From Natural to Industrial: How Biocoagulants Can Revolutionize Wastewater Treatment
Abstract
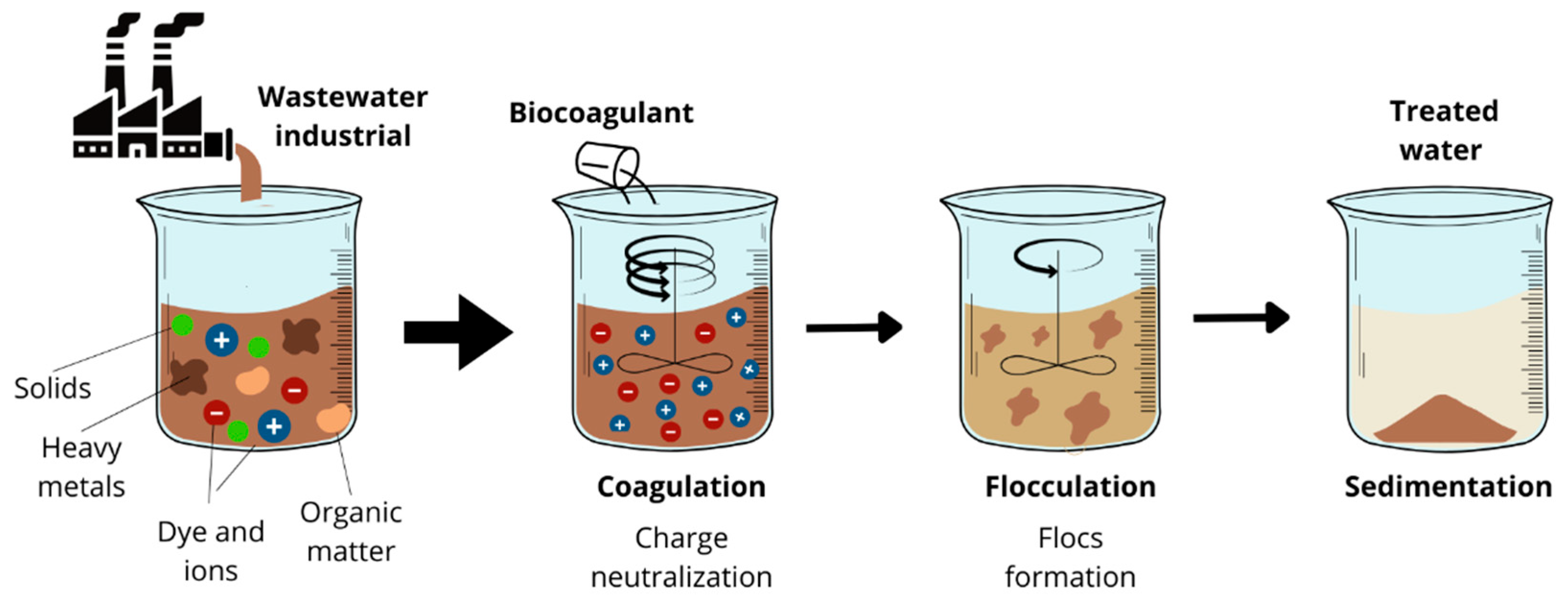
1. Introduction
2. Methodology
3. Biocoagulants
3.1. Main Sources of Biocoagulants and Production Methods
3.1.1. Vegetable Origin
3.1.2. Animal Origin
3.1.3. Microbial Origin
3.1.4. Extraction and Purification Techniques
3.2. Application of Biocoagulants in Industrial Wastewater Treatment
4. Sustainability and Environmental Impacts
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SDGs | Sustainable Development Goals |
| −OH | Hydroxyl |
| −COOH | Carboxyl |
| −NH2 | Amino |
| CO | Carbonyl |
| EPSs | Extracellular Polymeric Substances |
| TSS | Total Suspended Solids |
| COD | Chemical Oxygen Demand |
| BOD | Biological Oxygen Demand |
| Fe3O4 | Magnetite powder |
| PZC | Point of Zero Charge |
| Fe2+ and Fe3+ | Iron ions |
| Al3+ | Aluminum ion |
| Al(OH)₃ | Aluminum hydroxide |
| NaCl | Sodium chloride |
| KCl | Potassium chloride |
| H+ | Proton |
| Cr+6 | Chromate ion |
| HS− | Sulfides |
| Cl− | Chloride ion |
References
- Owodunni, A.A.; Ismail, S. Revolutionary Technique for Sustainable Plant-Based Green Coagulants in Industrial Wastewater Treatment—A Review. J. Water Process Eng. 2021, 42, 102096. [Google Scholar] [CrossRef]
- Panhwar, A.; Sattar Jatoi, A.; Ali Mazari, S.; Kandhro, A.; Rashid, U.; Qaisar, S. Water Resources Contamination and Health Hazards by Textile Industry Effluent and Glance at Treatment Techniques: A Review. Waste Manag. Bull. 2024, 1, 158–163. [Google Scholar] [CrossRef]
- Jiang, J.Q. The Role of Coagulation in Water Treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Sillanpaa, M.; Ncibi, M.C.; Matilainen, A.; Vepsalainen, M. Removal of Natural Organic Matter in Drinking Water Treatment by Coagulation: A Comprehensive Review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Zourif, A.; Benbiyi, A.; Kouniba, S.; El Guendouzi, M. Avocado Seed as a Natural Coagulant for Removing Dyes and Turbidity from Wastewater: Behnken Box Design, Sustainable Reuse, and Economic Evaluation. Sustain. Chem. Pharm. 2024, 39, 101621. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Ni, Y.; Kokot, S. Effects of Aluminum on Amyloid-Beta Aggregation in the Context of Alzheimer’s Disease. Arab. J. Chem. 2019, 12, 2897–2904. [Google Scholar] [CrossRef]
- Cojbasic, S.; Turk Sekulic, M.; Pap, S.; Taggart, M.A.; Prodanovic, J. Nature-Based Solutions for Wastewater Treatment: Biodegradable Freeze-Dried Powdered Bio-Flocculant. J. Water Process Eng. 2024, 65, 105863. [Google Scholar] [CrossRef]
- Shen, M.; Liu, S.; Hu, T.; Zheng, K.; Wang, Y.; Long, H. Recent Advances in the Research on Effects of Micro/Nanoplastics on Carbon Conversion and Carbon Cycle: A Review. J. Environ. Manag. 2023, 334, 117529. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Chik, C.E.N.C.E.; Owodunni, A.A.; Ahmad, A.; Alnawajha, M.M.; Rahim, N.F.M.; Said, N.S.M.; Abdullah, S.R.S.; Kasan, N.A.; et al. What Compound inside Biocoagulants/Bioflocculants Is Contributing the Most to the Coagulation and Flocculation Processes? Sci. Total Environ. 2022, 806, 150902. [Google Scholar] [CrossRef]
- Ahmad, A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Exploring the Extraction Methods for Plant-Based Coagulants and Their Future Approaches. Sci. Total Environ. 2022, 818, 151668. [Google Scholar] [CrossRef]
- United Nations Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/publications/transforming-our-world-2030-agenda-sustainable-development-17981 (accessed on 24 May 2025).
