of impurities and their amounts are shown in Table 6.
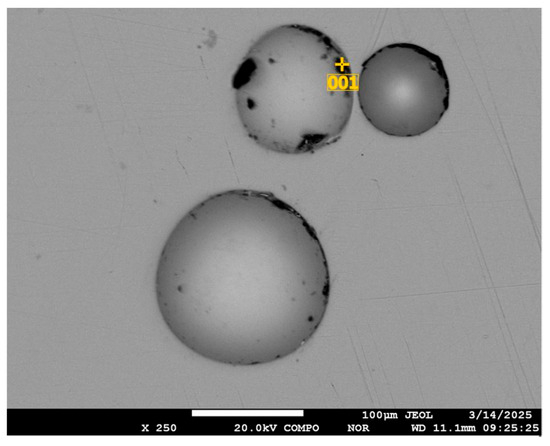
Figure 12.
Voids and concentration of impurities in them in selenium granules.

Table 6.
Composition and amount of elements in the voids of selenium granules.
The amount of carbon in the lower (Figure 12) void increases according to microprobe analysis data up to 44.71 wt. %.
In the granules there are local places of concentration of impurity elements, as well as in the initial selenium (Figure 13).
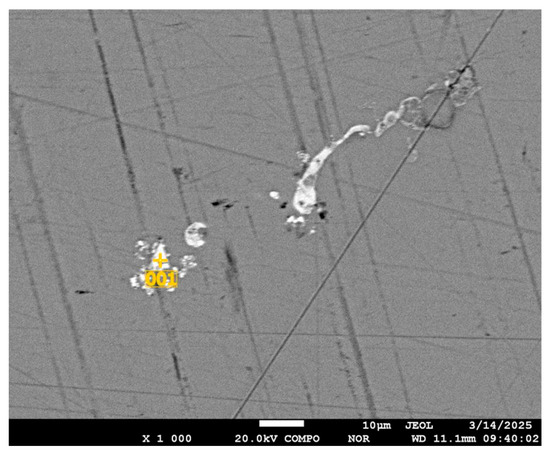
Figure 13.
Impurity elements in the mass of selenium in granules.
The composition and amount of impurity elements in the sampling point in Figure 13 is shown in Table 7; the right formation (Figure 13) of impurity elements is shown in Table 8.

Table 7.
Composition and amount of localized impurity elements in the granules.

Table 8.
Composition and amount of localized impurity elements in the right formation in the granule.
Thus, spherical and similar void formations were discovered in granulated selenium. Analyzing the data of the study, the increase in the number of voids in the granulated selenium and localization of impurities at the boundary of selenium and void formation should be noted. Impurities, apparently represented by selenides of elements, are distributed in the mass of selenium in local areas with a non-repeating composition. The amount and elemental composition of the impurity concentration regions vary randomly.
5. Investigation of the Aqueous Composition After Selenium Granulation
The possible presence of selenium dioxide on the surface of granules or in the gas space above the water tank, where the granules fall, was the basis for the IR spectroscopy of the cooling liquid.
The object of the study was water after granulation. The mass ratio of cooled granules and water was 2:1. The comparison was made with distilled water.
The spectrum (Figure 14) shows absorption bands of valence ν(OH)—3307.32 cm−1, deformation δHOH—1637.27 cm−1 and liberation νL H2O with a maximum at the wave number of 579.504 cm−1 of vibrations of molecular water [29].
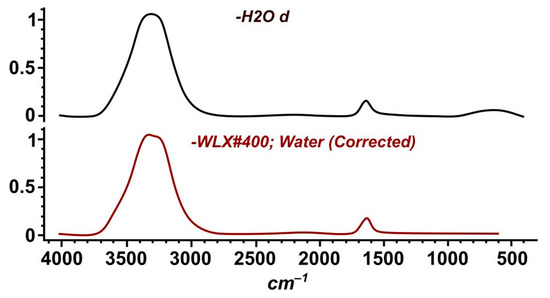
Figure 14.
IR spectrograms of water: upper—distilled water; lower—cooling water during granulation.
As a result, it was found that the water after cooling the granules by 96.37% corresponds to distilled water. At the same time, the qualitative analysis established the value of pH = 6, which corresponds to a weakly acidic reaction. The latter, apparently, is caused by an insignificant amount of selenic acid, H2SeO3.
6. Conclusions
At the request of a company producing selenium, and due to the lack of published data, patented technical and technological solutions, work was carried out to create a design for the granulation process of a rare element.
