1. Introduction
Nosocomial infections, or hospital-acquired infections, are a significant global health concern. These infections are often caused by antibiotic-resistant pathogens, making them challenging to treat with conventional antibacterial agents. Several nosocomial pathogens, including
S. aureus, methicillin-resistant
S. aureus (MRSA),
S. epidermidis,
B. subtilis,
P. aeruginosa, and
Escherichia coli, are notorious for their ability to form biofilms [
1,
2,
3,
4,
5,
6]. Biofilms can form on medical devices and within human and animal tissues [
7]. The development of these biofilms is associated with a range of factors, such as altered gene expression, a decrease in bacterial metabolism, and a shift in bacterial growth patterns, making these infections especially resilient to traditional antibiotic treatments [
8,
9,
10,
11,
12,
13,
14,
15,
16].
The increasing resistance of these pathogens to traditional antibiotics has spurred the need for alternative therapeutic approaches, particularly those derived from natural products. Among these, essential oils (EOs) have gained considerable attention due to their broad-spectrum antibacterial properties [
17,
18,
19,
20,
21,
22,
23]. EOs derived from various medicinal plants have been extensively studied for their antibacterial properties, including activity against key bacterial pathogens such as
S. aureus, MRSA, and
P. aeruginosa. For instance, it was reported that thyme and oregano essential oils were effective against
S. aureus [
24]. Although there have been significant advances, most studies still focus on a limited range of commonly used essential oils, often without detailed phytochemical analysis or evaluation under standardized conditions [
20,
21,
23,
25,
26,
27,
28]. In contrast, our study investigates the antibacterial potential of essential oils extracted from plant species belonging to several families—
Lauraceae,
Lamiaceae,
Myrtaceae, and
Pinaceae. Furthermore, we integrate comprehensive GC-MS profiling to correlate chemical composition with bioactivity, offering deeper mechanistic insights. By targeting the same clinically relevant pathogens and applying a standardized comparative approach, our work not only confirms but also expands the current understanding of essential oil efficacy. This approach underscores the originality of our research and its potential applications in developing alternative antibacterial agents.
Despite the extensive research on EOs, there remains a gap in understanding how their effects vary across different bacterial species, particularly in the context of biofilm formation. Biofilms are highly structured communities of microorganisms encased in extracellular matrices, which confer protection against antibacterial agents and are implicated in persistent infections. While several studies have assessed the antibacterial effects of EOs against planktonic bacteria, fewer have evaluated their ability to disrupt biofilm formation or target bacteria within biofilms, particularly in clinical settings.
EOs, which are volatile compounds extracted from plants, have been traditionally used in various medicinal and therapeutic applications [
24,
25,
26]. It is well established that EOs exhibit differential antibacterial activity against Gram-positive and Gram-negative bacteria, including nosocomial pathogens such as
S. aureus, MRSA,
P. aeruginosa, and
Salmonella spp. This variation is primarily attributed to structural and functional differences in their cell envelopes. Gram-positive bacteria have a thick but porous peptidoglycan layer that allows for relatively easy penetration of hydrophobic compounds such as terpenes, aldehydes, and phenolic constituents commonly found in EOs. These components can interact with intracellular targets or disrupt membrane integrity, leading to leakage of cellular contents and eventual cell death. In contrast, Gram-negative bacteria possess a more complex and resistant outer membrane composed of lipopolysaccharides, which acts as a permeability barrier against many hydrophobic substances. This outer layer impedes the diffusion of EO components into the periplasmic space and cytoplasm. Additionally, Gram-negative bacteria often express efflux pumps (e.g., AcrAB-TolC) and degradative enzymes that actively expel or neutralize antibacterial agents, including EO constituents. Additionally, EOs are known for their low toxicity to human cells, making them an attractive alternative or adjunct to conventional antibiotics [
21,
25,
26,
27,
28,
29,
30].
While EOs show promise in inhibiting bacterial growth, their impact on human cells and tissues must be carefully evaluated to ensure that they do not cause adverse effects when used as antibacterial agents [
31]. Balancing the antibacterial efficacy with their cytotoxicity is critical for determining their suitability for treating nosocomial infections [
32,
33].
