Abstract
Natural phosphate ores frequently contain calcium–magnesium carbonate minerals as gangue components. Their separation from target phosphate minerals poses significant challenges due to analogous surface characteristics. The flotation differentiation between fluorapatite and dolomite remains a key research focus in mineral processing. In conventional collector systems, selective depressants critically govern separation efficiency, as their interfacial specificity directly determines beneficiation outcomes. The selective depression behavior of fulvic acid (FA) in modulating fluorapatite–dolomite separation efficiency within oleate-dominated flotation systems was elucidated through micro-flotation experiments, complemented by zeta potential measurements, contact angle analysis, Fourier-transform infrared spectroscopy (FTIR), and molecular dynamics (MD) simulations. The findings revealed that fluorapatite and dolomite both exhibit high floatability under NaOl-mediated collector systems in the absence of depressant additives, leading to negligible selectivity in the differential separation of the mineral pair. However, the float of fluorapatite particles in weakly acidic conditions was strongly depressed when a small amount of FA was added as a depressant, while exerting minimal impact on dolomite’s floatability. In binary artificial mixed-mineral flotation systems, under optimized flotation conditions (pH 5.0, 60 mg/L NaOl, and 15 mg/L FA), the concentrate achieved a P2O5 grade of 33.86% with a fluorapatite recovery rate of 92.36%, demonstrating significant selective separation of fluorapatite from dolomite. Subsequent analysis revealed that FA competitively chemisorbs with NaOl on fluorapatite surfaces, selectively reducing the hydrophobicity of the fluorapatite surface and suppressing fluorapatite floatability, thereby enabling effective differential liberation of the mineral pair.
1. Introduction
Phosphate mineral deposits constitute essential feedstocks that have been universally adopted in many important fields such as agriculture, medicine, and the military [1,2,3]. As a strategically crucial mineral resource, these deposits are pivotal for sustaining industrial development in China [4,5]. In recent years, high-grade phosphate ore resources suitable for direct use have exhibited a declining trend alongside the progressive advancement of phosphorus-based chemical production. Consequently, the focus of phosphate ore development and utilization has gradually turned to complex and refractory low-grade phosphate ores, which typically contain associated calcium (magnesium) and siliceous gangue minerals in addition to phosphate minerals. These gangue minerals significantly impact subsequent wet phosphoric acid preparation [6,7,8]; for example, excessive magnesium impurities will significantly increase acid consumption, reduce product quality, and adversely affect phosphorus chemical production [9]. Among gangue constituents in phosphate-bearing deposits, dolomite predominates as a prevalent phase requiring consideration. Nevertheless, the surface property similarities between dolomite and fluorapatite lead to mutual selectivity impairment in conventional fatty-acid-based collector systems, creating significant barriers to efficient mineral separation in phosphate ore beneficiation [10,11,12].
Flotation reagents are key to the flotation process, with collectors and inhibitors exerting the most direct effect on mineral separation [13,14]. The strategic application of selective depressants enables differential regulation of collector adsorption dynamics at target mineral interfaces, while process-contingent modifications demonstrating enhance interfacial selectivity in phosphate beneficiation systems [15]. Thus, depressant selection is critical to achieving efficient separation of fluorapatite from dolomite. Previous studies have shown that carboxymethyl cellulose (CMC) [16], sodium alginate (SA) [17], gum Arabic [18] and pectin [19] serve as depressants of carbonate minerals in phosphate ore flotation system, all being non-toxic and degradable [20]. In reverse beneficiation circuits targeting phosphatic matrices, conventional acidic modifiers (H2SO4/H3PO4) are strategically employed as selective depressants of phosphate minerals [21]. Furthermore, researchers have identified hydroxyethyl idophosphate (HEDP) [22], amino trimethylene phosphonic acid (ATMP) [23], diethylene triamine-pentamethylene phosphonic acid (DTPMP) [24] and gallic acid [25] can be used as novel green, efficient phosphate depressants, noting their interaction with fluorapatite surfaces is remarkably stronger than with dolomite. Consequently, developing novel inhibitors for phosphate mineral flotation is essential and urgent.
FA, a structurally disordered humic substance, exhibits a polyfunctional molecular architecture containing electron-donating moieties (e.g., carboxyl (-COOH), carbonyl (C=O), and phenolic -OH groups) that govern its chelating capacity in mineral flotation [26]. Beyond its interfacial activity, FA’s environmental compatibility positions it as a sustainable depressant. FA originates from microbial breakdown and transformation of plant residues and exhibits inherent biodegradability. Additionally, FA possesses high water solubility and physiological activity, finding applications in medicine, agriculture, etc. [27,28,29]. Given its structural properties, FA is theoretically well-suited for application in phosphate ore flotation separation; however, no prior studies report this application. Based on the characteristics of fluorapatite and dolomite, this study explored the effect of FA as a depressant using pure mineral flotation tests within the NaOl collector system for fluorapatite–dolomite separation, combined with zeta potential, contact angle, FTIR analysis, and MD simulations the selective depression mechanism. The research provides fundamental insights and practical strategies for achieving differential beneficiation of the isostructural calcium-bearing mineral pairs via froth flotation.
2. Materials and Methods
2.1. Materials
Hand-selected high purity fluorapatite and dolomite mineral crystals were crushed, ground, and sieved to obtain pure mineral samples in the size fraction of 38–75 μm. The fraction less than 38 μm was used for subsequent detection and analysis. The pH value was adjusted using HCl and Na2CO3, while sodium oleate (NaOl) served as the collector. All chemical reagents (analytical grade) were procured from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without further purification. FA (≥98%) was obtained from Hefei Qiansheng Biotechnology Co., Ltd. (Hefei, China), and used as the depressant. All solutions and slurries in the experiments were prepared using 18.2 MΩ·cm resistivity ultrapure water.