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-Based Coagulants/Flocculants as Sustainable Market-Valued Products for Industrial Wastewater Treatment: A Review of Recent Developments. RSC Adv. 2023, 13, 19335–19355. [Google Scholar] [CrossRef] [PubMed]
- Soldani, J.; Tamburri, D.A.; Heuvel, W.-J. Van Den The Pains and Gains of Microservices: A Systematic Grey Literature Review. J. Syst. Softw. 2018, 146, 215–232. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ahmad, A.; Said, N.S.M.; Gustinasari, K.; Abdullah, S.R.S.; Imron, M.F. The Influence of Preparation and Pretreatment on the Physicochemical Properties and Performance of Plant-Based Biocoagulants in Treating Wastewater. Environ. Adv. 2023, 14, 100441. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.‘I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Saleem, M.; Bachmann, R.T. A Contemporary Review on Plant-Based Coagulants for Applications in Water Treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Johnson, I.; Abubakar, M.; Ali, S.; Kumar, M. Cyanobacteria/Microalgae for Distillery Wastewater Treatment- Past, Present and the Future. In Microbial Wastewater Treatment; Elsevier: Amsterdam, The Nederlands, 2019; pp. 195–236. ISBN 9780128168097. [Google Scholar] [CrossRef]
- Zourif, A.; Kouniba, S.; El Guendouzi, M. Valorization of Palm Petiole Waste as Natural Biocoagulants: Optimizing Coagulation-Flocculation for Sustainable Wastewater Treatment and Advancing Circular Economy in Agriculture. Biocatal. Agric. Biotechnol. 2025, 63, 103473. [Google Scholar] [CrossRef]
- Alnawajha, M.M.; Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R. Plant-Based Coagulants/Flocculants: Characteristics, Mechanisms, and Possible Utilization in Treating Aquaculture Effluent and Benefiting from the Recovered Nutrients. Environ. Sci. Pollut. Res. 2022, 29, 58430–58453. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Ferreira, R.M.; Boaventura, R.A.R.; Botelho, C.M.S. Natural Coagulants from Chestnut Shells: A Sustainable Approach for Textile Wastewater Treatment. Chemosphere 2025, 376, 144286. [Google Scholar] [CrossRef]
- Getahun, M.; Befekadu, A.; Alemayehu, E. Coagulation Process for the Removal of Color and Turbidity from Wet Coffee Processing Industry Wastewater Using Bio-Coagulant: Optimization through Central Composite Design. Heliyon 2024, 10, e27584. [Google Scholar] [CrossRef]
- Benalia, A.; Baatache, O.; Derbal, K.; Khalfaoui, A.; Amrouci, Z.; Pizzi, A.; Panico, A. The Use of Central Composite Design (CCD) to Optimize and Model the Coagulation-Flocculation Process Using a Natural Coagulant: Application in Jar Test and Semi-Industrial Scale. J. Water Process Eng. 2024, 57, 104704. [Google Scholar] [CrossRef]
- Mirbahoush, S.M.; Chaibakhsh, N.; Moradi-Shoeili, Z. Highly Efficient Removal of Surfactant from Industrial Effluents Using Flaxseed Mucilage in Coagulation/Photo-Fenton Oxidation Process. Chemosphere 2019, 231, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Adimachukwu, A.M.; Okey-Onyesolu, C.F.; Ejimofor, M.I.; Onukwuli, O.D. Management of Aquaculture Effluent Using Cyperus Esculentus as a Natural Coagulant: Coagulation Kinetics and Mass Transfer Modeling. Next Res. 2025, 2, 100267. [Google Scholar] [CrossRef]
- Varsani, V.G.; Vyas, S.J.; Dudhagara, D.; Chudasama, T.; Gadhvi, K. Unlocking the Potential of Lignocellulosic Waste: A Kinetic Modeling Approach for Bio-Coagulants in Sewage Water Treatment. Environ. Technol. Innov. 2024, 33, 103486. [Google Scholar] [CrossRef]
