Enhancing Biomethane Yield from Microalgal Biomass via Enzymatic Hydrolysis: Optimization and Predictive Modeling Using RSM Approach
Abstract
1. Introduction
2. Material and Methods
2.1. Substrate, Inoculum and Used Enzyme
2.2. Experimental Setup
2.2.1. Enzymatic Hydrolysis and Experimental Design
2.2.2. Experimental Procedure for Biomethane Production (BMP Test)
- The pretreated substrate: microalgal biomass subjected to the optimized enzymatic hydrolysis conditions determined earlier in this study;
- The control: microalgal biomass under the same operational conditions (pH, temperature, S/I ratio, inoculum, nutrient solution, incubation time) but without adding any enzyme, so no enzymatic pretreatment was applied.
2.3. Analytical Methods
2.4. Data Analysis
3. Results and Discussion
3.1. Experimental Results of Enzymatic Hydrolysis
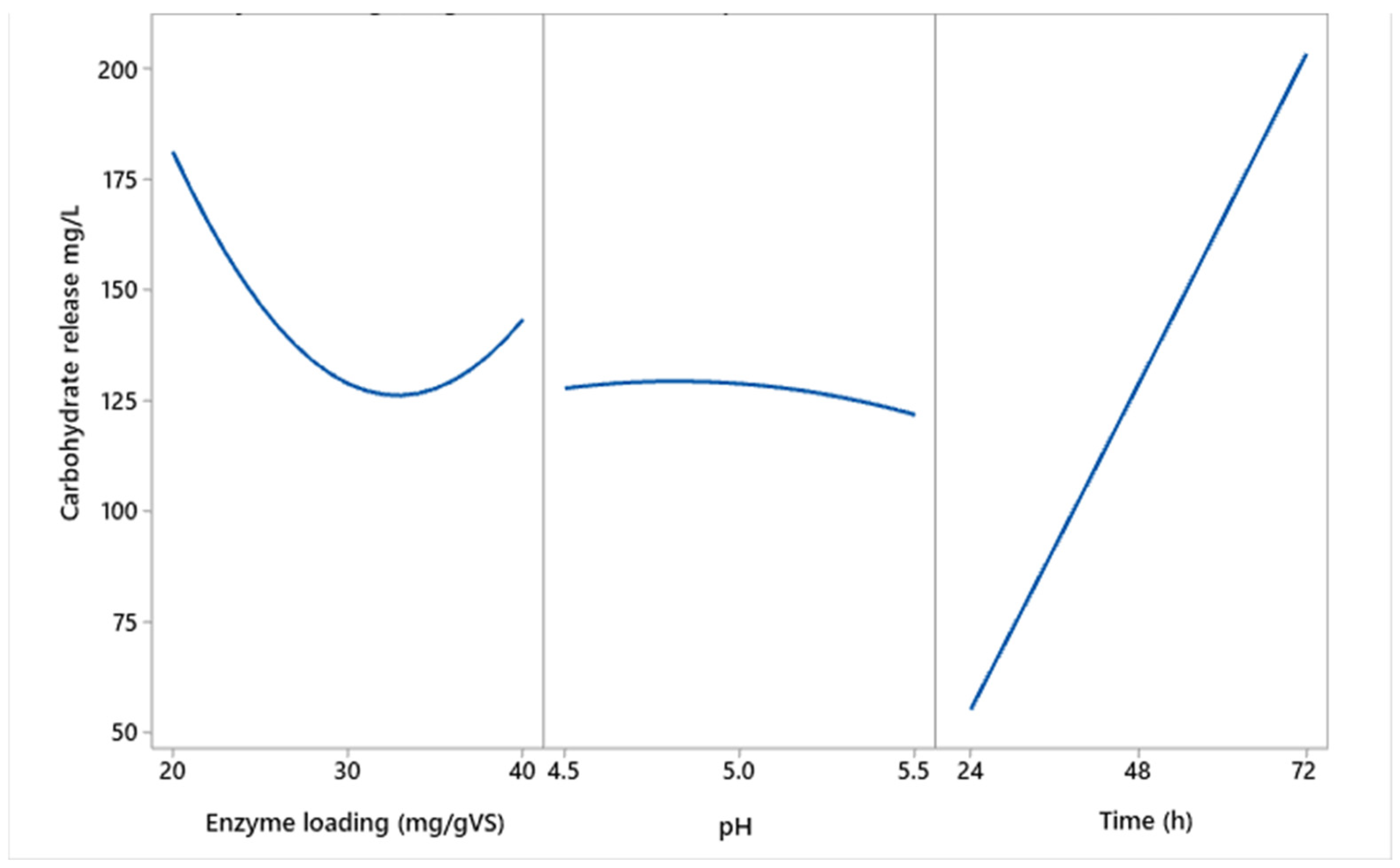
Effect of Enzymatic Hydrolysis and Main Process Factors on Carbohydrate Release
3.2. Model Development and Statistical Analysis
3.2.1. Model Fitting and ANOVA Results
3.2.2. Response Surface Analysis and Factors Interactions
3.2.3. Model Validation
3.3. Enzymatic Hydrolysis Anaerobic Digestion Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TS | Total solids |
| TVS | Total volatile solids |
| S | Substrate |
| I | Inoculum |
| CODt | Total chemical oxygen demand |
| CODs | Soluble chemical oxygen demand |
| TKN | Total Kjeldahl nitrogen |
| RSM | Response surface methodology |
| BBD | Box–Behnken Design |
| AD | Anaerobic digestion |
References
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment Methods to Enhance Anaerobic Digestion of Organic Solid Waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance Evaluation of Anaerobic Digestion Technology for Energy Recovery from Organic Fraction of Municipal Solid Waste: A Review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Threedeach, S.; Chiemchaisri, W.; Watanabe, T.; Chiemchaisri, C.; Honda, R. Antibiotic Resistance of Escherichia coli in Leachates from Municipal Solid Waste Landfills: Comparison between Semi-Aerobic and Anaerobic Operations. Bioresour. Technol. 2012, 113, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Sriwuryandari, L.; Priantoro, E.A.; Sintawardani, N.; Astuti, J.T.; Nilawati, D.; Putri, A.M.H.; Mamat; Sentana, S.; Sembiring, T. The Organic Agricultural Waste as a Basic Source of Biohydrogen Production. AIP Conf. Proc. 2016, 1711, 080002. [Google Scholar] [CrossRef]
