1. Introduction
Infections represent preventable factors contributing to cancer in humans, with approximately 15% of malignancies attributed to infectious origins [
1,
2]. Microbial infections play a significant role in promoting carcinogenesis. Several studies have recognized pelvic inflammatory disease as a potential risk factor for epithelial ovarian cancer. Consequently, it is suggested that microbial infections could have a contributory impact on the development of ovarian cancer. Microbial dysbiosis has been identified as a significant contributor to the etiology of various cancers, including ovarian cancer. The interaction between the host and microbiome can disrupt immune regulation, leading to oxidative stress and inflammation, which are closely intertwined in a cycle of mutual influence [
2,
3]. Prolonged oxidative stress induces DNA damage, thereby increasing the accumulation of mutations and the risk of carcinogenesis. Concurrently, chronic inflammation generates free radicals that directly contribute to tumorigenesis [
4,
5].
Certain bacteria, archaea, and viruses elevate the risk of cancer in the female genital tract, particularly affecting the tubes, ovaries, and endometrium, influencing the heterogeneity of these cancers and their response to therapies. The bacterial mechanisms involved in carcinogenesis encompass the secretion of toxins that directly damage DNA, the promotion of chronic inflammation, and the inhibition of immune cell activation. Notably, bacteria such as
Acinetobacter lwoffii, as well as species from genera such as
Shewanella,
Burkholderia, and others, have been identified within the ovarian cancer microbiome [
6,
7,
8]. Pathogen array and next-generation sequencing analyses revealed a distinct microbial signature in ovarian cancer tumor samples. Among these microbes,
Burkholderia cepacia demonstrated a prominent presence, emphasizing its potential role in the ovarian cancer tumor microenvironment [
9].
Ovarian cancer represents a significant public health challenge worldwide, ranking eighth in both incidence and mortality among women globally [
9]. It is the second most common female malignancy after breast cancer [
10]. Its non-specific symptoms and silent progression often lead to late diagnoses, resulting in limited treatment options and low survival rates. Ovarian cancer is the most lethal gynecological malignancy, with an estimated 66% mortality rate among the more than 300,000 women diagnosed in 2020 [
11]. Despite increased awareness, there has been little improvement in cure and survival rates in recent years. Effective treatments remain elusive, highlighting the urgent need for novel therapeutic strategies. Exploring unconventional factors potentially associated with tumor development and addressing secondary elements, such as the role of the microbiome, are crucial to preventing cancer emergence and progression. In this context, ovarian cancer remains a significant challenge due to its high mortality rate and the limited efficacy of existing therapies. The complexity of its tumor microenvironment, influenced by factors such as oxidative stress, inflammation, and microbial dysbiosis, underscores the need for integrative approaches targeting multiple pathways involved in tumor progression.
Brazilian red propolis (BRPE) emerges as a potential therapeutic agent capable of addressing these challenges. This natural product, renowned for its diverse bioactive compounds—including flavonoids, phenolic acids, and isoflavonoids—has demonstrated a wide range of pharmacological activities, such as anti-inflammatory, antioxidant, and antimicrobial effects. Importantly, BRPE has shown promising antitumor properties by inhibiting cell proliferation, angiogenesis, and metastasis while inducing apoptosis in cancer cells. Our previous research further supports this potential, demonstrating the in vitro efficacy of BRPE as an antitumoral agent against the ovarian cancer cell line OVCAR-3 [
12]. These findings position BRPE as a promising candidate for integrative cancer therapies [
13].
In this study, we evaluated the efficacy of BRPE in both its free form and encapsulated within polymeric nanoparticles (NCBRPE) against bacterial species commonly associated with the ovarian cancer microenvironment. The potential of these nanoparticles to reduce reactive oxygen species (ROS) levels was also investigated, highlighting their antioxidant properties and their indirect contribution to anti-inflammatory effects. Given the intricate relationship between oxidative stress, chronic inflammation, and cancer progression, this dual-action approach emphasizes the importance of combining antioxidant and antimicrobial activities to target key pathways involved in tumor development and improve therapeutic outcomes.
2. Materials and Methods
The BRPE was supplied by the Laboratory of Pharmacognosy at the School of Pharmaceutical Sciences, University of São Paulo, Brazil. The extract was collected in Canavieiras, Bahia, by the beekeepers’ association COAPER and is registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SISGEN) under the code AF234D8. The processing and characterization of BRPE were performed as described by Aldana-Mejía et al. (2021) [
14].
2.1. Phytochemical Profile and Chemical Characterization of the Main Biomarkers of Brazilian Red Propolis Extract
The chromatographic method [
14] was performed using a Waters 2695 high-performance liquid chromatography (HPLC) system, integrated with a 2998 photodiode array detector (PDA), a 1525 binary solvent delivery unit, and a 2707 autosampler. The Empower 3 software was utilized to control the analytical system and process the resulting data. All analyses were conducted in triplicate.
Chromatographic separations were performed on a Supelco Ascentis Express C-18 column (150 × 4.6 mm, 2.7 μm). The mobile phase consisted of water (A) containing 0.1% formic acid and acetonitrile (B) under a gradient program as follows: 20 → 50% B over 40 min; 50 → 100% B at 41 min; maintaining 100% B until 45 min; followed by a return to 20% B at 46 min to re-establish initial conditions by 50 min. The chromatography was carried out at a flow rate of 1 mL/min, with the column maintained at 40 °C, and an injection volume of 10 μL. The PDA was configured to scan wavelengths from 220 to 500 nm, with chromatograms monitored at 275 and 315 nm. Data acquisition was performed at specific wavelengths (249, 254, 260, 276, 280, 285, 376), chosen based on the maximum absorption of each compound.
The quantification of biomarkers formononetin, biochanin A, and vestitol was performed using ultra-performance liquid chromatography coupled with mass spectrometry (UPLC-MS) system equipped with a simple Quadrupole detector (SQ Detector 2, Waters, Milford, MA, USA). The system operated in negative electrospray ionization mode (ESI-) and used an Acquity UPLC BEH C18 column (100 × 2.1 mm, 1.7 μm particle size, Waters, Milford, MA, USA) at a flow rate of 0.4 mL/min and a temperature of 40 °C. The mobile phase consisted of ultrapure water with 0.1% ammonium acetate (C) and acetonitrile with 0.1% ammonium acetate (D), following a gradient elution as detailed: 90% D (0 min); 90% D → 30% D (2 min); 30% D → 2% D (3 min); 2% D → 2% D (6 min); 2% D → 0% D (6.5 min); 0% D → 0% D (8.5 min); 90% D → 10% D (9 min); and 90% D → 10% D (11 min). Each sample (10 μL, 0.1–50 μg/mL) was injected under these conditions. In the mass spectrometry system, the gas flow rate was set to 600 L/min for desolvation, with a gas temperature of 450 °C. The ionization source operated at a temperature of 500 °C, with a capillary voltage of 5.5 kV and a cone voltage of −44 V. These parameters ensured optimal ionization and detection of the analytes [
15] (
Figure S1, Supplementary Material).
