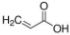
Abstract
This study analyzes the risks posed by high-temperature summer conditions to atmospheric storage tanks containing acrylic acid and proposes mitigation measures. Recent increases in heat waves and tropical nights have led to an increase in the temperatures of acrylic acid storage tanks. This temperature increase results in higher vapor pressure and promotes spontaneous polymerization, thereby increasing the risk of explosions in atmospheric storage tanks. Hazard and operability (HAZOP) analysis identified explosions due to pressure buildup as a major risk scenario. To mitigate this risk, a spray-tower system was introduced through a layer of protection analysis (LOPA), which effectively reduced the hazards associated with atmospheric storage tanks. Additionally, the removal of flame-arrester replacement operations not only achieves economic benefits, such as reduced replacement costs and labor time, but also enhances safety by eliminating worker exposure to hazardous chemicals. These findings have significant implications for improving safety at industrial sites and highlight the potential economic benefits of preventing chemical accidents.
1. Introduction
Atmospheric storage tanks are representative process facilities used in industrial sites to store hazardous materials, which serve as raw materials for product manufacturing. These storage tanks are designed considering the properties of the stored substances and are recognized as appropriate facilities for safely storing hazardous materials. The safety of atmospheric storage tanks is influenced not only by the chemical properties of the stored substances but also by external environmental changes such as heatwaves, typhoons, and earthquakes.
In recent years, rapid industrialization and urbanization have accelerated global warming, leading to an increase in the frequency of abnormal weather events [1]. Previous studies have shown that the occurrence of natural disasters due to abnormal weather has increased over the past few decades, prompting extensive research on large-scale disasters such as the Great East Japan earthquake, tsunamis, and floods [2,3,4]. In particular, there is a growing interest in the impact of climate change on social infrastructure and safety systems, as accidents caused by abnormal weather can lead to large-scale damage and are difficult to predict, increasing the likelihood of the collapse of industrial facilities and infrastructure [5].
In the chemical industry, the frequency of accidents related to atmospheric storage tanks has been increasing, with incidents reported not only due to natural disasters such as hurricanes, lightning, and storms, but also due to various causes such as bund wall failures and vacuum loss [6,7,8,9,10,11,12,13].
Analysis of previous studies indicates that research on the impact of abnormal weather has primarily focused on large-scale disasters such as earthquakes and floods, whereas studies on the impact of temperature variations, such as abnormal temperatures, on industrial facility safety remain insufficient [6]. While some studies have analyzed the impact of extreme temperatures, such as cold waves and heat waves, on the chemical industry, most have focused on accident analysis and countermeasures related to cold waves. In contrast, studies on the factors by which heat waves increase risk in the chemical industry remain relatively limited [7,8].
Therefore, this study aims to investigate the impact of elevated summer temperatures, particularly heatwaves, on the safety of industrial facilities. The frequency of chemical accidents involving atmospheric storage tanks tends to rise during the summer, and abnormal temperature conditions are considered a major contributing factor [5,14]. Notable examples include a temperature-induced overpressure incident at a waste acid storage tank in Gyeongju (2017) and a vapor leak involving styrene monomers in Hwaseong (2015) [15,16]. Given this context, acrylic acid was selected for detailed analysis due to its sensitivity to temperature. Acrylic acid must be strictly maintained within a controlled temperature range, as increased ambient heat can accelerate spontaneous polymerization, raise internal pressure, and potentially lead to explosion hazards [17,18,19].
In addition to the risks of pressure buildup and spontaneous polymerization, elevated temperatures can increase the vapor concentration of acrylic acid, potentially leading to the formation of flammable mixtures in the tank headspace. This additional flammability hazard should be carefully considered in the safety design and risk assessment of atmospheric storage tanks.
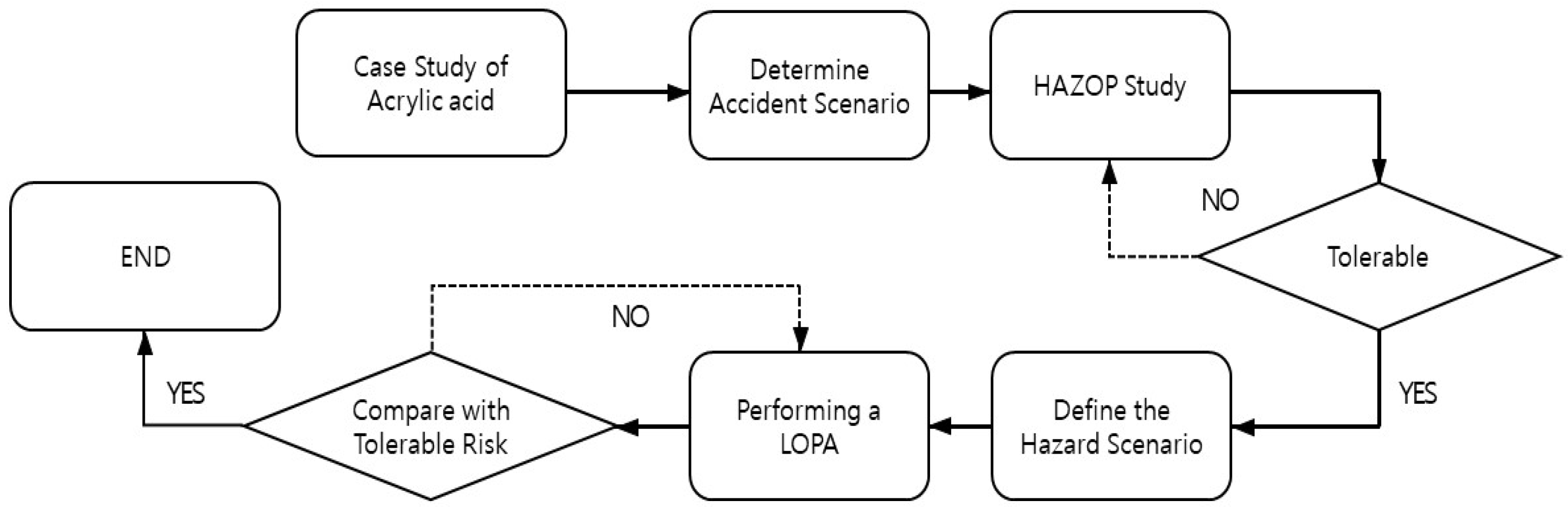
In this study, the hazard and operability (HAZOP) study methodology was applied to acrylic acid atmospheric storage tanks. The scenario with the highest risk was identified as a situation in which spontaneous polymer formation at the vent of the atmospheric storage tank rapidly increased owing to high summer temperatures, preventing the maintenance of atmospheric pressure and leading to an explosion. Subsequently, a layer of protection analysis (LOPA) was conducted on a scenario involving a tank explosion owing to high temperature. The initiating events were defined, and the protective layers were analyzed to quantitatively assess the risk. Based on this assessment, facility improvement measures were proposed to ensure the safe operation of atmospheric storage tanks. As illustrated in Figure 1, this study proceeded in the following sequence: investigation of domestic and international acrylic acid-related accident cases, risk assessment of acrylic acid storage tanks, and the establishment of mitigation measures. This research is expected to contribute to the quantitative assessment of the impact of abnormal temperatures on industrial facilities and the development of countermeasures to ensure the safety of chemical storage facilities. This will help proactively analyze risk factors in industrial sites that may increase due to heatwaves and tropical nights and aid in establishing practical safety measures.
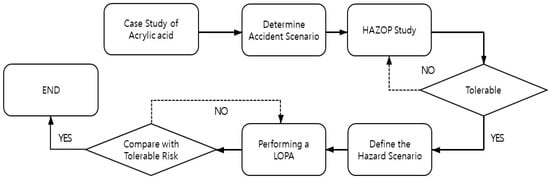
Figure 1.
Study Flow Chart.
2. Characteristics of Target Substance
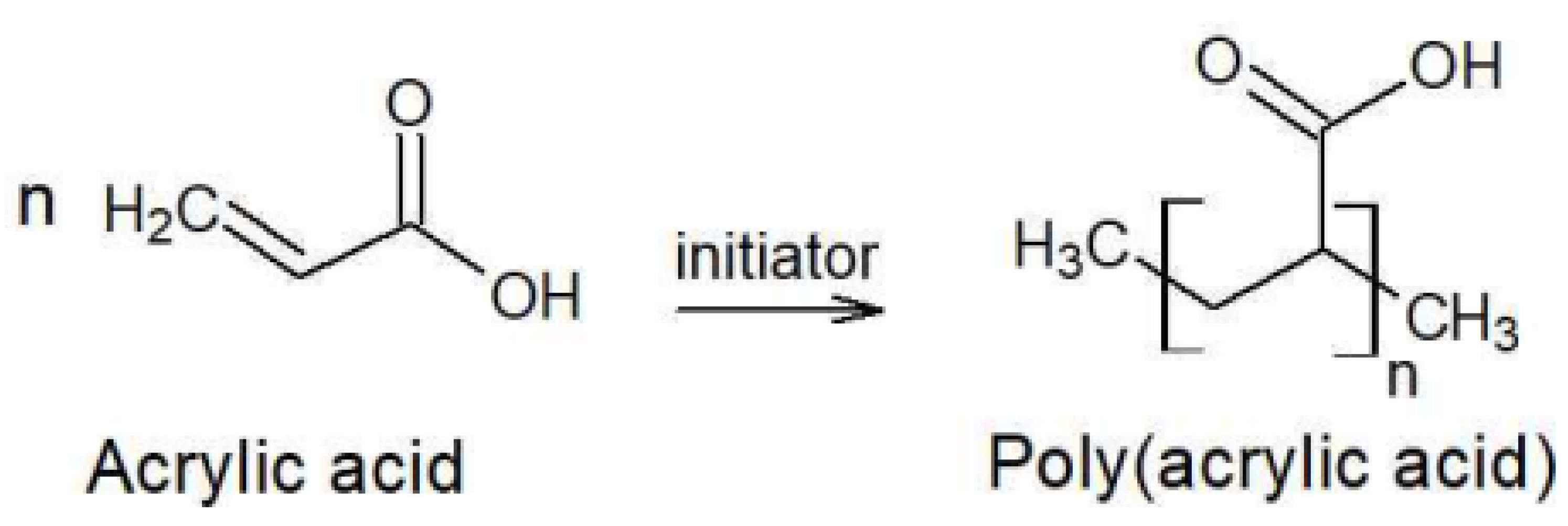
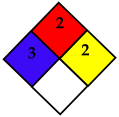
Acrylic acid undergoes a spontaneous Michael Addition Reaction (MAR) during storage, leading to the formation of dimers, which is one of the factors contributing to the temperature rise. The rate of dimer formation increases with increasing temperature and water content. At elevated temperatures, MAR accelerates, further increasing the temperature of the acrylic acid. As shown in Figure 2, acrylic acid can undergo polymerization and be converted into a high-molecular-weight polymer. If this reaction is not properly controlled, it poses the risk of runaway polymerization [17]. Acrylic acid is a flammable liquid that is classified as a regulated substance under various safety regulations. It is a process safety management (PSM) reportable substance and is designated as a toxic substance and an accident preparedness substance under the Chemical Substances Control Act. Additionally, under the Hazardous Materials Safety Control Act, acrylic acid falls under Class 4 (Flammable Liquids) and Type 2 petroleum (Water-Soluble Liquids) with specified quantity regulations. If handled in quantities exceeding the designated amount, legal requirements regarding storage and handling facilities must be met, including obtaining necessary permits. The hazard classification of acrylic acid according to the National Fire Protection Association (NFPA 704) is presented in Table 1 [17]. The handling precautions suggested by domestic acrylic acid manufacturers state that the storage temperature in tanks should be maintained below 25 °C, while also preventing freezing by keeping it above 15 °C. If crystallization occurs, it should be thawed by stirring in warm water below 40 °C, avoiding localized heating [7]. The physicochemical properties of acrylic acid are summarized in Table 2 [17,19]. Atmospheric storage tanks for flammable liquids must be equipped with flame arresters, vent valves, and flame traps to ensure tank safety [20]. In the gaseous state, acrylic acid does not contain polymerization inhibitors, and as the ambient temperature increases, polymer deposits easily form in flame arresters, vent valves, and flame traps. This can disable the pressure maintenance function of atmospheric storage tanks, potentially leading to tank rupture, pipeline rupture, and major industrial accidents [18,19,20,21].
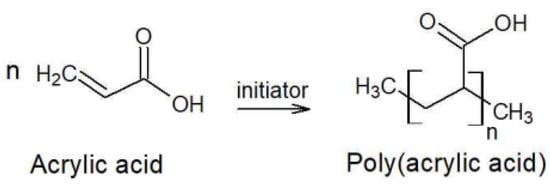
Figure 2.
Acrylic Acid Polymerization Reaction.

