Abstract
The increasing presence of emerging contaminants in aquatic environments, particularly endocrine disruptors (EDs), has raised significant environmental and public health concerns due to their toxicity, persistence, and ability to interfere with the endocrine systems of both aquatic organisms and humans. Among these compounds, the steroid hormones 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) stand out, as they are frequently detected in wastewater, even after conventional treatment processes, which often exhibit limited removal efficiency. In this context, advanced oxidation processes (AOPs), especially those based on the generation of sulfate radicals (SO4•−), have emerged as promising alternatives due to their high redox potential, extended half-life, and broad effectiveness across various pH levels. This work reviews recent advances in AOPs for the degradation of E2 and EE2, focusing on sulfate radical-based processes. The main degradation mechanisms, operational parameters, removal efficiency, challenges for large-scale application, and gaps in the current literature are discussed. The analysis indicates that despite their high effectiveness, sulfate radical-based processes still require further investigation in real wastewater matrices, the assessment of the toxicity of by-products, and the optimization of operational variables to be established as viable and sustainable technologies for wastewater treatment.
1. Introduction
With population growth, the demand for new substances to facilitate industrial, domestic, and personal practices has increased significantly in recent years. As a result, new substances have been introduced into the environment. Some pollutants are present in water bodies in trace concentrations (mg/L or µg/L). These pollutants are called contaminants of emerging concern (CEC), emerging contaminants or micropollutants, and there are intense concerns about their environmental effects and treatment challenges [1,2].
Among the micropollutants, endocrine disruptor (ED) compounds deserve special attention. According to the World Health Organization (WHO), “an endocrine disruptor (ED) is an exogenous substance or mixture that alters the function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations” [3], and according to the U.S. Environmental Protection Agency (EPA), is “an agent that interferes with the synthesis, secretion, transport, binding or elimination of natural hormones in the body that are responsible for maintaining homeostasis, reproduction, development and/or behavior” [4].
Many substances can affect the endocrine system, such as dioxins, which are found in the environment as a result of burning waste; bisphenol A, a xeno-estrogen commonly used in the manufacture of polycarbonate plastic and resin coatings for food and drink; and phthalates, commonly used as plasticizers in PVC and as solvents and fixatives in fragrances [5].
Pharmaceuticals, pesticides, disinfectants, and synthetic hormones are among the main routinely used products that can cause endocrine dysfunction. In addition, personal care products can also contain compounds classified as endocrine disruptors. Studies have also reported the presence of endocrine disruptors in aqueous matrices such as sewage and drinking water [6,7,8]. Among the endocrine disruptors, estrogen hormones are the main ones responsible for estrogenic activity. For this reason, although they are found in water bodies in low concentrations (ng/L to µg/L), they can cause biological effects [9,10].
Endocrine disruptors can act in the body through different mechanisms: by mimicking the natural hormone produced in the endocrine glands and promoting signaling pathways after binding to hormone receptors (agonistic effect), or by blocking receptors, impairing the action of the natural hormone (antagonistic effect) [5]. In addition, EDs interfere with hormone biosynthesis by inhibiting or stimulating enzymes essential to produce hormones, altering their concentrations in the body [11,12].
Some of the consequences of the effects of EDs on human health are thyroid problems, obesity, endometriosis, infertility, congenital disabilities, neurological disorders or disorders of sexual organ development, adverse immunological effects, cardiovascular diseases, and various types of cancer [11,12]. The effects can be particularly severe during critical periods of development, such as pregnancy and childhood, when the endocrine system is most vulnerable [5,11].
The natural hormones estrone (E1), 17β-estradiol (E2), and estriol (E3) and the synthetic hormone 17α-ethinylestradiol, widely used in contraceptive pills, have a high estrogenic potential and are released into the environment through sewage and industrial effluents. 17β-Estradiol has the highest estrogenic activity among the natural hormones, and 17α-ethinylestradiol also exhibits strong estrogenic potential [13,14,15].
When not produced internally and when in contact with the body, estrogen hormones can act as endocrine disruptors, potentially interfering with the synthesis, secretion, transport, binding, or elimination of natural hormones in the body that are responsible for maintaining homeostasis, reproduction, development, and/or behavior [4].
Wastewater is reported to be the main source of emerging contaminants in environmental matrices. Several reports in the literature exist on micropollutants in influent and effluents from wastewater treatment plants and in surface water, groundwater, and drinking water in various countries [6,7,8,16]. 17β-Estradiol (E2) and 17α-ethinylestradiol (EE2) can be biologically persistent and toxic and are capable of compromising essential functions for the maintenance and survival of aquatic biota including reproductive processes and the development of various species [17,18,19,20,21].
Due to their biological persistence, conventional wastewater treatment processes generally cannot remove all micropollutants efficiently. In addition, a fraction of the endocrine disruptors can adsorb to the sludge, resulting in a secondary environmental problem [12,22,23]. Given this scenario, demand is growing for technologies that are capable of promoting the effective removal of EDs including hormones.
Advanced oxidative processes (AOPs) are based on the formation of hydroxyl radicals (•OH), which promote the oxidation of the target contaminant, resulting in smaller molecules or even complete mineralization [24]. AOPs have been applied to remove emerging contaminants from aqueous matrices. For removing estrogen hormones, such as 17β-estradiol and 17α-ethinylestradiol, several studies that applied different types of AOPs can be found in the literature like photocatalysis, ozonation, and Fenton [15,25,26].
The study of oxidative processes with the formation of sulfate radicals has gained significant importance in removing endocrine disruptors from wastewater in the last decade. Research has been directed toward developing advanced oxidative processes based on photoactivated persulfate and peroxymonosulfate, either by ultraviolet (UV/PS), visible radiation, or sunlight irradiation [27,28].
This review work aimed to explore advances in advanced oxidative processes (AOPs) for the removal of endocrine disruptors, with a focus on the estrogenic hormones 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) using the activation of persulfate (PS) and peroxymonosulfate (PMS). The state-of-the-art advanced oxidation processes (AOPs) for E2 and EE2 removal, focusing on sulfate radical-based oxidation, are presented. It highlights the high efficiency of these processes in degrading steroid hormones, the main degradation mechanisms, key operational parameters, and the current challenges for large-scale implementation. Furthermore, this review sought to identify existing gaps in the literature and explore perspectives for advancing and optimizing these technologies.
2. Background of Steroid Hormones
Among the endocrine disrupting substances are natural hormones (estrone, 17β-estradiol and estriol) and synthetic hormones (17α-ethinylestradiol). Although some of these hormones are naturally present in the human body, overexposure to these compounds can cause a hormonal imbalance. For this reason, they appear on the United Kingdom Environment Agency’s (UKEA) list of EDs [13,14].
Hormones are chemical substances produced and secreted by the endocrine glands in different body areas such as the thyroid, pituitary, gonads and adrenal glands. When released into the bloodstream, they coordinate the functioning of the entire organism [29,30]. These messengers perform essential functions for the human body’s survival such as controlling organ activities; the levels of salts, sugars, and liquids in the blood; using and accumulating energy; and acting in the growth, development and reproduction of organisms [29,30].
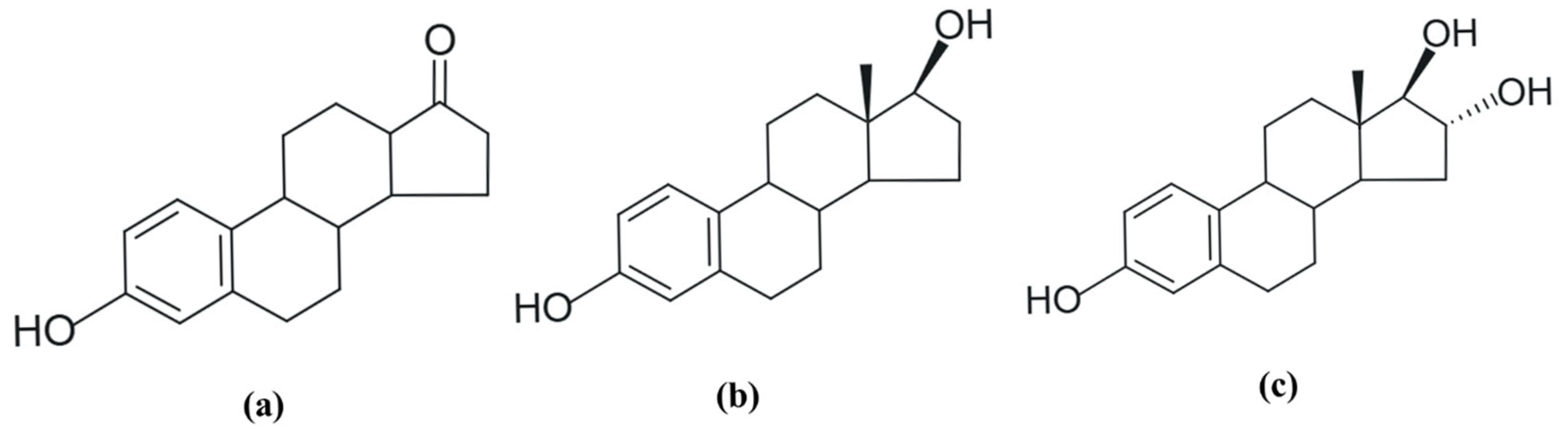
Estrogens have a phenolic group in their structure, and in some cases, an aliphatic hydroxyl group [29]. They can be called C18 steroids because they have 18 carbon atoms in their structure. There are three natural forms of estrogen often found in female excretions: estrone (E1), 17β-estradiol (E2), and estriol (E3) (Figure 1). Of these, 17β-estradiol or E2 has the most significant biological and estrogenic activity [29,30,31].
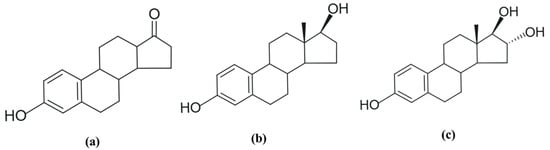
Figure 1.
Molecular formula of natural hormones: (a) estrone (E1); (b) 17β-estradiol (E2), and (c) estriol (E3). Adapted from [29]. Prepared using https://freetrial.marvin.cxn.io/ (accessed on 15 June 2025).
17β-Estradiol can act as an endocrine disruptor in the environment, affecting plant growth and the reproductive development of wildlife in small amounts [20,21]. Even at concentrations of ng/L, E2 can induce the production of vitellogenin (VTG) (a protein of female gametes) in male fish, causing changes in their reproductive characteristics and reduced survival rates in several fish species [21,32,33]. Furthermore, a small concentration between 10 and 100 ng/L can cause transcriptional changes in immune systems and protein degradation pathways in Daphnia magna [34], thus disrupting food chains in aquatic environments.
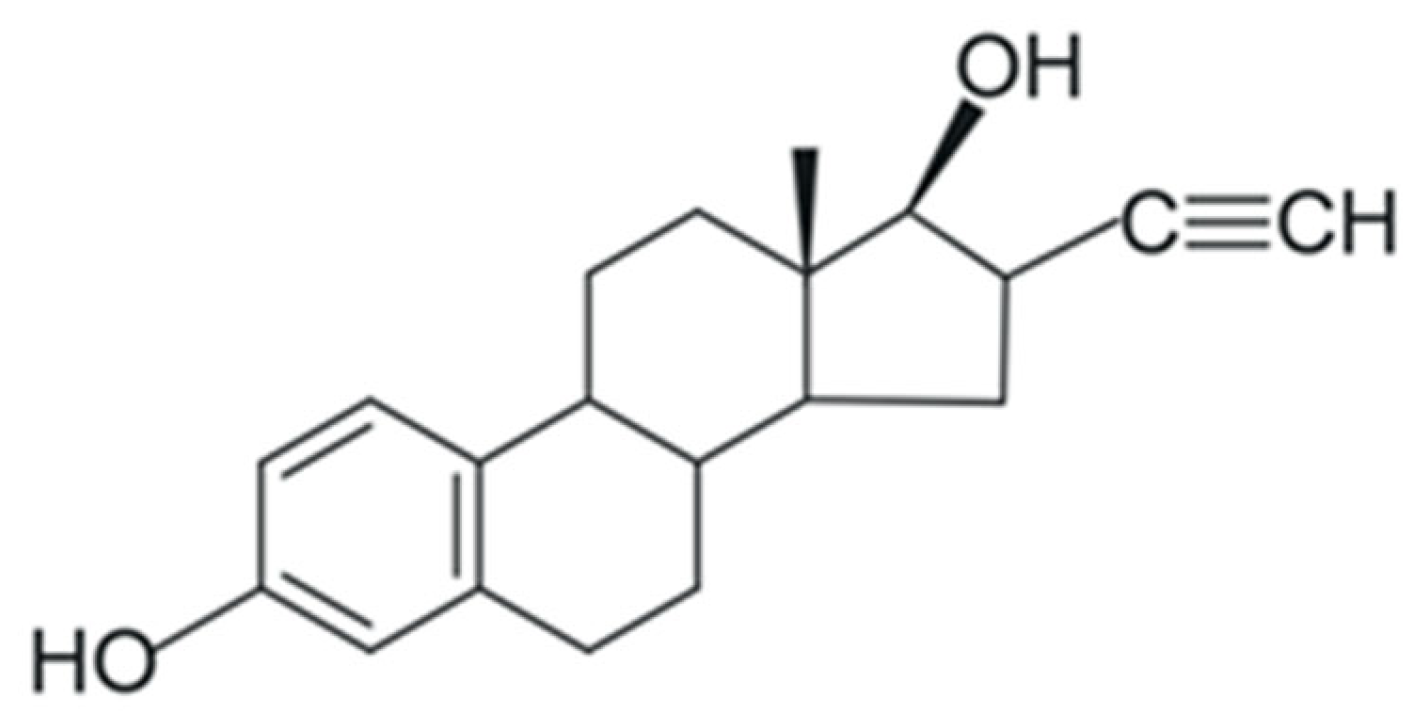
As they are primarily responsible for the growth and reproduction of animal species including humans, synthetic derivatives of natural hormones are commonly used as contraceptives in the control of symptoms surrounding menopause, physiological disorders, and in the treatment of prostate and breast cancer [29,35,36]. 17α-Ethinylestradiol (EE2) (Figure 2), a synthetic hormone derived from the natural hormone E2, is widely used in these drugs [29,35]. EE2 can affect activities vital for the maintenance and survival of aquatic biota such as the growth of bacteria, algae, and hydrophytes [17]. Additionally, in concentrations between 1.2 and 1.6 ng/L, EE2 can promote several effects in Danio rerio like persistent behavioral and fertility changes—offspring behavior affected by parental exposure (transgenerational effects) [37]. Table 1 shows the physicochemical properties of E2 and EE2.
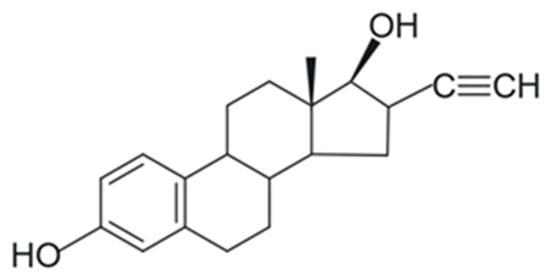
Figure 2.
Molecular formula of the synthetic hormone 17α-ethinylestradiol (EE2). Adapted from [29]. Prepared using https://freetrial.marvin.cxn.io/ (accessed on 15 June 2025).

Table 1.
Physicochemical properties of 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) [38,39,40].
2.1. Sources and Occurrence of Hormones in Water
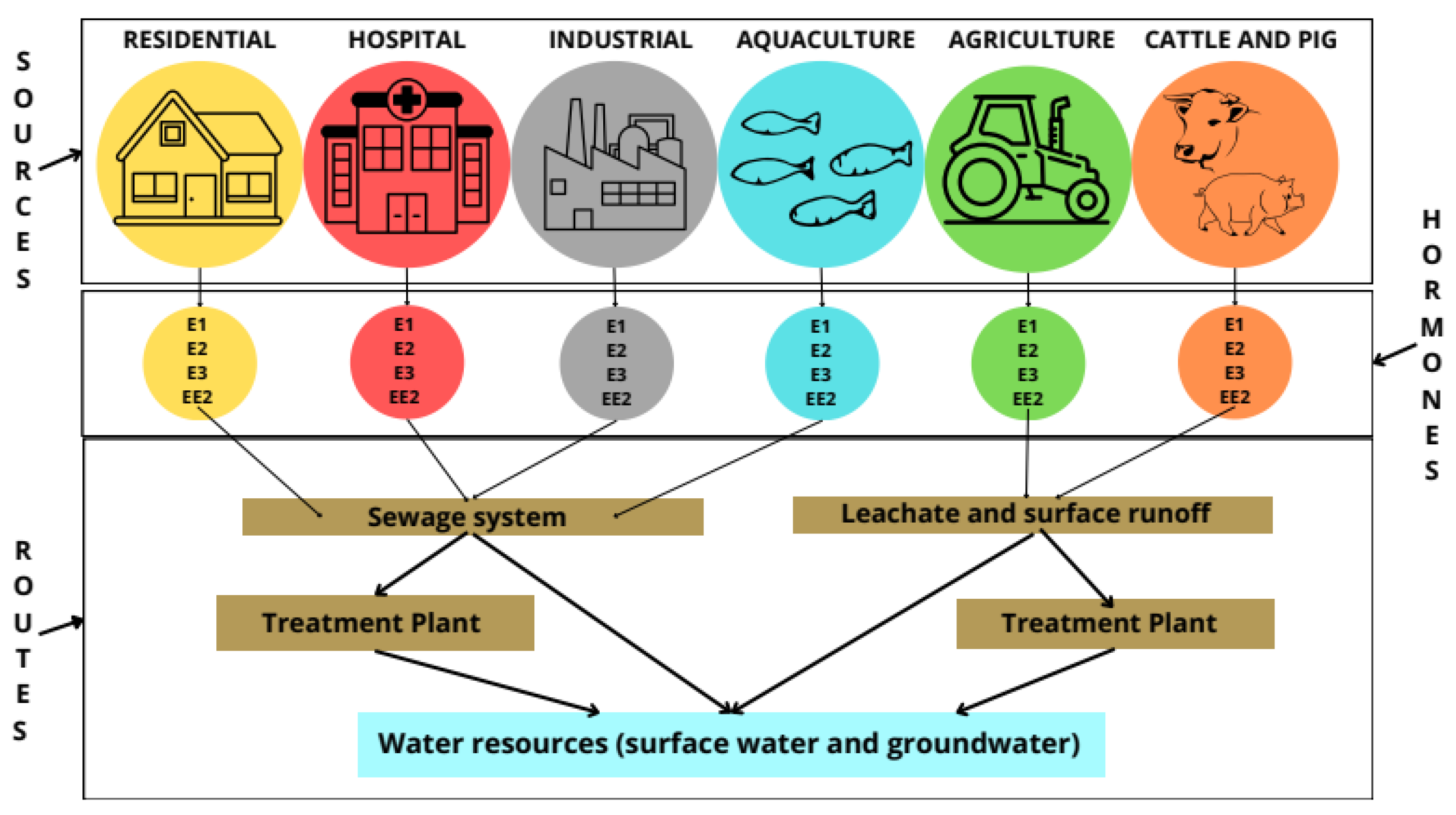
Steroid hormones and other endocrine disruptors can contaminate water resources by discharging raw wastewater (from urban, hospital, or industrial sources) and effluents from wastewater treatment plants (WWTPs). Most wastewater treatment plants are not designed to remove these micropollutants [12,33,41]. As a result, EDs are discharged along with the effluents. In addition, endocrine disruptors can reach water bodies by leaching waste from landfills, agricultural surface drains, and cattle and pig farms (Figure 3) [12,33,41].