2.2. Determination of Flavonoid Content in BRPE
The flavonoid content in BRPE was quantified using a modified version of the method described by Funari and Ferro (2006) [
16]. Ethanol was employed as the organic solvent, and the assay parameters included the use of 5% AlCl
3 (Sigma-Aldrich, St. Louis, MO, USA) and a reaction time of 30 min. Quercetin (Sigma-Aldrich, Burlington, MA, USA) at a concentration of 20 µg/mL (λ = 425 nm) served as the standard for constructing the calibration curve. The assay is based on the formation of stable aluminum–flavonoid complexes, which cause a characteristic shift in the flavonoid’s absorbance peak to 425 nm.
An ethanolic solution of BRPE (2 mg/mL) was prepared and mixed with 500 µL of 5% AlCl3, followed by dilution with ethanol to a final volume of 5 mL. After incubation at room temperature for 30 min, the absorbance was measured at 425 nm using a UV–Vis spectrophotometer (NanoPhotometer UV-Vis, Implen, Munich, Germany). The calibration curve (R2 = 0.9999, y = 0.043x + 0.0021) was generated using quercetin as the standard.
2.3. Total Phenolic Content in BRPE
Total phenolic content in BRPE was determined using the Folin–Denis method, as outlined by Funari and Ferro (2006) [
16]. Gallic acid (Sigma-Aldrich, Burlington, MA, USA) (λ = 760 nm) served as the standard for the calibration curve. For the assay, 100 µL of a 2 mg/mL BRPE solution was combined with 250 µL of the Folin–Denis reagent (Sigma-Aldrich, Burlington, MA, USA) in a 5 mL volumetric flask and diluted with water. After a 2 min reaction, 500 µL of 20% sodium carbonate solution was added to ensure an alkaline pH, and the flasks were left at room temperature for 30 min. Absorbance was measured at 760 nm using a UV–Vis spectrophotometer (NanoPhotometer UV-Vis, Implen, Munich, Germany).
The Folin–Denis reagent, containing molybdenum and tungsten acids, reacts with phenolic compounds in an alkaline environment. At this pH, phenolic compounds dissociate a proton to form phenolate ions, which reduce the reagent to produce blue-colored oxides. The intensity of this color, proportional to the phenolic content, was quantified via absorbance. A calibration curve using gallic acid as the standard was constructed (R2 = 0.9994, y = 0.0791x − 0.0963). This curve was applied to calculate the total phenolic content in BRPE.
2.4. Development and Characterization of the Polymeric Nanoparticles
Nanocapsules were prepared using the nanoprecipitation method, which is suitable for encapsulating lipophilic compounds. The organic phase (composed of acetone, ethanol, polylactic acid (Corbion N.V., Amsterdam, Netherlands), tributyrin (Thermo Scientific Chemicals, Waltham, MA, USA), and Brazilian red propolis extract) was poured into the aqueous phase (composed of Poloxamer P407 (BASF, Ludwigshafen, Germany) and phosphate buffer, pH 7.4) under magnetic stirring to facilitate the evaporation of the organic solvent over 24 h, resulting in Brazilian-red-propolis-loaded nanocapsules (NCBRPE) as described by Justino et al. (2023) [
12].
The encapsulation efficiency (EE) was determined using an indirect method. The concentration of biomarkers in the formulation (biochanin A, vestitol, and formononetin) was quantified via UPLC-MS. The method involved filtering a small volume (200 µL) of the nanocapsule formulation through Microcon filters (Millipore®, Darmstadt, Germany) equipped with an ultrafiltration membrane (10,000 g/mol cut-off) and centrifugation at 5000× g for 10 min to separate free (non-encapsulated) compounds from the nanocapsules. The filtrate, containing the non-encapsulated biomarkers, was diluted in methanol and analyzed.
2.5. Evaluation of Antioxidant Activity Using the DPPH Radical Reduction Method
The antioxidant activity of BRPE was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) (Sigma-Aldrich, Burlington, MA, USA) radical reduction method. Methanol was used as the organic solvent for the preparation of BRPE, DPPH, and ascorbic acid solutions, while citrate buffer was prepared in water. Solutions containing BRPE (1–60 μg/mL), 50 µL of DPPH solution (333 µM), and 50 µL of citrate buffer (pH 4.5) were added to 96-well plates. Ascorbic acid (Sigma-Aldrich, Burlington, MA, USA) was used as an antioxidant standard at concentrations ranging from 1 to 20 µg/mL. After incubating the plates in the dark for 30 min, absorbance readings were taken at 517 nm using a microplate reader (SPECTRAMax, Molecular Devices, San Jose, CA, USA). The reduction of the DPPH radical was calculated based on Aa% (the percentage of antioxidant activity) (Equation (1)), and the antioxidant potential was expressed as EC50 (the concentration at which the extract achieves half of its maximum response), determined using GraphPad Prism
® 8 software.
where Aa% = percentage of antioxidant activity [
17].
2.6. Evaluation of the Efficacy of BRPE, NCBRPE, and NC in Bacteria of the Ovarian Cancer Tumor Microenvironment
The minimum inhibitory concentration (MIC) was determined for selected bacterial strains, which were chosen based on their relevance, as reported in the literature, to infections associated with cancer patients and their potential presence in tumor microenvironments. While these strains were procured from the ATCC, they serve as representative models rather than direct isolates from the ovarian tumor microenvironment. This approach allows for controlled, reproducible experiments to evaluate the antimicrobial potential of the treatments.
The effectiveness of BRPE, NCBRPE, and NC formulations was tested against these bacteria. The effectiveness of BRPE, NCBRPE, and NC formulations was tested via the broth microdilution method according to the guidelines established by the Clinical and Laboratory Standards Institute (CLSI) [
18], using bacterial species including
Escherichia coli ATCC
® 25922™,
Klebsiella pneumoniae ATCC
® 13883™,
Pseudomonas aeruginosa ATCC
® 27853™,
Acinetobacter baumannii ATCC
® 14293™,
Burkholderia cepacia ATCC
® 25416™,
Stenotrophomonas maltophilia ATCC
® 13673™, and
Proteus mirabilis EW672.
From solutions BRPE, NCBRPE, and NC at 2 mg/mL, serial dilutions (2–1024 mg/L) were performed in 96-well plates (K12-096, Kasvi, Pinhais, PR, Brazil) using BBL™ Mueller Hinton II Broth (cation-adjusted) (CAMHB; BD, Franklin Lakes, NJ, USA) with final volumes of 190 μL. From colonies grown on Mueller Hinton agar (MHA; Kasvi, Pinhais, PR, Brazil) at 35 ± 2 °C overnight, bacterial suspensions equivalent to 0.5 McFarland (1 × 108 UFC/mL) scale were prepared. Then, the scale was adjusted to 5 × 105 UFC/mL, and 10 μL was added to 96-well plates. The plates were incubated at 35 ± 2 °C overnight. Finally, 20 μL of a sterile resazurin solution (0.02% v/v) was added to the wells, and the plates were incubated at 35 ± 2 °C for 1 h. The MIC was considered the first concentration capable of fully inhibiting bacterial growth.
