Efficiency of a New Biochar Made from Agave Bagasse to Remove Conventional Pollutants in Samples from Laguna de Bustillos, Chihuahua, Mexico, and Pharmaceutical Derivatives in Synthetic Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
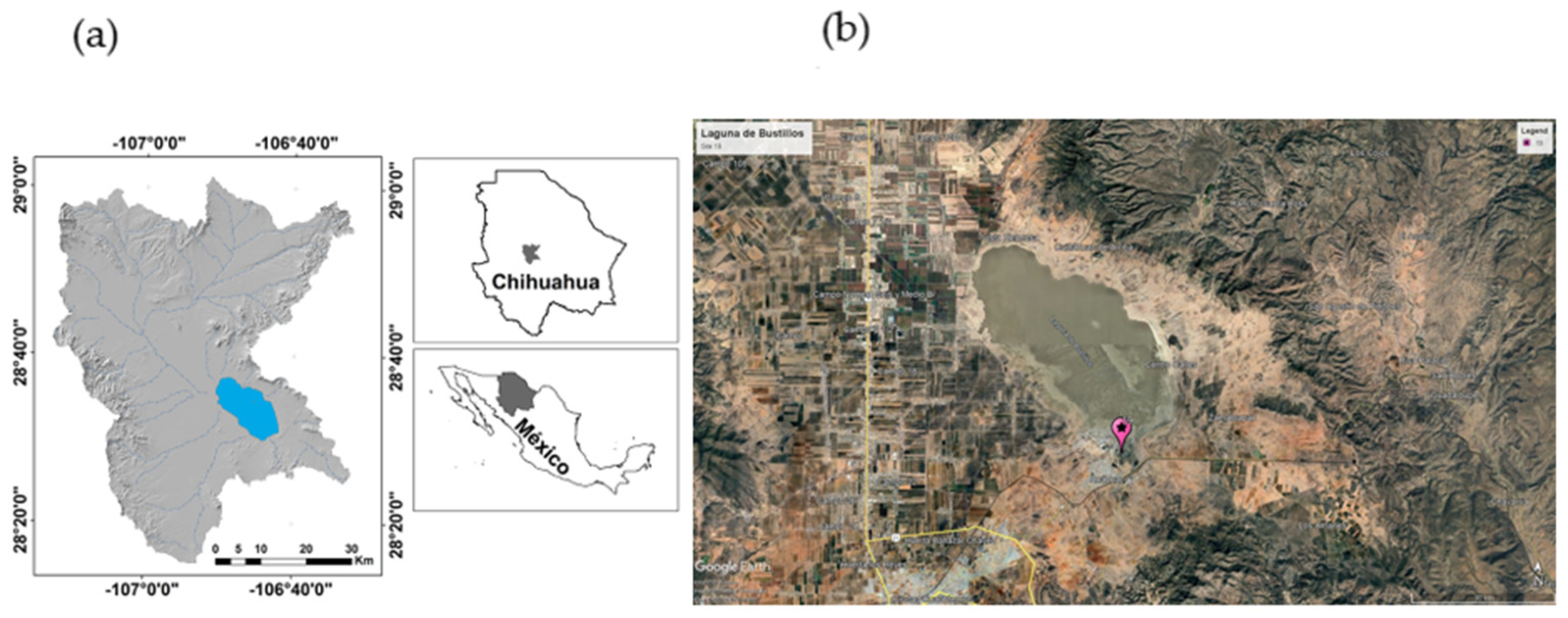
2.2. Materials
2.3. Water Sample Collection and Analysis
2.4. Biochar Synthesis
2.5. Biochar Characterizations
2.6. Potentiometric Titration Procedure of Biochars
3. Results and Discussion
3.1. BBAF1 Synthesis
3.2. Structural Characteristics of the BBAF1, CACCF2, and CVCF3 Biochars
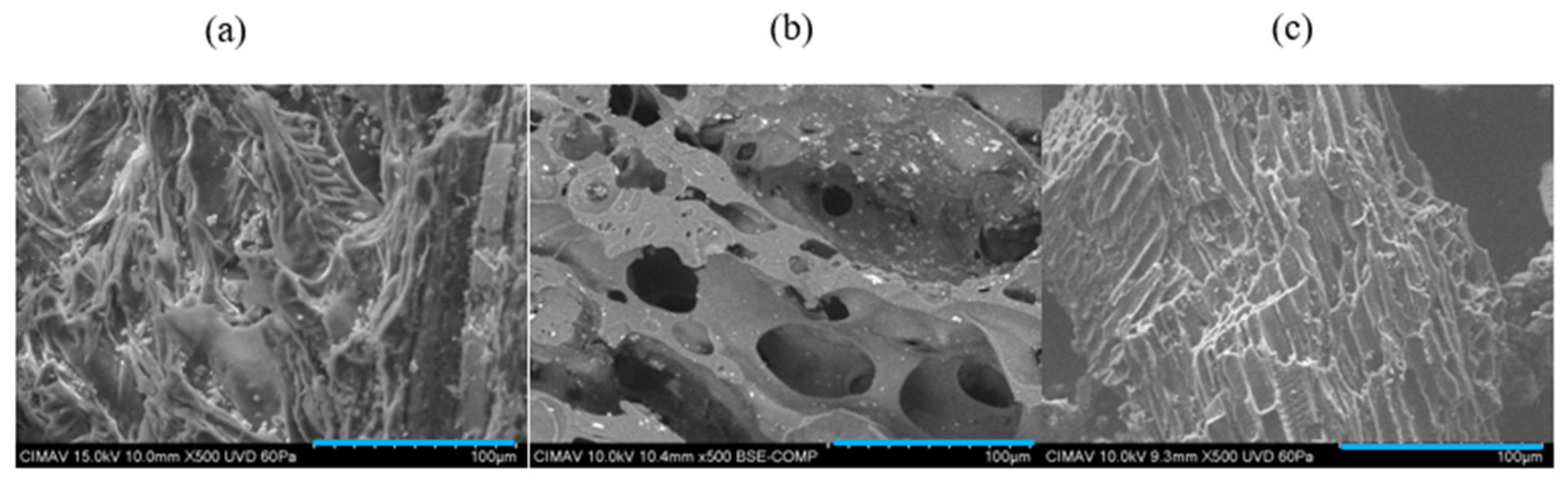
3.2.1. SEM
3.2.2. FTIR
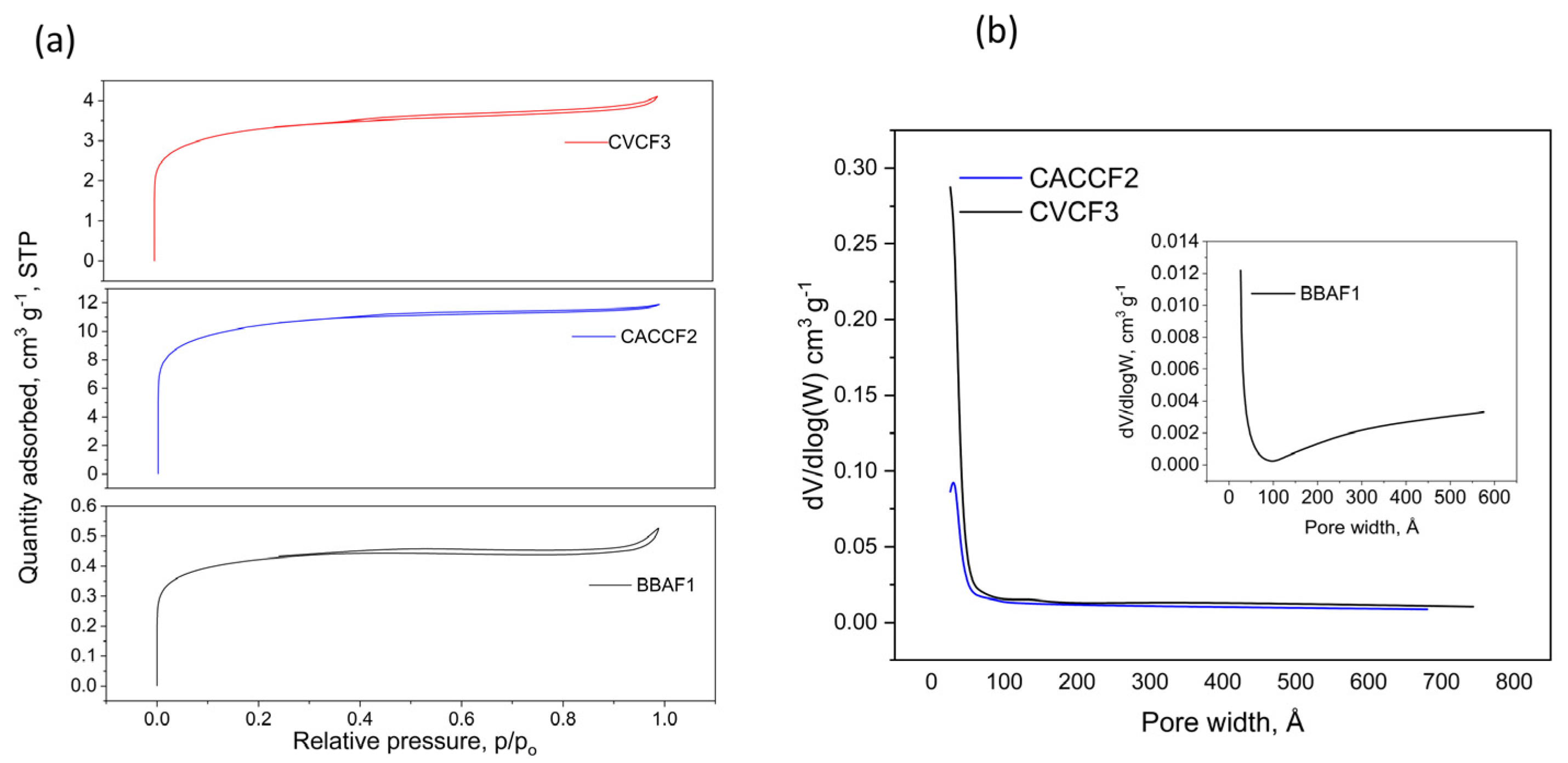
3.2.3. N2 Adsorption Isotherm Results
3.2.4. XRD and ζ-Potential Results
3.3. Pollutant Removal from Laguna Bustillos
3.4. Act and Dfc Removal in Packed Columns
3.5. Potentiometric Titration Results
3.6. Act and Dfc Removal
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Act | Acetaminophen |
| Dfc | Diclofenac |
| BBAF1 | Biochar Bagasse Agave Filter 1 |
