Biodegradable Mg-Zn-MgO Composites for Locking Compression Fixation Plates for Pediatric Orthopedics: Improved Mechanical Properties and Corrosion Resistance
Abstract
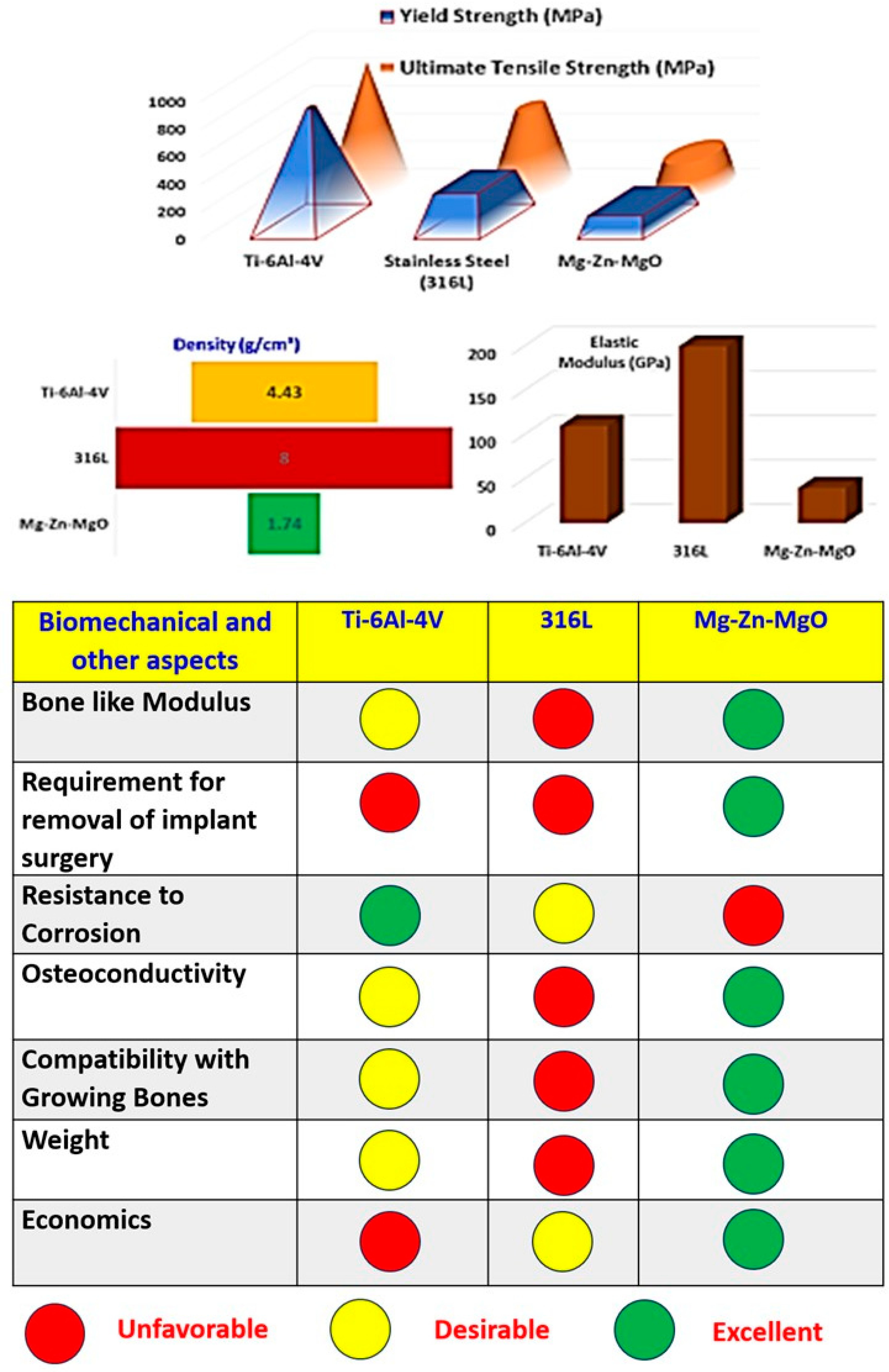
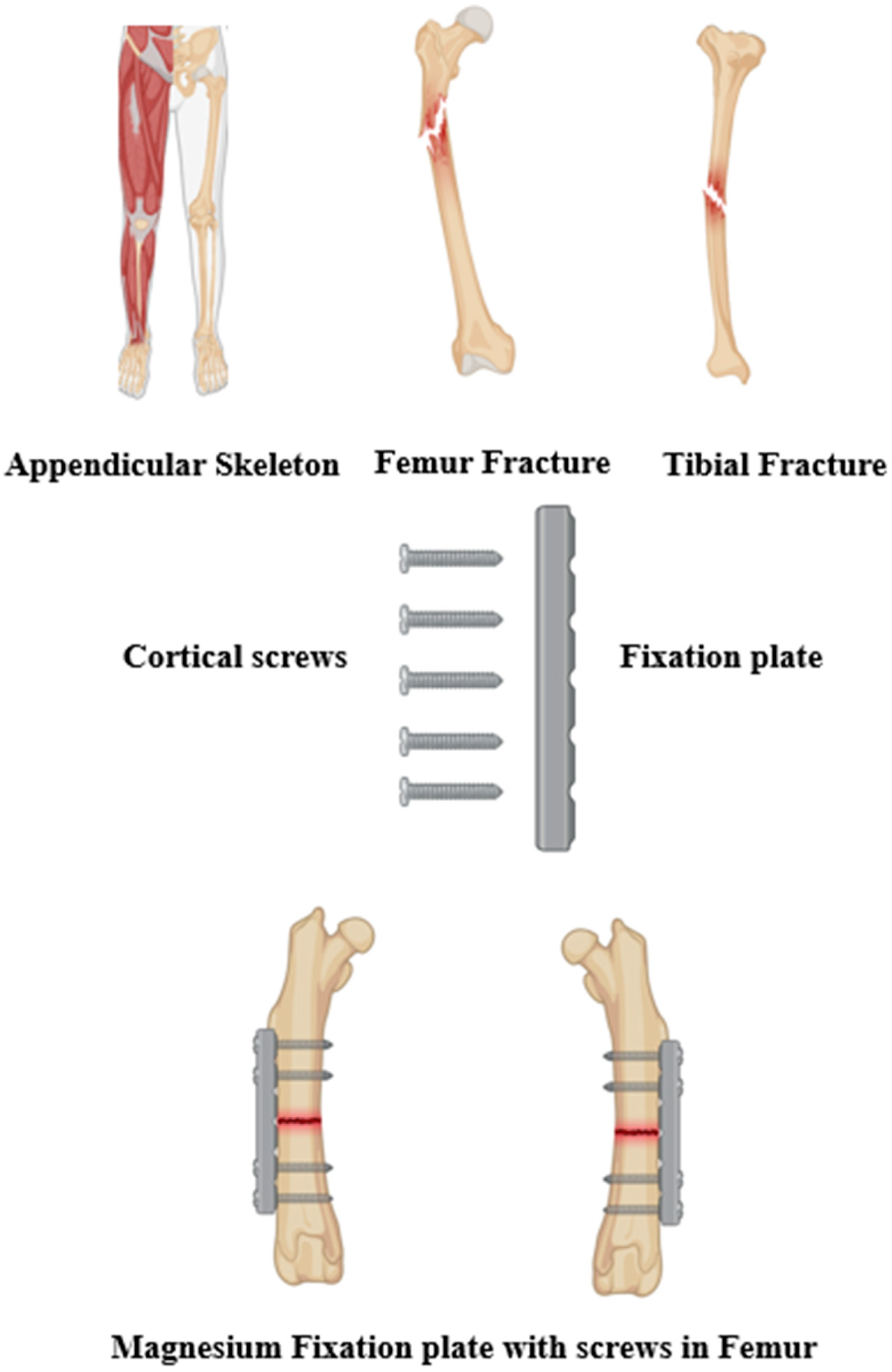
1. Introduction
2. Materials and Methods
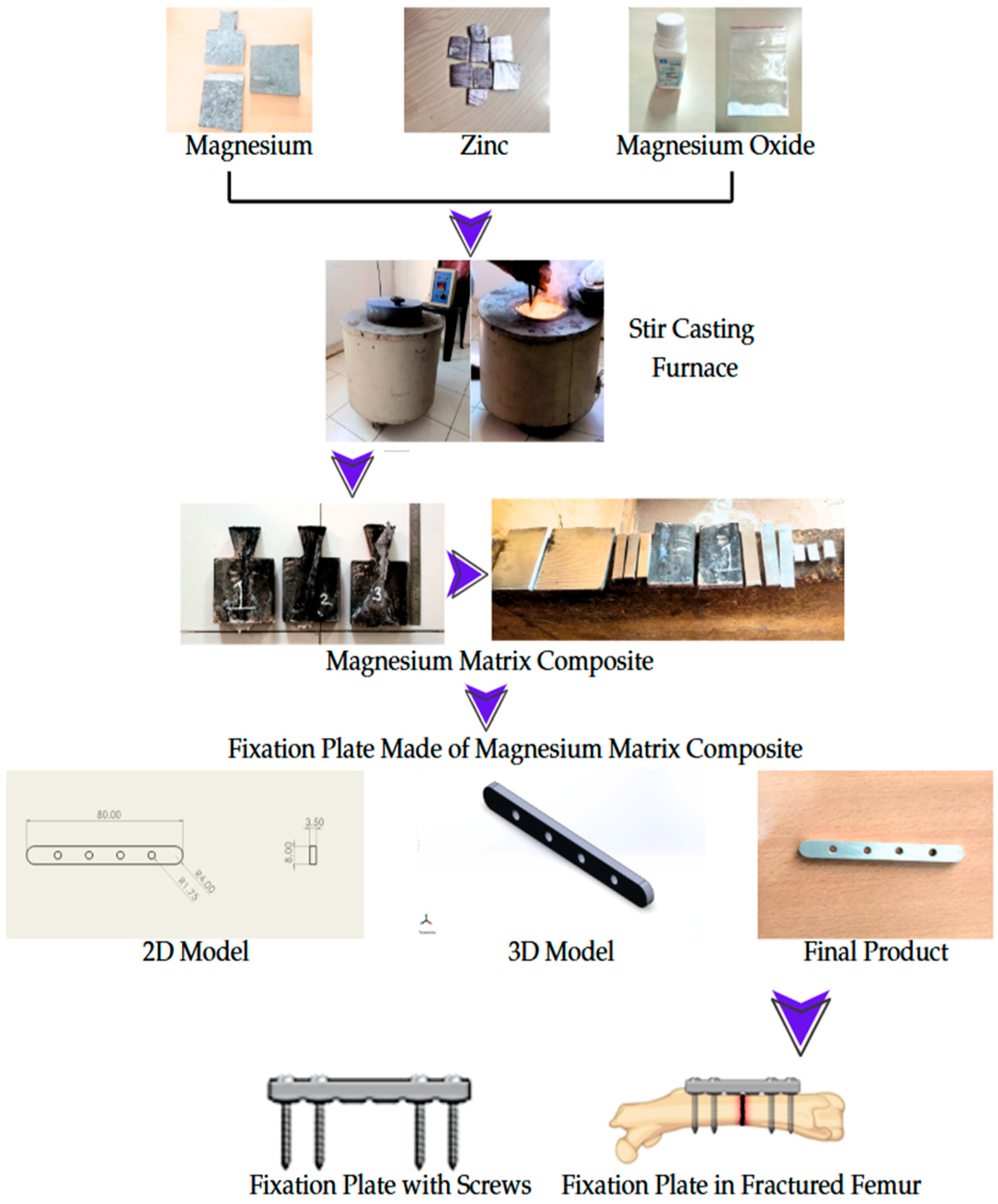
2.1. Constituents of Magnesium Matrix Composite
2.2. Preparation of Magnesium Matrix Composite
2.3. Locking Compression Plate
2.4. Mechanical Testing
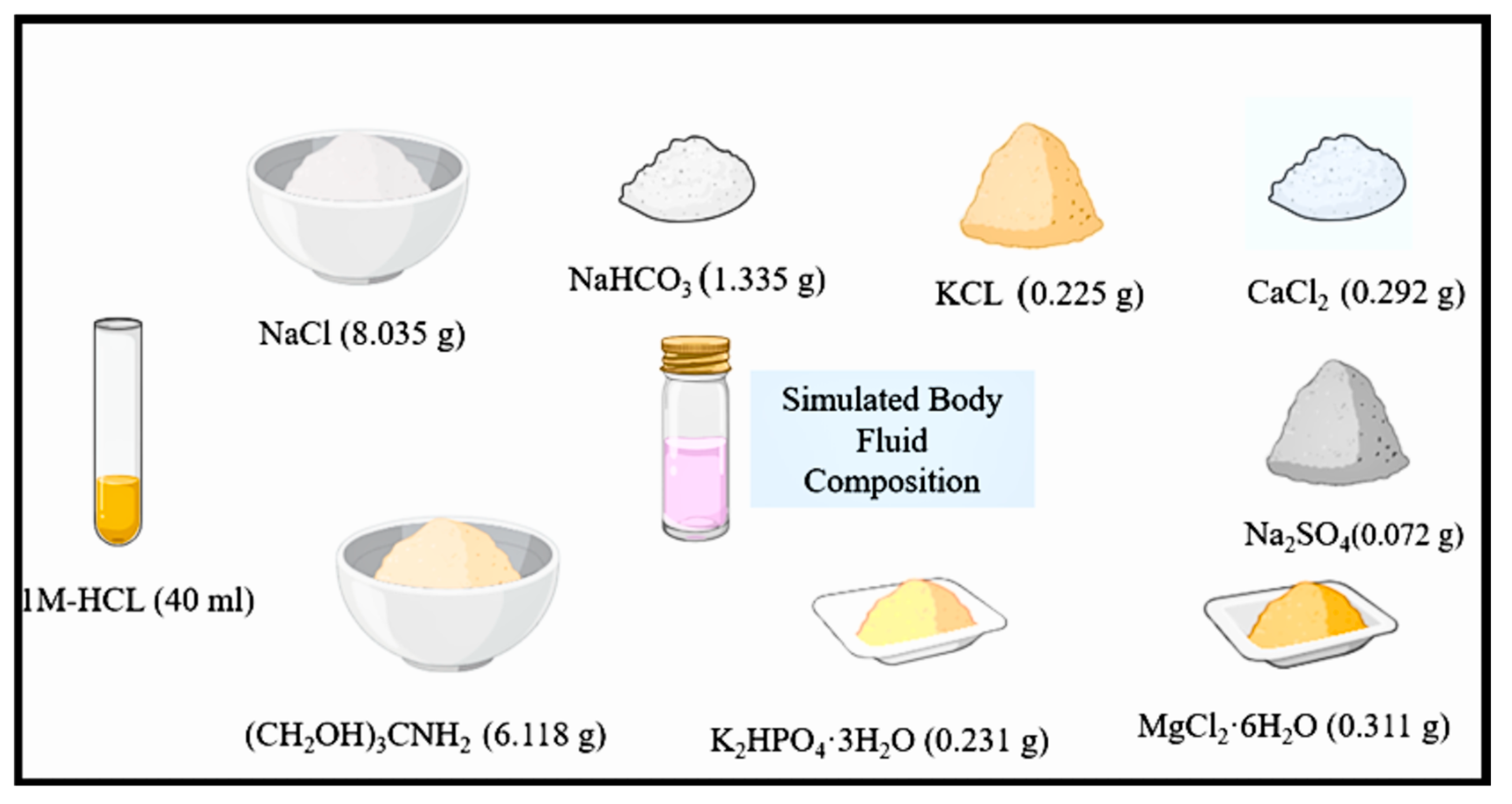
2.5. Simulated Body Fluid
2.6. Statistical Analysis
3. Results and Discussion
3.1. Tensile Test
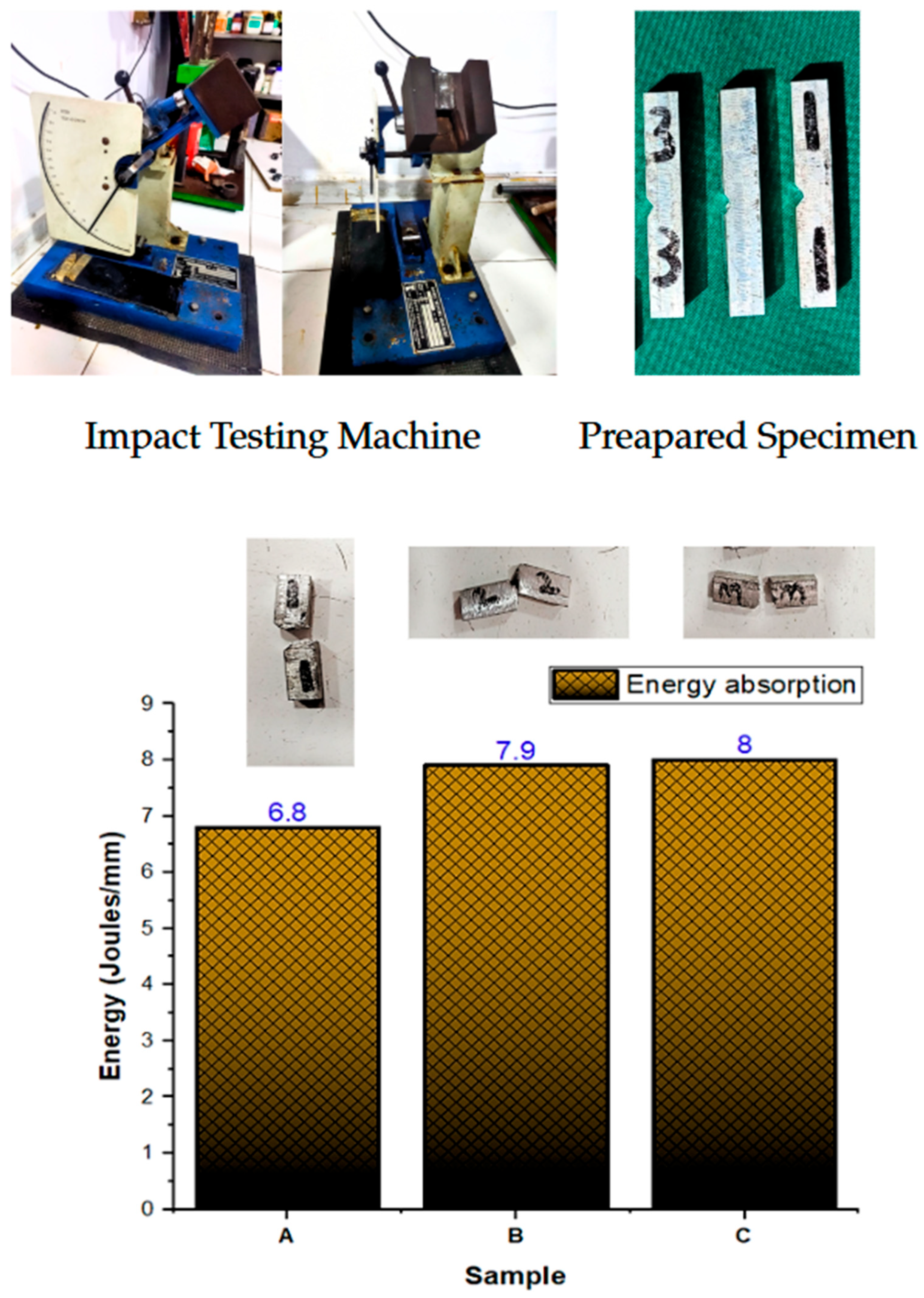
3.2. Impact Test
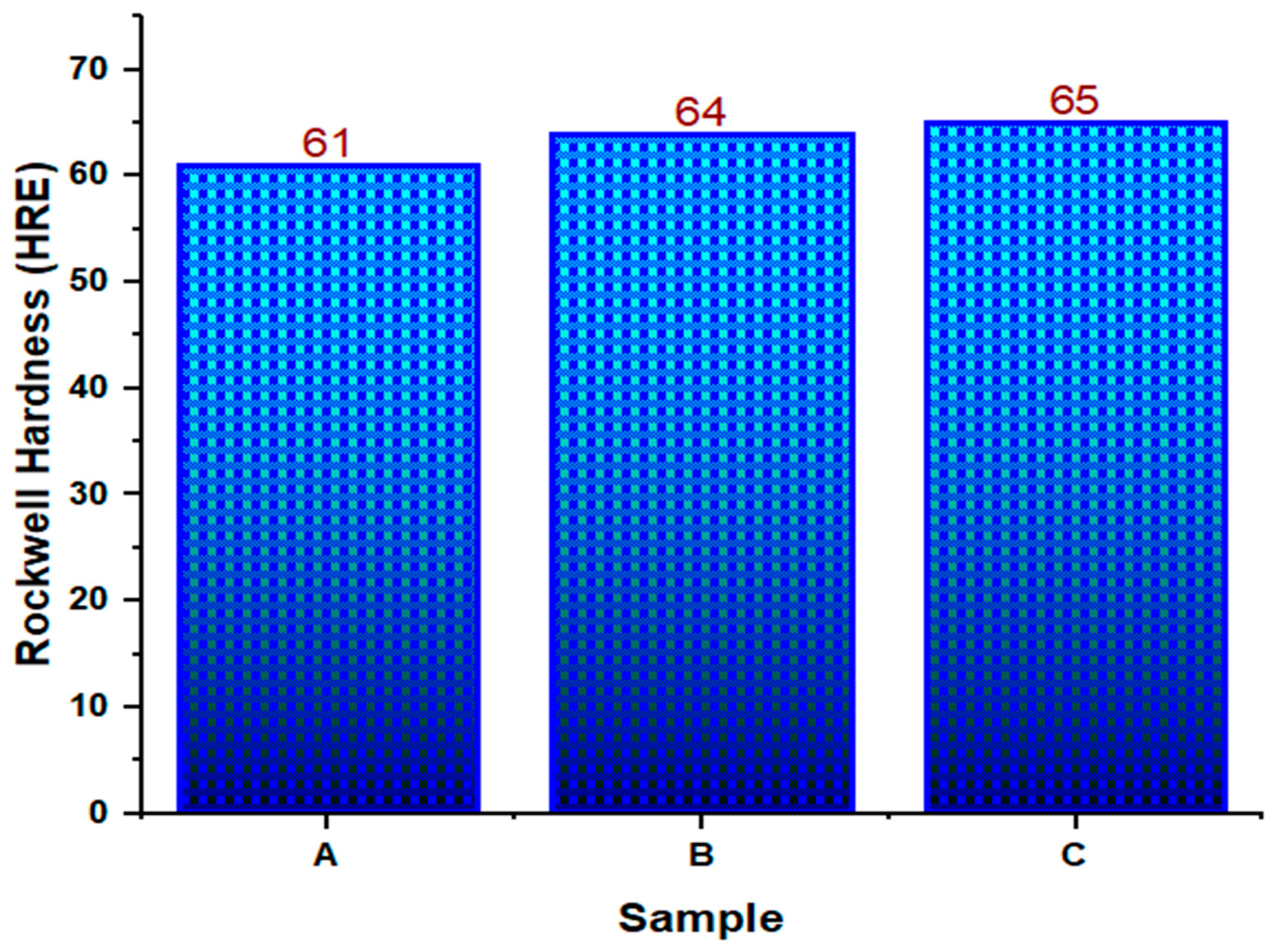
3.3. Hardness Test
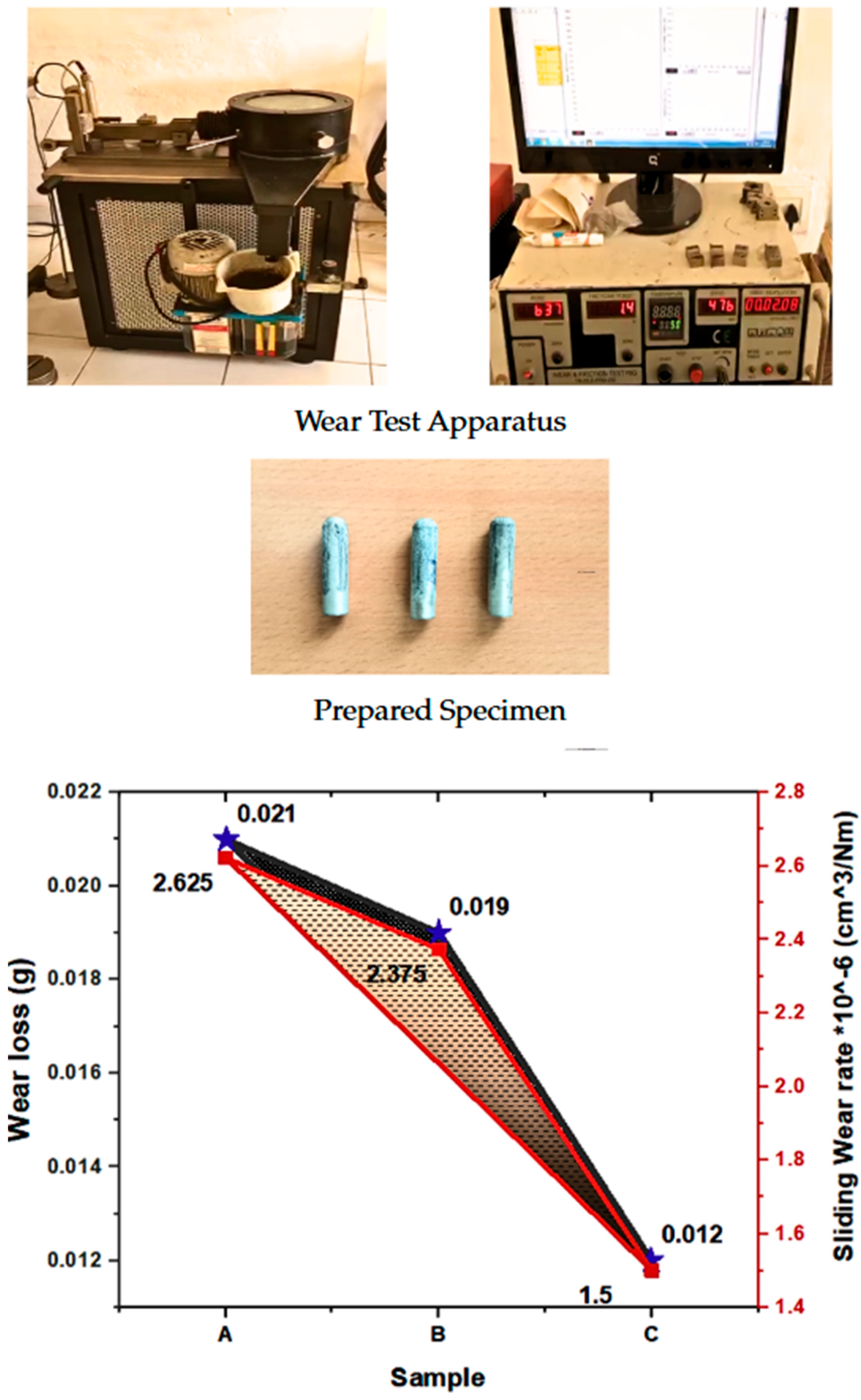
3.4. Wear Test
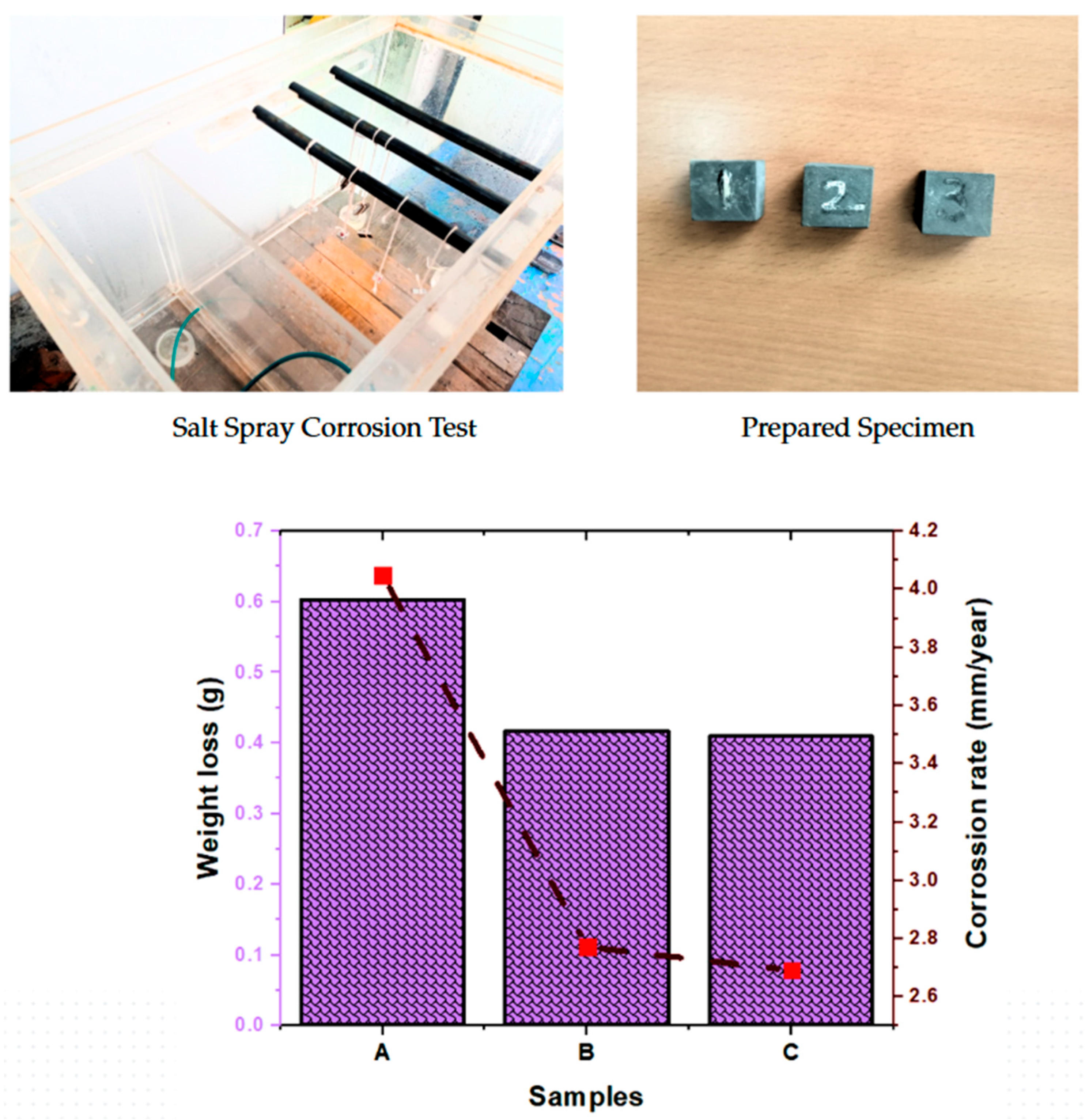
3.5. Corrosion Test
3.6. Morphology Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pereira, D.; Cachinho, S.; Ferro, M.C.; Fernandes, M.H.V. Surface Behaviour of High MgO-Containing Glasses of the Si-Ca-P-Mg System in a Synthetic Physiological Fluid. J. Eur. Ceram. Soc. 2004, 24, 3693–3701. [Google Scholar] [CrossRef]
- Gurunathan, M.K.; Hynes, N.R.J.; Al-Khashman, O.A.; Brykov, M.; Ganesh, N.; Ene, A. Study on the Impact and Water Absorption Performance of Prosopis Juliflora & Glass Fibre Reinforced Epoxy Composite Laminates. Polymers 2022, 14, 2973. [Google Scholar] [CrossRef]
- Fukuda, H.; Szpunar, J.A.; Kondoh, K.; Chromik, R. The Influence of Carbon Nanotubes on the Corrosion Behaviour of AZ31B Magnesium Alloy. Corros. Sci. 2010, 52, 3917–3923. [Google Scholar] [CrossRef]
- Velu, P.S.; Vignesh, N.J.; Hynes, N.R.J. Joining of Metal Matrix Composites. In Joining Processes for Dissimilar and Advanced Materials; Woodhead Publishing: Sawston, UK, 2021. [Google Scholar]