2.7. Optimization of Biofilm Formation Conditions and Biofilm Quantification
For optimizing biofilm formation, the incubation time and glucose concentrations were tested. The incubation period is important to allow bacteria time for adherence and synthesis of extracellular matrix components [
19], while glucose concentration plays a critical role in regulating quorum-sensing molecules that facilitate biofilm formation [
20]. Biofilm formation was assessed using crystal violet dye.
The conditions for formation of the biofilm of
B. cepacia were optimized, and the biofilm inhibitory concentration (BIC) was determined. In order to achieve optimum biofilm formation, different growth times and glucose (Merck, Darmstadt, Germany) concentrations were tested. Bacterial cultivation and preparation of suspensions were performed as mentioned before. The serial dilutions were carried out in 96-well plates using CAMHB supplemented with different glucose concentrations [0.5 and 2% (
v/
v)], and the plates were incubated at 35 ± 2 °C for 48 h and 72 h to evaluate the biofilm formation. After these periods, the medium was removed; the wells were gently washed three times with 200 μL of phosphate-buffered saline (PBS) (Sigma-Aldrich, Burlington, MA, USA); and the biofilms were stained with 2% crystal violet (Sigma-Aldrich, Burlington, MA, USA). After 30 min, the dye was resuspended in 95% ethanol, and the optical density (OD) was measured at 570 nm. The results were interpreted according to the guidelines established by Stepanović (2007) [
21] and Pavão and collaborators (2021) [
22]. Cut-off values were calculated using the negative control (ODc) according to Equation (2):
where ODc: OD of negative control; SDc: standard deviation of ODc.
Biofilm formation was evaluated following the criteria below [
22]:
2.8. Clonogenic Assay
To perform the clonogenic assay, OVCAR-3 cells, obtained from Rio de Janeiro Cell Bank (Brazil), were plated at a density of 2500 cells per well in a 6-well plate containing 3 mL of complete RPMI-1640 medium (Sigma-Aldrich, Burlington, MA, USA) supplemented with 20% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). After 24 h of incubation at 37 °C, 5% CO
2, and controlled humidity, the medium was removed from the wells, which were then washed and treated with the same concentrations of free BRPE and NCBRPE (99 µg/mL, equivalent to 4.69 × 10
10 nanoparticles/mL). The treatment lasted for 24 h. Subsequently, the wells were washed with PBS 1X (Sigma-Aldrich, St. Louis, MO, USA), and 3 mL of complete RPMI-1640 medium was added. The plate was incubated, with the medium changed every 3 days. After 15 days, colony formation (defined as a cluster of 50 or more cells) was visible in all wells corresponding to different treatments and the negative control. These colonies were manually counted and subsequently stained with 1% (
w/
v) crystal violet solution (Sigma-Aldrich, St. Louis, MO, USA). After solubilizing the dye in 33% (
v/
v) acetic acid solution, absorbance was measured at 595 nm using a plate reader (Biotek Synergy, Winooski, VT, USA). To calculate the plating efficiency (PE) and survival fraction (SF), the following Equations (3) and (4) were used [
23]:
2.9. Evaluation of Cell Death Type via Flow Cytometry
Cell death evaluation was performed in OVCAR-3 cells, obtained from Rio de Janeiro Cell Bank (Brazil), using flow cytometry with the FACSCanto instrument (BD, Franklin Lakes, NJ, USA) equipped with FACSDiva software version 6.3.1 (BD, Franklin Lakes, NJ, USA). The markers employed were Annexin V-FITC (Apoptosis Detection Kit) (Thermo Fisher®, Waltham, MA, USA) and Fixable Viability Dye eFluor (FVD—Thermo Fisher®, Waltham, MA, USA).
Cells were plated in 6-well plates containing 3 mL of complete RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 20% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), at a density of 6 × 105 cells/well. After 24 h of incubation at 37 °C, 5% CO2, and controlled humidity, the medium was removed, and cells were treated with BRPE and NCBRPE (99 µg/mL corresponding to 4.69 × 1010 nanoparticles/mL), diluted in complete RPMI-1640 medium, and further incubated for 24 h under the same conditions.
After the 24 h treatment period, cells were trypsinized with trypsin 2X (Trypsin solution 10X, Sigma-Aldrich, St. Louis, MO, USA), centrifuged (1700 rpm for 10 min), transferred to Falcon tubes, and washed twice with ice-cold PBS 1X (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, cells were resuspended in 1 mL of complete RPMI-1640 medium containing 1 µL of FVD and incubated in the dark on ice for 30 min. The washing procedure was repeated two more times, and finally, cells were resuspended in 200 µL of 1X binding buffer (BD Biosciences®, Franklin Lakes, NJ, USA) supplemented with 2 µL of Annexin V (BD Biosciences®, Franklin Lakes, NJ, USA) 15 min prior to analysis.
As negative controls, cells without markers, cells labeled only with Annexin V, and cells labeled only with FVD were used. Cell analysis was performed using excitation–emission wavelengths of 490/525 nm for Annexin V and 405/450 nm for FVD.
2.10. Erythrocyte Sedimentation Rate (ESR) Assay
Samples of peripheral human blood were collected for erythrocyte sedimentation rate and neutrophil oxidative burst assays. The experimental design of this study was based on the recommendations of the Ethical Principles of Experimentation and approved by the Ethics Committee at the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo (number 64521922.8.0000.5403).
The ESR assay measures the distance in millimeters that red blood cells travel over one hour. The selection criteria for the test included women aged 18–40 years who had not taken antibiotics or anti-inflammatory medications in the past 7 days. Eight milliliters of blood was collected from 10 volunteers in tubes containing ethylenediaminetetraacetic acid (EDTA) (Greiner bio-one, Americana, SP, Brazil). NCBRPE (2000 µg/mL) and NC (11.28 × 10
11 NC/mL) dispersions were pre-diluted in PBS (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, 990 µL of blood was mixed with 110 µL of the respective treatments, resulting in final concentrations of 200, 100, and 50 µg/mL of BRPE. An equal volume of blood mixed with 110 µL of PBS served as the experimental control. The blood samples were incubated with the different treatments for 1 h under gentle agitation at 37 °C. After incubation, the samples were homogenized, aspirated into glass pipettes measuring 200 mm in length and 2.55 mm in internal diameter, and vertically supported. After 1 h, the ESR was measured and expressed in mm/h [
24].