| CACCF2 | Coconut Shell Activated Carbon Filter 2 |
| CVCF3 | Comercial Vegetable Carbon Filter 3 |
| NO3 | Nitrates |
| NO2 | Nitrites |
| NH3 | Ammonia |
| TN | Total Nitrogen |
| COD | Chemical Oxygen Demand |
| TP | Total Phosphorus |
| PO4 | Phosphate |
| LB | Bustillos Lagoon |
| km | Kilometer |
| L | Liter |
| FTU | Formazin Turbidity Units |
| PtCo | Platinum-Cobalt scale |
| pH | Potential of hydrogen |
| UV-Vis | ultraviolet and visible |
| SEM | Scanning Electron Microscope |
| Pa | Pascal |
| SE | Secondary electron |
| BSE-COMP | Backscattered electron |
| FTIR-ATR | Fourier transform infrared attenuated total reflection spectra |
| BET | Brunauer–Emmett–Teller |
| CO2 | Carbon dioxide |
| BJH | Barret-Joyner-Halenda |
| N | Normality |
| M | Molarity |
| NaNO3 | Sodium Nitrate |
| HNO3 | Nitric Acid |
| Å | Angstrom |
References
- The United Nations World Water Development Report 2020: Water and Climate Change. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000372985.locale=en (accessed on 5 November 2024).
