Study on the Influence of Network Characteristics of Artificial Main Fractures and Natural Fractures on the Effectiveness of Acid Fracturing
Abstract
1. Introduction
2. Mathematical Models
- (1)
- Pressure equation of acid fracturing The acid used in the acid fracturing process is assumed to be an incompressible fluid. Thus, the pressure equation for the matrix and fracture can be expressed [8,30]:where and are the permeabilities of matrix and fracture (), respectively. and are the viscosity of liquid in the matrix and the fracture (Pas), respectively. is the core function of the EDFM model, and specific calculation method can be found in Lee’s paper [31]. and are the pressure field of the matrix and the fracture to be solved (Pa), respectively.
- (2)
- Velocity equation of acid fracturing
- (3)
- Acid concentration equation () of acid fracturingBased on the TSC and EDFM model, we can derive the transport equation containing the material exchange between the matrix and the fracture [8,30]:where and are the porosity of the matrix and the fracture, respectively. and the mass concentration of acid () of the matrix and the fracture (kmol/), respectively. and are the effective dispersion tensor of the matrix and the fracture (kmol/(s)), respectively. and are the rock interfacial areas of the matrix and the fracture (/).
- (4)
- Chemical reaction process of the self-diverting acid and rock:We mainly discuss the acid fracturing process in carbonate reservoirs. The acid–rock reaction process is mainly the chemical reaction between and :where represents the acid fracturing reaction rate. Base on Equation (4), the chemical reaction terms of Equation (3) can be expressed as follows:where is the mass transfer coefficient (m/s), is the reaction rate constant, R is the gas constant, is the, m is the asymptotic Sherwood number, is the ratio of the pore length to pore diameter, is the pore Reynolds number, and is the average pore radius (m).
- (5)