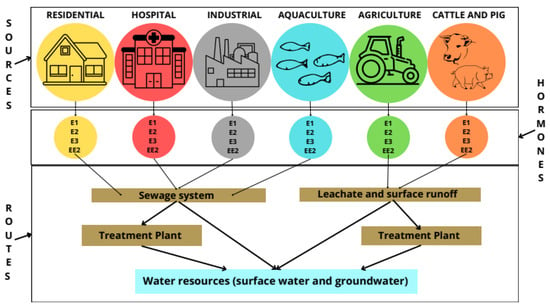
Figure 3.
Contamination routes of steroidal estrogens in groundwater and surface water bodies. Adapted from [41].
The environmental and human health problem related to the presence of endocrine disruptors in water is a topic of great relevance. However, there is evidence of the presence of hormones in water as far back as 1999, when concentrations of 0.021 µg/L of EE2 and 0.040 µg/L of estrone were quantified in the raw effluent of a sewage treatment plant located in Rio de Janeiro State (Brazil) [42].
Steroid hormones are recalcitrant substances, and conventional treatments applied in sewage treatment plants are generally inefficient for their complete removal [12,22,23]. In this way, hormones accumulate in the environment and are detected in surface waters, sediments, wastewater, and landfill leachate worldwide [19,43,44]. The concentration of hormones in surface waters varies depending on the water body and the source of pollution to which it is exposed (Table 2).

Table 2.
The concentration of E2 and EE2 in surface waters in different countries.
In response to the increasing contamination of water resources by endocrine-disrupting compounds (EDs), the European Union has established regulatory frameworks to control and monitor these substances in surface waters. Directive 2013/39/EU, which amends Directive 2008/105/EC, introduced Environmental Quality Standards (EQS) for priority substances including steroid hormones. As part of these efforts, Commission Decision 2015/495/EU outlined a Watch List of substances requiring priority monitoring, notably including 17β-estradiol (E2) and 17α-ethinylestradiol (EE2). To safeguard aquatic biota, the European Commission recommended maximum allowable concentrations of 0.4 ng/L for E2 and 0.035 ng/L for EE2, reflecting the high estrogenic potency and ecological relevance of these compounds [25,52,53,54].
Beyond the ecological implications, the WHO has also acknowledged the potential human health risks associated with estrogens in drinking water. The WHO recommends a provisional guideline value of 1 ng/L for E2 in water intended for human consumption based on toxicological data and exposure assessments. These regulatory benchmarks underscore the persistence and bioactivity of EDs and the pressing need for the advancement of water and wastewater treatment technologies, as conventional processes often fail to achieve the complete removal of these micropollutants [25,52,53,55].
2.2. Removal of Hormones from Wastewater
The removal of steroid hormones from wastewater has become a growing concern due to the environmental impact of these compounds. Thus, various technologies have been developed and improved to treat these substances including physical, chemical, and biological processes and their combinations [56]. The removal of EDs in conventional activated sludge systems is mainly based on the mechanisms of biodegradation and sorption to biomass [57,58]. As a result, only the transfer of pollutants from the liquid to the solid phase can occur, which turns the sludge into a potential secondary vector of contamination, with additional risks to public health and the environment, especially when there is no proper treatment or disposal [22,23].
Several studies have proposed using other biological technologies for the degradation of EDs such as membrane bioreactors (MBRs) [22,59,60,61]. MBRs can effectively remove a wide range of EDs including compounds resistant to biodegradation. The removal efficiency of E2 and EE2 by MBRs was reported by [62], where the authors achieved an efficiency removal of 89.0% and 70.9%, respectively. However, many variables need to be controlled, as the removal of EDs by MBRs can be affected by the sludge age, concentration of the target contaminants, the existence of anoxic and anaerobic compartments, wastewater composition, operating temperature, pH, and conductivity [22,59,60,61].
There are also studies of hormone removal by applying biological treatment technologies using an adhered biomass such as biofilm [62,63,64]. In this case, biomass adhered to a support becomes more resistant than in suspension, as in activated sludge. For example, the moving bed biofilm reactor (MBBR) is a technology used to degrade toxic compounds, such as phenol, showing good removals for this compound [65]. E2 removal values of over 99% were achieved using the MBBR, but 65 days of operation were required to reach this degradation [63].
Table S1 (Supplementary Material) shows that studies involving biological processes achieved a good removal efficiency for the hormones E2 and EE2. Similar results have been reported in other investigations that employed different biological treatment technologies to remove micropollutants [66,67,68,69,70,71].
However, it is important to highlight that several factors often limit the effectiveness of biological treatment. These include the complexity of the microbial degradation pathways, the need for extended hydraulic retention times, and the strong dependence on environmental parameters such as temperature, dissolved oxygen, pH, and nutrient availability. Additionally, the low concentrations of endocrine-disrupting compounds in wastewater can further hinder their biodegradation. As a result, many studies on this topic are relatively outdated, as recent research has increasingly focused on alternative and more efficient technologies for removing these persistent contaminants.
Besides biological processes, some studies have used physical processes to remove endocrine disruptors from wastewater such as adsorption and membrane filtration techniques (ultrafiltration, microfiltration and nanofiltration) [70,71,72]. The adsorption process on activated carbon has been applied to remove EDs, as this adsorbent has advantages such as a high surface area and pore structure, the possibility of regeneration and reuse, and low acquisition cost [12].
The removal of E2 and EE2 using activated carbon was evaluated, and adsorption efficiencies of approximately 50% for E2 and 40% for EE2 were reported [73]. Chemical modifications were made to the activated carbon using nitric acid, increasing the adsorption efficiencies of approximately 80% for E2 and 70% for EE2. Despite the removal rates obtained, activated carbon adsorption does not achieve degradation. Instead, the hormones are merely transferred from the aqueous phase to the adsorbent material, as this is a phase transfer process. If the adsorbent cannot be regenerated, its disposal becomes a new environmental problem [12].
In membrane separation processes, the effectiveness of removing endocrine disruptors is directly related to the size of the molecules of interest and the pore size and physicochemical properties of the membranes used, depending on the material composition, surface charge, and hydrophobicity [12,71,72]. Due to their larger diameter pores, microfiltration membranes have limitations in retaining these molecules. In contrast, studies have indicated that nanofiltration, ultrafiltration, and reverse osmosis can efficiently remove EDs in aqueous matrices [12,71,72].
However, the high operating cost associated with membrane separation technologies must be considered due to the frequent need to replace membranes due to fouling (fouling and clogging of pores), significantly reducing their useful life. In addition, it is important to note that, as with adsorption processes, membrane separation is a phase transfer method (i.e., the contaminants are only removed from the liquid phase and transferred to the membrane matrix without being degraded) [12,71,72].
The context outlined above underscores the need for complementary strategies to ensure the mineralization or transformation of endocrine disruptors (EDs) into less toxic and environmentally concerning by-products. In this regard, chemical treatment processes can remove EDs, such as steroid hormones, from water. Techniques such as ozonation and advanced oxidation processes (AOPs) have demonstrated high efficiency and have been the focus of recent research efforts [25,74,75]. Unlike physical treatment methods, these processes do not merely promote the phase transfer of the contaminants, but enable their effective degradation.
3. Bibliometric Survey on Scientific Literature Regarding Steroidal Hormone Removal in Wastewater
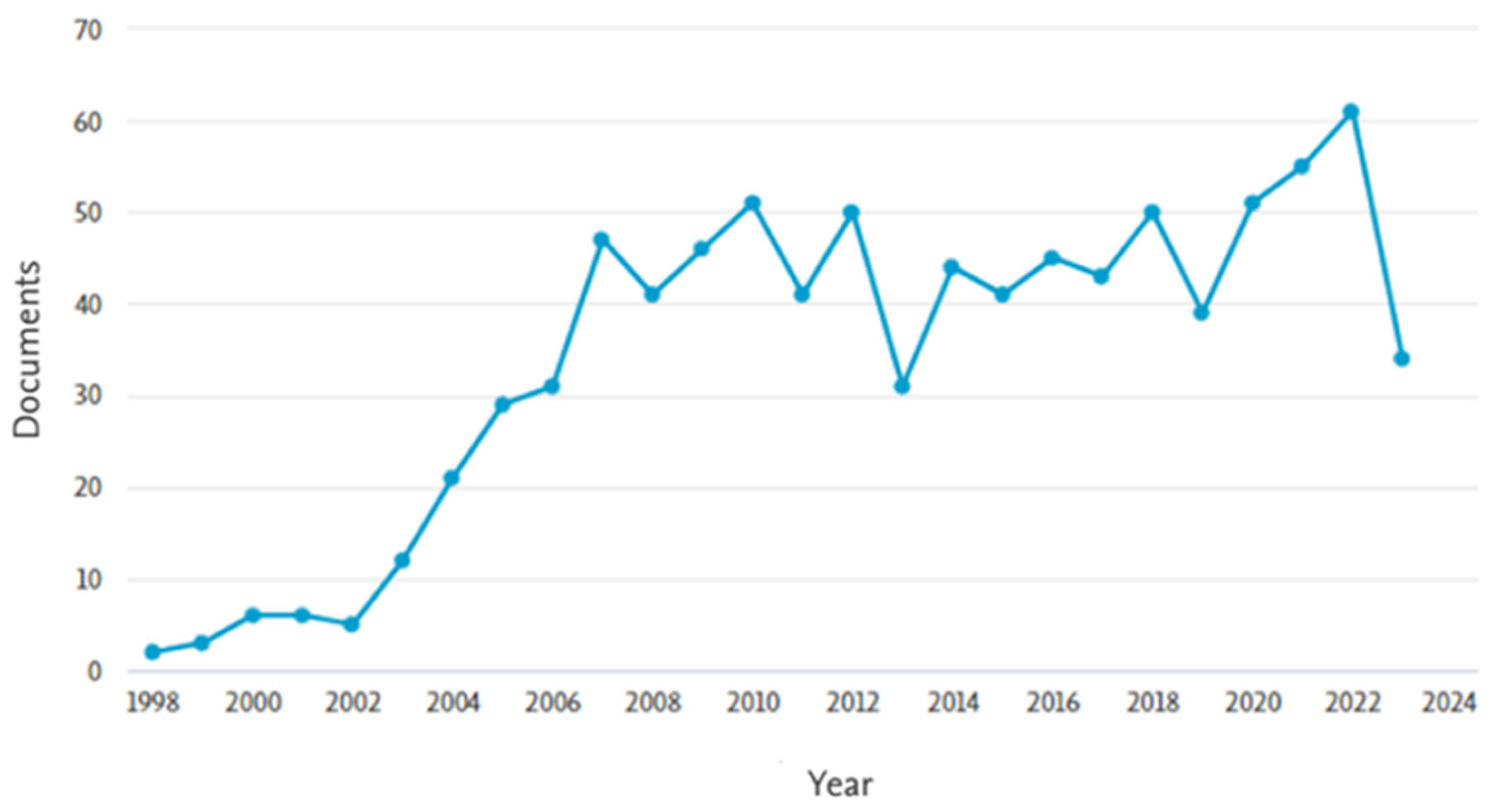
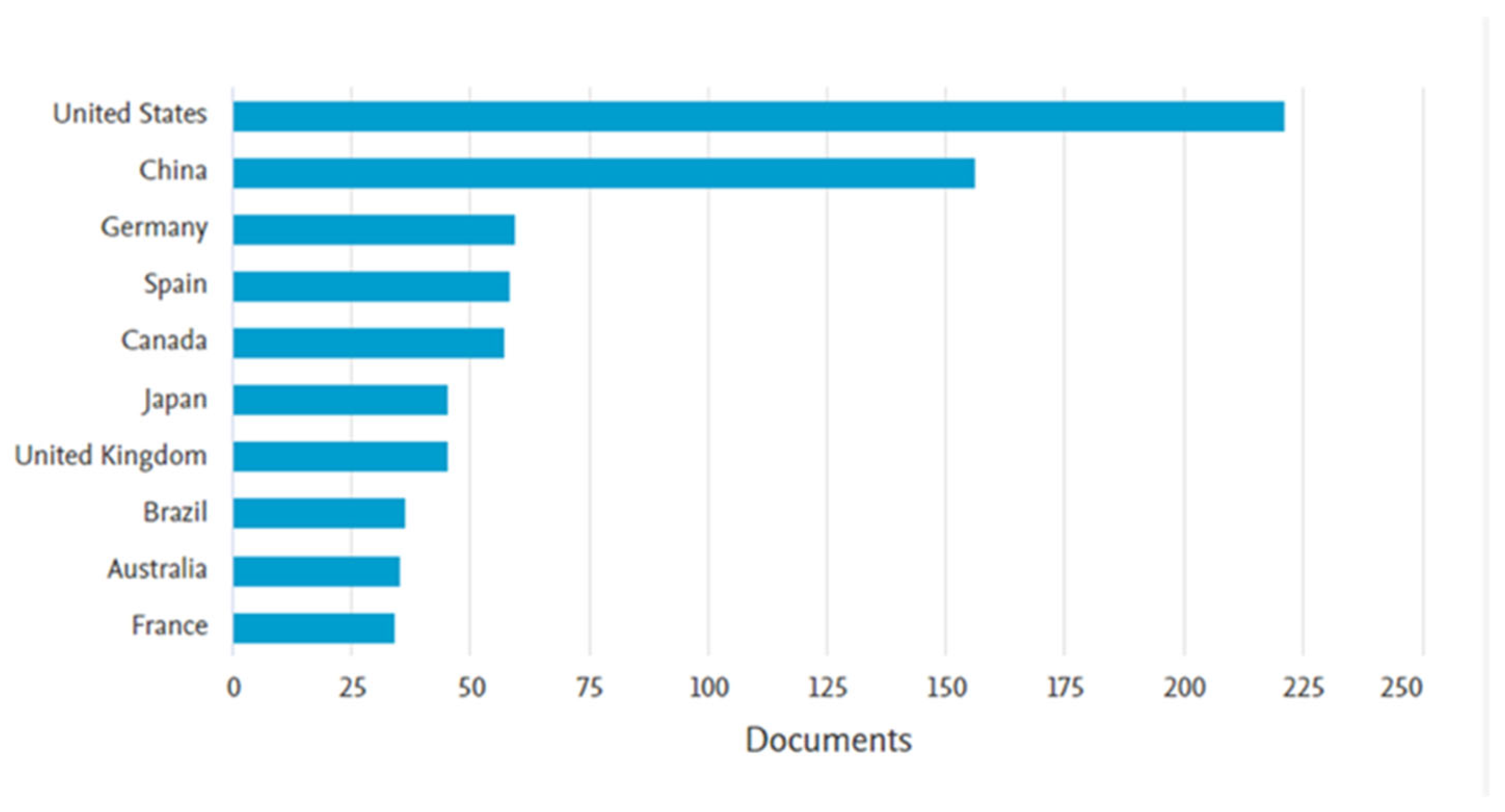
To highlight the relevance of the topic “removal of steroidal hormones from wastewater”, a bibliographic search was conducted using the Scopus database. The keywords “17β-estradiol or 17α-ethinylestradiol and wastewater” were applied to article titles, abstracts, and keywords, with the results filtered up to the year 2023 and restricted to the subject areas of engineering, chemical engineering, chemistry, environmental science, and agricultural and biological sciences. The search returned 885 documents, with the earliest publication dating to 1998. A marked increase in publications was observed since 2002 (Figure 4), indicating growing scientific interest in the topic. Regarding the geographical distribution of publications, the United States led with 221 documents, followed by China with 156. Brazil ranked eighth, with 36 publications (Figure 5). These data reinforce the importance of studying the occurrence of hormones in wastewater and underscore the ongoing need to develop effective technologies for their removal.
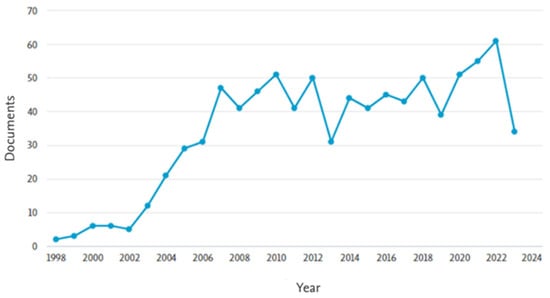
Figure 4.
Evolution of the number of scientific publications on treating steroidal hormones (E2 and EE2) in the wastewater theme.
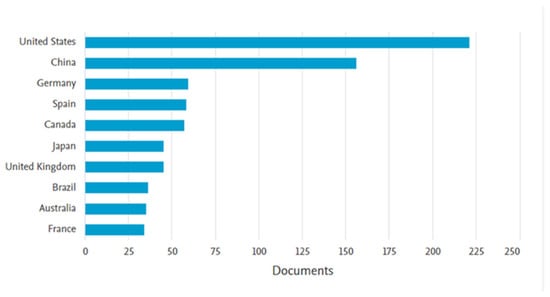
Figure 5.
Leading countries in scientific publications on treating steroidal hormones (E2 and EE2) in the wastewater theme.
To deepen the understanding of the application of advanced oxidation processes (AOPs) for the removal of steroidal hormones—specifically 17β-estradiol (E2) and 17α-ethinylestradiol (EE2)—from wastewater, a bibliographic survey was conducted using the Scopus database. The search encompassed publications from the past decade (2013–2023), limited to article titles, abstracts, and keywords. Only documents classified under engineering, chemical engineering, chemistry, environmental science, agricultural, and biological sciences were considered as a filtering criterion.