- Wang, X.; Liu, J.; Yu, Z.; Li, Y. Functionalized carbon-based nanomaterials for wastewater treatment. Mater. Adv. 2021, 2, 31–49. [Google Scholar]
- Feng, Y.; Zhang, J.; Zhao, W. Carbon-based adsorbents for emerging contaminant removal from water: Recent advances and prospects. Environ. Chem. Eng. 2024, 12. [Google Scholar]
- You, X.; Liu, M.; Chen, X.; Li, Y.; Yang, Y. Mechanistic insight into the simultaneous removal of Cr(VI) and phosphate by a novel versatile bimetallic material. J. Environ. Chem. Eng. 2024, 12. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, F.; Li, H.; Cui, J.; Ren, Y.; Yu, X. Recent Progress in Biochar-Based Photocatalysts for Wastewater Treatment: Synthesis, Mechanisms, and Applications. Appl. Sci. 2020, 10, 1019. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Saadi, W.; Ruiz, B.; Najar-Souissi, S.; Ouederni, A.; Fuente, E. High-pressure gas adsorption on activated carbons from pomegranate peels biochar: A promising approach for biogas purification. Biomass Bioenergy 2024, 186, 107258. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Yalasangi, V.; Mayilswamy, N.; Kandasubramanian, B. Biochar-derived adsorbents for removal of Rhodamine B from wastewater. Bioresour. Technol. Rep. 2024, 28, 101987. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: Science, technology and implementation. In Biochar for Environmental Management, 3rd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2024; pp. 222–591. [Google Scholar]
- García, A.P.G.; Hernández, S.C.; Jiménez, L.D. Agave lechuguilla waste can be applied as biochar-adsorbent to remove arsenic from water. Int. J. Environ. Sci. Technol. 2024, 22, 9193–9208. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U., Jr. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2017, 210, 26–32. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Thomas, H.; Cavalcante, J.D.N.; Araújo, P.C.E.; Silvino, D.S.E.; Perin, G.F.; da Silva, R.L.A.; Torres, S.A.; Cezar, M.R.S.; Dutra, D.E. Valorization of lignocellulosic agave residues via pyrolysis and its use as adsorbent for methylene blue removal. Biomass Conv. Bioref. 2024, 15, 6743–6756. [Google Scholar] [CrossRef]
- Salcedo-Chitica, A. Assessment of the Eutrophication Status in Laguna de Bustillos, Anáhuac, Mexico; Geological Engineering, Autonomous University of Chihuahua: Chihuahua, Mexico, 2015. [Google Scholar]
- CONABIO – AvesMX. 2015. Available online: https://avesmx.conabio.gob.mx/AICA.html (accessed on 1 November 2024).
- Vidal, R.; Berlanga, H.; Del Coro Arizmendi, M. Important Bird Areas Americas—Priority sites for biodiversity conservation. BirdLife International (BirdLife Conservation Series No.16). Quito, Ecuador. Available online: https://www.researchgate.net/publication/343558933_Important_bird_areas_Americas_-_Priority_sites_for_biodiversity_conser-vation_BirdLife_International_BirdLife_Conservation_Series_No_16_Quito_Ecuador#fullTextFileContent (accessed on 4 June 2025).
- Rubio-Arias, H.; Saucedo, R.A.; Lara, C.R.; Wood, K.; Jiménez, J. Water quality in the Laguna de Bustillos of Chihuahua, México. WIT Trans. Ecol. Environ. 2005, 80, 155–160. [Google Scholar]
- Quintana, M.R.M.; Soto, C.G.; Rubio, A.H.; Espinoza, P.J.R.; Holguín, L.C.; Camarillo, A.J. Evaluation of physicochemical parameters and metal content in sediments of the Laguna de Bustillos in Chihuahua. Sci. Front. UACJ Sci. Technol. Mag. 2014, 12, 37–45. [Google Scholar]
- Ochoa-Rivero, J.M.; Reyes-Fierro, A.V.; Peralta-Pérez, M.D.R.; de la Serna, F.J.Z.-D.; Ballinas-Casarrubias, L.; Salmerón, I.; Rubio-Arias, H.; Rocha-Gutiérrez, B.A. Levels and Distribution of Pollutants in the Waters of an Aquatic Ecosystem in Northern Mexico. Int. J. Environ. Res. Public Health 2017, 14, 456. [Google Scholar] [CrossRef]
- Peña, L.; Torres, M.; Alatorre, L.; Sáenz, A.; Aguilar, S.A.; Sánchez-Flores, E. Land use changes and impacts on runoff in a watershed in Chihuahua, Mexico: Modeling from a hydrofunctional perspective. In Investigación Interdisciplinaria una Mirada Desde el Norte de México, 2015; Chávez, J., Olivas, I.R.Á., Eds.; Universidad Autónoma de Ciudad Juárez: Chihuahua, México, 2015. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- López-Covarrubias, M.; Aguilar-Juárez, O. Treatment of tequila vinasses using biochar derived from agave bagasse. Trends Renew. Energy Sustain. (TERYS) 2023, 2, 327–333. [Google Scholar]
- Davies, C.W. Ion Association; Butterworths: London, UK, 1962; pp. 37–53. [Google Scholar]
- Jagiełło, J.; Bandosz, T.; Schwarz, J. Carbon surface characterization in terms of its acidity constant distribution. Carbon 1994, 32, 1026–1028. [Google Scholar] [CrossRef]
- Jagiello, J. Stable numerical solution of the adsorption integral equation using splines. Langmuir 1994, 10, 2778–2785. [Google Scholar] [CrossRef]
- Hidalgo-Reyes, M.; Caballero-Caballero, M.; Hernández-Gómez, L.H.; Urriolagoitia-Calderón, G. Chemical and morphological characterization of Agave angustifolia bagasse fibers. Botanical Sci. 2015, 93(4), 807–817. [Google Scholar] [CrossRef]
- Application of magnetic biochar derived from sugarcane bagasse in water treatment. In Water Reuse Desalination; IWA Publishing: London, UK, 2022; Volume 14, pp. 613–625.
