1. Introduction
The rise in industrial activities has caused a substantial increase in pollution of the environment, mostly due to the heavy metal wastewater released from factories into water bodies [
1,
2]. Recent studies have emphasized the need for efficient treatment strategies to remove toxic heavy metals such as lead and cadmium from industrial wastewater using sustainable biosorbents [
3,
4]. Various toxic metals, such as copper (Cu) and cadmium (Cd), may gather in living bodies, causing major dangers to human life and the environment [
5,
6]. The release of such toxic metals into the environment is a result of activities such as electroplating, mining, and the production of electronic goods [
7]. Removing them from water and waste is very important for environmental management.
Conventional methods, including chemical precipitation [
8], ion exchange, and membrane filtration [
9,
10], are common in the elimination of heavy metals. Adsorption is distinguished as an appealing solution among the existing technologies because of its affordable cost, excellent removal efficiency, and minimal generation of secondary waste [
11,
12,
13]. Several studies have confirmed the potential of low-cost natural adsorbents as effective and economical alternatives for removing heavy metals from wastewater [
14]. Advanced materials such as poly (gamma-glutamic acid)-based hydrogels have shown promising adsorption capabilities for heavy metals like Cu and Cd from aqueous environments [
15]. The use of advanced nanomaterials, such as electrospun zeolite/MWCNT nanofibers, has also been investigated for efficient heavy metal removal from wastewater [
16]. Nanocomposites and bio-nanocomposites have recently gained attention for their superior efficiency in removing heavy metals from wastewater [
17]. Therefore, in an attempt to produce sustainable adsorbents, researchers have turned to renewable resources for this purpose. A critical review of adsorbents for heavy metal removal has highlighted the efficacy of diverse materials, including agricultural waste, in eliminating toxic metals from effluents [
18]. Nature-based solutions, such as constructed wetlands with tropical plants, have also proven effective in heavy metal removal from wastewater [
19]. Dead fungal biomass derived from industrial effluents has also been proven effective in biosorption of heavy metals from aqueous solutions [
20].
The juice extraction from citrus fruit produces enormous quantities of waste peels, which account for more than half of the total mass of the fruit [
21]. Even though citrus wastes from major producers such as India, Brazil, and China contain rich compounds and multiple functional groups, the production of this waste is still increasing. Orange shell from citrus sinensis contains cellulose, pectin, and lignin as well as carboxyl, hydroxyl, and phenolic groups that allow heavy metal adsorption via ion exchange and complexation [
22]. Fruit peels, including citrus varieties, have been widely studied for their biosorption potential due to their abundance, cost-effectiveness, and high content of active functional groups [
7]. Due to these features, citrus peel becomes a more economic biosorbent option compared to traditional materials like activated carbon [
23,
24,
25]. Additionally, modified biochar has gained attention for its enhanced adsorption properties and effectiveness in removing heavy metals from both water and soil [
26].
This research studies the efficiency of orange peel powder (OPP) and a mixed adsorbent (activated charcoal combined with bone charcoal) in removing Cu (II) and Cd (II) from industrial wastewater. The research aims to achieve the following:
Calculate the degree of adsorption under various physicochemical conditions.
Evaluate the adsorption efficiency in comparing OPP and the mixed adsorbent system.
Study the reusability of the adsorbents after regeneration to minimize waste.
The use of agricultural waste for environmental remediation is the primary focus of this research, working on issues of waste management and water cleansing needs. The results could provide a sustainable strategy that aids circular economy practices, which can be an environmentally friendly means for industries to treat heavy metals and process citrus waste [
20,
26].
The adsorbents used in the current study are orange peel powder and a novel mixed adsorbent made of bone and activated charcoal. The surface texture of orange peel, enriched in cellulose, pectin, and lignin, allows it to act as a natural adsorption site, while activated charcoal provides a larger surface, and bone charcoal filled with hydroxyapatite ensures good ion exchange. Researchers typically use just one type of adsorbent, such as activated carbon [
27,
28] or orange peel cellulose [
29], rather than considering mixed adsorbents. This study addresses this gap by using a low-cost, renewable biosorbent, combined with a mixed charcoal system to enhance efficiency while maintaining sustainability. This is in a good agreement with reviews that show the necessity for cost-effective alternatives [
30].
When chemical binding and physical adsorption are used together, the mixed adsorbent system is likely to work more efficiently. The present study reveals that, at 0.2 M HCl, OPP and the composite sorbent can be used and reused over 9-10 times, with 90% for Cu (II) and 94% for Cd (II) desorption efficiency. For example, a review of regeneration found that many studies can only produce 3–5 regeneration cycles with decreasing efficiency [
30]. Reusing the adsorbent leads to less waste and is also economically beneficial, in keeping with sustainability aims.
In this study, both OPP and the mixed adsorbent are evaluated using different sets of physicochemical conditions (like pH, the amount of adsorbent used, and time). Research that examines only one variable or adsorbent offers less opportunity to compare the materials than this broader study does. For instance, orange peel was studied to remove Cd (II) through adsorption, though it was not compared to other materials used in similar studies [
31]. This work focuses on utilizing waste products such as orange peel and follows circular economy principles using juice extraction byproducts. This topic of sustainability is not stated and investigated in other studies, but it is becoming increasingly clear that it is essential to search for sustainable sources. For example, a study on thiol-lignocellulose sodium bentonite nanocomposites did not discuss agricultural waste; instead, it directed its focus on using advanced materials [
32]. Although Saudi Arabia orange peels are used, this study can be adapted for places where lots of citrus waste is generated.
2. Materials and Methodology
The following subsections describe the methodology for preparing the orange peel and mixed adsorbents, along with the analysis conducted using the atomic absorption spectrophotometer (Thermo Scientific™ with model number iCE™ 330, MA, USA), Fourier Transform Infrared (Thermo Nicolet spectrometers with model numbers Nicolet iS50/iS50R, USA), and scanning electron microscopy (JEOL-JSM 7600F, JEOL Ltd, Tokyo, Japan).
2.1. Preparation of the Orange Peel Adsorbent
Most of the orange peels used in this work came from nearby juice shops in Saudi Arabia. After washing the samples with tap water, they were rinsed with deionized water to remove any remaining contaminants. To protonate the functional groups, the peels were treated with a 0.1 N HNO3 solution for 5 h. After treatment, the peels were sun-dried for 5 days, followed by further drying in an oven at 105 °C for 3 h to remove all moisture. Finally, the dried peels were crushed, ground, and passed through a 1 mm sieve to obtain a uniform-sized adsorbent.
2.2. Preparation of the Mixed Adsorbent
The mixed adsorbent used in this study consists of equal quantities of activated charcoal (AC) and bone charcoal (BC). To determine the particle size distribution, the Malvern Particle Size Analyzer (Malvern Instruments Ltd., Malvern, UK) was employed. The resulting particle sizes for the mixed adsorbent and orange peel powder are 572.2 nm and 634.4 nm, respectively.
Table 1 lists the physical properties of the mixed adsorbent, such as surface area, particle size, adsorbent charge, and % of moisture.
2.3. Characterization of the Mixed Adsorbent
Because the mixed adsorbent is introduced in the present study as a new adsorbent for removing heavy metals from wastewater, a full characterization of this novel biosorbent will be provided in the following subsections.