- Okolo, B.I.; Adeyi, O.; Oke, E.O.; Agu, C.M.; Nnaji, P.C.; Akatobi, K.N.; Onukwuli, D.O. Coagulation Kinetic Study and Optimization Using Response Surface Methodology for Effective Removal of Turbidity from Paint Wastewater Using Natural Coagulants. Sci. Afr. 2021, 14, e00959. [Google Scholar] [CrossRef]
- Tomé, A.G.; Ribeiro, E.A.M.; Lima, M.; Brocenschi, R.F.; Ribeiro, L.N.M.; Amaral, e.F.A. Biorefinery of Peanut Shell Agroindustrial Lignocellulosic Waste for Synthesis of a Natural Coagulant Applied in the Treatment of Dairy Wastewater. J. Environ. Chem. Eng. 2023, 11, 111535. [Google Scholar] [CrossRef]
- Kumar, V.; Al-Gheethi, A.; Asharuddin, S.M.; Othman, N. Potential of Cassava Peels as a Sustainable Coagulant Aid for Institutional Wastewater Treatment: Characterisation, Optimisation and Techno-Economic Analysis. Chem. Eng. J. 2021, 420, 127642. [Google Scholar] [CrossRef]
- Bello, A.; Leiviskä, T. Sustainable Tannin-Based Coagulants Synthesized through Mannich Reaction Using Melamine as an Amine Source for Water Treatment Applications. Ind. Crops Prod. 2022, 176, 114336. [Google Scholar] [CrossRef]
- Pacheco, H.G.J.; Elguera, N.Y.M.; Mamani, M.R.A.; Alvarez, N.P.L.; Almeida, V. de C. Treatment of Textile Wastewater by Electrocoagulation Process Assisted with Biocoagulant Obtained from the Pitahaya Peels. Desalination Water Treat. 2023, 283, 1–10. [Google Scholar] [CrossRef]
- Boakye, A.; Attiogbe, F.; Emahi, I. Crescentia Cujete Fruit Shell as Green and Efficient Coagulant for Water Purification. Clean. Water 2024, 1, 100009. [Google Scholar] [CrossRef]
- El Gaayda, J.; Titchou, F.E.; Barra, I.; Karmal, I.; Afanga, H.; Zazou, H.; Yap, P.S.; Abidin, Z.Z.; Hamdani, M.; Akbour, R.A. Optimization of Turbidity and Dye Removal from Synthetic Wastewater Using Response Surface Methodology: Effectiveness of Moringa oleifera Seed Powder as a Green Coagulant. J. Environ. Chem. Eng. 2022, 10, 106988. [Google Scholar] [CrossRef]
- Garde, W.K.; Buchberger, S.G.; Wendell, D.; Kupferle, M.J. Application of Moringa oleifera Seed Extract to Treat Coffee Fermentation Wastewater. J. Hazard. Mater. 2017, 329, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.Q.; Ghazali, S.B. Dataset on Physicochemical Properties of Particle-Sized Moringa oleifera Seed Cake and Its Application as Bio-Coagulants in Water Treatment Application. Chem. Data Collect. 2019, 24, 100284. [Google Scholar] [CrossRef]
- Taiwo, A.S.; Adenike, K.; Aderonke, O. Efficacy of a Natural Coagulant Protein from Moringa oleifera (Lam) Seeds in Treatment of Opa Reservoir Water, Ile-Ife, Nigeria. Heliyon 2020, 6, e03335. [Google Scholar] [CrossRef]
- Varsani, V.; Vyas, S.J.; Dudhagara, D.R. Development of Bio-Based Material from the Moringa oleifera and Its Bio-Coagulation Kinetic Modeling–A Sustainable Approach to Treat the Wastewater. Heliyon 2022, 8, e10447. [Google Scholar] [CrossRef]
- Lester-Card, E.; Smith, G.; Lloyd, G.; Tizaoui, C. A Green Approach for the Treatment of Oily Steelworks Wastewater Using Natural Coagulant of Moringa oleifera Seed. Bioresour. Technol. Rep. 2023, 22, 101393. [Google Scholar] [CrossRef]
- Mohamed Noor, M.H.; Mohd Azli, M.F.Z.; Ngadi, N.; Mohammed Inuwa, I.; Anako Opotu, L.; Mohamed, M. Optimization of Sonication-Assisted Synthesis of Magnetic Moringa oleifera as an Efficient Coagulant for Palm Oil Wastewater Treatment. Environ. Technol. Innov. 2022, 25, 102191. [Google Scholar] [CrossRef]