- Pecorini, I.; Ferrari, L.; Baldi, F.; Albini, E.; Galoppi, G.; Bacchi, D.; Vizza, F.; Lombardi, L.; Carcasci, C.; Ferrara, G.; et al. Energy Recovery from Fermentative Biohydrogen Production of Biowaste: A Case Study Based Analysis. Energy Procedia 2017, 126, 605–612. [Google Scholar] [CrossRef]
- Zhao, C.H. Production of Cellulase by Trichoderma Reesei from Pretreated Straw and Furfural Residues. RSC Adv. 2018, 8, 36233–36238. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Microalgae conversion to biogas: Thermal pretreatment contribution on net energy production. Environ. Sci. Technol. 2014, 48, 7171–7178. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Blanco, S.; Ballesteros, M.; González-Fernández, C. Protease cell wall degradation of Chlorella vulgaris: Effect on methane production. Bioresour. Technol. 2014, 171, 421–427. [Google Scholar] [CrossRef]
- Córdova, O.; Chamy, R.; Guerrero, L.; Sánchez-Rodríguez, A. Assessing the Effect of Pretreatments on the Structure and Functionality of Microbial Communities for the Bioconversion of Microalgae to Biogas. Front. Microbiol. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Liu, D.; Zhao, X. Pretreatment of Lignocellulosic Biomass for Efficient Enzymatic Saccharification of Cellulose; INC: Singapore, 2019; ISBN 9780128159361. [Google Scholar]
- Quintero, R. Recent Advancements in Pretreatment Technologies of Biomass to Produce Bioenergy. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 57–69. [Google Scholar] [CrossRef]
- Hangri, S.; Derbal, K.; Policastro, G.; Panico, A.; Contestabile, P.; Pontoni, L.; Race, M.; Fabbricino, M. Combining Pretreatments and Co-Fermentation as Successful Approach to Improve Biohydrogen Production from Dairy Cow Manure. Environ. Res. 2024, 246, 118118. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef]
- Minitab, LLC. Minitab Statistical Software, Release 19.1.0; Minitab, LLC.: State College, PA, USA, 2024; Available online: https://www.minitab.com (accessed on 10 March 2025).
