Optimization and Mechanistic Investigation of Coal Gangue–Blast Furnace Slag Composite Geopolymers
Abstract
1. Introduction
2. Experimental Program
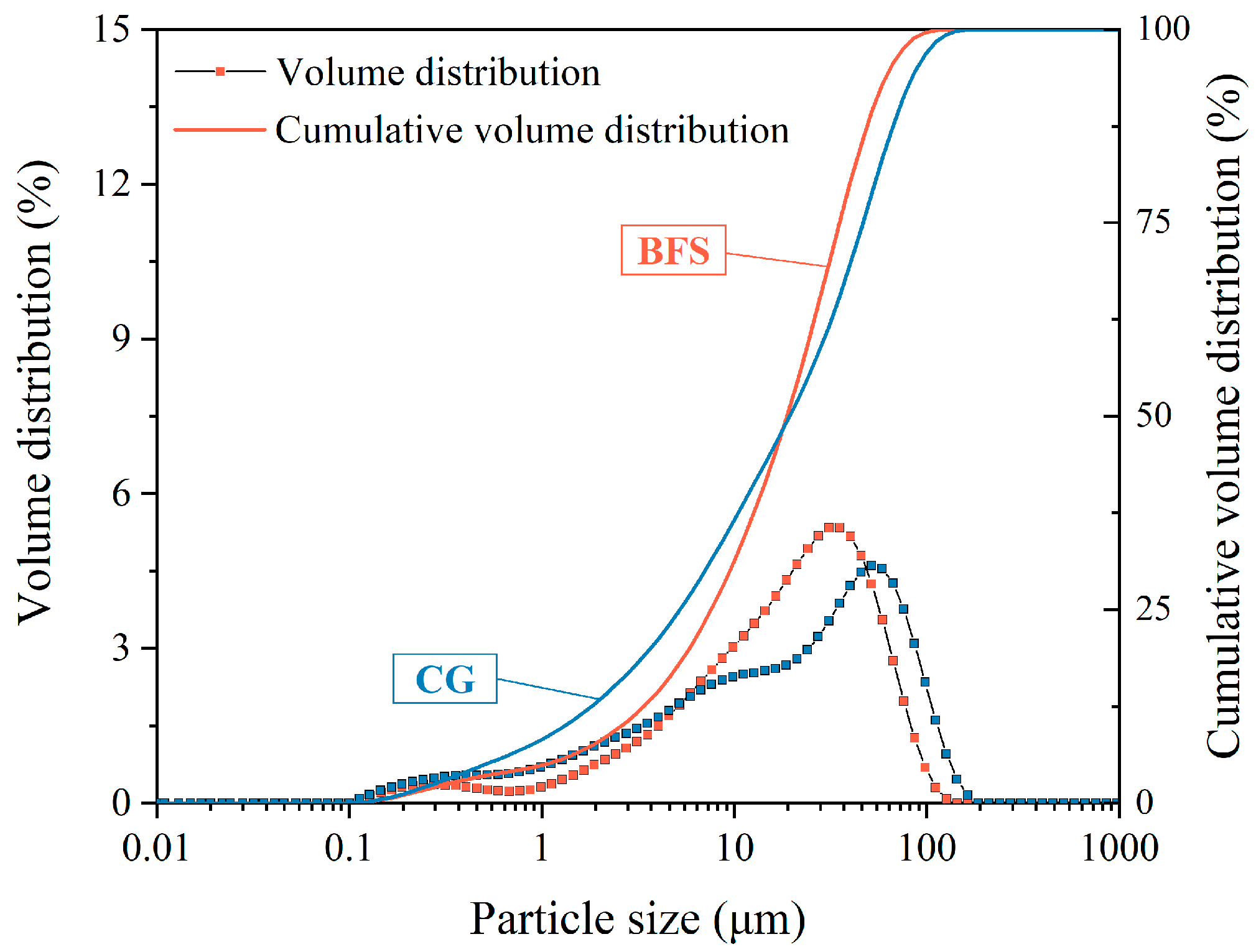
2.1. Materials
2.2. Experimental Methods and Testing
2.2.1. Sample Preparation Process
2.2.2. Mixing Ratio Design
2.2.3. Testing Methods
3. Results and Discussion
3.1. Single-Factor Experimental Study on Preparation of Composite Geopolymer
3.1.1. The Ratio of Liquid to Solid
3.1.2. Alkali Activator Dosage
3.1.3. Waterglass Modulus
3.1.4. BFS Content
3.1.5. Curing Temperature
3.1.6. One-Way ANOVA Test
3.2. RSM Experimental Study on the Preparation of Composite Geopolymer
3.2.1. Establish Function Model
3.2.2. Interaction Analysis
3.2.3. Optimal Scheme Prediction and Validation
3.3. Microstructural Analysis
4. Conclusions
- (1)
- The results from the single-factor experiments indicate that compressive strength increases monotonically with BFS content from 10% to 90%, reaching its peak at 90%. Conversely, as the waterglass modulus increases from 0.6 to 1.4, compressive strength gradually decreases, with the highest strength observed at a modulus of 0.6. The liquid-to-solid ratio (0.21–0.29) initially enhances compressive strength, peaking at 0.27, before declining at higher ratios. Similarly, the alkali activator dosage (5–15%) follows a trend of increasing strength up to 10%, after which strength decreases. Furthermore, raising the curing temperature from 30 °C to 70 °C leads to a continuous decline in strength, with the highest value recorded at 30 °C.
- (2)
- Building on the single-factor experiments, a three-factor, three-level RSM optimization was conducted to refine the composite geopolymer’s preparation parameters. A second-order regression model was established to describe the relationship between the response and influencing factors, with the ANOVA confirming its validity and statistical significance. The optimal parameters were identified to be a waterglass modulus of 1.06, an alkali activator dosage of 13.81%, and an initial 24 h curing temperature of 30 °C, yielding a maximum compressive strength of 91.13 MPa. XRD and SEM-EDS analyses further revealed the existence of partially unreacted CG particles in the geopolymer matrix. The primary reaction products were qualitatively identified as an amorphous mixture of C-S-H, C-A-S-H, and N-A-S-H, indicating a complex gel-phase structure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CG | Coal gangue |
| BFS | Blast furnace slag |
| RSM | Response surface methodology |
| XRD | X-ray diffraction |
| SEM-EDS | Scanning electron microscopy with energy dispersive spectroscopy |
| CCD | Central composite design |
| N-A-S-H | Sodium aluminosilicate hydrate |
| C-A-S-H | Calcium aluminosilicate hydrate |
| C-S-H | Hydrated calcium silicate |
References