- MedCrave (online). Biochar from sugarcane bagasse for reactive dye adsorption considering a circular economy approach. Eng. Fash. Technol. 2020, 6, 65–70. [Google Scholar]
- Hernández-Ramírez, J.; Salazar-Rábago, J.J.; Labrada-Delgado, G.J.; Aragón-Piña, A. Evaluación de la capacidad adsorbente del biocarbón producido a partir de hojas de agave. Rev. Int. Contam. Ambient. 2020, 36, 79–91. [Google Scholar]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Granados, P.; Mireles, S.; Pereira, E.; Cheng, C.-L.; Kang, J.J. Effects of Biochar Production Methods and Biomass Types on Lead Removal from Aqueous Solution. Appl. Sci. 2022, 12, 5040. [Google Scholar] [CrossRef]
- Bavariani, M.Z.; Ronaghi, A.; Ghasemi, R. Influence of Pyrolysis Temperatures on FTIR Analysis, Nutrient Bioavailability, and Agricultural use of Poultry Manure Biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Armynah, B.; Atika; Djafar, Z.; Piarah, W.H.; Tahir, D. Analysis of Chemical and Physical Properties of Biochar from Rice Husk Biomass. J. Phys. Conf. Ser 2018, 979, 012038. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the bet equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Barquilha, C.E.; Braga, M.C. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Yang, J.; Wei, Q.; Li, D.; Yu, J.; Cai, Z. Study on adsorption performance of manganese titanium oxide composite biochar for removal of Rhodamine B. J. Indian Chem. Soc. 2023, 100, 100958. [Google Scholar] [CrossRef]
- Van Thinh, N.; Yusuke, S.; Tu, P.H.; Kuwahara, Y. Novel co-doped iron oxide and graphitic carbon nanosheets on biochar for arsenite removal from contaminated water: Synthesis, applicability and mechanism. Bioresour. Technol. Rep. 2022, 17, 100929. [Google Scholar] [CrossRef]
- Siqueira, T.C.A.; da Silva, I.Z.; Rubio, A.J.; Bergamasco, R.; Gasparotto, F.; Paccola, E.A.d.S.; Yamaguchi, N.U. Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. Int. J. Environ. Res. Public Health 2020, 17, 526. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z.; Chau, H.W. Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: Characterization and mechanism. Ecotoxicol. Environ. Saf. 2017, 144, 514–521. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Zhang, Y.; Lin, T.; Zeng, G.; Zhang, S.; Wang, Y.; He, W.; Zhang, M.; Long, H. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour. Technol. 2019, 288, 121511. [Google Scholar] [CrossRef]