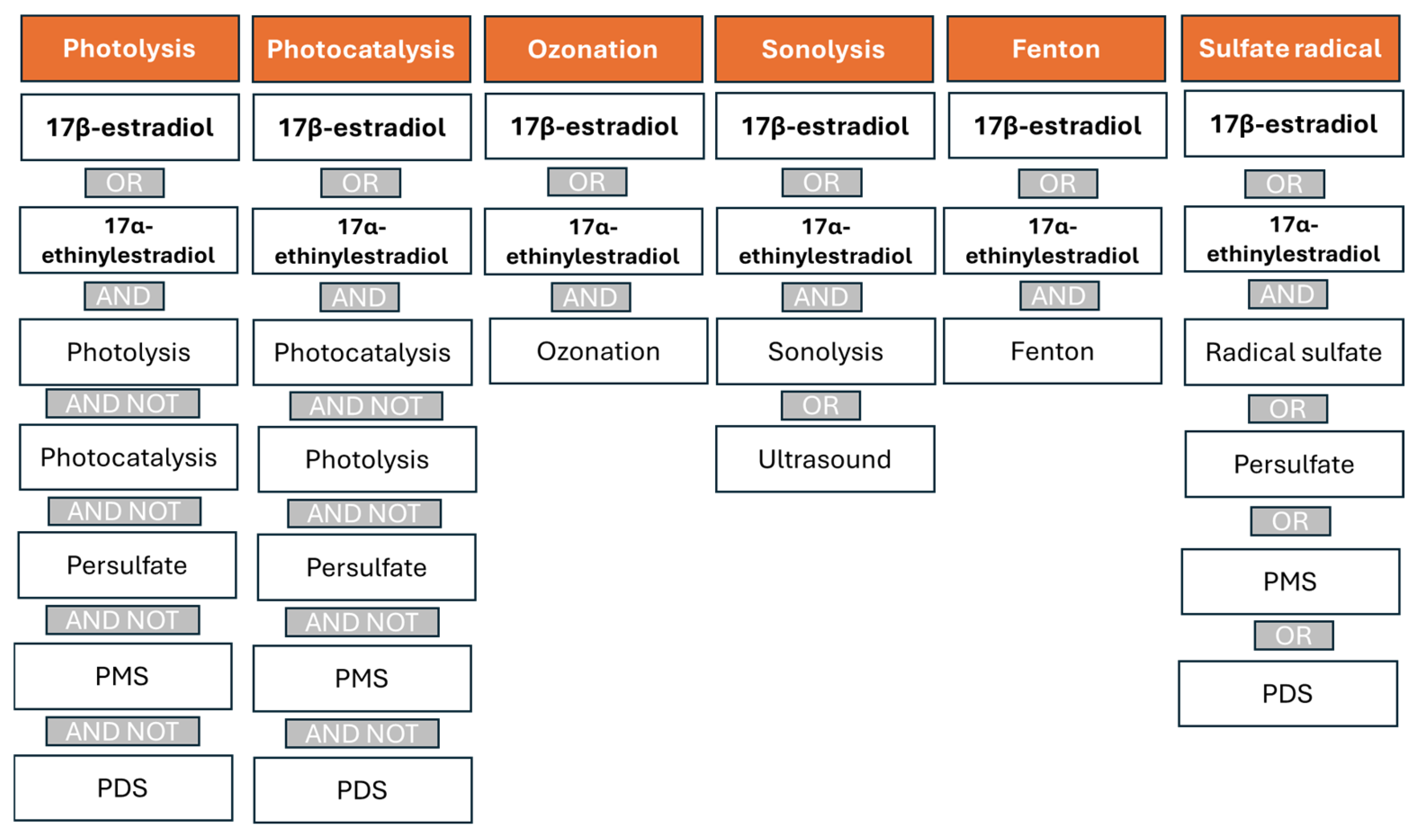
The search strategy was designed to specifically identify studies addressing the removal of these hormones through AOPs based on photolysis, photocatalysis, ozonation, sonolysis, Fenton processes, and technologies involving the generation of sulfate radicals (Figure 6). To avoid the duplication of records across overlapping techniques, control measures were implemented. For instance, since sulfate radical-based processes often involve activation by photolysis or transition metals in combination with light—thus overlapping with photocatalysis—the keywords “persulfate”, “PMS”, and “PDS” were excluded from the queries related to photolysis and photocatalysis.
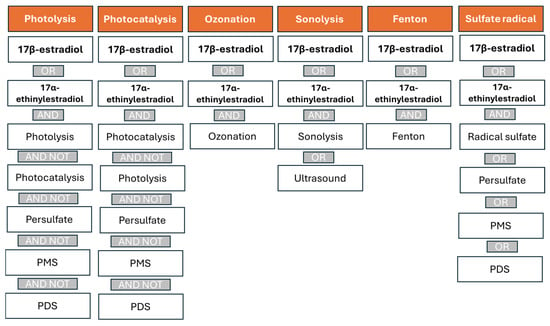
Figure 6.
Strategies used in the Scopus database search to identify articles related to removing E2 and EE2 using advanced oxidation processes (AOPs).
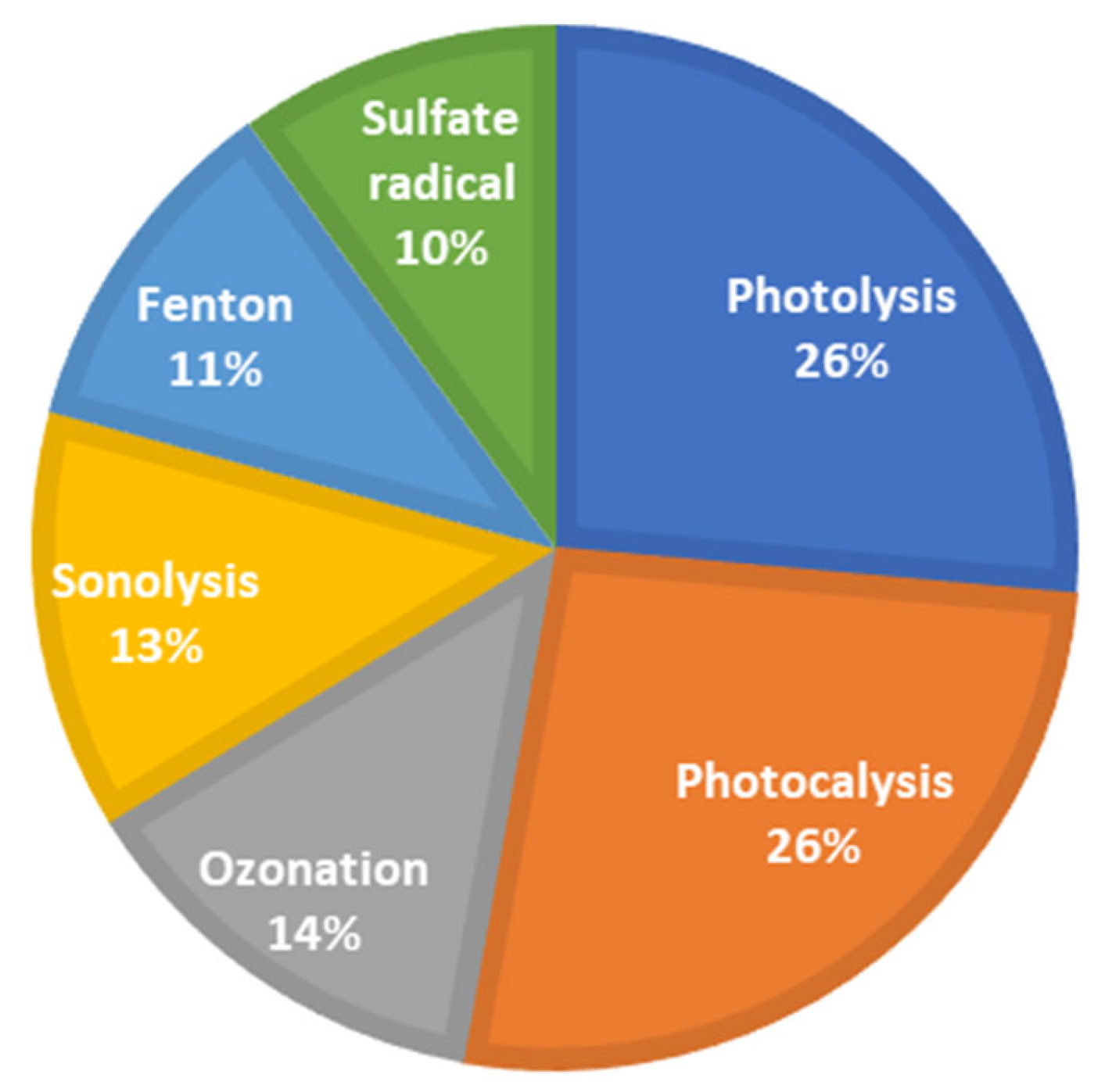
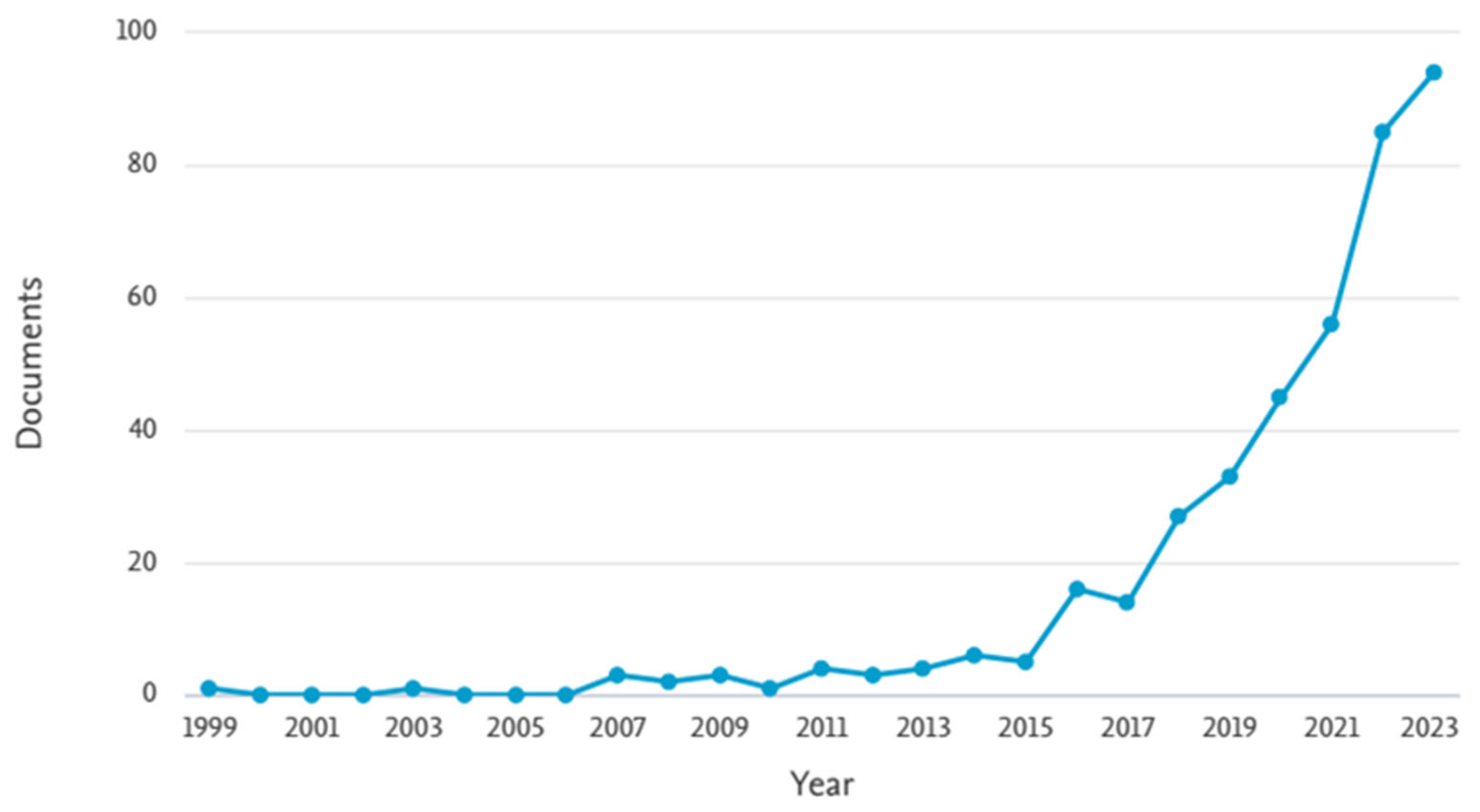
The search yielded a total of 229 articles related to the application of advanced oxidation processes (AOPs) for the removal of 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) from wastewater. A predominance of studies based on photolysis and photocatalysis was observed, accounting for more than 50% of the identified publications (Figure 7). Studies on sulfate radical-based processes have been published since 1999 and showed a steady increase beginning in 2015 (Figure 8). This progressive growth in scientific output suggests a rising interest within the academic community and indicates that these processes hold untapped potential for further exploration.
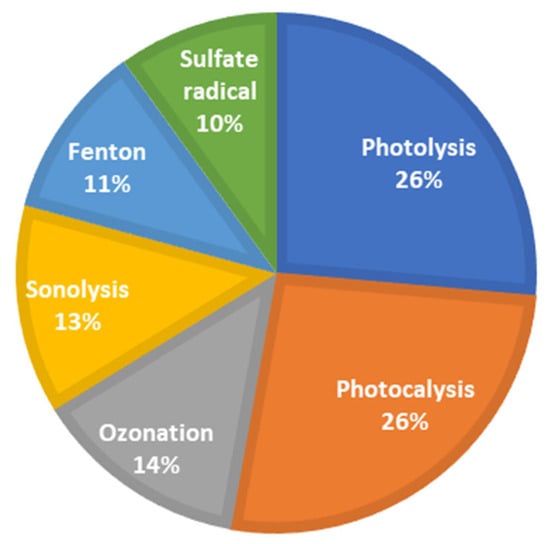
Figure 7.
Trends in applying advanced oxidation processes for E2 and EE2 removal (2013–2023).
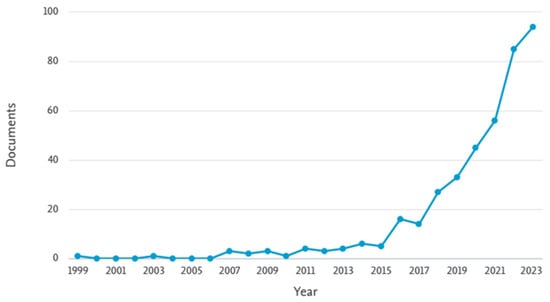
Figure 8.
The annual number of publications indexed in Scopus containing the keywords “AOP and sulfate radical or persulfate” (1999–2023).
4. Advanced Oxidation Processes (AOPs)
AOPs are based on the oxidation of organic compounds through the action of highly reactive radicals, primarily the hydroxyl radical (•OH), whose generation characterizes most of these processes. In addition to hydroxyl radicals, AOPs may also involve other oxidative species such as sulfate radicals (SO4•−) and chlorine radicals (Cl• and Cl2•−) [76,77,78,79,80,81].
4.1. Main AOP Applied to the Removal of E2 and EE2: Observations and Trends
Standard oxidation processes reported in the literature generate hydroxyl radicals through photolysis, photocatalysis, sonolysis, ozonation, and Fenton reactions. Several studies have also explored combining these techniques to enhance the removal efficiency of contaminants [77,79,80].
4.1.1. Photolysis-Based Processes
Endocrine disruptor (ED) photodegradation can occur via two pathways: direct photolysis or indirect photolysis. In direct photolysis, the ED molecule absorbs a photon, promoting bond cleavage or a molecular rearrangement, which converts the compound into simpler fragments. In contrast, indirect photolysis involves the absorption of incident light by photosensitizing agents (such as hydrogen peroxide, sodium persulfate, chlorine, or chromophoric groups of dissolved organic matter), leading to the formation of reactive radicals responsible for the degradation of target compounds [40,76,81]. It is important to note that indirect photolysis, which involves the generation of oxidizing radicals, is classified as an advanced oxidation process (AOP), whereas direct photolysis is not [26,40].
Photolysis has been widely investigated for removing steroid hormones (E2 and EE2) in aqueous matrices. Several studies have explored the use of photosensitizers, such as hydrogen peroxide and chlorine, in photolysis processes aimed at treating these hormones in synthetic solutions, drinking water, and wastewater, intending to generate oxidative radicals to degrade endocrine disruptors [26,40]. Some researchers have reported the complete removal (100%) of E2 and EE2 through indirect photolysis using photosensitizers (Table S2—Supplementary Materials).
Another strategy to enhance the efficiency of ultraviolet (UV)-based processes is to replace photosensitizers with a light source and a metal oxide semiconductor, which facilitates the generation of oxidative radicals responsible for hormone degradation. This process is known as photocatalysis [25,82].
4.1.2. Photocatalysis-Based Processes
Photocatalysis can be understood as the acceleration of a photochemical transformation by a metal oxide semiconductor, such as TiO2, ZnO, CdS, Fe2O3, and WO3, which acts as a catalyst. The activation of the semiconductor is triggered by artificial light sources (typically ultraviolet lamps) or natural sunlight [25,82]. A suitable catalyst for the degradation of endocrine-disrupting compounds is titanium dioxide (TiO2), which has been extensively studied and applied in advanced oxidation processes (AOPs), especially in photocatalysis, standing out among the most widely used catalysts due to its cost-effectiveness, chemical, thermal and photostability, high photocatalytic activity, and low toxicity [25,82].
Some criteria are essential for evaluating the most suitable catalyst for degrading ECs:
- -
- Photocatalytic efficiency: To evaluate the most suitable catalyst for degrading ECs. The degradation performance of the catalyst under specific conditions, like UV wavelength, intensity and water flux, must be quantified. For example, increasing the Ti surface content from 4% to 6.5% improved the E2 removal from 46% to 81% [25].
- -
- Stability and binding to support: The catalyst must remain stable during operation. PVDF membranes modified via e-beam-induced graft polymerization improved the TiO2 adhesion and minimize leaching [25].
- -
- Advanced oxidative potential: TiO2, when exposed to UV, generates hydroxyl radicals (•OH), which are powerful oxidants [81,82].
Photocatalysis is one of the most extensively studied advanced oxidation processes for the degradation of hormones in water (Figure 7), and one of the main challenges associated with using photocatalysts lies in the recovery of nanoparticles from the aqueous matrix after treatment. As a result, there has been growing research interest in developing membranes embedded with TiO2 nanoparticles [25,83]. Immobilized TiO2 on a poly(vinylidene fluoride) (PVDF) membrane has been successfully used in photocatalytic membrane reactors (PMRs) for the degradation of steroid hormones, such as 17β-estradiol (E2), at environmental concentrations (e.g., 100 ng/L), achieving over 96% removal under optimal UV irradiation conditions [25]. The selection of membrane materials is critical in ensuring stability and durability, and is a significant focus of the current investigations.
Research is also directed toward identifying alternative semiconductor materials that enhance hormone removal efficiency. Catalysts besides TiO2 are used in AOPs for degrading emerging contaminants. Fe2O3 and Fe3O4 are effective in (photo-)Fenton processes but require an acidic pH [15]; Al-ZnO is efficient under UV and returned a 98.28% efficiency removal for E2 [74]; N20BiOBr under UV returned an efficiency removal of 53.5% for E2 and EE2 [84]. Several studies have reported removal rates exceeding 98% for E2 and EE2 using photocatalytic processes using TiO2 and other catalysts (Table S2—Supplementary Materials).