- Ezemagu, I.G.; Ejimofor, M.I.; Menkiti, M.C. Turbidimetric Study for the Decontamination of Paint Effluent (PE) Using Mucuna Seed Coagulant (MSC): Statistical Design and Coag-Flocculation Modelling. Environ. Adv. 2020, 2, 100023. [Google Scholar] [CrossRef]
- Ancy, J.; Vasanthy, M.; Thamarai selvi, C.; Ravindran, B.; Chung, W.J.; Chang, S.W. Treatment of Coffee Cherry Pulping Wastewater by Using Lectin Protein Isolated from Ricinus communis L. Seed. J. Water Process Eng. 2021, 39, 101742. [Google Scholar] [CrossRef]
- DePaolis, M.; De Respino, S.; Samineni, L.; Brighton, S.; Kumar, M. Cottonseed Extract as a Coagulant for Water Treatment. Environ. Sci. Adv. 2022, 2, 227–234. [Google Scholar] [CrossRef]
- El Mouhri, G.; Elmansouri, I.; Amakdouf, H.; Belhassan, H.; Kachkoul, R.; El oumari, F.E.; Merzouki, M.; Lahrichi, A. Evaluating the Effectiveness of Coagulation–Flocculation Treatment on a Wastewater from the Moroccan Leather Tanning Industry: An Ecological Approach. Heliyon 2024, 10, e27056. [Google Scholar] [CrossRef]
- Garomsa, F.S.; Mehari, Y.; Desta, W.M.; Bidira, F.; Asaithambi, P. Removal of NO3−, PO3−, and Color from Brewery Wastewater by the Use of Indigenous Bio-Coagulant-Assisted Electrocoagulation. Prog. Eng. Sci. 2024, 1, 100032. [Google Scholar] [CrossRef]
- El Gaayda, J.; Titchou, F.E.; Karmal, I.; Barra, I.; Errami, M.; Yap, P.S.; Oh, W.D.; Iqbal, A.; Sillanpää, M.; Hamdani, M.; et al. Application of Grape Seed and Austrocylindropuntia mucilage for the Simultaneous Removal of Azo Dye and Turbidity from Synthetic Wastewater: Optimizing Experimental Conditions Using Box-Behnken Design (BBD). J. Water Process Eng. 2024, 58, 104718. [Google Scholar] [CrossRef]
- Husen, A.K.; Bidira, F.; Mekonin Desta, W.; Asaithambi, P. COD, Color, and Turbidity Reduction from Surface Water Using Natural Coagulants: Investigation and Optimization. Prog. Eng. Sci. 2024, 1, 100007. [Google Scholar] [CrossRef]
- Mathupreetha, S.; Wijekoon, W.M.A.P.; Dayanthi, W.K.C.N.; Nusrath, L.F.U. Laboratory and Pilot Scale Experiments with Seeds Extracts of Yard Long Beans (Vigna unguiculata subsp. sesquipedalis) and Snake Gourd (Trichosanthes Cucumerina) as Efficient Coagulants for Water Treatment. Total Environ. Eng. 2025, 2, 100009. [Google Scholar] [CrossRef]
- Shah, R.A.R.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N. ‘Izzati Prospective Plants as Biocoagulant-Flocculants for Removal of Total Suspended Solids in Coffee Effluent Treatment. Chem. Eng. Res. Des. 2023, 198, 282–295. [Google Scholar] [CrossRef]
- Khalid Salem, A.; Fadhile Almansoory, A.; Al-Baldawi, I.A. Potential Plant Leaves as Sustainable Green Coagulant for Turbidity Removal. Heliyon 2023, 9, e16278. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Salas-Mellado, M.D.L.M. Addition of Chia Seed Mucilage for Reduction of Fat Content in Bread and Cakes. Food Chem. 2017, 227, 237–244. [Google Scholar] [CrossRef]
- Javed, S.; Zulfiqar, Z.; Fatima, Z.; Muhammad, G.; Hussain, M.A.; Mushtaq, M.; Haseeb, M.T. A Comprehensive Review of Plant-Based Mucilages as Promising Candidates for Water Remediation. J. Environ. Chem. Eng. 2024, 12, 114035. [Google Scholar] [CrossRef]
- Adjeroud, N.; Elabbas, S.; Merzouk, B.; Hammoui, Y.; Felkai-Haddache, L.; Remini, H.; Leclerc, J.P.; Madani, K. Effect of Opuntia ficus indica Mucilage on Copper Removal from Water by Electrocoagulation-Electroflotation Technique. J. Electroanal. Chem. 2018, 811, 26–36. [Google Scholar] [CrossRef]