- Bakkar, A.; Neubert, V. Corrosion Characterisation of Alumina-Magnesium Metal Matrix Composites. Corros. Sci. 2007, 49, 1110–1130. [Google Scholar] [CrossRef]
- Sundar, G.; Navasingh, R.J.H. Corrosion Issues in Metal Matrix Composites &Bi-Metals. In Proceedings of the AIP Conference, Bahal, India, 7–9 February 2019; Volume 2142. [Google Scholar]
- Fontanella, J.; Andeen, C.; Schuele, D. Low-Frequency Dielectric Constants of α-Quartz, Sapphire, MgF2, and MgO. J. Appl. Phys. 1974, 45, 2852–2854. [Google Scholar] [CrossRef]
- Ambat, R.; Aung, N.N.; Zhou, W. Evaluation of Microstructural Effects on Corrosion Behaviour of AZ91D Magnesium Alloy. Corros. Sci. 2000, 42, 1433–1455. [Google Scholar] [CrossRef]
- Sundar, G.; Navasingh, R.J.H. Reinforcement in Aluminium Metal Matrix Composites. In Proceedings of the AIP Conference Proceedings, Bahal, India, 7–9 February 2019; Volume 2142. [Google Scholar]
- Hynes, N.R.J.; Sankaranarayanan, R.; Tharmaraj, R.; Pruncu, C.I.; Dispinar, D. A Comparative Study of the Mechanical and Tribological Behaviours of Different Aluminium Matrix–Ceramic Composites. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 330. [Google Scholar] [CrossRef]
- Goh, C.S.; Gupta, M.; Wei, J.; Lee, L.C. Characterization of High Performance Mg/MgO Nanocomposites. J. Compos. Mater. 2007, 41, 2325–2335. [Google Scholar] [CrossRef]
- Udhayan, R.; Bhatt, D.P. On the Corrosion Behaviour of Magnesium and Its Alloys Using Electrochemical Techniques. J. Power Sources 1996, 63, 103–107. [Google Scholar] [CrossRef]
- Raj, B.A.; Jappes, J.T.W.; Khan, M.A.; Dillibabu, V.; Navasingh, R.J.H. Studies on Mechanical Attrition and Surface Analysis on Heat-Treated Nickel Alloy Developed through Additive Manufacturing. Adv. Mater. Sci. Eng. 2022, 2022, 4861346. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Kumar, R. Electrochemical Machining of Aluminium Metal Matrix Composites. Surf. Eng. Appl. Electrochem. 2018, 54, 367–373. [Google Scholar] [CrossRef]
