Enhancing the Removal Efficiency of Rhodamine B by Loading Pd onto In2O3/BiVO4 Under Visible Light Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Photocatalyst Synthesis
2.1.1. Synthesis of In2O3
2.1.2. Synthesizing Heterojunction of In2O3/BiVO4
2.1.3. Preparation of Palladium Doped In2O3/BiVO4 Heterojunctions
2.2. Characterization of Prepared Samples
2.3. Evaluation of Photocatalytic Performance
3. Results and Discussion
3.1. Characterization
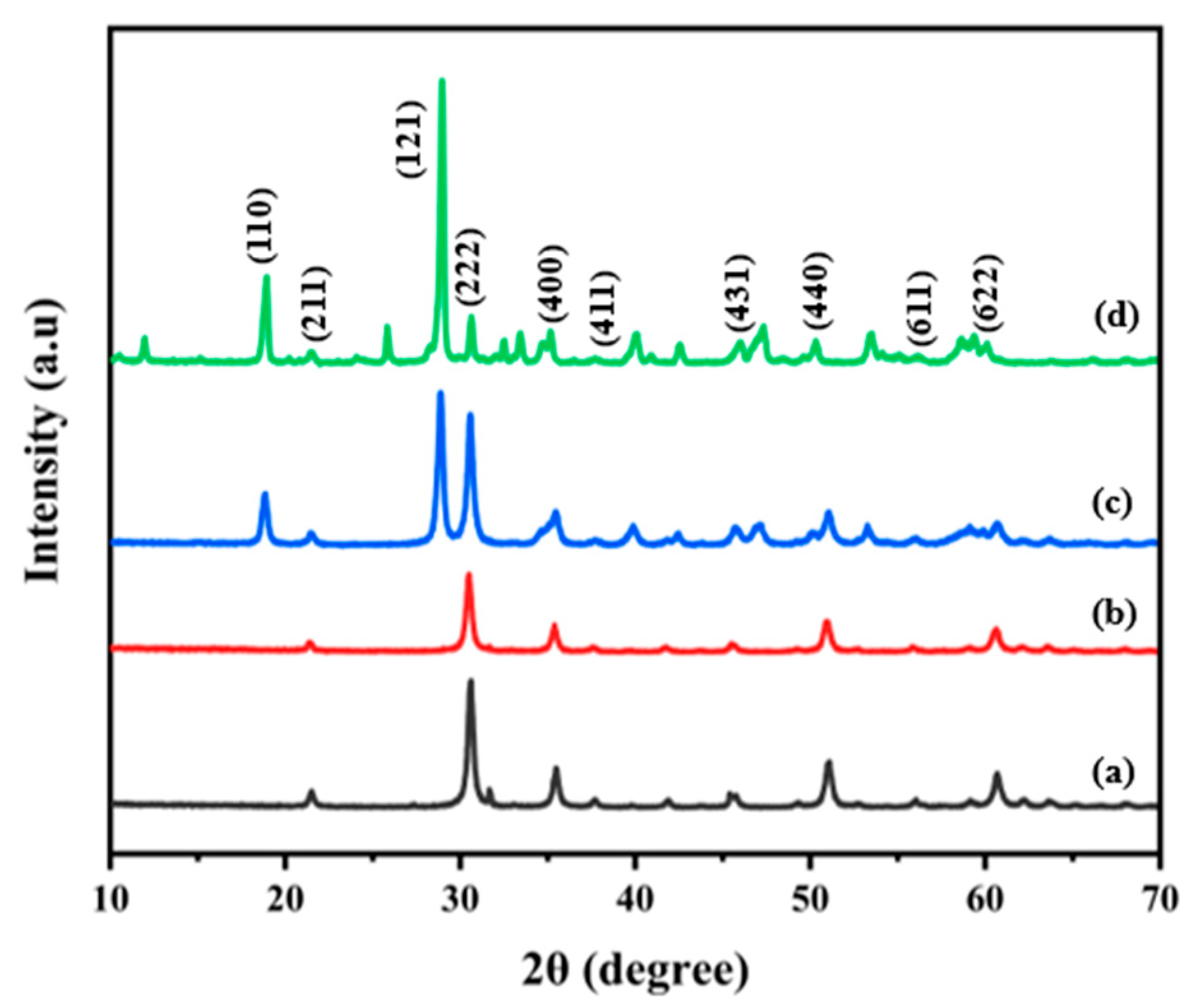
3.1.1. XRD
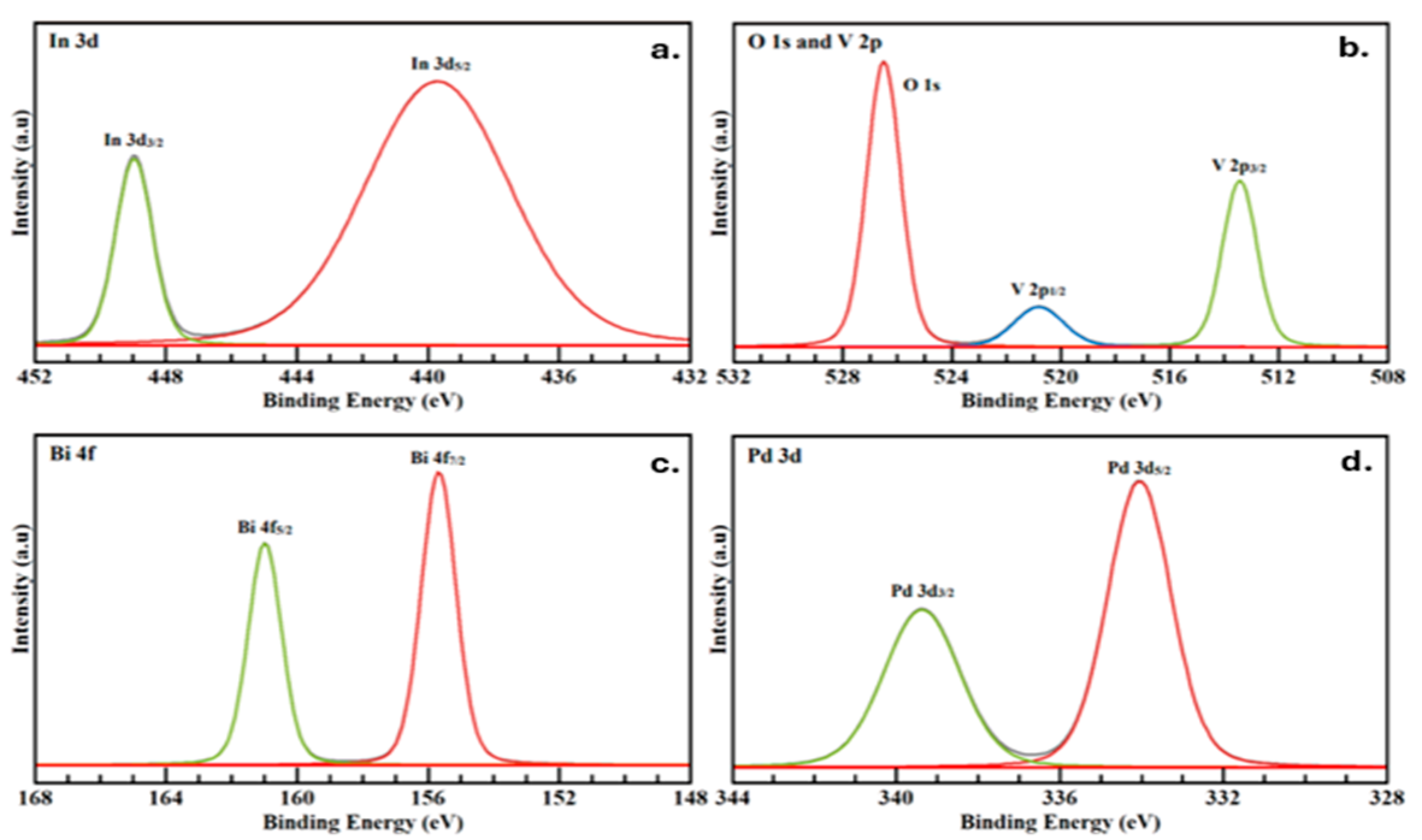
3.1.2. XPS
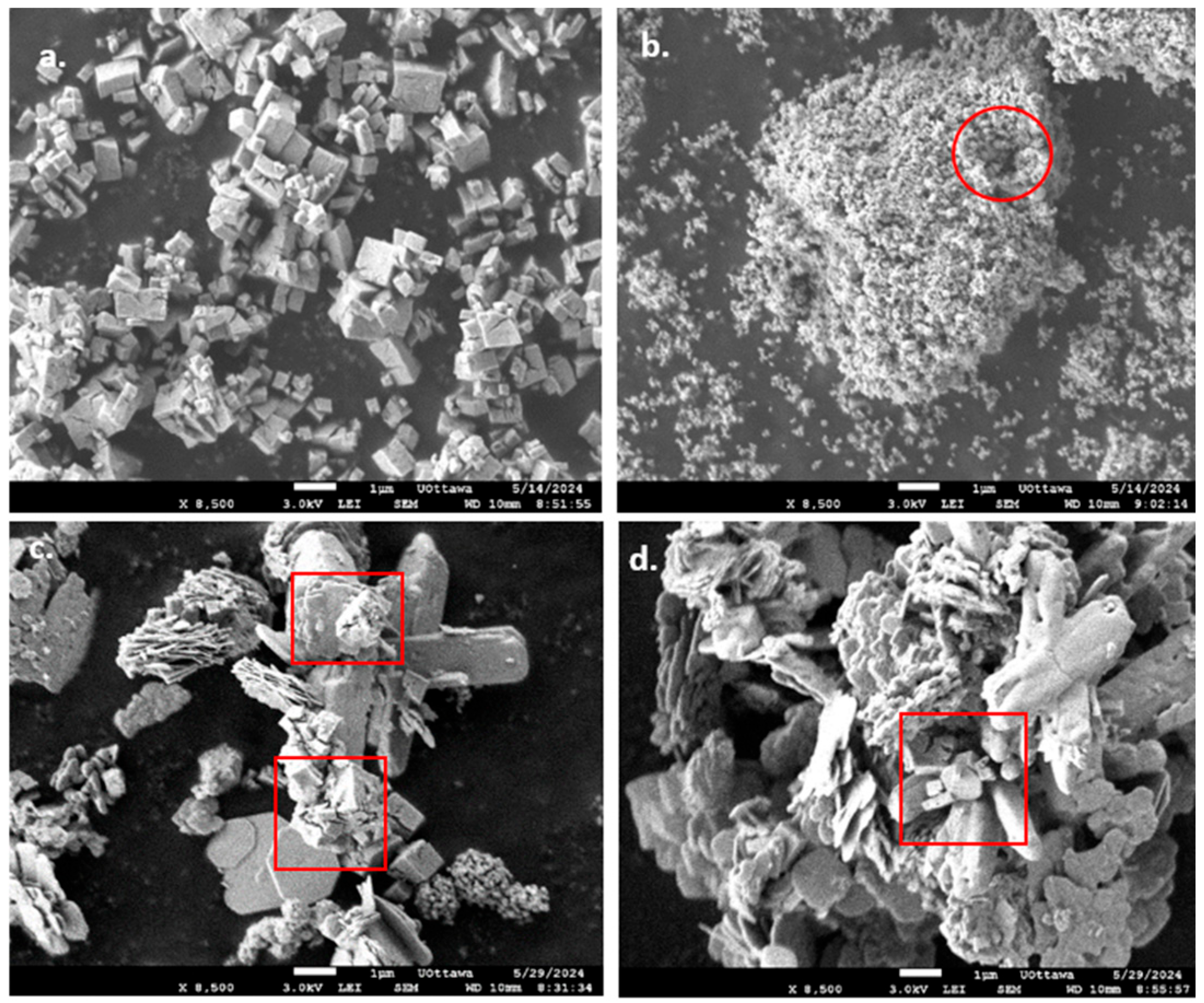
3.1.3. SEM
3.1.4. UV–Vis-NIR Spectroscopy
3.2. Assessment of the Photocatalytic Properties of the Developed Photocatalysts
3.2.1. Influence of Doping Metal
3.2.2. Effect of Pd-In2O3/BiVO4 Loading on Photocatalytic Degradation Efficiency
3.2.3. Recyclability and Durability of Pd-In2O3/BiVO4
3.2.4. RhB Degradation Pathway
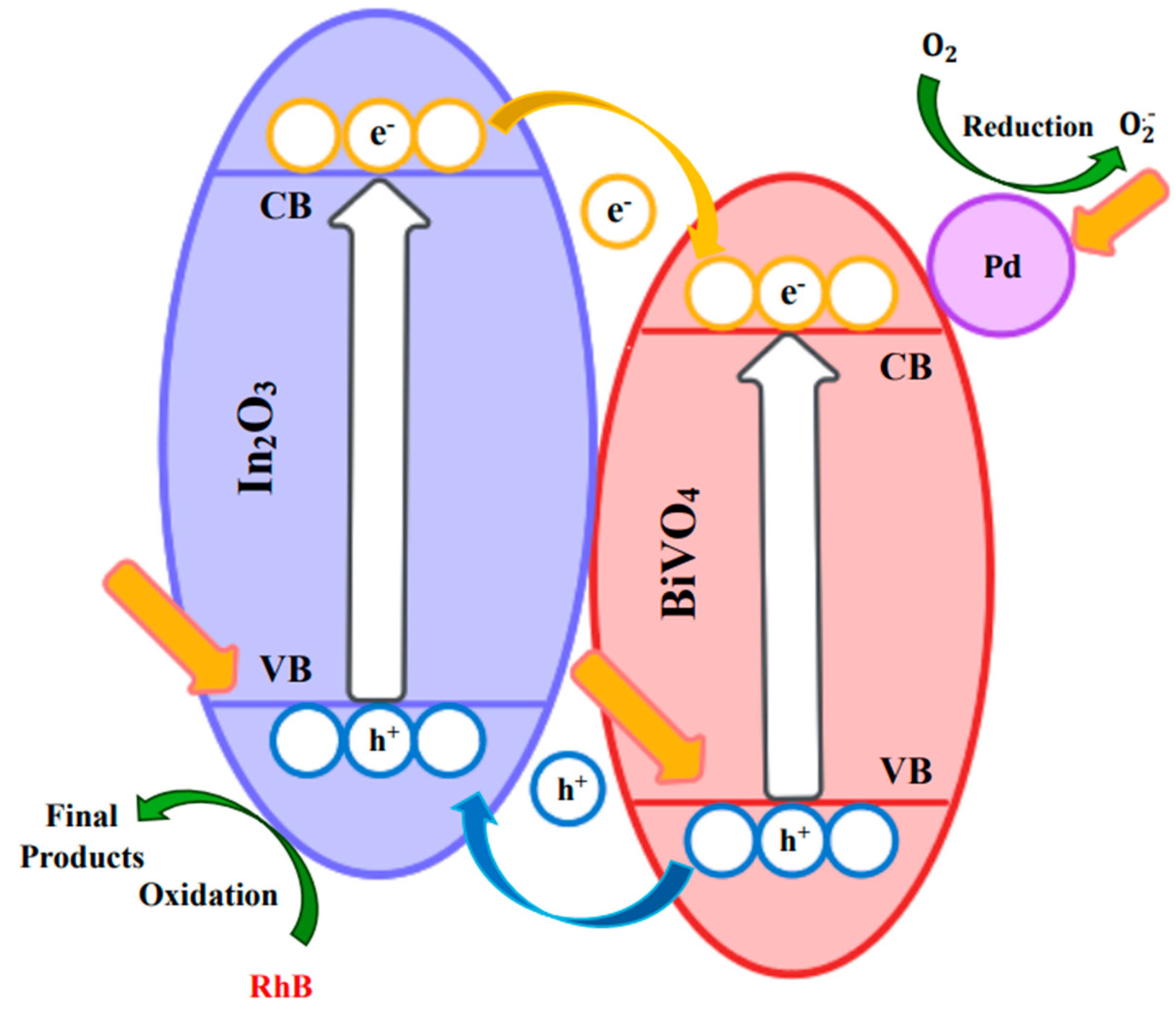
3.2.5. Proposed Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Liu, H.; Chen, M.; Zhang, H.; Wang, B.; Peng, J.; Liu, G. One-Step Synthesis of Hierarchical Flower-like SnO2/BiOCOOH Microspheres with Enhanced Light Response for the Removal of Pollutants. Langmuir 2020, 36, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, N.; Fan, Z.; Hu, Z.T.; Fan, L.; Zhou, J.; Huang, X. On-site H2O2 electro-generation process combined with ultraviolet: A promising approach for odorous compounds purification in drinking water system. Chem. Eng. J. 2022, 430, 132829. [Google Scholar] [CrossRef]
- Rui, J.; Deng, N.; Zhao, Y.; Tao, C.; Zhou, J.; Zhao, Z.; Huang, X. Activation of persulfate via Mn doped Mg/Al layered double hydroxide for effective degradation of organics: Insights from chemical and structural variability of catalyst. Chemosphere 2022, 302, 134849. [Google Scholar] [CrossRef]