- Martínez-Cruz, A.; Rojas Valencia, M.N.; Araiza-Aguilar, J.A.; Nájera-Aguilar, H.A.; Gutiérrez-Hernández, R.F. Leachate Treatment: Comparison of a Bio-Coagulant (Opuntia ficus Mucilage) and Conventional Coagulants Using Multi-Criteria Decision Analysis. Heliyon 2021, 7, e07510. [Google Scholar] [CrossRef]
- Tawakkoly, B.; Alizadehdakhel, A.; Dorosti, F. Evaluation of COD and Turbidity Removal from Compost Leachate Wastewater Using Salvia hispanica as a Natural Coagulant. Ind. Crops Prod. 2019, 137, 323–331. [Google Scholar] [CrossRef]
- Fard, M.B.; Hamidi, D.; Yetilmezsoy, K.; Alavi, J.; Hosseinpour, F. Utilization of Alyssum Mucilage as a Natural Coagulant in Oily-Saline Wastewater Treatment. J. Water Process Eng. 2021, 40, 101763. [Google Scholar] [CrossRef]
- Fard, M.B.; Hamidi, D.; Alavi, J.; Jamshidian, R.; Pendashteh, A.; Mirbagheri, S.A. Saline Oily Wastewater Treatment Using Lallemantia Mucilage as a Natural Coagulant: Kinetic Study, Process Optimization, and Modeling. Ind. Crops Prod. 2021, 163, 113326. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Tran, L.N.; Doan, V.T.; Luu, L.M.; Nguyen, Q.T.; Van Pham, Q.; Van Ngo, A.; Le, O.T.H. Coagulation and Flocculation of Dye Wastewater by FeCl3 and Mucilage Extracted from Dragon Fruit Peel (Hylocereus undatus) in Regard of Side Effects Caused by the Use of PACl and PAM. Desalination Water Treat. 2022, 250, 181–188. [Google Scholar] [CrossRef]
- Figueiredo, F.F.; Freitas, T.K.F.d.S.; Dias, G.G.; Geraldino, H.C.L.; Scandelai, A.P.J.; Vilvert, A.J.; Garcia, J.C. Textile-Effluent Treatment Using Aloe Vera Mucilage as a Natural Coagulant Prior to a Photo-Fenton Reaction. J. Photochem. Photobiol. A Chem. 2022, 429, 113948. [Google Scholar] [CrossRef]
- Lim, K.S.; Sethu, V.; Selvarajoo, A. Natural Plant Materials as Coagulant and Flocculants for the Treatment of Palm Oil Mill Effluent. Mater. Today Proc. 2021, 48, 871–887. [Google Scholar] [CrossRef]
- Lau, S.Y.; Tien, P.T.K.; Choy, S.Y.; Jeevanandam, J.; Show, P.L.; Lam, M.K.; Tan, Y.H.; Lim, S. Unleashing the Power of Plant-Based Modified Starch as a Game-Changing Natural Coagulant. Process Biochem. 2024, 147, 213–227. [Google Scholar] [CrossRef]
- Leal Castañeda, E.J.; Meléndez-Estrada, J.; Toscano-Flores, L.G. Dodecanoyl Chloride Modified Starch Particles: A Candidate for the Removal of Hydrocarbons and Heavy Metals in Wastewater. Environ. Adv. 2023, 11, 100333. [Google Scholar] [CrossRef]
- Salehin, M.; Khoshbouy, R.; Fatehifar, E. Development and Evaluation of Amine-Functionalized β-Cyclodextrin Grafted Starch as a Natural Flocculant for Turbidity Removal in Water Treatment. Int. J. Biol. Macromol. 2024, 280, 136118. [Google Scholar] [CrossRef]
- Kebaili, M.; Djellali, S.; Radjai, M.; Drouiche, N.; Lounici, H. Valorization of Orange Industry Residues to Form a Natural Coagulant and Adsorbent. J. Ind. Eng. Chem. 2018, 64, 292–299. [Google Scholar] [CrossRef]
- Ayat, A.; Arris, S.; Bencheikh-Lehocine, M.; Meniai, A.H. Landfill Leachate Pretreatment by Biocoagulation/Bioflocculation Process Using Plant-Based Coagulant (Optimization by Response Surface Methodology). Desalination Water Treat. 2021, 235, 66–79. [Google Scholar] [CrossRef]