- Ocampo-Perez, R.; Padilla-Ortega, E.; Medellin-Castillo, N.; Coronado-Oyarvide, P.; Aguilar-Madera, C.; Segovia-Sandoval, S.; Flores-Ramírez, R.; Parra-Marfil, A. Synthesis of biochar from chili seeds and its application to remove ibuprofen from water. Equilibrium and 3D modeling. Sci. Total Environ. 2019, 655, 1397–1408. [Google Scholar] [CrossRef]
- Beyan, S.M.; Prabhu, S.V.; Sissay, T.T.; Getahun, A.A. Sugarcane bagasse based activated carbon preparation and its adsorption efficacy on removal of BOD and COD from textile effluents: RSM based modeling, optimization and kinetic aspects. Bioresour. Technol. Rep. 2021, 14, 100664. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Comparison of the Surface Features of Two Wood-Based Activated Carbons. Ind. Eng. Chem. Res. 2000, 39, 301–306. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Jagiello, J.; Contescu, C.; Schwarz, J.A. Characterization of the surfaces of activated carbons in terms of their acidity constant distributions. Carbon 1993, 31, 1193–1202. [Google Scholar] [CrossRef]
- Bandosz, T.J. Surface chemistry of carbon materials. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- March, J.; Smith, M. Organic Chemistry, 6th ed.; J. Wiley and Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Schecher, W.D.; McAvoy, D.C. MINEQL+ V5.0: A Chemical Equilibrium Program for Personal Computers; Procter & Gamble Company: Cincinnati, OH, USA, 2023. [Google Scholar]
- Prescott, L.F. Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmac. 1980, 10, 2915–2985. [Google Scholar] [CrossRef] [PubMed]
- Settimo, L.; Bellman, K.; Knegtel, R.M.A. Comparison of the Accuracy of Experimental and Predicted pKa Values of Basic and Acidic Compounds. Pharm. Res. 2013, 31, 1082–1095. [Google Scholar] [CrossRef]
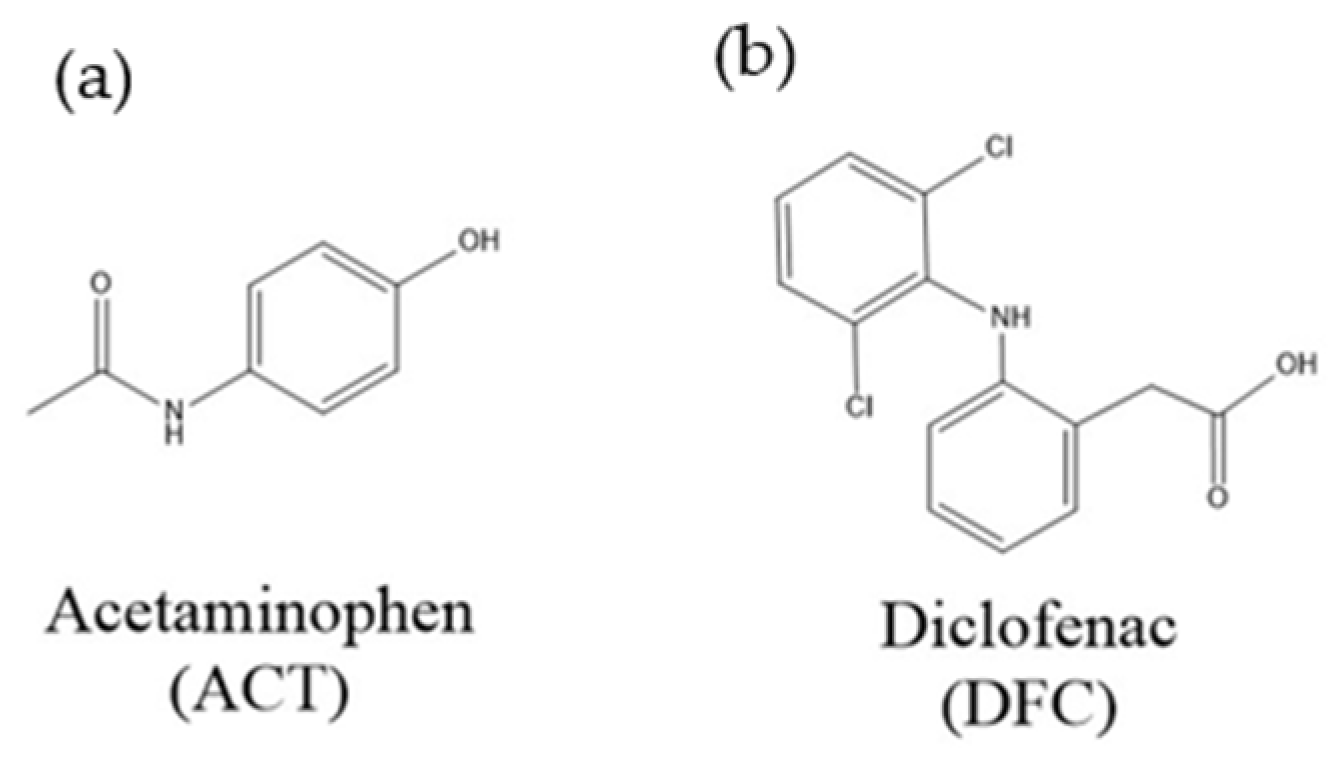





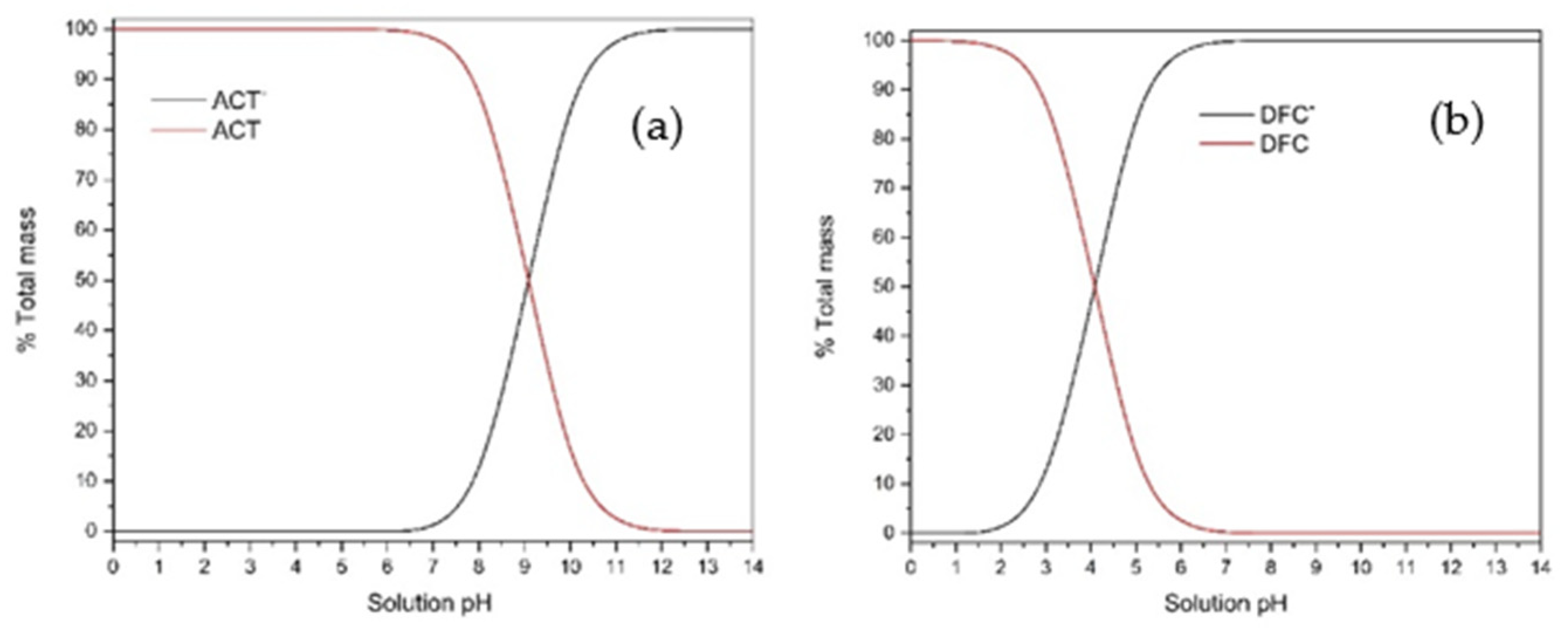
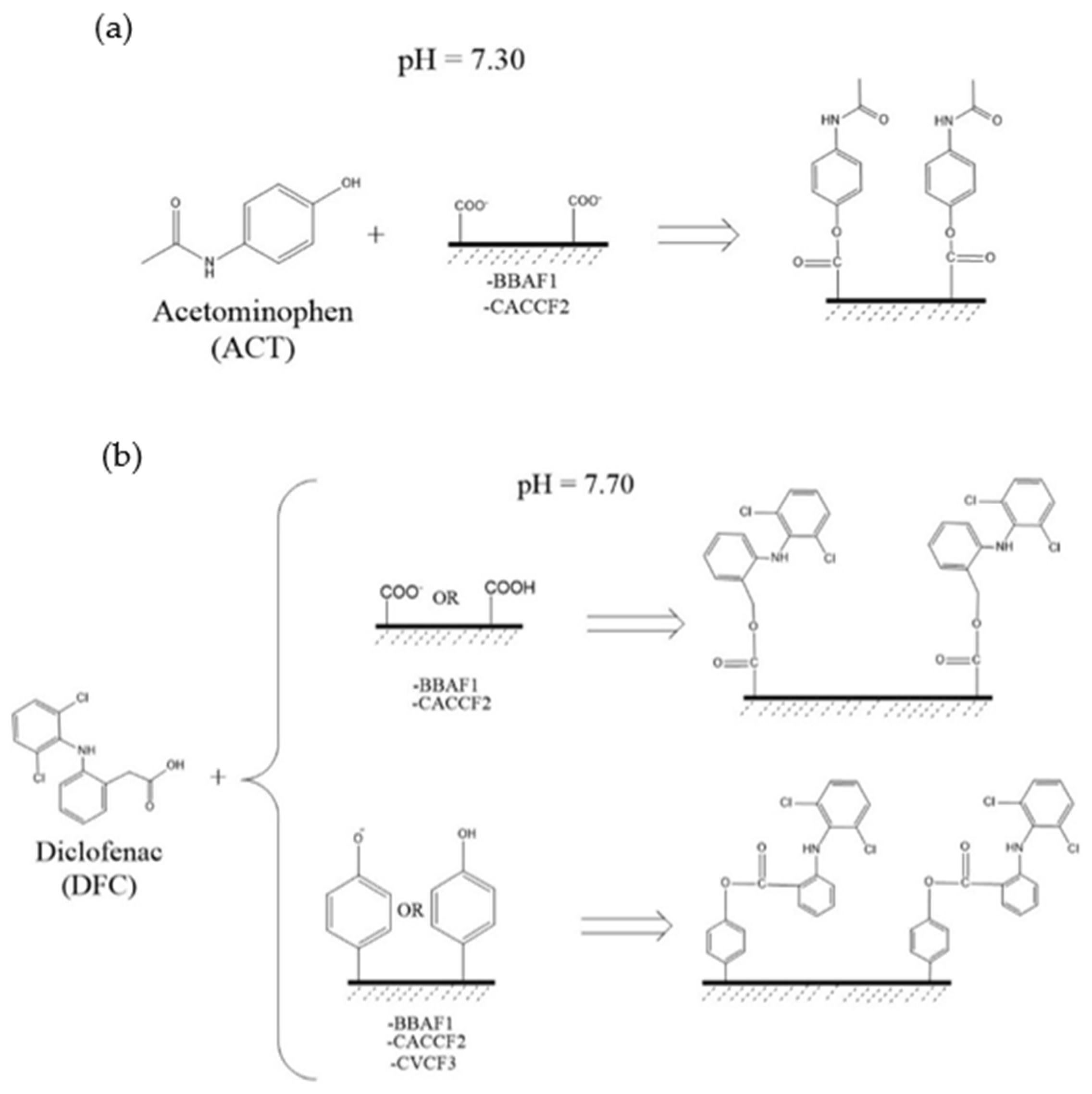
| Parameter | Concentration (mg L−1) | Parameter | Concentration (mg L−1) | Parameter | Concentration (mg L−1) |
|---|---|---|---|---|---|
| COD | 414 | NO2 | 0.139 | Turbidity | 214 FTU |
| NO3 | 1.3 | PO4 | 6.1 | Apparent Color | 646 PtCo |
| NH3 | 2.2 | NT | 8.63 | True color | 18 PtCo |
| pH | 7.8 |
| Temperature (°C) | Pyrolysis Time (min) | Initial Weight (g) | Final Weight (g) | Recovery Percentage (%) |
|---|---|---|---|---|
| 250 | 15 | 15.7 | 8.4 | 53.5 |