2.11. Oxidative Burst Assay in Neutrophils
The production of ROS was assessed using the luminol-dependent chemiluminescence (CL) method [
25]. Neutrophils were purified from blood samples using 2.5% gelatin, as described previously [
26], and were adjusted to a concentration of 5 × 10
6 cells/mL in Hanks gel (Sigma-Aldrich, Burlington, MA, USA) with 0.003 M luminol (Sigma-Aldrich, St. Louis, MO, USA) and 10
−6 M PMA (phorbol 12-myristate 13-acetate) (Sigma-Aldrich, St. Louis, MO, USA) added for stimulation, along with treatments at concentrations of 10, 25, and 50 µg/mL of NCBRPE and NC. Following exposure to the treatments, CL was measured using an AutoLumat Plus luminometer (Berthold, Bad Wildbad, Germany) immediately after nanoparticle addition and again 30 min after plate incubation at 37 °C. In both cases, the CL was registered for 15 min. For the negative control (spontaneous reaction), samples were incubated solely with luminol, while for the positive control, cells were exposed to PMA without treatments. The results were quantified by calculating the integrated area under the CL profile and considering the positive control as 100% of the production of ROS by neutrophils. The points in each group represent the means of the percentage of the area under the curve values relative to the positive control of each independent assay conducted in triplicate with neutrophils.
2.12. Statistical Evaluation Methods
All studies were conducted in triplicate, and the results are expressed as mean ± standard deviation where applicable. Statistical significance was analyzed using one-way ANOVA, followed by Dunnett’s test when comparisons were made with a specific group, Tukey’s test when comparisons were made between multiple groups, and/or Sidak’s test when groups were compared pairwise. Differences were considered significant at p < 0.05.
3. Results
3.1. Biomarker Quantification of BRPE via UPLC-MS
Formononetin, vestitol, and biochanin A were identified and quantified in BRPE, with formononetin and vestitol showing the highest concentrations (
Table 1).
3.2. Determination of Flavonoid Content in BRPE
The flavonoid content in BRPE was quantified using the aluminum chloride method, yielding a total flavonoid content of 50.05 mg of flavonoids per gram of BRPE.
3.3. Total Phenolic Content in BRPE
Using the Folin–Denis’s method, the total phenolic content of BRPE was quantified as 16.41%, corresponding to 164 mg of total phenolics per 1 g of BRPE.
3.4. Nanoparticle Characterization
The NC exhibited sizes of 190.9 ± 9.1 nm, a PdI of 0.08, and a zeta potential of −8.6 ± 0.7 mV, and the NCBRPE showed sizes of 178.3 ± 3.3 nm, a PdI of 0.06, and a zeta potential of −7.7 ± 0.3 mV (
Figure 1A and
Figure 1B, respectively). The encapsulation efficiency of BRPE markers (biochanin A, vestitol, and formononetin) exceeded 97%, as verified by UPLC-MS, demonstrating a strong affinity of these compounds for the oily core of the nanoparticles.
Figure 1A,B illustrate the size distribution profiles of NC and NCBRPE, respectively. The nanoparticles showed spherical morphology, with NCBRPE presenting sizes of 168.6 nm ± 12.7 (
n = 256) and NC of 190.4 nm ± 20.3 (
n = 185), respectively, using Image J (1.53k) software (
Figure 1C,D).
3.5. Determination of MIC for Bacteria of the Tumor Microenvironment
The MIC values for BRPE, NCBRPE, and NC formulations against the selected bacteria are presented in
Table 2.
3.6. Optimization of Biofilm Formation Conditions for B. cepacia and Biofilm Quantification
Biofilms cultivated with 2% glucose demonstrated initial formation at 48 h, with increased coverage and density by 72 h. However, biofilm formation was not uniformly distributed across the well surface.
Figure 2 illustrates biofilm formation at both time points and their respective negative controls.
The cut-off value for distinguishing biofilm formation was set at 0.149. Based on the measurements, an OD between 0.149 and 0.298 is classified as weak biofilm formation; an OD ranging from 0.289 to 0.596 indicates intermediate biofilm formation; and finally, an OD greater than 0.596 is classified as strong biofilm formation. Quantification revealed an average OD of 1.967 for B. cepacia ATCC® 25416™, classifying it as a strong biofilm former.
Statistical analysis (ANOVA followed by Dunnett’s post hoc test) revealed significant differences between the biofilms and their respective negative controls (
p < 0.05), as well as between the biofilms formed at 48 h and those formed at 72 h. The biofilms formed at 72 h exhibited higher density, as shown in
Figure 3. Despite some visual variability, the biofilms demonstrated statistical consistency across the samples.
3.7. Evaluation of Biofilm Inhibitory Concentration (BIC) for Biofilms Formed by B. cepacia
After confirming biofilm formation, the BIC assay was conducted. The BIC for NCBRPE was found to be 128 mg/L, consistent with the MIC test result. In contrast, the BRPE required a concentration of 64 mg/L for BIC, which was twice as high as its MIC (32 mg/L).
3.8. Clonogenic Assay
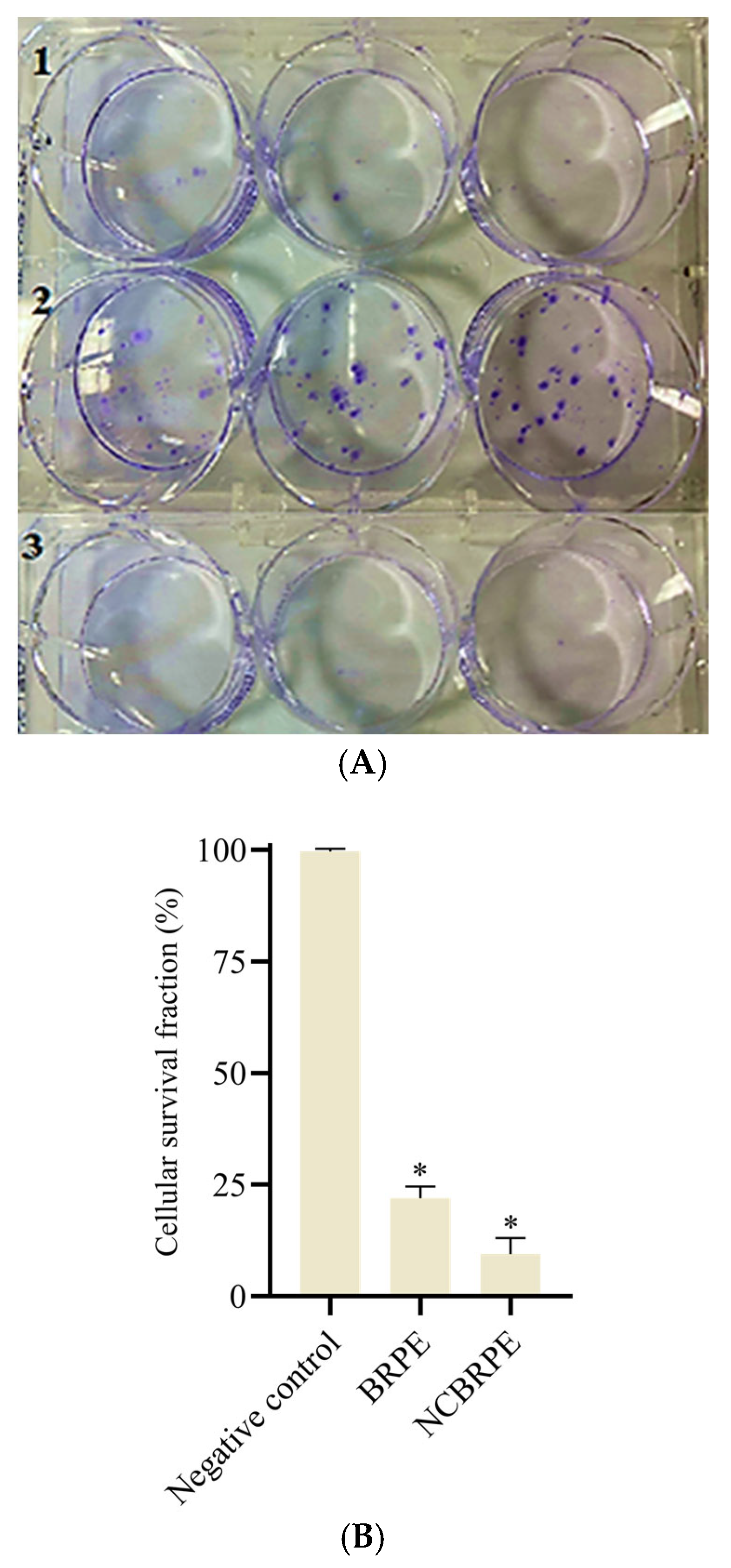
Figure 4A shows the OVCAR-3 cell colonies formed after 24 h of treatment (BRPE 99 µg/mL, NCBRPE 4.69 × 10
10 nanoparticles/mL) and subsequent 15-day incubation in complete RPMI-1640 culture medium.