- Rock quantity updating:Based on the TSC model, the acid–rock reaction process can change the pore quantity of rock (matrix and fracture). The updated calculation formula is as follows [14]:where is the dissolving power of the acid. The updated calculation formula for the quantity at the poro-scale is [14]:where the superscript i represents the quantity after reaction, and the subscript 0 represents the initial quantity at the beginning of the simulation. Further, represents the diffusion coefficient (m/s), and the subscripts L and T represent the x and y directions, respectively. and are 0.5 and 0.25 in this paper, which are related to the pore characteristics [14].
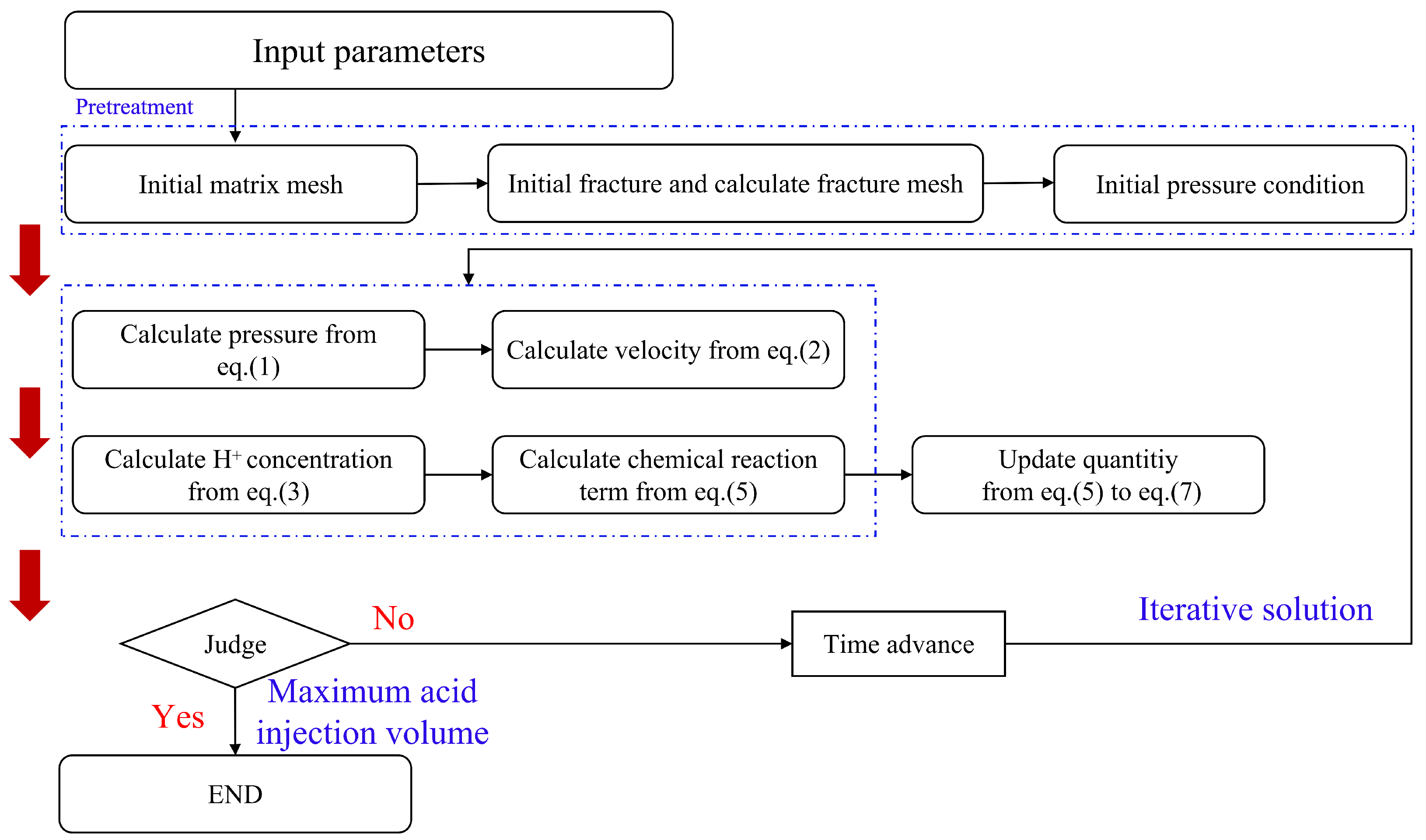