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Deeper insights into the effects of substrate to inoculum ratio selection on the relationship of kinetic parameters, microbial communities, and key metabolic pathways during the anaerobic digestion of food waste. Bioresour. Technol. 2022, 353, 127055. [Google Scholar] [CrossRef]
- Mahfouf Bouchareb, E.; Derbal, K.; Bedri, R.; Menas, S.; Bouchareb, R.; Dizge, N. Enhanced fermentative hydrogen production from potato waste by enzymatic pretreatment. Environ. Technol. 2024, 45, 1801–1809. [Google Scholar] [CrossRef]
- Ergüder, T.H.; Güven, E.; Demirer, G.N. Anaerobic Treatment of Olive Mill Wastes in Batch Reactors. Process Biochem. 2000, 36, 243–248. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; American Public Health Association (APHA) (Eds.) Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 2012; ISBN 978-087553-0130. [Google Scholar]
- Kumar, A.; Prasad, B.; Mishra, I.M. Optimization of process parameters for acrylonitrile removal by a low-cost adsorbent using Box-Behnken design. J. Hazard. Mater. 2008, 150, 174–182. [Google Scholar] [CrossRef]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge: Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Padil, M.D.; Dharma Putra, M.; Hidayat, M.; Kasiamdari, R.S.; Mutamima, A.; Iwamoto, K.; Darmawan, M.A.; Gozan, M. Mechanism and kinetic model of microalgal enzymatic hydrolysis for prospective bioethanol conversion. RSC Adv. 2023, 13, 21403–21413. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.A.; Tan, K.M.; Serri, N.A. Enzymatic hydrolysis of dilute alkaline pretreated Chlorella sp. biomass for biosugar production and fed-batch bioethanol fermentation. Asia-Pac. J. Sci. Technol. 2022, 27, 134699. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Himmel, M.E.; Mielenz, J.R. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, N.; Kucuker, M.A.; Kuchta, K. Fermentative hydrogen and methane production from microalgal biomass (Chlorella vulgaris) in a two-stage combined process. Appl. Energy 2014, 132, 108–117. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Tomás-Pejó, E.; Morales, M.M.; Ballesteros, M.; González-Fernández, C. Influence of enzymatic hydrolysis on the biochemical methane potential of Chlorella vulgaris and Scenedesmus sp. J. Chem. Technol. Biotechnol. 2015, 90, 2008–2014. [Google Scholar] [CrossRef]
- Passos, F.; Hom-Diaz, A.; Blánquez, P.; Vicent, T.; Ferrer, I. Improvement of microalgae anaerobic digestion by enzymatic pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef]




| Parameters | Unit | Substrate | İnoculum |
|---|---|---|---|
| pH | - | 6.7 | 7.5 |
| Total alkalinity | mg CaCO3/L | 12,000 | 1429 |
| Alkalinty | mg CaCO3/L | 4000 | NA |
| TS | g/L | 83.73 | 51.0 |
| VS | g/L | 43.12 | 29.0 |
| VS/TS | % | 51.49 | 56.86 |
| CODt | g/L | 17.77 | 16.35 |
| CODs | g/L | NA | 4.08 |
| CODs/CODt | % | NA | 24.95 |
| Carbohydrate | g/L | 0.196 | 0.72 |
| Protein | g/L | 0.0325 | NA |
| Coded Values | Factors | Coded Level | |||
|---|---|---|---|---|---|