- Baril, G.; Pébère, N. Corrosion of Pure Magnesium in Aerated and Deaerated Sodium Sulphate Solutions. Corros. Sci. 2001, 43, 471–484. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.H.; Meratian, M. Bio-Corrosion Behavior of Magnesium-Fluorapatite Nanocomposite for Biomedical Applications. Mater. Lett. 2010, 64, 2487–2490. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A Survey of Bio-Corrosion Rates of Magnesium Alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Vignesh, N.J.; Velu, P.S.; Navasingh, R.J.H. Physicochemical Analysis of Biobased Composites. In Biobased Composites: Processing, Characterization, Properties, and Applications; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.R.J.; Vignesh, N.J.; Barile, C.; Velu, P.S.; Ali, M.A.; Raza, M.H.; Pruncu, C.I. Mechanical and Microstructural Characterization of Hybrid Fiber Metal Laminates Obtained through Sustainable Manufacturing. Arch. Civ. Mech. Eng. 2022, 22, 35. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.; Fechner, D.; Störmer, M.; Blawert, C.; Witte, F.; Vogt, C.; Drücker, H.; Willumeit, R.; Kainer, K.U.; et al. Magnesium Alloys as Implant Materials-Principles of Property Design for Mg-RE Alloys. Acta Biomater. 2010, 6, 1714–1725. [Google Scholar] [CrossRef]
- Jerome, J.; Navasingh, R.J.H.; Sankaranarayanan, R. Mechanical Behavioural Testing of Fibre Metal Laminate Composites. In Proceedings of the AIP Conference, Bikaner, India, 14–15 October 2019; Volume 2220. [Google Scholar]
- Sankaranarayanan, R.; Navasingh, R.J.H.; Li, D.; Chrysanthou, A. Electromagnetic Riveting Technique of Joining Metals to Polymer Composites in Hybrid Multi-Material Aerospace Structures. Trans. Indian Inst. Met. 2021, 74, 2909–2924. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, J.; Yang, K.; Zhang, B.; Yao, Z.; Wang, H. Study of Bio-Corrosion of Pure Magnesium. Jinshu Xuebao/Acta Metall. Sin. 2005, 41, 1228–1232. [Google Scholar]