2.2. Micro-Flotation Experimental Method
Bench-scale flotation experiments were conducted in a custom-designed mini flotation cell. For each test, 2.0 g mineral sample and 70 mL ultrapure water were added to the cell. Pulp temperature was maintained at 25 ± 0.5 °C. A magnetic impeller (Shanghai Lichen Instrument Technology Co., Ltd. Shanghai, China) with the speed set at 900 rpm was used to agitate the pulp in flotation vessel. The pulp was continuously stirred for 2 min to achieve uniform mixing. Then, the slurry pH was adjusted with HCl/Na2CO3 solutions, followed by depressant (FA) and collector (NaOl) addition at specified intervals. After the flotation reagents were mixed homogeneously, air was blown into the flotation cell at a determined flow rate, and the froth was continuously scraped for 3 min. Froth products and pulp residues were then filtered, dried, and weighed to determine recovery.
Fluorapatite–dolomite systems were homogenously mixed at 1:1 mass ratio to constitute the feed material (theoretical P2O5 content of 20.13%) for the binary artificial mixture flotation experiments. The beneficiation protocol for the fluorapatite–dolomite binary system mirrored the benchmark conditions established for the single mineral flotation test. After the flotation was completed, final concentrates and rejection products were subjected to sequential vacuum filtration, and the P2O5 content in the flotation products was analyzed and measured through acid-base titration method. That is, in the nitric acid medium, ammonium molybdate reacted with phosphorus to form a yellow ammonium phosphomolybdate precipitate, and this precipitate was dissolved using a standard sodium hydroxide solution after filtration, with phenolphthalein as the indicator. Subsequently, the excess sodium hydroxide was titrated using a standard nitric acid solution to determine the phosphorus content. The flotation separation effect could then be calculated and evaluated using relevant formulas [30].
2.3. Zeta Potential and Contact Angle Measurements
This study systematically examines the electrokinetic behavior of mineral particles before and after reagent interaction to elucidate fundamental interaction mechanisms regulating flotation-based mineral–reagent interfaces. A 0.3 g aliquot of −5 μm particles was dispersed in 70 mL ultrapure water with 5 mL 1 × 10−3 mol/L KCl solution. After pH value adjustment using HCl/Na2CO3 solutions, 15 mg/L FA and 60 mg/L NaOl were introduced sequentially. Mechanical agitation was maintained for three consecutive minutes and then the mixture was allowed to settle for 10 min. The colloidal supernatant obtained through phase separation was subjected to zeta potential determination employing a Malvern Zetasizer Nano ZS90 system (Malvern Panalytical, Worcestershire, UK). The mean zeta potential value was calculated from three independent experimental runs with standard deviation analysis.
Concurrently, the dominant mechanisms of flotation reagents on mineral surface wetting characteristics, as reflected through contact angle variations, were systematically investigated to elucidate hydrophobicity modulation patterns. The contact angle quantifies surface hydrophobicity and provides critical insights into reagent-induced selectivity variation in mineral flotation systems [31,32]. Contact angle evolution was monitored following reagent introduction. 5.0 g mineral was agitated in 70 mL water (800 rpm, 2 min). After pH stabilization (at about 5.0), FA was added and the mixture was stirred for 10 min, followed by NaOl (10 min stirring). Treated samples were filtered, dried, pelleted, and analyzed via the sessile drop method (JC2000D3M goniometer, Shanghai, China) Measurements from three pellet areas (triplicate each) yielded arithmetic means.
2.4. FTIR Detection
The surface properties of minerals before and after interaction with reagents were characterized using the Fourier-transform infrared spectrometer (Nicolet 380, Thermo Fisher Scientific, Waltham, MA, USA) to elucidate the interfacial interactions between flotation depressants/collectors and heterogeneous mineral substrates. Sample preparation adhered to the established flotation parameters: reagent concentration thresholds and stage-wise addition sequences. For each test, 1.0 mg aliquot of the analyte was homogenously blended with 100 mg potassium bromide matrix and pelletized. The infrared spectral scan range was set from 400 to 4000 cm−1, with 4 cm−1 scan resolution.
2.5. Molecular Dynamic Simulation
The adsorption behavior of FA on fluorapatite and dolomite surfaces was investigated through MD simulations performed using Materials Studio 2017 (BIOVIA, San Diego, CA, USA). Crystallographic models of both minerals were initially constructed and subsequently subjected to systematic geometry optimization via the CASTEP module to achieve minimum energy configurations [33], following computational parameters established in prior methodology. Subsequent refinement of the mineral–FA adsorption system was implemented through energy minimization procedures within the Forcite module [34], with detailed simulation protocols consistent with our previous investigations [35]. The interfacial interaction strength was quantitatively evaluated through adsorption energy (ΔEads) calculations according to established thermodynamic principles [36]:
where Ecom, Emin, and EFA represent the total energies of the optimized adsorption complex, pristine mineral surface, and the isolated FA molecule, respectively. A more negative ΔEads value signifies the enhanced thermodynamic stability of the adsorption system, which is a fundamental criterion for assessing reagent–mineral affinity in froth flotation systems.
