1. Introduction
Niobium (Nb) is a rare metal prized for its high melting point, corrosion resistance, and ability to enhance alloy strength and heat resistance. These properties make it essential for producing high-strength steels, high-temperature alloys for turbines, and superconducting materials used in aerospace, nuclear power, and electronics. The primary natural sources of niobium are pyrochlore ((Na,Ca)
2Nb
2O
6(OH,F)) and columbite–tantalite ((Fe,Mn)(Nb,Ta)
2O
6), found in pegmatites, carbonatites, and alkaline rocks [
1]. Growing global demand for niobium, driven by high-tech industries, necessitates increased production and the exploration of alternative sources, such as secondary raw materials [
2].
Traditional processing of niobium-containing ores involves hydrometallurgical and pyrometallurgical techniques optimised for primary deposits like pyrochlore and columbite–tantalite [
3]. The process typically includes crushing and grinding to liberate niobium minerals, followed by froth flotation or magnetic separation to produce a concentrate. The concentrate is then subjected to acid leaching, often with hydrofluoric acid or mixed acids, to dissolve the niobium oxides, followed by solvent extraction or precipitation to recover high-purity niobium compounds [
4,
5,
6]. This acid leaching step is pivotal, as it enables efficient extraction of niobium from refractory oxides. Global niobium production is approximately 70,000–80,000 tons annually, with over 90% sourced from pyrochlore deposits in Brazil and Canada [
7]. Primary sources are limited by geographically concentrated reserves, high mining costs, and environmental impacts from ore extraction. Secondary sources, such as metallurgical by-products, are underexploited due to their low niobium content (typically <5%) and complex mineralogy, which complicates recovery. For instance, niobium accumulates in waste products like the dust chamber sublimations from titanium chlorinators, generated during titanium slag chlorination at the Ust-Kamenogorsk Titanium and Magnesium Plant JSC (Kazakhstan), a major titanium producer. These wastes offer significant potential for secondary niobium recovery, reducing reliance on primary deposits and mitigating environmental impacts.
Wastes from titanium–magnesium production, including fine dust from titanium raw material processing, have been investigated for their resource potential. For example, Ultarakova et al. (2022) demonstrated that dust from ilmenite concentrate smelting for titanium dioxide production contains valuable metals, highlighting its recovery potential [
8]. Similarly, Toishybek et al. (2023) underscored the feasibility of using such wastes as secondary sources of rare metals [
9]. These studies confirm the prospects for processing these wastes within resource-saving technologies.
Modern hydrometallurgical methods for niobium recovery employ various acids, each with distinct efficiencies and limitations. Sulphuric acid (H
2SO
4) alone achieves low niobium recovery from columbite–tantalite minerals, hindered by the low solubility of Nb
2O₅, which requires high acid concentrations, extended leaching times, and a high temperature of 200 °C in an autoclave for complete extraction, thereby increasing costs and environmental risks [
10]. Hydrofluoric acid (HF) alone, as shown by Rodriguez et al. (2015) and Gupta and Suri (1994), yields over 90% recovery from ferrocolumbite and pyrochlore due to the formation of stable fluoride complexes, but its toxicity, corrosiveness, and high reagent volumes pose significant safety and waste management challenges [
5,
6]. Hydrochloric acid (HCl) leaching, as demonstrated by Toromanoff and Habashi (1983), achieves 85–90% recovery from low-grade pyrochlore at 200 °C, but high energy demands and poor selectivity for complex matrices limit its applicability [
11]. Mixed-acid systems, such as HF with nitric acid (HNO
3), have been explored for columbite–tantalite ores to separate niobium from impurities like iron and manganese oxides, improving purification efficiency [
12]. The latest study on TiO
2 production residues demonstrated the potential of HCl leaching for niobium recovery, achieving a high recovery rate (>90%) within 60 min using 4 M HCl at 70 °C, with optimised parameters such as temperature (25–90 °C), HCl concentration (0.5–4 M), and stirring speed (100–500 rpm) [
13]. The kinetics followed a random pore model with pore diffusion as the limiting stage and a low activation energy of 16.8 ± 1.2 kJ/mol, offering a milder, cost-effective alternative to traditional HF or high-temperature methods, improving the economics of TiO
2 production and waste management. However, the reported complete dissolution of niobium at temperatures as low as 90 °C in HCl alone raises doubts, as Nb
2O
5’s low solubility necessitates fluoride ions to form soluble fluoro-complexes for effective recovery. Despite these advances, secondary sources like TiO
2 residues and titanium–magnesium production wastes remain underexplored due to their heterogeneous mineralogy and limited kinetic data [
14,
15]. This gap underscores the need for research into mixed-acid systems, such as HF + H
2SO
4, to enhance niobium recovery from man-made raw materials.
The purpose of this study is to investigate the kinetics of acid niobium leaching from niobium-containing middlings obtained through the water treatment of dust chamber sublimations of titanium chlorinators—waste products from UKTMP JSC (Ust-Kamenogorsk, Kazakhstan). A mixture of hydrofluoric and sulphuric acids was used as the leaching system. Kinetic analysis was conducted using mathematical models to determine key process parameters, including velocity constants, activation energy, and the limiting stage of interaction.