Table 1.
NFPA for acrylic acid [17].

Table 2.
Acrylic acid physical properties.
3. Analysis of Temperature Effects on Acrylic Acid in Atmospheric Storage Tanks Under Abnormal Weather Conditions
3.1. Current Status of Abnormal Weather Occurrences
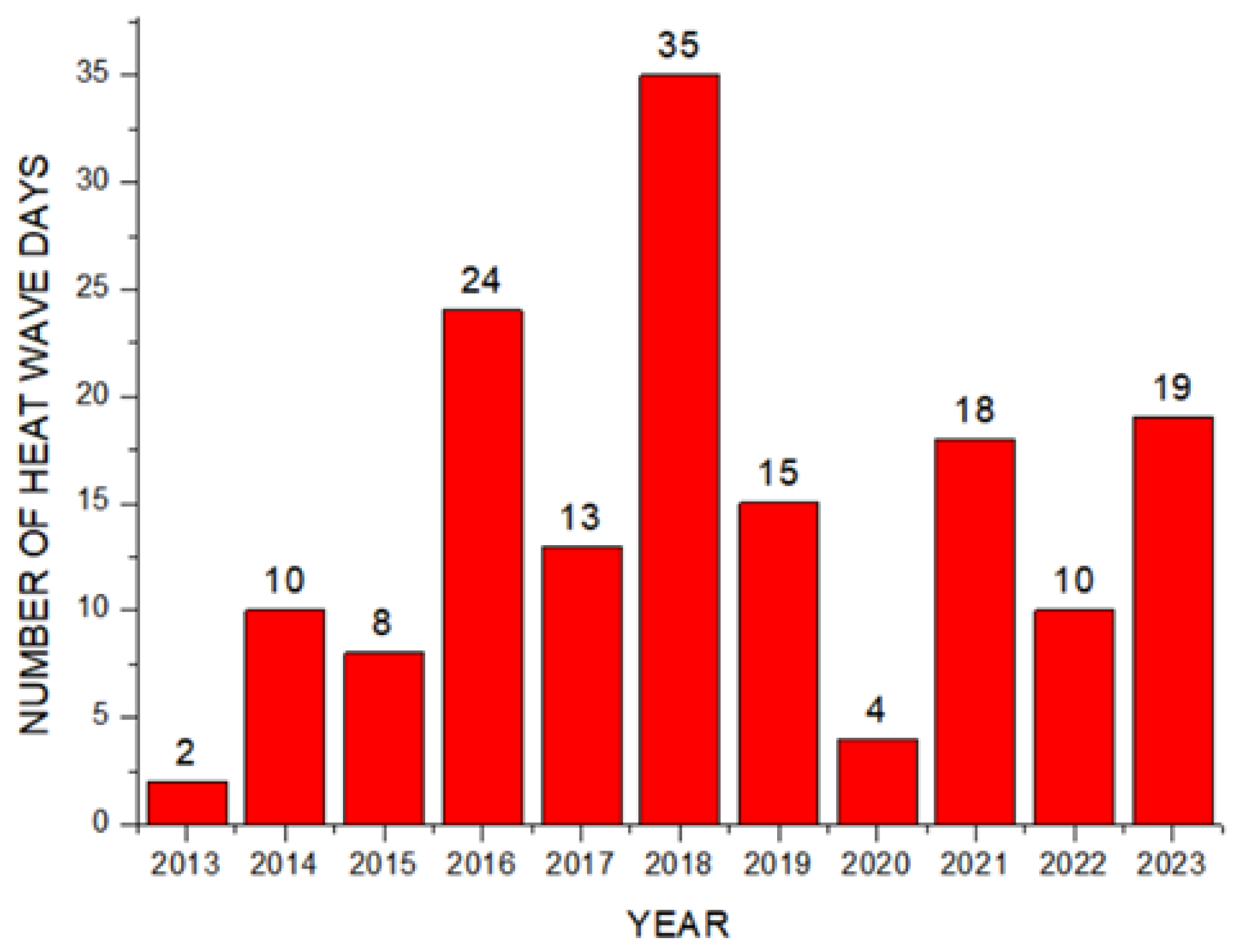
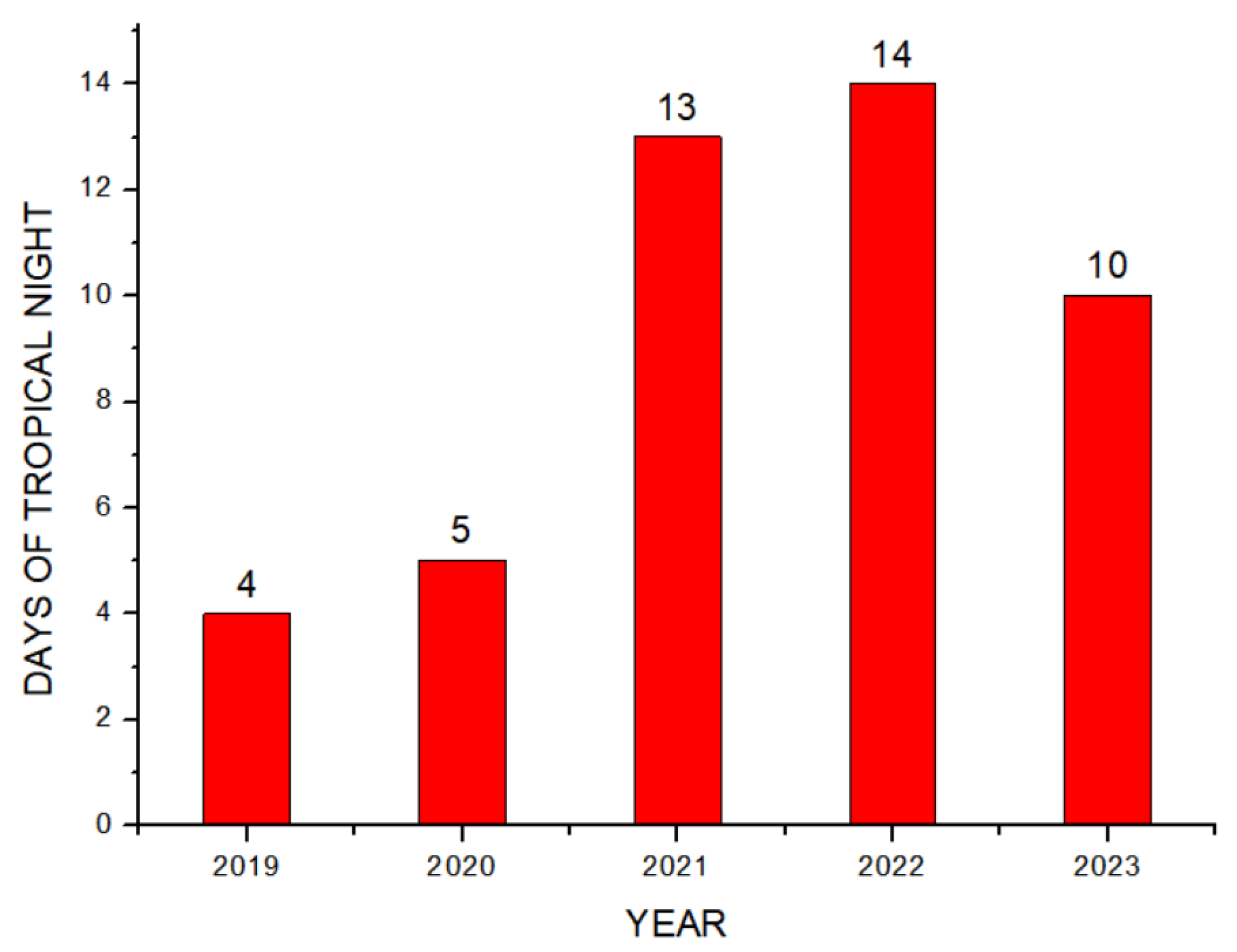
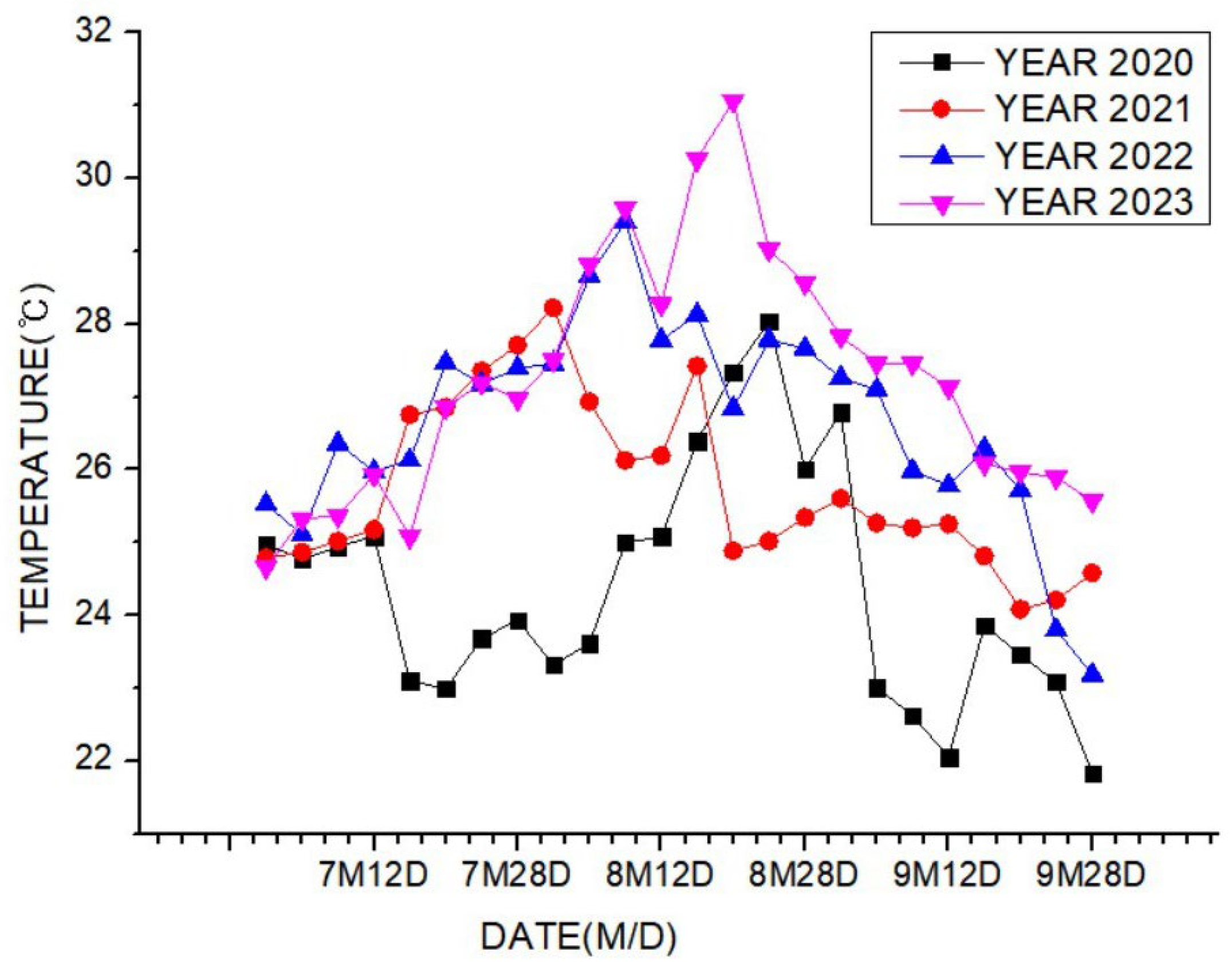
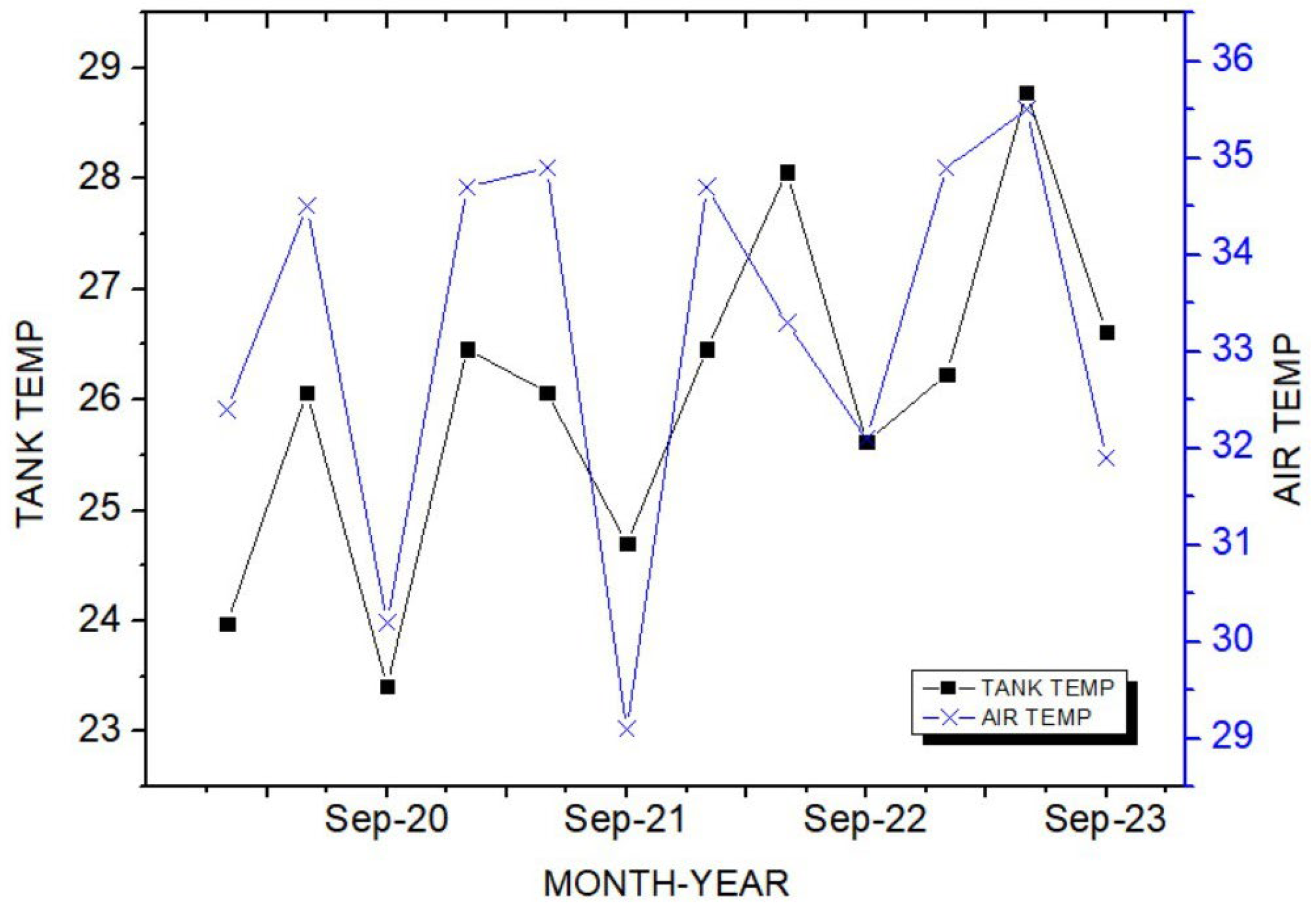
Theorem Climate change is progressing rapidly worldwide. In particular, the number of heatwave days during the summer has been continuously increasing, making abnormal temperature phenomena more evident. The results of the investigation of the occurrence of heat waves in Seoul, Republic of Korea, over the past ten years are shown in Figure 3. The number of heatwave days fluctuated between 2 and 35 days per year without displaying a specific pattern. However, a comparison of the year with the fewest occurrences, 2013 (2 days), to the year with the most occurrences, 2018 (35 days), showed an increase of approximately 17 times. Tropical nights are another factor contributing to abnormally high temperatures. An analysis of the number of tropical night occurrences in Seoul over the past five years showed an increasing trend, as shown in Figure 4. The duration of tropical nights increased from four days in 2019 to 14 days in 2022, representing approximately a 3.5-fold increase. Although heatwaves and tropical nights are classified as abnormal summer climate phenomena, they do not follow a specific pattern; a clear increasing trend was observed compared to 10 and 5 years ago. Research on securing the safety of chemical plants against abnormal summer climates has been difficult to find through prior studies. Although measures such as cooling systems and water spray facilities are currently being implemented to prepare for high-temperature environments, the implementation of additional measures to respond to extreme summer temperatures is urgently needed, especially considering that the increase in summer atmospheric temperatures leads to a higher risk of fire and explosion accidents in atmospheric storage tanks containing hazardous materials. This indicates that additional mitigation measures must be established to ensure safety [5]. To analyze the impact of high summer temperatures on the storage temperature of atmospheric storage tanks, a study was conducted on an acrylic acid storage tank located in Chung-ju over four years from 2020 to 2023. From October to May of the following year, when temperatures dropped, there was