| Unit | −1 | 0 | +1 | ||
| X1 | pH | - | 4.5 | 5 | 5.5 |
| X2 | Enzyme loading | mg/gVS | 24 | 48 | 72 |
| X3 | Time | hours | 20 | 30 | 40 |
| Exp No | Parameters | Response | |||
|---|---|---|---|---|---|
| pH | Enzyme Loading (mg/gVS) | Time (h) | Carbohydrate Release (mg/L) Experimental | Carbohydrate Release (mg/L) Predicted | |
| 1 | 4.5 | 20 | 48 | 183.958 | 180.211 |
| 2 | 5.5 | 20 | 48 | 183.180 | 179.166 |
| 3 | 4.5 | 40 | 48 | 133.180 | 143.582 |
| 4 | 5.5 | 40 | 48 | 133.013 | 136.760 |
| 5 | 4.5 | 30 | 24 | 52.180 | 34.149 |
| 6 | 5.5 | 30 | 24 | 79.180 | 69.627 |
| 7 | 4.5 | 30 | 72 | 210.000 | 221.375 |
| 8 | 5.5 | 30 | 72 | 160.000 | 178.031 |
| 9 | 5 | 20 | 24 | 38.147 | 59.924 |
| 10 | 5 | 40 | 24 | 110.424 | 118.053 |
| 11 | 5 | 20 | 72 | 313.013 | 305.385 |
| 12 | 5 | 40 | 72 | 190.000 | 168.222 |
| 13 | 5 | 30 | 48 | 126.000 | 128.898 |
| 14 | 4.5 | 20 | 48 | 134.513 | 128.898 |
| 15 | 5.5 | 20 | 48 | 126.180 | 128.898 |
| Term | Coefficient | Student Test (T) | Probability Value (p) |
|---|---|---|---|
| Constant | 128.90 | 15.07 | 0.000 |
| X1 | −3.10 | −4.65 | 0.579 |
| X2 | −24.34 | −0.59 | 0.006 |
| X3 | 68.64 | 13.10 | 0.000 |
| X1 × X1 | 1.30 | 0.17 | 0.873 |
| X2 × X2 | 26.87 | 3.49 | 0.018 |
| X3 × X3 | −3.37 | −0.44 | 0.680 |
| X1 × X2 | 1.42 | 0.19 | 0.856 |
| X1 × X3 | −17.76 | −2.40 | 0.062 |
| X2 × X3 | −59.32 | −8.01 | 0.000 |
| Source | Degree of Freedom | Sum of Square | Mean Square | Fisher Test (F) | Probability Value (p) |
|---|---|---|---|---|---|
| Model | 9 | 60,631.6 | 6736.8 | 30.69 | 0.001 |
| Linear | 3 | 42,509.9 | 14,170.0 | 64.56 | 0.000 |
| Square | 3 | 2776.7 | 2666.4 | 4.22 | 0.078 |
| Interactions | 3 | 15,345.0 | 5115.0 | 23.30 | 0.002 |
| Error | 5 | 1097.4 | 219.5 | ||
| Lack of fit | 3 | 1050.1 | 350.0 | 14.80 | 0.064 |
| Total | 14 | 24,560.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hangri, S.; Derbal, K.; Benalia, A.; Policastro, G.; Panico, A.; Pizzi, A. Enhancing Biomethane Yield from Microalgal Biomass via Enzymatic Hydrolysis: Optimization and Predictive Modeling Using RSM Approach. Processes 2025, 13, 2086. https://doi.org/10.3390/pr13072086
Hangri S, Derbal K, Benalia A, Policastro G, Panico A, Pizzi A. Enhancing Biomethane Yield from Microalgal Biomass via Enzymatic Hydrolysis: Optimization and Predictive Modeling Using RSM Approach. Processes. 2025; 13(7):2086. https://doi.org/10.3390/pr13072086
Chicago/Turabian StyleHangri, Souhaila, Kerroum Derbal, Abderrezzaq Benalia, Grazia Policastro, Antonio Panico, and Antonio Pizzi. 2025. "Enhancing Biomethane Yield from Microalgal Biomass via Enzymatic Hydrolysis: Optimization and Predictive Modeling Using RSM Approach" Processes 13, no. 7: 2086. https://doi.org/10.3390/pr13072086
APA StyleHangri, S., Derbal, K., Benalia, A., Policastro, G., Panico, A., & Pizzi, A. (2025). Enhancing Biomethane Yield from Microalgal Biomass via Enzymatic Hydrolysis: Optimization and Predictive Modeling Using RSM Approach. Processes, 13(7), 2086. https://doi.org/10.3390/pr13072086