However, the clinical application of EOs is often limited due to their volatility, poor water solubility, and instability, which reduce their bioavailability and effectiveness. Encapsulation of EOs into nano-sized carriers (such as liposomes, nanoparticles, or solid lipid nanoparticles) is one strategy that can enhance their stability, control their release, and improve their antibacterial activity [
34]. Encapsulating EOs can also minimize their cytotoxicity, ensuring a safer application in therapeutic settings [
18,
35,
36].
The novelty of the present study lies in its focus on the ability of EOs, specifically cinnamaldehyde (a major component of cinnamon bark oil), to disrupt biofilm formation and exert antibacterial activity against both Gram-positive and Gram-negative nosocomial pathogens, including MRSA and P. aeruginosa. Unlike previous studies, this work also investigates the impact of EOs on bacteria within biofilms, offering new insights into their potential as alternative treatments for chronic infections associated with biofilm-forming pathogens. In addition, we provide a comprehensive comparison between the antibacterial activities of cinnamaldehyde and its emulsion form. By addressing these issues, the present study contributes to a deeper understanding of the potential of EOs in combating bacterial biofilms and antibiotic-resistant pathogens, providing a foundation for future therapeutic applications. The evaluation of free and encapsulated EOs will provide insights into the potential of these natural compounds as viable alternatives or complementary treatments for hospital-associated infections. Furthermore, this research will explore the advantages of encapsulation techniques in overcoming the limitations of EOs, focusing on improving their therapeutic efficacy and safety profiles.
In this study, EOs, which are known for their antibacterial properties, and emulsions encapsulating these oils were tested against common nosocomial pathogens, such as S. aureus (ATCC 9144), MRSA (ATCC 43300), S. epidermidis (ATCC 14990), B. subtilis (ATCC 6051), P. aeruginosa (ATCC 10145), and S. Enteritidis (ATCC 8739). As part of this study, the cytotoxicity of all selected EO and EOEs was evaluated in vitro using Vero cells. These cells are widely employed in cytotoxicity assays as a model system to assess the potential toxic effects of substances on human cells.
The present investigation aimed to evaluate the antibacterial and cytotoxic activities of EOs and their emulsions against nosocomial infection pathogens in vitro.
4. Discussion
This study highlights the potent antibacterial, antibiofilm, and cytotoxic activities of selected EOs and their emulsified forms, with mechanistic insights that align with the existing literature. The primary antibacterial action of EOs is linked to their lipophilic components—notably phenols and terpenes—which disrupt bacterial membranes by integrating into the lipid bilayer. This leads to increased permeability, leakage of cytoplasmic contents, and collapse of the proton motive force [
46,
47]. Secondary mechanisms include inhibition of key enzymes (e.g., ATPase by eugenol) and interference with cell wall synthesis (e.g., citral), contributing to overall bacterial cell death. EOs also interfere with quorum sensing, the regulatory system for bacterial communication and biofilm formation. Certain EO constituents downregulate genes like
luxS and
icaA, suppressing EPS production and adherence [
48]. Emulsification further enhances this effect by improving EO solubility and biofilm penetration, resulting in better disruption of biofilm architecture [
49]. EOs exert anticancer effects primarily through oxidative stress, mitochondrial dysfunction, and apoptosis induction. Compounds such as limonene and α-pinene promote ROS generation, leading to mitochondrial depolarization and activation of caspase-mediated apoptosis [
50]. Emulsified EOs, due to enhanced bioavailability and cellular uptake, showed stronger cytotoxic responses, potentially via endocytic uptake mechanisms.
Valuable insights have been gained into the antibacterial properties of EOs and their bioactive components, particularly on cinnamaldehyde, the primary compound in cinnamon oil. The findings demonstrate that cinnamon oil possesses broad-spectrum antibacterial activity, showing marked efficacy against several clinically significant nosocomial pathogens. Cinnamaldehyde, in particular, exhibited strong inhibitory effects on both Gram-positive and Gram-negative bacteria, including S. aureus, MRSA, and P. aeruginosa.