3. Numerical Methods
3.1. Discretization Method
- (1)
- Pressure Equation
- (2)
- Acid concentration equation ():
3.2. Computational Methods and Simulation Parameters
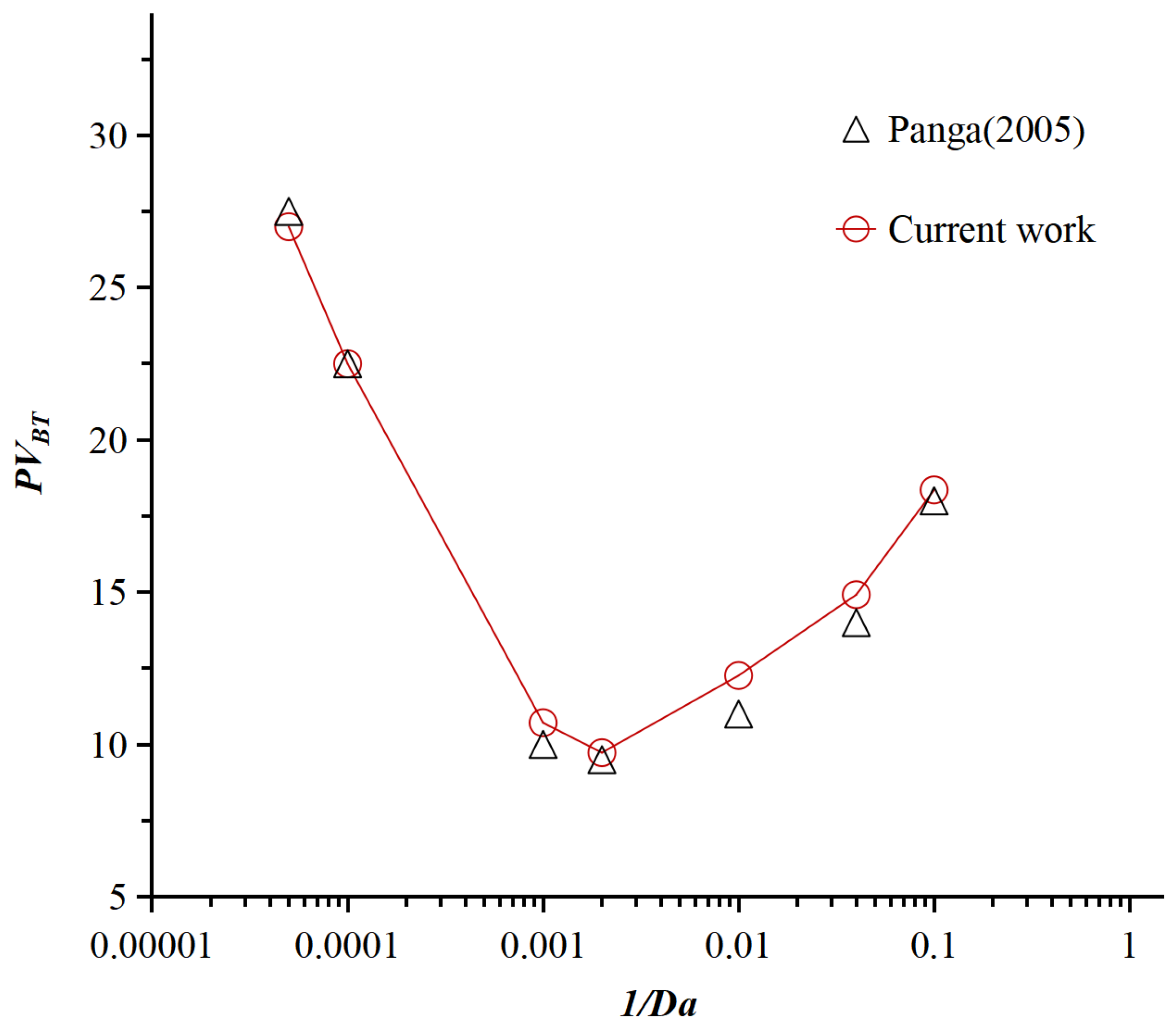
3.3. Model Validation
4. Results and Analysis
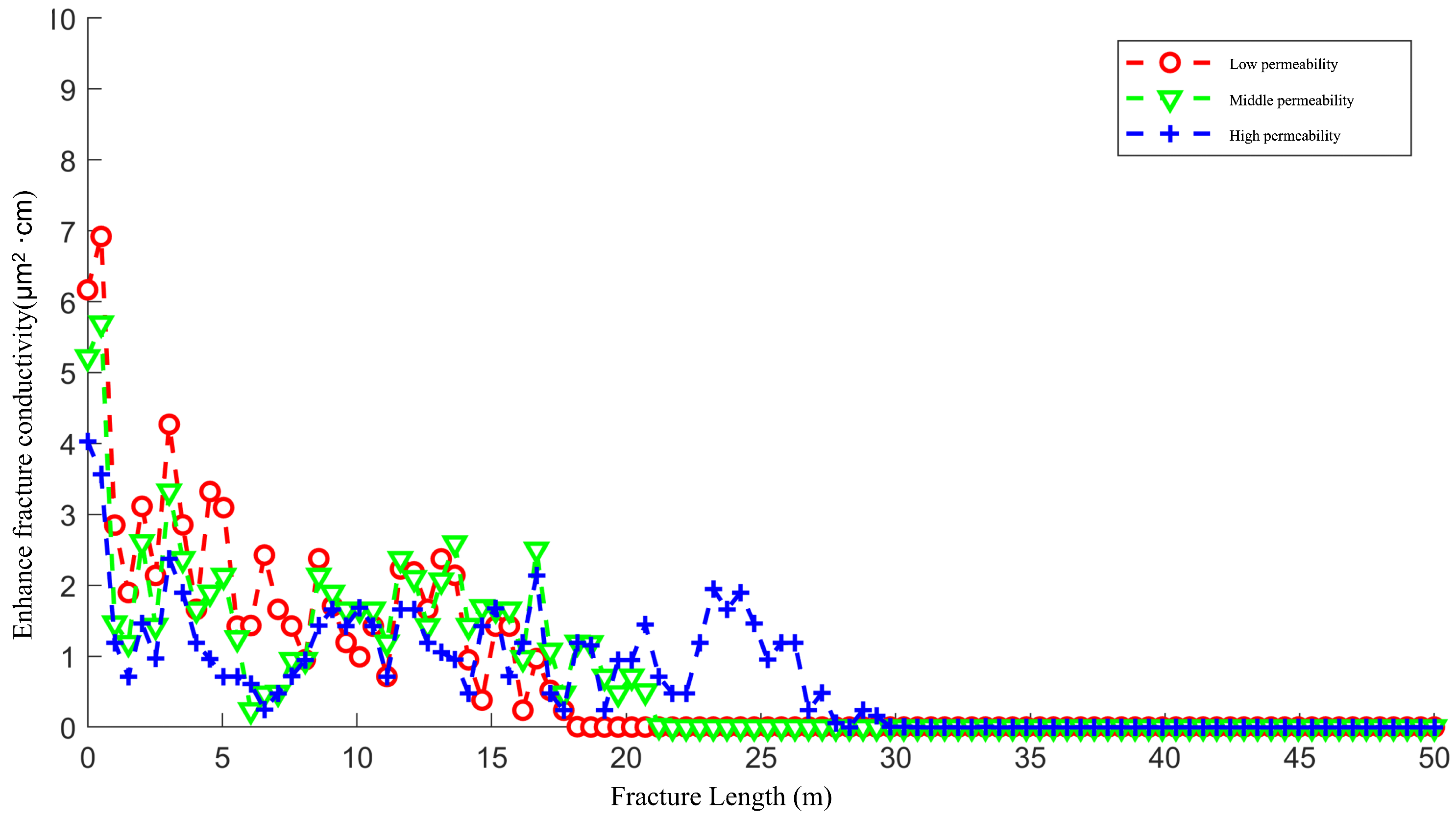
4.1. The Effect of the Permeability of Artificial Main Fractures on the Acid Fracturing Effect
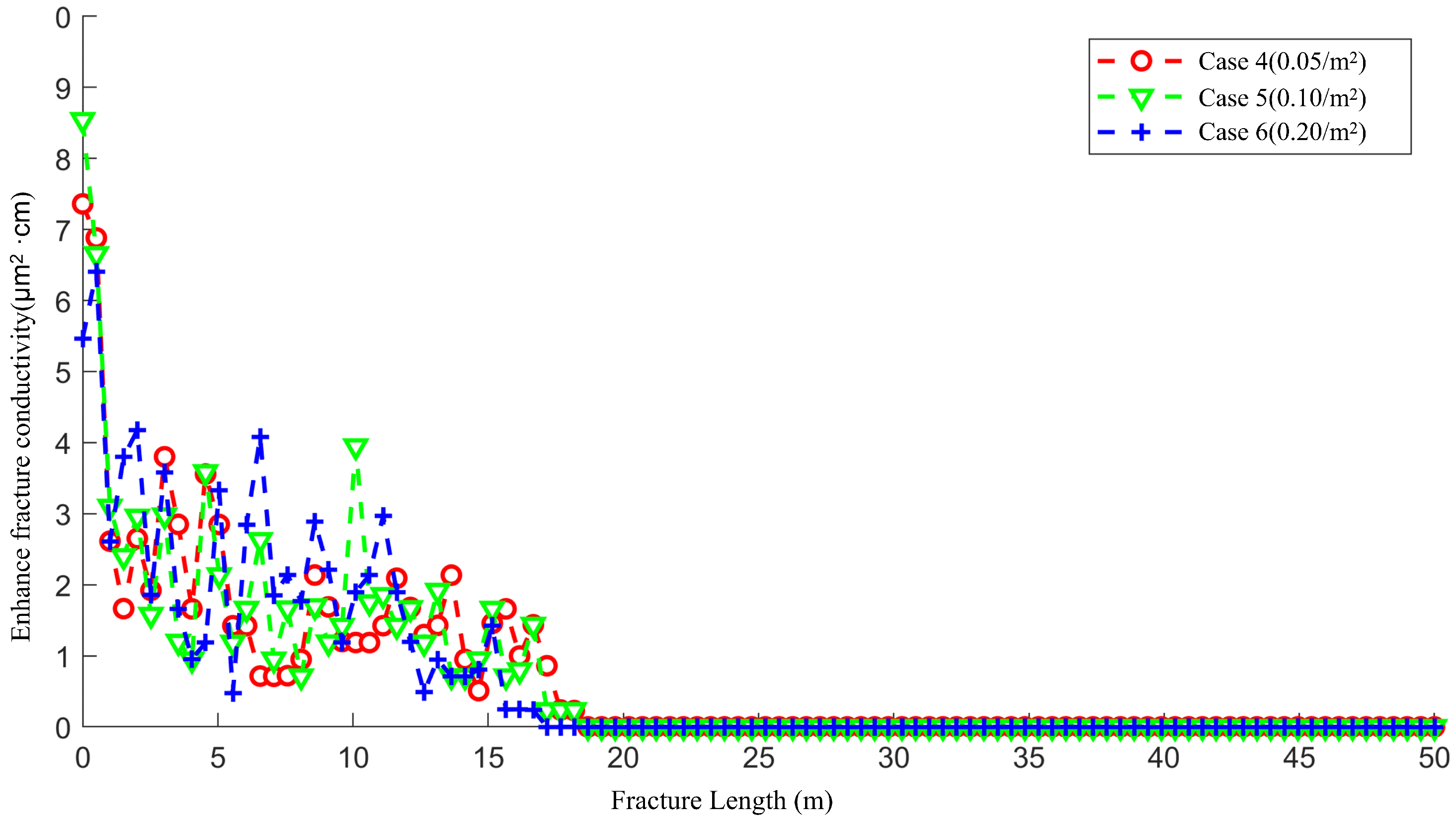
4.2. The Effect of Natural Fracture Density on the Acid Fracturing Effect of Low-Permeability Main Fractures
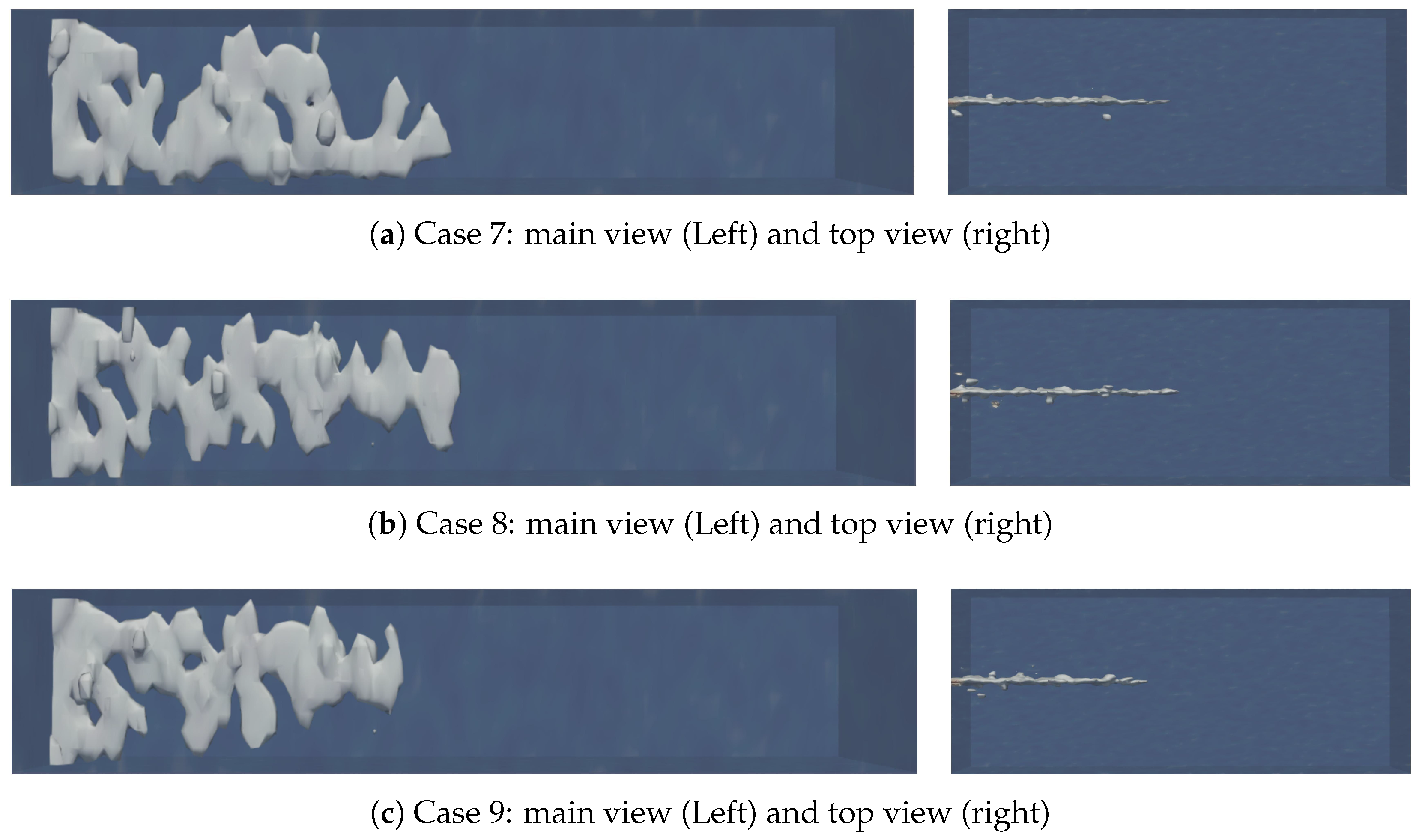
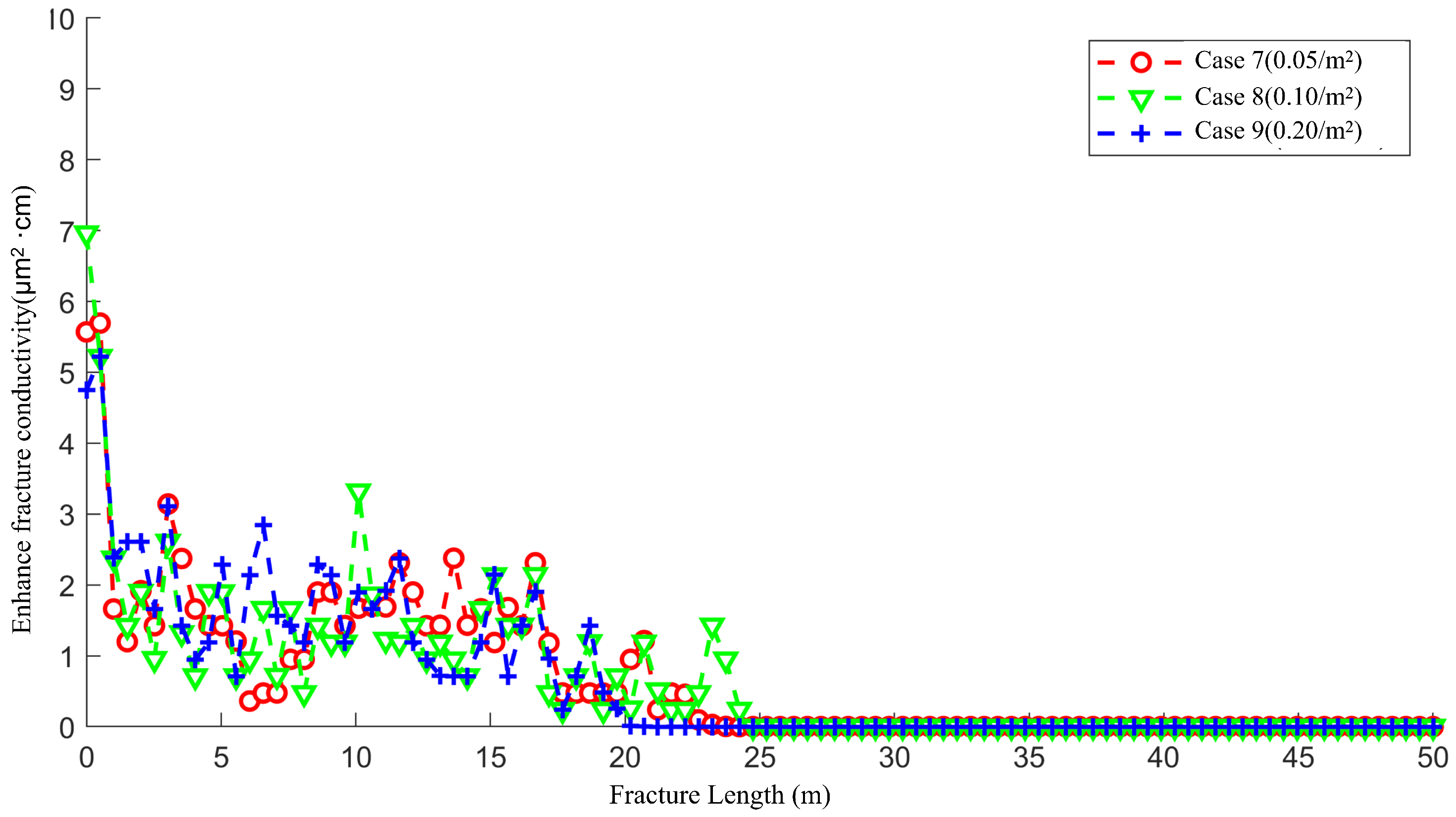
4.3. The Effect of Natural Fracture Density on the Acid Fracturing Effect of Middle-Permeability Main Fractures
