Production of Biochar from Plantain Rachis and Cassava Peel Towards Sustainable Management of Caribbean Agricultural Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of Biomasses
2.3. Treatment of Biomass
2.4. Production of Biochar
2.5. Characterization of Biomass and Biochar
3. Results and Discussion
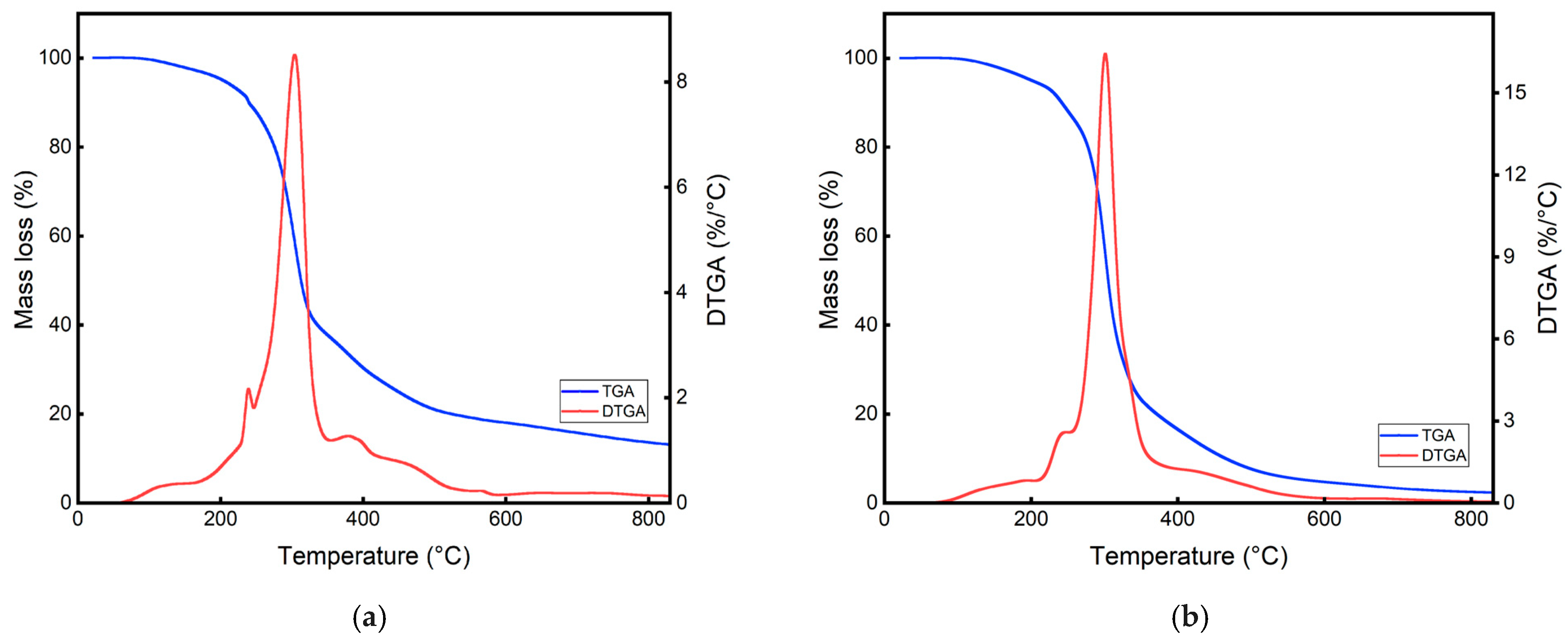
3.1. Degradation Process of Biomass