- Lanan, F.A.B.M.; Selvarajoo, A.; Sethu, V.; Arumugasamy, S.K. Utilisation of Natural Plant-Based Fenugreek (Trigonella foenum-graecum) Coagulant and Okra (Abelmoschus escluentus) Flocculant for Palm Oil Mill Effluent (POME) Treatment. J. Environ. Chem. Eng. 2021, 9, 104667. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia ficus indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Pine Cone as Bio-Coagulant for Heavy Metal Removal from Industrial Wastewater: Use of Box–Behnken Design. Ind. Crops Prod. 2024, 210, 118185. [Google Scholar] [CrossRef]
- Prasetyo, S.; Santos, C.A.; Sugih, A.K.; Kristianto, H. Utilization of Chitosan as a Natural Coagulant for Polyethylene Microplastic Removal. Sustain. Chem. Environ. 2025, 9, 100225. [Google Scholar] [CrossRef]
- Ejimofor, M.I.; Ezemagu, I.G.; Ugonabo, V.I.; Nnaji, P.C.; Anadebe, V.C.; Diyoke, C.; Menkiti, M.C. Adsorption Kinetics, Mechanistic, Isotherm and Thermodynamics Study of Petroleum Produced Water Coagulation Using Novel Egeria Radiate Shell Extract (ERSE). J. Indian Chem. Soc. 2022, 99, 100357. [Google Scholar] [CrossRef]
- Menkiti, M.C.; Ezemagu, I.G. Sludge Characterization and Treatment of Produced Water(PW) Using Tympanotonos Fuscatus Coagulant (TFC). Petroleum 2015, 1, 51–62. [Google Scholar] [CrossRef]
- Ejimofor, M.I.; Ezemagu, I.G.; Menkiti, M.C. Physiochemical, Instrumental and Thermal Characterization of the Post Coagulation Sludge from Paint Industrial Wastewater Treatment. S. Afr. J. Chem. Eng. 2021, 37, 150–160. [Google Scholar] [CrossRef]
- Ejimofor, M.; Menkiti, M.; Ezemagu, I. Integrated Treatment of Paint Wastewater Using Helix Pometia Shell Coagulant and Sludge Conversion to Biogas: Process Thermodynamics and Biogas Energy Content. Int. J. Plant Anim. Environ. Sci. 2021, 11, 391–422. [Google Scholar] [CrossRef]
- Okey-Onyesolu, C.F.; Chukwuma, E.C.; Okoye, C.C.; Umobi, C.O. Application of Fish Bone Chitosan-Protein Bio-Coagulant for Abattoir Wastewater Treatment: Comparative Process Optimization and Evaluation. Waste Manag. Bull. 2023, 1, 49–59. [Google Scholar] [CrossRef]
- Nouj, N.; Majbar, Z.; Abelouah, M.R.; Ben Hamou, A.; Chaoui, A.; Hafid, N.; Benafqir, M.; El Alem, N.; Jada, A.; Ouachtak, H.; et al. Eco-Friendly Wastewater Treatment Using a Crab Shell-Based Liquid Bio-Coagulant: Multi-Criteria Decision Analysis Related to Different Pollutants Separation. J. Environ. Chem. Eng. 2024, 12, 112318. [Google Scholar] [CrossRef]
- Iber, B.T.; Torsabo, D.; Chik, C.E.N.C.E.; Wahab, F.; Abdullah, S.R.S.; Hasan, H.A.; Kasan, N.A. Optimization of Chitosan Coagulant from Dry Legs of Giant Freshwater Prawn, Macrobrachium Rosenbergii in Aquaculture Wastewater Treatment Using Response Surface Methodology (RSM). J. Environ. Chem. Eng. 2023, 11, 109761. [Google Scholar] [CrossRef]
- Eamrat, R.; Rujakom, S.; Pussayanavin, T.; Taweesan, A.; Witthayaphirom, C.; Kamei, T. Optimizing Biocoagulant Aid from Shrimp Shells (Litopenaeus vannamei) for Enhancing Microplastics Removal from Aqueous Solutions. Environ. Technol. Innov. 2024, 33, 103457. [Google Scholar] [CrossRef]
- Esmaeili Nasrabadi, A.; Eydi, M.; Bonyadi, Z. Utilizing Chlorella Vulgaris Algae as an Eco-Friendly Coagulant for Efficient Removal of Polyethylene Microplastics from Aquatic Environments. Heliyon 2023, 9, e22338. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Hasan, H.A. Treatment of Real Aquaculture Effluent Using Bacteria-Based Bioflocculant Produced by Serratia Marcescens. J. Water Process Eng. 2022, 47, 102708. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Coagulation–Flocculation of Aquaculture Effluent Using Biobased Flocculant: From Artificial to Real Wastewater Optimization by Response Surface Methodology. J. Water Process Eng. 2023, 53, 103869. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Imron, M.F.; Ismail, N.I.; Ahmad, A.; Hasan, H.A. Isolation and Characterisation of Bioflocculant-Producing Bacteria from Aquaculture Effluent and Its Performance in Treating High Turbid Water. J. Water Process Eng. 2021, 42, 102194. [Google Scholar] [CrossRef]