- Meenashisundaram, G.K.; Nai, M.H.; Almajid, A.; Gupta, M. Development of High Performance Mg-TiO2 Nanocomposites Targeting for Biomedical/Structural Applications. Mater. Des. 2015, 65, 104–114. [Google Scholar] [CrossRef]
- Xiong, G.; Nie, Y.; Ji, D.; Li, J.; Li, C.; Li, W.; Zhu, Y.; Luo, H.; Wan, Y. Characterization of Biomedical Hydroxyapatite/Magnesium Composites Prepared by Powder Metallurgy Assisted with Microwave Sintering. Curr. Appl. Phys. 2016, 16, 830–836. [Google Scholar] [CrossRef]
- Sharan, V.; Rajesh Jesudoss Hynes, N.; Sankaranarayanan, R. Biodegradable Cutting Fluids for Manufacturing Processes. In Proceedings of the AIP Conference, Bikaner, India, 14–15 October 2019; Volume 2220. [Google Scholar]
- Fulmer, M.T.; Ison, I.C.; Hankermayer, C.R.; Constantz, B.R.; Ross, J. Measurements of the Solubilities and Dissolution Rates of Several Hydroxyapatites. Biomaterials 2002, 23, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Arockia Winston, J.; Navasingh, R.J.H.; Sankaranarayanan, R. Risk Assessment of Corrosion Inhibitors of Magnesium and Its Alloys. In Proceedings of the AIP Conference, Bikaner, India, 14–15 October 2019; Volume 2220. [Google Scholar]
- Driessens, F.C.M. Probable Phase Composition of the Mineral in Bone. Z. Naturforschung Sect. C J. Biosci. 1980, 35, 357–362. [Google Scholar] [CrossRef]
- Biber, R.; Pauser, J.; Geßlein, M.; Bail, H.J. Magnesium-Based Absorbable Metal Screws for Intra-Articular Fracture Fixation. Case Rep. Orthop. 2016, 2016, 9673174. [Google Scholar] [CrossRef] [PubMed]