Based on the analysis of the results obtained (
Figure 4B), it can be suggested that both BRPE and NCBRPE can reduce the persistent cell population with the ability to multiply. The average colony formation in the negative control was 52.7, approximately 5 times higher than for BRPE and 10 times higher than for NCBRPE.
The difference in cell survival fractions was statistically significant (* p < 0.05), demonstrating the enhanced efficacy of both BRPE and NCBRPE in limiting cell replication.
3.9. Evaluation of Cell Death Type via Flow Cytometry in OVCAR-3
The flow cytometry analysis showed that both forms of BRPE, free and nanoencapsulated, induce cell death mainly through apoptosis, with the majority of cells in the negative control remaining viable (98.06% ± 2.2).
For BRPE, the combined early and late apoptosis accounted for 23.3% ± 1.5 of the total population, while necrosis represented 2.4% ± 0.1. For NCBRPE, early and late apoptosis together constituted 29% ± 0.4 of the total population, with necrosis reaching 4.2% ± 0.5 (
Figure 5). The nanocarrier did not appear to influence the observed type of cell death.
3.10. Erythrocyte Sedimentation Rate Assay
The results for the ESR assay are presented in
Table 3 and
Table 4. The nanoparticles NC and NCBRPE were tested in whole blood samples to assess their influence on ESR.
Figure 6 shows a comparison of the treatments with NC and NCBRPE at different concentrations.
The International Committee for Standardization in Hematology (ICSH) establishes a reference value for the erythrocyte sedimentation rate (ESR) in women under 50 years of age as 20 mm/h. In 83% of the samples, ESR values did not exceed a 20 mm/h change after treatment. A standard deviation of ±2 mm/h was used to evaluate whether post-treatment values with NC fell within the established range (0–22 mm/h). The results were as follows: for NC 1, eight values fell within this range; for NC 2, nine values; and for NC 3, seven values. For samples treated with NCBRPE, six values fell within the range for both NCBRPE 100 µg/mL and 200 µg/mL, while nine values were within the range for NCBRPE 50 µg/mL.
Overall, the variability across concentrations, along with many points deviating from the mean, suggests inconsistent trends. No clear flat or upward trend in ESR was observed following NCBRPE treatment. Additionally, when comparing ESR values between the NC as a control and NCBRPE-treated blood, no statistically significant difference (*
p < 0.05) was detected between treatments, as shown in
Figure 6A–C.
A similar result was observed when using the ESR of nanoparticle-free blood as a control for comparison with samples treated with NCBRPE; across all nanoparticle concentrations, no statistically significant difference (* p < 0.05) was found between treatments.
3.11. Neutrophil Burst Oxidative Assay and Antioxidant Effect of Nanoparticles
The production of ROS was evaluated in neutrophils to assess the effects of NCBRPE treatment in both stimulated and unstimulated conditions.
Figure 7 presents ROS production results at two time points: immediately after PMA stimulation (0 min,
Figure 7A) and following stimulation with an incubation period (30 min,
Figure 7B).
In
Figure 7A, in response to PMA stimulation, ROS production was significantly lower in neutrophils treated with NCBRPE compared to those treated with equivalent concentrations of NC and the positive control (PMA without nanoparticles,
p < 0.05). However, in the absence of PMA, there was no difference in ROS production between neutrophils treated with either nanoparticle (NC or NCBRPE) and the spontaneous control.
Figure 7B shows that spontaneous ROS production (without PMA) in neutrophils pretreated with NC for 30 min was significantly higher compared to the negative control (neutrophils without nanoparticles). This suggests that the exposure to NC before stimulation may have induced a state of pre-activation in the neutrophils. However, NCBRPE significantly reduced ROS production compared to NC (
p < 0.05), with levels similar to those observed in the negative control. When PMA was used as a stimulus, ROS production was significantly reduced in neutrophils treated with NCBRPE compared to those treated with the corresponding concentrations of NC and the positive control (PMA without nanoparticles,
p < 0.05).
To evaluate the antioxidant effects of the treatments, a DPPH assay was conducted for BRPE, NCBRPE, and NC (
Figure 8). NC exhibited a maximum of 10% antioxidant activity within the tested concentration range. However, both BRPE and NCBRPE exhibited high antioxidant activity, with no significant difference between them. The significance was assessed using ANOVA with Sidak post hoc analysis.
4. Discussion
4.1. Role of Formononetin, Biochanin A, and Vestitol in BRPE
Formononetin, biochanin A, and vestitol, compounds found in BRPE, exhibit significant biological activities relevant to cancer and inflammatory diseases. Formononetin, an isoflavone, shows notable anticancer effects by inhibiting the growth of various cancer cells, including breast and prostate cancer, through apoptosis induction and cell cycle arrest [
27]. Additionally, it exerts anti-inflammatory effects by modulating key signaling pathways, such as NF-κB and PI3K/Akt, which are frequently dysregulated in cancer and inflammatory conditions [
28,
29]. Formononetin also has strong antioxidant activity, helping to neutralize free radicals and protect cells from oxidative stress—a key factor in chronic disease progression—by activating the Nrf2 pathway, which enhances the expression of antioxidant enzymes [
30,
31].