a risk of acrylic acid freezing at night, requiring the application of a hot water insulation system to the storage tank, which made it difficult to determine the direct impact of external temperatures. During the summer months from July to September, when there are no external influences other than ambient temperature, optimal conditions for observing storage temperature changes were provided. In the graphs, M represents the month and D represents the date. As shown in Table 3, the storage temperature of the acrylic acid storage tank has increased each year, which is also clearly observed in the annual temperature graphs presented in Figure 5. The minimum and maximum storage temperatures recorded for each year were 21.7 °C and 28.1 °C in 2020, 23.9 °C and 28.2 °C in 2021, 23.2 °C and 29.0 °C in 2022, and 24.6 °C and 31.1 °C in 2023, respectively. This demonstrates that the maximum storage temperature of the acrylic acid storage tank in 2023 increased by approximately 1.1 times compared to that of 2020, suggesting a strong correlation between the rise in summer atmospheric temperature and the increase in storage temperature, thus emphasizing the necessity of implementing additional safety measures to ensure the stability of atmospheric storage tanks under high-temperature conditions. The comparison between the maximum ambient temperature in the Chung-ju region and the storage tank temperature during the high-temperature summer months from July to September over the four-year period is presented in Figure 6. It can be observed that the fluctuation patterns of the storage tank temperature and ambient temperature exhibit a similar trend, as the maximum ambient temperature increased from 34.5 °C in August 2020 to 35.5 °C in August 2023, reflecting a 1 °C rise. During the same period, the storage tank temperature increased from 26.1 °C to 28.8 °C, showing a more significant rise of 2.7 °C. This indicates that the optimal storage temperature for acrylic acid, which is 25 °C, was exceeded by both the ambient temperature and storage temperature throughout the four-year investigation, thereby suggesting that the increase in ambient temperature caused by extreme weather conditions, such as summer heatwaves, leads to a rise in the storage temperature of acrylic acid, ultimately acting as a factor that increases the risk of accidents involving storage tanks each year.
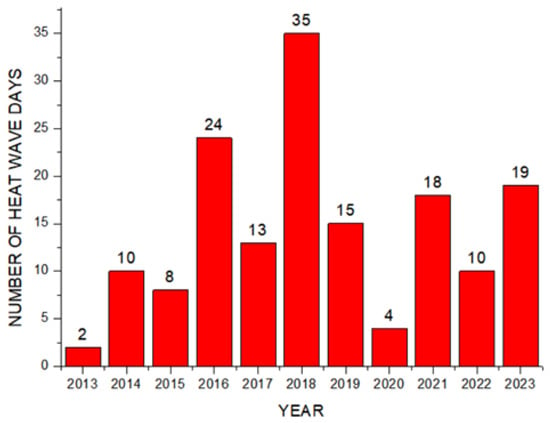
Figure 3.
Number of heatwave days by year.
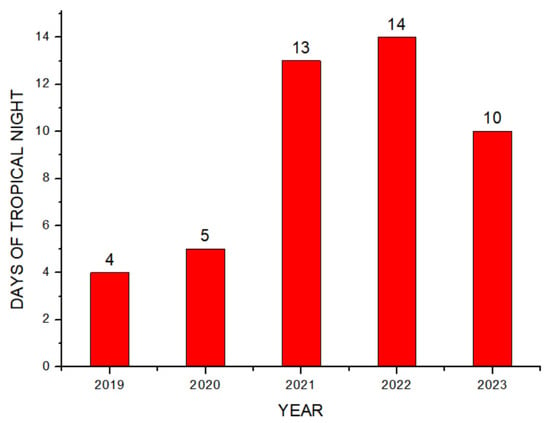
Figure 4.
Number of tropical nights.