2. Materials and Methods
Materials: Mineral acids used include hydrofluoric acid (extra pure grade) and sulphuric acid (chemically pure grade). The material studied is a niobium-containing intermediate product obtained after water treatment of sublimations from dust chambers of titanium chlorinators. Prior to analysis and experimental studies, the sample was ground to a particle size class of no more than 0.1 mm. Based on the results of X-ray fluorescence and chemical analysis, the composition of the studied material is presented in
Table 1 below.
Equipment. Velp Scientifica LS overhead mixer (Usmate Velate, Italy), LT-111a circulating thermostat (Saint Petersburg, Russia), Stuart water bath RE400DB (Stone, Staffordshire, UK), and an SNOL drying oven (Utena, Lithuania).
Experimental method. The kinetics of the interaction between the niobium-containing material and a solution of HF (18 M) and H
2SO
4 (7 M) was investigated under agitation in a temperature-controlled PTFE cell at 25, 45, 65, and 90 °C for leaching durations of 60, 120, 180, and 240 min. A 50 g sample was contacted with 150 mL of acid solution at a solid-to-liquid ratio (S:L) of 1:3, with a mixing speed of 300 rpm, ensuring particle suspension and uniform solution flow. Temperatures, leaching durations, and mixing speed were selected based on preliminary tests and literature analysis [
16]. For leaching, solutions of HF (18 M) and H
2SO
4 (7 M) were used in equal volume proportions, determined through calculations of the stoichiometric acid requirement, considering the chemical composition of the niobium-containing intermediate. The HF concentration, exceeding the stoichiometric requirement by 1.5 times, ensured selective niobium dissolution, while H
2SO
4 provided an optimal acidic environment and catalysed the decomposition of complex mineral phases, enhancing dissolution efficiency. The leaching equipment, shown in
Figure 1, consisted of a mixing device with a propeller, a vessel containing the solution and sample, a thermometer for temperature monitoring, a laboratory bath for maintaining specified temperatures, and a cooling refrigerator for condensing vapours generated during the process.
The leaching kinetics of niobium from a niobium-containing product were studied using various theoretical models describing the behaviour of the system during the dissolution process. The purpose of the study was to identify the limiting stages of the process, such as the rate of the chemical reaction on the surface of the particles, the diffusion of the reagent through the liquid film, the resistance of the layer of reaction products, and the possibility of the combined control of these factors.
The recovery degree of niobium in solution was calculated using the following equation:
where C
Nb is the niobium concentration, g/L; V
s is the solution volume, L; W
Nb is a mass fraction of niobium in the middlings; and M
a is the weight of the sample, g.
Analysis Methods. Chemical analysis was conducted with the use of the Optima 2000 DV inductively coupled plasma atomic emission spectrometer (Perkin Elmer Inc., Waltham, MA, USA). The D8 ADVANCE X-ray diffractometer (Bruker, Karlsruhe, Germany) was used for structural analysis, and the Venus 200 X-ray fluorescence spectrometer (PANalytical B.V., Almelo, The Netherlands) was used for elemental analysis. An electron scanning microscope with a JEOL JXA-8230 analyser (JEOL, Akishima, Tokyo, Japan) was used to map the elemental composition of samples using an energy-dispersive X-ray spectrometer (EDX). The STA 449 F3 Jupiter synchronous thermal analyser (NETZSCH, Selb, Bavaria, Germany) was used for thermal analysis with mass and phase transition measurements.
Analysis of the elemental composition shows that titanium and aluminium have the highest content among metals. The oxygen content is 45.68%. It indicates the predominant presence of oxide forms of compounds. The main components of the object of study also include iron, zirconium, and niobium.
