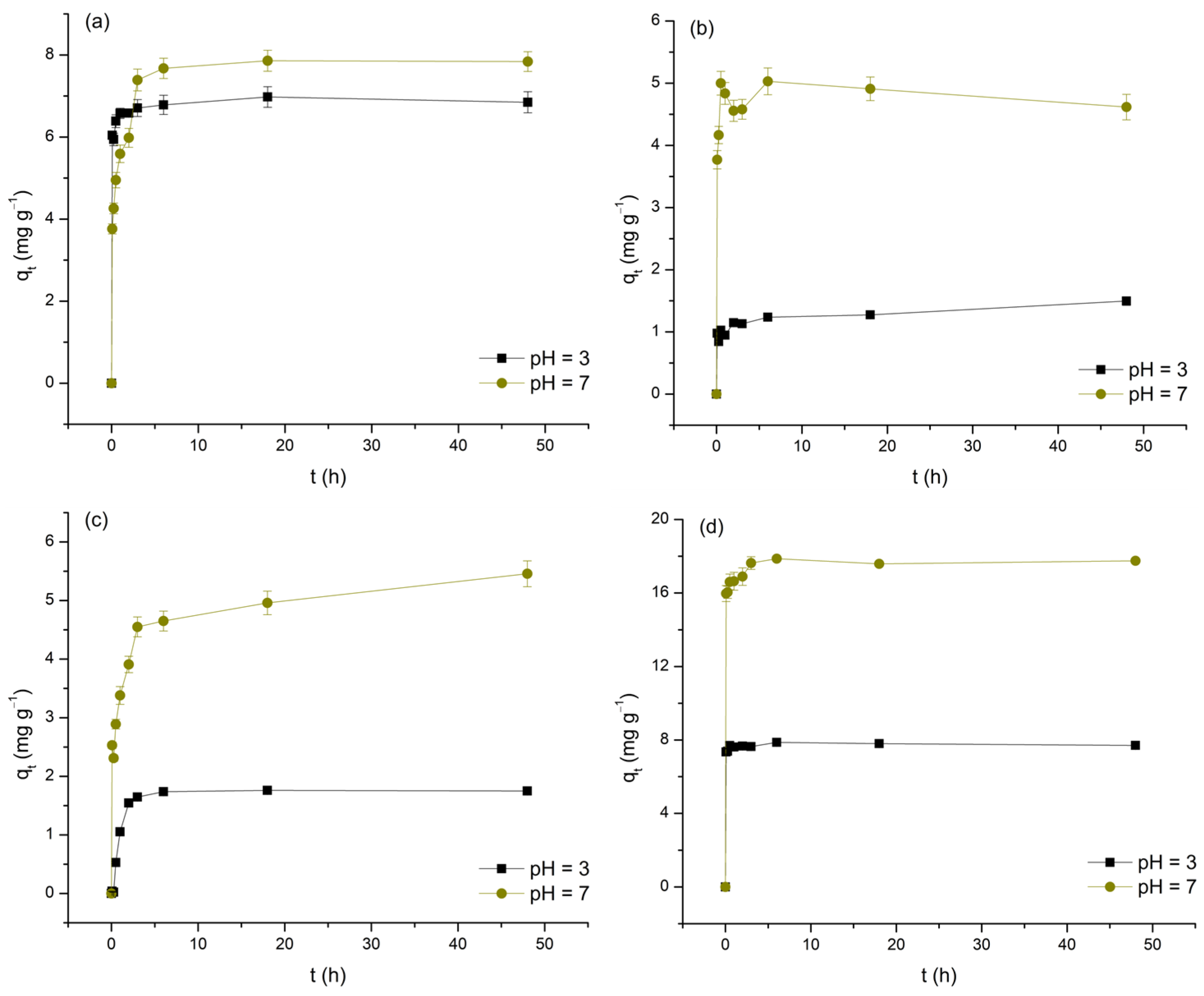
3.2. Influence of Contact Time and Investigation of Adsorption Kinetics
The impact of contact time on the removal of As(V) and selected M(II) by TiO
2, expressed as a function of
qt over
t, is shown in
Figure 6. To ensure consistency when identifying
t associated with the occurrence of equilibrium periods, the following criterion was introduced: adsorption equilibrium is considered to be established if the change in
qt with further increases in
t does not exceed ±10% of the presumed limiting value.
As clearly observed in the data, the increase in
t, to varying degrees, positively affects the levels of As(V) and M(II) removal. Furthermore, by dividing the investigated time intervals into distinct segments, it is concluded that, with the exception of the Cu(II)/pH 3 system, the initial 5 min phase exhibits the highest adsorption rates (11.72 to 191.65 mg min
−1) and the highest contributions to the average equilibrium
qt values (48.89 to 96.21%). This finding supports the hypothesis that physisorption predominantly contributes to the examined processes, likely due to the development of appropriate attractive forces between As(V) and M(II) and the initially large number of available adsorption sites on the TiO
2 surface. Based on the aforementioned criterion, it follows that, depending on the type of adsorbate and the pH of the medium, equilibrium is reached within a period of 5 min to 6 h. It should be emphasized that, in the case of the Cd/pH 3 and Cu/pH 7 systems, the deviation of the last points (48 h) compared to the preceding ones by more than 10% in terms of
qt raises questions about the completeness of adsorption equilibrium (despite this uncertainty, in determining the adsorption isotherms,
t was fixed at 24 h). The determined equilibrium times, with the exception of the Pb/pH 3 system, ≥30 min, indicate a reduction in the initial intensity of physisorption, likely caused by the decrease in concentration gradients and changes in the surface charge state of TiO
2 due to continuous saturation with As(V) and M(II) ions. These observations also suggest the potential contribution of other, less dominant adsorption mechanisms, such as appropriate forms of surface complexation. The recorded differences in equilibrium
t are a consequence of the dependence of this parameter on the physicochemical properties of the examined adsorbates, as well as the compatibility of their charges with those displayed by TiO
2 under the conditions of the medium (further discussion on this topic will be provided in
Section 3.5.). Finally, by examining the average equilibrium
qt values (
Table 4), the first information about the affinity of TiO
2 towards As(V) and M(II) is obtained, demonstrating the following trends: Pb(II) > As(V) > Cu(II) > Cd(II) and pH 7 > pH 3 (the observed trends should not be considered absolutely reliable, given that the experimental systems were not designed based on the principle of maintaining identical
m/V ratios and
C0).