4.1.3. Ozonation-Based Processes
Ozone can oxidize organic pollutants due to its high oxidation potential (E0 = 2.08 V) and has been widely applied in wastewater treatment. Ozonation can be enhanced through its combination with ultraviolet (UV) radiation, hydrogen peroxide (H2O2), or photocatalysis, which promotes the generation of reactive radicals and thereby intensifies the degradation of organic molecules [41,85].
The ozonation process has demonstrated high efficacy in removing pharmaceutical compounds, personal care products, and endocrine-disrupting substances in surface waters and effluents [85]. Increasing interest is in advancing ozonation techniques to enhance the removal efficiency for steroid hormones such as estradiol (E2) and ethinylestradiol (EE2). Among the recent approaches is the use of microbubbles, which aim to increase the contact area between the ozone and the aqueous phase, thereby improving its solubility and the overall degradation of organic contaminants [86]. Another strategy involves using ozone-loaded nonpolar solvents to enhance the solubility and stability of ozone in water [87]. As a result, several studies have reported removal efficiencies for E2 and EE2 exceeding 98% (Table S2).
4.1.4. Sonolysis-Based Processes
In aqueous matrices, sonolysis is based on applying sound waves at specific frequencies, which produce cycles of compression and rarefaction, leading to the formation of cavitation bubbles. These bubbles grow through the diffusion of vapor or gas from the surrounding liquid until they reach a critical size and collapse violently. This implosion generates extremely high local temperatures and pressures—approximately 4200 K and 975 bar—resulting in “hot spots”. These hot spots promote the dissociation of water molecules, generating hydroxyl radicals (•OH) [88,89,90].
Ultrasonic irradiation has gained increasing attention for removing endocrine-disrupting compounds from wastewater, as it is considered as an environmentally friendly approach that avoids using chemical reagents for pollutant degradation. Furthermore, since no sludge or residual waste is generated after contaminant degradation, sonolysis presents a promising alternative [88,90,91]. For the removal of steroid hormones such as E2 and EE2, studies have proposed the combined use of oxidants with ultrasound (e.g., US and KMnO4/US) to enhance the removal efficiencies [92]. Consequently, removal efficiencies approaching 100% for E2 and EE2 have been reported in the literature through sonolysis (Table S2—Supplementary Materials).
4.1.5. Fenton-Based Processes
The Fenton process is based on redox reactions in which ferrous iron (Fe2+) is first oxidized to ferric iron (Fe3+) in the presence of hydrogen peroxide (H2O2), which is also oxidized by the iron ion under acidic conditions [81,82,93]. Fenton reactions are most effective at low pH, with optimal values between 2 and 4. At higher pH levels, the degradation rates of target compounds tend to decrease [41,81,82]. A significant drawback of the conventional Fenton process is using iron salts as catalysts, which leads to the generation of large volumes of metallic sludge as waste. This limitation has driven the development of novel systems aimed at minimizing the presence of dissolved iron species and reducing sludge production without significantly compromising process efficiency [85].
Several strategies are being implemented to enhance Fe2+ regeneration, reduce the need for continuous iron addition, and minimize sludge formation, making the process more cost-effective. For the removal of endocrine-disrupting compounds, particularly the hormones E2 and EE2, studies have explored the use of light-assisted Fenton processes (photo-Fenton) [15,94], heterogeneous Fenton systems [44,95], and Fenton processes combined with biological treatments for high-COD effluents [96,97,98,99,100,101,102,103]. These studies aimed to enhance the efficiency of hormone removal, with reported removal rates of up to 100% (Table S2).
4.2. Key Factors Influencing the Efficiency of AOPs for Hormone Removal
It is important to emphasize that all technologies present strengths and drawbacks, and their performance varies according to the characteristics of the matrix and the target compounds. Therefore, selecting the most appropriate process for hormone removal must consider factors such as hormone concentration, organic matter content, wastewater turbidity, and operational costs.
It is essential to assess not only the percentage of removal, but also the residual hormone concentrations. Even with high removal rates, the remaining concentrations may still exhibit estrogenic activity and pose toxicological risks to aquatic biota. Furthermore, many studies in Table S2 (Supplementary Materials) utilized hormone concentrations that were not representative of environmentally relevant conditions (Table 2). Specifically, the reported concentrations in these studies ranged from micrograms per liter (µg/L) to milligrams per liter (mg/L), with the lowest observed concentration being approximately 10 µg/L. These findings indicate that advanced oxidation processes (AOPs), such as photocatalysis, Fenton, ozonation, and sonolysism, effectively remove endocrine-disrupting compounds (EDCs), particularly steroid hormones, within this concentration range. However, this also underscores the necessity for future studies to adopt initial contaminant concentrations in the nanogram per liter (ng/L) range to better reflect the real environmental scenarios and accurately assess the efficiency of AOPs under realistic conditions.
In addition to the need to evaluate the removal of hormones by advanced oxidation processes (AOPs) at environmentally relevant concentrations, it is also essential to investigate the influence of the water matrix. Existing pollutants can interfere with the degradation of emerging contaminants (ECs) during AOPs, primarily through competition for reactive species and light absorption, which can significantly reduce the treatment efficiency [25,81]. Natural organic matter (NOM), bicarbonates, and chlorides may react with hydroxyl radicals (•OH), decreasing their availability to oxidize target contaminants [81]. Furthermore, in photocatalytic processes, turbidity, dyes, and other background substances can absorb or scatter UV radiation, limiting the activation of photocatalysts such as TiO2 and consequently reducing the generation of reactive radicals [25].
Another important variable for removing hormones through light-based advanced oxidation processes is the choice of photoreactor, which significantly influences the removal efficiency. Photoreactors equipped with low-pressure mercury vapor ultraviolet lamps have been widely used, operating at a wavelength of 254 nm (UVC radiation) [78]. For photocatalysis, for example, UV/TiO2-type photoreactors are commonly employed due to their operational simplicity, high efficiency in generating oxidizing radicals, and compatibility with low-pressure lamps. On the other hand, reactors that utilize solar or visible light activation—especially in combination with sensitizers such as dyes or metal-ligand complexes—have gained attention as sustainable and energy-efficient alternatives [78,81].
4.3. Comparison of the Main AOPs Applied to the Removal of E2 and EE2: Advantages and Limitations of the Processes
Table S3 (Supplementary Materials) shows a comparative analysis of different technologies and operational conditions, facilitating the identification of the specific advantages and limitations of each advanced oxidation process. Given that all processes have inherent advantages and disadvantages, combining different oxidants and treatment strategies has been widely reported in the literature to enhance pollutant removal and overcome the individual limitations of each process. It is common to find studies that combine processes based on photolysis, photocatalysis, ozonation, sonolysis, and Fenton reactions to increase the process efficiency, reduce the energy costs and reagent demands, and the treatment of possible generated residues.
Thus, it is possible to find research on the treatment of micropollutants and endocrine disruptors that combine different processes, such as ozonation and photolysis (O3/UV, H2O2/O3/UV) [104,105], ozonation and photocatalysis [98], sonolysis and ozonation [99], and sonolysis and Fenton [100], among other possibilities. Combined reactor systems, such as UV/H2O2, UV/Fenton, and photoelectron-Fenton, have proven effective in optimizing the removal of EDCs, and are particularly suitable for effluents with complex characteristics [81,101].
Another widely reported combination in the literature involves the use of photolysis, photocatalysis, and sonolysis techniques associated with the addition of salts, which when activated, generate sulfate radicals responsible for the oxidation and degradation of micropollutants [78]. These sulfate radical-based processes, like the previously described AOPs, can be applied to removing the E2 and EE2 hormones and offer the advantage of releasing sulfate radicals, which have a higher oxidation potential than hydroxyl radicals [106,107].
5. Sulfate Radical-Based Processes for Hormone Removal
The study of oxidative processes involving the formation of sulfate radicals has gained significant importance in removing endocrine disruptors from wastewater over the past decade (Figure 8). Degradation occurs mainly through forming sulfate radicals (SO4•−), which trigger an electron transfer mechanism that breaks down the contaminant into smaller molecules. In addition to sulfate radical formation, the activation of persulfate or peroxymonosulfate can also generate secondary oxidants, such as hydroxyl radicals, superoxide radicals, or singlet oxygen, which may influence the transformation efficiency and by-product formation [79,101].
Compared with hydroxyl radicals, sulfate radicals offer several advantages: high redox potential (E0 = 2.5–3.1 V, compared with 1.9–2.7 V for •OH); longer half-life (30–40 μs, approximately 7.5 times the half-life of •OH); and less dependence on operational conditions (broader pH tolerance range) [78,104]. Moreover, compared with processes that use hydrogen peroxide (H2O2) to generate •OH radicals, persulfate salts present several advantages. These salts are relatively low cost (USD 0.93–2.65/kg), are widely available on the market, and are easier to transport and store than H2O2 [78,104].
Studies have confirmed the advantage of using sulfate radical-generating salts over hydrogen peroxide (H2O2) to remove endocrine disruptors. A comparative kinetic study between different widely used hydroxyl radical-based advanced oxidation technologies was conducted by [105] such as UV–Vis/H2O2/Fe(II) (Fenton) and UV/TiO2 (photocatalysis), and sulfate radical-based oxidative technologies (using peroxymonosulfate—PMS) such as UV–Vis/PMS/Fe(II). The results indicated that the process involving sulfate radicals demonstrated a higher removal efficiency in a shorter time for 17β-estradiol (E2) compared with traditional AOPs. With sulfate radical formation, E2 removal exceeded 80% in approximately 7 min, and estrogenic activity was eliminated. Regarding the reaction kinetics, the UV–Vis/PMS/Fe(II) process (k = 0.305) showed a degradation rate nearly four times faster compared with UV/TiO2 (k = 0.079).
Similarly, the steroid hormone E2 removal in ultrapure water using the UVA/UVB/H2O2 and UVA/UVB/PS systems was compared [103]. The study showed that using UVB/PS resulted in higher removal efficiencies. The removal efficiency exceeded 99% in 45 min with UVB + 5 mM H2O2 and in just 5 min with UVB + 5 mM S2O82−. In addition to hormone degradation, the processes could completely remove estrogenic activity. Table 3 summarizes the advantages of sulfate radical-based processes reported by the cited authors.

Table 3.
Comparison of sulfate radicals to hydroxyl radical-based processes [103,105].
5.1. Use of Peroxydisulfate (PDS) and Peroxymonosulfate (PMS) in AOPs for Hormone Removal
The use of PDS can be considered more economically viable than PMS in advanced oxidation processes (AOPs). For this reason, this salt has been more commonly employed in research involving the oxidation of hormones through sulfate radicals [106,108]. However, some studies have confirmed that when using a heterogeneous activation system, peroxymonosulfate (PMS) has been proven to be more effective than peroxydisulfate (PDS) in the oxidation of organic compounds and steroid hormones due to electron transfer-mediated mechanisms [75,109].
When applied in advanced oxidation processes (AOPs) for hormone removal, each salt presents specific advantages and disadvantages, as listed in Table 4. The selection of the most suitable salt depends on process variables such as the pH of the medium, the type of activation used, and financial feasibility.

Table 4.
Comparison of the PMS and PDS characteristics for use in advanced oxidation processes [75,101,102,104,109,110].
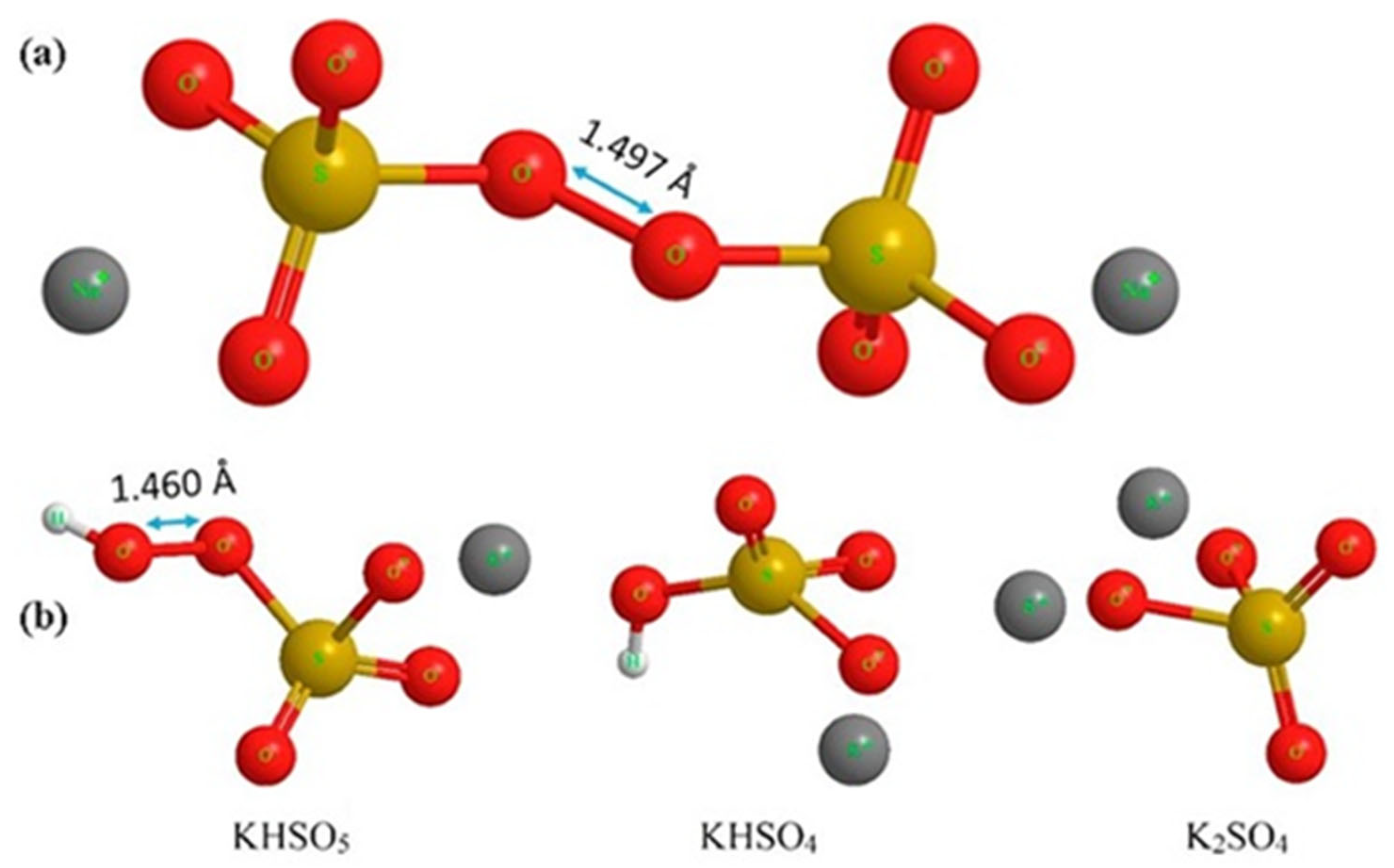
Persulfate (PS), also referred to as peroxydisulfate (PDS), is derived from peroxydisulfuric acid (H2S2O8) and can be found in three salt forms: sodium (Figure 9a), potassium, and ammonium. Potassium PDS has low water solubility (50 g/kg H2O at 25 °C), while ammonium PDS may release residual ammonia into the water. Sodium PDS (Na2S2O8), on the other hand, has high solubility—730 g/kg H2O at 25 °C—making it the first choice for oxidative treatment in wastewater. The bond dissociation energy of the peroxide bond in PDS is 140 kJ mol−1 [101,111,112].
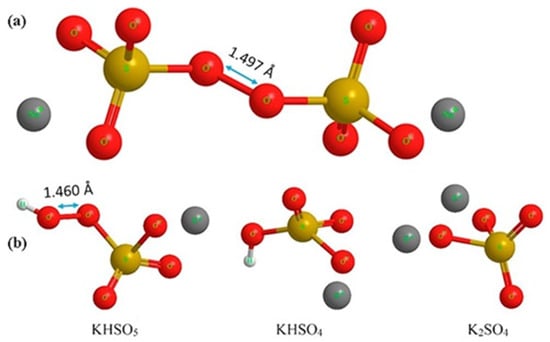
Figure 9.
(a) Structural formula of sodium peroxydisulfate (PDS), also known as sodium persulfate, with the peroxide bond at 1.497 Å. (b) Structural formula of potassium peroxymonosulfate (2KHSO5·KHSO4·K2SO4) [101]. With permission of Elsevier (license 6023120378404).
In addition to PS, there is also peroxymonosulfate (PMS or monopersulfate), derived from persulfuric acid. PMS is typically found in a more stable triple salt form, such as potassium PMS or Oxone® (chemical formula: 2KHSO5·KHSO4·K2SO4, Figure 9b), which has a high solubility in water (298 g/kg H2O). The energy required to break the peroxide bond in PMS is greater than in PDS, amounting to 377 kJ/mol. PMS has a shorter bond length than PDS [101,107].
5.2. Activation Methods of PMS and PDS
Activating the salts is necessary to increase the reaction rate of PDS or PMS with endocrine-disrupting compounds. The oxidation removal of phenolic compounds using PS without a catalyst or other activation method was reported to be less than 5% after 24 h of testing, demonstrating the need for PS activation for phenolic compound oxidation [113]. A comparative study with PS and UV-activated PS analyzed the efficiency of amoxicillin removal and proved that the removal was higher with UV-activated PS [114].
PDS and PMS can be activated to form sulfate radicals through different mechanisms: photoactivation (photolysis), heat (thermolysis), ultrasound (sonolysis), homogeneous catalysis, or heterogeneous catalysis (with oxides of Fe, Co, Mn, and Ti) (Figure S1—Supplementary Materials) [101,104,115].
5.3. Direct Activation by Ultraviolet Irradiation
For large-scale applications in water and wastewater treatment plants, activation through heat or ultrasound may render the process economically unfeasible due to the high energy demand involved [27,28]. Photoactivation requires a lower energy consumption, and because of this, recent research has been directed toward developing PS-based AOPs using photoactivation, whether through ultraviolet radiation, visible light, or sunlight irradiation [27,28].
Persulfate photoactivation can involve two mechanisms: the homolysis of the peroxide bond, which occurs through direct UV activation (photoactivation), or electron transfer reactions via photocatalytic activation (homogeneous or heterogeneous). In the activation by homolysis of the peroxide bond, sulfate and hydroxyl radicals are predominantly formed. In contrast, activation via electron transfer mechanisms predominantly leads to the formation of sulfate radicals, hydroxyl radicals, and superoxide radicals [78,116].