These pathogens are major contributors to hospital-acquired infections and are well-known for their multidrug resistance, highlighting the urgent need for alternative or complementary antibacterial therapies. Moreover, results from the biofilm assays reinforce the potential of cinnamaldehyde, as it effectively disrupted established biofilms formed by MRSA, S. aureus, and P. aeruginosa. Given that biofilm formation is a critical virulence factor in chronic infections and significantly enhances antibiotic resistance, cinnamaldehyde’s anti-biofilm activity presents it as a promising candidate for use in strategies aimed at preventing or treating persistent, biofilm-related infections.
Further research is warranted to investigate the synergistic effects of cinnamaldehyde with conventional antibiotics, its efficacy in vivo, and the potential benefits of using EO-based emulsions or delivery systems to enhance bioavailability and therapeutic outcomes. Additionally, a deeper understanding of the molecular mechanisms by which cinnamaldehyde exerts its antibacterial and anti-biofilm effects could guide the development of novel therapeutics targeting resistant bacterial strains.
However, the application of free EOs could be restricted due to poor solubility in water, low chemical stability, and high volatility, which leads to a reduction in long-lasting bioactivity. Encapsulation is a promising tool to overcome the various limitations of EO formulations, improve their functionality, and protect them from external environmental conditions. Natural biopolymers are non-toxic and biodegradable substances and can be used as carrier materials in the encapsulation of volatile bioactive compounds. Polysaccharides such as starch, chitosan, carrageenan, and cellulose can be used as matrixes for the various biologically active substances for the development of different forms such as emulsions, powders, coatings, films, patches, and wound dressings. For the past decade, materials based on biopolymers with immobilized EOs have been developed and applied using various technologies to increase food safety, to construct new medical devices, cosmetic, and personal care products. Therefore, using EOs as active ingredients in emulsions is an effective strategy for improving their solubility, stability, and controlled release. The emulsions prepared in this study were designed to encapsulate seven EOs derived from plants, including cinnamon EO and its bioactive component, cinnamaldehyde, to enhance their applicability in therapeutic and industrial applications.
Hydrophobically modified waxy maize starch, specifically OSA-starch, was employed as the stabilizing agent in this study. OSA-starch is a food-grade material, recognized for its safety and approval as a food additive, and it has recently emerged as a potential stabilizer for emulsions due to its ability to form stable structures in aqueous environments [
51]. The use of OSA-starch in the emulsions allowed for the incorporation of a range of EOs, including basil EO, cinnamon EO, cinnamaldehyde, eucalyptus EO, lavender EO, pine EO, rosemary EO, and tea tree EO, all of which have demonstrated antibacterial and therapeutic properties. The preparation of emulsions using OSA starch as a stabilizer proved to be an effective method for encapsulating EOs, ensuring their stability and controlled release. The emulsions demonstrated good homogeneity, stability, and appropriate droplet size distribution, which are critical for the successful delivery of the bioactive compounds [
35,
52]. Continued research on the long-term stability and biological effects of starch-based emulsions is essential to optimize their application in clinical settings. Recent studies have demonstrated the promising antibacterial potential of such systems. For example, an emulsion based on octenyl succinic anhydride (OSA) starch incorporating thymol showed strong bactericidal effects against
E. coli and
S. aureus, effectively targeting both planktonic cells and biofilms [
47]. Similarly, Sharif et al. [
53] investigated black cumin EO nanoemulsions stabilized by OSA-modified starch, reporting significantly enhanced antimicrobial activity compared to the pure EO. Moreover, it was demonstrated that the blank emulsion did not exhibit any antimicrobial activity, as would be expected given the nature of the polysaccharide matrix used to stabilize the emulsion. These findings underscore the potential of OSA starch-based emulsions as effective delivery systems to boost the antimicrobial efficacy of EOs, supporting their further development for clinical and pharmaceutical applications.