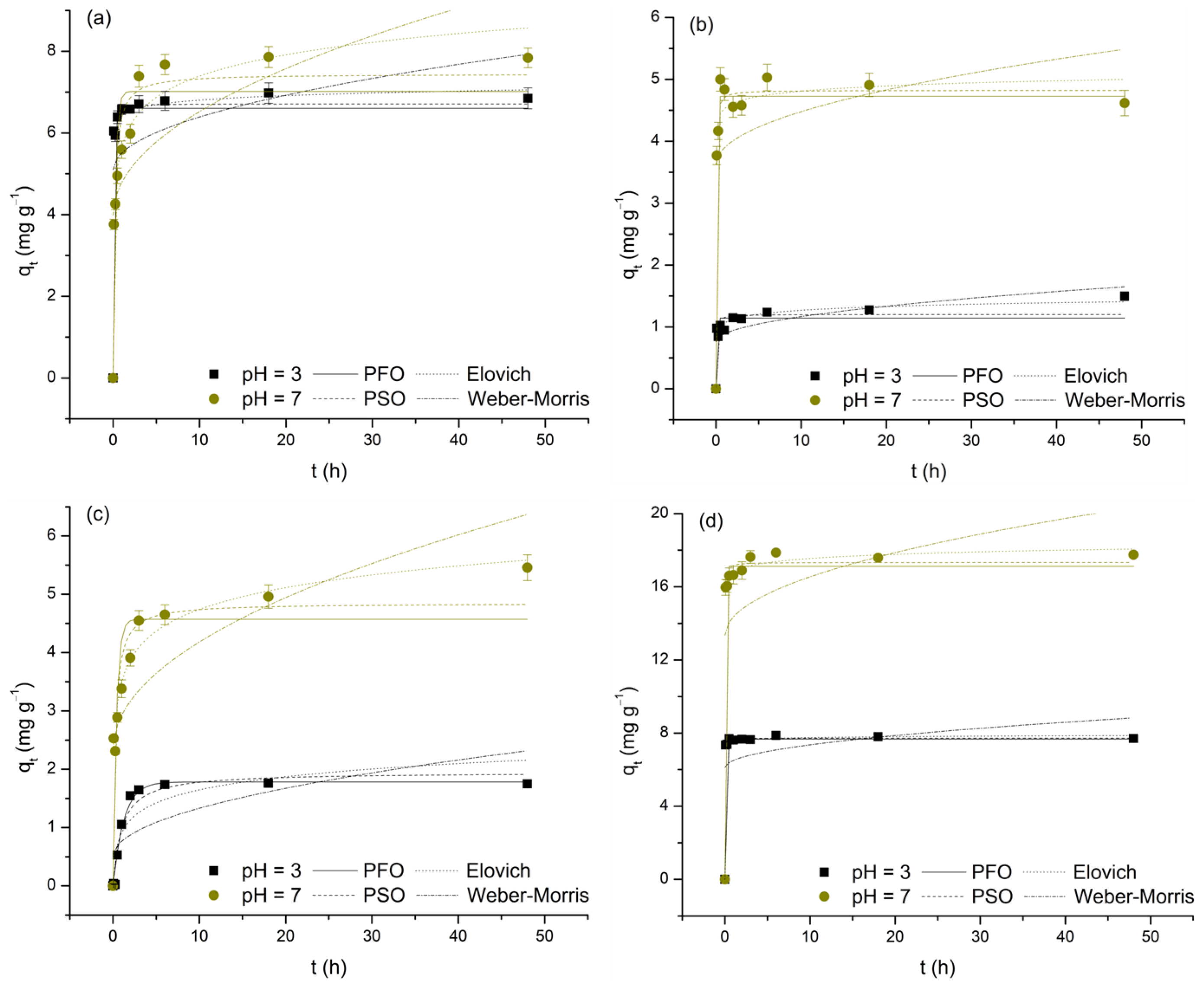
The adsorption kinetics of As(V) and M(II) on TiO
2 were investigated by analyzing the
qt vs.
t dependence using nonlinear forms of the pseudo-first-order, pseudo-second-order, Elovich, and Weber–Morris models (
Figure 7). The obtained values for the characteristic parameters and validity indicators of the applied functions are presented in
Table 5. Before proceeding with their interpretation, it is important to highlight that these values should be taken with some caution, as the analyzed datasets do not meet the criterion that the majority of points should not be too close to or included within the equilibrium state. In such cases, model specificity is lost, and the risk of unreliable estimation of kinetic parameters becomes significant [
60,
61].