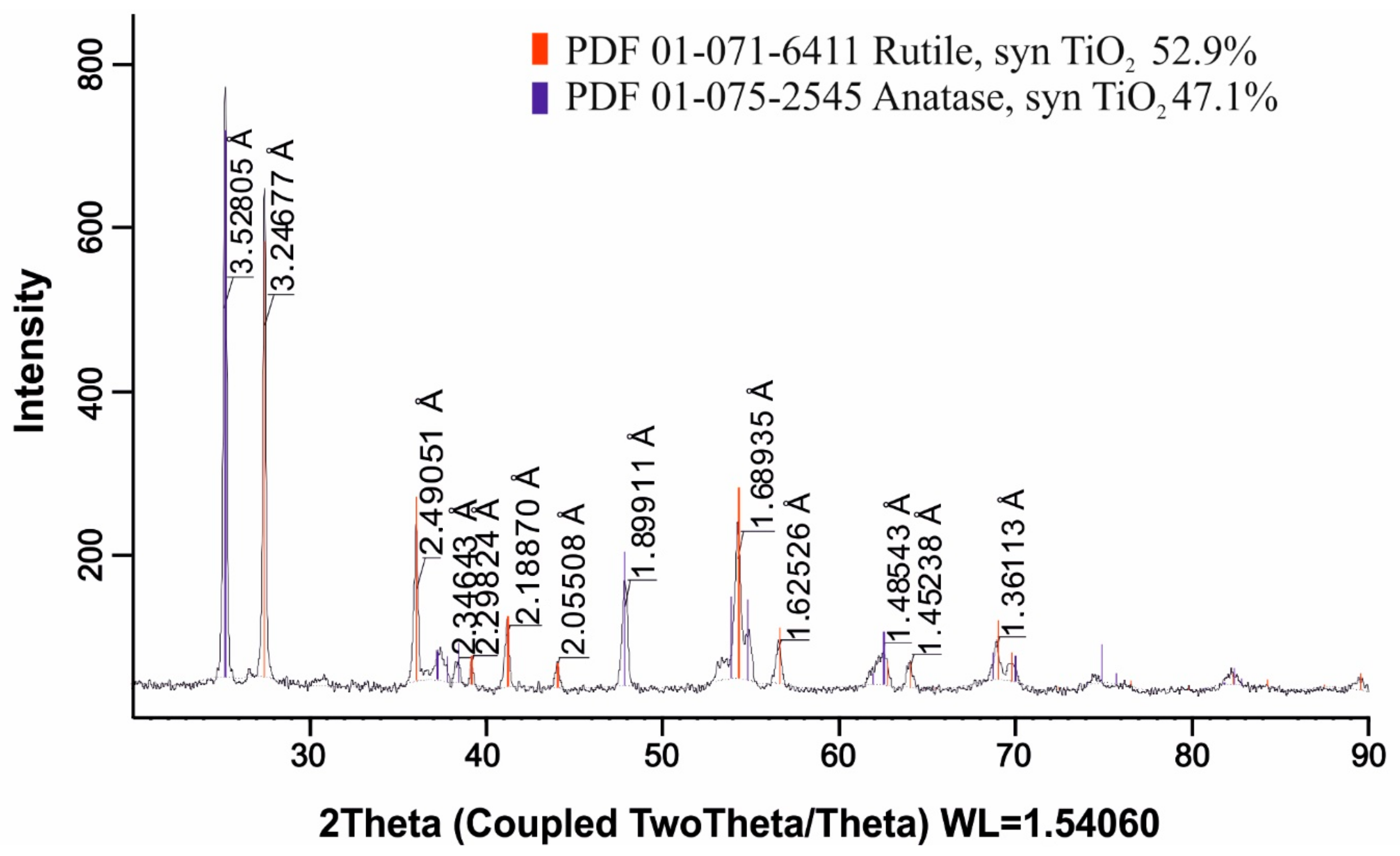
Figure 2 shows the results of the X-ray phase analysis.
X-ray phase analysis confirmed the presence of the following crystal phases: anatase (TiO2), titanium–aluminium–niobium complex oxide (Ti0.8Al0.1Nb0.1O2), titanium oxide (Ti2O3), hematite (Fe2O3), and zirconium–titanate (ZrTiO4). The predominance of titanium-containing phases indicates its key role in the product structure. The discovered compounds of different natures and compositions indicate a multiphase heterogeneous structure and a rather complex mineralogy of the material.
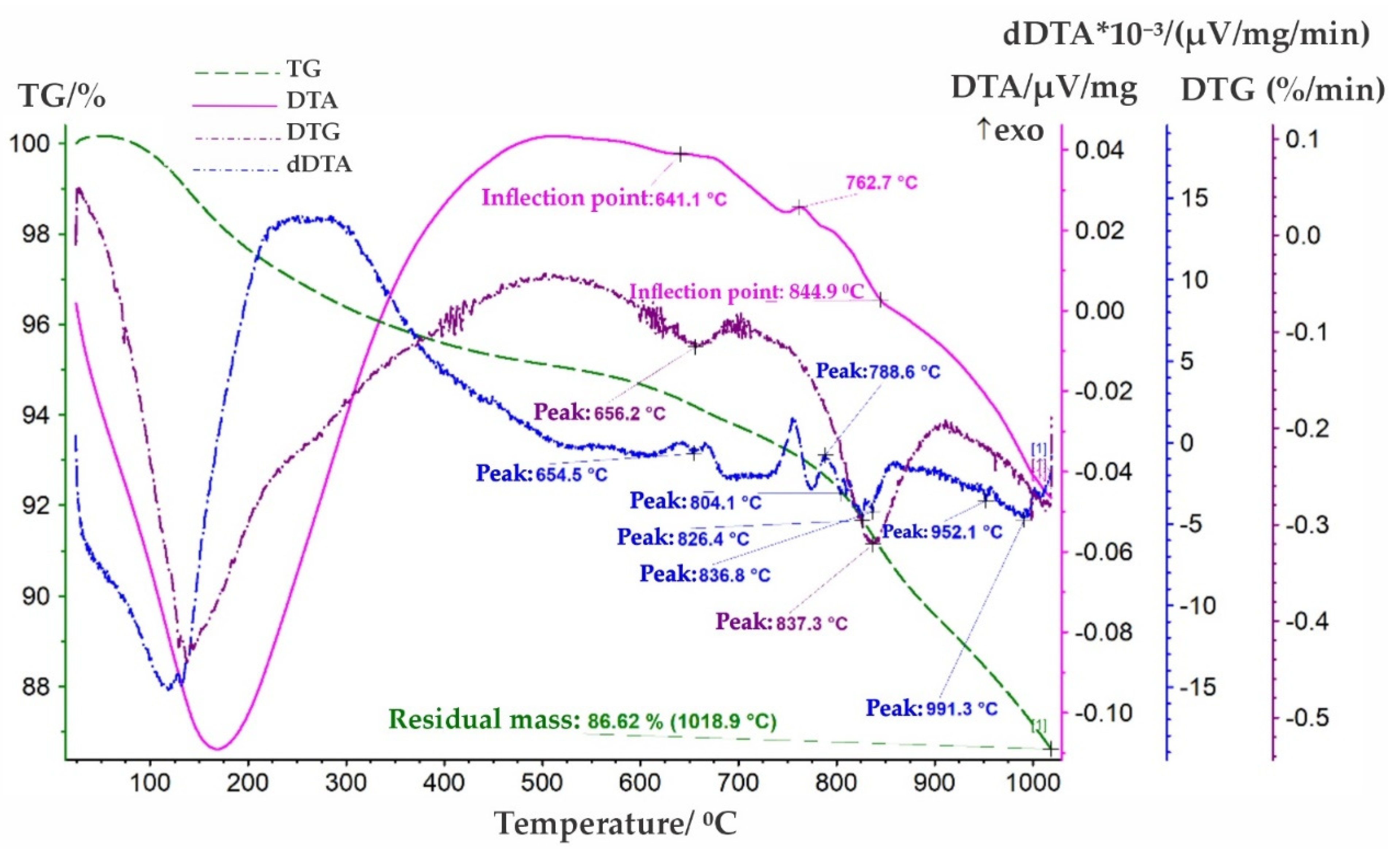
The thermal behaviour of niobium-containing middlings was investigated using synchronous thermogravimetric analysis (TG/DTA) with a NETZSCH STA 449 F3 Jupiter analyser. Peak temperatures were determined based on the NETZSCH Proteus software 6.1 database. The derivatogram (
Figure 3) reveals weak endothermic effects at temperatures of 654.5, 804.1, 826.4, 836.8, 952.1, and 991.3 °C, alongside an exothermic effect peaking at 788.6 °C. The endothermic effect at 654.5 °C is likely associated with the phase transition of NaNbO
3 from a rhombic to a cubic structure. The peak at 804.1 °C may reflect a polymorphic transformation of NbO
2. The transition of Nb
2O
5 from a low-temperature to a medium-temperature modification was recorded at 836.8 °C. The effect at 991.3 °C is presumed to correspond to the transition from α-TiO
2 to β-TiO
2 or the possible melting of a Na
5NbO
5 impurity. The exothermic peak at 788.6 °C may be attributed to the oxidation of Fe
2⁺ or a change in the valence state of titanium oxides. Minima on the DTG curve at 656.2 and 837.3 °C are accompanied by mass loss, with the latter likely related to the reduction of iron oxides. Mass losses at 656.2 °C and 837.3 °C indicate minor decomposition, probably associated with the release of volatile substances (e.g., chlorides or water, as per the sample composition) or the reduction of iron oxides [
17,
18]. Thermal analysis confirms the multiphase composition of the middlings, including NaNbO
3, Nb
2O
5, titanium oxides, and CaZrTi
2O
7, highlighting their mineralogical complexity and potential for further processing.
