A Comparative Study of Cyanide and Thiosulfate for Silver Leaching from Tailings: A Kinetics Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Materials
2.2. The Experimental Equipment
- An X-ray diffractometer (XRD PHILIPS X’PERT, Eindhoven, The Netherland) for mineralogical characterization of the samples; and the indexing for the obtained diffractograms was carried out with the MATCH version 1.1 software (developed by Crystal Impact, Bonn, Germany).
- An atomic absorption spectrometer (AAS- Perkin Elmer Model 2100, Waltham, MA, USA) for elemental quantification of the samples;
- A 1000 mL glass reactor with a flat bottom, mounted onto a heating plate with magnetic stirring;
- A thermometer to control the system temperature;
- An analytical digital balance (OHAUS Analytical Plus AP10S, Parsippany, NJ, USA) with a precision of 0.001 g (1 mg) for weighing the samples and reagents.
2.3. The Experimental Methods
2.4. Monitoring and Analysis
3. Results and Discussion
3.1. Characterization of the Material
3.2. Kinetic Studies of Cyanidation in an Alkaline Medium
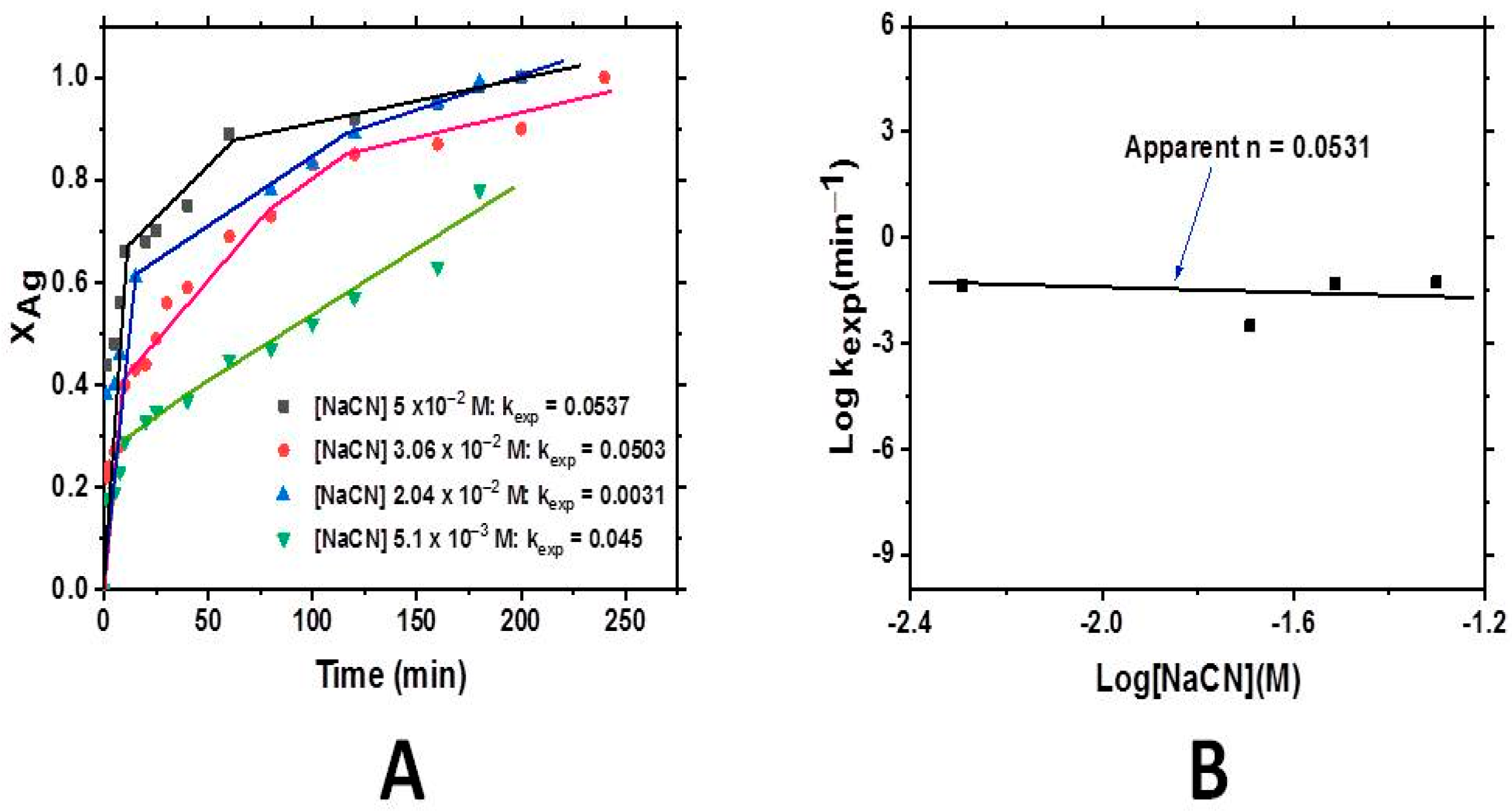
3.2.1. The Effect of Cyanide Concentration
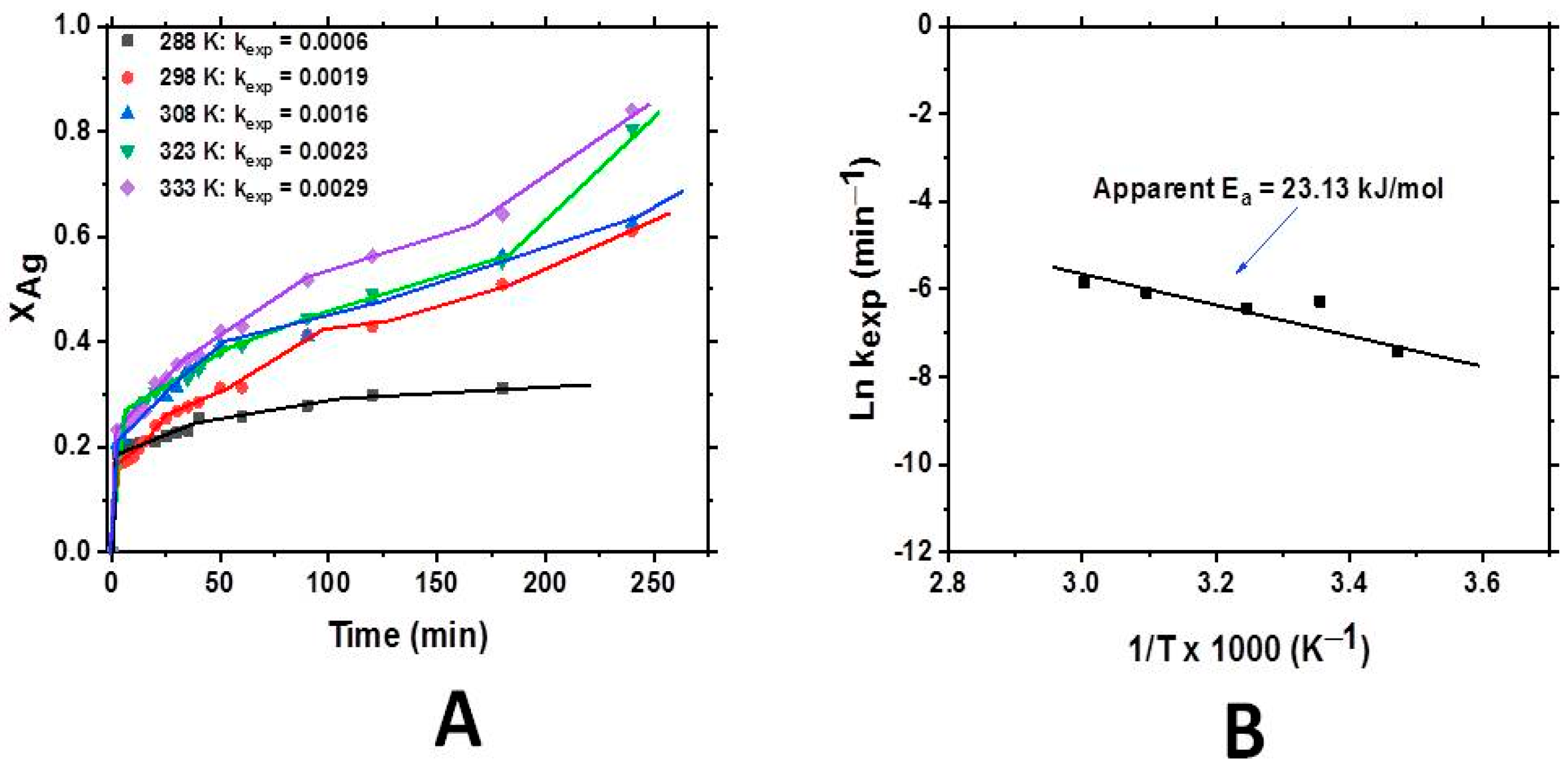
3.2.2. The Effect of Temperature on Cynide Leaching
3.2.3. The Effect of OH− Concentration
3.3. Kinetic Studies of Alkaline Leaching in a Thiosulfate Medium
3.3.1. The Effect of Thiosulfate Concentration
3.3.2. The Effect of Temperature on Thiosulfate Leaching
3.3.3. The Effect of pH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juan, H.A.; Eleazar, S.R.; Francisco, P.C.; Isauro, R.L.; Martín, R.P.; Miguel, P.L.; César, J.T.; Eduardo, C.S. Characterization of Burrows from Mining District of Pachuca–Real Del Monte, in Hidalgo State and Viability Study to Use These Residues as Alternate Industrial Material. In EPD Congress; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 73–80. [Google Scholar]