- National Bureau of Statistics of China. China Statistical Yearbook 2024; China Statistics Press: Beijing, China, 2024. [Google Scholar]
- Xu, Z.P.; Qian, Y.H.; Hong, X.P.; Luo, Z.G.; Gao, X.L.; Liang, H.D. Contamination characteristics of polycyclic aromatic compounds from coal sources in typical coal mining areas in Huaibei area, China. Sci. Total Environ. 2023, 873, 162311. [Google Scholar] [CrossRef]
- Wang, S.B.; Luo, K.L.; Wang, X.; Sun, Y.Z. Estimate of sulfur, arsenic, mercury, fluorine emissions due to spontaneous combustion of coal gangue: An important part of Chinese emission inventories. Environ. Pollut. 2016, 209, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Zhang, K.; Wang, Q.B.; Yang, K.; Yao, C.F.; Tan, X.Y. Application of modified solidified soil in in-situ backfilling of coal gangue: Evaluation of arsenic stabilization effect and mechanism study. Environ. Geochem. Health 2025, 47, 57. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, J.Y.; Huang, L.J.H.; Huang, W.F.; Duan, S.Y.; Ji, S.Y.; Zhang, G.X.; Ma, J.; Deng, J.S. Full-components utilization: Study on simultaneous preparation of sodalite and separation of yttrium from coal gangue by chlorination roasting process. Sep. Purif. Technol. 2024, 332, 125802. [Google Scholar] [CrossRef]
- Albidah, A.S. Effect of partial replacement of geopolymer binder materials on the fresh and mechanical properties: A review. Ceram. Int. 2021, 47, 14923–14943. [Google Scholar] [CrossRef]
- Norton, M.G.; Provis, J.L. 1000 at 1000: Geopolymer technology-the current state of the art. J. Mater. Sci. 2020, 55, 13487–13489. [Google Scholar] [CrossRef]
- He, M.; Yang, Z.B.; Li, N.; Zhu, X.H.; Fu, B.; Ou, Z.H. Strength, microstructure, CO2 emission and economic analyses of low concentration phosphoric acid-activated fly ash geopolymer. Constr. Build. Mater. 2023, 374, 130920. [Google Scholar] [CrossRef]
- Lim, J.N.; Liew, Y.M.; Heah, C.Y.; Tan, W.H.; Ken, P.W.; Pakawanit, P.; Tee, H.W.; Hang, Y.J.; Ong, S.W.; Ooi, W.E. Unveiling physico-mechanical and acoustical characteristics of fly ash geopolymers through the synergistic impact of density and porosity. J. Build. Eng. 2024, 91, 109684. [Google Scholar]
- Li, Z.P.; Zhang, J.Y.; Lei, Z.X.; Gao, M.S.; Sun, J.B.; Tong, L.H.; Chen, S.M.; Wang, Y.F. Designing low-carbon fly ash based geopolymer with red mud and blast furnace slag wastes: Performance, microstructure and mechanism. J. Environ. Manag. 2024, 354, 120362. [Google Scholar] [CrossRef]
- Ouda, A.S.; Gharieb, M. Behavior of alkali-activated pozzocrete-fly ash paste modified with ceramic tile waste against elevated temperatures and seawater attacks. Constr. Build. Mater. 2021, 285, 122866. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Mechanical and microstructural evolutions of fly ash/slag-based geopolymer at high temperatures: Effect of curing conditions. Ceram. Int. 2023, 49, 2091–2101. [Google Scholar] [CrossRef]