As a result of technological research and technical solutions for process design, the equipment was developed and the process of granulation of liquid selenium into water was implemented. When running the process of selenium granulation with a temperature of 225–250 °C through unheated sieves with the diameter of holes of 1–2 mm, the specific productivity up to 180 kg/(dm2 × h) was achieved. The latter corresponds to the productivity of industrial production and entails a reduction in operating costs, simplification of manipulations with dispersed material and dosing in processes where a rare element is used.
Electron microscopy studies revealed the localization of impurity elements in both the raw and granulated selenium. Uniform distribution of impurities on the mass of selenium was not found. The composition and content of elements in localized areas, even located next to each other, is arbitrary. Thus, one localization contains, wt. %: C—7.64; S—6.04; As—4.85; and Pb—40.72. Another, located nearby, contained C—4.68; Te—45.42; and Pb—12.67. In granulated selenium an increase in the number of spherical or close to spherical in shape voids was noted, on the boundaries of which impurity elements are concentrated. The change in chemical composition of granules in comparison with the initial selenium was not observed.
The composition was found to coincide with distilled water by 96.37%, which indicates a weak oxidation of the processed selenium when studying the composition of water used for selenium granulation.
The developed technology and its technical design can be used for dispersing other low-melting elements.
Author Contributions
Conceptualization, V.N.V. and S.A.T.; methodology, V.N.V.; investigation, B.K.K. and S.A.T.; data curation, V.N.V., A.V.N. and X.A.L.; writing—original draft preparation, V.N.V., S.A.T., B.K.K., A.V.N., B.M. and X.A.L.; writing—review and editing, S.A.T., V.N.V., A.V.N., B.M. and X.A.L.; visualization, A.V.N., S.A.T. and X.A.L.; project administration, S.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant AP19676703).
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Zainullin, L.A.; Sharanov, M.A.; Shulmeister, A.Y.; Dubichev, V.R. Installation for Melt Granulation. Copyright Certificate of the USSR No. 1662971, 15 July 1991. Available online: https://patents.su/2-1662971-ustanovka-dlya-granulyacii-rasplavov.html#text (accessed on 13 January 2025).
- Zainullin, L.A.; Greznev, V.G.; Mekhryakov, D.V.; Zainullin, R.V. Installation for Granulation of Slag Melt and Method of Granulation with Obtaining a Dry Product. Russian Federation Patent No. 2717322, 20 February 2020. [Google Scholar]
- Sinitsyn, N.N.; Dontsova, Y.V. Method of Granulation of Slag Melts. Russian Federation Patent No. 2790646, 28 February 2023. [Google Scholar]
- Zherdev, A.V.; Weintraub, S.S.; Rybinov, V.A.; Mosiashvili, V.V.; Tokmakov, M.K. Method of Granulation of Metallic Melts. Copyright Certificate of the USSR No. 263639, 10 November 1970. Available online: https://patents.su/1-263639-263639.html (accessed on 13 January 2025).
- Sadovnikova, R.A.; Koprov, M.Y.; Girshov, V.L.; Fomichev, K.M.; Anastasiadi, G.P.; Lebedev, V.N.; Sigachev, Y.N. Device for Granulation of Metallic Melts. Copyright Certificate of the USSR No. 709246, 15 January 1980. Available online: https://patentdb.ru/patent/709246 (accessed on 13 January 2025).
- Stepanova, T.A.; Lisitsyn, A.O. Drum-Type Granulator for Metal Melt. Russian Federation Patent No. 172045, 27 June 2017. [Google Scholar]
- Miryuk, O.A.; Zagorodnyuk, L.H. Granular materials based on expanded sands and their production waste. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2022, 321, 14–21. [Google Scholar] [CrossRef]
- Miryuk, O.A. Granular magnesia compositions. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2021, 316, 32–39. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Trebukhov, S.A.; Volodin, V.N.; Trebukhov, A.A.; Tuleutay, F.H. Selenium extraction out of metallurgical production middlings. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2018, 307, 56–64. [Google Scholar] [CrossRef]