- Velu, P.S.; Vignesh, N.J.; Hynes, N.R.J. Processing Methods for Manufacture of Biobased Composites. In Biobased Composites: Processing, Characterization, Properties, and Applications; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on Microstructure, Mechanical Properties and Corrosion Behavior of Mg-Zn Alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, C.; Huo, K.; Tang, G.; Tian, X.; Chu, P.K. Corrosion Behavior of ZrN/Zr Coated Biomedical AZ91 Magnesium Alloy. Surf. Coat. Technol. 2009, 203, 2554–2557. [Google Scholar] [CrossRef]
- Su, J.; Teng, J.; Xu, Z.; Li, Y. Biodegradable Magnesium-Matrix Composites: A Review. Int. J. Miner. Metall. Mater. 2020, 27, 724–744. [Google Scholar] [CrossRef]
- Lin, G.; Liu, D.; Chen, M.; You, C.; Li, Z.; Wang, Y.; Li, W. Preparation and Characterization of Biodegradable Mg-Zn-Ca/MgO Nanocomposites for Biomedical Applications. Mater. Charact. 2018, 144, 120–130. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, Y.; Chen, J.; Han, J.; Mao, Z.; Chen, M. Fabrication and Characterization of Friction Stir–Processed Mg-Zn-Ca Biomaterials Strengthened with MgO Particles. Int. J. Adv. Manuf. Technol. 2021, 117, 919–932. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Tang, C.; Pang, R.; Zhang, X.; Li, C.; Zhang, W.; Zhou, F.; Xue, F.; Lyu, S.; et al. In Vitro and Vivo Biocompatibility and Degradability of Low-Alloyed Mg-Zn-Ca-MgO Composite as Bone Repair Biomaterials. J. Met. Mater. Miner. 2024, 34, 1910. [Google Scholar] [CrossRef]
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Zeller-Plumhoff, B.; Gile, M.; Priebe, M.; Slominska, H.; Boll, B.; Wiese, B.; Würger, T.; Willumeit-Römer, R.; Meißner, R.H. Exploring Key Ionic Interactions for Magnesium Degradation in Simulated Body Fluid—A Data-Driven Approach. Corros. Sci. 2021, 182, 109272. [Google Scholar] [CrossRef]
- Suchý, T.; Bartoš, M.; Sedláček, R.; Šupová, M.; Žaloudková, M.; Martynková, G.S.; Foltán, R. Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds. Materials 2021, 14, 4388. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Pochert, A.; Beck, M.; Fiedler, R.; Gruber, J.; Lindén, M. Dissolution Kinetics of Mesoporous Silica Nanoparticles in Different Simulated Body Fluids. J. Sol-Gel Sci. Technol. 