- Muracchioli, M.; Menardi, G.; D’Agostini, M.; Franchin, G.; Colombo, P. Modeling the compressive strength of metakaolin-based geopolymers based on the statistical analysis of experimental data. Appl. Clay Sci. 2023, 242, 107020. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.H.; Ong, D.E.L.; Wang, S.; Yang, Y.; Dinh, H.L.; Zi, G. Correlation between dissolubilities of Si, Al, and Fe from aluminosilicate precursor and strength of fly ash-based geopolymer. Constr. Build. Mater. 2023, 393, 132107. [Google Scholar] [CrossRef]
- Li, X.Y.; Qiao, Y.J.; Shao, J.H.; Bai, C.Y.; Li, H.Q.; Lu, S.; Zhang, X.H.; Yang, K.; Colombo, P. Sodium-based alkali-activated foams from self-ignition coal gangue by facile microwave foaming route. Ceram. Int. 2022, 48, 33914–33925. [Google Scholar] [CrossRef]
- Ekinci, E.; Türkmen, I.; Birhanli, E. Mechanical and durability characteristics of GGBS-based self-healing geopolymer mortar produced using by an endospore-forming bacterium. J. Build. Eng. 2022, 57, 104944. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, A.G.; Zhu, Y.C.; Dai, J.G.; Xu, Q.; Liu, K.W.; Hao, F.J.; Sun, D.S. Manufacturing ultra-high performance geopolymer concrete (UHPGC) with activated coal gangue for both binder and aggregate. Compos. Part. B-Eng. 2024, 284, 111723. [Google Scholar] [CrossRef]
- Fei, E.; Zhang, X.D.; Su, L.J.; Liu, B.N.; Li, B.T.; Li, W.L. Analysis of calcination activation modified coal gangue and its acid activation mechanism. J. Build. Eng. 2024, 95, 109916. [Google Scholar]
- Zhang, W.; Lang, L.; Dong, C.X.; Qi, Z.; Zhang, Z.R.; Li, J.S. Comprehensive study on coal gangue-based geopolymer activated by phosphoric acid: From macroscale properties to molecular simulation. Constr. Build. Mater. 2024, 438, 137271. [Google Scholar] [CrossRef]
- Zhong, Q.Y.; Nie, H.; Xie, G.L.; Peng, H. Experimental study on the characteristics, rheological factors, and flowability of MK-GGBFS geopolymer slurry. J. Build. Eng. 2023, 76, 107300. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Ma, T.; Gu, G.H.; Chen, C.L.; Hu, J.Y. Understanding the changes in engineering behaviors and microstructure of FA-GBFS based geopolymer paste with addition of silica fume. J. Build. Eng. 2023, 70, 106450. [Google Scholar] [CrossRef]
- Sun, C.L.; Lin, S.J.; Yang, L.; Rao, F.; Zheng, Y.J. Immobilization of fluorinion in phosphorus tailings-blast furnace slag based geopolymers activated by waste alkalis. Cement Concrete Comp. 2024, 146, 105407. [Google Scholar] [CrossRef]
- Yang, X.K.; Wu, S.P.; Xu, S.; Chen, B.Y.; Chen, D.Y.; Wang, F.S.; Jiang, J.; Fan, L.L.; Tu, L.L. Effects of GBFS content and curing methods on the working performance and microstructure of ternary geopolymers based on high-content steel slag. Constr. Build. Mater. 2024, 410, 134128. [Google Scholar] [CrossRef]