ΔEads = Ecom − (Emin + EFA)
3. Results and Discussion
3.1. Mineral Composition Analysis
Chemical multi-element analysis and X-ray powder diffraction (XRD) analysis were used to analyze the chemical and mineral composition of pure fluorapatite and dolomite samples, with corresponding data presented in Table 1 and Figure 1. As shown in Table 1, fluorapatite comprises 40.26% P2O5 and 53.21% CaO, while dolomite comprises 52.64% CaO and 45.60% MgO, with minor contributions from other components. Combining these results with Figure 1, both minerals exhibit >95% purity, satisfying the purity requirements for flotation tests. The representative molecular structure of FA is depicted in Figure 2, which is consistent with the literature reports [26].

Table 1.
Chemical elemental analysis results of fluorapatite and dolomite samples.
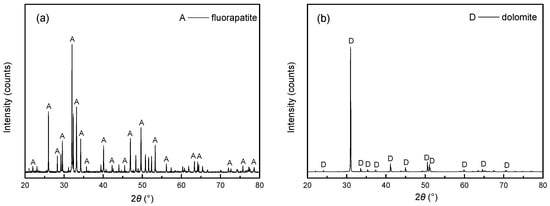
Figure 1.
XRD analysis results of (a) fluorapatite and (b) dolomite.
Figure 2.
Chemical structures of FA [26] (O, C, and H are color-coded as red, gray, and white, respectively).
3.2. Micro-Flotation Experimental Results
(1) Single mineral flotation
The flotation response of fluorapatite and dolomite to NaOl concentration gradients was quantitatively assessed under ambient pH and controlled slurry temperature (25 ± 0.5 °C), with separation efficiencies comprehensively documented in Figure 3a. As shown in Figure 3a, both fluorapatite and dolomite exhibited comparable hydrophobicity responses to NaOl under natural pH conditions. The recovery profiles showed concentration dependence, with recoveries for both species exceeding 90% at the critical concentration (60 mg/L). A slight enhancement in recovery was observed when increasing NaOl dosage to 80 mg/L, indicating reagent saturation at mineral surfaces. It can be seen that the apparent floatability similarity precludes effective separation through collector dosage modulation alone in the absence of selective depressants. The optimal NaOl concentration was therefore determined as 60 mg/L for subsequent separation studies, balancing recovery efficiency with reagent consumption minimization.
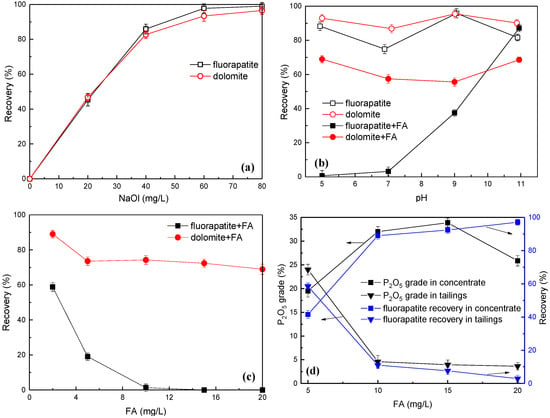
Figure 3.
Effect of NaOl dosage (a), pulp pH value (b), and FA dosage (c) on the flotation recovery of fluorapatite and dolomite and effect of FA dosage on the flotation separation efficiency (d).
Under controlled conditions (25 ± 0.5 °C, 60 mg/L NaOl), the effects of pH variation on the selective separation behavior between fluorapatite and dolomite were rigorously examined with/without FA, as quantitatively illustrated in Figure 3b. Figure 3b demonstrates that under depressant-free conditions, pulp pH exerted negligible influence on fluorapatite and dolomite floatability, with sustained recoveries above 70% and the difference in recovery rate between the two minerals was not significant. Consequently, sole modulation of pulp pH failed to establish sufficient selectivity between the two minerals, precluding their separation through pH adjustment alone, which is consistent with prior findings [37]. Upon the introduction of 10 mg/L FA, dolomite retained recoveries exceeding 55% across the tested pH range, whereas fluorapatite underwent significant suppression, particularly under acidic conditions, where near-complete inhibition was achieved. Maximum selectivity occurred at pH 5.0, demonstrating FA’s preferential adsorption on fluorapatite surfaces. The optimal performance at pH 5.0 can be attributed to the favorable ionization state of the collector, which enhances its interaction with the mineral surface. At pH 5.0, the target mineral achieves a surface charge that promotes selective adsorption, while competing ions remain less active, thereby improving overall selectivity and efficiency. Thus, it can be concluded that FA serves as a selective depressant capable of differential interfacial modification, strong chemisorption on fluorapatite contrasts with weak physical adsorption on dolomite, enabling effective separation through pH-controlled depression strategies.
The differential depression efficacy of FA was systematically evaluated under optimized flotation parameters (25.0 ± 0.5 °C, 60 mg/L NaOl, pH 5.0), as quantitatively detailed in Figure 3c. As illustrated by Figure 3c, with the increase in FA dosage, the recovery rate of fluorapatite significantly decreased. The experimental results demonstrated concentration-dependent inhibition effects on mineral recovery rates. When FA concentration exceeded 10 mg/L, fluorapatite recovery was effectively suppressed to near-zero levels. For dolomite, a gradual decline in recovery was observed (from 85.4% to 72.1%) with increasing FA concentrations up to 5 mg/L, beyond which no statistically significant variation occurred. Maximum differential recovery between the two minerals was achieved at 10 mg/L FA, which was established as the optimal inhibitor dosage through systematic evaluation of separation efficiency. Unlike CMC and alginate, which require alkaline conditions (pH > 9) for efficacy, FA achieves selective depression at pH 5.0. This enables integration into existing acidic flotation circuits without pH adjustment costs. Moreover, FA’s dosage (15 mg/L) is 40–50% lower than gallic acid (25–30 mg/L) for comparable P2O5 recovery [16,17,25]. Unlike plant-derived gallic acid, FA sourced from abundant lignite deposits significantly reduces reagent expenses while maintaining superior selectivity.