For this reason, with the exception of the Weber–Morris model (R2: 0.011–0.548, χ2: 0.160–2.573; low χ2, but also low R2, values indicate that this model, although successful in predicting experimentally obtained data, does not adequately describe their variability), it follows that all three remaining functions can be considered suitable for interpreting the investigated adsorption processes (R2: 0.763–0.998, χ2: 0.010–1.838). Therefore, it is only reasonable to assume that the adsorption kinetics of the observed systems are not controlled by intraparticle diffusion but are primarily the result of the rate at which the appropriate surface interactions are established, as evidenced by the relatively short times required to reach equilibrium. Although not ideal for direct comparison, the validity parameters of the applied reaction models provide grounds for partial generalization, i.e., concluding that the adsorption processes of interest are best described by the Elovich equation (deviations from this rule occur only in the case of the Cd(II)/pH 7 and, to a greater extent, Cu(II)/pH 3 systems; these deviations may be a result of the previously mentioned lack of model specificity or may stem from changes in the adsorption mechanisms influenced by the pH of the medium). Thus, based on the assumptions of the prioritized model, it appears that the involvement of chemisorption, occurring on the heterogeneous TiO2 surface through the progressive increase in activation energy caused by the reduction in the number of highly active adsorption sites over time, cannot be neglected. However, it is important to refrain from drawing conclusions regarding the dominance of physisorption or chemisorption. Observing the values of the α parameter, as experimental data suggest, it is evident that the initial adsorption rate, regardless of pH, is highest in systems where Pb(II) serves as the adsorbate (9.85 × 1043 and 3.76 × 1021 mg g−1 h−1) and, conversely, the lowest in systems characterized by the presence of Cu(II) (5.05 and 3.58 × 102 mg g−1 h−1; for the sake of comparison, the α values, except for the Cu(II)/pH 3 system, are drastically higher compared to the corresponding h values of the pseudo-second-order model). Regarding the β parameter, an indicator of the level of surface energy heterogeneity, the change in pH results in a loss of the observed consistency, as selected adsorbates form completely different relationships (Pb(II) > Cd(II) > As(V) > Cu(II)—pH 3 and Cd(II) > Pb(II) > Cu(II) > As(V)—pH 7). However, a subsequent trend is recorded: pH 3 > pH 7. All this indicates that the behavior of the examined adsorption systems is the result of the simultaneous interaction of two key factors: on the one hand, the type and distribution of adsorption sites on the TiO2 surface, and on the other, the specific properties of As(V) and the selected M(II) (e.g., electronegativity, ionic radius, hydration energy, etc.). Finally, it is important to note that the α and β values should not be considered reliable, which is particularly noticeable in the case of α, which is most likely overestimated by the model and therefore lacks physical significance.
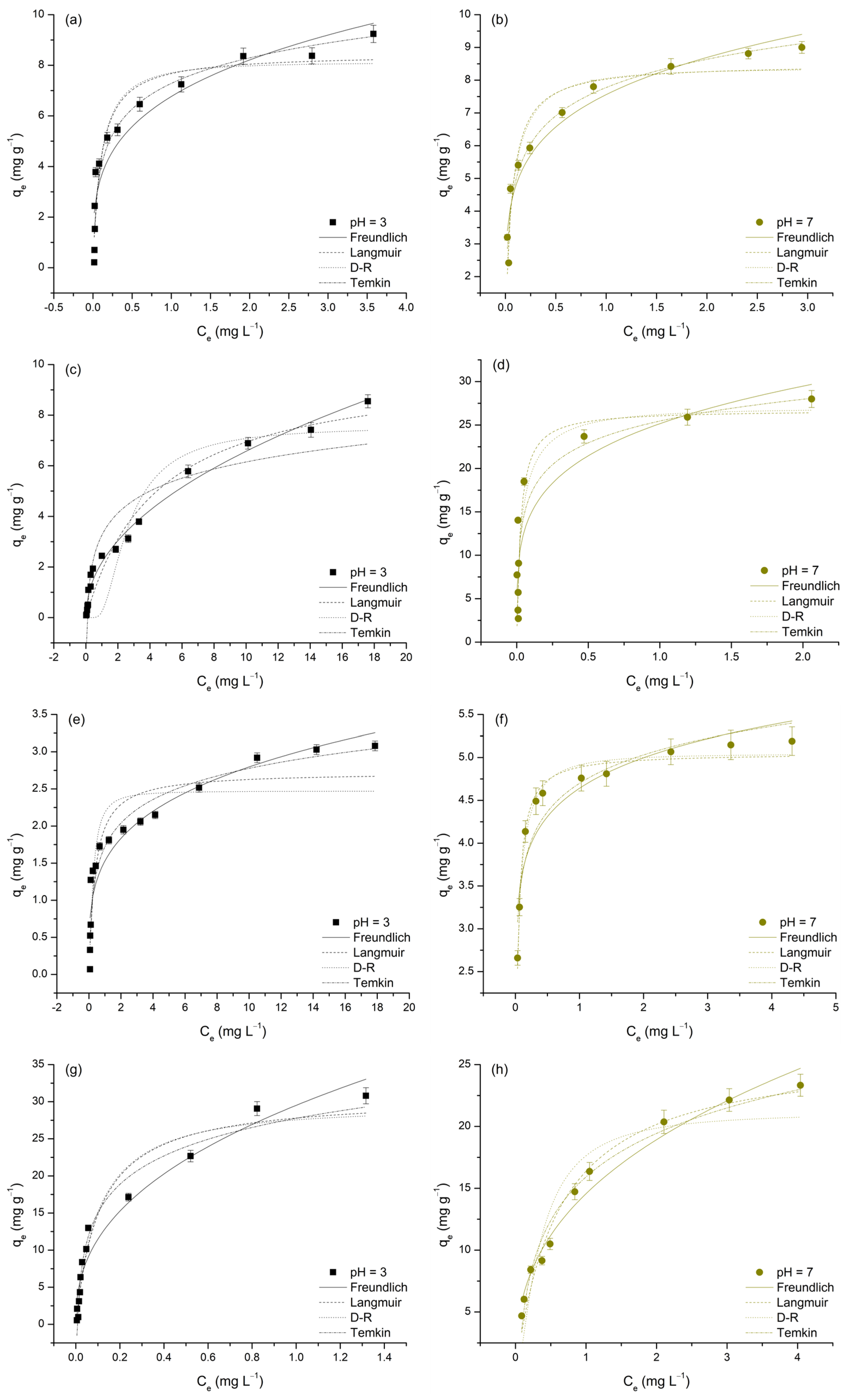
3.3. Influence of Initial Concentration and Investigation of Adsorption Isotherms
The initial concentration of metal ions is a key factor influencing the adsorption process, as it directly affects both the
qe and
REe. To better understand this influence on the adsorption performance of TiO
2, the initial concentrations of M(II) and As(V) were varied within the range of 50 to 5000 µgL
−1 at pH 3 and from 300 to 5000 µgL
−1 at pH 7. The obtained results are presented in
Figure 8 and
Table 6.