2016, 79, 319–327. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and Assessment of Revised Simulated Body Fluids. J. Biomed. Mater. Res. A 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef]
- Thanh Hoai, T.; Kim Nga, N. Synthesis and Investigation into Apatite-Forming Ability of Hydroxyapatite/Chitosan-Based Scaffold. VNU J. Sci. Nat. Sci. Technol. 2019, 35, 86–94. [Google Scholar] [CrossRef]
- Aviles, T.; Hsu, S.M.; Clark, A.; Ren, F.; Fares, C.; Carey, P.H.; Esquivel-Upshaw, J.F. Hydroxyapatite Formation on Coated Titanium Implants Submerged in Simulated Body Fluid. Materials 2020, 13, 5593. [Google Scholar] [CrossRef]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical Development of Simulated Body Fluids Used in Biomedical Applications: A Review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Ali, M.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-Based Composites and Alloys for Medical Applications: A Review of Mechanical and Corrosion Properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of Alloying Elements on the Corrosion Behavior and Biocompatibility of Biodegradable Magnesium Alloys: A Review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An Innovative Specimen Configuration for the Study of Mg Corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- ASTM G99-23; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2023.
- Haghshenas, M. Mechanical Characteristics of Biodegradable Magnesium Matrix Composites: A Review. J. Magnes. Alloys 2017, 5, 189–201. [Google Scholar] [CrossRef]