(2) Artificial mixed minerals flotation
Systematic investigations were conducted to characterize FA-mediated separation dynamics in binary mineral systems. Flotation tests using artificial mixed fluorapatite–dolomite were performed under controlled conditions: pulp temperature (25.0 ± 0.5 °C), 60 mg/L NaOl collector, and pH 5.0. As shown in Table 2 and Figure 3d, the concentrate recovery exhibits monotonic enhancement with increasing FA concentration, while P2O5 grade followed a parabolic profile peaking at 33.86% (15 mg/L FA). This extremum corresponded to optimal separation performance, yielding 55.80% mass recovery with exceptional P2O5 upgrading efficiency (recovery of 92.36%). The inverse relationship between recovery and grade beyond the critical 15 mg/L threshold suggested competitive adsorption mechanisms requiring further surface analysis.

Table 2.
Flotation performance of artificial mixed minerals under varying FA dosages.
3.3. Electrokinetics Behavior Analysis
Surface electrokinetic modulations are intrinsically linked to reagent adsorption phenomena at mineral interfaces. Zeta potential analysis provides mechanistic insights into pH-dependent reagent–mineral interfacial interactions [15]. A systematic investigation of pH-modulated zeta potential evolution was conducted for reagent-treated fluorapatite and dolomite systems, with comparative data presented in Figure 4.
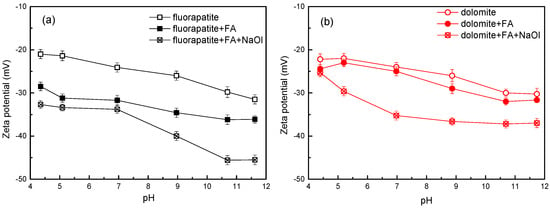
Figure 4.
Zeta potential results of (a) fluorapatite and (b) dolomite before and after being treated with flotation reagents.
As shown in Figure 4a, fluorapatite maintained a negative zeta potential across the experimental pH conditions, showing a progressive decline at higher pH values, consistent with prior observations [38,39]. Following the addition of FA, the zeta potential of fluorapatite exhibited a pronounced negative shift, likely attributable to preferential FA adsorption on its surface. Studies have demonstrated that FA predominantly dissociates into anionic species within weakly acidic to alkaline media [28]. The anionic FA can undergo chemical adsorption on the negatively charged surface of fluorapatite. Furthermore, limited zeta potential modulation upon NaOl introduction implied that FA-derived colloidal films dominate fluorapatite surfaces through competitive adsorption, physically blocking NaOl interfacial interactions. Increasing solution alkalinity enhanced fluorapatite’s negative charge, indicating competitive adsorption between NaOl anions and FA on the fluorapatite surface, where the inhibitor fails to suppress NaOl deposition [40]. As shown in Figure 4b, dolomite exhibited consistently negative zeta potentials that decreased monotonically with increasing pH throughout the investigated range, aligning with published reports [41]. Following FA treatment, dolomite showed minimal zeta potential modulation, suggesting limited adsorption efficacy on its surface. Furthermore, persistent NaOl adsorption on FA-treated dolomite surfaces was evidenced by pH-dependent zeta potential modifications, indicating the inhibitor film permits collector penetration [42]. In summary, under weakly acidic conditions, the inhibitor FA selectively interacted on different mineral surfaces; however, in neutral–alkaline media, competitive adsorption between NaOl collector and FA inhibitor on fluorapatite compromises sustained flotation inhibition, thereby corroborating prior experimental data.
3.4. Contact Angle Analysis
Under controlled experimental conditions, the wettability behavior of fluorapatite and dolomite surfaces was systematically investigated under varying reagent regimes, including the presence and absence of inhibitors and collectors. These comparative analyses, detailed in Figure 5, elucidate the distinct responses of the mineral surfaces to reagent interactions. As illustrated in Figure 5, NaOl treatment induces significant hydrophobicity in both minerals, with contact angles of 113.5° (fluorapatite) and 117.0° (dolomite) [43]. In contrast, when only FA is introduced, the contact angles sharply decrease to 20.5° (fluorapatite) and 15.0° (dolomite), demonstrating that FA effectively preserves the inherent hydrophilicity of both mineral surfaces. Notably, sequential treatment with FA followed by NaOl revealed distinct mineral-specific behaviors: fluorapatite exhibited a marked reduction in hydrophobicity (contact angle: 78.3°), whereas dolomite retained its hydrophobic state (117.0°). These results demonstrate that FA selectively suppresses fluorapatite the hydrophobization while minimally interfering with dolomite’s response to NaOl. This differential wettability modulation aligns with flotation outcomes, where dolomite demonstrated superior floatability over fluorapatite. Thus, FA enabled effective separation by selectively tailoring surface hydrophobicity, highlighting its potential as a targeted depressant in mineral processing systems.
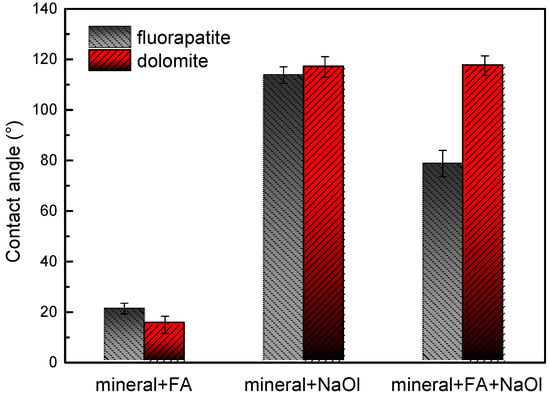
Figure 5.