- Guo, N.; Liu, H.; Fu, Y.; Hu, J. Preparation of Fe2O3 nanoparticles doped with In2O3 and photocatalytic degradation property for rhodamine B. Optik 2020, 201, 163537. [Google Scholar] [CrossRef]
- Xiao, C.; Tan, Z.; Wang, C.; Yang, X.; Zhang, G.; Pan, H. Fabrication of In2O3/TiO2 nanotube arrays hybrids with homogeneously developed nanostructure for photocatalytic degradation of Rhodamine B. Mater. Res. Bull. 2018, 106, 197–203. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Sridevi, A.; Ramji, B.R.; Prasanna Venkatesan, G.K.D.; Sugumaran, V.; Selvakumar, P. A facile synthesis of TiO2/BiOCl and TiO2/BiOCl/La2O3 heterostructure photocatalyst for enhanced charge separation efficiency with improved UV-light catalytic activity towards Rhodamine B and Reactive Yellow 86. Inorg. Chem. Commun. 2021, 130, 108715. [Google Scholar] [CrossRef]
- Chen, N.; Liu, B.; Zhang, P.; Wang, C.; Du, Y.; Chang, W.; Hong, W. Enhanced photocatalytic performance of Ce-doped SnO2 hollow spheres by a one-pot hydrothermal method. Inorg. Chem. Commun. 2021, 132, 108848. [Google Scholar] [CrossRef]
- Nie, M.; Liao, J.; Cai, H.; Sun, H.; Xue, Z.; Guo, P.; Wu, M. Photocatalytic property of silver enhanced Ag/ZnO composite catalyst. Chem. Phys. Lett. 2021, 768, 138394. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Yin, C.; Jiao, L.; Ding, L. WO3 nanocrystal prepared by self-assembly of phosphotungstic acid and dopamine for photocatalytic degradation of Congo red. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 147–151. [Google Scholar] [CrossRef]