Additionally, the complex composition of EOs was considered in this study. EOs can contain a mixture of volatile compounds, including major components (20–95%), minor compounds (1–20%), and trace compounds (<1%). These bioactive molecules are responsible for the therapeutic and antibacterial properties of the EOs, and their incorporation into emulsions may enhance the stability and activity of the oils. For example, cinnamaldehyde, the primary component in cinnamon EO, is known for its strong antibacterial properties, and its encapsulation in an emulsion may help to maintain its potency over time [
54,
55,
56,
57]. Other major compounds found in the EOs, such as eucalyptol in eucalyptus oil and linalool in lavender oil, also contribute to the therapeutic effects of the emulsions, particularly in applications like wound healing and infection control [
15,
27,
57,
58,
59,
60,
61].
Cinnamaldehyde maintained significant antibacterial activity in the emulsified form, although its effect was less pronounced than pure oil. This reduction in effectiveness could be attributed to the solubilization process in the emulsion, which might influence the bioavailability and direct contact of the active compound with the bacterial cell wall [
52]. However, it is notable that the cinnamaldehyde emulsion still showed inhibition against all bacterial strains tested, except
P. aeruginosa. Moreover, it should be stressed that the OSA-starch matrix used to emulsify EOs does not possess antibacterial activity, as demonstrated by Sharif et al. [
53]. This suggests that emulsions can still be an effective delivery method for EOs in clinical or therapeutic settings, especially when combined with other bioactive substances [
14].
Interestingly, basil and rosemary oils exhibited minimal antibacterial activity, aligning with previous reports that have described the relatively weaker antibacterial properties of basil EO [
43].
The GC-MS results revealed that cinnamon oil (
C. verum) contained a high concentration of eugenol (32.3%). Cinnamaldehyde, which constitutes 88.6% of the composition, is the dominant compound, along with other compounds. These findings corroborate the work of [
62], who noted that cinnamaldehyde is a significant contributor to the antibacterial activity of cinnamon oil. Additionally, the findings on the composition of basil, eucalyptus, lavender, pine needle, rosemary, and tea tree oils align with the existing literature, where methyl chavicol (from basil), 1,8-cineole (from eucalyptus and tea tree oils), and linalool (from lavender oil) have been reported as key antibacterial agents [
63].
The antibacterial properties of EOs and their major bioactive compounds, particularly cinnamaldehyde, were evaluated against both Gram-positive and Gram-negative nosocomial pathogens. The results suggest that the EOs and compounds tested possess varying degrees of antibacterial activity, with cinnamon EO and cinnamaldehyde exhibiting the most potent effects. These findings are consistent with previous research that has highlighted the antibacterial potential of EOs derived from various plant sources, especially against hospital-acquired pathogens [
28,
60,
64]. Cinnamaldehyde, in particular, exhibited the highest antibacterial activity among all the oils and compounds tested. The inhibition zones observed in the study (ranging from 24.37 mm to 50.22 mm) against both Gram-positive and Gram-negative bacteria indicate a broad-spectrum antibacterial effect [
65]. These results are in agreement with those of [
66], who demonstrated the effectiveness of cinnamaldehyde against a variety of bacterial strains, including hospital-associated pathogens such as
S. aureus and
E. coli. Furthermore, the antibacterial effects of cinnamon oil, eucalyptus oil, and pine needle oil were also noted against most bacterial strains tested, except
P. aeruginosa [
20], which highlighted the resistance of
P. aeruginosa to several EOs [
67,
68]. Cinnamaldehyde disrupts biofilms mainly by inhibiting quorum sensing in
P. aeruginosa, downregulating genes like
lasI,
lasR,
rhlI, and
rhlR, and interfering with biofilm maturation. Its lipophilic nature increases membrane permeability and promotes biofilm dispersal. It may also impair energy metabolism by inhibiting ATPase. Emulsified forms enhance these effects by improving solubility and penetration.
P. aeruginosa shows strong resistance to EOs due to its impermeable outer membrane, active efflux pumps (e.g., MexAB-OprM), and dense biofilm matrix. Its adaptability and oxidative stress tolerance further reduce EO effectiveness. Overcoming this resistance is crucial, highlighting the importance of advanced delivery systems like nanoemulsions to boost EO efficacy [
11,
69].