Contact angle between fluorapatite and dolomite before and after being treated with flotation reagents.
3.5. FTIR Analysis
Delineating pre-adsorption (untreated) and post-adsorption (FA/NaOl-modified) surface chemistries of target minerals, FTIR was employed to elucidate the interfacial interactions between flotation reagents and calcium-containing minerals (fluorapatite/dolomite). Figure 6 shows comparative spectra of fluorapatite and dolomite under pre-treatment (untreated) and post-treatment (flotation-reagent-exposed) conditions.
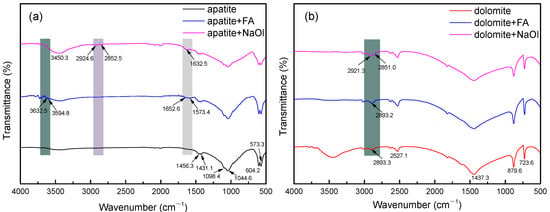
Figure 6.
FTIR spectra results of (a) fluorapatite and (b) dolomite before and after being treated with flotation reagents.
Figure 6a reveals fluorapatite’s diagnostic FTIR signatures at 1098.4 and 1044.6 cm−1, assigned to ν3(PO43−) asymmetric stretching modes. Features at 604.2 and 573.3 cm−1 correspond to ν4(PO43−) bending vibrations, while dual absorption features at 1456.3 and 1431.1 cm−1 indicate ν(C-O) asymmetric stretching. After interaction with FA, the infrared spectral characteristics of fluorapatite changed, with a peak at 1652.6 cm−1 likely attributed to the -COOH group in FA [44]. Broad absorption between 3750 and 3560 cm−1 suggests FA–Ca complexation at surface coordination sites [45]. After the addition of NaOl, the infrared spectrum of fluorapatite showed a sharpening and broadening of the absorption peak around 3450.3 cm−1, and the peaks at 2924.6 cm−1 and 2852.5 cm−1 were the symmetric stretching vibration absorption peaks of the -C-H bonds in methyl and methylene groups [46], all indicating a significant chemisorption of NaOl on fluorapatite surfaces. Figure 6b displays dolomite’s spectral fingerprints: ν(C=O) at 2527.1 cm−1, ν3(CO32−) at 1437.3 cm−1, with additional ν2(CO32−) and ν4(CO32−) modes at 879.6 and 723.6 cm−1 respectively [47]. Dolomite surfaces showed negligible FA-induced spectral changes, suggesting minimal adsorbate–surface interactions below detection limits. FA-treated dolomite maintained spectral congruence with untreated specimens. NaOl-exposed dolomite exhibited distinct ν(CH2/CH3) signatures at 2921.3 and 2851.0 cm−1 [48], demonstrating strong surfactant anchoring through hydrocarbon interactions [49].
3.6. MD Simulation Results
The predominant crystallographic surfaces of fluorapatite and dolomite are presented in Figure 7, demonstrating their characteristic surface orientations [50,51]. Then, MD simulations were conducted to determine the optimal binding orientations for FA–mineral interactions in single-point adsorption models. As evidenced by Figure 8 and Figure 9, the MD simulation results confirmed the preferential adsorption of FA molecules onto the (001) surface of fluorapatite compared with the (104) surface of dolomite. This surface attachment implies potential hydrophilicity enhancement through specific interfacial interactions between FA and the mineral substrates. MD simulations revealed that FA predominantly adsorbed onto fluorapatite (001) surfaces via chemisorption between its deprotonated carboxyl groups (-COO−) and surface Ca2+ sites (Figure 8b). The optimized adsorption configuration showed multiple coordination bonds with O atoms of FA forming stable bonds to Ca atoms, characteristic of strong ionic interactions. In contrast, FA adsorption on dolomite (104) surfaces involved weaker adsorption, with Ca2+/Mg2+ sites exhibiting longer bonding distances. As shown in Table 3, the adsorption energy (ΔEads) for FA–fluorapatite (−454.65 kcal/mol) was significantly more negative than for FA–dolomite (−152.84 kcal/mol), confirming thermodynamically favored chemisorption on fluorapatite. This energy difference arose from FA’s anionic functional groups (-COO−, phenolic -OH) selectively complexed with Ca2+ on fluorapatite. The more negative adsorption enthalpy observed for fluorapatite correlates with its selective flotation depression in experimental trials. These molecular-level insights elucidate the mechanistic basis for fluorapatite/dolomite separation selectivity.
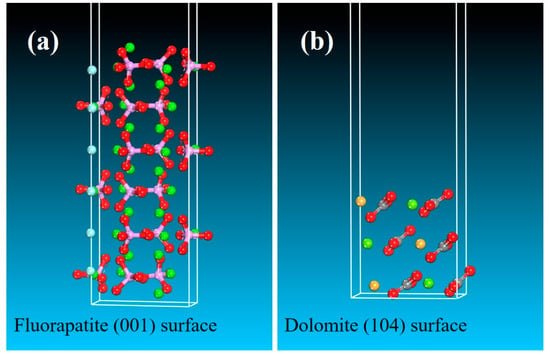
Figure 7.