Table 3.
Yearly temperatures of atmospheric tanks in Chung-Ju, Korea.
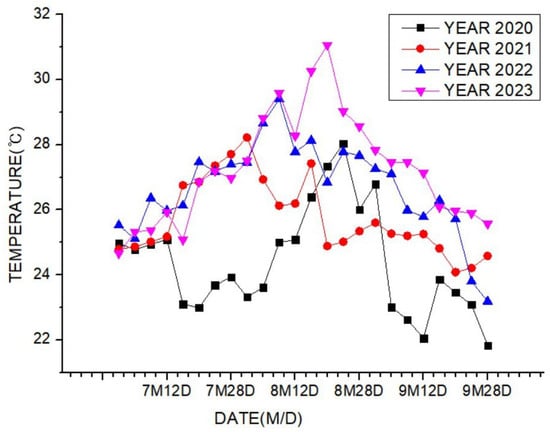
Figure 5.
Temperature trend for acrylic acid storage tanks during 4 years.
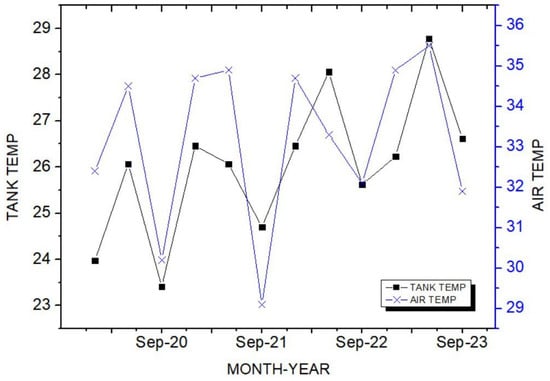
Figure 6.
Comparison of air and tank temperature trends.
3.2. Case Studies of Accidents in Atmospheric Storage Tanks Due to Abnormal Weather Conditions
Acrylic acid is a highly reactive substance with significant potential for chemical accidents owing to polymerization reactions. In particular, the depletion of polymerization inhibitors or the absence of oxygen can accelerate free-radical polymerization, leading to dimerization and rapid polymerization in both the liquid and gaseous states [6]. This free radical reaction involves the continuous bonding of acrylic acid molecules to form polyacrylic acid. Even in the presence of polymerization inhibitors, once free-radical polymerization begins, the reaction rate can increase rapidly and become a major cause of runaway reactions. Owing to these characteristics, failure to properly control the pressure and temperature in atmospheric storage tanks can result in severe explosion accidents. A survey of domestic and international accident cases related to acrylic acid was conducted. The domestic cases are summarized in Table 4, and the international cases are presented in Table 5. In domestic cases, incidents include the release of acrylic acid vapor due to a reactor explosion during synthetic resin manufacturing, leakage caused by an exothermic reaction during the mixing of acrylic acid and resin, smoke generation due to an abnormal reaction during the mixing of epoxy and acrylic acid, overheating caused by an exothermic reaction during mixing, injuries resulting from an abnormal reaction during the disposal of acrylic acid waste, and an explosion leading to leakage when the top cover of the methacrylic acid storage tank fails because of high temperatures [22]. An analysis of domestic accident cases revealed that out of a total of six incidents, five occurred because of the failure to properly control the exothermic reaction during the synthesis of acrylic acid and the resin. Most of these accidents involved vapor leakage caused by excessive pressure generated during the reaction, indicating that the pressure buildup leading to vapor leakage was a common factor. The primary cause of these accidents was identified as a lack of understanding of the characteristics of acrylic acid, which resulted in unsuccessful temperature control during the synthesis reactions. International accidents involving acrylic acid include a wide range of incidents, such as runaway reactions due to reactor overheating, explosions caused by repeated partial melting and solidification of stored drums, accidents triggered by power failures, runaway reactions due to abnormal feedstock input, runaway reactions caused by overheating, explosions resulting from cooling system failure, runaway reactions due to insufficient cooling capacity, and runaway polymerization in storage tanks. A comprehensive analysis of these cases indicated that most accidents occurred because of failures in temperature management or control during the storage and synthesis of acrylic acid. These findings suggest that precise temperature control is a critical factor for ensuring safe handling and synthesis of acrylic acid. An investigation of domestic and international accidents related to acrylic acid confirmed that risk mitigation measures for acrylic acid are urgently needed. Additionally, cases of atmospheric storage tank accidents caused by abnormal temperatures were examined. In 2017, a leakage accident occurred in a nitrocellulose (NC) waste acid storage tank in Gyeongju because of the rupture of an overpressure relief vent. The cause of the accident was determined to be an increase in vapor pressure due to rising external temperatures, an increase in vapor pressure caused by the reaction heat of the NC, and a pressure rise due to the reaction byproducts of the NC [15]. In August 2015, a vapor leak of styrene monomers occurred through the vent piping of an underground storage tank in Hwaseong. This accident took place during a period of sustained high temperatures, with an average summer temperature ranging from 26 to 31 degrees Celsius, and was presumed to have resulted from the initiation of a polymerization reaction due to the temperature rise of the storage tank [16]. To manage the temperature of atmospheric storage tanks, improvement measures, such as the installation of internal coils, internal or external heat exchangers, and mixers or agitators, are necessary to maintain a stable temperature for the stored substances [20]. These accident cases highlight the impact of rising external temperatures on the internal pressure and chemical reactions in storage tanks, confirming the necessity of establishing measures to ensure safety. In this study, improvement measures are proposed to prevent chemical accidents in acrylic acid storage tanks caused by rising ambient temperature. As the external temperature increases, the vapor pressure inside the storage tank increases, leading to a higher proportion of acrylic acid in the gaseous phase. Because polymerization inhibitors are not present in gaseous acrylic acid, the risk of polymerization reactions increases, making it essential to establish safety measures to mitigate these risks [5].

Table 4.
Domestic Accident cases involving Acrylic acid [17].

Table 5.
Overseas Accident cases involving Acrylic acid [17].
4. Risk Assessment
In line with ISO 31000, which emphasizes a systematic and structured approach to risk management, the HAZOP method was adopted as a qualitative technique for identifying potential process deviations and hazards [23,24]. The HAZOP study results served as the primary basis for conducting a risk assessment of acrylic acid atmospheric storage tanks [24]. In Layer of Protection Analysis (LOPA), a single-impact accident identified through the HAZOP study is used as an example to illustrate the LOPA procedure. As described in prior studies, LOPA should begin by reviewing the results of the HAZOP study and extracting relevant scenarios [24,25].
4.1. Process Details of Acrylic Acid Storage Tank
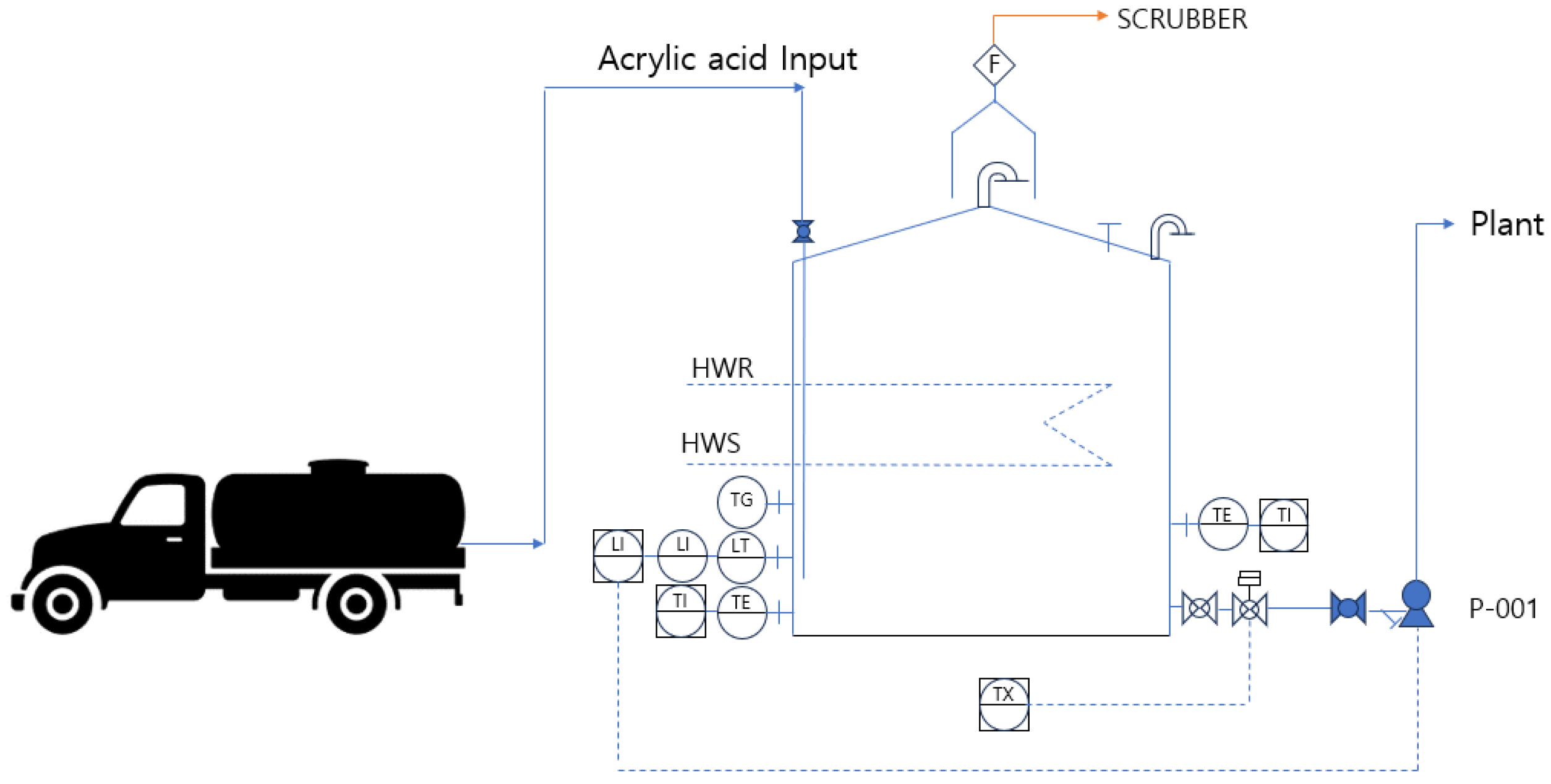
The acrylic acid transported by tank lorries is stored in atmospheric storage tanks through an acrylic acid input system and transferred to the plant using P-001. The storage tank maintains atmospheric pressure and a temperature range of 10 to 30 °C while storing acrylic acid before it is transported to the plant. During winter, the tank is heated using hot water supplied through hot water supply (HWS) and hot water return (HWR) systems, whereas heating is not applied during summer. The storage tank is equipped with instruments to measure the temperature and liquid level. An emergency shut-off valve is installed, which is interlocked with a gas-detection system to respond to potential leaks. The top of the atmospheric storage tank is fitted with a vent line and a flame arrester, and a flame trap is installed as a safety device. The emissions from the tank pass through a scrubber before the final gas is incinerated in a regenerative thermal oxidizer (RTO) for treatment. The process flow diagram of the acrylic acid atmospheric storage tank is shown in Figure 7, and the corresponding data are summarized in Table 6.
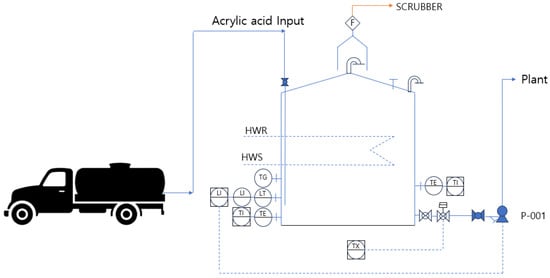
Figure 7.
PFD for storage tanks.