- Jabeen, S.; Iqbal, J.; Arshad, A.; Awan, M.S.; Warsi, M.F. (In1-xFex)2O3 nanostructures for photocatalytic degradation of various dyes. Mater. Chem. Phys. 2020, 243, 122516. [Google Scholar] [CrossRef]
- Chang, P.; Wang, Y.; Wang, Y.; Zhu, Y. Current trends on In2O3 based heterojunction photocatalytic systems in photocatalytic application. Chem. Eng. J. 2022, 450, 137804. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, M.; Xiao, L.; Mou, S.; Zhao, X.; Xie, Y.; Jiang, G.; Jiang, X.; Dong, F. Unraveling the Influence of Oxygen Vacancy Concentration on Electrocatalytic CO2 Reduction to Formate over Indium Oxide Catalysts. ACS Catal. 2023, 13, 4021–4029. [Google Scholar] [CrossRef]
- Nagata, T. Indium Oxide: In2O3. In Single Crystals of Electronic Materials: Growth and Properties; Woodhead Publishing: Sawston, UK, 2019; pp. 523–546. [Google Scholar] [CrossRef]
- King, P.D.C.; Veal, T.D.; Fuchs, F.; Wang, C.Y.; Payne, D.J.; Bourlange, A.; Zhang, H.; Bell, G.R.; Cimalla, V.; Ambacher, O.; et al. Band gap, electronic structure, and surface electron accumulation of cubic and rhombohedral In2O3. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 79, 205211. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, F.; Zhong, Y.; Xiao, T.; Yu, Q.; Zhu, X.; Feng, W.; Qi, Z. Preparation and Photocatalytic Performance of In2O3/Bi2WO6 Type II Heterojunction Composite Materials. Molecules 2024, 29, 4911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Song, X.; Liu, X.; Zhou, W.; Wang, H.; Huo, P. Au nanoparticles-modified S-scheme In2O3/H1.5CN heterojunction with enhanced photocatalytic CO2 reduction activity. Appl. Surf. Sci. 2022, 601, 154246. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Z.; Zhao, H. Enhancement of photocatalytic degradation performance under visible-light by the construction of heterogeneous structures of BiVO4-G/FTO worm-like bilayer films. J. Solid. State Chem. 2024, 339, 124903. [Google Scholar] [CrossRef]