2.3.1. FTIR Spectroscopy Analysis
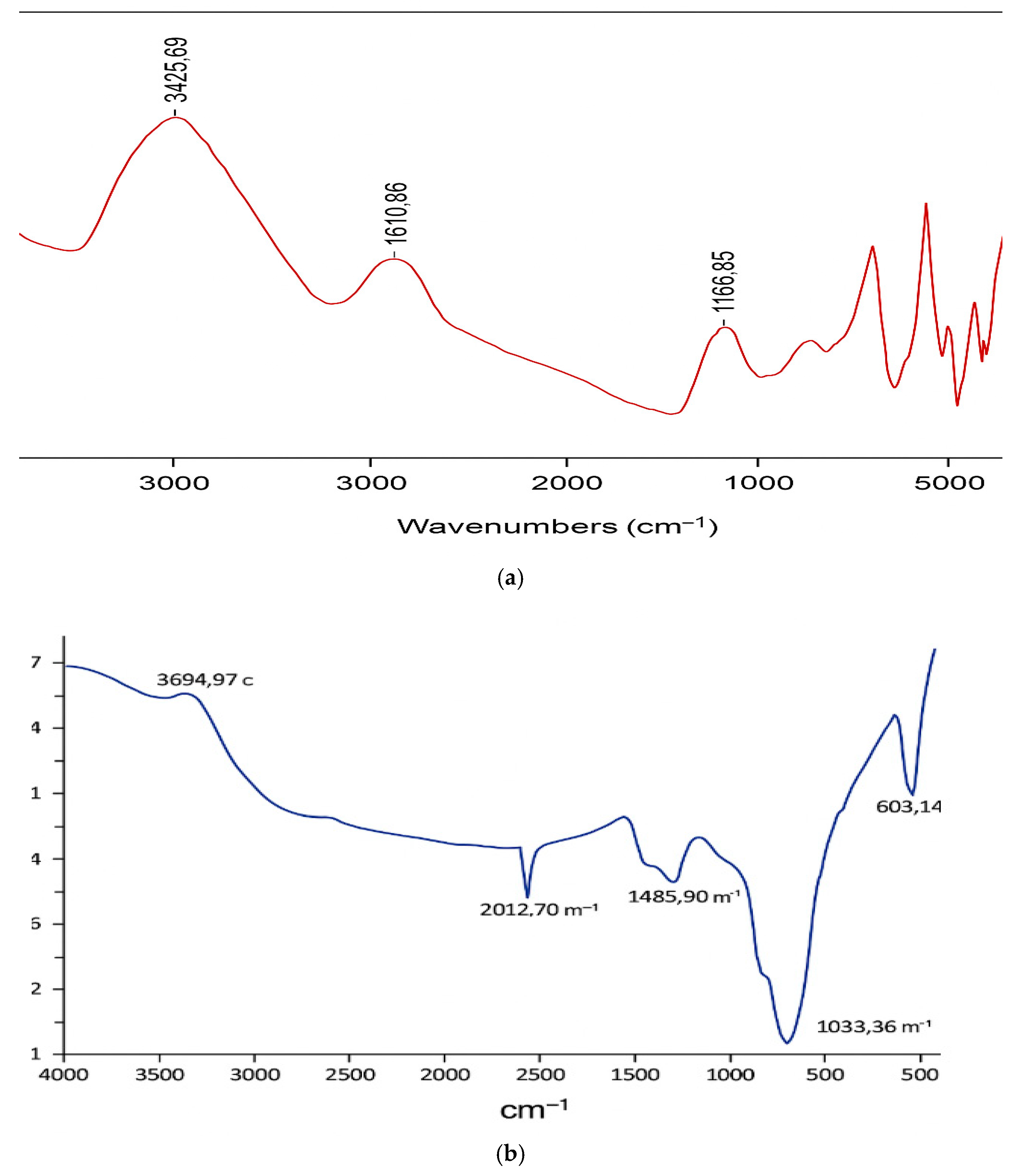
Fourier Transform Infrared (FTIR) examination of the mixed adsorbent was carried out to see if any functional groups changed due to Cu (II) adsorption. All measurements were collected on a Thermo Scientific Nicolet iS50 FTIR spectrometer over the wavenumber region of 540–3400 cm
−1. In the spectrum before adsorption, as shown in
Figure 1a, several absorption bands could be seen for hydroxyl (-OH), amino (-NH), aliphatic (-CH), carbonyl (-C=O), and ether (C–O) groups. The absorption bands include the following:
3600–3000 cm−1: O–H and N–H stretching vibrations;
2900–2800 cm−1: Symmetric and asymmetric C–H stretching of aliphatic hydrocarbons;
1700–1600 cm−1: C=O stretching (carbonyl/ketone groups);
1550–1300 cm−1: C–H bending and C=C stretching;
1106–1024 cm−1: C–O stretching and S=O symmetric vibrations.
The peaks for these functional groups shifted and reduced in intensity after adsorption, as illustrated in
Figure 1b, suggesting their participation in binding Cu (II) ions. In particular, the shifts seen in the region of 3425–1025 cm
−1 indicate that metal ions interacted with the surface-active sites of the considered adsorbent by electrostatic interaction, ion exchange, or by forming complexes. Samples were scanned four times at a resolution of 32 cm
−1 to obtain transmission spectra. The illustrated spectrum presents the groups responsible for the adsorption of Cu (II) ions onto the investigated mixed adsorbent. The strong transmittance peak at 3694.97 cm
−1 is linked to –OH and –NH stretching vibrations, meaning that hydroxyl and amine groups are present. Also, it is noticed that the symmetric and asymmetric C–H vibrations of aliphatic acids at 2012.70 cm
−1. The stretching vibration at 1468.90 cm
−1 is probably due to aromatic ketone groups. Spectrum from 1033.36 cm
−1 to 603.14 cm
−1 covers C–O stretching, aromatic C–H asymmetric bending, and the symmetric vibrations of S=O. The presence of these many small peaks shows that different functional groups help the adsorbent bind with Cu (II) using methods such as exchange, attraction and complexation. Resulting from the spectra analysis, it is indicated that the binding of Cu (II) ions to the adsorbent occurred by means of physisorption and chemisorption, showing its overall effectiveness.
2.3.2. SEM Analysis
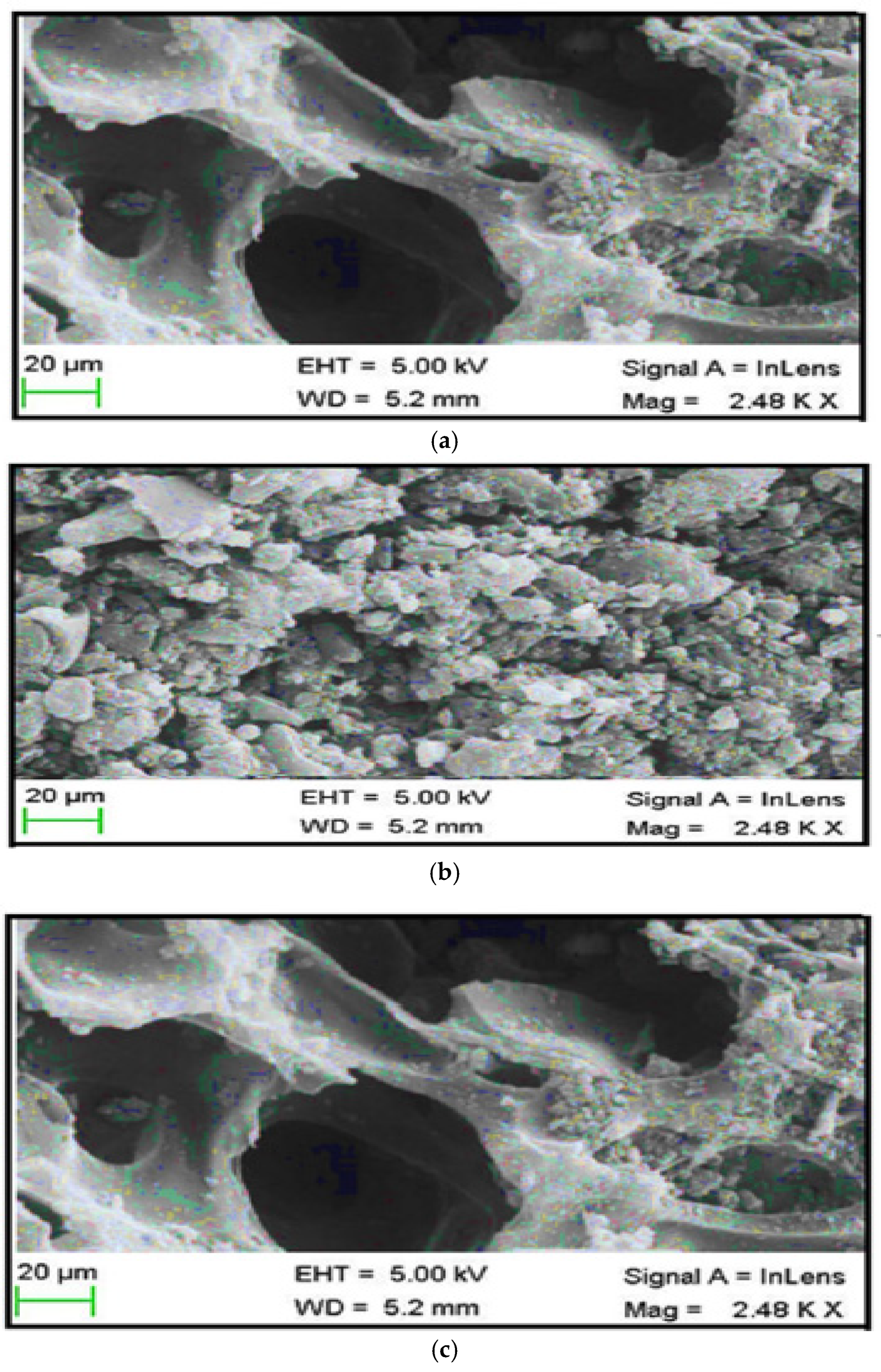
Scanning electron microscopy (SEM, JEOL-JSM 7600F) was used to examine the micrography of the mixed adsorbents, and the results are presented in
Figure 2a–c.