Biochanin A, another isoflavone found in plants and BRPE, demonstrates significant antioxidant and anti-inflammatory effects. It mitigates oxidative stress by increasing the activity of antioxidant enzymes like superoxide dismutase and catalase and activating the Nrf2 pathway, thereby protecting cells from oxidative damage linked to cancer [
32,
33]. Additionally, biochanin A inhibits cancer cell proliferation and induces apoptosis, supporting its role in cancer therapy by targeting multiple pathways involved in cancer progression and cell survival [
34]. Biochanin A’s antioxidant and anti-inflammatory effects complement those of formononetin, further protecting cells from oxidative damage and supporting inflammation control.
Vestitol, an isoflavonoid, shares similar anti-inflammatory and antioxidant properties. It reduces pro-inflammatory cytokines and reactive oxygen species (ROS) implicated in the pathogenesis of cancer and inflammatory diseases. Vestitol modulates immune responses by inhibiting neutrophil migration, reducing inflammatory chemokines like CXCL1/KC and CXCL2/MIP-2, and suppressing nitric oxide production in macrophages [
29,
30]. These actions enhance the anti-inflammatory effects of formononetin and biochanin A, suggesting their potential as a synergistic approach to cancer therapy by collectively reducing tumor growth and inflammation. These activities provide the rationale for selecting these markers for quantification.
4.2. Concentrations of Phenolic Acids and Flavonoids in BRPE
After evaluating the amount of the biological markers, the study proceeded with assays to determine the concentrations of phenolic and flavonoid compounds in BRPE and NCBRPE. Using the established method, the flavonoid content in BRPE was quantified, revealing a substantial concentration of 50.5 mg/g BRPE.
Flavonoid levels ranging from 27 mg/g to 43 mg/g have been documented for various types of Brazilian propolis [
34]. Andrade et al. (2017) [
35] found levels of 31.48 mg/g, while Righi et al. (2011) [
36] reported levels of 32.9 mg/g. According to the Ministry of Agriculture’s normative instruction “Technical Regulation for the Fixation of Identity and Quality of Propolis” (2001) [
37], propolis extracts with a flavonoid content exceeding 2% (
w/
w) are classified as high-content, with a minimum quality requirement of 0.5% (
w/
w). Thus, the extract used in this study surpasses the legislated minimum for flavonoid content. This high flavonoid concentration in BRPE not only meets quality standards but also indicates potential health benefits due to its antioxidant properties.
The total phenolic content of BRPE in this study revealed a significant concentration of 164 mg/g BRPE. Other studies have determined concentrations ranging from 120 to 240 mg/g [
38,
39]. The Brazilian normative instruction from the Ministry of Agriculture [
37] specifies that the concentration of phenolic compounds in propolis samples must be at least 5% (
w/
w), a standard exceeded by the extract used in this study. The high phenolic content in BRPE not only meets but surpasses these quality standards, highlighting its potential health benefits due to its strong antioxidant and anti-inflammatory capacities.
4.3. Nanoencapsulation of BRPE
Nanoencapsulation of BRPE offers significant advantages in biomedical applications by enhancing the stability, solubility, and bioavailability of its bioactive compounds. Natural extracts like BRPE often face challenges such as degradation due to environmental factors and limited absorption in biological systems. By encapsulating BRPE into nanoparticles, the bioactive compounds are protected from environmental degradation and enzymatic breakdown, thereby improving their stability. This approach enhances therapeutic efficacy while minimizing potential side effects [
40].
A distinctive aspect of this study is the encapsulation of the entire BRPE, preserving the natural synergistic interactions among its multiple bioactive components. This contrasts with existing studies that focus on isolating and encapsulating individual compounds, which may overlook the combined effects that contribute to the extract’s overall bioactivity. This strategy not only preserves the chemical diversity of the extract but also maximizes its biofunctional properties, addressing a gap in current research. By maintaining the integrity of the whole extract, the nanoencapsulation strategy employed here aims to retain and potentially amplify the antimicrobial, antioxidant, and anticancer properties inherent to BRPE [
41].
Nanoprecipitation ensures high encapsulation efficiency and stability of the nanoparticles. Compared to conventional encapsulation systems, the nanoscale formulation developed offers an increased surface area and enhanced interaction with biological targets, which is particularly beneficial for biomedical applications. This approach aligns with advancements in nanotechnology aimed at improving the delivery and efficacy of bioactive compounds [
42].
Encapsulating isolated bioactive compounds or whole extracts is a fundamental consideration in nanoparticle development, as it significantly impacts the biological and therapeutic outcomes. Isolated compounds allow for precise dosing and targeted therapeutic effects, often simplifying the formulation process and enabling more controlled studies of specific bioactivities. However, this approach may overlook potential synergistic interactions between the diverse constituents of natural extracts, which can enhance their overall efficacy. In contrast, nanoencapsulation of whole extracts, such as Brazilian red propolis extract (BRPE), preserves the natural complexity and synergy of bioactive compounds, potentially amplifying their therapeutic potential [
43].
The encapsulation of the entire BRPE in this study was prioritized to leverage these synergistic effects, offering a multi-functional therapeutic approach that targets both antimicrobial and anticancer pathways.
4.4. Antibacterial Effects of BRPE and NCBRPE
The antibacterial effects of BRPE are notable [
44,
45], and based on the results, it is possible to observe that BRPE was more efficient than NCBRPE in all cases and that
B. cepacia and
S. maltophilia were the species in which the lowest concentrations of BRPE and NCBRPE were needed to cause growth inhibition. NC had no effect on any bacteria evaluated.
According to CLSI, sulfamethoxazole and ceftazidime exhibit MICs of 38 mg/L and 64 mg/L, respectively, when tested against
B. cepacia ATCC
® 25416™ [
46], indicating that BRPE is more effective than these two established antimicrobials. Additionally, BRPE was also highly effective against
A. baumannii ATCC
® 14293™.
On the other hand, NCBRPE demonstrated lower effectiveness against the tested bacteria, and several factors related to both the bacteria and the nanoparticles may have contributed to this outcome. Specifically, the passive transport of nanoparticles to infection sites can be influenced by factors such as hydrophobicity, Van der Waals forces, and electrostatic attraction. Passive transport may be enhanced when there is an interaction between the negatively charged bacterial outer membrane and positively charged particles [
47]. Nevertheless, in this case, NCBRPE exhibited a negative surface charge, similar to the external membranes of the tested bacteria, which likely resulted in repulsion between the nanoparticle and the bacterial membrane. Additionally, NCBRPE encounters steric hindrance due to the presence of the polymeric surfactant Poloxamer 407, which may further hinder interactions between nanoparticles and bacteria, complicating uptake. This barrier could help explain the reduced effectiveness of NCBRPE compared to BRPE against the tested bacterial species. Another important factor is the prolonged release of BRPE compounds from the nanoparticles, which may affect the biological impact in short-term evaluation assays [
48].