3.2. Biochar Production
3.3. Characterization of Biochar
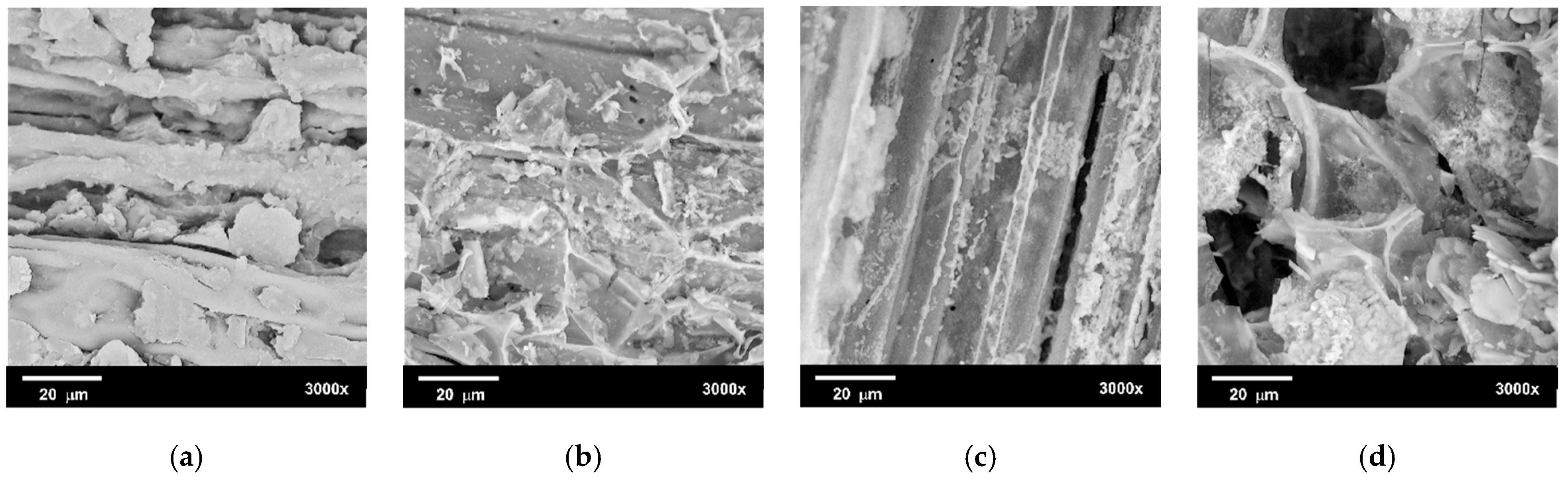
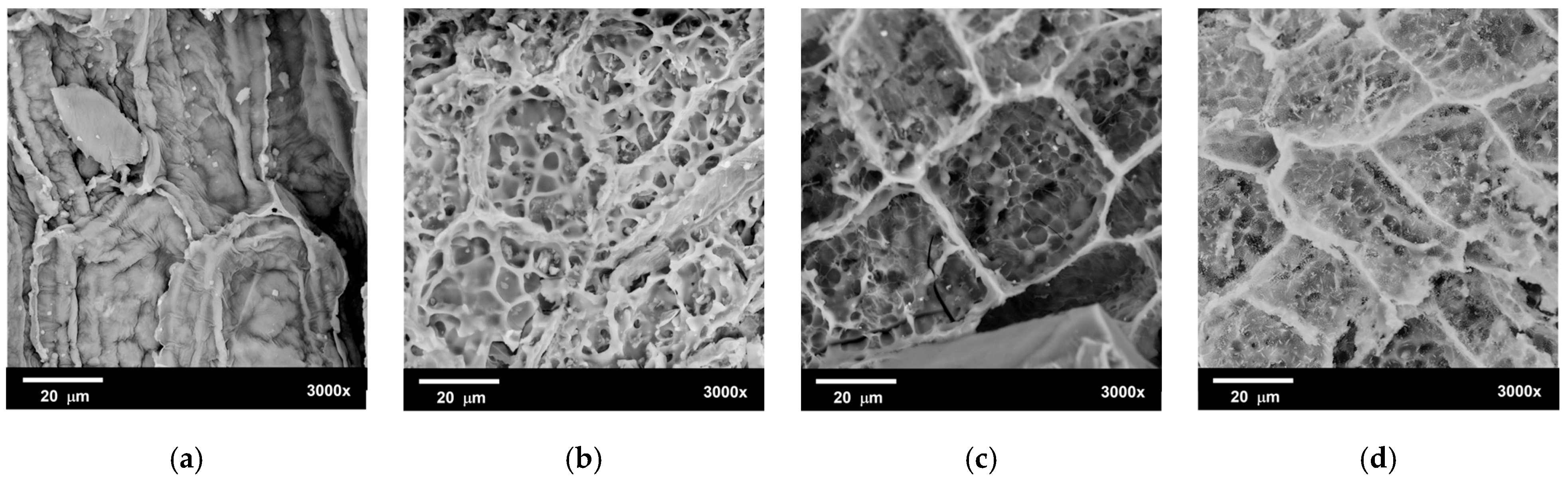
3.3.1. Scanning Electron Microscopy Analysis
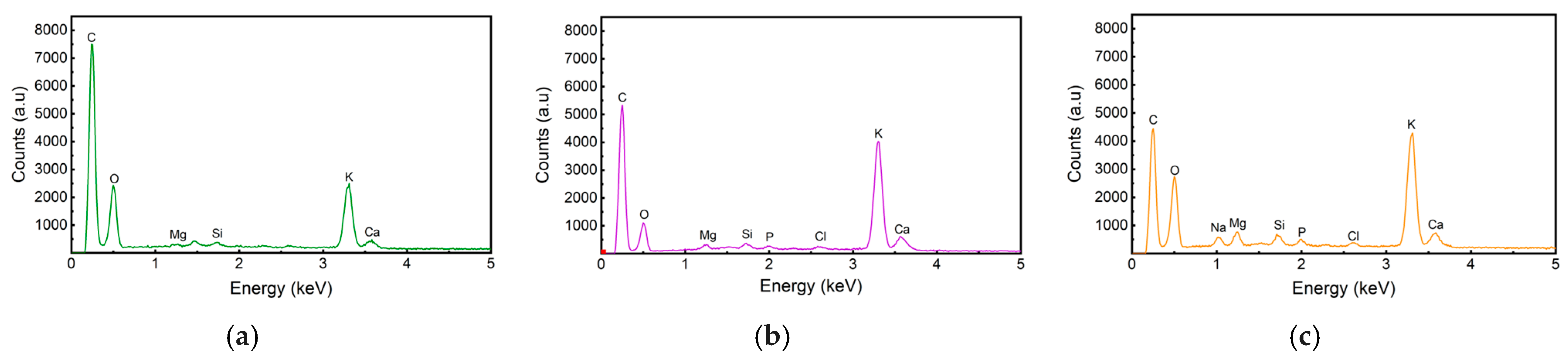
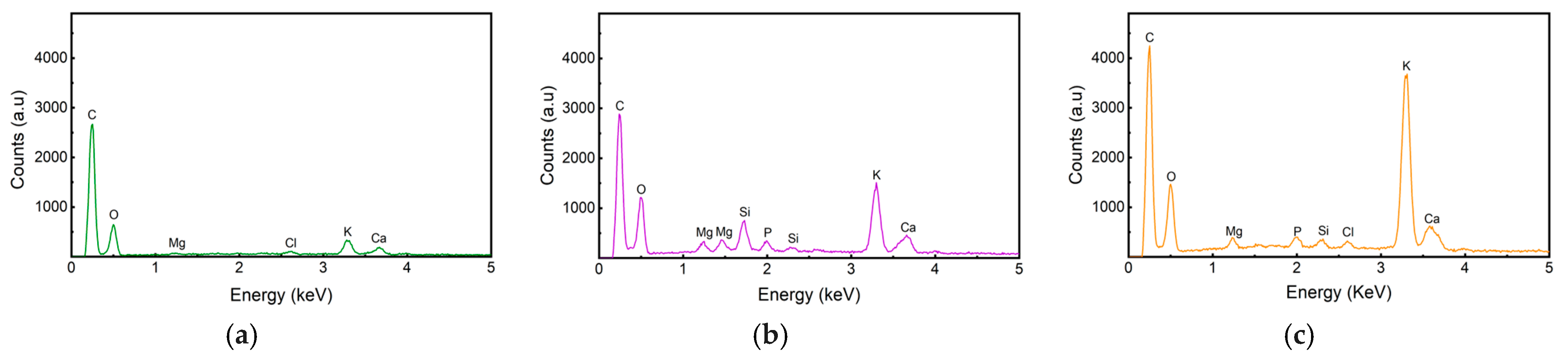
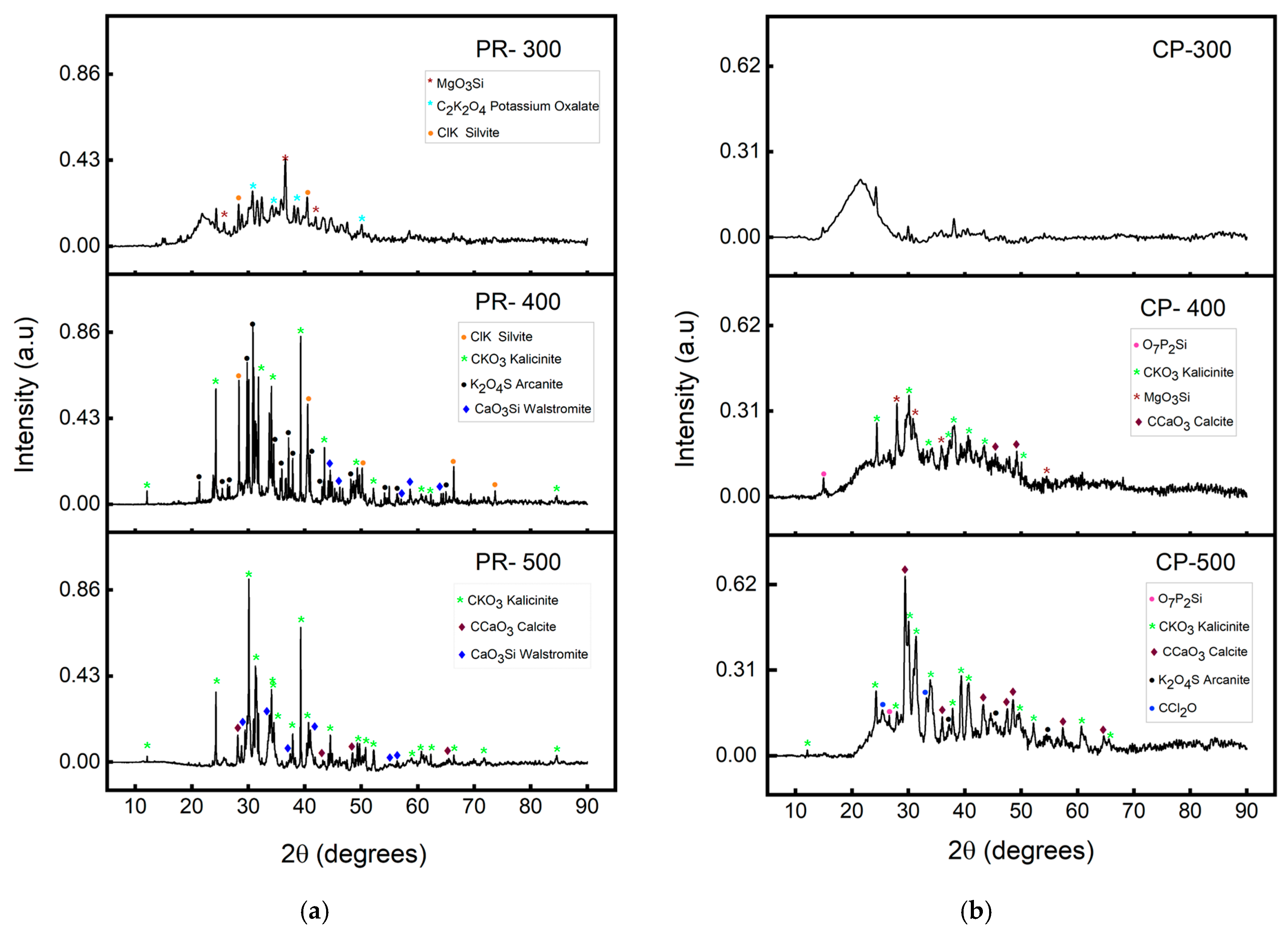
3.3.2. Energy Dispersive X-Ray (EDX) and X-Ray Diffraction Analysis
3.3.3. Fourier Transform Infrared Spectroscopy Analysis
3.4. Applications of Biochar
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cervigón, F. Variability and sustainability of the Southern Subarea of the Caribbean Sea large marine ecosystem. Environ. Dev. 2017, 22, 30–41. [Google Scholar] [CrossRef]
- Schuhmann, P.W.; Mahon, R. The valuation of marine ecosystem goods and services in the Caribbean: A literature review and framework for future valuation efforts. Ecosyst. Serv. 2015, 11, 56–66. [Google Scholar] [CrossRef]
- Ecological Threat Report 2024: Analysing Ecological Threats, Resilience & Peace, Sydney, October 2024. Institute for Economics & Peace. Available online: http://visionofhumanity.org/resources (accessed on 14 October 2024).