Table 6.
Node list of the HAZOP study.
4.2. Hazard and Operability (HAZOP) Study
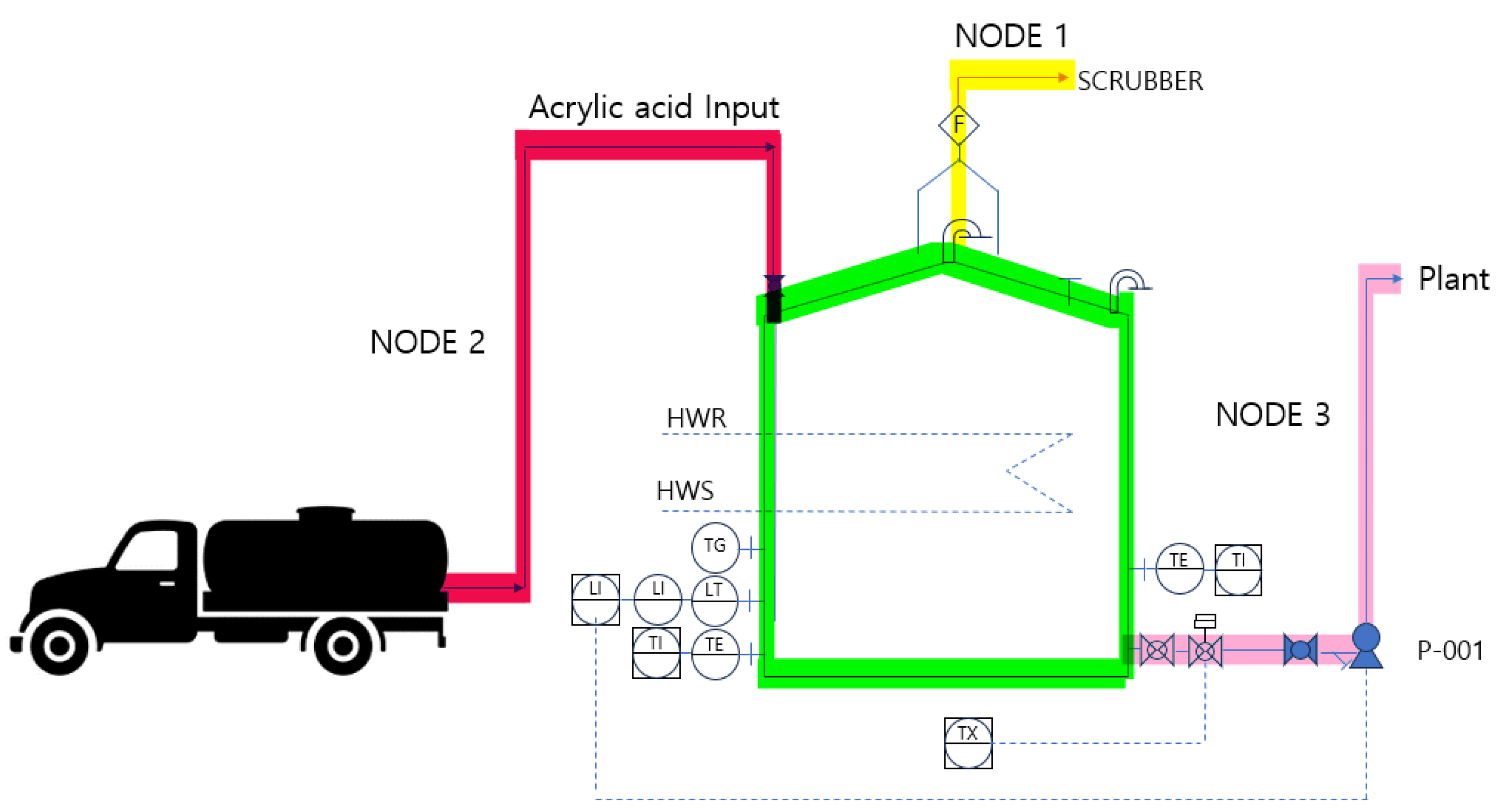
The HAZOP study was conducted by dividing the system into three NODES for risk assessment using equipment-to-equipment connections as criteria. NODE 1 covered the section from the atmospheric storage tank to the scrubber, NODE 2 examined the process from the tank lorry to the outdoor storage tank input, and NODE 3 reviewed the pipeline transporting acrylic acid from the outdoor storage tank to the plant. The results of this assessment are presented in Table 7 and Figure 8.

Table 7.
Node list of the HAZOP study.
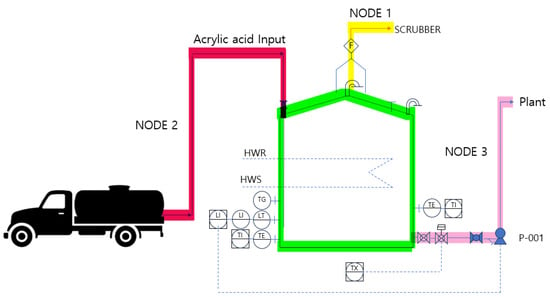
Figure 8.
NODE diagram.
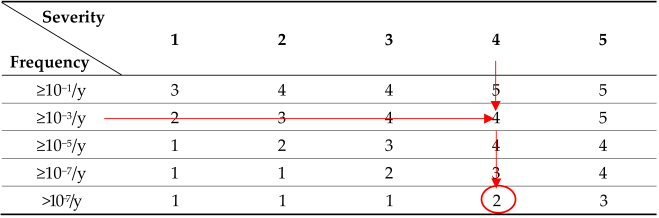
The risk matrix for the HAZOP study was evaluated by categorizing the severity and frequency into four and five levels, respectively, as shown in Table 8. The overall risk was classified into five levels based on the product of the severity and frequency scores. A score of 1–3 corresponds to Level 1, which represents negligible risk and can be disregarded. A score of 4–6 falls under Level 2, indicating minor risk, where work can proceed in its current state while accepting risk. A score of 8–11 is classified as Level 3, representing significant risk, whereas a score of 12–15 is categorized as Level 4, indicating major risk. For both levels 3 and 4, work may proceed conditionally; however, risk mitigation plans must be established to improve safety conditions. Finally, a score of 16–20 is designated as Level 5, which represents an unacceptable risk, meaning that the task cannot be permitted under any circumstances, and immediate corrective actions must be taken. The results of the HAZOP study are summarized in Table 9. The deviation matrix was evaluated primarily based on pressure, weight, sequence, and temperature, with pressure identified as the most critical risk factor. The most hazardous scenario was determined to be the rapid increase in vapor pressure inside the storage tank owing to high internal temperatures, which accelerated the formation of polymer deposits in the scrubber and vent piping. The consequence of this accident was the blockage of the scrubber and vent piping owing to polymer deposits, leading to a failure to maintain atmospheric pressure and resulting in a rupture caused by pressure buildup. The current safety measure identified in the risk assessment was the emergency vent, and the risk was assessed with a frequency rating of 2 and a severity rating of 4, giving a total score of 8, which classifies it as a Level 3 risk. The recommended improvement measure is to identify and implement a method to relieve the pressure at high temperatures to prevent the risk from escalating.

Table 8.
HAZOP Study.