Figure 2a revealed that before adsorption, the spherical particles were randomly spread, forming irregular groups of spheres [
33]. As shown in
Figure 2b,c, at a magnification of 2480 times, both copper and cadmium showed visible clusters of tiny particles on their surfaces. Therefore, the prepared adsorbents were predicted to exhibit better adsorption due to the clear attachment and visibility of the Cu and Cd metals on their surface [
34]. The same SEM pattern, with irregular aggregates of spherical clusters, was detected for the synthesized Cu-MOF adsorbents at a high magnification of 10,000. This can be attributed to the agglomeration of the small particles, which improves the material’s ability to adsorb substances [
35].
2.4. Heavy Metal Analysis
To control the pH, a standard pH meter was used for the simulated metal ion solution and after the adsorption process, the final concentration of Cu (II) and Cd (II) ions was measured with an atomic absorption spectrometer (Thermo Scientific ICE 3000 series) before and after the adsorption process. Batch adsorption experiments were performed using industrial effluents in 250 mL conical flasks. The industrial effluent was diluted from 350 to 50 ppm by adding 14.28 mL of effluent to 85.72 mL of deionized water. The pH of the resulting solution was adjusted by adding 0.1 N NaOH or 0.1 N HCl, and the metal ion concentration was fixed at 50 ppm. The pH was varied at values of 2, 4, 6, and 8. The adsorbent doses of 0.5, 1, 2, 3, and 5 g/L were added at each pH value, and the flasks were agitated in an orbital shaker at 180 rpm for about 1 h. At different time intervals (ranging from 15 min to approximately 3.5–4 h), the solutions were filtered, and the supernatant was analyzed for the final residual Cu (II) and Cd (II) concentration using the Atomic Absorption Spectrometer (AAS).
The adsorption capacity and percentage removal were calculated using the following formulas:
where
qe is the amount adsorbed in mg/g,
Co is the initial metal ion concentration in ppm,
Ce is the final metal ion concentration in ppm,
V is the volume of the solution in mL, and
m is the mass of the adsorbent in g.
3. Results and Discussion
The subsequent sections analyze the impact of pH, adsorbent dosage, and contact time on the removal efficiency of Cu (II) and Cd (II) from industrial effluents. In addition, a comparative study for evaluating the effectiveness of the introduced mixed adsorbent and orange peel powder adsorbent for removing these metals from industrial effluents will be discussed.
3.1. Copper Removal Using Orange Peel and Mixed Adsorbents
3.1.1. Influence of pH on Copper Removal
The adsorption process is primarily influenced by the type and ionization state of the functional groups (ligands) present in the biomass. Adsorption efficiency is decreased at lower pH levels because hydronium ions predominate on the adsorbent surface, generating repulsive forces that prevent metal ions from interacting with the orange peel’s adsorption sites [
29]. As the pH increases, the competition between hydrogen ions decreases, allowing more metal-binding sites to be available for adsorption. Consequently, at higher pH values, the surface charge of the orange peel becomes more negative, enhancing the adsorption process [
36]. This behavior is attributed to the deprotonation of functional groups such as carboxyl and hydroxyl groups, which facilitate electrostatic attraction with positively charged metal ions [
37]. However, experiments above pH 8 were not conducted, as copper hydroxide precipitates may form, interfering with the adsorption process and the accuracy of the biosorption studies [
38].
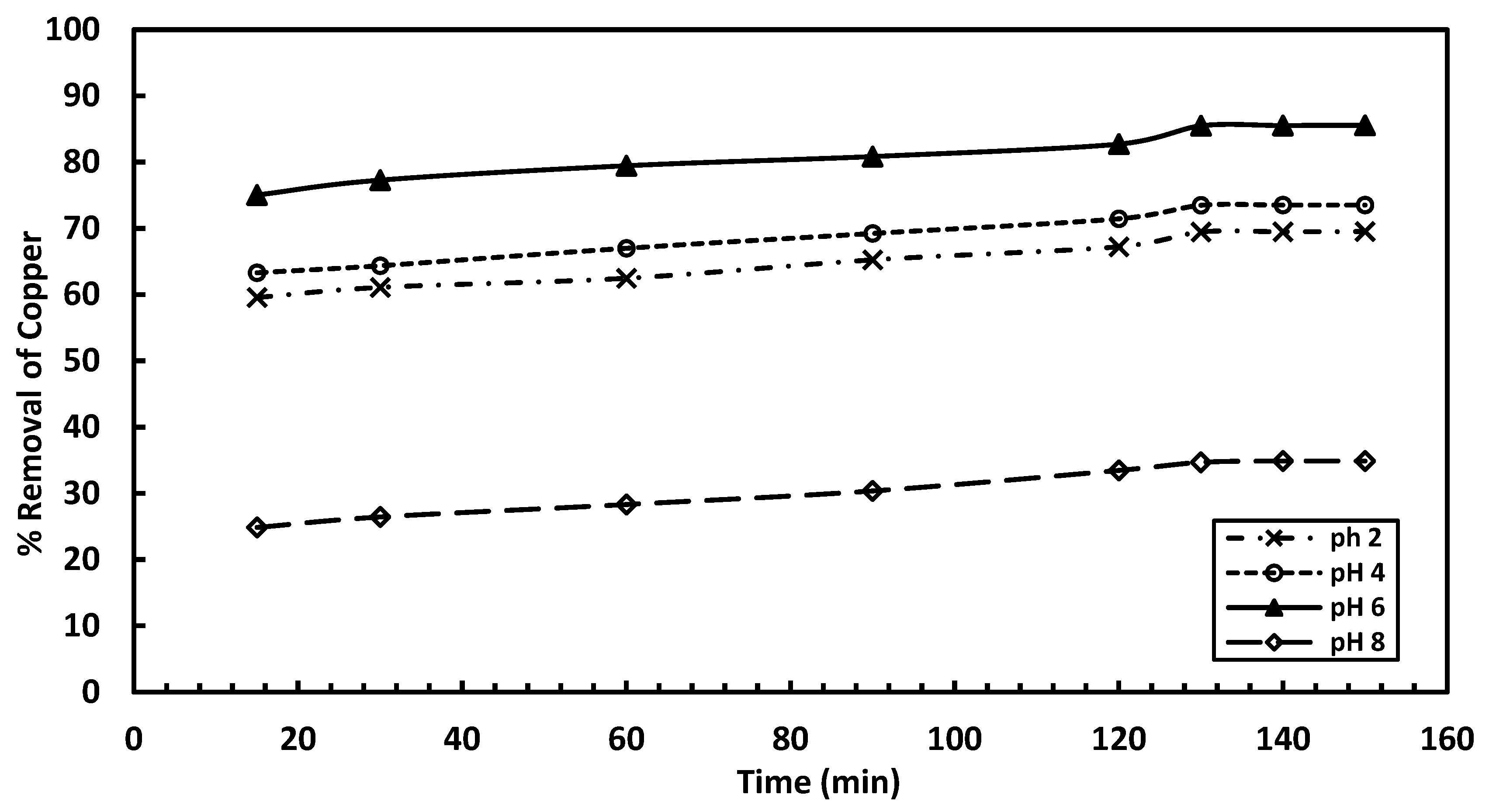
The experiments were conducted at 25 °C, using a metal ion concentration of 50 mg/L, with an adsorbent dosage of 0.5 g/L and agitation for 60 min. Samples were collected every 30 min for up to 2 h to allow for saturation, and residual concentrations were measured using Atomic Absorption Spectroscopy (AAS). As shown in
Figure 3, the highest copper removal efficiency (75% to 85.6%) for orange peel is observed at pH 6. At pH 4, the removal efficiency ranges from 63.32% to 73.54%, while at pH 2, the efficiency ranges from 59.6% to 69.54%. At pH 8, copper removal is reduced to be within the range of 24.9–35%.