It is evident that increasing the concentration of the analyzed metals in the liquid phase leads to a rise in
qe, while simultaneously causing a decrease in
REe values, as illustrated in
Figure 9 and
Table 7. The observed increase in adsorption capacity (
qe) with higher initial metal concentrations (
Ct) is attributed to the enhanced concentration gradient, which provides a stronger driving force for mass transfer, thereby facilitating adsorption. This trend has been consistently reported in studies where higher initial metal concentrations resulted in increased adsorption capacities, due to the greater availability of metal ions for interaction with the adsorbent surface [
62]. Conversely, the decrease in removal efficiency with increasing
C0 can be attributed to the gradual saturation of the TiO
2 surface, which has a limited number of active sites. As these sites become occupied, the adsorbent’s ability to remove additional metal ions diminishes, resulting in lower removal efficiencies [
35]. This behavior is particularly relevant at high initial concentrations, where the limited availability of adsorption sites leads to reduced removal performance [
30,
63,
64].
To gain a deeper understanding of the adsorption behavior of M(II) and As(V) on TiO
2, and to evaluate its adsorption affinities and capacities for these metals, the experimental data were further analyzed using four commonly applied isotherm models: Freundlich, Langmuir, Dubinin–Radushkevich (D–R), and Temkin (
Figure 9). The parameters obtained from these models are presented in
Table 7.
The adsorption data were well represented by all four isotherm models, as indicated by the high R
2 values (0.802–0.985) and relatively low χ
2 values, suggesting a good fit in most cases. According to Simonin [
61], models with R
2 > 0.85 can be considered to show a good fit and are generally reliable for predicting adsorption behavior. Among the applied models, the Langmuir model generally provided the best fit, with R
2 values above 0.90 for most metals, except for Cu(II) at pH 3 (R
2 = 0.879) and Cd(II) at pH 7 (R
2 = 0.819), where the fit was slightly weaker. The Freundlich model showed particularly good performance for Cd(II) at pH 3 (R
2 = 0.985) and Pb(II) at pH 7 (R
2 = 0.975), indicating its suitability under these conditions. The Temkin model best described the adsorption of Pb(II) at pH 7 (R
2 = 0.975) and As(V) at pH 3 (R
2 = 0.949), highlighting the relevance of adsorbate–adsorbent interactions in these cases. Conversely, the D–R model exhibited lower R
2 values in some instances, particularly for Cd(II) at pH 7 (R
2 = 0.825) and Cu(II) at pH 3 (R
2 = 0.830). Although the D–R model provided a good fit for Cu(II) at pH 7 (R
2 = 0.981), its overall performance was generally weaker than that of the Langmuir and Freundlich models (
Table 7). The following sections will provide an in-depth discussion of each applied model in relation to the adsorption behavior of the studied metals.
The Freundlich model describes adsorption on heterogeneous surfaces, where the Freundlich constant (
KF) indicates adsorption capacity and the adsorption intensity parameter (
n) reflects surface heterogeneity and binding strength [
31,
65]. The variation in
KF values across investigated metals and pH levels can provide insights into adsorption efficiency. However, a direct comparison of
KF values is not possible due to their different units, which stems from the nonlinearity of the adsorption isotherms. Instead, a qualitative assessment of the relative adsorption affinity among different metal ions can be made based on the observed trends. At pH 3, the order of
KF values was as follows: Pb(II) > As(V) > Cd(II) > Cu(II) (
Table 7). This trend indicates that Pb(II) exhibited the highest affinity toward TiO
2, likely due to its larger ionic radius and strong electrostatic interactions. As(V) also showed significant adsorption, which can be attributed to the specific interactions between arsenate species and surface functional groups of TiO
2 [
66].
At pH 7, the
KF values follow a different trend: Cd(II) > Pb(II) > As(V) > Cu(II). This shift suggests that Cd(II) adsorption increased significantly at neutral pH, likely due to reduced proton competition and more pronounced surface-related interactions, such as electrostatic and/or complexation effects. Meanwhile, Pb(II) still exhibited a relatively high
KF value, although lower than at pH 3, while As(V) adsorption remained relatively stable across both pH levels, likely due to the ability of arsenate species to interact with the TiO
2 surface through ligand exchange mechanisms, even under neutral conditions [
66]. In contrast, Cu(II) consistently displayed the lowest
KF values across both pH levels, indicating a weaker affinity for TiO
2 [
63].