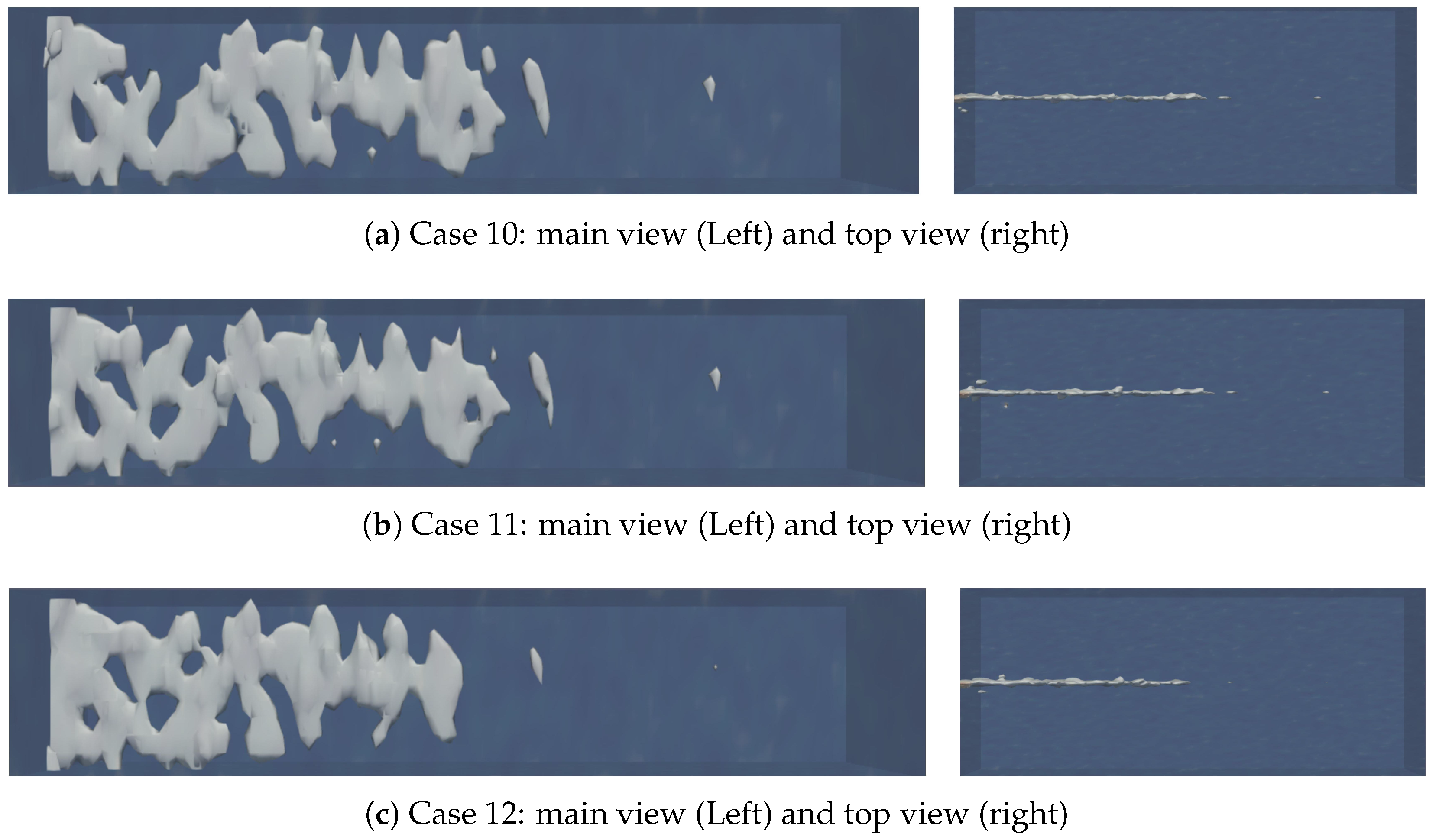
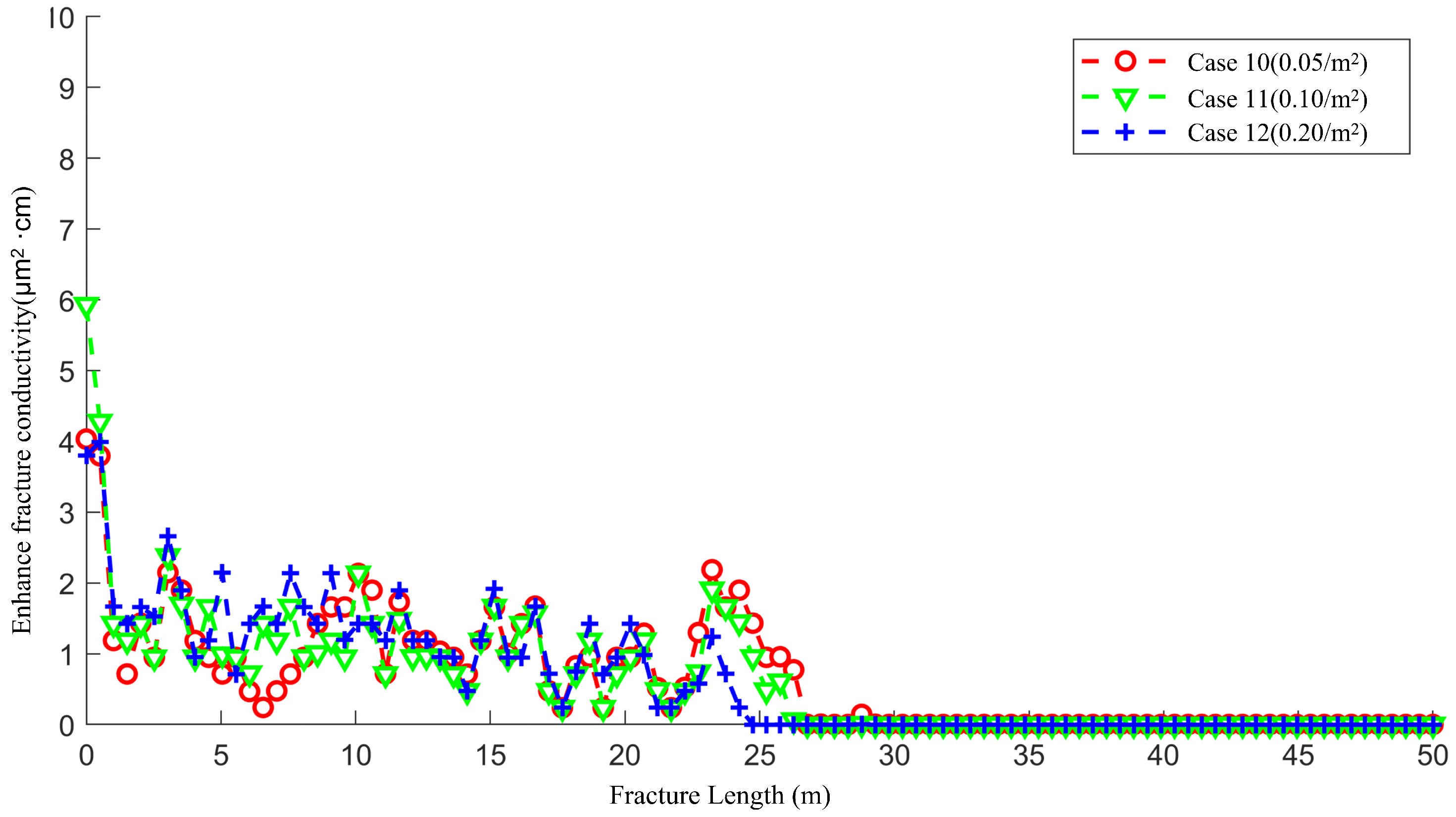
4.4. The Effect of Natural Fracture Density on the Acid Fracturing Effect of High-Permeability Main Fractures
5. Conclusions
- (1)
- Under the same acid injection volume conditions, the higher the permeability of the artificial main fracture, the longer the length of the wormhole formed, and the less the acid leakage. Moreover, the higher the permeability of the main fracture, the less the improvement in fracture conductivity near the wellbore after acid fracturing, but the greater the improvement in average fracture conductivity.