- Chang, J.W.; Guo, X.W.; Fu, P.H.; Peng, L.M.; Ding, W.J. Effect of Heat Treatment on Corrosion and Electrochemical Behaviour of Mg-3Nd-0.2Zn-0.4Zr (Wt.%) Alloy. Electrochim. Acta 2007, 52, 3160–3167. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In Vitro and in Vivo Corrosion Measurements of Magnesium Alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.C.; Schmutz, P.; Brunner, S.; Liu, M.; Song, G.; Atrens, A. An Exploratory Study of the Corrosion of Mg Alloys during Interrupted Salt Spray Testing. Corros. Sci. 2009, 51, 1277–1292. [Google Scholar] [CrossRef]
- Song, G. Control of Biodegradation of Biocompatable Magnesium Alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Wang, X.; Nie, Q.; Ma, X.; Fan, J.; Yan, T.; Li, X. Microstructure and Properties of Co-Continuous (β-TCP+MgO)/Zn-Mg Composite Fabricated by Suction Exsorption for Biomedical Applications. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2017, 27, 1996–2006. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; He, D.; Shi, J. Synthesis and Characterization of Mesoporous CaO-MO-SiO2-P2O5 (M = Mg, Zn, Cu) Bioactive Glasses/Composites. J. Mater. Chem. 2008, 18, 4103–4109. [Google Scholar] [CrossRef]
- Tang, C.; Lyu, S.; Zhao, Z.; Chen, M. Effects of MgO Nano Particles on the Mechanical Properties and Corrosion Behavior of Mg–Zn–Ca Alloy. Mater. Chem. Phys. 2023, 297, 127380. [Google Scholar] [CrossRef]
- Deng, C.J.; Wong, M.L.; Ho, M.W.; Yu, P.; Ng, D.H.L. Formation of MgO and Mg-Zn Intermetallics in an Mg-Based Composite by in Situ Reactions. Compos Part A Appl. Sci. Manuf. 2005, 36, 551–557. [Google Scholar] [CrossRef]



| Sample | Mg (%) | Zn (%) | MgO (%) | Total Density (g/cm3) | Total Mass (g) |
|---|---|---|---|---|---|
| A | 96 | 4 | 0 | 1.997 | 718.92 |
| B | 95.6 | 4 | 0.4 | 2.004 | 721.44 |
| C | 95.4 | 4 | 0.6 | 2.008 | 722.88 |
| Ion | Concentration (mM) |
|---|---|
| Na+ | 142 |
| K+ | 5 |
| Mg2+ | 1.5 |
| Ca2+ | 2.5 |
| Cl− | 147.8 |
| HCO3− | 4.2 |
| HPO42− | 1 |
| SO42− | 0.5 |
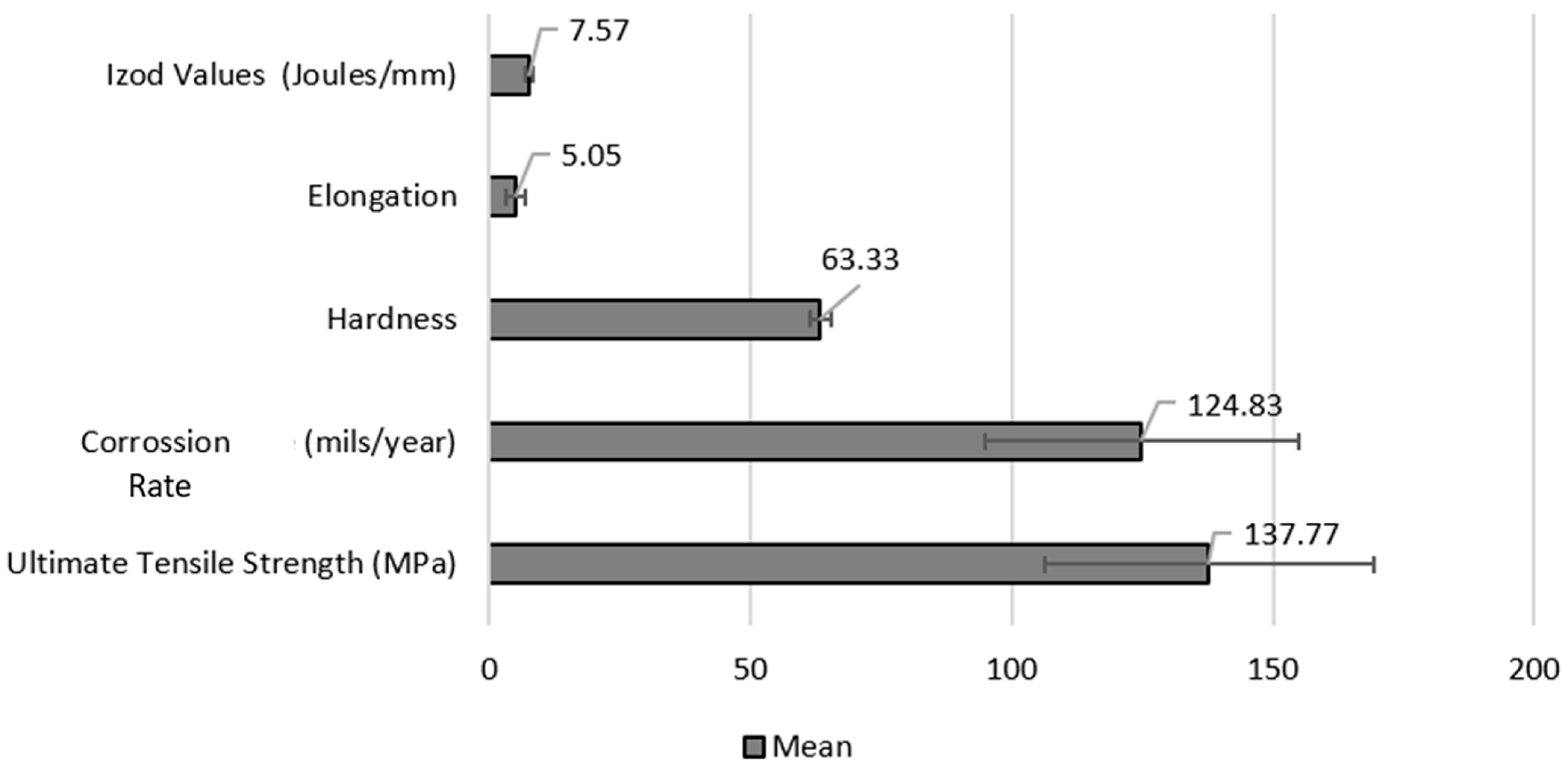
| Property | Mean (%) | Standard Deviation (SD) | Standard Error (SE) | 95% Confidence Interval (±) |