These results underline the importance of chemical composition in determining the efficacy of EOs. For example, basil oil was dominated by methyl chavicol (53.4%), which, despite its prominent concentration, might not be as effective against the tested bacterial strains compared to other constituents like cinnamaldehyde in cinnamon oil or 1,8-cineole in eucalyptus oil.
Furthermore, the variability in the antibacterial activity of EOs, particularly the lack of activity in certain oils like basil and rosemary, highlights the need for further exploration into the synergistic effects of EOs when used in combination. The synergy between EOs or between EOs and conventional antibiotics could offer a promising approach to overcoming bacterial resistance [
70,
71,
72].
The ability of
S. aureus, MRSA, and
P. aeruginosa to form biofilms is a well-documented mechanism that significantly contributes to the persistence and chronicity of infections. The biofilm formation not only shields the bacteria from the host immune response but also renders standard antibiotic therapies ineffective, making biofilm-related infections particularly challenging to treat [
73]. The biofilm matrix acts as a protective barrier, limiting the penetration of antibiotics and reducing the metabolic activity of the bacteria. This reduction in metabolic rate is one of the key factors that enhance bacterial resistance, allowing biofilm-associated bacteria to tolerate antibiotic concentrations up to 1000 times higher than their planktonic counterparts [
15,
74,
75]. Therefore, targeting biofilm formation or disrupting established biofilms represents a crucial strategy in combating chronic infections caused by these pathogens.
In the present study, the disruption of biofilms by cinnamaldehyde was striking, as evidenced by the complete absence of biofilm layers on the tube walls following a 1 h treatment using the tube method. This result highlights the potential of cinnamaldehyde as an effective biofilm-disrupting agent [
76]. The findings are consistent with previous studies reporting the anti-biofilm properties of cinnamaldehyde and other EOs [
77,
78]. For instance, it was found that cinnamaldehyde effectively inhibits biofilm formation in various pathogens, including
S. aureus and
P. aeruginosa [
79,
80,
81]. Similarly, studies demonstrated the biofilm-disrupting activity of EOs, including cinnamaldehyde, against
S. aureus and other biofilm-forming bacteria [
76,
79,
80,
81,
82,
83]. We agree that using absorbance measurements could provide more quantitative data and strengthen the results. We will consider incorporating this method in future studies as a replacement for the tube method. It is also important to note that the concentration of cinnamaldehyde used in this study to remove pre-formed biofilms was five times higher than MBC. This higher concentration is necessary because biofilm-associated bacteria are inherently more resistant to antibacterial agents due to the protective biofilm matrix. The need for higher concentrations of biofilm-disrupting agents has been widely recognized in the literature [
80]. Agents like cinnamaldehyde require significantly higher concentrations to penetrate and eradicate biofilms compared to their effect on planktonic bacteria [
55,
72,
81,
82]. This increased concentration reflects the enhanced resistance of biofilm-embedded bacteria and underscores the challenge of effectively targeting biofilm-related infections.