The adsorption intensity parameter, n, remained below 1 for all metals, confirming a favorable adsorption process. Lower n values were observed for As(V) (0.28 at pH 3, 0.20 at pH 7) and Cu(II) (0.26 at pH 3, 0.11 at pH 7), indicating stronger binding interactions and a highly heterogeneous adsorption surface. For Cd(II) (0.48, 0.23) and Pb(II) (0.41, 0.38), the adsorption process is still favorable, but the lower heterogeneity may indicate more uniform binding sites for these ions.
The Langmuir model assumes monolayer adsorption on a homogenous surface, characterized by
qm (maximum adsorption capacity) and
KL (Langmuir constant related to binding strength) [
65]. At pH 3, the order of
qm values was as follows: Pb(II) > Cd(II) > As(V) > Cu(II) (2.73 mg·g
−1). The high adsorption capacity of Pb(II) is consistent with the Freundlich model results, reinforcing its strong affinity for TiO
2. Cd(II) and As(V) also exhibited notable adsorption capacities, whereas Cu(II) had the lowest
qm value, indicating weak interaction with the adsorbent surface. The highest uptake for Pb(II), followed by Cd(II) and Cu(II), using composites synthesized from titanium dioxide and carbon spheres (TNT/CS) were reported by Ade et al. [
67], which is in agreement with our work.
At pH 7, the order of
qm values changed to Cd(II) > Pb(II) > As(V) > Cu(II). The substantial increase in Cd(II) adsorption at pH 7 aligns with the Freundlich trend, confirming that its adsorption is highly pH-dependent. Similarly, Cu(II) adsorption also improved, suggesting that, at higher pH, the adsorption of Cu(II) becomes more pronounced. This observation was also reported by Chen et al. [
68], who investigated metal adsorption on titanium dioxide-based nanomaterials and found that increasing pH enhanced Cu(II) uptake due to reduced proton competition and increased availability of negatively charged surface sites. In contrast, Pb(II) adsorption capacity showed a slight decrease at pH 7 compared to pH 3, which was also consistent with the Freundlich model. Despite this moderate reduction, Pb(II) still demonstrated a strong adsorption capacity, indicating its significant affinity for TiO
2 under both acidic and neutral conditions [
69,
70].
The Langmuir constant (
KL), which represents the binding affinity of the adsorbate, showed distinct trends across the investigated pH levels. At pH 3, As(V) (9.82 L mg
−1) and Pb(II) (9.26 L mg
−1) had the highest
KL values, indicating strong adsorption affinity, while Cd(II) (0.22 L mg
−1) exhibited the weakest binding. This suggests that Pb(II) and As(V) interact more effectively with TiO
2 under acidic conditions. At pH 7, a substantial increase in
KL was observed for Cd(II) (37.64 L mg
−1) and Cu(II) (29.61 L mg
−1), suggesting that their adsorption became more favorable at neutral pH likely due to enhanced electrostatic and/or complexation interactions. In contrast, the
KL value for Pb(II) (1.67 L mg
−1) sharply decreased, while As(V) maintained a relatively high
KL (16.96 L mg
−1), indicating its continued affinity for TiO
2 across both pH levels. The separation factor (
RL, where 0 <
RL < 1) confirmed favorable adsorption conditions for all metal ions, with lower
RL values at pH 7 suggesting enhanced adsorption under neutral conditions. A similar observation was reported in another similar study [
71].
The Dubinin–Radushkevich (D-R) isotherm model, which describes adsorption through a pore-filling mechanism rather than monolayer adsorption, provided theoretical saturation capacities (
qd) [
72]. Pb(II) exhibited the highest
qd (29.11 mg g
−1 at pH 3 and 21.13 mg g
−1 at pH 7), confirming its strong adsorption affinity, as also indicated by the Langmuir and Freundlich models. In contrast, Cu(II) showed the lowest
qd (2.47–5.04 mg g
−1), suggesting weak adsorption (
Table 7). Cd(II) and As(V) (7.56–26.94 mg g
−1) displayed pH-dependent adsorption, particularly Cd(II), which exhibited a significant increase at pH 7, likely due to stronger surface interactions. The mean free energy (
Ed), which determines the nature of the adsorption mechanism, ranged from 0.65 to 7.47 kJ/mol, indicating that adsorption was predominantly physisorption-driven (
Ed < 8 kJ/mol). According to the D-R model, adsorption processes with
Ed < 8 kJ/mol are classified as physisorption, while values exceeding this threshold indicate chemisorption [
31]. The
Ed values confirmed that all metal ions were primarily adsorbed via physisorption, except for Cd(II), which had an
Ed value of 7.32 kJ/mol, approaching the chemisorption threshold, suggesting additional interactions. The R
2 values for the D-R model were generally lower than those of the Langmuir and Freundlich models, indicating that pore filling may not fully explain adsorption for all metal ions. However, for Cu(II) at pH 7 (R
2 = 0.981), the strong model fit suggests that surface porosity played a significant role in its adsorption behavior.
The Temkin isotherm model, which accounts for indirect adsorbate-adsorbent interactions and assumes a linear decrease in adsorption energy with increasing surface coverage, provided further insights into the thermodynamic behavior of the adsorption process [
72]. In this model, the equilibrium binding constant (
KT) reflects the strength of the adsorbate–adsorbent interaction, while the binding energy constant (
bt) is directly related to the heat of adsorption and thus indicative of the thermodynamic nature of the process. As shown in
Table 7, both
KT and
bt values exhibited clear pH-dependent trends. Pb(II) exhibited the highest
KT at pH 3 (1.52 × 10
2 L g
−1), indicating strong affinity under acidic conditions; however, this value declined significantly at pH 7 (23.07 L g
−1), suggesting reduced interaction strength at higher pH.