|---|---|---|---|---|
| Ultimate Tensile Strength (MPa) | 137.77 | 31.01 | 17.91 | 77.09 |
| Elongation (%) | 5.05 | 1.69 | 0.97 | 4.17 |
| Izod Impact (J/mm) | 7.57 | 0.64 | 0.37 | 1.61 |
| Wear Loss (g) | 0.0173 | 0.0046 | 0.0026 | 0.0111 |
| Weight Loss (g) | 0.4763 | 0.111 | 0.0641 | 0.276 |
| Corrosion Rate (mils/year) | 124.83 | 29.29 | 16.91 | 72.84 |
| Hardness (HRE) | 63.33 | 2.08 | 1.2 | 5.17 |
| Applied Load (N) | Sliding Velocity (m/s) | Sliding Distance (mm) | Sliding Diameter (mm) | R.P.M | Time (s) |
|---|---|---|---|---|---|
| 20 | 1 | 400 | 40 | 478 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navasingh, R.J.H.; Amos, D.A.; Gurunathan, M.K.; Nikolova, M.P. Biodegradable Mg-Zn-MgO Composites for Locking Compression Fixation Plates for Pediatric Orthopedics: Improved Mechanical Properties and Corrosion Resistance. Processes 2025, 13, 2077. https://doi.org/10.3390/pr13072077
Navasingh RJH, Amos DA, Gurunathan MK, Nikolova MP. Biodegradable Mg-Zn-MgO Composites for Locking Compression Fixation Plates for Pediatric Orthopedics: Improved Mechanical Properties and Corrosion Resistance. Processes. 2025; 13(7):2077. https://doi.org/10.3390/pr13072077
Chicago/Turabian StyleNavasingh, Rajesh Jesudoss Hynes, Daniel Asirvatham Amos, Manoj Kumar Gurunathan, and Maria P. Nikolova. 2025. "Biodegradable Mg-Zn-MgO Composites for Locking Compression Fixation Plates for Pediatric Orthopedics: Improved Mechanical Properties and Corrosion Resistance" Processes 13, no. 7: 2077. https://doi.org/10.3390/pr13072077
APA StyleNavasingh, R. J. H., Amos, D. A., Gurunathan, M. K., & Nikolova, M. P. (2025). Biodegradable Mg-Zn-MgO Composites for Locking Compression Fixation Plates for Pediatric Orthopedics: Improved Mechanical Properties and Corrosion Resistance. Processes, 13(7), 2077. https://doi.org/10.3390/pr13072077