The direct application of peroxydisulfate (PDS) or peroxymonosulfate (PMS) without activation results in a slow reaction kinetics, generally insufficient for the effective removal of pollutants [114]. Direct ultraviolet (UV) irradiation, within the wavelength range of 200 to 400 nm, is considered as a sustainable activation method since it avoids generating sludge from metal catalysts [78,101]. Within this range, a wavelength of 254 nm—emitted by low-pressure mercury lamps—is commonly used, providing adequate energy for homolytic cleavage of the peroxide bond [78,104].
LED lamps have emerged as a more sustainable alternative due to their higher energy efficiency, lower energy dissipation as heat, and significantly longer lifespan. Low-intensity UVC-LED lamps (with UV irradiance below 5 W/m2) have effectively treated micropollutants in wastewater [27,117,118]. However, studies regarding the use of UVC-LED lamps for activating PDS or PMS in sulfate radical-based processes are scarce. The limited research has primarily focused on removing antibiotics [118,119,120].
In the direct activation of PDS or PMS with UV radiation, pollutant degradation occurs via two mechanisms:
- (i)
- Photolysis, in which molecules are broken down by direct light absorption (Equation (1));
- (ii)
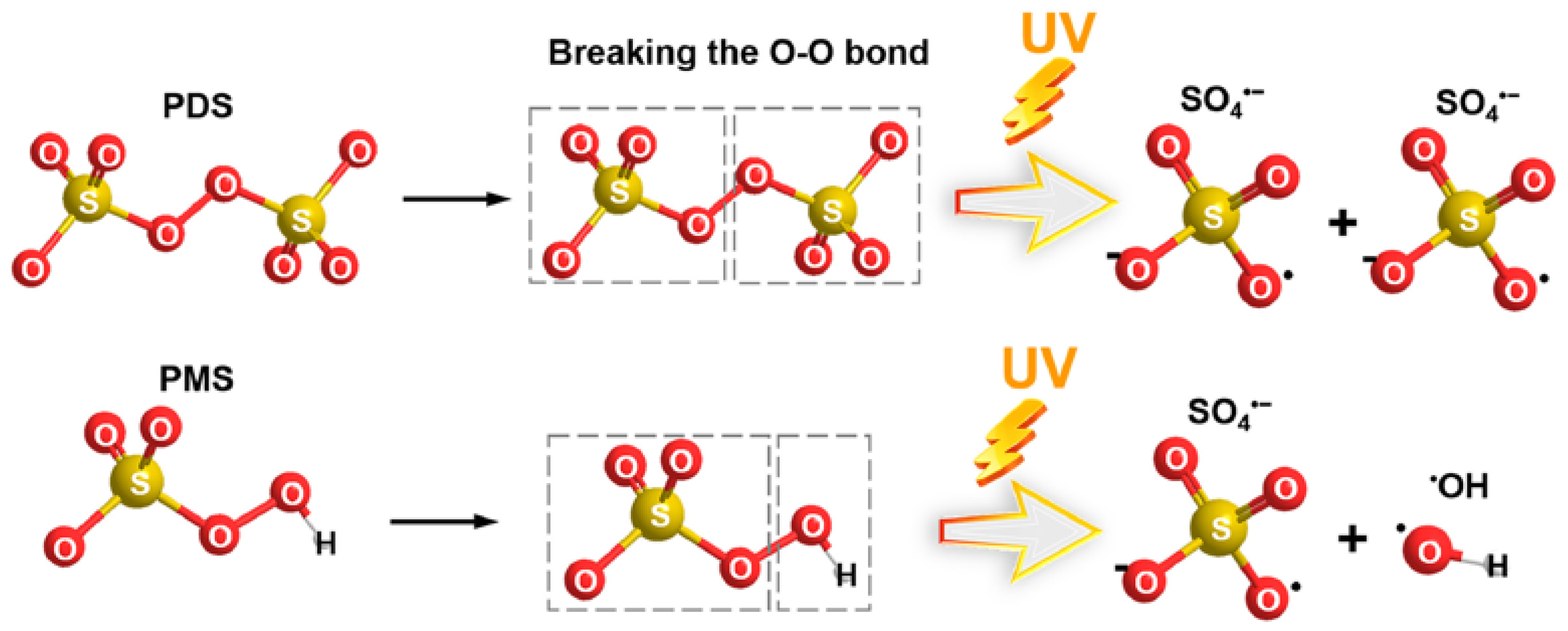
- The formation of active oxidizing radicals resulting from the cleavage of the peroxide bond (–O–O) in PDS and PMS (Equations (2) and (3), respectively) (Figure 10). Sulfate radicals can react with water or hydroxide ions, generating hydroxyl radicals (Equations (4) and (5), respectively). Finally, both SO4•− and •OH radicals promote the degradation of pollutants (Equation (6)), leading to smaller transformation products or complete mineralization into CO2 and water [78,104].
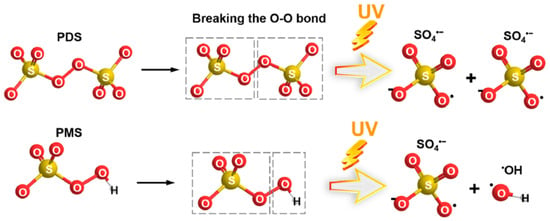 Figure 10. Activation of PDS and PMS by ultraviolet light. Adapted from [78] with permission of Elsevier (license 6023150870371).
Figure 10. Activation of PDS and PMS by ultraviolet light. Adapted from [78] with permission of Elsevier (license 6023150870371).
Pollutants + hν → Products
S2O82− + hν → 2 SO4•−
HSO5− + hν → SO4•− + •OH
SO4•− + H2O → SO42− + •OH + H+
SO4•− + OH− → SO42− + •OH
SO4•− or •OH + Pollutants → Products
6. Degradation of E2 and EE2 by Sulfate Radical-Based Processes
Several recent studies have investigated the removal of steroid hormones (E2 and EE2) through advanced oxidation processes (AOPs) involving persulfate (PDS) or peroxymonosulfate (PMS) (Table S4—Supplementary Materials) using the different activation methods previously discussed (light radiation, heat, ultrasound, and transition metals). The available studies revealed the broad effectiveness of these processes when properly activated. A significant portion of the studies employed metals (such as MnFe2O4, zero-valent iron, γ-MnOOH, or TiO2) to activate the oxidant, often in combination with UV or visible light. Examples include [32,75,105,106,121], where metal activation was combined with UV/PMS or UV/PDS for hormone degradation.
Some studies used UV radiation alone to activate persulfate (PDS) without needing metallic catalysts. Notable examples include [102,103,104], in which persulfate was activated solely by UV radiation (UVC, UVA, or UVB). The absence of metal catalysts in these systems avoids the generation of secondary metal residues, eliminating the need for the recovery and treatment of metals in the reaction medium. However, the literature still lacks more comprehensive studies focused on removing steroid hormones using processes based solely on photoactivation (such as UV/PS and UV/PMS), which limits a more robust assessment of this approach’s potential environmental and economic benefits.
Processes combining PDS with UV radiation (254–365 nm), hydrodynamic cavitation, or zero-valent iron (nZVI) have shown strong performance, achieving removal rates of up to 100% in treatment times ranging from 4 s to 60 min. PMS has also demonstrated high efficiency, yielding similarly notable results, including the complete removal of estrogenic activity, as reported in [105,121].
The diversity of tested matrices, including deionized water, treated wastewater, and animal farm effluents, demonstrates the robustness of sulfate radical-based AOPs under various operational conditions. These results highlight the potential of these processes as promising technologies for effectively removing estrogenic hormones from different types of wastewater.
The studies in Table S4 (Supplementary Materials) utilized hormone concentrations that were not representative of environmentally relevant conditions. Specifically, the reported concentrations in these studies ranged from micrograms per liter (µg/L) to milligrams per liter (mg/L). These findings indicate that sulfate radical-based processes effectively remove endocrine-disrupting compounds (EDCs), particularly steroid hormones, within this concentration range. However, this also underscores the need for future studies to adopt initial contaminant concentrations in the nanogram per liter (ng/L) range to better reflect the real environmental scenarios.
6.1. Main Operational Factors Influencing Hormone Removal from Wastewater by Advanced Oxidation Processes with Sulfate Radical Generation
Several variables are critical to the efficiency of sulfate radical-based processes, including the pH, concentration of certain anions, presence of organic matter, and the sample matrix. Some substances can act as scavengers of oxidizing radicals, negatively affecting the reaction. The concentrations of hormones and persulfate (PS) also significantly influence the reaction rate and hormone removal efficiency.
6.1.1. pH
pH directly affects hormone removal by influencing the generation of radicals (SO4•− and •OH). The influence of pH on removing 17β-estradiol using UV/PS in deionized water at pH values of 5, 7, 9, and 11 was studied [104]. A decrease in the kinetic rate constant (k) with increasing pH was observed, and the highest k was obtained at pH 5. Alkaline pH reduced the redox potential (E0) of the hydroxyl radical and decreased the efficiency of the E2-UV/PS reaction, which can be partially attributed to the deprotonation process of E2, whose pKa is 10.7.
17β-Estradiol removal using UV/PS under acidic and basic conditions, varying the pH from 3 to 11, was tested [102]. Hormone removal efficiency decreased as pH increased. The highest degradation rate was found at pH 3. Therefore, an acidic pH is recommended for UV/PS processes to enhance the removal efficiency. Additionally, the authors reported that under acidic conditions, SO4•− is the predominant radical species, and the faster degradation of E2 is due to the higher generation rate of SO4•− at lower pH.
Regarding PMS, E2 removal in deionized water via the Vis/PMS/Fe(II) process at pH 3, 5, 7, 8, and 11 was investigated [75]. The highest observed degradation rate constant (kobs) occurred at pH 3 and the lowest at pH 9. At pH 11, the kobs increased again, showing that for PMS, under the activation system used by the authors, an alkaline pH (11) can yield a degradation rate close to that observed at pH 3.
PMS activation using phosphorus-doped cow manure biochar (PBC500) for E2 degradation was examined in deionized water [110]. Experiments were conducted over a pH range of 3 to 11. Results showed that the E2 degradation efficiency decreased from 98.91% to 89.65% over 90 min as the pH increased from 3 to 11. Despite the decrease, the removal efficiency remained relatively high even at pH 11, indicating that the PBC500/PMS system was also effective under alkaline conditions.
In summary, pH is crucial in improving the advanced oxidation processes based on sulfate radicals for hormone removal. Acidic conditions generally enhance the generation of SO4•− radicals, leading to higher degradation rates of 17β-estradiol, as observed in multiple cited studies. Although some systems maintain a relatively high efficiency even at alkaline pH, such as PMS activated with specific catalysts like PBC500, the optimal removal is typically achieved under acidic conditions. Therefore, controlling the pH is essential to maximize the effectiveness of these treatment processes.
6.1.2. Temperature
The temperature dependence of E2 removal via UV/heat/PS was assessed using an E2 concentration of 3 mg/L and PS at 20 mg/L [102]. Five temperatures were tested (25, 35, 45, 55, and 65 °C). The authors showed that the degradation rate significantly increased with temperature, which can be expected following thermodynamic principles. At 25 °C, only 20% of E2 was degraded after 72 h (k = 0.0024 h−1), while complete degradation was achieved in 15 h at 65 °C (k = 0.4709 h−1). These results indicate that higher temperatures enhance SO4•− generation.
6.1.3. Ions Concentration
A significant positive impact on E2 degradation with PS in the presence of nitrate (NO3−) was reported [104]. At a concentration of 20 mg/L NO3−, the E2 degradation efficiency increased by 21.4% compared with the control. NO3− can generate oxygen radicals (O•−), which subsequently react with water to form hydroxyl radicals (•OH). Similarly, the increase in NO3− concentration enhanced the hormone degradation rates using PMS. Nitrate positively influenced E2 oxidation via SO4•− at concentrations up to 20 mg/L [75].
Regarding Cl−, low concentrations of Cl− (<500 mg/L) negatively affected the activity of both sulfate and hydroxyl radicals formed from PMS activation [75]. In contrast, there was a positive effect of Cl− at concentrations between 1000 and 1500 mg/L with PS, possibly due to the generation of Cl• radicals, which may eventually lead to the formation of •OH radicals [104]. However, at very high Cl− concentrations, complex chain reactions may be triggered, and Cl− may react simultaneously with radicals such as SO4•−, •OH, and Cl•, forming Cl2•−, which subsequently reacts to reform Cl−. Therefore, an adverse effect of Cl− may become noticeable at elevated concentrations [104].
6.1.4. Organic Matter Concentration
The E2, E1, and EE2 removal efficiency via UV/PS in two matrices, ultrapure water and wastewater effluent, was compared [103]. Dissolved carbon—particularly organic carbon in sewage—acted as the primary scavenger of hydroxyl and sulfate radicals, reducing the hormone removal efficiency. It was estimated that approximately 82% of hydroxyl radicals and 74% of sulfate radicals were consumed by organic matter in the effluent. This demonstrates that organic carbon in real matrices is the main factor reducing the efficiency of advanced oxidation processes.
The effect of natural organic matter (represented by humic acids) and turbidity on E2 removal via UV/PS at different concentrations (5 to 100 mg/L of humic acids and 5 to 100 NTU turbidity) was also assessed [104]. Both organic matter and turbidity can reduce the efficiency of E2 removal. The removal efficiency decreased by 43% at 100 NTU of turbidity, as turbidity inhibited E2 degradation by blocking UV light, thereby reducing SO4•− generation. Furthermore, at 100 mg/L of humic acids (approximately 100 NTU), the E2 removal rate dropped by about 98%.
Regarding the influence of organic matter on steroid hormone removal using PMS, humic acid concentrations up to 150 mg/L were tested to evaluate interference in E2 degradation via Vis/PMS/Fe(II) [75]. Results showed that increasing humic acid progressively reduced the hormone degradation efficiency. The inhibitory effect was caused by the competitive reaction between the steroid hormone and humic acids with reactive and non-radical species.
6.1.5. Oxidant-to-Hormone Ratio
PS concentrations ranging from 5 to 40 mg/L were tested for E2 removal via UV/PS, and it was found that increasing the PS concentration enhanced the E2 degradation rate [104]. Plotting k against PS concentration showed a linear increase in the constant rate with rising PS levels. Furthermore, keeping the E2 concentration fixed at 3 mg/L while increasing the PS from 5 to 40 mg/L led to a removal efficiency increase from 30% to 98% within 20 min. When varying the E2 concentration from 1 to 3 mg/L at a constant 40 mg/L PS, a decrease in the degradation rate was observed with rising hormone levels. The condition that yielded the highest E2 degradation was 40PS:1E2. PS concentrations ranging from 20 to 200 mg/L were tested for the treatment of 3 mg/L of E2 using a heat/photoactivation system, and a nearly complete removal was reported under the condition 200PS:3E2, equivalent to 66.67PS:1E2 [102]. Some studies have employed higher oxidant-to-hormone ratios that sometimes exceeded values of 100:1 [32,103,106,121].
6.1.6. UV Radiation Dose
Studies employing ultraviolet (UV) activation have tended to use lower oxidant-to-hormone ratios compared with those utilizing metal catalysts as activators. This can be considered as an advantage of light-based activation due to the reduced need for chemical reagents. However, to ensure that radiation use translates into cost benefits—especially concerning energy consumption—optimizing the light intensity and exposure time (i.e., the irradiation dose) is necessary for effective hormone degradation.
Another study used a significantly lower dose of just 45 mJ/cm2 [106]. This wide variation highlights the need for optimization studies of the UV/PS and UV/PMS irradiation doses to ensure efficient steroid hormone removal with minimal energy consumption and to avoid unnecessary costs.
7. Integrated Analysis of the Efficiency of Oxidative Processes with Sulfate Radical Formation for the Removal of E2 and EE2
7.1. Identification of Degradation Products
The identification of transformation products (TPs) formed during sulfate radical-based advanced oxidation processes in association with by-products, estrogenic activity, and toxicity evaluation is essential to ensure the environmental safety of treated water because while hormones like E2 and EE2 may be efficiently degraded, their by-products can exhibit estrogenic activity or present unknown toxic effects [105,122]. Identifying TPs also helps clarify the degradation mechanisms and optimize treatment conditions [75,105]. Nevertheless, despite this and the growing application of AOPs for the degradation of steroid hormones, few studies have provided detailed information on the degradation mechanisms and transformation products (TPs) generated during these processes.
This gap is partly attributed to the analytical challenges associated with the low concentrations, instability, and structural complexity of the transformation intermediates. The identification and structural elucidation of these compounds requires high-cost analytical tools, such as tandem LC-MS/MS and QTOF-MS (quadrupole time-of-flight mass spectrometry), which are essential for accurate characterization [75,87]. In the absence of such analyses, the actual effectiveness and safety of the treatment remain uncertain—especially when considering water reuse or discharge into sensitive environments—highlighting a critical and unresolved issue in the current literature.
Among the literature found on E2 degradation via sulfate radicals, the degradation mechanisms of 17β-estradiol (E2) using a MnFe2O4/g-C3N4 system activated by peroxymonosulfate (PMS) under visible light (MnF/PMS/Vis) was investigated, employing LC-MS/MS-QTOF analysis. After 30 to 45 min of oxidation, three main E2 transformation pathways were identified: (1) conversion to estrone (E1, m/z 270) via the dehydrogenation of ring A; (2) formation of 2-hydroxy estradiol (m/z 288) via hydroxylation at the C-2 position of the aromatic ring; and (3) generation of a ketone intermediate (m/z 285) through hydroxyl group oxidation [75].
Fourteen transformation products (TPs) were identified during the degradation of E2 using the Vis/PMS/Fe(II) system and high-resolution mass spectrometry (LC-HR-MS). However, only six key TPs were definitively identified due to multiple isomers, which hindered precise structural assignments. Although structural elucidation was partial, the study revealed that E2 degradation occurred through reactions promoted simultaneously by sulfate radicals (SO4•−) and hydroxyl radicals (•OH), both generated during PMS activation. These radicals mainly acted via hydrogen abstraction at allylic positions of the E2 aliphatic ring, forming ketones, hydroquinones, and quinones and promoting ring-opening reactions and subsequent mineralization [105].