Structures of the optimized mineral surface of (a) fluorapatite and (b) dolomite (pink, cyan, green, and brown atoms represent P, F, Mg and Ca, respectively).
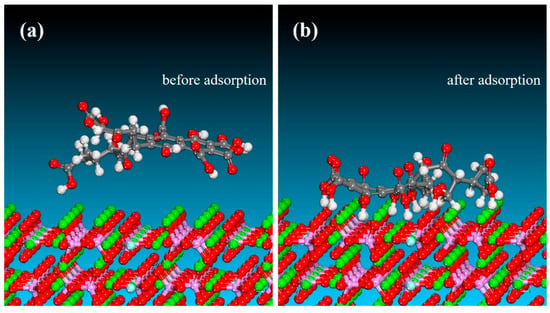
Figure 8.
Molecular models of the fluorapatite (001) surface before (a) and after (b) FA adsorption.
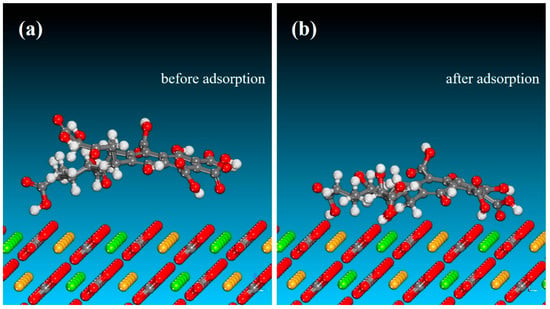
Figure 9.
Molecular models of the dolomite (104) surface before (a) and after (b) FA adsorption.

Table 3.
Adsorption energies between FA and the surfaces of fluorapatite and dolomite.
4. Implications
FA serves as an effective, green depressant for fluorapatite–dolomite separation. Under weakly acidic conditions (pH 5.0) and a low dosage (15 mg/L), it enables high-purity phosphate concentrate recovery (P2O5 33.86%, 92.36%) while reducing energy/chemical use and offering a non-toxic alternative. Its biodegradability, potential use of natural sources, and minimized acid consumption enhance environmental sustainability. Fundamentally, FA’s strong, selective chemisorption on fluorapatite (ΔE = −454.65 kcal/mol) provides a template for designing humic-based depressants, potentially extendable to other calcium minerals (e.g., calcite-fluorite), with MD simulations validating the adsorption selectivity for reagent development.
5. Conclusions
The strategic integration of FA as a selective depressant with NaOl collector enabled precision separation of fluorapatite from dolomite through optimized flotation protocols. Mechanistic insights into FA’s depression behavior were systematically elucidated via multi-dimensional surface characterization, including zeta potential analysis, wettability profiling, FTIR, and MD simulations. Critical findings emerging from this investigation are summarized below:
- (1)
- Single-mineral flotation tests revealed that under optimized conditions (25 ± 0.5 °C, pH 5.0), fluorapatite exhibited near-complete depression (negligible floatability) with 10 mg/L FA and 60 mg/L NaOl, whereas dolomite achieved a recovery of 74.26%. These results confirm FA’s selective inhibitory effect on fluorapatite.
- (2)
- Artificial mixed-ore flotation tests under identical conditions with 15 mg/L FA and 60 mg/L NaOl yielded final concentrate assaying 33.86% P2O5 with 92.36% metallurgical recovery. This demonstrated FA’s remarkable separation efficiency in selectively enriching dolomite while suppressing fluorapatite.
- (3)
- Zeta potential measurements indicated a pronounced negative shift for fluorapatite after FA treatment, confirming substantial FA adsorption on its surface. Wettability analysis revealed that fluorapatite’s hydrophobicity under NaOl activation remained significantly lower than that of dolomite. FTIR spectra confirmed chemisorption as the dominant FA–fluorapatite interaction mechanism. The greater negative adsorption energy magnitude signifies stronger FA–mineral interactions and preferential fluorapatite surface adherence.
- (4)
- In weakly acidic environments, FA selectively adsorbed onto fluorapatite surfaces via chemisorption, effectively suppressing its floatability while minimally interfering with dolomite’s natural response to NaOl. The observed adsorption selectivity enabled benchmark separation efficiency between fluorapatite and dolomite through interfacial engineering, establishing a novel paradigm for strategic mineral beneficiation. Future work will focus on kinetic adsorption analysis to elucidate the rate-controlling mechanisms of FA–mineral interactions and to improve the predictive modeling of adsorption dynamics under varying operational conditions.
Author Contributions
Conceptualization, Y.T.; methodology, D.H.; software, Q.L. and H.F.; investigation, M.W. and W.Y.; data curation, Q.L.; writing—original draft preparation, Q.L. and Y.F.; writing—review and editing, Z.L.; supervision, Y.T.; funding acquisition, Y.T. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Open Project of National Engineering and Technology Research Center for Development & Utilization of Phosphate Resources (NECP2023-14), National Natural Science Foundation of China (52104263), Open Project of Changjiang River Scientific Research Institute (CKWV20231183/KY), Natural Science Foundation of Hubei Province (2023BCB079), and Yunnan Provincial Technology Innovation Center Project (202305AK340002).
Data Availability Statement
All data can be provided as required.