- Salinas, R.E.; Patiño, C.F.; Hernández, A.J.; Hernández, C.L.E.; Rivera, L.I. La Problemática de los Jales en el Estado de Hidalgo. In Memoria del IV Congreso Nacional de Metalurgia y Materiales; Secretaría de Educación Pública: Mexico City, Mexico, 2006; pp. 237–328. [Google Scholar]
- Hernandez, J.; Salinas, E.; Patino, F.; Rivera, I.; Flores, J.; Trapala, N.; MiguelPérez, L.; Mizraim, U.; Flores, G.; Ivan, A.; et al. Tile Production Using Wastes from Mining Industry of the Mining District Pachuca and Real Del Monte. In AIP Conference Proceedings; American Institute of Physics: Melville, NY, USA, 2012. [Google Scholar]
- de Espinosa, L. Estudio de Ingeniería Conceptual Para la Instalación de una Planta de Beneficio Con Capacidad Nominal de 12,500 ton/día; Compañia Real del Monte y Pachuca, S.A. de C.V.: Pachuca, Mexico, 1984; 154. [Google Scholar]
- Geyne, A.R.; Fries, C.; Segerstrom, K.; Black, R.F.; Wilson, J.F. Geology and Mineral Deposits of the Pachuca—Real del Monte District, State of Hidalgo; Consejo de Recursos Naturales no Renovables: Mexico City, Mexico, 1963. [Google Scholar]
- Hernandez, J.; Patino, F.; Rivera, I.; Reyes, I.A.; Flores, M.U.; Juarez, J.C.; Reyes, M. Leaching kinetics in cyanide media of Ag contained in the industrial mining-metallurgical wastes in the state of Hidalgo, Mexico. Int. J. Min. Sci. Technol. 2014, 24, 689–694. [Google Scholar] [CrossRef]
- Juárez, J.C.; Rivera, I.; Patiño, F.; Reyes, M.I. Efecto de la Temperatura y Concentración de Tiosulfatos sobre la Velocidad de Disolución de Plata contenida en Desechos Mineros usando Soluciones S2O32−-O2-Zn2+. Inf. Technol. 2012, 23, 133–138. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Veglio, F. Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Barani, K.; Dehghani, M.; Azadi, M.R.; Karrech, A. Leaching of a polymetal gold ore and reducing cyanide consumption using cyanide-glycine solutions. Min. Eng. 2021, 163, 106802. [Google Scholar] [CrossRef]