In contrast, Cd(II) and Cu(II) displayed higher KT values at pH 7, aligning with Langmuir and Freundlich trends that indicated improved adsorption at neutral pH due to enhanced electrostatic and/or complexation interactions. Notably, all bt values were positive, indicating that the calculated enthalpy changes (ΔHads) are negative. This confirms that the adsorption processes are exothermic in nature, meaning they release energy and may become less favorable at elevated temperatures.
The exothermic nature of the process is particularly evident for Cu(II) at pH 7, which exhibited the highest bt value (5.18 kJ mol−1), corresponding to the most negative enthalpy change and indicating strong adsorbate-adsorbent interactions. Conversely, lower bt values observed for Pb(II) and As(V), especially under acidic conditions, suggest weaker adsorption interactions. These findings are consistent with the results of the Langmuir and Freundlich models, which showed that Pb(II) and As(V) adsorb more effectively under acidic conditions, while Cd(II) and Cu(II) adsorption is favored at neutral pH.
Overall, the Temkin model complements the results from other isotherm models by emphasizing the thermodynamic nature of adsorption and highlighting the role of pH in modulating interaction strength. The variations in KT and bt underscore the complex interplay between electrostatic attraction, surface complexation, and thermodynamic driving forces in controlling the adsorption of metal ions onto TiO2.
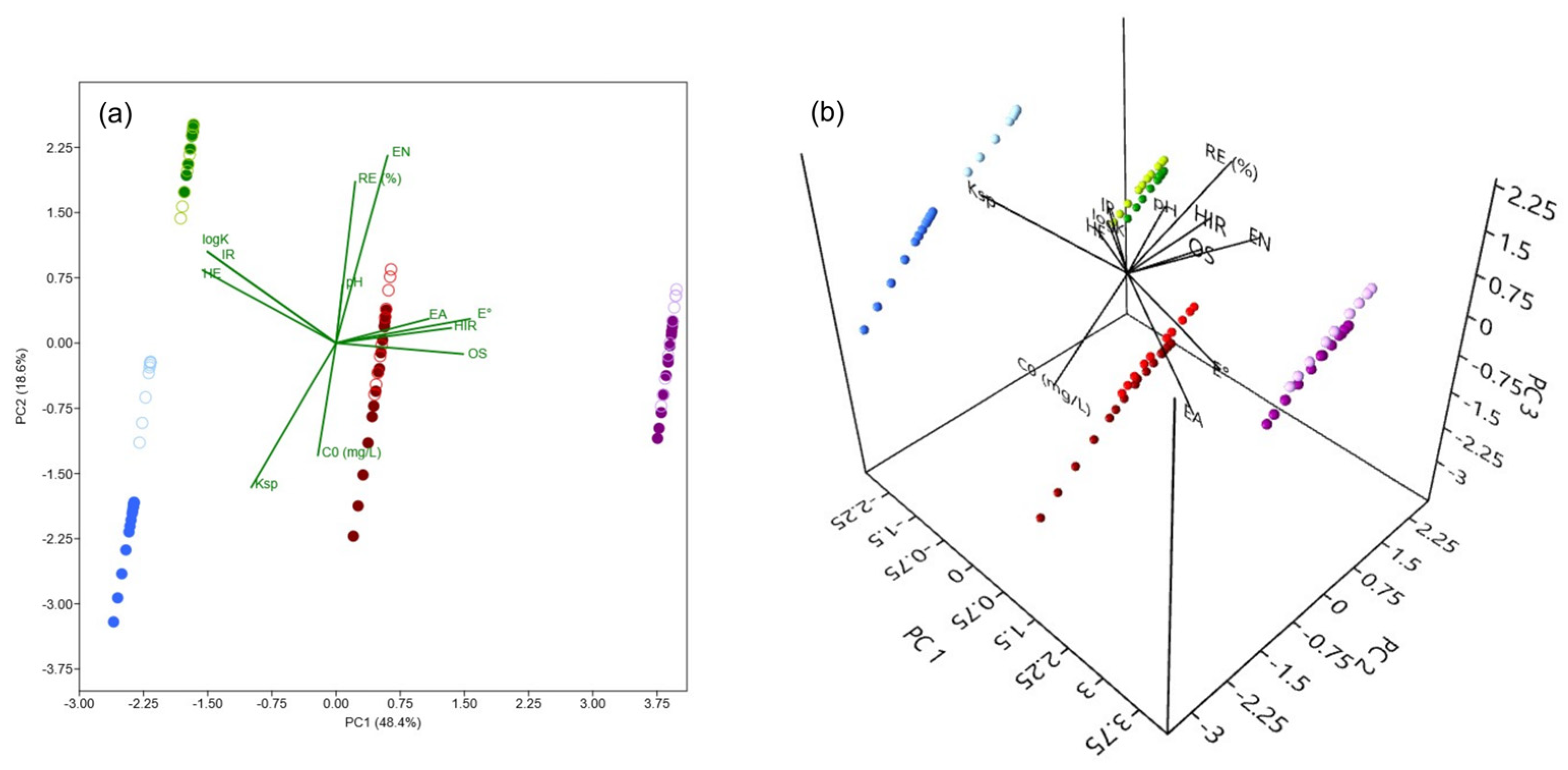
3.4. Principal Component Analysis
A PCA was conducted to investigate the influence of experimental conditions (
C0 and pH), along with the properties of As(V) and M(II) (oxidation state—
OS, ionic radius (pm)—
IR, hydrated ionic radius (pm)—
HIR, electronegativity (Pauling)—
EN, hydration energy (kJ mol
−1)—
HE, hydrolysis constant—
logK, solubility product constant—
Ksp, standard reduction potential (V)—
E°, and electron affinity (eV)—
EA;
Table 8) on the recorded
REe values [
73,
74,
75].