- Qasim, W.; Mane, A.V. Characterization and Treatment of Selected Food Industrial Effluents by Coagulation and Adsorption Techniques. Water Resour. Ind. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Noukeu, N.A.; Gouado, I.; Priso, R.J.; Ndongo, D.; Taffouo, V.D.; Dibong, S.D.; Ekodeck, G.E. Characterization of Effluent from Food Processing Industries and Stillage Treatment Trial with Eichhornia crassipes (Mart.) and Panicum maximum (Jacq.). Water Resour. Ind. 2016, 16, 1–18. [Google Scholar] [CrossRef]
- Pacheco, H.G.J.; Elguera, N.Y.; Ancco, M.; Castro, A.E.L.F.; Meza, M.E.B.; Almeida, V.C. Combined Coagulation-Electrocoagulation Process Using Biocoagulant from the Opuntia ficus-indica for Treatment of Cheese Whey Wastewater. Environ. Monit. Assess. 2023, 195, 491. [Google Scholar] [CrossRef]
- Ramadhani, A.N.; Sarosa, A.N.K.W.; Al Rosyad, L.H. The Potency of Microbial Flocculant Produced by B. Licheniformis Using Molasses as the Carbon Source and Its Application in Food Industry Wastewater Treatment. Mater. Today Proc. 2022, 63, S244–S247. [Google Scholar] [CrossRef]
- Marichamy, M.K.; Kumaraguru, A.; Jonna, N. Particle Size Distribution Modeling and Kinetic Study for Coagulation Treatment of Tannery Industry Wastewater at Response Surface Optimized Condition. J. Clean. Prod. 2021, 297, 126657. [Google Scholar] [CrossRef]
- Aguilera Flores, M.M.; Medellín Castillo, N.A.; Ávila Vázquez, V.; González García, R.; Cardona Benavides, A.; Carranza Álvarez, C. Evaluation of a Biocoagulant from Devilfish Invasive Species for the Removal of Contaminants in Ceramic Industry Wastewater. Sci. Rep. 2022, 12, 9917. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. A Comprehensive Study on Coagulant Performance and Floc Characterization of Natural Cassia Obtusifolia Seed Gum in Treatment of Raw Pulp and Paper Mill Effluent. Ind. Crops Prod. 2014, 61, 317–324. [Google Scholar] [CrossRef]
- Huang, J.; Shen, X.; Lin, J.; Zhou, J.; Li, Y. Synergistic Heavy Metal Sequestration from Acidic Metallurgical Wastewater: Unraveling Critical Chemical Bond-Mediated Adsorption Mechanisms in Vanadium Slag-Derived Hydroxyapatite. J. Water Process Eng. 2025, 72, 107629. [Google Scholar] [CrossRef]
- Chalaris, M.; Gkika, D.A.; Tolkou, A.K.; Kyzas, G.Z. Advancements and Sustainable Strategies for the Treatment and Management of Wastewaters from Metallurgical Industries: An Overview. Environ. Sci. Pollut. Res. Int. 2023, 30, 119627–119653. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the Art and Sustainability of Natural Coagulants in Water and Wastewater Treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass Waste Utilisation in Low-Carbon Products: Harnessing a Major Potential Resource. NJP Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Ho, Y.C. New Vegetal Biopolymeric Flocculant: A Degradation and Flocculation Study. Iran. J. Energy Environ. 2014, 5, 26–33. [Google Scholar] [CrossRef][Green Version]
- Abidin, Z.Z.; Madehi, N.; Yunus, R.; Derahman, A. Effect of Storage Conditions on Jatropha curcas Performance as Biocoagulant for Treating Palm Oil Mill Effluent. J. Environ. Sci. Technol. 2019, 12, 92–101. [Google Scholar] [CrossRef]