| 250 | 25 | 13.5 | 6.8 | 50.3 |
| 250 | 30 | 19.3 | 8.2 | 42.4 |
| 300 | 15 | 15.1 | 7.7 | 50.9 |
| Sample | Feedstock | Pyrolysis Conditions | Morphology Description | Reference |
|---|---|---|---|---|
| BBAF1 | Agave tequilana Weber bagasse | 250 °C, 20 min | Fibrous structure derived from cellulose chains in agave cores; moderate surface porosity with visible microcavities. | This study |
| Biochar from Agave angustifolia | Agave angustifolia bagasse | 400 °C 1 h | Rough surface with heterogeneous porosity; partial preservation of fibrous structure. | [31] |
| Magnetic biochar from sugarcane bagasse | Sugarcane bagasse | 500 °C, 2 h | Highly porous surface with lamellar structures; pore formation due to multicellular thermal degradation. | [32] |
| Activated biochar from sugarcane bagasse | Sugarcane bagasse | 600 °C, 1.5 h | Presence of microtubes (0.6–1.0 µm) and cavities <0.5 µm; highly porous, enhancing adsorption. | [33] |
| Biochar from agave leaves | Agave spp. leaves | 400 °C, 1 h | Porous structure with wide pore size distribution (0.01–300 µm); porosity results from volatile component loss. | [34] |
| Sample | SBET (m2 g−1) | CBET | Pore Size, Dp (Å) | Vt (cm3 g−1) | Vmic (cm3 g−1) | Vmeso a (cm3 g−1) | Vmic/Vt % | Vmeso/Vt % | Sexto (m2 g−1) | Smic (m2 g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| BBAF1 | 34.70 ± 0.14 | 1410.0 | 65.4 | 0.018 | 0.008 | 0.009 | 45.56 | 54.29 | 14.24 | 20.45 |
| CACCF2 | 855.24 ± 6.72 | 1042.8 | 38.3 | 0.409 | 0.198 | 0.211 | 48.55 | 51.44 | 374.43 | 480.81 |
| CVCF3 | 269.35 ± 3.88 | 689.58 | 48.3 | 0.139 | 0.058 | 0.081 | 42.04 | 58.39 | 127.36 | 141.98 |
| Day | Column No. | Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COD | NO3 | NO2 | NH3 | NT | PO4 | Turbidity | Apparent Color | True Color | ||
| 1 | 1 | 217 | 0.8 | 0.048 | 0.342 | 2.64 | 4.2 | 8.9 | 160 | 18 |
| 2 | 220 | 0.6 | 0.039 | ---- | 2.93 | 5.9 | 18 | 49 | 15 | |
| 3 | 308 | 0.7 | 0.048 | ---- | 3.3 | 5.9 | 22 | 83 | 19 | |
| 2 | 1 | 138 | 0.7 | 0.044 | ---- | 2.83 | 4.34 | 7.42 | 120 | 19 |
| 2 | 202 | 0.7 | 0.043 | ---- | 3.14 | 6.1 | 4.4 | 50 | 10 | |
| 3 | 391 | 2.1 | 0.057 | ---- | 5.31 | 7.2 | 25 | 136 | 13 | |
| 3 | 1 | 185 | 0.634 | 0.032 | 0.246 | 3.45 | 1.53 | 2.03 | 62 | 15 |
| 2 | 289 | 0.62 | 0.036 | 0.272 | 2.94 | 3.89 | 2.79 | 37 | 12 | |
| 3 | 308 | 0.877 | 0.065 | 0.195 | 4.94 | 8.4 | 25.9 | 202 | 15 | |