- Wu, M.C.; Lee, P.H.; Lee, D.L. Enhanced photocatalytic activity of palladium decorated TiO2 nanofibers containing anatase-rutile mixed phase. Int. J. Hydrogen Energy 2015, 40, 4558–4566. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, H.; Zhang, P.; Xu, T. Pt deposited TiO2 films with exposed {0 0 1} facets for photocatalytic degradation of a pharmaceutical pollutant. Appl. Catal. A Gen. 2016, 521, 75–82. [Google Scholar] [CrossRef]
- Arab Chamjangali, M.; Bagherian, G.; Javid, A.; Boroumand, S.; Farzaneh, N. Synthesis of Ag–ZnO with multiple rods (multipods) morphology and its application in the simultaneous photo-catalytic degradation of methyl orange and methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Sheikhshoaie, I.; Ramezanpour, S.; Khatamian, M. Synthesis and characterization of thallium doped Mn3O4 as superior sunlight photocatalysts. J. Mol. Liq. 2017, 238, 248–253. [Google Scholar] [CrossRef]
- Qusti, A.H.; Malkhasian, A.Y.S.; Salam, M.A. Enhancement of CdS nanoparticles photocatalytic activity by Pt and In2O3 doping for the degradation of malachite green dye in water. J. Mol. Liq. 2018, 255, 364–369. [Google Scholar] [CrossRef]
- Yin, J.Z.; Huang, S.B.; Jian, Z.C.; Pan, M.L.; Zhang, Y.Q.; Fei, Z.B.; Xu, X.R. Enhancement of the visible light photocatalytic activity of heterojunction In2O3/BiVO4 composites. Appl. Phys. A Mater. Sci. Process. 2015, 120, 1529–1535. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A.; Shawky, A. Facile synthesis of Pt–In2O3/BiVO4 nanospheres with improved visible-light photocatalytic activity. J. Alloys Compd. 2019, 775, 542–548. [Google Scholar] [CrossRef]