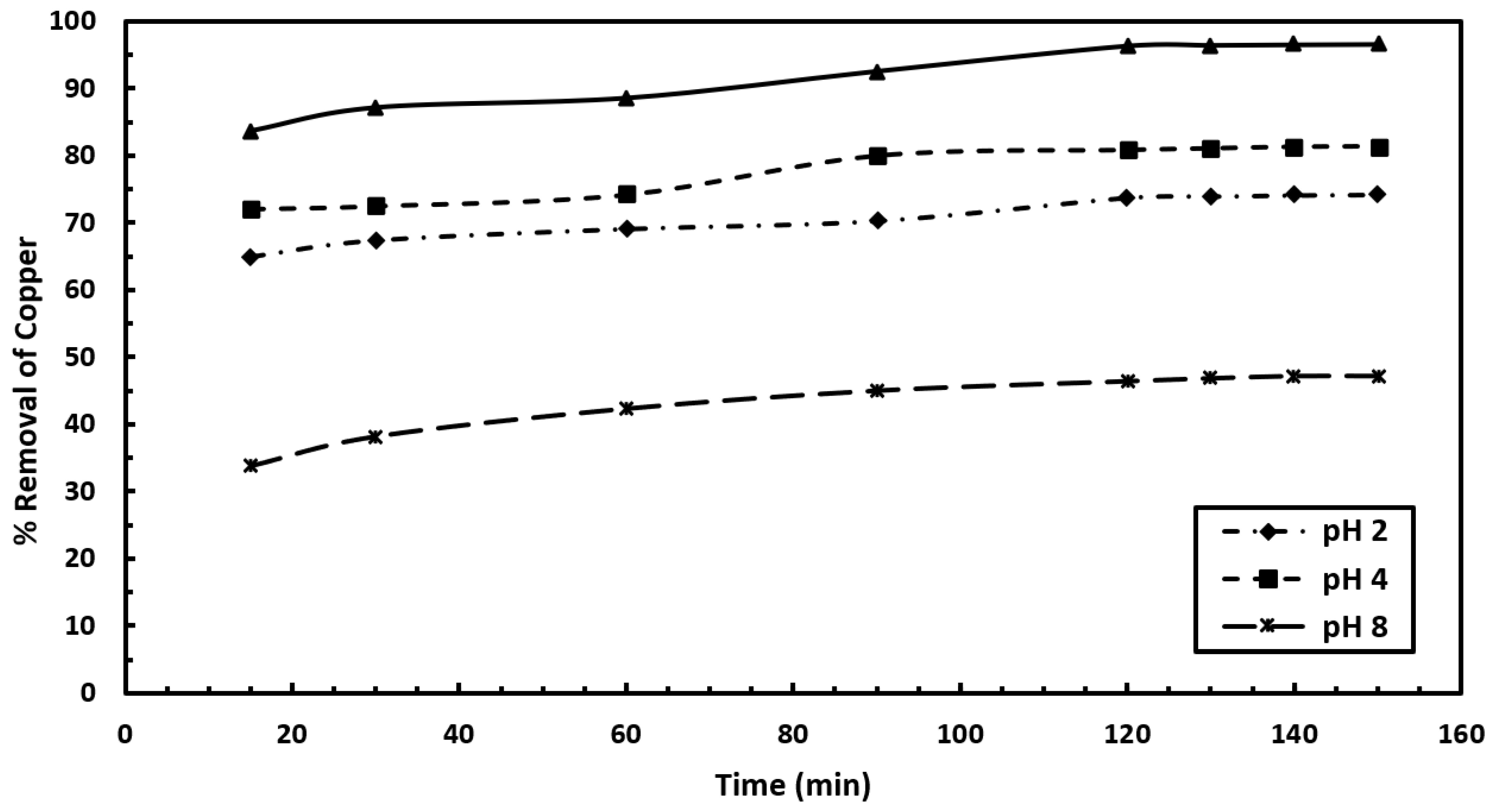
As shown in
Figure 4, for the mixed adsorbent (AC + BC), the copper removal efficiency is higher at all pH levels compared to orange peel. At pH 6, the removal ranges from 83.64% to 96.62%, at pH 4, it ranges from 72.1% to 81.42%, at pH 2, it ranges from 64.88% to 74.22%, and at pH 8, it ranges from 33.76% to 47.26%. The mixed adsorbent (AC + BC) exhibits better copper removal than orange peel. This improved performance of the mixed adsorbent can be attributed to the large surface area of activated charcoal (AC) and the negative charge from bone charcoal (BC), which work synergistically to enhance the adsorption of copper ions.
The adsorption capacities for copper removal using the mixed adsorbent and orange peel at pH values of 2, 4, 6, and 8, calculated using Equation (1), are as follows: 7.1 and 6.55 mg/g at pH 2, 7.8 and 6.95 mg/g at pH 4, 9.22 and 8.15 mg/g at pH 6, and 4.43 and 3.1 mg/g at pH 8 for the mixed and orange peel adsorbents, respectively. It is clear that the highest estimated adsorption capacities for copper removal are achieved at pH 6 for both adsorbents, which is in good agreement with the experimental results. Maximum adsorption for both adsorbents is possible at pH 6 because the surface is fully ionized, and other protons do not interfere. The mixed adsorbent exhibits a maximum adsorption capacity of 9.22 mg/g at pH 6, whereas orange peel exhibits an adsorption rate of 8.15 mg/g. This difference is consistent with the literature, where composite adsorbents often outperform single component biosorbents due to combined physical and chemical adsorption mechanisms.