- Öztürk, E.; Ateş, A.E.; Selçuk, H.; Ateş, S. Treatment of Real Pharmaceutical Industry Wastewater by Photo-Fenton Oxidation Using the Response Surface Methodology, Evaluation of Diclofenac Degradation and Toxicity. Eng. Sci. Technol. Int. J. 2025, 64, 102004. [Google Scholar] [CrossRef]



| Source of Industrial Effluent | Industrial Effluent | Biocoagulant | Optimal Dosage | Main Results | References |
|---|---|---|---|---|---|
| Agriculture and Food Processing | Wet coffee processing | Moringa stenopetala B. seed, Acanthus sennii C. stems, and Aloe vera L. | 750 mg L−1 | Color and turbidity removal rates of 99.9% and 98.7%, respectively | [21] |
| Palm oil mill | Moringa oleifera | 1000 mg L−1 | TSS, color, and COD removal rates of 83.0%, 35.6%, and 85.2%, respectively | [38] | |
| Cheese whey | Opuntia ficus-indica | 4400 mg L−1 | Turbidity and COD removal rates of 98.9% and 83.8%, respectively | [82] | |
| Fish processing | Protein-rich liquid from the chitin extraction process | 17.5 mL L−1 | Turbidity, BOD, and COD removal rates of 98.9%, 92.1%, and 78.9%, respectively | [73] | |
| Tofu industry | B. licheniformis | 20 mg L−1 | Turbidity, COD, and BOD removal rates of 70.0%, 75.9%, and 80.2%, respectively | [83] | |
| Brewery | Custard apple seed | 2000 mg L−1 | Nitrate, color, and phosphates removal rates of 98.0%, 99.6%, and 97.8%, respectively | [43] | |
| Compost leachate (from organic fertilizer production) | Salvia hispanica mucilage | 40,000 mg L−1 | COD and turbidity removal rates of 39.8% and 62.4% | [53] | |
| Aquaculture | Serratia marcescens | 10 mg L−1 | Turbidity and TSS removal rates of 80.1% and 92.2%, respectively | [78] | |
| Textile and Chemical | Textile industry | Chestnut shell | 100 mg L−1 | Color and aluminum removal rates of 31% and 15%, respectively | [20] |
| Car wash | Flaxseed mucilage | 100 mg L−1 | Surfactant and COD removal rates of 80.8% and 57.0%, respectively | [23] | |
| Paint industry | Hibiscus esculentus, Detarium microcarpum, Xanthosoma | 100 mg L−1 | Turbidity removal rates of 84–95% | [26] | |
| Tannery | Moringa oleifera | 2000 mg L−1 | TSS removal rate of 89.9% | [84] | |
| Metallurgical and Heavy Industry | Steel processing | Moringa oleifera | 10 mg L−1 | Turbidity removal rate of 90%. | [37] |
| Ceramic industry | Devilfish | 200 mg L−1 | Turbidity, COD, and TSS removal rates of 67.4%, 56.9%, and 50%, respectively | [85] | |
| Pulp, Paper, and Packaging | Raw pulp and paper mill | Cassia obtusifolia seed gum | 750 mg L−1 | TSS and COD removal rates of 86.9% and 36.2%, respectively | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.M.P.; de Farias, B.S.; Fernandes, S.S. From Natural to Industrial: How Biocoagulants Can Revolutionize Wastewater Treatment. Processes 2025, 13, 1706. https://doi.org/10.3390/pr13061706
da Silva RMP, de Farias BS, Fernandes SS. From Natural to Industrial: How Biocoagulants Can Revolutionize Wastewater Treatment. Processes. 2025; 13(6):1706. https://doi.org/10.3390/pr13061706
Chicago/Turabian Styleda Silva, Renata Machado Pereira, Bruna Silva de Farias, and Sibele Santos Fernandes. 2025. "From Natural to Industrial: How Biocoagulants Can Revolutionize Wastewater Treatment" Processes 13, no. 6: 1706. https://doi.org/10.3390/pr13061706
APA Styleda Silva, R. M. P., de Farias, B. S., & Fernandes, S. S. (2025). From Natural to Industrial: How Biocoagulants Can Revolutionize Wastewater Treatment. Processes, 13(6), 1706. https://doi.org/10.3390/pr13061706