However,
B. cepacia possesses a unique lipopolysaccharide structure that decreases the negative charge of its cellular envelope, rendering it inherently resistant to cationic antimicrobials [
49]. This decrease in negative charge may have heightened the susceptibility of this bacterial species to NCBRPE, as it could have lessened the repulsion between the nanoparticles and the outer membrane of the bacteria. Similarly,
S. maltophilia has a cell membrane positively charged at physiological pH, and a similar effect on susceptibility to NCBRPE may have occurred for this species [
50].
4.5. Biofilm Inhibitory Concentration for B. cepacia
According to the MIC results,
B. cepacia ATCC
® 25416™ was selected to continue the experiments due to the superior performance of NCBRPE compared to other bacteria studied and its high prevalence in the ovarian cancer microenvironment.
B. cepacia can form biofilms not only on abiotic surfaces like glass and plastic but also on biotic surfaces, such as epithelial cells. This bacterium is known for causing invasive infections by colonizing epithelial cells and macrophages [
51], leading to severe inflammation, which emphasizes its significant role in the tumor development environment. Following confirmation of biofilm formation, the BIC assay was performed.
The BIC for NCBRPE matched its MIC result, indicating consistent efficacy across both tests. In contrast, BRPE required a concentration twice as high for BIC compared to its MIC. The consistent concentration of NCBRPE required to inhibit bacterial growth in both MIC and BIC tests may be attributed to the charges of the biofilm matrix components. Biofilm matrix charges vary, ranging between negative and positive values depending on pH levels. At lower pH, positive charges in the biofilm matrix are commonly observed [
52]. Given the 72 h incubation period established for biofilm formation, metabolic by-products from bacteria can acidify the medium, potentially resulting in a positively charged biofilm matrix. This environment may facilitate the dispersion of NCBRPE within the matrix, thereby explaining why its BIC remains unchanged relative to its MIC for this formulation. Furthermore, due to its nanoparticle size, NCBRPE can typically penetrate the biofilm more effectively [
53].
The increase in BIC for BRPE compared to MIC is typical because the bacteria within biofilms exhibit greater resistance to antimicrobial agents. This resistance arises from challenges in dispersing these agents within the polymeric matrix of the biofilm, as well as reduced bacterial metabolism [
54]. However, there is currently no standardized establishment of antimicrobial sensitivity/resistance thresholds specifically for biofilms [
51].
4.6. Ovarian Cancer and B. cepacia Biofilm
Building on the understanding of antimicrobial resistance within biofilms, the discussion further integrates the cytotoxicity effects observed in a 3D ovarian cancer cell model (OVCAR-3), expanding the analysis by connecting our previous findings with the BIC results obtained in the present study.
Considering that in vivo, the interaction between tumor cells and microenvironment bacteria occurs in a three-dimensional manner, the further discussion focuses on the cytotoxicity results in the 3D model of ovarian cancer cells (OVCAR-3) from previous studies, alongside the results obtained for BIC in the current study. The IC50 of NCBRPE in the OVCAR-3 3D model was 137.2 µg/mL [
12], which was slightly higher than the BIC for
B. cepacia (128 µg/mL). Thus, NCBRPE demonstrates efficacy in targeting both ovarian cancer cells and bacteria from the tumor microenvironment. Therefore, an additional perspective on utilizing the NCBRPE formulation could involve its application as a preventive and/or adjuvant treatment for ovarian cancer.
4.7. Clonogenic Assay
This study also examined the impact of BRPE and NCBRPE on the long-term proliferative potential of ovarian cancer cells (OVCAR-3) using the clonogenic assay, which measures the capacity of cells to retain proliferative potential after cytotoxic treatment [
23,
55]. While residual tumor cells may persist after therapy, only a fraction retain the potential for multiplication. In ovarian cancer, this subset can include stem-cell-like properties that support self-renewal and possible tumor recurrence [
56]. The clonogenic assay results showed that both BRPE and NCBRPE effectively reduced the long-term proliferative capacity of OVCAR-3 ovarian cancer cells.
The difference in cell survival fractions was statistically significant (* p < 0.05), with NCBRPE showing greater effectiveness than BRPE in reducing colony-forming ability. The nanoparticle NCBRPE was shown to be twice as effective as BRPE in inhibiting the reproductive potential of cells, which positions NCBRPE as a promising agent for limiting tumor growth and recurrence by effectively targeting cancer cell proliferation.
Other studies have demonstrated similar results, with BRPE and propolis from stingless bees (
Scaptotrigona sp.) tested on Hep-2 laryngeal tumor cells and U251, U343, and MCR-5 glioblastoma cells, respectively, showing a consistent decrease in colony-forming capacity across these cancer cell lines [
57,
58].
4.8. Flow Cytometry Assay: Type of Cell Death Induced by BRPE and NCBRPE
To further investigate the mechanisms of cell death induced by BRPE and NCBRPE, flow cytometry was employed, elucidating the pathways through their impact on cancer cell viability. Cell death occurs in various forms, distinguished by the type of stimulus, molecular mechanisms, and morphological changes. The main categories are apoptosis, autophagy, and necrosis, and cell death can be classified as either programmed or non-programmed [
59].
The flow cytometry analysis revealed that both BRPE and NCBRPE primarily induce cell death through apoptosis rather than necrosis. This apoptotic pathway suggests a controlled mechanism of cell elimination that avoids the inflammatory responses typically associated with necrosis. Apoptosis is generally advantageous in therapeutic settings because it prevents the release of intracellular contents, thereby reducing inflammation and limiting a microenvironment conducive to tumor progression [
57]. These findings highlight the ability of NCBRPE to effectively target cancer cells while potentially mitigating inflammatory responses within the ovarian cancer microenvironment.
Necrosis, in contrast, is a less efficient form of cell death that often leaves behind cells with damaged genomes capable of further proliferation. This process stimulates inflammation, creating a microenvironment conducive to tumor growth. Necrosis is an unprogrammed, inflammatory form of cell death triggered by factors such as infection, toxins, injury, or environmental stresses like high pressure and oxygen deprivation. It is characterized by cytoplasmic swelling, plasma membrane rupture, organelle degradation, and random DNA fragmentation, which collectively recruit immune cells and promote inflammation [
60,
61,
62].
Previous studies [
63,
64] have also demonstrated the ability of BRPE to induce cell death through apoptosis. The first study investigated bladder cancer cells of the 5637 lineage, while the second focused on breast cancer cells MCF-7. These findings underscore the consistent effects of BRPE across different cancer types and highlight its therapeutic potential.
4.9. Erythrocyte Sedimentation Rate Assay
The impacts of BRPE and NCBRPE on the ESR and reactive oxygen species (ROS) generation were evaluated, providing a clearer understanding of their influence in controlling the inflammation within the tumor microenvironment.