- Yasmeen, S.; Burratti, L.; Duranti, L.; Sgreccia, E.; Prosposito, P. Photocatalytic Degradation of Organic Pollutants—Nile Blue, Methylene Blue, and Bentazon Herbicide—Using NiO-ZnO Nanocomposite. Nanomaterials 2024, 14, 470. [Google Scholar] [CrossRef]
- Khaokhajorn, C.; Amornpitoksuk, P.; Randorn, C.; Rattana, T.; Suwanboon, S. One-pot synthesis of In2O3/ZnO nanocomposite photocatalysts and their enhanced photocatalytic activity against cationic and anionic dye pollutants. Inorg. Chem. Commun. 2023, 157, 111392. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, G.; Yin, L.; Liu, W.; Zhang, B.; Feng, S.; Bi, Y.; Liu, Y.; Liu, T.; Wang, Z.; et al. Full-spectrum broad-spectrum degradation of antibiotics by BiVO4@BiOCl crystal plane S-type and Z-type heterojunctions. Chem. Eng. J. 2023, 467, 143450. [Google Scholar] [CrossRef]
- Helal, A.; Yu, J.; Eid, A.I.; El-Hakam, S.A.; Samra, S.E.; El-Sheikh, S.M. Influence of a hole inversion layer at the In2O3/BiVO4 interface on the high-efficiency photocatalytic performance. Surf. Interfaces 2021, 25, 101148. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, S.; Yang, X.; Guo, W.; Guo, Y.; Huo, M. Fabrication of metallic platinum and indium oxide codoped titania nanotubes for the simulated sunlight photocatalytic degradation of diethyl phthalate. Catal. Commun. 2012, 24, 75–79. [Google Scholar] [CrossRef]
- Luo, W.; Li, Z.; Yu, T.; Zou, Z. Effects of surface electrochemical pretreatment on the photoelectrochemical performance of Mo-doped BiVO4. J. Phys. Chem. C 2012, 116, 5076–5081. [Google Scholar] [CrossRef]