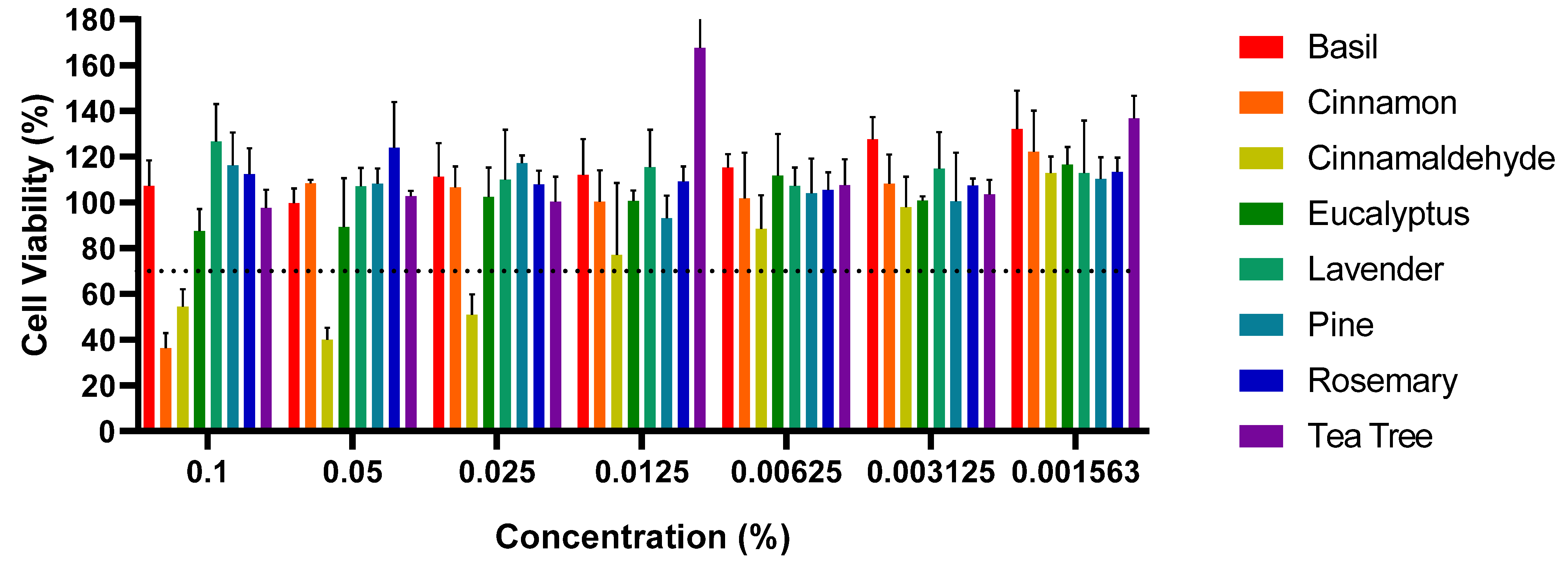
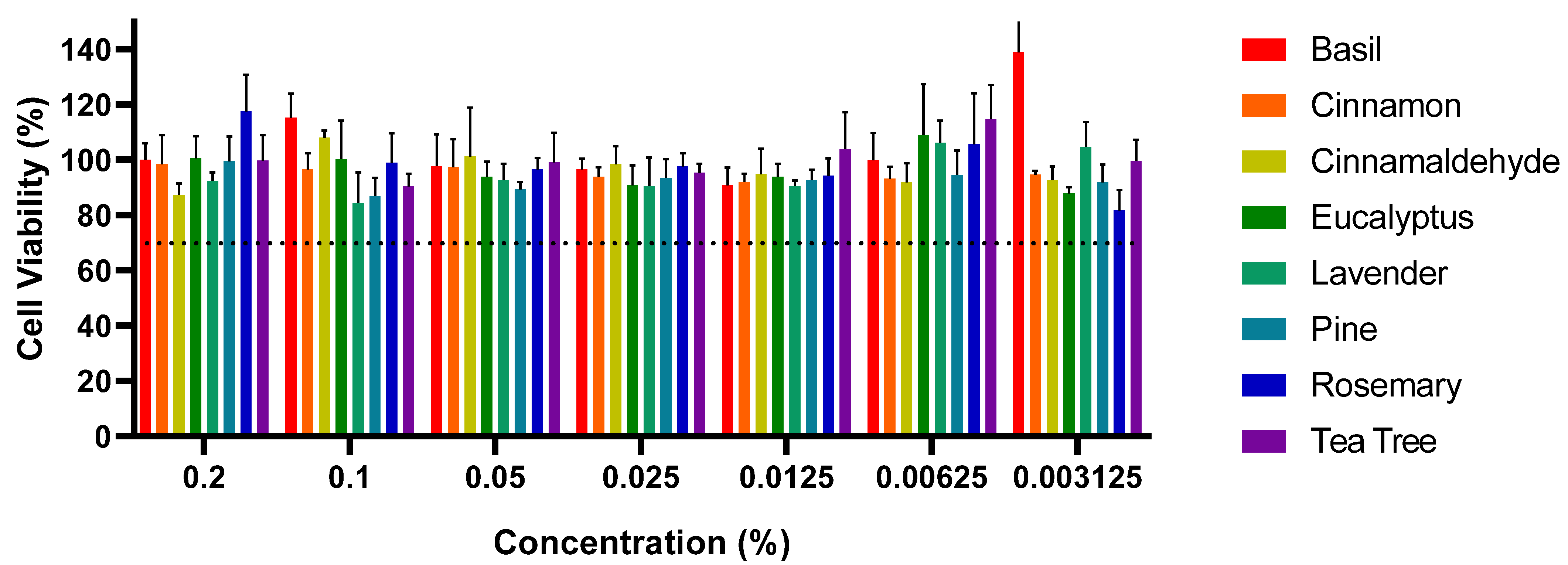
The results from this study highlight the cytotoxic effects of certain EOs and EOEs on Vero cells, specifically cinnamon EO and cinnamaldehyde; meanwhile, other EOs, such as basil and eucalyptus, did not exhibit significant cytotoxicity at the selected concentrations, with cell viability remaining above 70% [
45]. The observed cytotoxicity of cinnamon EO and cinnamaldehyde is consistent with previous reports in the literature, where various EOs and their bioactive compounds demonstrated cytotoxic effects at higher concentrations, typically above 0.02%. For example, studies have shown that EOs like clove, cinnamon, and eucalyptus exhibit varying degrees of toxicity on different cell lines at high concentrations [
50]. In this study, cinnamaldehyde demonstrated cytotoxicity at concentrations ranging from 0.0125% to 0.1%, consistent with previous research suggesting its toxicity at higher levels. Likewise, cinnamon EO displayed cytotoxicity at a concentration of 0.1%, aligning with prior studies that demonstrated its antibacterial efficacy and potential cytotoxicity at elevated concentrations [
56,
57,
58,
62,
68]. During the healing phase of an infected wound, the continuous recruitment of eukaryotic cells to the wound site minimizes the impact on non-target cells. Therefore, although high concentrations of these EOs can cause cytotoxic effects, the wound environment will likely ensure minimal damage to the healthy eukaryotic cells involved in tissue repair [
84,
85,
86]. Previous studies have shown that EOs at appropriate concentrations can be safe and effective for wound healing by promoting antibacterial activity and stimulating tissue repair without significant cytotoxicity [
87,
88,
89]. Further research is needed to determine the optimal concentrations for various applications in order to fully understand their potential for clinical use, particularly in the management of nosocomial pathogens [