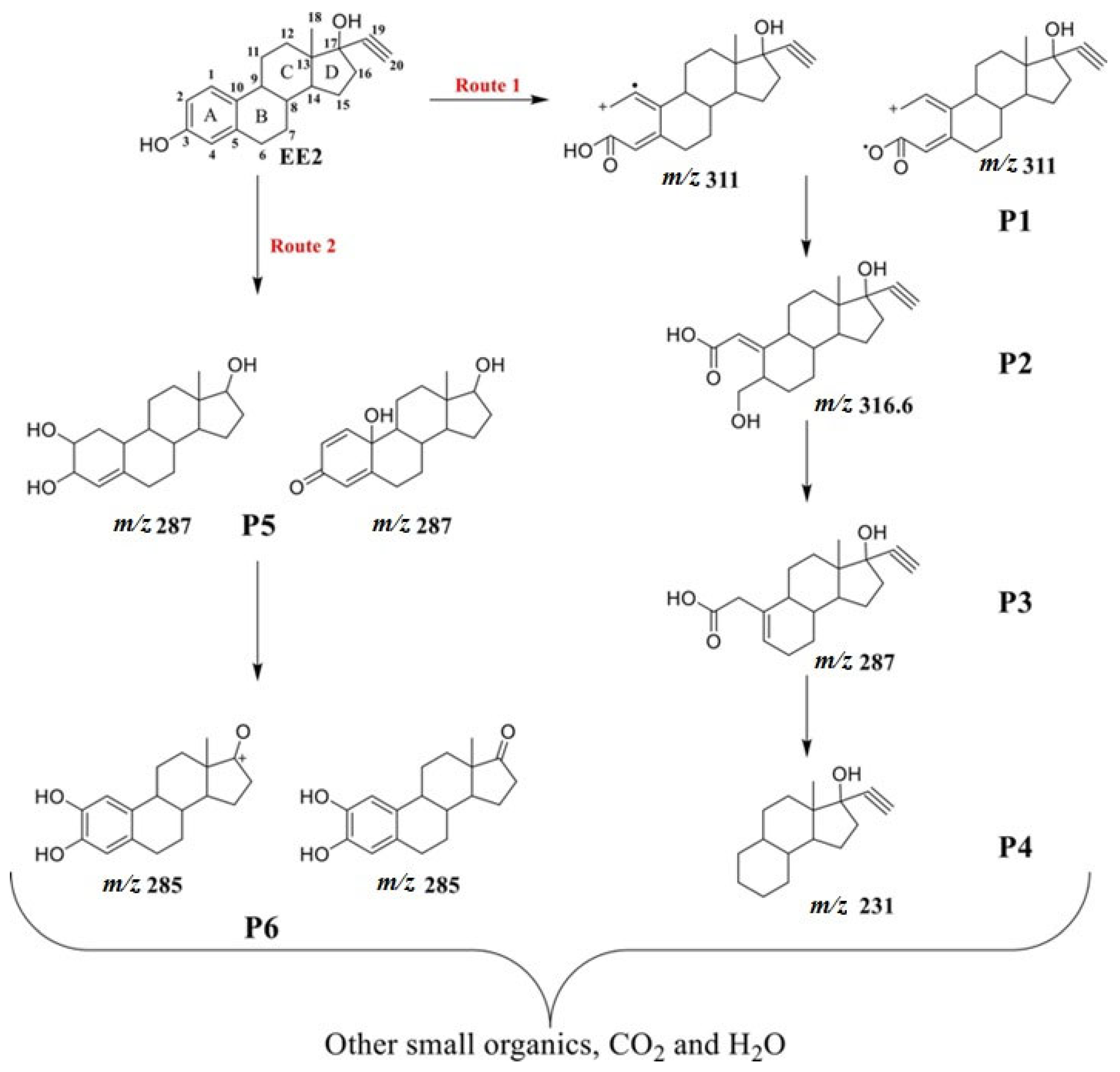
For EE2, degradation intermediates were investigated using persulfate activated with Pg-C3N4/RGO/BiOI under visible light. High-resolution mass spectrometry (Waters Acquity UPLC I-Class with Xevo G2-XS QTOF) was employed, identifying six intermediates. Based on the reactive species and degradation products, two predominant degradation pathways for EE2 were proposed (Figure 11). In the first route, oxidants attack C-2—a highly electron-dense site on the EE2 molecule—leading to the formation of an initial intermediate (P1, m/z 311), which is subsequently oxidized and undergoes dehydration and decarbonylation reactions, forming lower molecular weight compounds (P2 to P4). In the second pathway, the initial oxidative attack produces a hydroxylated intermediate that generates P5 (m/z 287) and P6 (m/z 285) after successive dehydrogenation and dealkylation steps. These intermediates are eventually mineralized into CO2 and H2O [122].
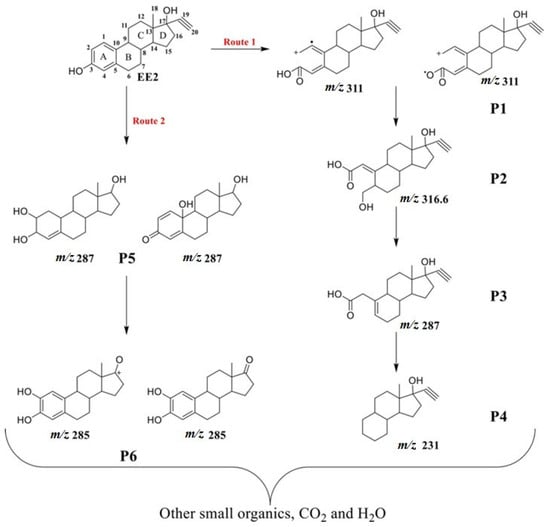
Figure 11.
Degradation pathways for EE2 by oxidation using persulfate activated with Pg-C3N4/RGO/BiOI under visible light [122]. With permission of Elsevier (license 6027760431042).
The results reported in this section collectively emphasize the essential role of identifying transformation products (TPs) in evaluating the actual effectiveness and safety of sulfate radical-based advanced oxidation processes (SR-AOPs) for hormone degradation. Key intermediates of E2 and EE2 were identified, and their formation was linked to the reduction in estrogenic activity [105,122]. Degradation pathways for E2 and 17α-methyltestosterone were proposed based on the identified TPs, and ecotoxicological evaluations were conducted, showing that the treated water had a reduced impact on seed germination and mammalian cell viability [75]. Together, these findings reinforce that degradation efficiency alone is not a sufficient metric of process success—comprehensive TP identification, estrogenic activity, and toxicity testing are critical to ensure that SR-AOPs do not generate persistent or harmful by-products.
7.2. Assessment of Estrogenic Activity
Quantifying the estrogenic activity after hormone degradation by sulfate radical-based advanced oxidation processes is essential to ensure that the transformation products no longer pose a hormonal risk. While parent compounds such as E2 and EE2 can be efficiently removed, their degradation may yield intermediates that retain or even enhance the endocrine-disrupting potential. Despite this, the literature has underexplored the evaluation of residual estrogenic activity. Many studies on hormone removal through sulfate radical formation processes did not assess the estrogenic activity of the transformation products formed [32,102,106,121]. Among the exceptions, one study stands out in which the estrogenic activity of intermediates generated during the oxidation of EE2 with persulfate activated by Pg-C3N4/RGO/BiOI under visible light was investigated. Using MCF-7 breast cancer cells, a complete elimination of estrogenic activity after treatment was reported, demonstrating the effectiveness of the process not only in removing the parent compound, but also in neutralizing its endocrine-disrupting potential [122].
The in vitro YES (yeast estrogen screen) assay was employed to monitor the reduction in estrogenic activity during E1, E2, and EE2 (0.5 µM each) degradation in ultrapure water using the UVA/UVB/PS system. A substantial decrease in estrogenic activity, greater than 97%, was reported after 3 h of treatment. The concentrations of hormones post-treatment accounted for approximately 3% of the initial estrogenic activity. It was impossible to determine whether this percentage was due to the persistence of estrogenic activity, possibly caused by degradation by-products, proximity to the quantification limit (~15 nM), or experimental contamination [103].
The estrogenic activity of E2 in effluent treated with UV–Vis/PMS/Fe(II) was also evaluated. Estrogenic activity was measured using cell lines expressing the estrogen receptor alpha (ERα). Initially, a preliminary PMS/Fe(II) test without ultraviolet radiation was conducted. After 15 min of oxidation, the estrogenic activity corresponding to the concentration resulting in 50% of the maximum luciferase activity (EC50 = 1.5 × 10−10 M) remained close to that of the initial solution (EC50 = 6 × 10−10 M). The authors concluded that oxidation without UV radiation was ineffective in reducing estrogenic activity, even though 35% of the initial E2 concentration had already degraded. This suggests that one or more E2 degradation by-products maintained an estrogenic activity level similar to the parent compound [105].
The phenolic group is essential for receptor binding and the estrogenic activity of all steroidal estrogens. Thus, the results reported indicate that the phenolic moiety of E2 is preserved, which may have hindered the conversion of hydroquinone-like structures into quinones. In contrast, after a few minutes of irradiation, the luciferase activity dropped significantly, approaching the basal level of the cell line, suggesting that the treated effluent no longer exhibited estrogenic activity [105].
The efficiency of the Co–SA/CMN/PMS system in degrading 17β-estradiol (E2) and eliminating its residual estrogenic activity was evaluated. A yeast-based estrogen receptor assay showed reduced estrogenic activity from 24.7 to 0.2 ng-E2 eq/L after only 8 min of treatment, indicating the near-complete neutralization of endocrine-disrupting potential. This reduction was attributed to structural modifications in the transformation products, particularly in the phenolic region responsible for hormonal interaction [123].
The results presented in this section using bioassays, such as YES, MCF-7 cell assays, and HELN ERα, demonstrate that advanced oxidation processes based on sulfate radical formation can remove E2 and EE2 and significantly reduce the estrogenic activity in treated samples, allowing for a reliable confirmation of treatment effectiveness beyond chemical degradation.
7.3. Assessment of By-Product Toxicity
Evaluating the toxicity of degradation products formed during hormone oxidation is essential, as many by-products may retain or even increase their toxicity despite removing the parent compound [103]. Studies have shown that the oxidation of 17β-estradiol can lead to the formation of quinones, whose toxicity and hormonal impact are not yet fully understood [105]. Since hormones are active at very low concentrations, treatments must not generate compounds that remain biologically active or toxic [121].
Without a toxicity evaluation, the environmental safety of the process is compromised, limiting the practical application of advanced oxidation processes in real wastewater systems. Despite this, the evaluation of by-product toxicity following advanced oxidation processes (AOPs) based on sulfate radical generation remains scarce in the literature, as many studies have focused solely on quantifying the removal of the original pollutant without considering the potentially toxic effects of the transformation products formed during treatment [32,102,106,121], thus representing an important gap in the literature.
In this context, the residual toxicity after treating 17β-estradiol (E2) using the Co–SA/CMN/PMS system was assessed [123]. Chinese hamster ovary (CHO) cells were used as a biological model. An integrated approach based on cellular biomarkers was applied including cytotoxicity analysis, intracellular oxidative stress (via quantification of reactive oxygen species—ROS), DNA damage (through γ-H2AX expression), and oxidative DNA damage (measured by 8-OH(d)G). The results revealed that prior to treatment, E2 induced severe cytotoxic effects, reducing the cell viability to only 3%, along with high levels of ROS and DNA damage. After 8 min of treatment, the toxicity was drastically reduced, with the cell viability restored to 98%, a significant decrease in ROS levels, and virtually no evidence of genotoxic damage. These findings demonstrate that the process not only removed E2, but also eliminated its associated toxicity, highlighting the environmental and biological efficacy of the proposed system [123].
Residual toxicity following the MnF/PMS/Vis process was also evaluated. Two biological models were employed: rice seeds (Oryza sativa L.) and cell cultures including Eker leiomyoma tumor-3 (ELT3) and mouse Sertoli TM4 cells. In the germination assay, seeds were exposed to different concentrations of treated water, and parameters such as shoot and root length and seedling dry weight indicated that the treatment did not negatively affect plant development. In parallel, the in vitro cytotoxicity analysis using ELT3 (uterine tumor) and TM4 (mouse Sertoli) cells showed that the treated water did not cause significant toxic effects, with a high cell viability observed through optical density measurements and cellular morphology. The results indicate that the PMS-based process effectively removed contaminants and neutralized their residual toxicity [75].
The findings discussed in this section highlight that advanced oxidation processes involving sulfate radicals effectively eliminate E2 and EE2 and substantially minimize the toxicity of the treated samples.
8. Summary of Key Findings
- The steroid hormones 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) are emerging contaminants with high estrogenic potential and are frequently detected in aquatic environments, even after conventional wastewater treatment.
- Advanced oxidation processes (AOPs) are promising alternatives for the degradation of E2 and EE2, overcoming the limitations of conventional (biological) and physical treatment methods.
- Among the advanced oxidation processes (AOPs) investigated, those based on hydroxyl radicals, such as ozonation, photocatalysis, and Fenton reactions, demonstrated high hormone removal efficiencies, often exceeding 99%. These results, however, are strongly influenced by several operational and environmental variables including the oxidant concentration, pH, matrix composition, and the presence of organic matter, among others.
- AOPs involving sulfate radical (SO4•−) formation have gained increasing attention recently due to their higher redox potential (2.5–3.1 V), longer half-life, and effectiveness across a broader pH range than hydroxyl radicals. These processes employ activation methods such as UV radiation, heat, ultrasound, and transition metals to activate salts like persulfate (PDS) and peroxymonosulfate (PMS), generating SO4•− radicals capable of efficiently degrading persistent compounds such as E2 and EE2.
- Several studies have reported the 100% removal of E2 and EE2 using sulfate radical-based AOPs, demonstrating the potential of these processes to eliminate endocrine-disrupting compounds. Like in AOPs based on hydroxyl radicals, these results are strongly influenced by several operational and environmental variables including the oxidant concentration, pH, matrix composition, and the presence of organic matter, among others.
- Despite their potential, sulfate radical-based AOPs still represent a small portion of the published research but have consistently grown since 2015, indicating increasing academic interest and promising future applications.
9. Gaps in the Literature
- Although the photoactivation of persulfate (PS) and peroxymonosulfate (PMS) offers significant advantages, such as avoiding the generation of metal residues, few studies have assessed the exclusive use of photoactivation—without added metals—for the removal of the steroid hormones 17β-estradiol (E2) and 17α-ethinylestradiol (EE2).
- In the context of photoactivation, studies are not exploring alternative light sources beyond conventional mercury UV lamps such as UV-LEDs and solar energy.
- Key operational parameters directly influencing process efficiency, such as pH, oxidant-to-hormone ratio, radiation source selection (UVA, UVB, or UVC), and irradiation dose, have not been sufficiently investigated or optimized.
- Most studies on the degradation of E2 and EE2 were conducted using concentrations that are not environmentally relevant, typically in the mg/L or µg/L range. There is a notable scarcity of research addressing degradation at environmentally relevant levels (ng/L). This can be attributed to the need for more sensitive analytical methods to detect hormones at such low concentrations.
- Real-scale applications using complex real wastewater under varying operational conditions are scarce, limiting our understanding of the effectiveness of AOPs in the presence of dissolved organic matter, salts and pH fluctuations in real effluents.
- There is a significant gap in the integral evaluation of the processes: identifying degradation products and assessing the residual estrogenic activity and toxicity of the by-products, particularly in sulfate radical processes like UV/PS and UV/PMS.
- Although numerous studies have demonstrated the technical efficiency and kinetics of sulfate radical-based advanced oxidation processes (SR-AOPs) for the removal of hormones such as E2 and EE2, no comprehensive economic feasibility assessments—particularly at pilot or full scale—were identified in the existing literature for these processes. Economic and energy efficiency assessments of oxidative processes based on sulfate radicals are lacking, which hinders the evaluation of their feasibility for large-scale implementation in wastewater treatment plants.
10. Challenges and Prospects for Large-Scale Application
10.1. Future Research and Opportunities for Improvement
Technological advancements are essential for the large-scale application of sulfate radical-based advanced oxidation processes (SR-AOPs). Progress has been made in developing novel catalysts and reactor configurations at the laboratory scale, which aim to enhance the efficiency, scalability, and overall feasibility of these treatment systems. Transition metal oxides, such as MnFe2 O4 [75] and manganite (γ-MnOOH) [121], have been investigated and have shown high efficiency in PMS and PDS activation, achieving the effective degradation of estrogens like E2 and EE2.
Carbon-based composite materials, including phosphorus-doped biochar [110] and g-C3N4-based photocatalysts [75,122], have also been studied for their dual role as adsorbents and radical activators, with the added advantage of low metal leaching and a reduced toxicity of transformation products [75,110,122]. Furthermore, immobilized catalysts, such as TiO2 incorporated into PVDF membranes, have been proposed to enhance catalyst stability, reusability, and compatibility with membrane-based systems [106].
Regarding the reactor design, photocatalytic membrane reactors have been developed to integrate light irradiation and catalytic oxidation within a compact and continuous system, reducing catalyst loss [106]. UV-LED photoreactors are considered as a promising energy-efficient alternative to traditional mercury UV lamps and may offer potential for decentralized or small-scale water treatment applications involving UV/PMS or UV/PS systems, although their use in sulfate radical-based processes remains mainly unexplored [15,117]. In addition, solar-driven SR-AOP configurations have been proposed as low-cost and sustainable options, especially in regions with high solar irradiance, reducing the energy demands while maintaining high treatment efficiency [102,105].
These developments demonstrate that material innovations and reactor engineering are critical in advancing sulfate radical-based advanced oxidation processes (SR-AOPs) toward practical, full-scale implementation. Further research is needed to advance SR-AOPs, with opportunities for improvement in both the development of novel catalysts and catalytic membranes as well as in the design of photoreactors employing alternative, cost-effective, or renewable energy sources such as solar irradiation.
In addition, few studies have evaluated the formation of by-products and the removal of estrogenic activity and residual toxicity in an integrated manner. Some exceptions reported the identification of degradation products [75,122], the elimination of estrogenic activity following hormone degradation [103,123], and the reduction or elimination in toxicity associated with these compounds [75,123]. Most studies are still limited to removing the original compound, highlighting a significant gap in the comprehensive efficiency evaluation. This creates an excellent opportunity for new studies that provide a more integrated process assessment.
Furthermore, several studies employed varying oxidant-to-hormone ratios, irradiation doses, and pH ranges, therefore, a systematic approach to simultaneously optimize these parameters remains lacking. Such optimization is essential not only to maximize the removal efficiency of steroid hormones, but also to ensure the economic feasibility of the process. The absence of optimized strategies may lead to excessive reagent use and high energy consumption, particularly due to the reliance on artificial light sources. In this context, conducting optimization studies that integrate treatment efficiency with operational sustainability presents a valuable opportunity for enhancing advanced oxidation processes, thereby promoting their practical applicability on a large scale in wastewater treatment plants.
10.2. Difficulties in Implementation in Industrial Wastewater Treatment Plants
Despite the proven effectiveness of advanced oxidative processes based on sulfate radicals in the degradation of endocrine disruptors, such as 17β-estradiol (E2) and 17α-ethinylestradiol (EE2), on a laboratory scale, their application on a large scale faces challenges. One of the main obstacles is the complexity of real matrices such as effluents from industrial wastewater treatment plants. Some studies have observed that the degradation efficiency is significantly reduced in the presence of natural organic matter, turbidity, and inorganic salts—typical components of municipal and industrial effluents [75,104]. For example, the degradation rate of E2 was shown to decrease from 0.140 min−1 in deionized water to 0.001 min−1 in effluent from a wastewater treatment plant, indicating substantial matrix interference [104].