- Wuddivira, M.N.; De Gannes, V.; Meerdink, G.; Dalrymple, N.; Henry, S. Challenges and Opportunities for Food and Nutrition Security in the Americas: The View of the Academies of Sciences, 2017, The Inter-American Network of Academies of Sciences (IANAS-IAP). Available online: https://ianas.org/wp-content/uploads/2020/07/fnb02c-1.pdf (accessed on 14 July 2018).
- FAO. Latin America and the Caribbean Regional Synthesis for the State of the World’s Biodiversity for Food and Agriculture; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- UN Environment. Waste Management Outlook for Latin America and the Caribbean. United Nations Environment Programme, Latin America and the Caribbean Office. Panama City, Panama. 2018. Available online: https://www.unep.org/ietc/resources/publication/waste-management-outlook-latin-america-and-caribbean (accessed on 1 October 2018).
- UN Environment. Global Waste Management Outlook. United Nations Environment Programme. 2015. Available online: https://www.unep.org/ietc/resources/publication/global-waste-management-outlook-2015 (accessed on 6 March 2015).
- Manyuchi, M.M.; Mbohwa, C.; Muzenda, E. Potential to Use Municipal Waste Biochar in Wastewater Treatment for Nutrients Recovery. Phys. Chem. Earth Parts A/B/C 2018, 107, 92–95. [Google Scholar] [CrossRef]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in Water and Wastewater Treatment—A Sustainability Assessment. Chem. Eng. J. 2021, 420 Pt 1, 129946. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of Soil Quality Using Biochar and Brown Coal Waste: A Review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Santa-Olalla, A.; Campos, P.; López-Núñez, R.; González-Pérez, J.A.; Almendros, G.; Knicker, H.E.; Sánchez-Martín, A.; Fernández-Boy, E. Impact of Biochar Amendment on Soil Properties and Organic Matter Composition in Trace Element-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 2140. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hoang, A.T.; Nižetić, S.; Pandey, A.; Cheng, C.K.; Luque, R.; Ong, H.C.; Thomas, S.; Nguyen, X.P. Biomass-derived Biochar: From Production to Application in Removing Heavy Metal-Contaminated Water. Process Saf. Environ. Prot. 2022, 160, 704–733. [Google Scholar] [CrossRef]
- Sri Shalini, S.; Palanivelu, K.; Ramachandran, A.; Vijaya, R. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Conv. Bioref. 2021, 11, 2247–2267. [Google Scholar] [CrossRef]
- Rex, P.; Mohammed Ismail, K.R.; Meenakshisundaram, N.; Barmavatu, P.; Bharadwaj, A.V.S.L.S. Agricultural Biomass Waste to Biochar: A Review on Biochar Applications Using Machine Learning Approach and Circular Economy. ChemEngineering 2023, 7, 50. [Google Scholar] [CrossRef]
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A Review on Biochar Production from Different Biomass Wastes by Recent Carbonization Technologies and its Sustainable Applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. A review of the next-generation biochar production from waste biomass for material applications. Sci. Total Environ. 2023, 904, 167171. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Ogunsuyi, H.; Olawale, C. Evaluation of Plantain Biomass (Musa paradisiaca L.), as Feedstock for Bio-Ethanol Production. Green Sustain. Chem. 2021, 11, 59–71. [Google Scholar] [CrossRef]
- Mustofa, A. Bioethanol Production From Banana Stem By Using Simultaneous Saccharification and Fermentation (SSF). IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012004. [Google Scholar] [CrossRef]
- Castañeda-Niño, J.P.; Mina Hernandez, J.H.; Solanilla Duque, J.F. Potential of Plantain Pseudostems (Musa AAB Simmonds) for Developing Biobased Composite Materials. Polymers 2024, 16, 1357. [Google Scholar] [CrossRef]