| 4 | 1 | 193 | 0.99 | 0.028 | ---- | 3.21 | 2.52 | 2.9 | 72 | 13 |
| 2 | 206 | 0.549 | 0.04 | ---- | 3.53 | 6.17 | 2.05 | 82 | 18 | |
| 3 | 213 | 0.673 | 0.099 | ---- | 19.1 | 21.6 | 39 | 18 | 19 | |
| 5 | 1 | 191 | 0.664 | 0.03 | ---- | 4.06 | 2.77 | 3.45 | 74 | 15 |
| 2 | 211 | 0.429 | 0.037 | ---- | 4.11 | 6.68 | 3.14 | 42 | 19 | |
| 3 | 309 | 0.844 | 0.097 | ---- | 8.2 | 8.17 | 50 | 254 | 26 | |
| 6 | 1 | 235 | 0.861 | 0.204 | 1.1 | 0.984 | 3.1 | 3.22 | 65 | 17 |
| 2 | 230 | 0.424 | 0.093 | 2.1 | 2.48 | 5.91 | 3.04 | 51 | 13 | |
| 3 | 401 | 0.667 | 0.071 | 3.07 | 5.63 | 11.3 | 44 | 293 | 22 | |
| 16 | 1 | 384 | 1.1 | 0.144 | 1.92 | 8.35 | 4.1 | 9.2 | 88 | 25 |
| 2 | 299 | 0.633 | 0.097 | 1.02 | 8.11 | 3.3 | 8.1 | 73 | 21 | |
| 3 | 901 | 0.921 | 0.106 | 4.53 | 10.3 | 5.7 | 66 | 142 | 33 | |
| Pharmaceutical | pH | Removal mg g−1 (% in Parenthesis) | ||
|---|---|---|---|---|
| Column 1 | Column 2 | Column 3 | ||
| Act | 7.3 | 0.506 (59) | 0.67 (85) | 0.43 (48) |
| Dfc | 7.7 | 0.549 (64) | 0.624 (79) | 0.342 (37) |
| Feedstock | Adsorbate | Adsorption Capacity, mg g−1 | Reference |
|---|---|---|---|
| Banana peel biochar | Rhodamine B | 19.06 | [43] |
| Co-doped Fe2O3 and graphitic carbon nanosheets on biochar | Arsenite | 49.2 | [44] |
| Sugar cane bagasse | Methylene blue | 9.41 | [45] |
| Corn straws | As (III) | 2.9–8.3 | [46] |
| Camphor leaves | Ciprofloxacin | 379.7 | [47] |
| Chili seeds | Ibuprofen | 12.8–26.1 | [48] |
| Sugarcane bagasse-based activated carbon | DQO | 884 | [49] |
| Agave bagasse biochar | Act and Dfc | 0.67 and 0.62, respectively | This work |
| Agave bagasse biochar | DQO | 5.91 | This work |
| Sample | pKa | Assignment | Content of Groups, mmol/g | Ligand Density/nm2 |
|---|---|---|---|---|
| BBAF1 | 5.54 | Carboxyls | 0.17 | 2.95 |
| 6.73 | Carboxyls | 0.2 | 3.47 | |
| 9.91 | OHs | 0.65 | 11.28 | |
| CACCF2 | 6.3 | Carboxyls | 0.05 | 0.03 |
| 7.6 | OHs | 0.11 | 0.07 | |
| CVCF3 | 9.16 | OHs | 0.32 | 0.71 |
| Sample/Functional Group | pKa | Individual Functional Group Contents, % a | Total % | ||||
|---|---|---|---|---|---|---|---|
| -COO- | -COOH | PhO- | PhOH | Negative Functional Groups | Neutral Functional Groups | ||
| Act initial solution pH = 7.30 | |||||||
| BBAF1 | |||||||
| Carboxyls | 5.54 | 98.3 | 1.7 | -- | -- | 177.25 | 101.45 |
| Carboxyls | 6.73 | 78.7 | 21.3 | -- | -- | ||
| OH | 9.91 | -- | -- | 0.25 | 99.75 | ||