- Zulkifili, A.N.; Fujiki, A.; Kimijima, S. Flower-like BiVO4 microspheres and their visible light-driven photocatalytic activity. Appl. Sci. 2018, 8, 216. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV–Vis spectroscopy data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Luo, L.; Fu, L.; Liu, H.; Xu, Y.; Xing, J.; Chang, C.R.; Yang, D.Y.; Tang, J. Synergy of Pd atoms and oxygen vacancies on In2O3 for methane conversion under visible light. Nat. Commun. 2022, 13, 2930. [Google Scholar] [CrossRef]
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P. Photocatalytic performance of ZnO nanomaterials for self sensitized degradation of malachite green dye under solar light. Appl. Catal. A Gen. 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Gahlot, S.; Dappozze, F.; Mishra, S.; Guillard, C. High surface area g-C3N4 and g-C3N4-TiO2 photocatalytic activity under UV and Visible light: Impact of individual component. J. Environ. Chem. Eng. 2021, 9, 105587. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Li, Y.; Chen, J.; Yang, S.; Liu, R.; Xu, Z. Effects of structure and surface properties on the performance of ZnO towards photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2022, 599, 153898. [Google Scholar] [CrossRef]
- Jakimińska, A.; Pawlicki, M.; Macyk, W. Photocatalytic transformation of Rhodamine B to Rhodamine-110—The mechanism revisited. J. Photochem. Photobiol. A Chem. 2022, 433, 114176. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, X.L.; Liu, Z.Y.; Li, W.W.; Yuan, H.; Ma, X.B.; Li, L.X.; Yu, H.Q. Degradation of rhodamine B in a novel bio-photoelectric reductive system composed of Shewanella oneidensis MR-1 and Ag3PO4. Environ. Int. 2019, 126, 560–567. [Google Scholar] [CrossRef]