The ESR assay results provide insights into the inflammatory potential of BRPE and NCBRPE in the blood environment. The ESR test can reflect the pro-inflammatory nature of nanoparticles through interactions with blood that may induce the release of pro-inflammatory cytokines, resulting in alterations to blood protein profiles. Elevated ESR levels often indicate the onset of an inflammatory response, as inflammation typically increases acute-phase proteins like fibrinogen, leading to higher blood viscosity and accelerated erythrocyte sedimentation [
65]. In this study, neither BRPE nor NCBRPE significantly impacted the ESR, as most values remained within the reference range, suggesting these formulations do not exert pro-inflammatory effects.
Erythrocyte sedimentation is influenced by factors such as plasma’s ability to reduce erythrocyte surface charge, which leads to agglutination. This process depends on cellular characteristics, zeta potential, plasma viscosity, and the presence of bridging macromolecules [
60,
66]. Despite their sizes being compatible with macromolecules, NC and NCBRPE did not notably impact the proximity of red blood cells, as indicated by the stable ESR values. Their low negative charges likely minimized changes to the overall zeta potential, maintaining repulsive forces between red blood cells and preventing agglomeration. Another study found that polymeric nanoparticles do not significantly alter erythrocyte interactions, thereby maintaining an environment similar to blood plasma, with preserved erythrocyte distribution and aggregate size [
67].
The significant variability in ESR results suggests that responses to treatment are diverse, reflecting the complexity of the phenomenon under study, and highlights the need for more comprehensive investigations [
68]. Nevertheless, the ESR test, as an indirect inflammation marker, supports the non-pro-inflammatory nature of BRPE and NCBRPE, particularly relevant given that chronic inflammation induced by bacterial infections is a recognized factor in ovarian cancer progression. Antioxidants, such as those in BRPE, have been associated with reduced ESR values, indicating a decrease in inflammatory proteins within the blood. Thus, the lower ESR observed with NCBRPE treatment supports the hypothesis that antioxidants may mitigate chronic inflammation associated with ovarian cancer development, reinforcing their potential role in managing inflammation and reducing the risk of inflammation-driven tumor progression.
4.10. Oxidative Burst in Neutrophils and DPPH Assays
The analysis of oxidative burst in neutrophils was conducted to assess the oxidative-stress-modulating effects of NC and NCBRPE. The assay results indicate that NCBRPE effectively reduces ROS production in neutrophils, especially when compared to the control groups treated with either PMA alone or NC. This effect was observed both in spontaneous ROS production and in PMA-stimulated neutrophils. The reduction in ROS levels by NCBRPE highlights its antioxidant properties, which are attributed to the bioactive compounds in BRPE. This reduction in oxidative stress is particularly relevant for applications in the ovarian cancer microenvironment, where elevated ROS can contribute to tumor progression and tissue damage.
In ovarian cancer, the disease often progresses into a chronic state characterized by both immune activation and suppression, with neutrophils playing an important role in this dynamic. Within the tumor microenvironment, neutrophils interact with cancer and immune cells, contributing to cancer progression by generating ROS at inflammation sites, which can lead to endothelial dysfunction and tissue damage [
68,
69]. Elevated ROS levels are integral to carcinogenesis, as they participate in initiation and promotion processes, activate proto-oncogenes, and inactivate tumor suppressor genes, thereby destabilizing cellular function [
70]. Studies in human ovarian cancer models have shown that neutrophils release ROS to suppress T-cell proliferation, while mitochondrial DNA from necrotic cells further activates neutrophils, compounding oxidative stress and immunosuppression within the tumor environment [
71,
72]. The presence of oxidative stress markers in the serum of ovarian cancer patients supports these findings, underlining the role of ROS in disease progression [
73].
In general, the physicochemical properties of nanoparticles, while valuable in medical applications, may pose toxicological risks due to their potential to generate excessive ROS levels, leading to cellular damage, mutagenesis, and tissue injury [
74]. However, the current study shows that NCBRPE reduces ROS production in neutrophils, demonstrating its antioxidant potential and ability to modulate oxidative stress. This effect is particularly beneficial in the context of ovarian cancer, where decreasing ROS levels may alleviate tissue damage and ROS-mediated immunosuppression of T cells, potentially preventing bacterial infections and promoting a healthier microenvironment.
The literature further supports BRPE’s role in controlling oxidative stress, with reports demonstrating reduced ROS production in neutrophils following BRPE administration, including in vivo evidence. This antioxidant effect is attributed to the phenolic compounds, particularly isoflavonoids, abundant in BRPE, which help protect cells from damage induced by free radicals implicated in chronic diseases like cancer and inflammation [
75,
76,
77,
78]. The antioxidant activity of BRPE, associated with its flavonoid and phenolic compounds, functions by neutralizing free radicals generated during inflammation. These compounds are capable of donating electrons to free radicals, chelating metal ions that catalyze oxidation reactions, activating antioxidant enzymes, and inhibiting oxidases, thus playing a significant role in preventing DNA damage and carcinogenesis [
78,
79]. The antioxidant potential of BRPE was demonstrated through the DPPH assay, which also revealed that NCBRPE preserved most of the extract’s antioxidant capacity.
The findings of this study, coupled with existing literature, underscore the potential of BRPE and NCBRPE as therapeutic agents in managing oxidative stress and reducing ROS levels, which may have critical implications for controlling inflammation and limiting ovarian cancer progression.
5. Conclusions
The comprehensive findings of this study highlight the therapeutic potential of BRPE and its nanoencapsulated form. Both BRPE and NCBRPE demonstrated significant antimicrobial efficacy against Burkholderia cepacia, a prevalent bacterium associated with ovarian cancer, with NCBRPE showing enhanced and consistent activity against biofilm formation. This capability is crucial, as biofilms contribute to persistent infections and inflammation, potentially accelerating tumor progression.
The clonogenic assay further demonstrated that NCBRPE effectively limits the proliferation of cancer cells with long-term survival capacity, surpassing the efficacy of BRPE. This reduction in cell survival underscores NCBRPE’s potential to target cancer cells that contribute to tumor recurrence and growth.
Flow cytometry analysis revealed that both BRPE and NCBRPE primarily induce apoptosis—a form of programmed cell death that minimizes inflammation compared to necrosis. This finding highlights their therapeutic advantage in promoting controlled, non-inflammatory cell death, which is particularly beneficial for reducing tumor-supportive inflammation.
Additionally, the ESR assay results confirmed the non-pro-inflammatory nature of both BRPE and NCBRPE, with no significant ESR elevation observed, supporting their anti-inflammatory potential. The antioxidant properties of NCBRPE were further demonstrated by its ability to reduce ROS generation in neutrophils, thereby mitigating oxidative stress that is commonly associated with tumorigenesis and immune suppression.
In summary, the combined antibacterial, antioxidant, anti-inflammatory, and pro-apoptotic properties of NCBRPE position it as a promising candidate for preventive or adjunctive therapy in ovarian cancer treatment. By addressing both the cancer cells and the associated inflammatory and oxidative conditions of the tumor microenvironment, NCBRPE presents a comprehensive strategy for managing ovarian cancer and its progression.