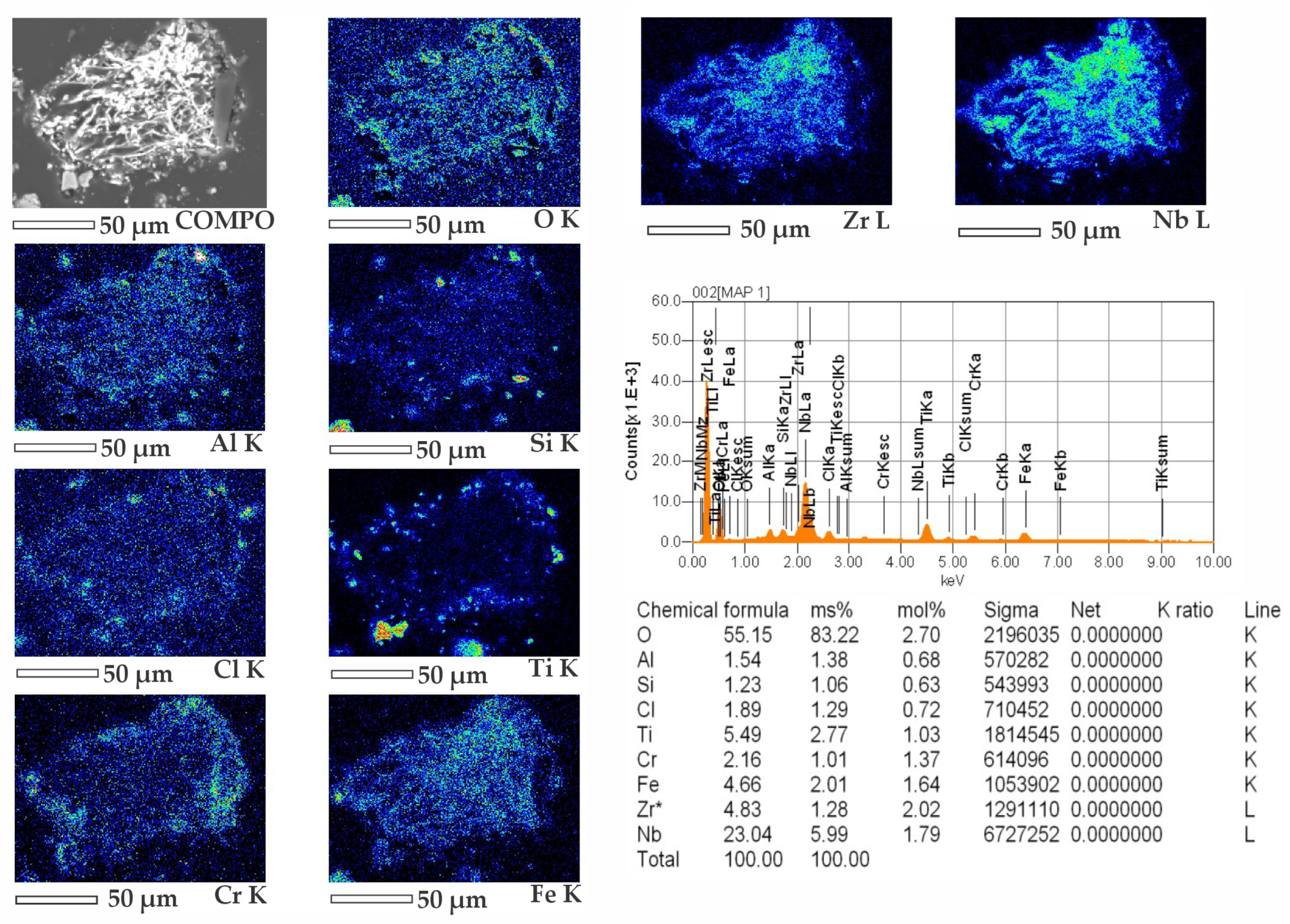
The mineral composition of the middlings was investigated using electron probe microanalysis on a JEOL JXA-8230 instrument. Elemental mapping (
Figure 4) revealed a significant niobium oxide content, spatially correlated with zirconium, suggesting the possible presence of a zirconium–niobium oxide phase, presumably ZrNb
2O
7. Given the low niobium content (2.06 wt.%), precise distribution within the matrix and confirmation of the phase composition require further analysis, such as examination of thin sections and SEM imaging of polished cross-sections. Niobium may also be present in other forms, such as NaNbO
3 and Nb
2O
5, as indicated by thermal analysis results. Trace amounts of titanium and silicon oxides were detected at grain boundaries, indicating the heterogeneous nature of the material.
Thus, the physical and chemical studies show that the niobium-containing middlings form a multicomponent system with a pronounced multiphase structure. The mineral composition of the sample is mainly represented by oxide compounds of titanium, aluminium, iron, zirconium, and niobium. The study object is characterised by mineralogical heterogeneity and is a rich source of such rare metals as titanium, niobium, and zirconium, which determines its potential value as a raw material for subsequent processing.
3. Results and Discussions
Leaching is a complex, heterogeneous process that ensures the transfer of valuable components from the solid phase to an aqueous solution. This process includes several stages, proceeding sequentially and/or in parallel. In the initial step, the reactant diffuses from the solution volume to the surface of the solid particle through the adjacent liquid boundary layer (external diffusion). Further, the reactant penetrates the particle through a layer of a solid reaction product or a porous structure (internal diffusion). Then, a chemical interaction occurs on the surface of the solid phase. The resulting soluble product migrates from the reaction surface to the outer boundary of the particle through the porous structure or layer of the reaction product. In the final stage, the solute diffuses through the boundary liquid layer back into the solution volume. The leaching rate is determined by the limiting stage. It can be external diffusion, internal diffusion, or a chemical reaction on the surface that determines the overall kinetics of the process.
The chemical behaviour of niobium oxide (Nb
2O
5) in acidic media is characterised by its low solubility in sulphuric acid (H
2SO
4) in the absence of complexing agents. This is due to the high chemical inertness of niobium pentoxide due to the Nb-O strong covalent mesh [
19]. When Nb
2O
5 reacts with H
2SO
4, sulphate complexes can be formed by the following reaction:
However, this reaction is hypothetical, and the equilibrium is significantly shifted towards the original reactants due to the low stability and solubility of NbO(SO
4)
2 [
20,
21]. As a result, pure H
2SO
4 is not efficient enough to leach Nb
2O
5 without additional agents.