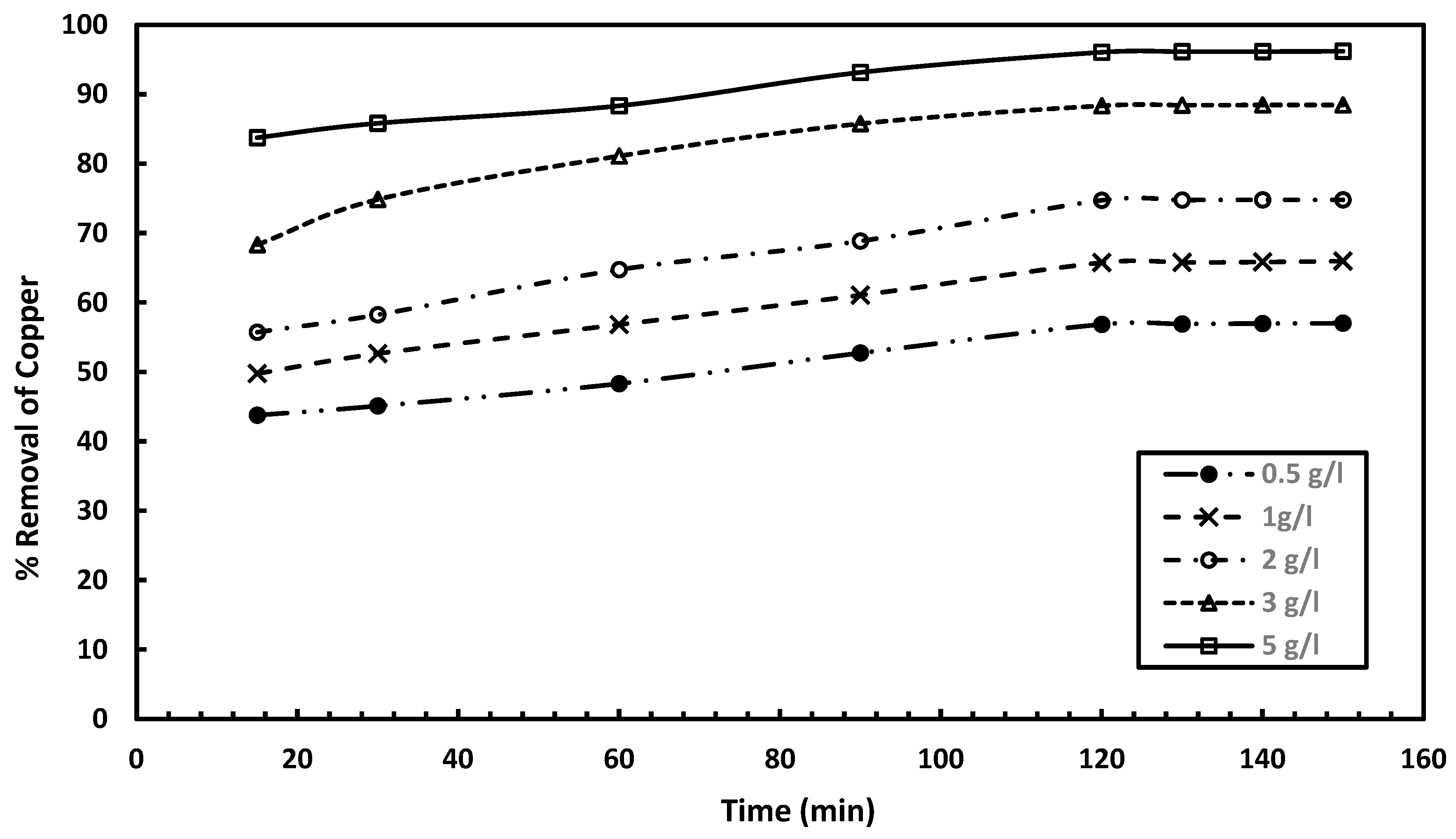
3.1.2. Influence of Adsorbent Dosage on Copper Removal
The effect of adsorbent dosage on copper removal was examined at different dosages of 0.5, 1, 2, 3, and 5 g/L, while maintaining constant experimental conditions. The experiments were performed at pH 6, 25 °C with a 50 mg/L metal ion concentration and agitation (180 rpm) for 60 min at the specified dosages. Samples were collected at 30 min intervals for 2 h to reach equilibrium, with residual concentrations measured using an Atomic Absorption Spectrophotometer (AAS). As presented in
Figure 5, the copper removal efficiency with orange peel ranges from 33.1% to 46.6% at 0.5 g/L, 39.1% to 51.4% at 1 g/L, 50.3% to 68.8% at 2 g/L, 65% to 77.4% at 3 g/L, and 74.9% to 85.18% at 5 g/L as adsorbent dosage.
As illustrated in
Figure 6, the copper removal efficiency using the mixed adsorbent ranges from 43.78% to 57% at 0.5 g/L, 49.76% to 66% at 1 g/L, 55.74% to 74.8% at 2 g/L, 68.32% to 88.48% at 3 g/L, and 83.76% to 96.2% at 5 g/L. The adsorption capacities for mixed adsorbent and orange peel at various dosages were calculated and the obtained results are as follows: at adsorbent concentration of 0.5 g/L, the adsorption capacity is 5.22 mg/g for mixed adsorbent and is 4.13 mg/g for orange peel; at 1 g/L, the adsorption capacities are 3.02 and 2.31 mg/g for the mixed adsorbent and orange peel, respectively; at 2 g/L, the capacities of 1.71 and 1.53 mg/g were determined by utilizing the mixed and orange peel adsorbents, respectively; at 3 g/L, the capacity is 1.38 mg/g for mixed adsorbent and is 1.21 mg/g using orange peel; and at 5 g/L, it is 0.92 mg/g for mixed adsorbent and is 0.81 mg/g in case of orange peel.
These results indicate that the mixed adsorbent consistently shows the highest copper removal efficiency. This enhanced performance of the mixed adsorbent can be attributed to the high surface area of the activated charcoal combined with the negative charge of bone charcoal, which together create a synergistic effect [
39,
40]. The large surface area of activated charcoal provides numerous adsorption sites, while the negatively charged surface of bone charcoal enhances electrostatic interactions with positively charged copper ions, thereby improving removal efficiency [
41]. Because of its increased surface area and ion-exchange properties, the mixed adsorbent routinely outperforms orange peel in terms of metal removal efficiency. According to these results, higher dosages are more feasible for attaining total metal ion removal in industrial applications, even though low dosages optimize capacity.
3.1.3. Impact of Contact Time on Copper Removal
Following the optimization of pH and adsorbent dosage, additional experiments were performed to assess the effect of contact time on copper removal efficiency. The tests were conducted at pH 6, 25 °C with a metal ion concentration of 50 mg/L and an adsorbent dosage of 5 g/L. The samples were agitated for 60 min at 180 rpm, and measurements were taken every 30 min for 150 min. The residual concentrations were analyzed using AAS. As illustrated in
Figure 7, the copper removal achieved by the mixed adsorbent ranges from 33.72% to 80.7% at 5 g/L, while for orange peel, it varies from 27.54% to 70.4% at the same adsorbent dosage. These results indicate that the mixed adsorbent (AC + BC) provided a higher copper removal efficiency. The calculated maximum adsorption capacities for the mixed adsorbent and orange peel at 5 g/L are 0.67 mg/g and 0.56 mg/g, respectively. According to the obtained data, the mixed adsorbent performs faster and more efficiently than orange peel, making it a better choice for time-sensitive applications. These results are consistent with earlier research on composite adsorbents, which frequently show better kinetics because of their heterogeneous structure [
42].
3.2. Cadmium Removal Using Orange Peel and Mixed Adsorbents
3.2.1. Effect of pH on Cadmium Removal
Cadmium removal experiments were conducted at 25 °C with an initial metal ion concentration of 50 mg/L, using an adsorbent dosage of 0.5 g/L and an agitation time of 60 min at 180 rpm. Samples were taken every 30 min for 2 h to monitor the adsorption process until saturation was reached. Residual cadmium concentrations were analyzed using Atomic Absorption Spectroscopy (AAS). As shown in
Figure 8, the mixed adsorbent achieves cadmium removal efficiencies ranging from 45.7% to 69.3% at pH 6, 38.26% to 57.92% at pH 4, 29.18% to 50.94% at pH 2, and from 23.46% to 38.4% at pH 8. Similarly, as illustrated in
Figure 9, cadmium removal using orange peel ranges from 39.8% to 53.74% at pH 6, 31.6% to 45.62% at pH 4, 24.88% to 40.67% at pH 2, and from 17.6% to 30.71% at pH 8. The highest cadmium removal (69.3%) is observed at pH 6 using the mixed adsorbent. As mentioned before, this enhanced performance of the mixed adsorbent can be attributed to the synergistic effect of the activated charcoal’s high surface area combined with the bone charcoal’s negative surface charge. The calculated adsorption capacities for the mixed and orange peel adsorbents are 4.36 and 3.55 mg/g at pH 2, 5.11 and 4.1 mg/g at pH 4, 5.98 and 4.85 mg/g at pH 6, and 3.33 and 2.61 mg/g at pH 8, respectively.