- Cerecedo-Sáenz, E.; Cardénas-Reyes, E.A.; Rojas-Calva, A.H.; Reyes-Valderrama, M.I.; Rodríguez-Lugo, V.; Toro, N.; Gálvez, E.; Acevedo-Sandoval, O.A.; Hernández-Ávila, J.; Salinas-Rodríguez, E. Use of the O2-Thiosemicarbzide System, for the Leaching of: Gold and Copper from WEEE & Silver Contained in Mining Wastes. Materials 2021, 14, 7329. [Google Scholar] [CrossRef]
- Larrabure, G.; Rodríguez-Reyes, J.C.F. A review on the negative impact of different elements during cyanidation of gold and silver from refractory ores and strategies to optimize the leaching process. Min. Eng. 2021, 173, 107194. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Breuer, P.L.; Choo, W.L. A kinetic study that compares the leaching of gold in the cyanide, thiosulfate and chloride systems. Metall. Mater. Trans. B 2002, 32B, 979–986. [Google Scholar] [CrossRef]
- Schmitz, P.A.; Duyvesteyn, S.; Johnson, W.P.; Enloe, L.; McMullen, J. Ammoniacal thiosulfate and sodium cyanide leaching of preg-robbing Goldstrike ore carbonaceous matter. Hydrometallurgy 2001, 60, 25–40. [Google Scholar] [CrossRef]
- Rivera, I.; Patiño, F.; Roca, A.; Cruells, M. Kinetics of silver leaching in the O2—Thiosulfte system. Hydrometallurgy 2015, 156, 63–70. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Wadsworth, M.E. Cinética de los Procesos de la Metalurgia Extractiva; Trillas: Mexico City, Mexico, 1986; pp. 141–148. [Google Scholar]
- de la Torre-Miranda, N.; Eloy, P.; de la Torre, E.; Steenhaut, T.; Polenius, C.; Herman, S. Activted carbon functionalized with amine sites as an efficient alternative for gold thiosulfate recovery. Mater. Chem. Phys. 2024, 312, 128657. [Google Scholar] [CrossRef]
- Rivera, I.; Roca, A.; Cruells, M.; Patiño, F.; Salinas, E. Study of silver precipitation in thiosulfate solutions using sodium dithionite. Application to an industrial effluent. Hydrometallurgy 2007, 89, 89–98. [Google Scholar] [CrossRef]
- Islas, H.; Flores, M.U.; Juárez, J.C.; Reyes, M.; Blanco, A.; Gutiérrez, E.J.; Aguilar, J.; Nolasco, M.C.; Rodríguez, I.; Reyes, I.A. Silver leaching from jarosite-type compounds using cyanide and non-cyanide lixiviants. A kinetic approach. Min. Eng. 2021, 174, 107250. [Google Scholar] [CrossRef]