A significant acceleration of the dissolution process is achieved with the introduction of hydrofluoric acid (HF), which contributes as follows to the formation of stable fluoride complexes, mainly hexafluoro niobates:
This process is key in the industrial processing of niobium ores such as pyrochlore and columbite, where excess HF (10–15 mol) ensures complete dissolution of the oxide [
6]. Studies also indicate the possibility of [NbF
7]
2− formation at higher HF concentrations. The formation of fluoro-oxoniobates is assumed in mixed systems, including HF and H
2SO
4:
Such complexes as [NbOF
5]
2− are well known in niobium and tantalum chemistry, and the addition of H
2SO
4 reduces the need for HF and stabilises solutions through the presence of hydrosulphate ions. The exact stoichiometric composition of products can vary depending on the ratio of acids, including such forms as Hₓ[NbOₓFγ]. The high coordination capacity of the fluoride ion (F
−) compared to the sulphate ion (SO
42−) explains the predominance of fluoride complexes, while H
2SO
4 acts as a protonation catalyst, increasing the solubility of Nb
2O
5 [
22]. Therefore, effective leaching requires an integrated approach that combines kinetic models with the chemical specifics of the reagents used.
Based on the described mechanism, kinetic leaching curves of niobium were obtained in the coordinates of α-τ (fraction of recovery time) at various temperatures (
Figure 5). To enhance the reliability of the data, 16 experiments were conducted across four temperatures (25, 45, 65, and 90 °C) and four leaching durations (60, 120, 180, and 240 min), with each experiment performed in two parallel replicates, resulting in a total of 32 experiments. The average value of the niobium recovery degree from the replicates was used to construct the curves, with relative standard deviations (RSD) ranging from 2.1% to 4.3%, indicating good repeatability. Higher variability was observed at 25 °C for shorter durations (60 and 120 min), likely due to slower reaction kinetics. Potential sources of error include minor variations in sample composition, temperature control inaccuracies (±0.5 °C), and analytical errors in niobium concentration measurements (±2% precision). These factors contributed to an estimated uncertainty of ±6.5% in the velocity constants derived from the mixed kinetic model, ensuring robust modelling results. Analysis of the data shows a significant influence of temperature on the process: with an increase in temperature from 25 to 90 °C, the time to reach equilibrium decreases from 240 to 120 min, and the niobium recovery degree into solution increases from 35.25 to 93.5%. These results indicate thermal activation of the process and highlight the need to take temperature parameters into account to develop efficient niobium recovery technologies.
An assessment of the limiting step in the process is required to understand the factors that determine the leaching rate further. It is achieved by systematically processing kinetic data using mathematical models of heterogeneous reactions. The kinetics of dissolution of solid particles in a liquid medium are due to a complex interaction of such physical and chemical factors as the solubility of the reaction products, the dynamics of changes in particle size, and the influence of surface layers on mass transfer. Fundamental models are used to describe these processes. The following approaches stand out among them: the Shrinking Particle Model, where the reaction takes place on the outer surface, the products completely pass into the solution, and the particle radius gradually decreases [
23,
24]; the Shrinking Core Model—Constant Size, characterised by the preservation of the external dimensions of the particle while moving the reaction inward and forming a layer of products around the shrinking nucleus [
25,
26]; and the Shrinking Core Model—Shrinking Particle, that describes the simultaneous contraction of the nucleus and the overall particle geometry in the presence of process-influencing products [
27]. These models provide a theoretical basis for the analysis of the mechanisms controlling the leaching rate and allow for the optimisation of hydrometallurgical processes for niobium recovery based on the identified patterns.
The model of a contracting nucleus enables the description of the leaching process through analytical equations. They correspond to a specific stage that limits the reaction rate. These dependencies help us understand whether a chemical reaction or diffusion slows down the process and provide a basis to optimise it.
If the leaching rate is determined by a chemical reaction on the surface of the unreacted nucleus, the process is expressed by the following equation:
where α is the fraction of leached matter (from 0 to 1), τ is the time, and k is the velocity constant that depends on the reactant activity and the material properties. In this case, the reactant quickly reaches the nucleus surface, and the slowdown is associated only with the reaction itself. This mode is more common at high temperatures or for small particles, where diffusion does not create significant obstacles.
When the velocity is limited by the diffusion of the reactant through the liquid boundary film around the particle, this equation applies:
Here, k depends on the rate of mass transfer through the film, and the process is slowed down due to weak mixing of the solution or low convection, making it difficult to deliver the reagent to the surface.
If the main obstacle is diffusion through the layer of reaction products accumulated around the nucleus, the following equation is used:
In this case, k is related to the permeability of the layer of products, and the deceleration is caused by their density and thickness. This mode is typical when solid residues are formed in the process, blocking the reagent’s access to the nucleus.