- Zhao, J.H.; Tong, L.Y.; Li, B.E.; Chen, T.H.; Wang, C.P.; Yang, G.Q.; Zheng, Y. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Muraleedharan, M.; Nadir, Y. Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: A review. Ceram. Int. 2021, 47, 13257–13279. [Google Scholar] [CrossRef]
- Kul, A.; Ozcelikci, E.; Ozel, B.F.; Ilcan, H.; Sahin, O.; Gunal, M.F.; Yildirim, G.; Sahmaran, M. Optimizing mechanical performance of geopolymers produced from construction and demolition waste: A comparative study of materials from different origins. Constr. Build. Mater. 2024, 426, 136171. [Google Scholar] [CrossRef]
- Küçükyıldırım, E.; Yorulmaz, H.; Durak, U.; Ilkentapar, S.; Uzal, B.; Karahan, O.; Atis, C.D. Reaction kinetics and properties of pumice-based geopolymer systems cured at room temperature. Constr. Build. Mater. 2023, 409, 134074. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Nie, Q.; Jia, X. A high-strength red mud–fly ash geopolymer and the implications of curing temperature. Powder Technol. 2023, 416, 118242. [Google Scholar] [CrossRef]
- Xu, L.Y.; Lao, J.C.; Qian, L.P.; Khan, M.; Xie, T.Y.; Huang, B.T. Low-carbon high-strength engineered geopolymer composites (HS-EGC) with full-volume fly ash precursor: Role of silica modulus. J. CO2 Util. 2024, 88, 102948. [Google Scholar] [CrossRef]
- Liu, Y.W.; Lu, C.F.; Hu, X.; Shi, C.J. Effect of silica fume on rheology of slag-fly ash-silica fume-based geopolymer pastes with different activators. Cement Concrete Res. 2023, 174, 107336. [Google Scholar] [CrossRef]
- Amiri, H.; Azadi, S.; Karimaei, M.; Sadeghi, H.; Dabbaghi, F. Multi-objective optimization of coal waste recycling in concrete using response surface methodology. J. Build. Eng. 2022, 45, 103472. [Google Scholar] [CrossRef]
- Ja’e, I.A.; Salih, A.R.; Syamsir, A.; Min, T.H.; Itam, Z.; Amaechi, C.V.; Anggraini, V.; Sridhar, J. Experimental and predictive evaluation of mechanical properties of kenaf-polypropylene fibre-reinforced concrete using response surface methodology. Dev. Built Environ. 2023, 16, 100262. [Google Scholar]
- Dihaji, H.; Azerkane, D.; Bih, L.; Essaddek, A.; Haily, E.L. Comparative study of geopolymers synthesized with alkaline and acid reactants at various liquid-to-solid ratios using Moroccan kaolin clay. Constr. Build. Mater. 2025, 468, 140453. [Google Scholar] [CrossRef]
- Salim, M.U.; Moro, C. Towards sustainable construction: Performance evaluation of slag-cenosphere geopolymers under different NaOH concentrations. J. Build. Eng. 2024, 91, 109605. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Ma, C.Z.; Huang, B.S.; Lu, X.B. Development of alkali activated cementitious material from sewage sludge ash: Two-part and one-part geopolymer. J. Clean. Prod. 2023, 384, 135547. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Zhang, D.S.; Zhu, T.; Yang, Q.N.; Vandeginste, V.; Li, J.B. Influence of ground granulated blast furnace slag on recycled concrete powder-based geopolymer cured at ambient temperature: Rheology, mechanical properties, reaction kinetics and air-void characteristics. Constr. Build. Mater. 2024, 438, 137190. [Google Scholar] [CrossRef]