Table 9.
Severity for flash point and discharge rate.
4.3. Improvement in Risk Assessment
The risk assessment results indicated that the acrylic acid atmospheric storage tank experienced an increase in internal temperature and vapor pressure owing to high ambient temperatures in the summer. As a result, excessive amounts of acrylic acid in the gaseous state accumulate inside the tank and are discharged through vent piping or scrubber piping in proportion to the increased vapor pressure. Because gaseous acrylic acid does not contain polymerization inhibitors, polymerization can easily occur, particularly in vent piping and scrubber piping equipped with flame arresters, where the polymerization of gaseous acrylic acid is accelerated. If the vent piping is blocked by polymer deposits, the resulting pressure buildup in the storage tank may lead to rupture, making it essential to manage the storage tank temperature and promptly eliminate any factors that could induce polymerization.
Atmospheric storage tanks are equipped with cooling and insulation systems. However, if the internal temperature of the tank rises sharply owing to extreme heat, the cooling systems may fail to function effectively, potentially leading to catastrophic rupture. To prevent such accidents, measures must be devised and implemented to relieve the pressure generated at high temperatures.
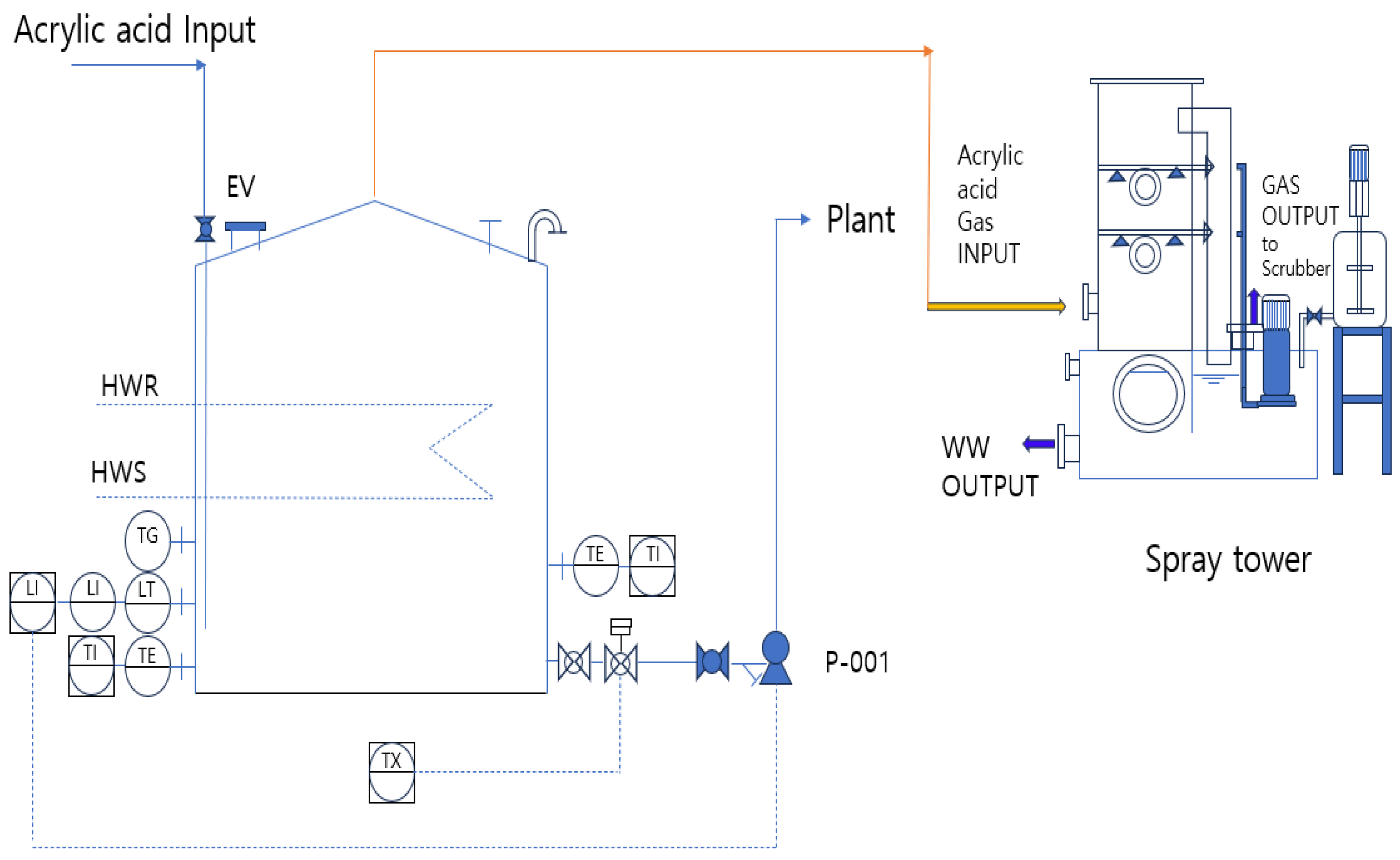
To reduce this risk, it is necessary to facilitate a smooth flow of gas inside the storage tank to minimize the residence time of the gaseous acrylic acid and reduce the likelihood of polymerization. The gaseous acrylic acid passes through the spray tower and scrubs the shortest possible distance. Figure 9 illustrates an improved design in which the flame arrester and flame trap, which contribute to polymerization in the upper section of the atmospheric storage tank, were removed, and the vent was directly connected to the spray tower to enhance circulation in the gaseous phase.
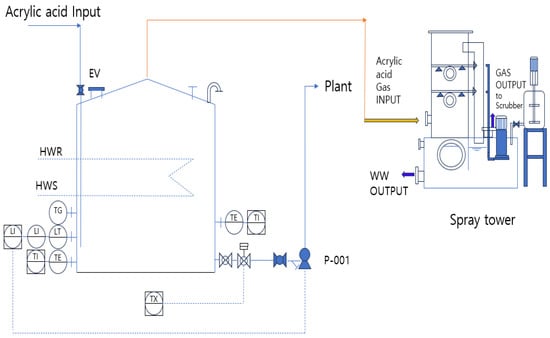
Figure 9.
PFD for an improved storage tank featuring a Spray Tower.
In the spray tower, a neutralizing agent was used to spray and neutralize acrylic acid in the gaseous state while maintaining atmospheric pressure at the gas outlet. As acrylic acid continuously accumulates in spray towers, wastewater treatment must be performed at regular intervals.
4.4. Layer of Protection Analysis (LOPA)
A pressure-rise case identified in the HAZOP study was selected as the scenario and reassessed through LOPA to evaluate the impact of abnormal temperatures. The scenario considered is that a rise in ambient temperature leads to an increase in the temperature of the acrylic acid atmospheric storage tank, which in turn causes an increase in the internal vapor pressure. This results in the accumulation of emissions in localized exhaust systems, such as scrubbers, where polymerization occurs in the gaseous phase, leading to an overpressure event and rupture of the atmospheric storage tank.
To evaluate the risk of this scenario, the LOPA method was applied based on the simplified Process Risk Assessment framework proposed by the Center for Chemical Process Safety (CCPS) [26]. The severity assessment followed the CCPS methodology, utilizing flashpoints and release quantities rather than considering worker injuries. The frequency assessment measures the likelihood of an incident using the following equation, which accounts for initiating events and failure rates [26]:
- F: Accident frequency ()
- f: initial accident rate ()
- PFDi = Probability of Failure on Demand ()
The severity was structured as a matrix based on the flash point and leakage rate of the flammable liquid, as shown in Table 8. The flash point was categorized into five levels, ranging from 21 °C to 250 °C, while the leakage rate was also divided into five levels, covering a range from 1 to 1000 tons. The leakage rate was determined based on the transfer pump leakage rate, which is 1.19 m3/min. Over one hour of leakage, the total volume was calculated to be 71.5 m3, which falls under the category of less than 100 tons. Acrylic acid has a flash point of 49 °C, and since the leaked amount is below 100 tons, the severity level according to the matrix was determined to be 4.
The frequency of the initiating event is determined using the failure rate applied by the CCPS [26]. Since the initiating event involves the failure of an atmospheric storage tank, the failure frequency was assessed as 10−3 per year (10−3/y) as summarized in Table 10.

Table 10.
Frequency Value, Assigned to Initiating Events [26].
In the LOPA, independent protection layers (IPLs) are selected to reduce the frequency of an initiating event, leading to an accident through independent protection devices [26,27]. An IPL is a device, system, or measure that is used to prevent accidents. Although all IPLs function as safeguards, not all safeguards qualify as IPLs [7]. Examples of IPLs include process design, basic process control systems (BPCS), alarm systems, interlocks, physical protection devices, post-leak protection systems, onsite emergency responses, and external emergency responses. The frequency reduction values of both active and passive IPLs used in this analysis are shown in Table 11.

Table 11.
Frequency Value of Active IPLs and Passive IPLs [26].
A manual IPL was selected as the open-venting system. The open-venting system functions as an emergency vent, with a frequency of 10−2/y. Consequently, the total accident frequency was reduced to 10−5/y. Even if the pipeline connected to the scrubber is blocked, the emergency vent functions as a separate safety device, making it a suitable manual IPL. As an additional manual IPL measure, the upper vent piping of the acrylic acid storage tank was modified into a direct piping system to immediately remove the vaporized acrylic acid.
The key difference between Figure 7 and Figure 9 is the improvement in the upper section of the storage tank by replacing the flame arrester and trap with a spray tower. The gaseous acrylic acid removed from the upper vent was neutralized in a spray tower before being transferred to the final scrubber.
A spray tower is a system that neutralizes acrylic acid during a spraying process because of its high solubility in water. The LOPA was considered an inherently safe design, and its probability of failure on demand (PFD) was determined to be 10−2/y. Because the spray tower and its associated piping are independent and separate from the rupture scenario of the acrylic acid storage tank, they are suitable as IPLs. An additional active IPL involves installing a pressure transmitter (PT) in the spray-tower piping of the storage tank, as shown in Figure 9. Regular inspections are conducted if the pressure reaches a set threshold. In the LOPA analysis, this measure can be classified as a basic process control system (BPCS), and its PFD is determined to be 10−1/y. However, for a BPCS to be recognized as an IPL, it must be capable of operating independently, even in the event of system failure. If the system is not configured separately from an existing BPCS, it is unsuitable as an IPL.
Risk assessments before and after applying the initiating event, active IPL, and passive IPL were calculated, as shown in Table 12.