- Wang, C.; Guo, G.; Zhu, C.; Li, Y.; Jin, Y.; Zou, B.; He, H.; Wang, A. Facile Synthesis, Characterization, and Photocatalytic Evaluation of In2O3/SnO2 Microsphere Photocatalyst for Efficient Degradation of Rhodamine B. Nanomaterials 2022, 12, 3151. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Das, S.; Balan, R.; Lekshmi, I.C. Tuning donor-acceptor strength through preferential binding in mesoporous ZrO2-TiO2 nanocomposite as mechanistic approach for enhanced photocatalytic degradation of Alizarin Yellow GG dye. J. Alloys Compd. 2022, 898, 162769. [Google Scholar] [CrossRef]
- Ramzan, M.; Li, Y.; Ahuja, R. Electronic structure, mechanical and optical properties of In2O3 with hybrid density functional (HSE06). Solid State Commun. 2013, 172, 37–40. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Das, A.; Patra, M.; Kumar, P.M.; Bhagavathiachari, M.; Nair, R.G. Role of type II heterojunction in ZnO–In2O3 nanodiscs for enhanced visible-light photocatalysis through the synergy of effective charge carrier separation and charge transport. Mater. Chem. Phys. 2021, 263, 124431. [Google Scholar] [CrossRef]
- Mishra, R.; Bera, S.; Chatterjee, R.; Banerjee, S.; Bhattacharya, S.; Biswas, A.; Mallick, S.; Roy, S. A review on Z/S—Scheme heterojunction for photocatalytic applications based on metal halide perovskite materials. Appl. Surf. Sci. Adv. 2022, 9, 100241. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X. Recent development on palladium enhanced photocatalytic activity: A review. J. Alloys Compd. 2020, 830, 154669. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, H.; Chen, S.; Jia, Y.; Guo, L.; Wang, H.; Dai, W. Photoreduction of CO2 on Pd-In2O3: Synergistic optimization of progressive electron transfer via amorphous/crystalline Pd and oxygen vacancies. Chem. Eng. J. 2024, 492, 152394. [Google Scholar] [CrossRef]