- Evelyn, E.; Okewale, A.O.; Owabor, C.N. Optimization, Kinetics and thermodynamic modeling of pulp production from plantain stem using the kraft process. Clean. Chem. Eng. 2025, 11, 100129. [Google Scholar] [CrossRef]
- Kouteu Nanssou, P.A.; Jiokap Nono, Y.; Kapseu, C. Pretreatment of cassava stems and peelings by thermohydrolysis to enhance hydrolysis yield of cellulose in bioethanol production process. Renew. Energy 2016, 97, 252–265. [Google Scholar] [CrossRef]
- Hartulistiyoso, E.; Farobie, O.; Zaky, M. Delignification of Cassava Peel as Bioethanol Raw Material using Combined Alkali and Microwave Heating Methods. IOP Conf. Ser. Earth Environ. Sci. 2022, 1038, 012021. [Google Scholar] [CrossRef]
- Abdullah; Azzahra Eka, N. Kinetics Study of Bioethanol Production from Cassava Peels Waste using Saccharomyces diastaticus. Int. J. Chem. Biochem. Sci. (IJCBS) 2024, 25, 884–892. [Google Scholar] [CrossRef]
- Marvie, I.; Sitanggang, A.B.; Budijanto, S. Characterization of Insoluble Fiber in Cassava Peel and Its Hydrolyzate Potential as a Prebiotic for Lactobacillus Plantarum. In Proceedings of the 6th Food Ingredient Asia Conference—6th FiAC, Bogor, Indonesia, 14–16 October 2020; pp. 31–37. [Google Scholar] [CrossRef]
- Abdullah, N.; Taib, R.M.; Mohamad Aziz, N.S.; Omar, M.R.; Disa, N. Banana pseudo-stem biochar derived from slow and fast pyrolysis process. Heliyon 2023, 9, e12940. [Google Scholar] [CrossRef]
- Odeyemi, S.O.; Iwuozor, K.O.; Emenike, E.C.; Odeyemi, O.T.; Adeniyi, A.G. Valorization of waste cassava peel into biochar: An alternative to electrically-powered process. Total Environ. Res. Themes 2023, 6, 100029. [Google Scholar] [CrossRef]
- Hamissou, I.G.M.; Appiaha, K.E.K.; Sylviec, K.A.T.; Ousmaila, S.M.; Casimir, B.Y.; Benjamina, Y.k. Valorization of cassava peelings into biochar: Physical and chemical characterizations of biochar prepared for agricultural purposes. Sci. Afr. 2023, 20, e01737. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patra, B.R.; Podder, J.; Dalai, A.K. Synthesis of Biochar from Lignocellulosic Biomass for Diverse Industrial Applications and Energy Harvesting: Effects of Pyrolysis Conditions on the Physicochemical Properties of Biochar. Front. Mater. 2022, 9, 870184. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J.A. Review on pyrolysis of biomass constituents: Mechanism and composition of the products obtained from the conversion of cellulose, hemicellulose and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Chen, L.-H.; Jiang, J. Why fine tree roots are stronger than thicker roots: The role of cellulose and lignin in relation to slope stability. Geomorphology 2014, 206, 196–202. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 1312, 123614. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Zhu, Y.; Chen, J.; Lu, Y.; Li, S.; Zheng, Y.; Zheng, Z. Efficient ex-situ catalytic upgrading of biomass pyrolysis vapors to produce methylfurans and phenol over bio-based activated carbon. Biomass Bioenergy 2020, 142, 105794. [Google Scholar] [CrossRef]
- Setyawan, H.Y.; Safira, L.; Mulyarto, A.R.; Wijana, S.; Pranowo, D. The effect of pyrolysis temperature and ball-milling duration on characteristics of micro bio-char derived from oil palm empty fruit bunches. Sustain. Environ. 2023, 9, 2173041. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Lu, S. Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Samer, M. Pyrolysis; InTech: Vienna, Austria, 2017. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, C.; Hu, Z.; Zhou, Y.; Xiao, Y.; Wang, T. Pyrolysis of sewage sludge by electromagnetic induction: Biochar properties and application in adsorption removal of Pb(II), Cd(II) from aqueous solution. Waste Manag. 2019, 89, 48–56. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, Chemical, and Physical Properties of Wood and Perennial Grass Biochars for Possible Composite Application. BioRes 2015, 11, 1334–1348. [Google Scholar] [CrossRef]