- Shi, Y.X.; Zhao, Q.X.; Xue, C.H.; Jia, Y.L.; Guo, W.C.; Zhang, Y.Y.; Qiu, Y.X. Preparation and curing method of red mud-calcium carbide slag synergistically activated fly ash-ground granulated blast furnace slag based eco-friendly geopolymer. Cement Concrete Comp. 2023, 139, 104999. [Google Scholar] [CrossRef]
- Fan, L.D.; Wu, D.S.; Yu, Y.Q.; Yang, J.; Zhang, J.Y.; Li, P.T.; Guo, J.Q. Mechanical properties of Halloysites-based and Halloysites-modified slag/fly ash-based geopolymers. J. Build. Eng. 2024, 98, 111427. [Google Scholar] [CrossRef]
- Çelikten, S.; Saridemir, M.; Deneme, I.Ö. Mechanical and microstructural properties of alkali-activated slag and slag plus fly ash mortars exposed to high temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Evaluation of the suitability of ground granulated silico-manganese slag in Portland slag cement. Constr. Build. Mater. 2016, 125, 127–134. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, L.F.; Ma, X.; Wang, H. Compositional, microstructural and mechanical properties of ambient condition cured alkali-activated cement. Constr. Build. Mater. 2016, 113, 237–245. [Google Scholar] [CrossRef]








| SiO2 | CaO | MgO | Al2O3 | K2O | Na2O | Fe2O3 | P2O5 | TiO2 | SO3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| CG | 59.52 | 0.26 | 0.18 | 36.57 | 0.75 | - | 1.24 | 0.05 | 1.14 | 0.16 |
| BFS | 35.62 | 36.31 | 7.50 | 14.93 | 0.39 | 0.22 | 0.60 | - | 1.28 | 2.29 |
| Variables | Factors | Coded Levels of Variables | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | ||
| Waterglass module | X1 | 0.2 | 0.4 | 0.7 | 1 | 1.2 |
| Alkali activator dosage/% | X2 | 2.5 | 5.54 | 10 | 14.46 | 17.5 |
| Curing temperature/°C | X3 | 30 | 38 | 50 | 62 | 70 |
| Number | X1 | X2 | X3 | Number | X1 | X2 | X3 |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | −1 | 13 | −1 | −1 | 1 |
| 2 | −1.682 | 0 | 0 | 14 | 0 | 0 | 0 |
| 3 | 0 | −1.682 | 0 | 15 | 0 | 0 | 1.682 |
| 4 | 0 | 0 | 0 | 16 | −1 | 1 | −1 |
| 5 | −1 | 1 | 1 | 17 | 1 | 1 | 1 |
| 6 | 0 | 0 | 0 | 18 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 19 | 0 | 0 | 0 |
| 8 | 1.682 | 0 | 0 | 20 | 0 | 0 | 0 |
| 9 | 0 | 0 | −1.682 | 21 | −1 | −1 | −1 |
| 10 | 1 | −1 | 1 | 22 | 0 | 0 | 0 |
| 11 | 1 | −1 | −1 | 23 | 0 | 0 | 0 |
| 12 | 0 | 1.682 | 0 |
| Factor | Source | Sum of Square | d.f. | Mean Square | F | p-Value |
|---|---|---|---|---|---|---|
| Liquid-to-solid ratio | Between | 164.46 | 4 | 41.11 | 2.87 | 8.01 × 10−2 |