3.2.2. Effect of Adsorbent Dosage on Cadmium Removal
The influence of adsorbent dosage on cadmium removal was evaluated at concentrations of 0.5, 1, 2, 3, and 5 g/L under consistent experimental conditions. All tests were conducted at 25 °C, pH 6, with an initial cadmium concentration of 50 mg/L and agitation (180 rpm) for 60 min at the specified dosages. Samples were collected every 30 min over 2 h. As shown in
Figure 10, cadmium removal by orange peel increases from the range of 30.1–42.74% at 0.5 g/L to the range of 70.18–80.49% at 5 g/L.
Figure 11 demonstrates that the mixed adsorbent achieves higher removal efficiencies, ranging from 40.91 to 52.81% at 0.5 g/L to 81.32–89.68% at 5 g/L. These results indicate that cadmium removal is improved with increasing the adsorbent dosage, with a superior performance of the mixed adsorbent compared to the orange peel. The highest cadmium removal is achieved at a dosage of 5 g/L for both adsorbents.
The calculated adsorption capacities for cadmium removal using the mixed and orange peel adsorbents are 4.79 mg/g and 3.82 mg/g at 0.5 g/L, 2.7 mg/g and 2.12 mg/g at 1 g/L, 1.57 mg/g and 1.46 mg/g at 2 g/L, 1.25 mg/g and 1.12 mg/g at 3 g/L, and 0.87 mg/g and 0.78 mg/g at 5 g/L, respectively.
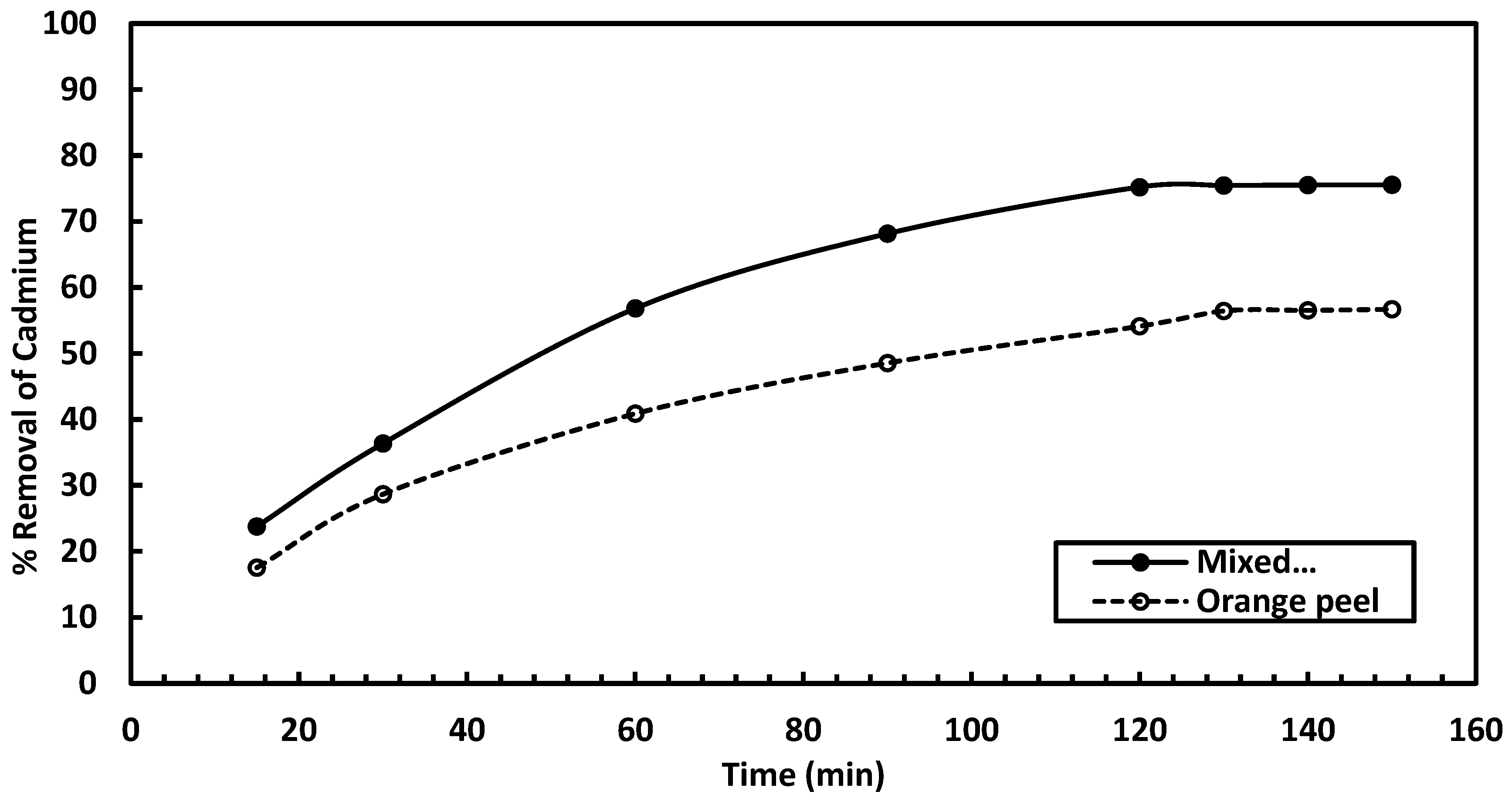
3.2.3. Influence of Contact Time on Cadmium Removal
The effect of contact time on cadmium removal was investigated for both studied adsorbents. The experiments were conducted at pH 6, 25 °C, with a metal ion concentration of 50 mg/l and a 60 min agitation (180 rpm) time, using an adsorbent dosage of 5 g/L. As shown in
Figure 12, the maximum cadmium removal for the mixed adsorbent ranges from 23.78% to 75.56%, while for the orange peel adsorbent, it ranges from 17.54% to 50.7%. The calculated adsorption capacities for the mixed and orange peel adsorbents at 5 g/L are 0.61 and 0.45 mg/g, respectively, demonstrating the higher efficiency of the mixed adsorbent for removing cadmium.
3.3. Adsorption Isotherm Modeling and Kinetic Analysis
The experimental data were collected from this study and analyzed to focus on equilibrium and kinetic aspects of removing Cu2+ and Cd2+ using OPP and mixed adsorbents. The information gathered for equilibrium data includes both adsorption capacities (qeq, mg/g) and equilibrium concentrations (Ceq, mg/L) at different pH values and adsorbent doses. The kinetic data provide adsorption amounts over time (qt, mg/g) for contact times of various durations. Under standard batch experiment procedures, the initial concentration of metal in the solution was assumed to be 50 mg/L, and the volume of solution was taken as 0.1 L.
When the pH was held at 6 to optimize adsorption, the equilibrium data were applied for isotherm modeling, and the C
eq values were determined using the results of the removal efficiency tests. A kinetic study was carried out using time points of up to 150 min for Cu
2+ and up to 60 min for Cd
2+. The model parameters were determined using linear regression, and model fit was evaluated using the coefficient of determination (R
2).
This study applies the Langmuir and Freundlich isotherm models to select the best isotherm model that expresses the experimental data. The Langmuir isotherm is presented in Equation (3), where qeq is the equilibrium adsorptive capacity, qmax (mg/g) represents the maximum adsorption, and KL (L/mg) is the Langmuir constant. However, the Freundlich isotherm follows Equation (4), where KF is the Freundlich constant (mg/g), and n is the adsorption intensity.
Using OPP and mixed adsorbents, the Langmuir and Freundlich isotherms were fitted to the equilibrium data for Cu
2+ and Cd
2+ and
Table 2 provides a summary of the parameters.