Table 12.
Risk Matrix for LOPA.
The severity is determined to be 4 based on the leakage rate and flash point, and since the initiating event has a frequency of 10−3/y, the risk level is determined to be 4 according to the risk matrix. When only the manual IPL, the emergency vent, is applied, the frequency is reduced to 10−5/y, maintaining a risk level of 4. When an additional manual IPL, the spray tower, is applied, the frequency is further reduced to 10−7/y, lowering the risk level to 3. If an additional active IPL, such as installing a PT and implementing continuous monitoring as an independent BPCS, is added, the frequency decreases to 10−8/y, bringing the risk level down to 2. Through the LOPA analysis, it is confirmed that by applying active and passive IPLs to the initiating event (10−3/y), the accident frequency of the acrylic acid storage tank can be reduced to 10−8/y or lower, thereby classifying the risk level as 2, which is considered a minor risk. In accordance with ISO 45001, which emphasizes a systematic approach to occupational health and safety, the risk assessment process in this study was aligned with the PDCA (Plan-Do-Check-Act) cycle. The HAZOP and LOPA analyses correspond to the “Do” phase, focusing on hazard identification and risk evaluation, while the proposal and review of improvement measures align with the “Check” phase for monitoring and continuous improvement.
4.5. Dispersion Distance Analysis
Through LOPA, it was confirmed that the accident frequency of the initiating event for the acrylic acid atmospheric storage tank decreased. Additionally, the impact range before and after facility improvements was analyzed. The dispersion distance was quantitatively assessed using Consequence Analysis (CA) as part of a quantitative risk assessment. This analysis was conducted using E-CA, an accident impact analysis support program [20]. To describe the atmospheric dispersion behavior, the Gaussian Plume Model was referenced, which estimates the concentration of toxic substances in the air using the following equation:
- C (x, y, z): Concentration of the substance at point (x, y, z)
- Q: Mass flow rate (kg/s)
- u: Wind speed (m/s)
- x: downwind distance (m)
- y: crosswind distance (m)
- z: vertical distance (m)
The temperature of the acrylic acid storage tank was divided into four stages, increasing in 10 °C increments from 10 °C to 40 °C, and the results were compared before and after the facility improvements. The meteorological conditions for the simulated scenario were based on 12 years of maximum temperature, average temperature, average humidity, and prevailing wind data for Chungju City, obtained from the Korea Meteorological Administration [22,28,29]. Furthermore, alternative conditions were applied by referring to technical guidelines on selecting worst-case and alternative scenarios [30]. The dispersion of toxic substances in the atmosphere can lead to exposure for workers and nearby residents [31]. The concentration that defines the human health impact based on the concentration and exposure time of toxic substances is ERPG-2, which for acrylic acid is 50 ppm [32]. ERPG-2 represents the threshold for serious but reversible health effects and is widely used in emergency risk assessments. Therefore, it was used as the reference value in this study.
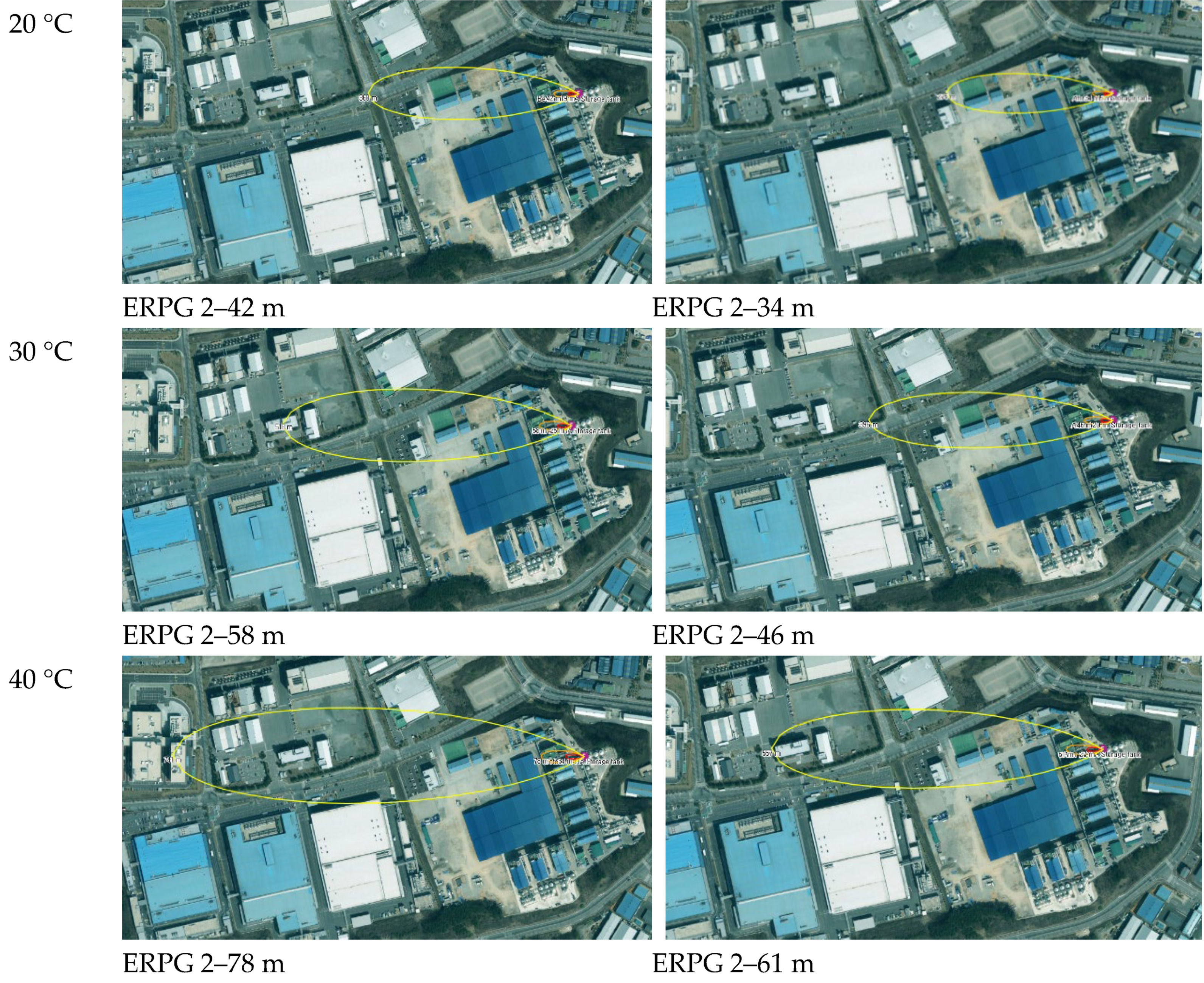
The results before and after facility improvements are shown in Table 13. As the temperature of the atmospheric storage tank increased from 10 °C to 40 °C, the dispersion distance corresponding to the ERPG-2 toxic concentration increased from 30 m to 78 m. After facility improvements, this range was reduced to 24 m to 61 m. This result is consistent with the LOPA risk assessment, reaffirming that facility improvements effectively reduced the dispersion distance of toxic substances.

Table 13.
Distance of Toxic Dispersion of e-CA.
Figure 10 shows the results of the dispersion distance analysis using CA before and after facility improvements. It can be observed that the dispersion of toxic substances has slightly decreased. In the event of an acrylic acid leakage accident, the majority of the impact is expected to occur within the facility premises.


Figure 10.
Distance of toxic dispersion of CA by temperature.
4.6. Discussion
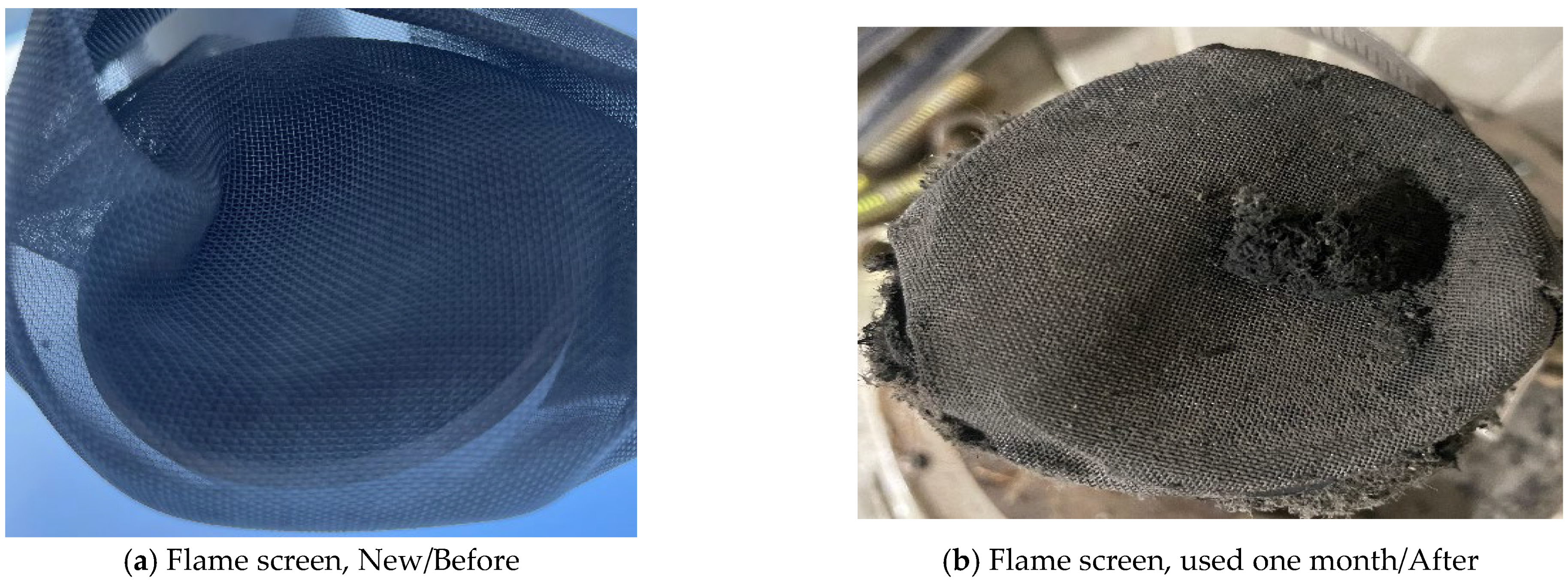
When flame arresters and flame traps are installed on the top of atmospheric storage tanks, connecting them to a Spray Tower can help reduce risk. Due to the characteristics of acrylic acid, the tank must be maintained at atmospheric pressure. Manufacturers recommend installing a pressure gauge to monitor whether overpressure is forming inside the tank due to polymerization reactions or other factors. Additionally, the installation location may become blocked due to the formation of polymer deposits caused by the condensation of acrylic acid vapor, so injecting instrument air to prevent nozzle clogging is necessary. In industrial settings, acrylic acid vapor can condense on the flame arresters and flame traps, leading to polymer formation and clogging of the mesh screen. To prevent this risk, regular inspections and replacement of flame arresters at least once a month are carried out. Figure 11 compares the condition of the flame arrester before and after replacement. Figure 11 shows that polymer deposits accumulate in the flame arrestor after one month of use, significantly reducing its operational efficiency. Clogging may lead to a dangerous pressure build-up, which supports the recommendation for removal or frequent maintenance. If maintenance is inadequate and replacement is not conducted in a timely manner, blockages similar to those seen in the post-replacement image may occur.
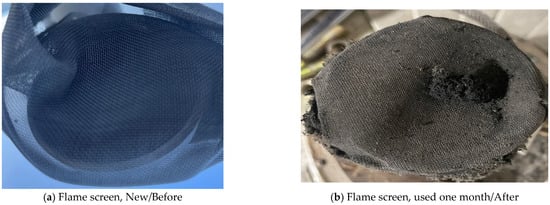
Figure 11.
Comparison of before/after use images of a flame screen.
When installing and operating a Spray Tower, it is essential to periodically check the maintenance of atmospheric pressure using a pressure gauge and monitor the wastewater management status of the Spray Tower. Additionally, during the initial operation, the formation of polymer deposits in the piping should be monitored monthly, and after evaluation, the inspection frequency can be gradually adjusted to a quarterly or semi-annual basis. Figure 12 demonstrates that critical issues may arise when Spray Tower maintenance is inadequate, emphasizing the importance of establishing proper management points.