The following kinetic equation is applicable to describe processes where the rate of reaction is determined by the combined effect of a surface chemical reaction and diffusion through the product layer [
28]:
This expression reflects the kinetics of processes with a mixed-velocity control mechanism, when neither of the factors (chemical reaction at the interface or diffusion through the resulting layer of products) is exclusively limiting. The use of this model is justified in cases where experimental data indicate the significant influence of temperature, which is a sign of chemical control, while the presence of a residual layer of products on the surface of particles indicates significant diffusion resistance.
Figure 6 shows graphical dependencies based on the kinetic equations presented, reflecting the kinetic patterns of the leaching process. Analysis of the data obtained makes it possible to determine the limiting stage of the reaction, identify the mechanism of interaction between the reactant and the solid phase, and calculate the velocity constant and its correlation coefficients, characterising the degree of compliance of the experimental data with the selected kinetic models.
Analysis of the data presented in
Table 2 shows that the highest values of the determination coefficient R
2 were obtained for Equation (8). This indicates a higher degree of correspondence of this model to experimental data in comparison with other equations considered. Based on this, the angular coefficients of the lines constructed in the coordinates of the equation that are numerically equivalent to the apparent temperature constants of the velocity are used to determine the apparent activation energy Ea (kJ/mol). The calculation is performed using the Arrhenius equation. It relates the reaction rate to temperature through an exponential relationship, allowing the energy barrier of the process to be estimated. This approach provides a reliable quantitative interpretation of kinetic parameters based on empirical data.
Further, the reaction rate constant k is expressed in terms of the Arrhenius equation in the following form:
where R is the universal gas constant (8.314 J/(mol·K)), T is the absolute temperature (K), A is the pre-exponential factor reflecting the frequency of particle collisions and steric factors, and Ea is the apparent activation energy (kJ/mol) characterising the energy barrier of the reaction.
For the convenience of analysis, Equation (9) is converted into a logarithmic form. It makes it possible to represent it as an equation of a line, as follows:
where the dependence in the coordinates ln k − 1/T takes on a linear form. The angle of inclination of such a line, determined by the ratio Ea/R, corresponds to the energy characteristics of the process, while the pre-exponential factor A can be calculated both mathematically and graphically, based on the value of the segment cut off by the experimental line on the vertical axis.
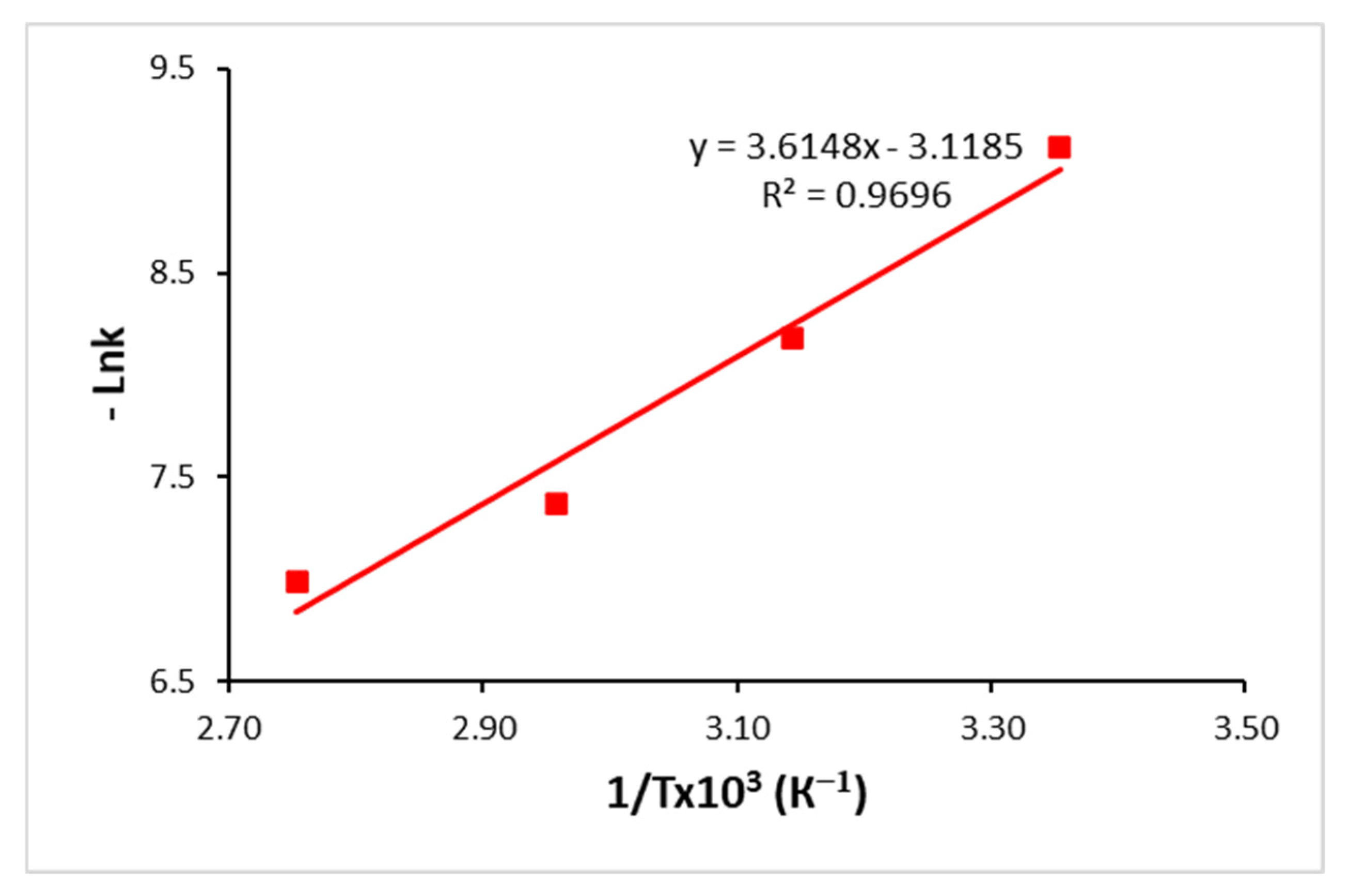
The dependences of the dissolution rate of niobium in the temperature range of 298–363 K are analysed in the coordinates of the Arrhenius equation (ln k of 1/T × 10
−3) and are presented in
Figure 7. The data obtained demonstrate the linear nature of the relationship and confirm the applicability of this model to describe the kinetics of the process within the specified temperature range.