- Li, X.; Han, P.-W.; Wang, Y.L.; Fu, G.-Y.; Zhi, S.; Ye, S.-F. Silver dissolution in a novel leaching system: Reaction kinetic study. Int. Miner. Metall. Mater. 2019, 26, 168. [Google Scholar] [CrossRef]
- Chen, J.; Xie, F.; Wang, W.; Fu, Y.; Wang, J. Leaching of Gold and Silver from a Complex Sulfide Concentrate in Copper-Tartrate-Thiosulfate Solutions. Metals 2022, 12, 1152. [Google Scholar] [CrossRef]
- Banijamali, S.H.; Raygan, S.; Amadeh, A.A. Study of silver extraction from Ag2S containing concentrate in the presence of copper sulfate, sodium thiosulfate, sodium metabisulfite, and ascorbic acid. Min. Eng. 2022, 183, 107607. [Google Scholar] [CrossRef]
- Konadu, K.T.; Sasaki, K. Sulfidic gold ore leaching by cysteine in the presence of Na2SO3. Hydrometallurgy 2023, 216, 106018. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Cao, H.; Wu, J.; Wang, F.; Wang, Y. The Crack Propagation Behaviour of CO2 Fracturing Fluid in Unconventional Low Permeability Reservoirs: Factor Analysis and Mechanism Revelation. Processes 2024, 13, 159. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wu, J.; Li, X.; Li, H.; Cheng, Y. Wellhead Stability During Development Process of Hydrate Reservoir in the Northern South China Sea: Evolution and Mechanism. Processes 2025, 13, 40. [Google Scholar] [CrossRef]
- Juárez-Tapia, J.C.; Patiño-Cardona, F.; Roca-Vallmajor, A.; Teja-Ruíz, A.M.; Reyes-Dominguez, I.A.; Reyes-Pérez, M.; Pérez-Labra, M.; Flores-Guerrero, M.U. Determination of Dissolution Rates of Ag Contained in Metallurgical and Mining Residues in the S2O32−-O2-Cu2+ system. Minerals 2018, 8, 309. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, E.; Hernández-Ávila, J.; Rivera-Landero, I.; Cerecedo-Sáenz, E.; Reyes-Valderrama, M.I.; Correa-Cruz, M.; Rubio-Mihi, D. Leaching of silver contained in mining tailings, using sodium thiosulfate. A kinetic study. Hydrometallurgy 2016, 160, 6–11. [Google Scholar] [CrossRef]