| Within | 143.12 | 10 | 14.31 | |||
| Total | 307.58 | 14 | ||||
| Alkali activator dosage | Between | 328.53 | 4 | 82.13 | 15.81 | 2.52 × 10−4 |
| Within | 51.96 | 10 | 5.20 | |||
| Total | 380.49 | 14 | ||||
| Waterglass modulus | Between | 480.11 | 4 | 120.03 | 35.06 | 7.40 × 10−6 |
| Within | 34.24 | 10 | 3.42 | |||
| Total | 514.35 | 14 | ||||
| BFS content | Between | 3038.50 | 4 | 759.62 | 32.73 | 1.02 × 10−5 |
| Within | 232.12 | 10 | 23.21 | |||
| Total | 3270.62 | 14 | ||||
| Curing temperature | Between | 3245.97 | 4 | 811.49 | 44.55 | 2.43 × 10−6 |
| Within | 182.16 | 10 | 18.22 | |||
| Total | 3428.13 | 14 |
| Mix | Variables | Response | ||
|---|---|---|---|---|
| Waterglass Module | Alkali Activator Dosage/% | Curing Temperature/°C | Compressive Strength/MPa | |
| 1 | 1 | 14.46 | 38 | 85.62 |
| 2 | 0.2 | 10 | 50 | 28.72 |
| 3 | 0.7 | 2.5 | 50 | 32.28 |
| 4 | 0.7 | 10 | 50 | 69.97 |
| 5 | 0.4 | 14.46 | 62 | 39.08 |
| 6 | 0.7 | 10 | 50 | 69.14 |
| 7 | 0.7 | 10 | 50 | 66.77 |
| 8 | 1.2 | 10 | 50 | 59.36 |
| 9 | 0.7 | 10 | 30 | 73.77 |
| 10 | 1 | 5.54 | 62 | 45.11 |
| 11 | 1 | 5.54 | 38 | 61.28 |
| 12 | 0.7 | 17.5 | 50 | 52.10 |
| 13 | 0.4 | 5.54 | 62 | 36.89 |
| 14 | 0.7 | 10 | 50 | 69.51 |
| 15 | 0.7 | 10 | 70 | 46.83 |
| 16 | 0.4 | 14.46 | 38 | 52.29 |
| 17 | 1 | 14.46 | 62 | 37.43 |
| 18 | 0.7 | 10 | 50 | 69.77 |
| 19 | 0.7 | 10 | 50 | 64.64 |
| 20 | 0.7 | 10 | 50 | 63.15 |
| 21 | 0.4 | 5.54 | 38 | 33.85 |
| 22 | 0.7 | 10 | 50 | 67.31 |
| 23 | 0.7 | 10 | 50 | 75.36 |
| Source | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|
| X1 | 1034.58 | 90.43 | <0.0001 | Yes |
| X2 | 365.20 | 31.92 | <0.0001 | Yes |
| X3 | 1051.79 | 91.94 | <0.0001 | Yes |
| X1X2 | 1.96 | 0.17 | 0.6855 | No |
| X1X3 | 367.23 | 32.10 | <0.0001 | Yes |
| X2X3 | 291.28 | 25.46 | 0.0002 | Yes |
| X12 | 1107.04 | 96.77 | <0.0001 | Yes |
| X22 | 1287.28 | 112.52 | <0.0001 | Yes |
| X32 | 107.20 | 9.37 | 0.0091 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Ma, T.; Li, D.; Xia, M. Optimization and Mechanistic Investigation of Coal Gangue–Blast Furnace Slag Composite Geopolymers. Processes 2025, 13, 1703. https://doi.org/10.3390/pr13061703
Zhao S, Ma T, Li D, Xia M. Optimization and Mechanistic Investigation of Coal Gangue–Blast Furnace Slag Composite Geopolymers. Processes. 2025; 13(6):1703. https://doi.org/10.3390/pr13061703
Chicago/Turabian StyleZhao, Shujie, Tian Ma, Dongwei Li, and Ming Xia. 2025. "Optimization and Mechanistic Investigation of Coal Gangue–Blast Furnace Slag Composite Geopolymers" Processes 13, no. 6: 1703. https://doi.org/10.3390/pr13061703
APA StyleZhao, S., Ma, T., Li, D., & Xia, M. (2025). Optimization and Mechanistic Investigation of Coal Gangue–Blast Furnace Slag Composite Geopolymers. Processes, 13(6), 1703. https://doi.org/10.3390/pr13061703