Figure 10 presents a PCA biplot, offering a visual representation of the correlations between these variables. The first two principal components, PC1 and PC2, explain 67% of the total variance (48.4% and 18.6%, respectively;
Figure 10a), providing insight into the primary factors governing the removal of As(V) and M(II) under varying conditions. Incorporating the third principal component, PC3 (
Figure 10b), which accounts for an additional 13.1% of the total variance, increases the cumulative explained variance to 80.1%, offering further insight into the influence of certain variables.
The PCA biplot clearly shows a strong correlation between the uptake of selected adsorbates and the experimental conditions, with REe positively correlating with the pH of the background solution. This can be attributed to the contribution of pH in promoting favorable electrostatic conditions within the systems of interest, as well as to the decrease in the solubility of the investigated species (primarily Cu(II) and Pb(II)), both of which typically have a positive effect on the achieved removals. This finding is consistent with the negative correlation observed between REe and Ksp. In the case of Cd(II), the PC1 × PC2 biplot shows a clear clustering of data points corresponding to different pH conditions, whereas such separation is not evident in the three other cases. However, by incorporating a third principal component, a distinction based on pH becomes observable for As(V), Cu(II), and Pb(II) as well. This suggests that, to varying extents, pH influences the removal of both As(V) and M(II) by the investigated adsorbent. A negative correlation between REe and C0 was also observed, indicating possible saturation of the TiO2 at higher concentrations. Finally, REe shows a strong association with EN, suggesting that adsorbates with a greater tendency to gain electrons exhibit a higher affinity for adsorption onto TiO2.
Distinct clustering of adsorbates is evident in the biplot, with As(V) (purple) positioned at the far right, clearly separated from Cd(II) (blue), Cu(II) (red), and Pb(II) (green), suggesting fundamentally different removal mechanisms. This separation aligns with the high E° of As(V), which may reflect its strong tendency to engage in surface complexation, i.e., ligand exchange with TiO2, in contrast to the predominantly electrostatic interactions governing the M(II) cationic species. In contrast, Pb(II) aligns with HE and logK, indicating that hydration and hydrolysis effects play a significant role in its removal. The central position is occupied by Cu(II), reflecting a moderate influence from multiple variables. Meanwhile, Cd(II), located at the far left, likely reflects its high solubility but still exhibits relatively high adsorption, possibly due to other factors.
Overall, PCA reveals that As(V) and M(II) removal is governed by a combination of solution chemistry (C0 and pH) and intrinsic adsorbates properties, with distinct trends observed for different elements. The clustering patterns suggest that treatment strategies should be tailored to the specific characteristics of each adsorbate and environmental conditions.
3.5. Adsorption Mechanisms
Since all adsorption experiments covered in this study were conducted at two pH values, 3 and 7, interpreting the effects of potentially involved adsorption mechanisms should be approached by considering the impact of the given environmental parameter, both on the electric state of the TiO2 surface and on the forms in which As(V) and M(II) are present.
Namely, based on the fact that the
pHpzc for TiO
2 is 4.34 ± 0.12, it is clear that under the monitored experimental conditions, both possible scenarios regarding the surface charge of the adsorbent are realized: at pH 3, the surface is predominantly positive (pH <
pHpzc), while at pH 7, it is predominantly negative (pH >
pHpzc). The reason for this lies in the interactions occurring between the amphoteric active sites of TiO
2, formed through its hydroxylation by water molecules, and the aqueous protons, which consequently result in protonation and deprotonation reactions, as shown by Equations (3) and (4) [
76,
77,
78]
where TiOH, TiOH
2+, and TiO