Using ultraviolet radiation sources, combined with high dosages of oxidants such as peroxymonosulfate (PMS) and persulfate (PDS), can significantly increase the operating and energy costs of the processes. In some studies, such as one in which radiation doses of up to 1800 J/cm2 were applied, significant challenges were highlighted regarding the feasibility of implementation in full-scale continuous systems [102]. Additionally, oxidant-to-hormone ratios exceeding 100:1 have been reported, with a ratio of 200:1 used in one case, indicating high reagent consumption [121]. Although catalytic systems with materials such as nZVI/PGBC or MnFe2O4/g-C3N4 are highly efficient at degrading steroid hormones, aspects such as the cost of synthesis, structural stability, and the reuse of catalysts still need to be improved [32,75]. Thus, it is essential to carry out studies that address the technical-economic optimization and energy efficiency of these processes to make their practical application in wastewater treatment plants feasible.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13071949/s1, Table S1. Biological processes used to remove E2 and EE2. Table S2. Summary of Advanced Oxidative Processes for degradation of E2 and EE2 in aqueous matrices. Table S3. Advantages and limitations of Advanced Oxidation Processes observed for the degradation of E2 and EE2. Table S4. Sulfate radical-based processes applied to E2 and EE2 degradation. Figure S1. Main activation mechanisms of PDS/PMS. Refs. [124,125,126,127,128,129] are cited in the Supplementary Materials.
Author Contributions
C.S.S.T.—Writing and Editing; D.M.B.—Supervision, Review and Editing; J.C.C.—Conceptualization, Supervision, Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AOP | Advanced oxidation process |
| Al-ZnO/Fe | Aluminum-Zinc Oxide with Iron |
| BSA | Bovine serum albumin |
| C/C0 | Final/initial concentration ratio |
| CAS | Chemical Abstracts Service (Registry Number) |
| COD | Chemical oxygen demand |
| Cl•, Cl2•− | Chlorine radicals |
| D5 | Decamethylcyclopentasiloxane |
| DEs | Endocrine disruptors |
| E1 | Estrone |
| E2 | 17β-Estradiol |
| E3 | Estriol |
| EE2 | 17α-Ethinylestradiol |
| ED | Endocrine disruptor |
| EDC | Endocrine-disrupting compound |
| EPA/USEPA | United States Environmental Protection Agency |
| EU | European Union |
| EfOM | Extracellular organic matter |
| Fe2+ | Ferrous iron |
| Fe3+ | Ferric iron |
| HO•/•OH | Hydroxyl radical |
| HRT | Hydraulic retention time |
| H2O2 | Hydrogen peroxide |
| KMnO4 | Potassium permanganate |
| LD | Limit of detection |
| LED | Light emitting diode |
| LQ | Limit of quantification |
| MBBR | Moving bed biofilm reactor |
| MBR | Membrane bioreactor |
| N20BiOBr | Nitrogen-doped bismuth oxybromide |
| NCBI | National Center for Biotechnology Information |
| NaAH | Sodium aldehyde |
| NaAg | Sodium alginate |
| O.M | Organic matter |
| PDS | Peroxydisulfate |
| PMS | Peroxymonosulfate |
| PS | Persulfate |
| ppmv | Parts per million by volume (GC-MS) |
| PVDF | Polyvinylidene fluoride |
| SO4•− | Sulfate radical |
| SR-AOPs | Sulfate radical-based advanced oxidation processes |
| TiO2 | Titanium dioxide |
| UKEA | United Kingdom Environment Agency |
| US | Ultrasound/sonolysis |
| UV | Ultraviolet |
| UVC-LED | Ultraviolet-C light emitting diode |
| VTG | Vitellogenin |
| WHO | World Health Organization |
| k | Reaction rate constant |
| mg/L, µg/L, ng/L | Concentration units (milligrams, micrograms, nanograms per liter) |
References
- Bila, D.M.; Dezotti, M. Desreguladores endócrinos no meio ambiente: Efeitos e conseqüências. Quim. Nova 2007, 30, 651–666. [Google Scholar] [CrossRef]
- Luo, L.; Meng, D.; He, L.; Wang, X.; Xia, L.; Pan, X.; Jiang, F.; Wang, H.; Dai, J. Photocatalytic activation of peroxydisulfate by a new porous g-C3N4/reduced graphene oxide/TiO2 nanobelts composite for efficient degradation of 17α-ethinylestradiol. Chem. Eng. J. 2022, 446, 137325. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Assessment of the State-of-the-Science of Endocrine Disruptors; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- US Environmental Protection Agency (USEPA). A Review of the Reference Dose and Reference Concentration Processes; USEPA: Washington, DC, USA, 2002. [Google Scholar]
- Interdonato, L.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R. Endocrine Disruptor Compounds in Environment: Focus on Women’s Reproductive Health and Endometriosis. Int. J. Mol. Sci. 2023, 24, 5682. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, X.; Lin, H.; Lin, X.; Mo, J.; Chen, C.; Dai, X.; Liao, D.; Gao, C.; Li, Y. Occurrence and distribution of natural and synthetic progestins, androgens, and estrogens in soils from agricultural production areas in China. Sci. Total Environ. 2021, 751, 141766. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Deich, C.; Frazão, H.C.; Appelt, J.S.; Li, W.; Pohlmann, T.; Waniek, J.J. Occurrence and distribution of estrogenic substances in the northern South China Sea. Sci. Total Environ. 2021, 770, 145239. [Google Scholar] [CrossRef]
- Cédat, B.; de Brauer, C.; Métivier, H.; Dumont, N.; Tutundjan, R. Are UV photolysis and UV/H 2 O 2 process efficient to treat estrogens in waters? Chemical and biological assessment at pilot scale. Water Res. 2016, 100, 357–366. [Google Scholar] [CrossRef]
- Gorito, A.M.; Pesqueira, J.F.J.R.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Nunes, O.C.; Almeida, C.M.R.; Silva, A.M.T. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 2021, 9, 10–14. [Google Scholar] [CrossRef]
- European Commission Community Strategy for Endocrine Disrupters—A Range of Substances Suspected of Interfering with the Hormone Systems of Humans and Wildlife. Community Strateg Endocr Disrupters a Range Subst Suspected Interf with Horm Syst Humans Wildlife Commun from Comm to Counc Eur Parliam COM 706 Final. 1999. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:51999DC0706&from=EN (accessed on 12 May 2025).
- Werkneh, A.A.; Gebru, S.B.; Redae, G.H.; Tsige, A.G. Removal of endocrine disrupters from the contaminated environment: Public health concerns, treatment strategies and future perspectives—A review. Heliyon 2022, 8, e09206. [Google Scholar] [CrossRef]
- Astuti, M.P.; Rangsivek, R.; Padhye, L.P. Laboratory and pilot-scale UV, UV/H2O2, and granular activated carbon (GAC) treatments for simultaneous removal of five chemicals of emerging concerns (CECs) in water. J. Water Process Eng. 2022, 47, 102730. [Google Scholar] [CrossRef]
- He, H.; Huang, B.; Gu, L.; Xiong, D.; Lai, C.; Tang, J.; Pan, X. Stimulated dissolved organic matter by electrochemical route to produce activity substances for removing of 17α-ethinylestradiol. J. Electroanal. Chem. 2016, 780, 233–240. [Google Scholar] [CrossRef]
- Silva, L.G.R.; Costa, E.P.; Starling, M.C.V.M.; dos Santos Azevedo, T.; Bottrel, S.E.C.; Pereira, R.O.; Sanson, A.L.; Afonso, R.J.C.F.; Amorim, C.C. LED irradiated photo-Fenton for the removal of estrogenic activity and endocrine disruptors from wastewater treatment plant effluent. Environ. Sci. Pollut. Res. 2021, 28, 24067–24078. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ouyang, T.; Lu, G.; Li, M.; Li, Y.; Hou, J.; He, C.; Gao, P. Ecosystem risk-based prioritization of micropollutants in wastewater treatment plant effluents across China. Water Res. 2024, 263, 122168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Tao, Y.; Wu, W.; Zeng, Y.; Liao, K.; Li, X.; Chen, L. Transcriptomic and Physiological Responses of Chlorella pyrenoidosa during Exposure to 17α-Ethinylestradiol. Int. J. Mol. Sci. 2022, 23, 3583. [Google Scholar] [CrossRef]
- Rodrigues, S.; Silva, A.M.; Antunes, S.C. Assessment of 17α-ethinylestradiol effects in Daphnia magna: Life-history traits, biochemical and genotoxic parameters. Environ. Sci. Pollut. Res. 2021, 28, 23160–23173. [Google Scholar] [CrossRef]
- Huang, B.; Tang, J.; He, H.; Gu, L.; Pan, X. Ecotoxicological effects and removal of 17β-estradiol in chlorella algae. Ecotoxicol. Environ. Saf. 2019, 174, 377–383. [Google Scholar] [CrossRef]
- Li, S.; Chu, R.; Hu, D.; Yin, Z.; Mo, F.; Hu, T.; Liu, C.; Zhu, L. Combined effects of 17β-estradiol and copper on growth, biochemical characteristics and pollutant removals of freshwater microalgae Scenedesmus dimorphus. Sci. Total Environ. 2020, 730, 138597. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, J.; Meng, S.; Chen, J.; Gu, Z. Testicular transcriptome alterations in zebrafish (Danio rerio) exposure to 17Β-estradiol. Chemosphere 2019, 218, 14–25. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Banihashemi, B.; Droste, R.L. Sorption-desorption and biosorption of bisphenol A, triclosan, and 17α-ethinylestradiol to sewage sludge. Sci. Total Environ. 2014, 487, 813–821. [Google Scholar] [CrossRef]
- Metcalf & Eddy, Inc. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Liu, S.; Véron, E.; Lotfi, S.; Fischer, K.; Schulze, A.; Schäfer, A.I. Poly(vinylidene fluoride) membrane with immobilized TiO2 for degradation of steroid hormone micropollutants in a photocatalytic membrane reactor. J. Hazard. Mater. 2023, 447, 130832. [Google Scholar] [CrossRef] [PubMed]
- Perondi, T.; Michelon, W.; Junior, P.R.; Knoblauch, P.M.; Chiareloto, M.; de Fátima Peralta Muniz Moreira, R.; Peralta, R.A.; Düsman, E.; Pokrywiecki, T.S. Advanced oxidative processes in the degradation of 17β-estradiol present on surface waters: Kinetics, byproducts and ecotoxicity. Environ. Sci. Pollut. Res. 2020, 27, 21032–21039. [Google Scholar] [CrossRef] [PubMed]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Bennett, G.F. Endocrine Disrupters in Wastewater and Sludge Treatment Processes. J. Hazard. Mater. 2003, 100, 317–318. [Google Scholar] [CrossRef]
- Lintelmann, J.; Katayama, A.; Kurihara, N.; Shore, L.; Wenzel, A. Endocrine disruptors in the environment: (IUPAC technical report). Pure Appl. Chem. 2003, 75, 631–681. [Google Scholar] [CrossRef]
- Darbre, P.D. What Are Endocrine Disrupters and Where Are They Found? Endocrine Disruption and Human Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 3–26. [Google Scholar] [CrossRef]
- Ding, J.; Xu, W.; Liu, S.; Liu, Y.; Tan, X.; Li, X.; Li, Z.; Zhang, P.; Du, L.; Li, M. Activation of persulfate by nanoscale zero-valent iron loaded porous graphitized biochar for the removal of 17β-estradiol: Synthesis, performance and mechanism. J. Colloid Interface Sci. 2021, 588, 776–786. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Z.; Luo, Z.; Li, H.; Chen, G. Endocrine disrupting chemicals in wild freshwater fishes: Species, tissues, sizes and human health risks. Environ. Pollut. 2019, 244, 462–468. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, J.; Gu, Z.; Yang, G.; Li, T.; Chen, J. Transcriptome alterations in female Daphnia (Daphnia magna) exposed to 17β-estradiol. Environ. Pollut. 2020, 261, 114208. [Google Scholar] [CrossRef]
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 2014, 69, 104–119. [Google Scholar] [CrossRef]
- Klaic, M.; Jirsa, F. 17α-Ethinylestradiol (EE2): Concentrations in the environment and methods for wastewater treatment—An update. RSC Adv. 2022, 12, 12794–12805. [Google Scholar] [CrossRef] [PubMed]
- Volkova, K.; Reyhanian Caspillo, N.; Porseryd, T.; Hallgren, S.; Dinnétz, P.; Porsch-Hällström, I. Developmental exposure of zebrafish (Danio rerio) to 17α-ethinylestradiol affects non-reproductive behavior and fertility as adults, and increases anxiety in unexposed progeny. Horm. Behav. 2015, 73, 30–38. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (NCBI). PubChem Compound Summary for CID 5757, Estradiol 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Estradiol (accessed on 12 May 2025).
- National Center for Biotechnology Information (NCBI). PubChem Compound Summary for CID 5991, Ethinyl Estradiol 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ethinyl-Estradiol (accessed on 12 May 2025).
- Bertoldi, C.; Rodrigues, A.G.; Fernandes, A.N. Removal of endocrine disrupters in water under artificial light: The effect of organic matter. J. Water Process Eng. 2019, 27, 126–133. [Google Scholar] [CrossRef]
- Bilal, M.; Rizwan, K.; Adeel, M.; Barceló, D.; Awad, Y.A.; Iqbal, H.M.N. Robust strategies to eliminate endocrine disruptive estrogens in water resources. Environ. Pollut. 2022, 306, 119373. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stumpf, M.; Mueller, J.; Haberer, K.; Wilken, R.D.; Servos, M. Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany, Canada and Brazil. Sci. Total Environ. 1999, 225, 81–90. [Google Scholar] [CrossRef]
- He, J.; Guo, J.; Zhou, Q.; Yang, J.; Fang, F.; Huang, Y. Analysis of 17A-ethinylestradiol and bisphenol A adsorption on anthracite surfaces by site energy distribution. Chemosphere 2019, 216, 59–68. [Google Scholar] [CrossRef]
- Karim, S.; Bae, S.; Greenwood, D.; Hanna, K.; Singhal, N. Degradation of 17A-ethinylestradiol by nano zero valent iron under different pH and dissolved oxygen levels. Water Res. 2017, 125, 32–41. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, K.; Deng, Y. Occurrence and photochemical degradation of 17α-ethinylestradiol in Acushnet River Estuary. Chemosphere 2006, 63, 1583–1590. [Google Scholar] [CrossRef]
- Aydin, E.; Talinli, I. Analysis, occurrence and fate of commonly used pharmaceuticals and hormones in the Buyukcekmece Watershed, Turkey. Chemosphere 2013, 90, 2004–2012. [Google Scholar] [CrossRef]
- Kiran Kumar, A.; Sarma, P.N.; Venkata Mohan, S.R. Incidence of selected endocrine disrupting estrogens in water bodies of Hyderabad and its relation to water quality parameters. Environ. Eng. Manag. J. 2016, 15, 315–325. [Google Scholar] [CrossRef]
- Martínez, N.A.; Schneider, R.J.; Messina, G.A.; Raba, J. Modified paramagnetic beads in a microfluidic system for the determination of ethinylestradiol (EE2) in river water samples. Biosens. Bioelectron. 2010, 25, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Teresa Lima do Nascimento, M.; Dalva de Oliveira Santos, A.; Lima da Cunha, D.; da Cruz Felix, L.; Gomes Moreira da Silva, G.; Ann Hauser-Davis, R.; Monteiro da Fonseca, E.; Maia Bila, D.; Antônio Baptista Neto, J. Estrogenic Activity and Endocrine Disruptor Compounds Determined in Guanabara Bay (Brazil) by Yeast Estrogen Screen Assays and Chemical Analyses Atividade Estrogênica e Desregulado. Anuário Do Inst. De Geociências Univ. Fed. Do Rio De Jan. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Montagner, C.C.; Jardim, W.F. Spatial and seasonal variations of pharmaceuticals and endocrine disruptors in the Atibaia River, São Paulo State (Brazil). J. Braz. Chem. Soc. 2011, 22, 1452–1462. [Google Scholar] [CrossRef]
- Gomes, J.P. Análise da Ocorrência do Hormônio Estrogênico, 17α-Etinilestradiol, no Lago Paranoá; Fundação Oswaldo Cruz, Escola Nacional de Saúde Pública Sergio Arouca: Brasília, Brazil, 2018. [Google Scholar]
- European Union. European Commission Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/ECof the European Parliament of the Council. Off. J. Eur. Union 2015, L78, 40–42. [Google Scholar]
- European Union. European Parliament and Council Directive 2013/39/EU of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, L226, 1–17. [Google Scholar]
- European Commission. Proposal for a Directive of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (Recast); European Commission: Brussels, Belgium, 2018. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st and 2nd addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Abdullah, M.S.; Goh, P.S.; Ismail, A.F.; Hasbullah, H. The Treatment of Endocrine-Disruptive Chemicals in Wastewater through Asymmetric Reverse Osmosis Membranes: A Review. Symmetry 2023, 15, 1049. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Saracevic, E.; Kreuzinger, N. Adsorption of bisphenol-A, 17β-estradiole and 17α- ethinylestradiole to sewage sludge. Chemosphere 2004, 56, 843–851. [Google Scholar] [CrossRef]
- Petrie, B.; McAdam, E.J.; Lester, J.N.; Cartmell, E. Assessing potential modifications to the activated sludge process to improve simultaneous removal of a diverse range of micropollutants. Water Res. 2014, 62, 180–192. [Google Scholar] [CrossRef]
- Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; bin Mohd Nor, M.Z.; binti Azis, R.S. Recent advances in the rejection of endocrine-disrupting compounds from water using membrane and membrane bioreactor technologies: A review. Polymers 2021, 13, 392. [Google Scholar] [CrossRef]