The data shows good agreement with the Langmuir model (R2 > 0.95) for both OPP and mixed adsorbents, indicating monolayer adsorption. Due to its large surface area and ion exchange properties, the mixed adsorbent exhibits higher maximum adsorption capacities than OPP: 10.0 mg/g for Cu2+ and 6.0 mg/g for Cd2+ for OPP, and 12.0 mg/g for Cu2+ and 7.0 mg/g for Cd2+ in the case of the mixed adsorbent. The data is also well-matched by the Freundlich model (R2 > 0.90), and n is also greater than 1, both of which indicate favorable adsorption. However, these results show that the experimental data are best fitted using the Langmuir model compared to the Freundlich model.
The current study used the pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetic models to determine which one best matched the experimental data. Equation (5) illustrates the linear form of physical adsorption assumed by the PFO model, where k
1 is the rate constant (min
−1) and q
t is the adsorption capacity at time t (mg/g). Equation (6), where k
2 is the rate constant (g/mg·min), represents the linear form of the PSO model’s assumption of chemisorption.
The kinetic data were fitted to the pseudo-first-order and pseudo-second-order models. As discussed in
Table 3, the results demonstrated that the pseudo-second-order model offered a better fit.
A higher fit (R
2 > 0.97) is observed for the pseudo-second-order model than for the pseudo-first-order model, indicating chemisorption is the limiting factor. The mixed adsorbent shows an increase in rate constants (k
2), suggesting that the synergy between activated charcoal and bone charcoal leads to faster adsorption on the surface. These results are consistent with those of Li et al. [
43], who found that modified orange peel exhibited pseudo-second-order kinetics for Ni
2+ and Cd
2+.
3.4. Statistical Analysis
To reflect data variability and support the reliability of the experimental results, statistical analysis using ANOVA and normality (Shapiro–Wilk) test analysis should be applied to the current study. To achieve this, as presented in
Section 3.1.1, the influence of pH on the copper removal efficiency using orange peel and mixed absorbents will be taken as an example to apply the statistical analysis. Each experiment was repeated four times to show the accuracy of the obtained results. It should be noticed that the replicate two data shown in
Table 4 represent the mean copper removal efficiency extracted from the data of
Figure 3 and
Figure 4 for orange peel and mixed adsorbent, respectively. The obtained data shows that the considered experimental results are very accurate with very low standard deviation (SD), which reflects the reliability of these results.
By applying the Two-Way ANOVA analysis to the data presented in
Table 4, the obtained results are listed in
Table 5. The ANOVA Parameters are SS (Sum of Squares), df (Degrees of Freedom), MS (Mean Square), F-value,
p-value, and Partial η
2. SS quantifies total variation, df is the number of independent comparisons, MS is used to calculate the F-value, which indicates the ratio of factor variance to error variance, p-value is the probability of observed results under the null hypothesis, and Partial η
2 (effect size) is the proportion of variance explained.
Many valuable observations about biosorbents’ effectiveness at different pH values are provided by the thorough analysis of copper removal by orange peel and mixed adsorbents. Supporting the strength of the results are high F-values and super low p-values (
p < 0.0001) for all ANOVA factors. Regarding the ANOVA analysis, adsorption efficiency mostly depends on pH, as shown by the extremely significant pH effect (F = 2986.72,
p < 0.0001, η
2 = 0.996). The parabolic response, which is best at pH 6, is typical of how most biosorbents function, where repulsion with contaminants is reduced at neutral pH values [
44]. The sudden decrease in efficiency seen at pH 8 of 60% for both adsorbents may lead to the formation of precipitates or the loss of active sites through deprotonation of functional groups [
45].
The fact that the mixed adsorbent outperformed consistently at all pH levels demonstrates a big advantage in using composite materials (+8.54% average benefit across pH levels). According to the present study, the synergistic effects between the two components of mixed adsorbent improve performance by increasing both surface area and the number of active sites [
46]. The greater pH 8 result (+10.93%) suggests that the mixed adsorbent works well in alkaline conditions because its cation exchange capacity is preserved [
47].
It is noticed that the pH effect varies significantly amongst adsorbents, as indicated by the strong interaction effect (F = 25.51,
p < 0.0001, η
2 = 0.705). While the performance of both best fits at pH 6, the mixed adsorbent achieves a 12.3% higher result from pH 2 to pH 6 than the result for orange peel. This means that the surface chemistry of the composite material has been changed to make it more sensitive to pH within the desired range [
48].
The normality (Shapiro–Wilk) test analysis shows test statistic W = 0.982, and p-value = 0.589, which indicates the residuals are
normally distributed (p > 0.05).
3.5. Mixed Adsorbent Regeneration and Reusability for Cu (II) and Cd (II) Removal
Disposing of used adsorbents is a significant challenge in adsorption processes, making the regeneration of these materials crucial from economic and environmental perspectives [
49]. Regenerating adsorbents not only reduces the need for new materials but also addresses the environmental concerns related to the disposal of spent adsorbents. Many regeneration techniques have been explored, each with varying degrees of success. These include solvent washing, thermal treatment, chemical processes, and electrochemical regeneration. Among these, solvent washing is particularly effective in recovering solutes. Common eluents used in desorption studies include dilute HNO
3, HCl, H
2SO
4, as well as mineral and organic acids [
50,
51]. The regeneration process followed in the present study is by washing the mixed adsorbent with a 0.2 M HCl solution. Using an orbital shaker, the desorption process was conducted for 60 min at a rotational speed of 150–180 rpm. Afterward, the adsorbent was rinsed with water to remove any residual metal ions, ensuring that the adsorbent could be reused effectively in subsequent cycles.
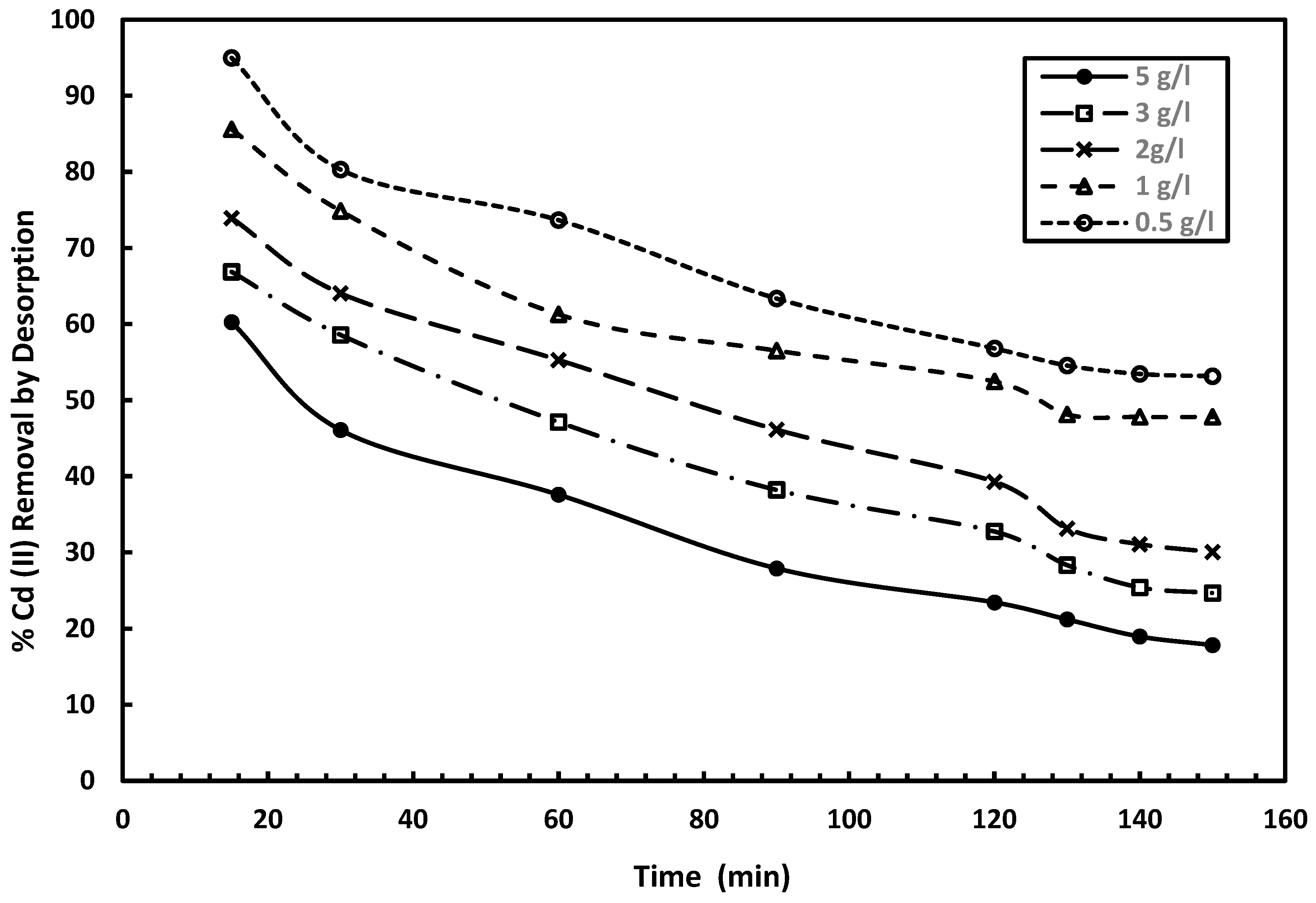
The desorption experiments for Cu (II) and CD II) were performed using five different mixed adsorbent dosages: 0.5 g/L, 1 g/L, 2 g/L, 3 g/L, and 5 g/L, as illustrated in
Figure 13 and
Figure 14. The desorption efficiency, which is the ratio of desorbed Cu(II) or Cd (II) to the total amount adsorbed on the adsorbent [
52,
53], is used to gauge the effectiveness of desorption over time. The percentage of adsorbed metal ions over time is plotted with % desorption on the