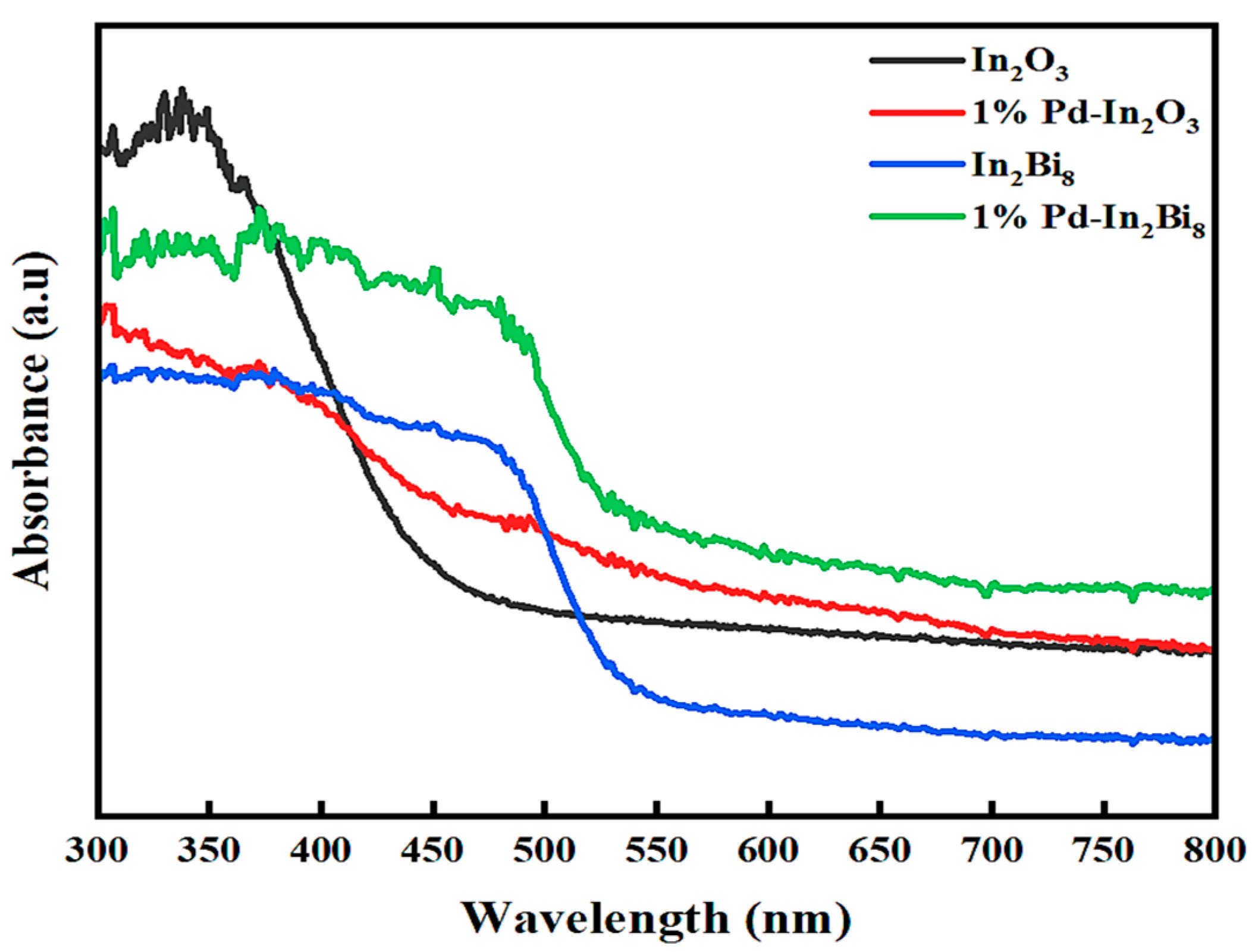
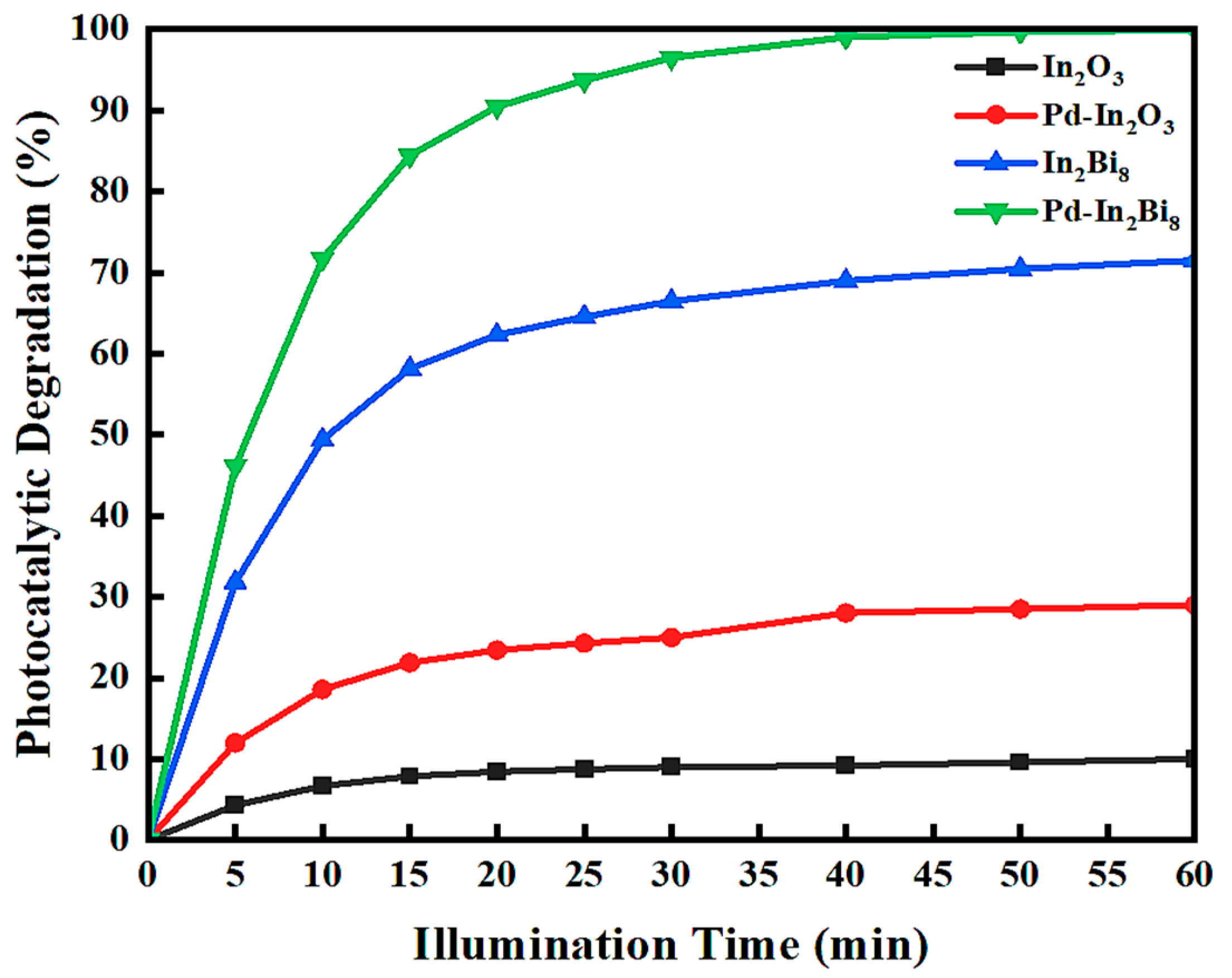
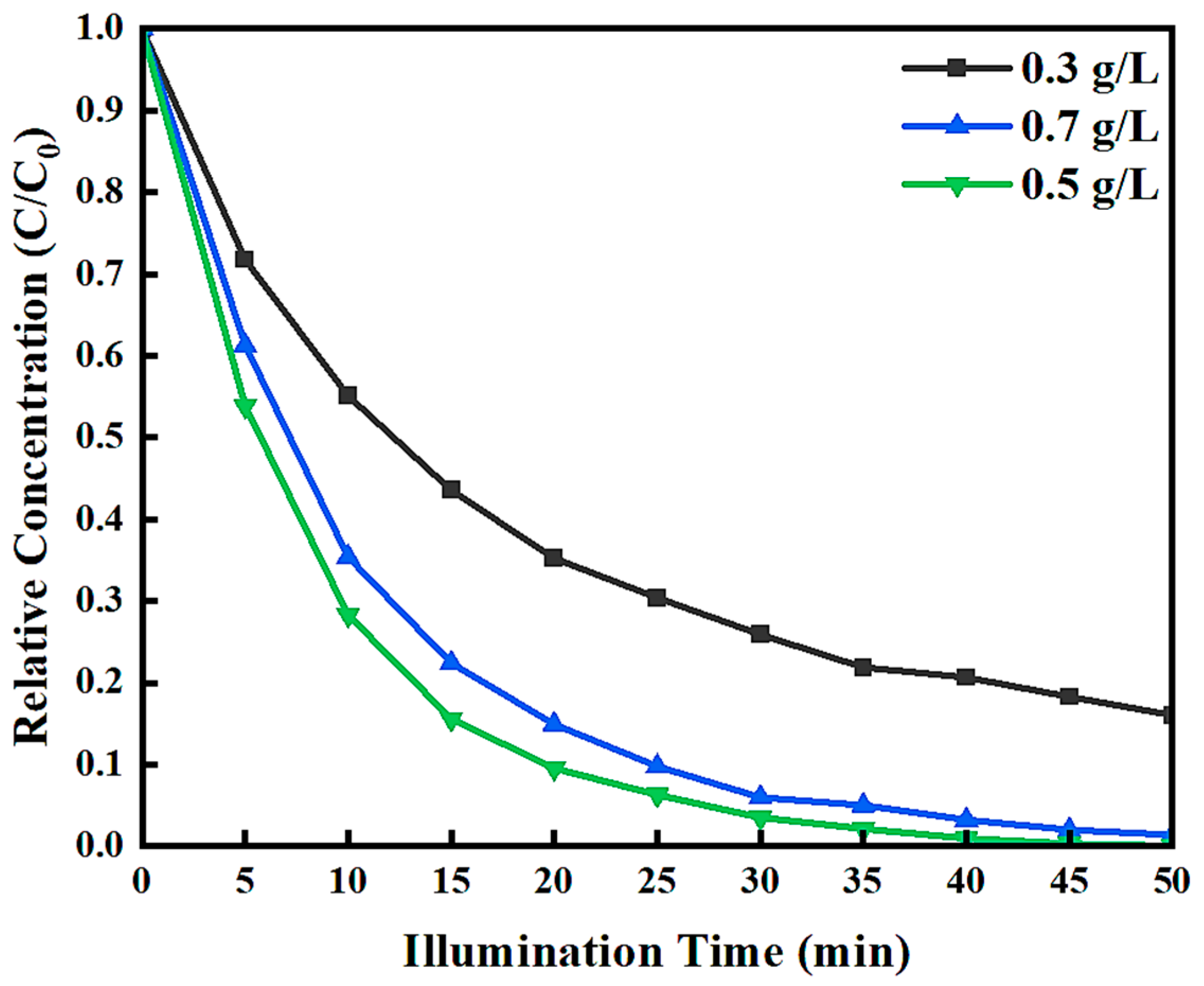
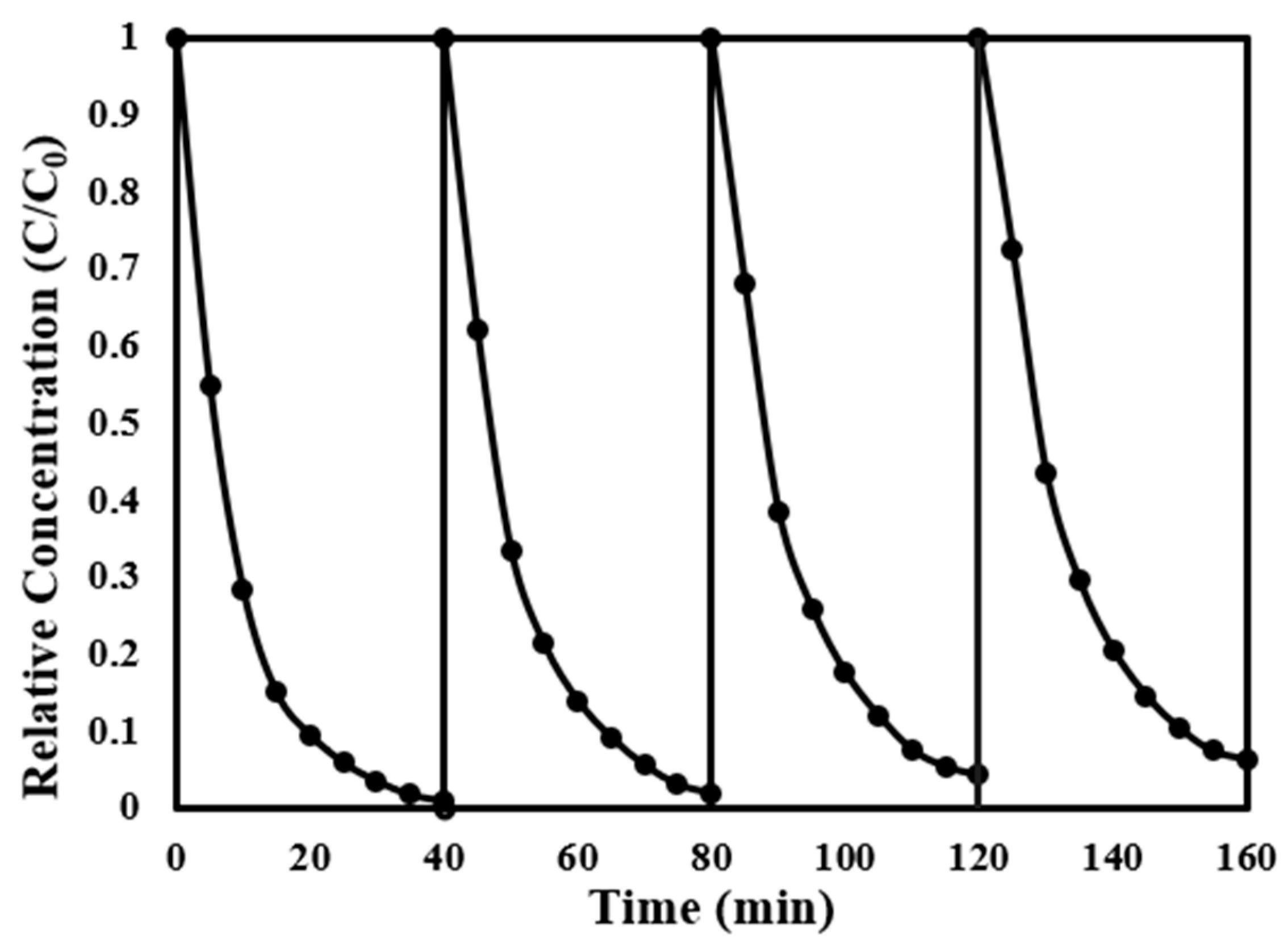
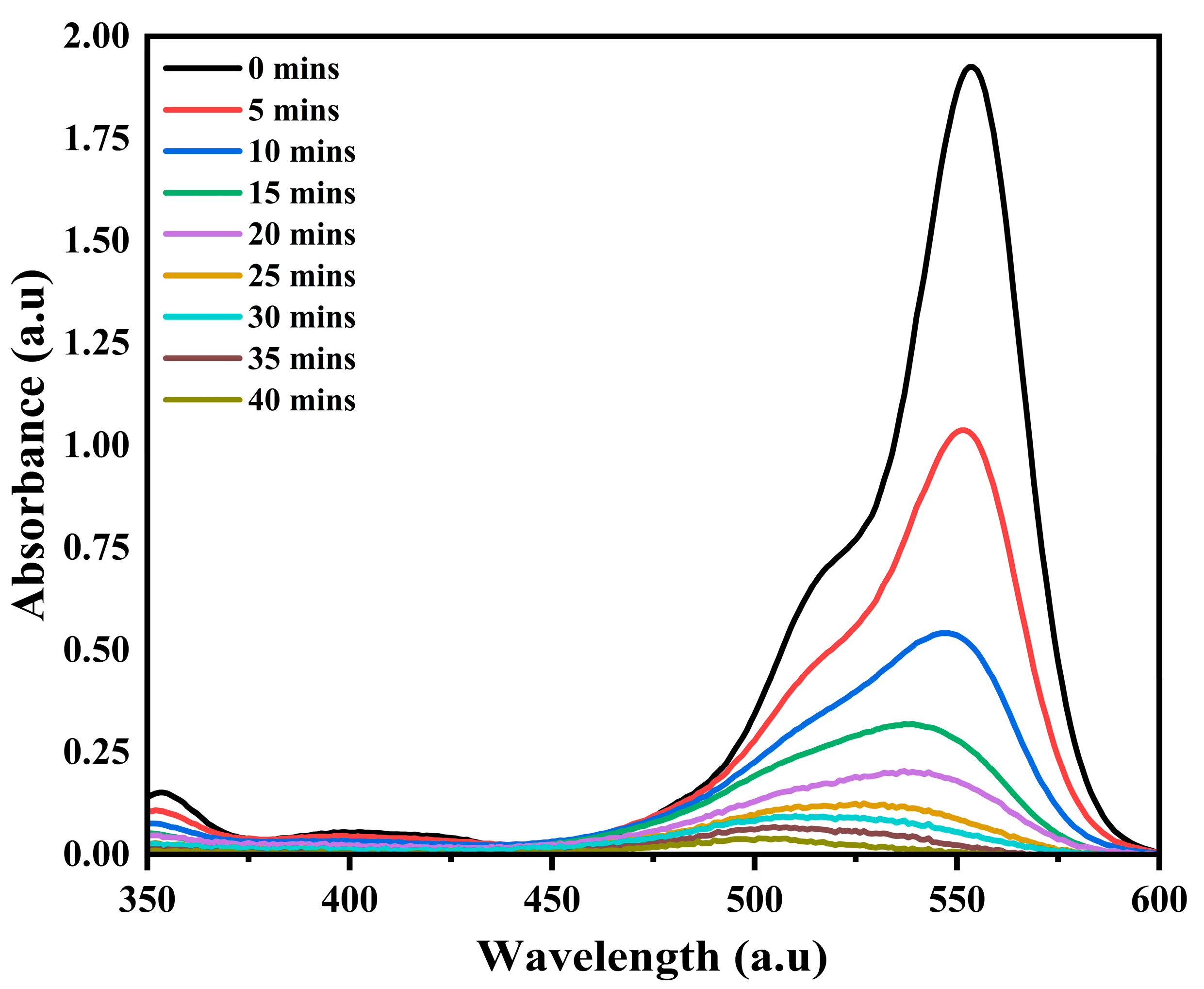
| Samples | Wavelength (nm) | Eg (eV) |
|---|---|---|
| In2O3 | 439.6 | 2.82 |
| Pd-In2O3 | 481.7 | 2.57 |
| In2O3/BiVO4 | 540.8 | 2.29 |
| Pd-In2O3/BiVO4 | 596.1 | 2.08 |
| Photocatalyst | χp (eV) | Ee (eV) | Eg (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|---|---|
| In2O3 | 5.27 | 4.50 | 2.82 | −0.63 | 2.18 |
| BiVO4 | 6.03 | 4.50 | 2.35 | 0.36 | 2.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Parekh, S.; Li, S.; Meng, X.; Zhang, Z. Enhancing the Removal Efficiency of Rhodamine B by Loading Pd onto In2O3/BiVO4 Under Visible Light Irradiation. Processes 2025, 13, 1983. https://doi.org/10.3390/pr13071983
Zhu Y, Parekh S, Li S, Meng X, Zhang Z. Enhancing the Removal Efficiency of Rhodamine B by Loading Pd onto In2O3/BiVO4 Under Visible Light Irradiation. Processes. 2025; 13(7):1983. https://doi.org/10.3390/pr13071983
Chicago/Turabian StyleZhu, Yuanchen, Shivam Parekh, Shiqian Li, Xiangchao Meng, and Zisheng Zhang. 2025. "Enhancing the Removal Efficiency of Rhodamine B by Loading Pd onto In2O3/BiVO4 Under Visible Light Irradiation" Processes 13, no. 7: 1983. https://doi.org/10.3390/pr13071983
APA StyleZhu, Y., Parekh, S., Li, S., Meng, X., & Zhang, Z. (2025). Enhancing the Removal Efficiency of Rhodamine B by Loading Pd onto In2O3/BiVO4 Under Visible Light Irradiation. Processes, 13(7), 1983. https://doi.org/10.3390/pr13071983