- Volodin, V.; Trebukhov, S.; Nitsenko, A.; Linnik, X.; Tuleutay, F. Thermodynamics of antimony—Selenium alloys formation and evaporation. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2023, 330, 13–21. [Google Scholar] [CrossRef]
- Bairwa, A.; Reddy, A.K.; Singh, G.; Sharma, V.K. Granulation and Atomization Process for Production of metal Granules and Powders. Int. J. Mech. Prod. Eng. 2018, 6, 24–28. [Google Scholar]
- Ahmed, M.J.; Schollbach, K.; van der Laan, S.; Brouwers, H.J.H. Reactivity of air granulated basic oxygen furnace steel and its immobilization of heavy metals. J. Build. Eng. 2024, 91, 109704. [Google Scholar] [CrossRef]
- Kazachkov, I.V. Granulation of Metals as One of the Methods for Production of the Unique New Materials. Mod. Appl. Mater. Sci. 2019, 1, 71–84. [Google Scholar] [CrossRef]
- Vesterberg, P.; Beskow, K.; Rick, C.-J. Granulation of Ferroalloys—Results from Industrial Operations and Comparative Study on Fines Generation: Efficient technologies in ferroalloy industry. In Proceedings of the Thirteenth International Ferroalloys Congress, Almaty, Kazakhstan, 9–12 June 2013; Volume 1, pp. 65–72. [Google Scholar]
- Polanco, F.H.; Damasceo, R. Granulation Methods for Metals and Ferroalloys. In Proceedings of the 72nd ABM Annual Congress, Sao Paulo, Brazil, 2–6 October 2017; Volume 72, pp. 400–410. [Google Scholar] [CrossRef]
- Glasunov, S.G.; Solonina, O.P.; Chereshneva, I.F. Oxidation-free granulation of metals. Met. Sci. Heat Treat. 1974, 16, 765–768. [Google Scholar] [CrossRef]
- Bobrovski, A.P.; Efimochkin, I.Y.; Bolshakova, A.N.; Khudnev, A.A. Production of spherical granules of a high-temperature metal based on molybenium. Proc. VIAM 2022, 1, 44–52. [Google Scholar] [CrossRef]
- Tan, P.; Jang, J.; Ji, J. Experimental simulation of the ancient production of gold granults. Herit. Sci. 2024, 12, 363. [Google Scholar] [CrossRef]
- Nechvoglod, O.V.; Sergeyeva, S.V.; Agafonov, S.N. Study of the Influence of the Granulation Modes of Cu2S Melt on the Granulometric and Structural Characteristics of Particles. Metallurgist 2021, 65, 82–89. [Google Scholar] [CrossRef]
- Ozerov, S.S.; Bogatyrev, D.M. Investigation of the process of copper–nickel matte granulation. Metallurgist 2023, 4, 71–77. [Google Scholar] [CrossRef]
- Zharov, M.V. Investigation of the properties of Al–Cu–Mg granulated materials pressed from the granules obtained by centrifugation technology at ultra-high cooling rates. Tekhnologiya Mashinostroyeniya 2021, 4, 5–9. Available online: https://elibrary.ru/item.asp?id=45699255 (accessed on 20 January 2025).
- Zharov, M.V. Processes of Obtaining Granular Materials from Al–Zn–Mg–Cu Aluminium Alloys Using Ultra–Fast Granular Cristallization. Metallurgist 2022, 66, 276–289. [Google Scholar] [CrossRef]
- Zharov, M.V. Production of quality NiAl granules: Study of the process peculiarities. Tsvetnye Met. 2022, 11, 50–56. [Google Scholar] [CrossRef]
- Filyashin, M.K.; Korolev, M.G.; Savchenko, V.I.; Astakhov, A.N.; Ilyukhin, V.I.; Bary-atinskii, V.P.; Yaroshenko, A.V.; Yevsyukov, V.N.; Bubnov, S.Y.; Polyakov, V.N. Device for Granulation of Aluminum Melts. Useful Model of the Russian Federation No. 16468, 10 January 2001. Available online: https://patents.s3.yandex.net/RU16468U1_20010110.pdf (accessed on 20 January 2025).
- Silayev, A.F.; Fishman, B.D. Dispersing of Liquid Metals and Alloys; Metallurgiia: Moscow, Germany, 1983; p. 144. [Google Scholar]
- Scherban, A.P.; Kovtun, G.P.; Gorbenko, Y.V.; Solopikhin, D.A.; Virich, V.D.; Pirozhenko, L.A. Preparation of high-purity granulated metals: Cadmium, zinc, lead. Tekhnologiya i Konstr. v Elektron. Appar. 2017, 55–60. [Google Scholar] [CrossRef]
- Hubertus, J.M.S.; Cornelis, H. Method of Obtaining Granulated Sulfur. Copyright Certificate of the USSR No. 1484293, 30 May 1989. Available online: https://patents.su/6-1484293-sposob-polucheniya-granulirovannojj-sery.html (accessed on 20 January 2025).
- Pestova, G.S. Modeling of the Granulation Process of Metallic Melts. Publishing House Education and Science s.r.o. Available online: http://www.rusnauka.com/8_NMIW_2008/Tecnic/27104.doc.htm (accessed on 15 March 2025).
- Nakamoto, K. IR and Raman Spectra of Inorganic and Coordination Compounds; Mir: Moscow, Germany, 1991; p. 536. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).