88].
5. Conclusions
This study investigated the antibacterial and cytotoxic properties of various EOs and their emulsified formulations against clinically relevant nosocomial pathogens. The findings highlight the considerable antibacterial and antibiofilm potential of EOs, particularly those containing cinnamaldehyde, in combating antibiotic-resistant and biofilm-forming bacteria.
Cinnamaldehyde exhibited the most potent antibacterial activity among the tested compounds, with MICs ranging from 1.31 to 2.62 mg/mL. It was effective against the tested Gram-positive and Gram-negative pathogens, including S. aureus, MRSA, S. epidermidis, B. subtilis, S. Enteritidis, and P. aeruginosa. Other EOs, such as cinnamon, eucalyptus, and pine, also demonstrated antibacterial activity, although their efficacy against P. aeruginosa was comparatively limited.
Notably, cinnamaldehyde showed significant biofilm-disrupting capabilities, completely eradicating biofilms formed by S. aureus, MRSA, and P. aeruginosa within one hour. This underscores its potential as a valuable therapeutic agent for treating persistent biofilm-associated infections, often resistant to conventional therapies.
This study also highlights the value of aqueous EO emulsions stabilized using OSA-starch as an effective strategy to enhance the use of plant-derived oils in various biomedical and industrial fields. Continued research into their long-term stability and broader biological effects will be crucial to harness their potential across different applications.
Cytotoxicity analyses revealed that cinnamaldehyde and cinnamon EO were cytotoxic at concentrations above 0.1%. In contrast, other EOs, including basil, eucalyptus, and lavender, did not exhibit significant cytotoxicity at the tested concentrations, indicating their potential safety for therapeutic use when applied at appropriate doses.