- Manzila, A.N.; Moyo, T.; Petersen, J. A Study on the Applicability of Agitated Cyanide Leaching and Thiosulfate Leaching of Gold Extraction in Artisanal and Small-Scale Gold Mining. Minerals 2022, 12, 1291. [Google Scholar] [CrossRef]
- Roldán-Contreras, E.; Salinas-Rodríguez, E.; Hernàndez-Ávila, J.; Cerecedo-Sáenz, E.; Rodríguez-Lugo, V.; Jeldres, R.I.; Toro, N. Leaching of Silver and Gold Contained in a Sedimentary Ore, Using Sodium Thiosulfate; A Preliminary Study. Metals 2020, 10, 159. [Google Scholar] [CrossRef]
- Turan, M.D.; Assemi, S.; Nadirov, R.K.; Karamyrzayev, G.A.; Baigenzhenov, O.; Toro, N. Selective Hydrocloric Acid Leaching of Zinc, Lead, and Silver from Mechanically Activated Zinc Plant Residues. J. Non-Ferr. Met. 2022, 63, 490–499. [Google Scholar] [CrossRef]
- Li, H.; Ma, A.; Srinivasakannan, C.; Zhang, L.; Li, S.; Yin, S. Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process. J. Alloys Compd. 2018, 763, 241–249. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Srinivasakannan, C.; Zhang, L.; Yin, S.; Yang, K.; Xie, H. Efficient cleaning extraction of silver from spent symiosis lead-zinc mine assisted by ultrasound in sodium thiosulfate system. Ultrason. Sonochem. 2018, 49, 118–127. [Google Scholar] [CrossRef]
- Kasaini, H.; Kasongo, K.; Naude, N.; Katabua, J. Enhanced leacheability of gold and silver in cyanide media: Effect of alkaline pre-treatment of jarosite minerals. Min. Eng. 2008, 21, 1075–1082. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Q.; Wang, Y.; Long, T.; Deng, S. Effects of pyrite, quartz and sodium sulfite on roasting of a refractory sulfide concentrate and gold, silver, copper leaching during cyanidation. Hydrometallurgy 2024, 226, 106306. [Google Scholar] [CrossRef]
- Yoğurtcuoğlu, E.; Alp, İ. Effect of Na2S + NaOH pre-identification leaching on oxidized refractory Au-Ag ores. J. Eng. Sci. 2024, 13, 440–447. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, G.; Kang, J.; Wang, C. Comprehensive recovery and recycle of jarosite residues from zinc hydrometallurgy. Chem. Eng. J. Adv. 2020, 3, 100023. [Google Scholar] [CrossRef]