y-axis and time on the
x-axis. As shown in
Figure 13, the desorption of Cu (II) decreases significantly with increasing mixed adsorbent dosage. Specifically, the copper removal for the desorption process drops from 90% to 44.2% at 0.5 g/L, 75.96% to 36% at 1 g/L, 68.17% to 17.11% at 2 g/L, 58.84% to 12.88% at 3 g/L, and drops from 53.72% to 10.4% at 5 g/L. This illustrates a clear trend: while the initial desorption efficiency is higher at lower adsorbent dosages, it decreases more rapidly over time. As the dosage increases, desorption starts at a lower percentage but declines more gradually. This suggests that higher adsorbent dosages result in reduced desorption efficiency, possibly due to stronger metal–adsorbent binding or a lower availability of desorbing agents compared to the increased adsorbent mass.
Figure 14 also reveals a marked reduction in the desorption arability of Cd (II), which is decreased from 95% to 53% at 0.5 g/L, 85.6% to 47.8% at 1 g/L, 73.94% to 30% at 2 g/L, 66.88% to 24.7% at 3 g/L, and from 60.26% to 17.8% at 5 g/L. The trend observed in the desorption of Cd (II) is similar to that of Cu (II). At lower adsorbent dosages, the desorption efficiency starts higher but declines rapidly over time. In contrast, at higher adsorbent dosages, the initial desorption percentage is small, and the rate of decrease is slower. This suggests that by increasing the adsorbent dosage, Cd (II) ions are more strongly retained due to enhanced adsorption capacity or stronger binding forces, resulting in reduced desorption efficiency over time.
Generally, the results show that the adsorption of Cu (II) and Cd (II) using the mixed adsorbent is largely reversible, with strong binding between the adsorbent and metal ions. The achieved desorption efficiencies of 90% for Cu (II) and 94% for Cd (II) suggest that these metal ions can be effectively removed. The regenerated adsorbent can be reused for 9–10 cycles before reaching saturation and being discarded. Regarding the desorption process, Cd (II) demonstrates higher desorption rates at all adsorbent dosages. These results prove the potential of adsorbent regeneration in wastewater treatment, allowing for the recovery of metal ions from treated adsorbents.
In comparison to adsorbents made from agricultural waste, the ones in this study have strong performance. Orange and banana peels were shown by Kelly-Vargas et al. [
3] to be effective in removing metals, but no details on restoring the metal-free material were provided. This study addresses the need pointed out by Kelly-Vargas et al. [
54] for sustainable reuse of adsorbents, as it focuses on 9–10 regeneration cycles that maintain high efficiency (90% for Cu
2+, 94% for Cd
2+).
It is evident from all pH, dosage, and contact time tests that mixed adsorbents (AC + BC) perform better than orange peel powder. The removal of these metals is enhanced by the presence of exchange sites in bone charcoal and a large surface area with physical adsorption in charcoal. Orange peel is less effective than the others, though, because of its smaller surface area and reliance only on carboxyl and hydroxyl groups for metal attachment. This study advances our understanding of low-cost adsorbents for the removal of heavy metals from industrial wastewater. Because it works faster and is more effective, the introduced mixed adsorbent may be a better option for large-scale wastewater treatment systems than relying solely on traditional adsorbents like activated carbon.
4. Conclusions
Industrial activities contribute significantly to environmental pollution, with untreated effluent discharges—especially those containing heavy metals like copper Cu (II) and cadmium Cd (II) posing substantial risks to public health and the environment. Addressing this issue requires effective and sustainable remediation methods. This study examines the use of natural source adsorbents, specifically the orange peel adsorbent and the innovative mixed adsorbent composed of equal parts of activated charcoal (AC) and bone charcoal (BC), to enhance the removal and recovery of heavy metals from industrial wastewater.
The current research work examines and evaluates the effect of various parameters on the adsorption efficiency for copper and cadmium, and the results show the following:
pH: Low pH levels hinder adsorption due to competition from hydrogen ions, whereas higher pH levels improve adsorption by increasing the availability of binding sites.
Adsorbent dosage: Increasing the dosage typically enhances metal ion removal, but excessive dosages can impede desorption efficiency.
Contact time: Sufficient contact time is necessary for optimal adsorption, as the process eventually reaches equilibrium.
The obtained optimal conditions for copper and cadmium removal are pH of 6, a dosage of 5 g/L, and a contact time of 60 to 150 min. Under these conditions, the mixed adsorbent’s removal efficiencies can reach 96.62% for Cu(II) and exceed 75.56% for Cd (II), while OPP is only able to remove 75–85.6% of Cu(II) and 50.7–75.56 % of Cd (II). The maximum adsorption capacity for Cu(II) and Cd (II) is 12.0 mg/g and 7.0 mg/g, respectively, using the mixed adsorbent compared to 10.0 mg/g and 6.0 mg/g in the case of OPP. The fast adsorption ability and surface charge of the mixed adsorbent, due to its high surface area and negative surface charge, underline the benefits of using mixed materials to enhance adsorption efficiency. At lower dosages (0.5 g/L) using 0.2 M HCl, both adsorbents demonstrated exceptional reusability, achieving desorption efficiencies of 90% for Cu(II) and 94% for Cd (II), permitting reuse for 9–10 cycles prior to saturation. This significantly extends the introduced adsorbents’ operational lifespan compared to previous studies reporting only 3–5 cycles. These results were verified by isotherm and kinetic modeling with the Langmuir model (R2 > 0.95), which suggested monolayer adsorption, and the pseudo-second-order model (R2 > 0.97), indicating chemisorption as the rate-limiting step.
Although orange peel is a cost-effective and accessible adsorbent, its lower efficiency and regeneration capacity than mixed adsorbents were noted. The mixed adsorbent showed better reusability and higher efficiency, making it a superior option for repeated cycles of metal recovery. This work highlights the potential of combining adsorbent regeneration, solute recovery, and reuse to enhance the economic viability of industrial effluent treatment. By reducing the cost and extensive use of adsorbent materials, the current study provides a sustainable, cost-effective solution for heavy metal remediation in the industrial wastewater treatment field. This study opens the door for creative solutions to heavy metal pollution, a major worldwide environmental issue, by utilizing inexpensive, environmentally friendly materials.