- Freitas, A.M.; Nair, V.D.; Harris, W.G. Biochar as Influenced by Feedstock Variability: Implications and Opportunities for Phosphorus Management. Front. Sustain. Food Syst. 2020, 4, 510982. [Google Scholar] [CrossRef]
- Qin, L.; Wu, Y.; Hou, Z.; Jiang, E. Influence of biomass components, temperature and pressure on the pyrolysis behavior and biochar properties of pine nut shells. Bioresour. Technol. 2020, 313, 123682. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Pelaez-Samaniego, M.R.; Garcia-Perez, M. Perspectives of Engineered Biochar for Environmental Applications: A Review. Energy Fuels 2022, 36, 7940–7986. [Google Scholar] [CrossRef]
- Rizwan, M.; Murtaza, G.; Zulfiqar, F.; Moosa, A.; Iqbal, R.; Ahmed, Z.; Irshad, S.; Khan, I.; Li, T.; Chen, J.; et al. Sustainable manufacture and application of biochar to improve soil properties and remediate soil contaminated with organic impurities: A systematic review. Front. Environ. Sci. 2023, 11, 1277240. [Google Scholar] [CrossRef]
- Zhu, H.; An, Q.; Mohd Nasir, A.S.; Babin, A.; Saucedo, S.L.; Vallenas, A.; Li, L.; Baldwin, S.A.; Lau, A.; Bi, X. Emerging applications of biochar: A review on techno-environmental-economic aspects. Bioresour. Technol. 2023, 388, 129745. [Google Scholar] [CrossRef]
- Sepúlveda-Cadavid, C.; Romero, J.H.; Torres, M.; Becerra-Agudelo, E.; López, J.E. Evaluation of a Biochar-Based Slow-Release P Fertilizer to Improve Spinacia oleracea P Use, Yield, and Nutritional Quality. J. Soil. Sci. Plant Nutr. 2021, 21, 2980–2992. [Google Scholar] [CrossRef]
- Cen, Z.; Wei, L.; Muthukumarappan, K.; Sobhan, A.; McDaniel, R. Assessment of a Biochar-Based Controlled Release Nitrogen Fertilizer Coated with Polylactic Acid. J. Soil. Sci. Plant Nutr. 2021, 21, 2007–2019. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, L.; Huang, Q.; Tan, Z. Development of a carbon-based slow release fertilizer treated by biooil coating and study on its feedback effect on farmland application. J. Clean. Prod. 2019, 239, 118085. [Google Scholar] [CrossRef]
- Amoakwah, E.; Shim, J.; Kim, S.; Lee, Y.; Kwon, S.; Sangho, J.; Park, S. Impact of silicate and lime application on soil fertility and temporal changes in soil properties and carbon stocks in a temperate ecosystem. Geoderma 2023, 433, 116431. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass derived N-doped biochar as efficient catalyst supports for CO2 methanation. J. CO2 Util. 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Gasim, M.F.; Choong, Z.-Y.; Koo, P.-L.; Low, S.-C.; Abdurahman, M.-H.; Ho, Y.-C.; Mohamad, M.; Suryawan, I.W.K.; Lim, J.-W.; Oh, W.-D. Application of Biochar as Functional Material for Remediation of Organic Pollutants in Water: An Overview. Catalysts 2022, 12, 210. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Ok, Y.S. Biochar as absorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wang, Y.; Zhang, L.; Huang, Q. Study of the mechanism of remediation of Cd-contaminated soil by novel biochars. Environ. Sci. Pollut. Res. 2017, 24, 24844–24855. [Google Scholar] [CrossRef] [PubMed]

| Income Level | Country or Territory |
|---|---|
| Low | Haiti |
| Lower–middle | El Salvador, Guatemala, Guyana, Honduras, Nicaragua |