- (2)
- The communication between natural fractures and artificial main fractures locally increases the filtration rate of acid into the matrix, guiding the formation of wormhole branches. However, at low natural fracture densities, there are fewer natural fractures communicating with the artificial main fracture, resulting in lateral filtration of the acid solution The trend has not been significantly altered by natural fractures, and there are fewer branches of wormholes formed.
- (3)
- For low-permeability main fractures, natural fractures lead to the longest and most numerous wormhole branches. As the permeability of artificial main fracture increases, the influence of natural fractures gradually decreases.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aljawad, M.S.; Aljulaih, H.; Mahmoud, M.; Desouky, M. Integration of field, laboratory, and modeling aspects of acid fracturing: A comprehensive review. J. Pet. Sci. Eng. 2019, 181, 106158. [Google Scholar] [CrossRef]
- Lo, K.; Dean, R. Modeling of acid fracturing. SPE Prod. Eng. 1989, 4, 194–200. [Google Scholar] [CrossRef]
- Hewett, T. Fractal distributions of reservoir heterogeneity and their influence on fluid transport. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 5–8 October 1986; p. SPE-15386-MS. [Google Scholar]
- Li, N.; Dai, J.; Liu, P.; Luo, Z.; Zhao, L. Experimental study on influencing factors of acid-fracturing effect for carbonate reservoirs. Petroleum 2015, 1, 146–153. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y. Triaxial experimental investigation into the characteristics of acid-etched fractures and acid fracturing. J. Pet. Sci. Eng. 2021, 202, 108431. [Google Scholar] [CrossRef]
- Ugursal, A.; Zhu, D.; Hill, A.D. Development of acid fracturing model for naturally fractured reservoirs. SPE Prod. Oper. 2019, 34, 735–748. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Y.; Peng, Y.; Zhao, J.; Qi, T.; Zeng, J.; Hou, P. Simulation and evaluation for acid fracturing of carbonate reservoirs based on embedded discrete fracture model. Nat. Gas Ind. B 2021, 8, 637–649. [Google Scholar] [CrossRef]
- Chang, T.; Jiang, Y.; Li, Y.; Chen, X.; Kang, X.; Mo, W. Study on the effect of natural fractures and temperature on the wormhole morphology formed by two-phase acidizing in carbonate rocks. Phys. Fluids 2024, 36, 083333. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Zhu, Y.; Liu, Y. Effects of acid–rock reaction heat on fluid temperature profile in fracture during acid fracturing in carbonate reservoirs. J. Pet. Sci. Eng. 2014, 122, 31–37. [Google Scholar] [CrossRef]
- You, J.; Lee, K.J. Analyzing the dynamics of mineral dissolution during acid fracturing by pore-scale modeling of acid-rock interaction. SPE J. 2021, 26, 639–652. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, H.; Chen, G.; Peng, J.; Han, H.; Qin, Y.; Wang, L.; Liu, D. Experimental study of the influences of different factors on the acid–rock reaction rate of carbonate rocks. J. Energy Storage 2023, 63, 107064. [Google Scholar] [CrossRef]
- Crowe, C.; Hutchinson, B.; Trittipo, B. Fluid-loss control: The key to successful acid fracturing. SPE Prod. Eng. 1989, 4, 215–220. [Google Scholar] [CrossRef]
- Bazin, B.; Roque, C.; Chauveteau, G.; Bouteca, M. Acid filtration in dynamic conditions to mimic fluid loss in acid fracturing. In Proceedings of the SPE European Formation Damage Conference and Exhibition, The Hague, The Netherlands, 2–3 June 1997; p. SPE-38168-MS. [Google Scholar]
- Panga, M.K.; Ziauddin, M.; Balakotaiah, V. Two-scale continuum model for simulation of wormholes in carbonate acidization. AIChE J. 2005, 51, 3231–3248. [Google Scholar] [CrossRef]
- Liu, H.; Baletabieke, B.; Wang, G.; Guo, J.; Xia, F.; Chen, Z. Influences of real-time acid–rock reaction heat on etched fracture dimensions during acid fracturing of carbonate reservoirs and field applications. Heliyon 2022, 8, e11659. [Google Scholar] [CrossRef]
- Guo, T.; Li, Y.; Ding, Y.; Qu, Z.; Gai, N.; Rui, Z. Evaluation of acid fracturing treatments in shale formation. Energy Fuels 2017, 31, 10479–10489. [Google Scholar] [CrossRef]
- Nierode, D.; Kruk, K. An evaluation of acid fluid loss additives retarded acids, and acidized fracture conductivity. In Proceedings of the Fall Meeting of the Society of Petroleum Engineers of AIME, Las Vegas, NV, USA, 30 September–3 October 1973; p. SPE-4549-MS. [Google Scholar]
- Nasr-El-Din, H.A.; Al-Driweesh, S.; Al-Muntasheri, G.A.; Marcinew, R.; Daniels, J.; Samuel, M. Acid fracturing HT/HP gas wells using a novel surfactant based fluid system. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003; p. SPE-84516-MS. [Google Scholar]
- Schriefer, F.E.; Shaw, M.S. Use of Fine Salt as a Fluid Loss Material in Acid Fracturing Stimulation Treatments. In Proceedings of the SPE Annual Fall Technical Conference and Exhibition, Houston, TX, USA, 1–3 October 1978; p. SPE-7570-MS. [Google Scholar]
- Zhang, L.; Mou, J.; Cheng, X.; Zhang, S. Evaluation of ceramsite loss control agent in acid fracturing of naturally fractured carbonate reservoir. Nat. Gas Ind. B 2021, 8, 302–308. [Google Scholar] [CrossRef]