Figure 12.
Comparison of Spray Towers.
The primary focus of this study is to mitigate the risks of acrylic acid storage tanks caused by high temperatures during summer. The proposed measures for effectively managing acrylic acid hazards are classified into technical measures and management measures. As a technical measure, this study directly connected the vent piping at the top of the atmospheric storage tank. Additionally, a Spray Tower was utilized to rapidly remove gaseous acrylic acid, and risk assessment confirmed its effectiveness in reducing hazards. Furthermore, installing a pressure transmitter (PT) is suggested as a method for continuously monitoring the atmospheric pressure state of the storage tank.
Three key management measures can be implemented. First, a periodic inspection method for directly connected piping should be established. Regular inspection and maintenance procedures must be established for all facilities related to atmospheric storage tanks, such as vent piping and the Spray Tower. These procedures should be strictly followed to detect and prevent unexpected failures or issues in advance. Standardizing inspection items and procedures is necessary to ensure systematic preventive maintenance and continuous performance monitoring of each facility. Second, a monitoring system should be implemented. A real-time monitoring system should be introduced to detect potential hazards in acrylic acid storage tanks, including temperature, pressure, flow rate, and leakage. The system should provide immediate alerts upon detecting abnormal conditions. If necessary, an interlock system with an automatic shutdown function should be implemented to further enhance safety. Third, worker education and training should be enhanced. Regular training and education programs should be strengthened to improve workers’ understanding of potential equipment hazards under high-temperature conditions and the operation of improved equipment. An emergency response manual should be developed, and periodic emergency drills should be conducted to ensure effective responses in case of actual incidents.
From an economic perspective, the frequency of flame arrester replacements has decreased, resulting in cost savings. However, wastewater treatment costs from the Spray Tower have increased, making the overall direct economic impact on maintenance costs relatively insignificant. Nevertheless, the systematic management approach has enhanced operational efficiency, leading to potential productivity gains. Productivity improvement has been achieved as the time required for flame arrester replacement has been reduced. Replacing the flame arrester once a month requires workers to wear protective clothing and perform the replacement, which takes approximately 0.5 h per operation. For one storage tank, this equates to an annual productivity improvement of six hours. By reducing the time required for flame arrester replacement, workers can focus on production activities, leading to economic benefits in terms of productivity.
Chemical exposure risks have been eliminated. During the flame arrester replacement process, workers are exposed to acrylic acid, a hazardous chemical. Although protective equipment is worn, the risk of exposure cannot be completely eliminated due to the continuous venting of acrylic acid vapor from the storage tank. The proposed facility improvements eliminate the need for flame arrester replacement, effectively reducing exposure occurrences from twelve times per year to zero times per year per storage tank.
The risk of chemical accidents has been reduced. The analysis of accident cases indicates that while acrylic acid-related incidents are infrequent, their impact can be severe. This study incorporated risk assessment techniques to evaluate even minor risk factors and verified that hazard reduction measures effectively lower the likelihood of accidents. If polymerization occurs in acrylic acid storage tanks, inadequate facility management can escalate into a major industrial disaster. However, by implementing the facility improvement measures proposed in this study, the frequency of polymerization events can be minimized, and real-time pressure monitoring enables overall risk reduction.
The most significant economic benefit is ensuring the long-term safety of acrylic acid storage tanks through continuous facility improvements and systematic management. This goes beyond simple cost savings, as preventing chemical accidents and securing facility safety hold substantial economic value in terms of risk mitigation and sustainable operational reliability.
5. Conclusions
Rapid industrialization has accelerated global warming, leading to frequent occurrences of extreme weather events. In Seoul, the number of tropical nights persisted for 14 consecutive days in 2022, while the duration of heatwaves peaked at 35 days in 2018. Compared to 2013, the number of heatwave days in 2023 increased more than eightfold. Analyzing the number of heatwave days over the past decade and the duration of tropical nights over the last five years shows an increasing trend in the persistence of abnormal temperature phenomena.
This study examined safety enhancement measures for acrylic acid, a substance requiring strict temperature control, under abnormal temperature conditions. The results of the HAZOP study indicated that polymer deposits from gaseous acrylic acid could easily form on the flame arrester, hindering the maintenance of atmospheric pressure in the storage tank. Based on these findings, a semi-quantitative risk assessment using the Layer of Protection Analysis (LOPA) method was conducted, revealing that the failure probability of atmospheric storage tanks was calculated as 10−3/y. As an additional risk reduction measure, a fixed independent protection layer in the form of an emergency vent was installed, reducing the failure probability to 10−5/y.
Further mitigation measures included the introduction of a Spray Tower system. The gaseous acrylic acid emitted from atmospheric storage tanks was treated with a neutralizing agent inside the Spray Tower, passed through a scrubber, and ultimately processed in a regenerative thermal oxidizer (RTO). In this process, the addition of inherent safety design elements to manual independent protection layers (IPL) reduced the probability by 10−2/y, bringing the probability of failure on demand (PFD) down to 10−7/y. Moreover, a pressure transmitter was installed in the piping connected to the Spray Tower to monitor real-time pressure increases, which, when operated as a standalone Basic Process Control System (BPCS), further reduced the probability by 10−2/y.
As a result, the overall risk level decreased from 4 to 2, indicating a reduction to a minor risk level. Additionally, an analysis of the impact range was conducted to examine whether the risk reduction observed in the LOPA analysis influenced the toxic substance dispersion range. The findings confirmed that after facility improvements, the dispersion range of toxic substances decreased slightly, thereby reducing the overall impact range.
While this study proposed risk mitigation measures through facility improvements, the inherent physical and chemical properties of acrylic acid still pose a high risk if the fraction increases or if temperature control fails. Therefore, workplaces must continuously strengthen their internal management systems to ensure stable operation of piping and facilities.
The storage temperature of atmospheric storage tanks has been gradually increasing due to extreme temperatures. As a result, it is necessary to design storage systems with internal coils or other mechanisms that enable cooling or heating during the initial design phase. However, if abnormal temperatures exceed the design limits, additional measures must be implemented to ensure safety.
Author Contributions
Conceptualization, G.J.; Software, M.N.; Validation, J.K. and B.Y.; Formal analysis, J.K.; Investigation, M.N.; Writing—original draft, G.J.; Writing—review & editing, G.J. and M.N; Supervision, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Catholic University of Louvain (UCL), EM-DAT: The Emergency Events Database, Centre for Research on Epidemiology of Disaster (CRED). Available online: https://www.emdat.be/ (accessed on 25 October 2019).
- Chakraborty, A.; Ibrahim, A.; Cruz, A.M. A study of accident investigation methodologies applied to the Natech events during the 2011 Great East Japan earthquake. J. Loss Prev. Process Ind. 2018, 51, 208–222. [Google Scholar] [CrossRef]
- Cozzani, V.; Campedel, M.; Renni, E.; Krausmann, E. Industrial accidents triggered by flood events Analysis of past accidents. J. Hazard. Mater. 2009, 175, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, V.; Antonioni, G.; Landucci, G.; Tugnoli, A.; Bonvicini, S.; Spadoni, G. Quantitative assessment of domino and NaTech scenarios in complex industrial areas. J. Loss Prev. Process Ind. 2013, 28, 10–22. [Google Scholar] [CrossRef]
- Ricci, F.; Moreno, V.C.; Cozzani, V. Analysis of NaTech Accidents Triggered by Extreme Temperatures in the Chemical and Process Industry. Chem. Eng. Trans. 2020, 82, 79–84. [Google Scholar]
- Ricci, F.; Moreno, V.C.; Cozzani, V. Natech accidents triggered by cold waves. Process Saf. Environ. Prot. 2023, 173, 107–119. [Google Scholar] [CrossRef]
- Yim, J.P.; Park, S.Y. A study on safety of Atmospheric storage tank through detailed analysis of accident case. J. Korean Soc. Saf. 2019, 34, 41–48. [Google Scholar]
- Chang, J.I.; Lin, C.C. A study of storage tank accidents. J. Loss Prev. Process Ind. 2006, 19, 51–59. [Google Scholar] [CrossRef]
- Qin, R.; Zhu, J.; Khakzad, N. Multi-hazard failure assessment of atmospheric storage tanks during hurricanes. J. Loss Prev. Process Ind. 2020, 68, 104325. [Google Scholar] [CrossRef]
- Necci, A.; Argenti, F.; Landucci, G.; Cozzani, V. Accident scenarios triggered by lightning strike on atmospheric storage tanks. Reliab. Eng. Syst. Saf. 2014, 127, 30–46. [Google Scholar] [CrossRef]
- Olivar, O.J.; Mayorga, S.Z.; Giraldo, F.M.; Sánchez-Silva, M.; Pinelli, J.P.; Salzano, E. The effects of extreme winds on atmospheric storage tanks. Reliab. Eng. Syst. Saf. 2020, 195, 106686. [Google Scholar] [CrossRef]
- Trávníček, P.; Junga, P.; Kudělka, J.; Kotek, L. Prevention of an atmospheric storage tank bund failure. J. Loss Prev. Process Ind. 2021, 70, 104438. [Google Scholar] [CrossRef]
- Griffin, M.L. Protecting atmospheric storage tanks against vacuum collapse. J. Loss Prev. Process Ind. 2000, 13, 83–89. [Google Scholar] [CrossRef]
- National Institute of Chemical Safety, Integrated Chemical Information System. Available online: https://icis.me.go.kr/pageLink.do (accessed on 3 February 2025).
- Korea Occupational Safety and Health Agency. Leak Accident Due to Rupture of Overpressure Vent of Waste Acid Storage Tank; Korea Occupational Safety and Health Agency: Incheon, Republic of Korea, 2018. [Google Scholar]
- Korea Occupational Safety and Health Agency. Thermal Risk Assessment of Styrene Monomer; Korea Occupational Safety and Health Agency: Incheon, Republic of Korea, 2015. [Google Scholar]
- KOSHA (Korea Occupational Safety and Health Agency); Industrial Safety and Health Agency. Thermal Risk Assessment of Acrylic Acid; Korea Occupational Safety and Health Agency: Incheon, Republic of Korea, 2022. [Google Scholar]
- Vince, I. Acrylic acid runaway. Loss Prev. Bull. 2020, 272, 13–16. [Google Scholar]
- LG Chem. Safe Handling and Storage of Acrylic Acid and Acrylates. Available online: https://www.lgchemon.com (accessed on 3 February 2025).
- Korea Occupational Safety and Health Agency. KOSHA Guide (D-35-2017). In Technical Guidelines for the Design of Atmospheric Pressure Storage Tanks; Korea Occupational Safety and Health Agency: Incheon, Republic of Korea, 2017. [Google Scholar]
- Seol, M.-s. A Study on Implications for the Prevention of Major Industrial. Korean J. Hazard. Mater. 2022, 10, 97–106. [Google Scholar] [CrossRef]
- Available online: https://data.kma.go.kr/climate/tropicalNight/selectTropicalNightChart.do (accessed on 3 February 2025).
- ISO 31000; Risk Management—Guidelines. International Organization for Standardization: Geneva, Switzerland, 2018.
- Available online: https://www.newspim.com/news/view/20220530000814 (accessed on 3 February 2025).
- Korea Occupational Safety and Health Agency. Technical Guidelines on the HAZOP Method for Risk and Operational Analysis of Batch Processes; Korea Occupational Safety and Health Agency: Incheon, Republic of Korea, 2017; p. 86. [Google Scholar]
- Willey, R.J. Layer of Protection Analysis. Procedia Eng. 2014, 84, 12–22. [Google Scholar] [CrossRef]
- Anato, L.; Carrero, L.; Brouillard, G.; Morar, C. Application and challenges of layers of protection analysis LOPA in mining processes: Insights into benefits and limitations. Process Saf. Prog. 2024, 43, 469–477. [Google Scholar] [CrossRef]
- Korea Meteorological Administration. Available online: https://www.weather.go.kr/w/index.do (accessed on 3 February 2025).
- Korea Meteorological Administration. Available online: https://data.kma.go.kr/cmmn/main.do (accessed on 3 February 2025).
- Center for Chemical Process Safety. Layer of Protection Analysis (Simplified Process Risk Assessment); Center for Chemical Process Safety: New York, NY, USA, 2001; pp. 31–149. [Google Scholar]
- Korea Occupational Safety and Health Agency. KOSHA Guide (P-113-2023). In Technical Guidelines for the Layers of Protection Analysis (LOPA) Technique; Korea Occupational Safety and Health Agency: Incheon, Republic of Korea, 2023. [Google Scholar]
- Korea Occupational Safety and Health Agency. KOSHA Guide (P-102-2012). In Technical Guideline for Accident Damage Prediction Technique; Korea Occupational Safety and Health Agency: Incheon, Republic of Korea, 2012. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).