– represent neutral, protonated, and ionized hydroxyl groups on the TiO
2 surface.
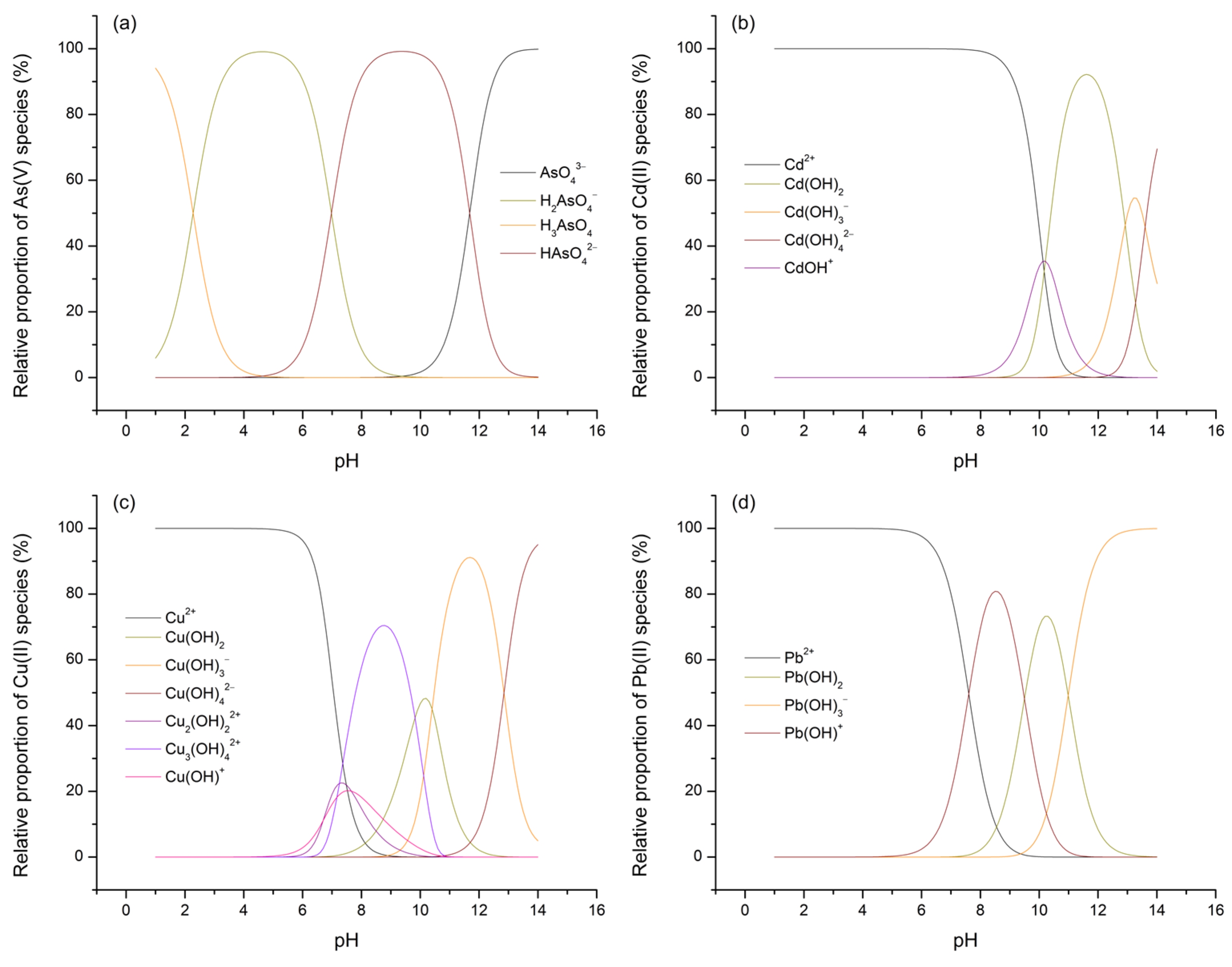
Considering that the potential for and extent of adsorption processes for As(V) and M(II) on TiO
2 are influenced not only by the electric state of the surface of the given metal oxide but also by the forms in which the investigated adsorbates are present within the aqueous phase, the concentrations of their species across the entire pH range were calculated using Visual MINTEQ software (version 3.1). The results obtained this way, expressed in terms of percentage distributions, are presented in
Figure 11 (species with a relative abundance of less than 5% are not shown or considered for further discussion).
By referring to the speciation diagrams, it is observed that, at pH 3, As(V) predominantly exists in the form of the H2AsO4– oxyanion, followed by a significantly smaller fraction of the neutral H3AsO4 acid, while all three M(II) ions predominantly exist as M2+ ([(M(H2O)6]2+). Upon reaching pH 7, As(V) is approximately evenly distributed between the H2AsO4– and HAsO42– oxyanions, while the range and distribution of the M(II) species now vary depending on the specific metal: Cd(II)—Cd2+; Cu(II)—Cu2+ > Cu2(OH)22+ > CuOH+ > Cu3(OH)42+; Pb(II)—Pb2+ > Pb(OH)+.
Having thus established a comprehensive understanding of the charge distribution in contact pairs, it is reasonable to expect a higher extent of As(V) adsorption at pH 3 and, conversely, a more pronounced removal of M(II) species at pH 7, particularly under the assumption that electrostatic interactions, i.e., physisorption, predominantly govern the processes of interest. This rationale stems from the fact that, at pH 3, electrostatic compatibility is achieved solely in the TiO
2/As(V) system (referring here to electrophilic TiOH
2+ surface sites and H
2AsO
4– ions), whereas in other cases, this compatibility is lost and is further accompanied by unfavorable competition between M
2+ ions and the highly mobile and abundant H
3O
+ ions for the same adsorption sites. On the other hand, at pH 7, due to the formation of certain nucleophilic centers on the TiO
2 surface (the precise nature of which is difficult to determine, as the
pKa2 of the –OH groups on TiO
2 typically ranges between 8 and 9), favorable conditions are established for the removal of various M(II) cationic species, though not for the adsorption of H
2AsO
4– and HAsO
42–, further hindered by the now significant participation of competing OH
– ions. Nevertheless, based on the values of the parameters
qm and
qd, which represent the adsorption capacities of TiO
2 for As(V) and M(II), the hypothesis holds true only for Cd(II) and Cu(II), considering that the increase in pH has little or even an unexpected effect on the removal levels of As(V) and Pb(II). Moreover, given that all four adsorbates exhibit substantial removal even under conditions generally considered unfavorable (e.g., at
C0 = 1,
REe ranges from 57.73 to 95.49%), it is justifiable to argue that the interpreted adsorption mechanisms, in addition to electrostatic interactions, also involve the contribution of other phenomena. It should be noted that, even under broadly unfavorable conditions, certain localized surface sites may retain compatibility with the species being removed, which is evident from the
pKa1 values, spanning the range of 2 to 4, that is, both below and above the
pHpzc. These additional contributions are understood to include processes such as ligand exchange, ion exchange, and hydrogen bonding. Of these, the first is most likely to occur in systems involving As(V), while the latter two are more prominent when M(II) species serve as adsorbates (some of the potentially relevant reaction pathways for these adsorption mechanisms are illustrated in Equations (5)–(9)). Therefore, it can be concluded that the removal of As(V) and M(II) by TiO
2, despite various observations and parameter values suggesting a predominance of either physisorption or chemisorption, is most appropriately characterized as a complex process involving the simultaneous action of both mechanisms (
Figure 12), with their mutual contribution being determined by the surrounding environmental conditions [
28,
70,
79,
80,
81,
82].