- Komesli, O.T.; Muz, M.; Ak, M.S.; Bakırdere, S.; Gökçay, C.F. Comparison of EDCs removal in full and pilot scale membrane bioreactor plants: Effect of flux rate on EDCs removal in short SRT. J. Environ. Manag. 2017, 203, 847–852. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, H.; Cicek, N. Removal mechanisms of 17β-estradiol and 17α-ethinylestradiol in membrane bioreactors. Water Sci. Technol. 2012, 66, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, Z.; Zhu, S.; Liu, D. 17β-Estradiol removal routes by moving bed biofilm reactors (MBBRs) under various C/N ratios. Sci. Total Environ. 2020, 741, 140381. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lan, L.; Tadda, M.A.; Zhu, S.; Ye, Z.; Liu, D. Interaction between 17β-estradiol degradation and nitrification in mariculture wastewater by Nitrosomonas europaea and MBBR. Sci. Total Environ. 2020, 705, 135846. [Google Scholar] [CrossRef]
- Tolêdo, C.S.S.; Matheus, M.C.; Fontoura, G.A.T.; Dezotti, M.; Fiaux, S.B. Impact of gradually-achieved high phenol loads on the nitrification and COD removal performance of an MBBR fed with synthetic wastewater. Environ. Technol. 2024, 45, 1326–1342. [Google Scholar] [CrossRef]
- Castellanos, R.M.; Bassin, J.P.; Bila, D.M.; Dezotti, M. Biodegradation of natural and synthetic endocrine-disrupting chemicals by aerobic granular sludge reactor: Evaluating estrogenic activity and estrogens fate. Environ. Pollut. 2021, 274, 116551. [Google Scholar] [CrossRef]
- da Mota Oliveira, J.L.; de Souza, T.C.; Gomes, L.C.A.; Saggioro, E.M. Removal of 17β-estradiol (E2) and 17α-ethynylestradiol (EE2) by a sequencing batch reactor following UV/H2O2 process. Int. J. Environ. Sci. Technol. 2024, 21, 7733–7748. [Google Scholar] [CrossRef]
- Faria, C.V.; Ricci, B.C.; Silva, A.F.R.; Amaral, M.C.S.; Fonseca, F.V. Removal of micropollutants in domestic wastewater by expanded granular sludge bed membrane bioreactor. Process Saf. Environ. Prot. 2020, 136, 223–233. [Google Scholar] [CrossRef]
- Moya-Llamas, M.J.; Trapote, A.; Prats, D. Removal of micropollutants from urban wastewater using a UASB reactor coupled to a MBR at different organic loading rates. Urban Water J. 2018, 15, 437–444. [Google Scholar] [CrossRef]
- Jun, B.M.; Hwang, H.S.; Heo, J.; Han, J.; Jang, M.; Sohn, J.; Park, C.M.; Yoon, Y. Removal of selected endocrine-disrupting compounds using Al-based metal organic framework: Performance and mechanism of competitive adsorption. J. Ind. Eng. Chem. 2019, 79, 345–352. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.; Liu, Y.; Liu, N.; Tan, X.; Jiang, L.; Wen, J.; Hu, X.; Yin, Z. Removal of 17β-estradiol from aqueous solution by graphene oxide supported activated magnetic biochar: Adsorption behavior and mechanism. J. Taiwan Inst. Chem. Eng. 2019, 102, 330–339. [Google Scholar] [CrossRef]
- Si, X.; Hu, Z.; Ding, D.; Fu, X. Effects of effluent organic matters on endocrine disrupting chemical removal by ultrafiltration and ozonation in synthetic secondary effluent. J. Environ. Sci. 2019, 76, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Prokić, D.; Vukčević, M.; Kalijadis, A.; Maletić, M.; Babić, B.; Đurkić, T. Removal of Estrone, 17β-Estradiol, and 17α-Ethinylestradiol from Water by Adsorption onto Chemically Modified Activated Carbon Cloths. Fibers Polym. 2020, 21, 2263–2274. [Google Scholar] [CrossRef]
- Majumder, A.; Bhatnagar, A.; Kumar Gupta, A. Simultaneous removal of sulfamethoxazole, 17β-estradiol, and carbamazepine from hospital wastewater using a combination of a continuous constructed wetland-based system followed by photocatalytic reactor. Chem. Eng. J. 2023, 466, 143255. [Google Scholar] [CrossRef]
- Poomipuen, K.; Sakulthaew, C.; Chokejaroenrat, C.; Angkaew, A.; Techauay, K.; Poompoung, T.; Teingtham, K.; Phansak, P.; Lueangjaroenkit, P.; Snow, D.D. Dual Activation of Peroxymonosulfate Using MnFe2O4/g-C3N4 and Visible Light for the Efficient Degradation of Steroid Hormones: Performance, Mechanisms, and Environmental Impacts. ACS Omega 2023, 8, 36136–36151. [Google Scholar] [CrossRef]
- Chaves, F.P.; Gomes, G.; Della-Flora, A.; Dallegrave, A.; Sirtori, C.; Saggioro, E.M.; Bila, D.M. Comparative endocrine disrupting compound removal from real wastewater by UV/Cl and UV/H2O2: Effect of pH, estrogenic activity, transformation products and toxicity. Sci. Total Environ. 2020, 746, 141041. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A review of pharmaceuticals and endocrine-disrupting compounds: Sources, effects, removal, and detections. Water. Air. Soil Pollut. 2013, 224, 1770. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Bhat, S.A.; Kumar, V.; Li, F.; Verma, P. Detection and Treatment of Emerging Contaminants in Wastewater; IWA Publishing: London, UK, 2024. [Google Scholar]
- Yang, S.; Yu, W.; Du, B.; Fan, G.; Zhang, Z.; Pu, G.; Yang, W.; Wang, Q.; Sun, W.; Tang, J. A citrate-loaded nano-zero-valent iron heterogeneous Fenton system for steroid estrogens degradation under different acidity levels: The effects and mechanisms. Chem. Eng. J. 2021, 421, 129967. [Google Scholar] [CrossRef]
- Vieira, W.T.; De Farias, M.B.; Spaolonzi, M.P.; Da Silva, M.G.C.; Vieira, M.G.A. Latest advanced oxidative processes applied for the removal of endocrine disruptors from aqueous media—A critical report. J. Environ. Chem. Eng. 2021, 9, 105748. [Google Scholar] [CrossRef]
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A Critical Evaluation of Advanced Oxidation Processes for Emerging Contaminants Removal. Environ. Process. 2017, 4, 283–302. [Google Scholar] [CrossRef]
- Castellanos, R.M.; Paulo Bassin, J.; Dezotti, M.; Boaventura, R.A.R.; Vilar, V.J.P. Tube-in-tube membrane reactor for heterogeneous TiO2 photocatalysis with radial addition of H2O2. Chem. Eng. J. 2020, 395, 124998. [Google Scholar] [CrossRef]
- López-Velázquez, K.; Guzmán-Mar, J.L.; Montalvo-Herrera, T.J.; Mendiola-Alvarez, S.Y.; Villanueva-Rodríguez, M. Efficient photocatalytic removal of four endocrine-disrupting compounds using N-doped BiOBr catalyst under UV-Vis radiation. J. Environ. Chem. Eng. 2021, 9, 106185. [Google Scholar] [CrossRef]
- Cuerda-correa, E.M.; Alexandre-franco, M.F.; Fern, C. Antibiotics from Water. An Overview. Water 2019, 12, 102. [Google Scholar] [CrossRef]
- Lee, Y.G.; Park, Y.; Lee, G.; Kim, Y.; Chon, K. Enhanced degradation of pharmaceutical compounds by a microbubble ozonation process: Effects of temperature, pH, and humic acids. Energies 2019, 12, 4373. [Google Scholar] [CrossRef]
- Ben Fredj, S.; Novakoski, R.T.; Tizaoui, C.; Monser, L. Two-Phase Ozonation for the Removal of Estrone, 17β-Estradiol and 17α-Ethinylestradiol in Water Using Ozone-Loaded Decamethylcyclopentasiloxane. Ozone Sci. Eng. 2017, 39, 343–356. [Google Scholar] [CrossRef]
- Boutamine, Z.; Hamdaoui, O.; Merouani, S. Sonochemical and photosonochemical degradation of endocrine disruptor 2-phenoxyethanol in aqueous media. Sep. Purif. Technol. 2018, 206, 356–364. [Google Scholar] [CrossRef]
- Camargo-Perea, A.L.; Rubio-Clemente, A.; Peñuela, G.A. Use of ultrasound as an advanced oxidation process for the degradation of emerging pollutants in water. Water 2020, 12, 1068. [Google Scholar] [CrossRef]
- Rao, Y.; Yang, H.; Xue, D.; Guo, Y.; Qi, F.; Ma, J. Sonolytic and sonophotolytic degradation of Carbamazepine: Kinetic and mechanisms. Ultrason. Sonochem. 2016, 32, 371–379. [Google Scholar] [CrossRef]
- Pokhalekar, P.; Chakraborty, M. Degradation of bisphenol A and 4-tert-octylphenol: A comparison between ultrasonic and photocatalytic technique. Desalin. Water Treat. 2016, 57, 10370–10377. [Google Scholar] [CrossRef]
- Deng, J.; Tang, K.; Zhu, S.; Ma, X.; Zhang, K.; Song, Y.; Li, X.; Li, Q.; Liu, Z.; Zhou, K. Competitive degradation of steroid estrogens by potassium permanganate combined with ultrasound. Int. J. Environ. Res. Public Health 2015, 12, 15434–15448. [Google Scholar] [CrossRef] [PubMed]
- Machulek, J.A.; Oliveira, S.C.; Osugi, M.E.; Ferreira, V.S.; Quina, F.H.; Dantas, R.F.; Oliveira, S.L.; Casagrande, G.A.; Anaissi, F.J.; Silva, V.O.; et al. Application of Different Advanced Oxidation Processes for the Degradation of Organic Pollutants. In Organic Pollutants-Monitoring, Risk and Treatment; INTECH: Bucheon-si, Republic of Korea, 2013; p. 142. [Google Scholar]
- Baycan, N.; Li Puma, G. Nanostructred catalysts for photo-oxidation of endocrine disrupting chemicals. J. Photochem. Photobiol. A Chem. 2018, 364, 274–281. [Google Scholar] [CrossRef]
- Yu, W.; Yang, S.; Du, B.; Zhang, Z.; Xie, M.; Chen, Y.; Zhao, C.; Chen, X.; Li, Q. Feasibility and mechanism of enhanced 17β-estradiol degradation by the nano Zero Valent Iron-citrate system. J. Hazard. Mater. 2020, 396, 122657. [Google Scholar] [CrossRef] [PubMed]
- López-Velázquez, K.; Villanueva-Rodríguez, M.; Mejía-González, G.; Herrera-López, D. Removal of 17α-ethinylestradiol and caffeine from wastewater by UASB-Fenton coupled system. Environ. Technol. 2021, 42, 3771–3782. [Google Scholar] [CrossRef]
- Sgroi, M.; Snyder, S.A.; Roccaro, P. Comparison of AOPs at pilot scale: Energy costs for micro-pollutants oxidation, disinfection by-products formation and pathogens inactivation. Chemosphere 2021, 273, 128527. [Google Scholar] [CrossRef]
- Alrousan, D.; Afkhami, A.; Bani-Melhem, K.; Dunlop, P. Organic degradation potential of real greywater using TiO2-based advanced oxidation processes. Water 2020, 12, 2811. [Google Scholar] [CrossRef]
- Siddique, Z.; Malik, A.U.; Asi, M.R.; Inam-ur-Raheem, M.; iqbal, M.; Abdullah, M. Impact of sonolytic ozonation (O3/US) on degradation of pesticide residues in fresh vegetables and fruits: Case study of Faisalabad, Pakistan. Ultrason. Sonochem. 2021, 79, 105799. [Google Scholar] [CrossRef]
- Patil, P.B.; Raut-Jadhav, S.; Pandit, A.B. Effect of intensifying additives on the degradation of thiamethoxam using ultrasound cavitation. Ultrason. Sonochem. 2021, 70, 105310. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Chokejaroenrat, C.; Satapanajaru, T.; Chirasatienpon, T.; Angkaew, A. Removal of 17β-Estradiol Using Persulfate Synergistically Activated Using Heat and Ultraviolet Light. Water. Air. Soil Pollut. 2020, 231, 247. [Google Scholar] [CrossRef]
- Gabet, A.; Métivier, H.; de Brauer, C.; Mailhot, G.; Brigante, M. Hydrogen peroxide and persulfate activation using UVA-UVB radiation: Degradation of estrogenic compounds and application in sewage treatment plant waters. J. Hazard. Mater. 2021, 405, 124693. [Google Scholar] [CrossRef] [PubMed]
- Angkaew, A.; Sakulthaew, C.; Satapanajaru, T.; Poapolathep, A.; Chokejaroenrat, C. UV-activated persulfate oxidation of 17β-estradiol: Implications for discharge water remediation. J. Environ. Chem. Eng. 2019, 7, 102858. [Google Scholar] [CrossRef]
- Brienza, M.; Mahdi Ahmed, M.; Escande, A.; Plantard, G.; Scrano, L.; Chiron, S.; A Bufo, S.; Goetz, V. Relevance of a photo-Fenton like technology based on peroxymonosulphate for 17β-estradiol removal from wastewater. Chem. Eng. J. 2014, 257, 191–199. [Google Scholar] [CrossRef]
- Castellanos, R.M.; Presumido, P.H.; Dezotti, M.; Vilar, V.J.P. Ultrafiltration ceramic membrane as oxidant-catalyst/water contactor to promote sulfate radical AOPs: A case study on 17β-estradiol and 17α-ethinylestradiol removal. Environ. Sci. Pollut. Res. 2022, 29, 42157–42167. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Ahmed, I. Journey describing applications of oxone in synthetic chemistry. Chem. Rev. 2013, 113, 3329–3371. [Google Scholar] [CrossRef]
- Přibilová, P.; Odehnalová, K.; Rudolf, P.; Pochylý, F.; Zezulka, Š.; Maršálková, E.; Opatřilová, R.; Maršálek, B. Rapid AOP Method for Estrogens Removal via Persulfate Activated by Hydrodynamic Cavitation. Water 2022, 14, 3816. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Yun, E.T.; Seo, J.W.; Lee, C.; Kim, S.H.; Kim, J.H.; Lee, J. Activation of Peroxymonosulfate by Surface-Loaded Noble Metal Nanoparticles for Oxidative Degradation of Organic Compounds. Environ. Sci. Technol. 2016, 50, 10187–10197. [Google Scholar] [CrossRef]
- You, W.; Fan, G.; Zhou, J.; Lin, R.; Cao, X.; Song, Y.; Luo, J.; Zou, J.; Hong, Z.; Xu, K.-Q.; et al. Activation of Peroxymonosulfate by P-Doped Cow Manure Biochar for Enhancing Degradation of 17β-Estradiol. Water 2024, 16, 1754. [Google Scholar] [CrossRef]
- Behrman, E.J.; Dean, D.H. Sodium peroxydisulfate is a stable and cheap substitute for ammonium peroxydisulfate (persulfate) in polyacrylamide gel electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 723, 325–326. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.D.; Liu, L.; Liu, Y.; Zhang, H.W.; Han, X. Efficient oxidation of phenol by persulfate using manganite as a catalyst. J. Mol. Catal. A Chem. 2016, 411, 264–271. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Janjani, H. Reclamation of hospital secondary treatment effluent by sulfate radicals based–advanced oxidation processes (SR-AOPs) for removal of antibiotics. Microchem. J. 2020, 153, 104430. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, H.; Liu, W.; Ao, Z.; Ji, H.; Liu, Y.; Su, S.; Yao, G.; Lai, B. Insights into heterogeneous catalytic activation of peroxymonosulfate by natural chalcopyrite: pH-dependent radical generation, degradation pathway and mechanism. Chem. Eng. J. 2020, 397, 125387. [Google Scholar] [CrossRef]
- Li, W.; Guo, H.; Wang, C.; Zhang, Y.; Cheng, X.; Wang, J.; Yang, B.; Du, E. ROS reevaluation for degradation of 4-chloro-3,5-dimethylphenol (PCMX) by UV and UV/persulfate processes in the water: Kinetics, mechanism, DFT studies and toxicity evolution. Chem. Eng. J. 2020, 390, 124610. [Google Scholar] [CrossRef]
- Carra, I.; Sánchez Pérez, J.A.; Malato, S.; Autin, O.; Jefferson, B.; Jarvis, P. Application of high intensity UVC-LED for the removal of acetamiprid with the photo-Fenton process. Chem. Eng. J. 2015, 264, 690–696. [Google Scholar] [CrossRef]
- Raikar, L.G.; Gandhi, J.; Gupta, K.V.K.; Prakash, H. Degradation of Ampicillin with antibiotic activity removal using persulfate and submersible UVC LED: Kinetics, mechanism, electrical energy and cost analysis. Chemosphere 2024, 349, 140831. [Google Scholar] [CrossRef]
- Verma, S.; Nakamura, S.; Sillanpää, M. Application of UV-C LED activated PMS for the degradation of anatoxin-a. Chem. Eng. J. 2016, 284, 122–129. [Google Scholar] [CrossRef]
- Ye, J.S.; Liu, J.; Ou, H.S.; Wang, L. lin Degradation of ciprofloxacin by 280 nm ultraviolet-activated persulfate: Degradation pathway and intermediate impact on proteome of Escherichia coli. Chemosphere 2016, 165, 311–319. [Google Scholar] [CrossRef]
- Jia, D.; Li, Q.; Hanna, K.; Mailhot, G.; Brigante, M. Efficient removal of estrogenic compounds in water by MnIII-activated peroxymonosulfate: Mechanisms and application in sewage treatment plant water. Environ. Pollut. 2021, 288, 117728. [Google Scholar] [CrossRef]
- Luo, L.; Cha, C.; Meng, D.; Zou, W.; Xia, L.; Jiang, F.; Ma, X.; Dai, J. Photocatalytic activation of persulfate for rapid degradation of 17α-ethinylestradiol over quasi-full-visible-responsive Pg-C3N4/RGO/BiOI. J. Environ. Chem. Eng. 2024, 12, 113489. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, W.; Wu, D.; Wu, Q.; Hu, H. Highly selective production of singlet oxygen by manipulating the spin state of single-atom Co–N moieties and electron localization. Appl. Catal. B Environ. 2023, 324, 122248. [Google Scholar] [CrossRef]
- Li, F.; Yuasa, A.; Obara, A.; Mathews, A.P. Aerobic batch degradation of 17-β estradiol (E2) by activated sludge: Effects of spiking E2 concentrations, MLVSS and temperatures. Water Res. 2005, 39, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, R.; Tavares, F.V.F.; Gómez, M.; Irusta, R.; Le-Clech, P. Removal of 17-β estradiol from wastewater: Comparison between a laboratory scale conventional activated sludge and a membrane bioreactor. Desalin Water Treat. 2013, 51, 2336–2342. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Onubogu, J.; Joyce, E.; Mason, T. Sonochemical degradation of endocrine disrupting chemicals 17β-estradiol and 17α-ethinylestradiol in water and wastewater. Int. J. Environ. Sci. Technol. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Pešoutová, R.; Stříteský, L.; Hlavínek, P. A pilot scale comparison of advanced oxidation processes for estrogenic hormone removal from municipal wastewater effluent. Water Sci. Technol. 2014, 70, 70–75. [Google Scholar] [CrossRef]
- Padovan, R.N.; de Carvalho, L.S.; de Souza Bergo, P.L.; Xavier, C.; Leitão, A.; dos Santos Neto, Á.J.; Lanςas, F.M.; Azevedo, E.B. Degradation of hormones in tap water by heterogeneous solar TiO2-photocatalysis: Optimization, degradation products identification, and estrogenic activity removal. J. Environ. Chem. Eng. 2021, 9, 106442. [Google Scholar] [CrossRef]
- Kim, S.H.; Tian, Q.; Fang, J.; Sung, S. Removal of 17-β estradiol in water by sonolysis. Int. Biodeterior Biodegrad. 2015, 102, 11–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).