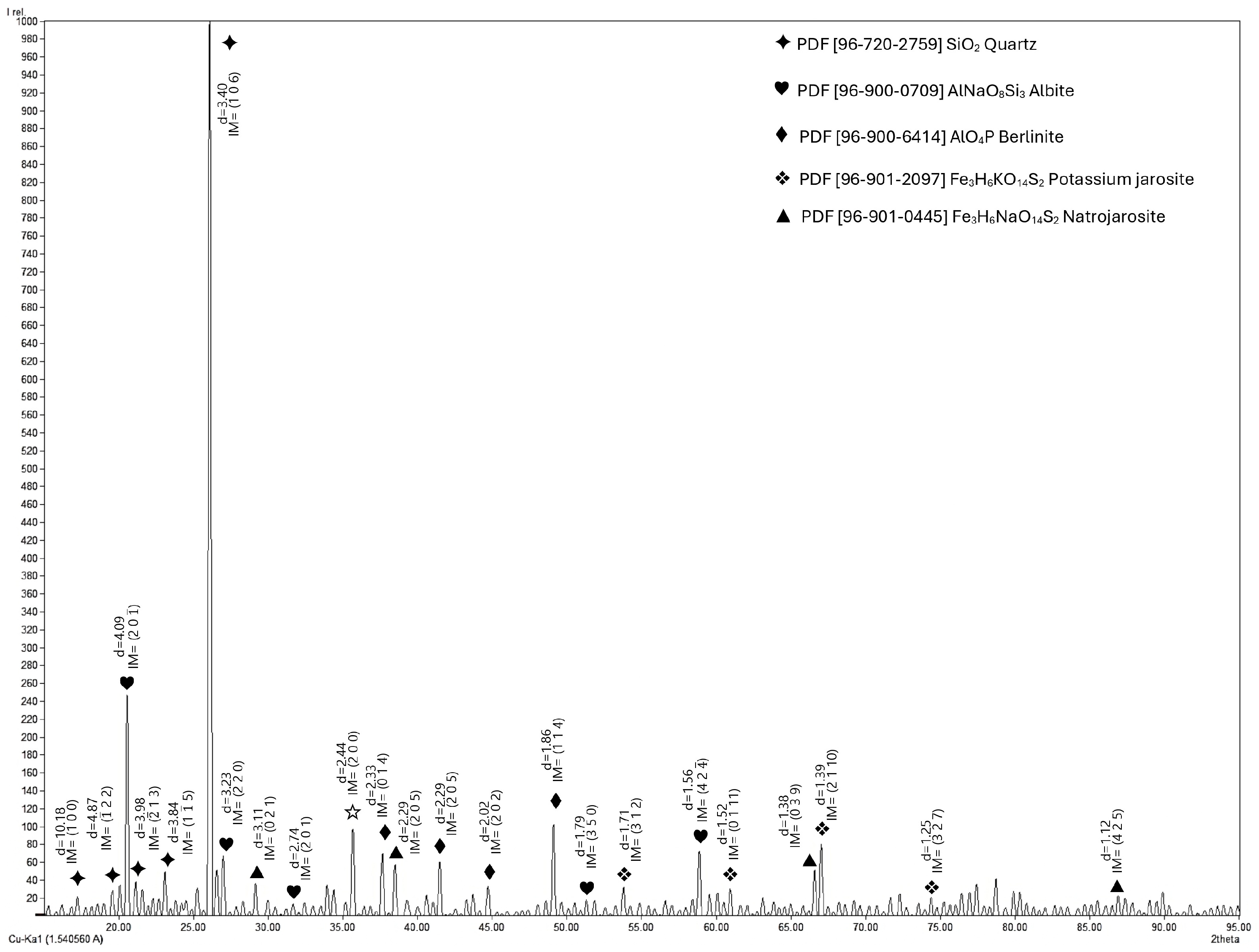





| Element | wt. % | Element | wt. % |
|---|---|---|---|
| Si | 29.32 | Ag | 83 1 |
| Al | 3.41 | Au | 0.28 1 |
| Ca | 2.69 | S | 0.38 |
| Fe | 2.41 | Ti | 0.16 |
| K | 2.40 | P | 0.031 |
| Mn | 1.21 | Zn | 0.036 |
| Mg | 0.70 | Pb | 0.031 |
| Na | 0.76 | Cu | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ávila, J.; Salinas-Maldonado, R.G.; García-Cerón, A.; Flores-Badillo, J.; Cerecedo-Sáenz, E.; Toro, N.; Saldana, M.; Gálvez, E.; Gutiérrez-Amador, M.P.; Salinas-Rodríguez, E. A Comparative Study of Cyanide and Thiosulfate for Silver Leaching from Tailings: A Kinetics Approach. Processes 2025, 13, 1522. https://doi.org/10.3390/pr13051522
Hernández-Ávila J, Salinas-Maldonado RG, García-Cerón A, Flores-Badillo J, Cerecedo-Sáenz E, Toro N, Saldana M, Gálvez E, Gutiérrez-Amador MP, Salinas-Rodríguez E. A Comparative Study of Cyanide and Thiosulfate for Silver Leaching from Tailings: A Kinetics Approach. Processes. 2025; 13(5):1522. https://doi.org/10.3390/pr13051522
Chicago/Turabian StyleHernández-Ávila, Juan, Ramón G. Salinas-Maldonado, Alondra García-Cerón, Javier Flores-Badillo, Eduardo Cerecedo-Sáenz, Norman Toro, Manuel Saldana, Edelmira Gálvez, M. P. Gutiérrez-Amador, and Eleazar Salinas-Rodríguez. 2025. "A Comparative Study of Cyanide and Thiosulfate for Silver Leaching from Tailings: A Kinetics Approach" Processes 13, no. 5: 1522. https://doi.org/10.3390/pr13051522
APA StyleHernández-Ávila, J., Salinas-Maldonado, R. G., García-Cerón, A., Flores-Badillo, J., Cerecedo-Sáenz, E., Toro, N., Saldana, M., Gálvez, E., Gutiérrez-Amador, M. P., & Salinas-Rodríguez, E. (2025). A Comparative Study of Cyanide and Thiosulfate for Silver Leaching from Tailings: A Kinetics Approach. Processes, 13(5), 1522. https://doi.org/10.3390/pr13051522