- Ugursal, A.; Schwalbert, M.P.; Zhu, D.; Hill, A.D. Acid fracturing productivity model for naturally fractured carbonate reservoirs. In Proceedings of the SPE International Hydraulic Fracturing Technology Conference and Exhibition, Muscat, Oman, 16–18 October 2018; p. SPE-191433-18IHFT-MS. [Google Scholar]
- Broaddus, G.; Knox, J.; Fredrickson, S. Dynamic etching tests and their use in planning acid treatments. In Proceedings of the SPE Oklahoma City Oil and Gas Symposium/Production and Operations Symposium, Stillwater, OK, USA, 25 October 1968; p. SPE-2362-MS. [Google Scholar]
- Pournik, M.; Zhu, D.; Hill, A. Acid-fracture conductivity correlation development based on acid-fracture characterization. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Scheveningen, The Netherlands, 27–29 May 2009; p. SPE-122333-MS. [Google Scholar]
- Deng, J.; Mou, J.; Hill, A.D.; Zhu, D. A new correlation of acid-fracture conductivity subject to closure stress. SPE Prod. Oper. 2012, 27, 158–169. [Google Scholar] [CrossRef]
- Al-Momin, A.; Zhu, D.; Hill, A. The effects of initial condition of fracture surfaces, acid spending and acid type on conductivity of acid fracture. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 25–28 March 2014; p. OTC-24895-MS. [Google Scholar]
- Asadollahpour, E.; Baghbanan, A.; Hashemolhosseini, H.; Mohtarami, E. The etching and hydraulic conductivity of acidized rough fractures. J. Pet. Sci. Eng. 2018, 166, 704–717. [Google Scholar] [CrossRef]
- Malagon, C.; Pournik, M.; Hill, A.D. The texture of acidized fracture surfaces: Implications for acid fracture conductivity. SPE Prod. Oper. 2008, 23, 343–352. [Google Scholar] [CrossRef]
- Ruffet, C.; Fery, J.; Onaisi, A. Acid-fracturing treatment: A surface-topography analysis of acid-etched fractures to determine residual conductivity. SPE J. 1998, 3, 155–162. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Du, J.; Liu, J.; Xiong, G.; Chen, P.; Li, Y.; Chen, X. Evaluation of acid retardation based on acid-etched fracture morphology. Geoenergy Sci. Eng. 2025, 244, 213492. [Google Scholar] [CrossRef]
- Chang, T.; Jiang, Y.; Zhao, H.; Chen, X.; Mo, W. Effect of two-phase viscosity difference and natural fractures on the wormhole morphology formed by two-phase acidizing with self-diverting acid in carbonate rocks. Phys. Fluids 2024, 36, 093623. [Google Scholar] [CrossRef]
- Moinfar, A.; Varavei, A.; Sepehrnoori, K.; Johns, R.T. Development of an efficient embedded discrete fracture model for 3D compositional reservoir simulation in fractured reservoirs. SPE J. 2014, 19, 289–303. [Google Scholar] [CrossRef]
- Carman, P.C. Flow of Gases Through Porous Media; Academic Press: Cambridge, MA, USA, 1956. [Google Scholar]
- Valdes-Parada, F.J.; Ochoa-Tapia, J.A.; Alvarez-Ramirez, J. Validity of the permeability Carman–Kozeny equation: A volume averaging approach. Phys. A Stat. Mech. Its Appl. 2009, 388, 789–798. [Google Scholar] [CrossRef]


















| Parameter | [kmol/m3] | K | ||
| Value | 4.4178 (15 wt% HCL) | Calculated | 0.01–0.04 | 0.90 |
| Parameter | [mPas] | [kg/kmol] | [m2/m3] | [m2/m3] |
| Value | 1 | 50 | 5000 | 500 |
| Specific Parameters | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Main fracture permeability [D] | 5 | 10 | 20 |
| Equivalent fracture width [m] | 7.746 × | 1.0954 × | 1.5492 × |
| Injection displacement [] | 1 | ||
| Specific Parameters | Case 4 | Case 5 | Case 6 |
|---|---|---|---|
| Natural fracture density [m−2] | 0.05 | 0.1 | 0.2 |
| Main fracture permeability [D] | 5 | ||
| Injection displacement [] | 1 | ||
| Specific Parameters | Case 7 | Case 8 | Case 9 |
|---|---|---|---|
| Natural fracture density [m−2] | 0.05 | 0.1 | 0.2 |
| Main fracture permeability [D] | 10 | ||
| Injection displacement [] | 1 | ||
| Specific Parameters | Case 10 | Case 11 | Case 12 |
|---|---|---|---|
| Natural fracture density [m−2] | 0.05 | 0.1 | 0.2 |
| Main fracture permeability [D] | 20 | ||
| Injection displacement [] | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhong, L. Study on the Influence of Network Characteristics of Artificial Main Fractures and Natural Fractures on the Effectiveness of Acid Fracturing. Processes 2025, 13, 1710. https://doi.org/10.3390/pr13061710
Jiang Y, Zhong L. Study on the Influence of Network Characteristics of Artificial Main Fractures and Natural Fractures on the Effectiveness of Acid Fracturing. Processes. 2025; 13(6):1710. https://doi.org/10.3390/pr13061710
Chicago/Turabian StyleJiang, Youshi, and Lirui Zhong. 2025. "Study on the Influence of Network Characteristics of Artificial Main Fractures and Natural Fractures on the Effectiveness of Acid Fracturing" Processes 13, no. 6: 1710. https://doi.org/10.3390/pr13061710
APA StyleJiang, Y., & Zhong, L. (2025). Study on the Influence of Network Characteristics of Artificial Main Fractures and Natural Fractures on the Effectiveness of Acid Fracturing. Processes, 13(6), 1710. https://doi.org/10.3390/pr13061710