Conflicts of Interest
Authors Yuan Tang, Menglai Wang and Wenquan Yang were employed by the Yunnan Phosphate Chemical Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ruan, Y.; He, D.; Chi, R. Review on beneficiation techniques and reagents used for phosphate ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; Hakkou, R.; Benzaazoua, M. Review of the main factors affecting the flotation of phosphate ores. Minerals 2020, 10, 1109. [Google Scholar] [CrossRef]
- Yu, L.; Yu, P.; Bai, S. A critical review on the flotation reagents for phosphate ore beneficiation. Minerals 2024, 14, 828. [Google Scholar] [CrossRef]
- Xie, S.; Yang, J.; Huang, R.; Lv, X.; Li, X.; He, X. A novel process for the separation and recovery of phosphorus and rare earth elements from associated rare earth phosphate ores. Sep. Purif. Technol. 2024, 340, 126687. [Google Scholar] [CrossRef]
- Lv, X.; Xiang, L.; Wang, T. Intensification of low-grade phosphate ore purification using a hydrocyclone with venturi-style inlet. J. Ind. Eng. Chem. 2024, 135, 257–269. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Q.; Chen, C.; Liu, H.; Yang, L. Separation of phosphoric acid and magnesium from wet process phosphoric acid by solvent extraction. Can. Metall. Q 2023, 62, 791–802. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Li, K.; Zhang, S.; Zhou, J.; Luo, W.; Liu, Z.; Qin, W.; Wang, H.; Hu, Y.; et al. Reverse flotation separation of quartz from phosphorite ore at low temperatures by using an emerging Gemini surfactant as the collector. Sep. Purif. Technol. 2020, 246, 116923. [Google Scholar] [CrossRef]
- Sajid, M.; Bary, G.; Asim, M.; Ahmad, R.; Ahamad, M.I.; Alotaibi, H.; Rehman, A.; Khan, I.; Yin, G. Synoptic view on P ore beneficiation techniques. Alex. Eng. J. 2022, 61, 3069–3092. [Google Scholar] [CrossRef]
- Lehr, J.R.; Frazier, A.W.; Smith, J.P. Phosphoric acid impurities, precipitated impurities in wet-process phosphoric acid. J. Agric. Food. Chem. 1966, 14, 27–33. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; El Amari, K.; Yaacoubi, A.; Abidi, A.; Etahiri, A.; Baçaoui, A. Influence of surface dissolution on reagents’ adsorption on low-grade phosphate ore and its flotation selectivity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127700. [Google Scholar] [CrossRef]
- Amankonah, J.O.; Somasundaran, P. Effects of dissolved mineral species on the electrokinetic behavior of calcite and apatite. Colloids Surf. 1985, 15, 335–353. [Google Scholar] [CrossRef]
- Amirech, A.; Bouhenguel, M.; Kouachi, S. Two-stage reverse flotation process for removal of carbonates and silicates from phosphate ore using anionic and cationic collectors. Arab. J. Geosci. 2018, 11, 593. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araujo, A.D.; Peres, A.E.C. Reagents in igneous phosphate ores flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Dong, L.; Wei, Q.; Jiao, F.; Qin, W. Utilization of polyepoxysuccinic acid as the green selective depressant for the clean flotation of phosphate ores. J. Clean. Prod. 2021, 282, 124532. [Google Scholar] [CrossRef]
- Feng, B.; Zhong, C.; Zhang, L.; Guo, Y.; Wang, T.; Huang, Z. Effect of surface oxidation on the depression of sphalerite by locust bean gum. Miner. Eng. 2020, 146, 106142. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; Abidi, A.; Yaacoubi, A.; El Amari, K.; Etahiri, A.; Baçaoui, A. Direct flotation of low-grade Moroccan phosphate ores: A preliminary micro-flotation study to develop new beneficiation routes. Arab. J. Geosci. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Tao, L.; Xiang, G.; Miao, Z.; Wang, J.; Wu, W.; Tian, M.; Jia, W.; Gao, Z. Method and mechanism of reverse flotation for dephosphorization of spodumene concentrate using sodium alginate as a depressant. J. Clean. Prod. 2024, 451, 142171. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, L.; Zhang, W.; Wang, H.; Gao, Z. Flotation separation of apatite and calcite using gum arabic as a depressant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127723. [Google Scholar] [CrossRef]
- El-Bahi, A.; Taha, Y.; Ait-Khouia, Y.; Hakkou, R.; Benzaazoua, M. Advancing phosphate ore minerals separation with sustainable flotation reagents: An investigation into highly selective biobased depressants. Adv. Colloid. Interface Sci. 2023, 317, 102921. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, Y.; Li, C.; Cheng, R. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. Int. J. Miner. Process. 2017, 167, 95–102. [Google Scholar] [CrossRef]
- Zheng, X.; Smith, R.W. Dolomite depressants in the flotation of apatite and collophane from dolomite. Miner. Eng. 1997, 10, 537–545. [Google Scholar] [CrossRef]
- Li, B.; Liu, D.; Shi, Q.; Zhang, G.; Zheng, H. Application of hydroxyethylidene diphosphonic acid as a depressant for efficient pyrite separation from serpentine: A performance and mechanistic study. Miner. Eng. 2023, 204, 108381. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, L.; Fu, W.; Chi, R.; Li, H.; Yang, S. Investigations of amino trimethylene phosphonic acid as a green and efficient depressant for the flotation separation of apatite from calcite. Miner. Eng. 2022, 181, 107552. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, C.; Chen, Q.; Li, Q.; He, D.; Li, Z.; Fu, Y. Effect of a novel environmental-friendly chelating depressant DTPMP on the flotation separation of magnesite and dolomite. Appl. Surf. Sci. 2025, 690, 162650. [Google Scholar] [CrossRef]
- Lan, S.; Shen, P.; Zheng, Q.; Qiao, L.; Dong, L.; Liu, D. Effective flotation separation of apatite from dolomite using a new eco-friendly depressant gallic acid. Green. Chem. 2024, 26, 1627–1636. [Google Scholar] [CrossRef]
- Tang, W.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Zhang, C.; Huang, B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef]
- Saar, R.A.; Weber, J.H. Fulvic acid: Modifier of metal-ion chemistry. Environ. Sci. Technol. 1982, 16, 510A–517A. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, S.; Hao, J.; Feng, Q. Flotation separation of chalcopyrite from pyrite using mineral fulvic acid as a selective depressant under weakly alkaline conditions. Trans. Nonferrous Met. Soc. China 2025, 35, 313–325. [Google Scholar] [CrossRef]
- Lan, S.; Dong, L.; Shen, P.; Su, H.; Mao, Y.; Shen, Z.; Liu, D. Application of waste gypsum as novel selective depressant in the flotation separation of apatite from dolomite. Chem. Eng. J. 2024, 498, 155567. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, W.; Kelebek, S. Selective flotation of magnesite from calcite using potassium cetyl phosphate as a collector in the presence of sodium silicate. Miner. Eng. 2020, 146, 106154. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Khoso, S.A.; Sun, W.; Hu, Y. Understanding the activation mechanism of Pb2+ ion in benzohydroxamic acid flotation of spodumene: Experimental findings and DFT simulations. Miner. Eng. 2019, 143, 106006. [Google Scholar] [CrossRef]
- BIOVIA Materials Studio 2017 (17.1.0.48). Available online: https://www.3ds.com/products/biovia/materials-studio (accessed on 12 May 2025).