| Upper–middle | Belize, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, Grenada, Jamaica, Mexico, Panama, Peru, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Venezuela. |
| High | Antigua and Barbuda, Bahamas, Barbados, Saint Kitts and Nevis, Trinidad and Tobago |
| Biomass | Mass Loss | Solid Product | ||
|---|---|---|---|---|
| First Stage | Second Stage | Third Stage | ||
| Plantain rachis | 3.16 (%) | 59.12 (%) | 19.90 (%) | 17.82 (%) |
| Cassava peel | 6.02 (%) | 72.93 (%) | 16.11 (%) | 4.94 (%) |
| Application | Hemicellulose (%) | Cellulose (%) | Lignin (%) | Reference |
|---|---|---|---|---|
| Bioethanol | 28.0 | 29.0 | 41.3 | [17] |
| Bioethanol | 19.4 | 44.6 | 36.0 | [18] |
| Paper pulp | 3.46 | 58.03 | 38.51 | [20] |
| Application | Hemicellulose (%) | Cellulose (%) | Lignin (%) | Reference |
|---|---|---|---|---|
| Bioethanol | 30.5 | 57.7 | 1.3 | [21] |
| Bioethanol | 15.66 | 59.01 | 12.79 | [22] |
| Bioethanol | 15.6 | 44.0 | 36 | [23] |
| Prebiotic | 4.49 | 55.79 | 7.32 | [24] |
| Parameters | PR-300 | PR-400 | PR-500 | CP-300 | CP-400 | CP-500 |
|---|---|---|---|---|---|---|
| Yield (%) | 65.7 | 45.6 | 33.7 | 62.0 | 37.5 | 25.4 |
| MC (%) | 8.9 | 7.1 | 5.1 | 10.3 | 10.3 | 9.2 |
| Compound | PR Biochar | CP Biochar | ||||
|---|---|---|---|---|---|---|
| 300 °C | 400 °C | 500 °C | 300 °C | 400 °C | 500 °C | |
| MgO3Si | ✓ | ✓ | ||||
| CCaO3 | ✓ | ✓ | ✓ | |||
| CKO3 | ✓ | ✓ | ✓ | ✓ | ||
| O7P2Si | ✓ | ✓ | ||||
| CCl2O | ✓ | |||||
| K2O4S | ✓ | |||||
| C2K2O4 | ✓ | |||||
| ClK | ✓ | ✓ | ||||
| CaO3Si | ✓ | ✓ | ✓ | |||
| Compound | PR Biochar | CP Biochar | ||||
|---|---|---|---|---|---|---|
| 300 °C | 400 °C | 500 °C | 300 °C | 400 °C | 500 °C | |
| 3200–3400 O–H stretching of H-bonded hydroxyl groups | ✓ | ✓ ↓ | – | ✓ | ✓↓ | – |
| 3000–3200 C-H bond of aliphatic functional groups | – | – | – | ✓ | ✓ ↑ | ✓↑ |
| 1700–1740 C=C and C=O stretching of carbonyl and carboxyl groups | ✓ | – | – | ✓ | ✓↓ | – |
| 1550–1650 C=C stretching of aromatic components, C=O stretching of conjugated ketones and quinones | ✓ | ✓ | ✓ | ✓ | ✓ ↓ | ✓↓ |
| 1470–1490 C=C asymmetric stretching of lignin and C-H2 stretching of lignin carbohydrate | – | ✓ ↓ | ✓↓ | ✓ | ✓ ↓ | ✓↓ |
| 1380 stretching in the benzene rings zone | ✓ | ✓ ↑ | ✓↑ | – | ✓ | ✓ |
| 1050–1250 C–O–C stretching of cellulose and hemicellulose | – | ✓ ↑ | ✓↑ | – | ✓ | ✓ |
| 600–832 C–H stretching of aromatic and heteroatomic compounds | – | ✓ ↑ | ✓↑ | – | – | ✓↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herazo, A.P.; Zambrano, A.; Marín, L.; Mass, J.; Montenegro, D.N. Production of Biochar from Plantain Rachis and Cassava Peel Towards Sustainable Management of Caribbean Agricultural Waste. Processes 2025, 13, 2059. https://doi.org/10.3390/pr13072059
Herazo AP, Zambrano A, Marín L, Mass J, Montenegro DN. Production of Biochar from Plantain Rachis and Cassava Peel Towards Sustainable Management of Caribbean Agricultural Waste. Processes. 2025; 13(7):2059. https://doi.org/10.3390/pr13072059
Chicago/Turabian StyleHerazo, Adriana Patricia, Alejandra Zambrano, Lorena Marín, Julio Mass, and Diana Nathalie Montenegro. 2025. "Production of Biochar from Plantain Rachis and Cassava Peel Towards Sustainable Management of Caribbean Agricultural Waste" Processes 13, no. 7: 2059. https://doi.org/10.3390/pr13072059
APA StyleHerazo, A. P., Zambrano, A., Marín, L., Mass, J., & Montenegro, D. N. (2025). Production of Biochar from Plantain Rachis and Cassava Peel Towards Sustainable Management of Caribbean Agricultural Waste. Processes, 13(7), 2059. https://doi.org/10.3390/pr13072059