It was established as a result of experimental studies that the apparent activation energy of the niobium dissolution process in a fluoride–sulphuric acid solution is 30.05 kJ/mol. The obtained value indicates a mixed mechanism of this process, where the dissolution rate is determined by the combined effect of two limiting stages: chemical interaction at the interface between solid niobium and solution, and diffusion transfer of reactants to the surface of the solid phase and reaction products from the surface to the volume of the solution. The moderate magnitude of the apparent activation energy indicates that none of these steps is exclusively dominant. This is typical for systems with complex kinetics, where the contribution of each of the mechanisms—chemical and diffusion—depends on such experimental conditions as temperature, reactant concentration, and mixing intensity.
The insoluble sludge (
Figure 8) formed in the process of fluoride–sulphuric acid leaching is characterised by a high titanium content. It makes it a promising material for the production of synthetic rutile or re-involvement in the technological cycle of titanium slag chlorination. At the same time, the niobium and zirconium that accumulate in the liquid phase of the solution can be further extracted using the solvent recovery method. Taking into account the significant volumes of waste generated in titanium–magnesium production, the development of integrated processing technology is a relevant direction.
This technology provides for the return of titanium to the chlorination process and the selective separation of rare metals. It helps to increase the efficiency of resource use and reduce the impact of accumulated waste on the environment.
4. Conclusions
Structural and chemical studies of the initial niobium-containing product showed its multiphase structure with a predominance of oxide compounds of titanium, aluminium, iron, zirconium, and niobium, emphasising the mineralogical complexity and potential of the raw material as a source of rare metals. The kinetics of niobium leaching from this product is subject to a mixed mechanism, where the process rate is determined by a chemical reaction on the surface of the particles and diffusion through the layer of products. This is confirmed by the correspondence of model data 1 − 2(1 − α)1/3 + (1 − α)2/3 = kτ. The apparent activation energy is 30.05 kJ/mol, indicating the combined influence of kinetic and diffusion factors in a heterogeneous system. An increase in temperature from 25 to 90 °C increases the niobium recovery rate from 35.25 to 93.5% and shortens the equilibrium time from 240 to 120 min, demonstrating thermal activation of the process. Chemical interaction on the surface of the particles prevails over diffusion through the liquid film or product layer, which plays a secondary role in these conditions. The insoluble residue beneficiated with titanium is suitable for the production of synthetic rutile or reuse in the technological cycle of chlorination of titanium slags. This increases the efficiency of raw material use and reduces the volume of waste generated. The liquid phase with niobium and zirconium is promising for solvent recovery. The data obtained form the basis for the development of technologies intended to process man-made raw materials, ensuring the sustainable use of resources and reducing the environmental burden.
The proposed HF + H2SO4 leaching system offers advantages over established methods. Unlike H2SO4 alone, which requires high temperatures (200 °C) and yields low niobium recovery, the HF + H2SO4 system achieves 93.5% recovery at 90 °C, reducing energy costs. Compared to HF alone, it uses less HF, lowering costs and risks. It also outperforms HCl-based methods by offering better selectivity and lower energy needs, making it ideal for complex secondary sources like titanium–magnesium production wastes.
For industrial scaling, pilot tests should optimise acid concentrations and mixing to maximise recovery and minimise reagent use. Solvent extraction for niobium and zirconium recovery can create a closed-loop process. Titanium-rich residues can be reused in chlorination cycles, cutting waste. Safety measures, including corrosion-resistant equipment and HF handling protocols, are critical. Environmental assessments and waste management plans are needed for regulatory compliance.
Economically, the process reduces costs via lower energy and HF use and adds value by recovering metals from waste. Environmentally, it cuts waste through residue reuse and solvent extraction but requires robust HF effluent treatment to prevent contamination, ensuring sustainability.