- Hu, Y.H.; Gao, Z.Y.; Sun, W.; Liu, X.W. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 415, 439–448. [Google Scholar] [CrossRef]
- Rai, B. Design of tailor-made surfactants for industrial applications using a molecular modelling approach. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 139–148. [Google Scholar]
- Tang, Y.; Yin, W.Z.; Kelebek, S. Molecular dynamics simulation of magnesite and dolomite in relation to flotation with cetyl phosphate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125928. [Google Scholar] [CrossRef]
- Jing, L.; Xue, K.; Tian, J.; Zhang, X.; Wang, D.; Guo, W.; Ma, Z.; Xu, L. Separation mechanism of apatite and dolomite with flotation depressant konjac glucomannan. Miner. Eng. 2024, 216, 108844. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, Y.; Lu, L.; Hu, X.; Li, S. Removal of dolomite and potassium feldspar from apatite using simultaneous flotation with a mixed cationic-anionic collector. Int. J. Min. Sci. Technol. 2023, 33, 783–791. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Q.; Wang, X. New insights on depressive mechanism of citric acid in the selective flotation of dolomite from apatite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130075. [Google Scholar] [CrossRef]
- Zeng, M.; Li, K.; Huang, L.; Bao, S.; Liu, C.; Yang, S. Interaction mechanism of interfacial nano-micro bubbles with collectors and its effects on the fine apatite flotation. Appl. Surf. Sci. 2025, 682, 161736. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Ren, Q.; Tu, R.; Qiu, F.; Gao, Z.; Xu, S.; Sun, W.; Tian, M. Innovating an amphoteric collector derived from dodecylamine molecular structure: Facilitating the selective flotation separation of apatite from quartz and dolomite. Miner. Eng. 2024, 215, 108819. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, H.; Huang, L.; Zhu, Y.; Li, F. Flotation separation of magnesite from dolomite with gellan gum as depressant and its depression mechanism. Miner. Eng. 2024, 212, 108718. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; El-Bahi, A.; Ait-Khouia, Y.; Benzaazoua, M.; Hakkou, R. Selective flotation of calcite and dolomite from apatite using bio-based alternatives to conventional collectors: Castor and mustard oils. Miner. Eng. 2024, 208, 108597. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Ding, Z.; Yu, A.; Zhang, Y.; Wen, S.; Bai, S. Influence and mechanism of new environmentally friendly depressant carboxymethyl-β-cyclodextrin on the flotation separation of chalcopyrite and pyrite. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134576. [Google Scholar] [CrossRef]
- Kaba, O.B.; Filippov, L.O.; Filippova, I.V.; Badawi, M. Interaction between fine particles of fluorapatite and phosphoric acid unraveled by surface spectroscopies. Powder Technol. 2021, 382, 368–377. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, X.; Yu, F.; Miller, J.D. States of coadsorption for oleate and dodecylamine at selected spodumene surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 313–321. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Ince, D.E.; Johnston, C.T.; Moudgil, B.M. Fourier transform infrared spectroscopic study of adsorption of oleic acid/oleate on surfaces of apatite and dolomite. Langmuir 1991, 7, 1453–1457. [Google Scholar] [CrossRef]
- Liu, W.; Mao, Y.; Zheng, J.; Wang, Z.; Shang, C.; Liu, W.; Zhao, Q.; Zhao, S.; Shen, Y. Enhancing the flotation separation of magnesite and dolomite by introducing a phosphonic acid depressant during grinding. Sep. Purif. Technol. 2025, 361, 131412. [Google Scholar] [CrossRef]
- Chen, A.; Wang, X.; Zhang, Q. Interaction and inhibition mechanism of sulfuric acid with fluorapatite (001) surface and dolomite (104) surface: Flotation experiments and molecular dynamics simulations. Minerals 2023, 13, 1517. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Mao, S.; Li, L.; Ke, B.; Zhang, Q. Effects of structure of fatty acid collectors on the adsorption of fluorapatite (001) surface: A first-principles calculations. Appl. Surf. Sci. 2018, 444, 699–709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).