| CACCF2 | |||||||
| Carboxyls | 6.3 | 90.9 | 9.1 | -- | -- | 124.2 | 75.8 |
| OH | 7.6 | -- | -- | 33.3 | 66.7 | ||
| CVCF3 | |||||||
| OH | 9.16 | -- | -- | 1.4 | 98.6 | 1.4 | 98.6 |
| Dfc initial solution pH = 7.70 | |||||||
| BBAF1 | |||||||
| Carboxyls | 5.54 | 99.31 | 0.69 | -- | -- | 190.22 | 109.78 |
| Carboxyls | 6.73 | 90.3 | 9.7 | -- | -- | ||
| OH | 9.91 | -- | -- | 0.61 | 99.39 | ||
| CACCF2 | |||||||
| Carboxyls | 6.3 | 96.15 | 3.85 | -- | -- | 152.05 | 47.95 |
| OH | 7.6 | -- | -- | 55.9 | 44.1 | ||
| CVCF3 | |||||||
| OH | 9.16 | -- | -- | 3.39 | 96.61 | 3.39 | 96.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Esparza, W.N.; Aguilar-Juárez, O.; Gómez-Salazar, S.; Morán-Salazar, R.; López-Covarrubias, M.; Leal-Quezada, L.O.; Del Real-Olvera, J.; Reyes-Gómez, V.M. Efficiency of a New Biochar Made from Agave Bagasse to Remove Conventional Pollutants in Samples from Laguna de Bustillos, Chihuahua, Mexico, and Pharmaceutical Derivatives in Synthetic Water. Processes 2025, 13, 1861. https://doi.org/10.3390/pr13061861
Medina-Esparza WN, Aguilar-Juárez O, Gómez-Salazar S, Morán-Salazar R, López-Covarrubias M, Leal-Quezada LO, Del Real-Olvera J, Reyes-Gómez VM. Efficiency of a New Biochar Made from Agave Bagasse to Remove Conventional Pollutants in Samples from Laguna de Bustillos, Chihuahua, Mexico, and Pharmaceutical Derivatives in Synthetic Water. Processes. 2025; 13(6):1861. https://doi.org/10.3390/pr13061861
Chicago/Turabian StyleMedina-Esparza, Wendy Nayely, Oscar Aguilar-Juárez, Sergio Gómez-Salazar, René Morán-Salazar, Montserrat López-Covarrubias, Luz Olivia Leal-Quezada, Jorge Del Real-Olvera, and Víctor Manuel Reyes-Gómez. 2025. "Efficiency of a New Biochar Made from Agave Bagasse to Remove Conventional Pollutants in Samples from Laguna de Bustillos, Chihuahua, Mexico, and Pharmaceutical Derivatives in Synthetic Water" Processes 13, no. 6: 1861. https://doi.org/10.3390/pr13061861
APA StyleMedina-Esparza, W. N., Aguilar-Juárez, O., Gómez-Salazar, S., Morán-Salazar, R., López-Covarrubias, M., Leal-Quezada, L. O., Del Real-Olvera, J., & Reyes-Gómez, V. M. (2025). Efficiency of a New Biochar Made from Agave Bagasse to Remove Conventional Pollutants in Samples from Laguna de Bustillos, Chihuahua, Mexico, and Pharmaceutical Derivatives in Synthetic Water. Processes, 13(6), 1861. https://doi.org/10.3390/pr13061861








