Abstract
This study investigates the potential of biochar derived from agricultural residues—corn cob and wheat straw—for removing polycyclic aromatic hydrocarbons (PAHs) from aqueous systems. Biochars were produced via pyrolysis at 700 °C and characterized using BET, SEM, EDS, FTIR, and pXRD to evaluate physicochemical properties. Adsorption experiments with naphthalene, fluorene, fluoranthene, and pyrene revealed high adsorption affinities (Log Kd = 4.35–5.69 L/kg), with Freundlich isotherm modeling indicating nonlinear behavior (n = 0.732–0.923), suggesting a combination of pore filling and chemical interactions such as π-π stacking and hydrogen bonding. Corn-cob biochar, rich in lignin, exhibited a higher surface area (111 m2/g) and greater affinity for fluorene, while wheat-straw biochar, with a higher oxygen content and more functional groups, performed better for naphthalene and pyrene. FTIR and pXRD confirmed aromatic and graphitic structures facilitating PAH interactions. These results underscore the importance of feedstock selection and pyrolysis conditions in tailoring biochar properties for specific pollutants. While both biochars compare favorably with conventional adsorbents like activated carbon, further research on long-term stability in complex matrices is needed. Overall, the findings support the development of cost-effective, scalable, and eco-friendly biochar-based technologies for water remediation.
1. Introduction
The increasing levels of environmental pollution caused by industrialization and agricultural activities have led to severe contamination of water bodies by hazardous organic pollutants [1]. Among these pollutants, polycyclic aromatic hydrocarbons (PAHs) present substantial environmental and health hazards because of their long-lasting nature, bioaccumulative nature, and toxic effects. PAHs such as naphthalene, fluorene, fluoranthene, and pyrene are widely found in aquatic ecosystems due to industrial discharges, fossil fuel combustion, and agricultural runoff. Their hydrophobic nature allows them to accumulate in sediments, making their removal from water sources a critical environmental challenge [2,3,4].
One promising approach for the remediation of PAH-contaminated water is the use of biochar derived from agricultural biomass [5]. Biochar, a carbon-rich material produced through the pyrolysis of biomass under oxygen-limited conditions, has gained attention for its high adsorption capacity, high porosity, and high number of surface functional groups that enable the sequestration of organic contaminants [6,7]. Corn residues, including corn stalks and straw, represent abundant and underutilized agricultural byproducts that can be converted into biochar for environmental applications [8]. However, despite biochar’s potential, several research gaps remain that hinder its widespread application. These include the need for field-scale studies, a deeper understanding of adsorption mechanisms in complex matrices, the standardization of production methods, and assessments of the long-term environmental impact and economic viability (Table 1). Addressing these gaps will be crucial for advancing biochar-based technologies in water remediation.

Table 1.
Summary of research gaps and corresponding insights.
The ability of biochar to remove PAHs from water is influenced by various factors, such as pyrolysis conditions, surface chemistry, and the mechanisms of adsorption [22]. Despite numerous studies on biochar production and application, significant gaps remain in understanding the interactions between biochar and PAHs in aqueous environments. Most previous research has focused on biochar derived from woody biomass [23,24], with limited exploration of agricultural residues such as corn stalks and straw. Additionally, there is a need to optimize pyrolysis parameters to enhance biochar’s adsorption capacity for PAHs and to investigate the long-term stability of adsorbed contaminants. In this study, adsorption experiments were conducted to evaluate the removal efficiency of PAHs using corn- and straw-based biochar. Freundlich isotherm modeling was applied to characterize the adsorption behavior, and the results were analyzed to assess the feasibility of biochar as a sustainable remediation material. Adsorption is a widely acknowledged and efficient method for eliminating PAHs from wastewater, utilizing various carbon-based adsorbents such as biochar, activated carbon, graphene, and carbon nanotubes [25].
The aim of this study is to address these research gaps by investigating the potential of biochar derived from corn and straw residues for the removal of PAHs from aquatic ecosystems. The specific objectives include (i) evaluating the adsorption efficiency of corn- and straw-based biochar for selected PAHs (naphthalene, fluorene, fluoranthene, and pyrene), (ii) applying Freundlich isotherm modeling to understand adsorption mechanisms, and (iii) identifying key factors influencing biochar’s performance in PAH removal. By providing insights into the adsorption characteristics of corn- and straw-derived biochar, this study contributes to the development of sustainable and cost-effective solutions for mitigating PAH pollution in water systems.
2. Materials and Methods
2.1. Feedstock and Biochar Preparation
Two types of biomasses—corn cob and wheat straw—were utilized as the feedstock for biochar (BC) production. The biomass was sourced from agricultural fields and forest areas in the Autonomous Province of Vojvodina, Republic of Serbia, immediately after harvest. The materials were oven-dried at 60 °C for 24 h before being placed in a Nabertherm furnace (Nabertherm, Germany). When oxygen was excluded from the furnace during the pyrolysis of raw materials, nitrogen gas was used to fill the furnace. The pyrolysis process involved sealing the furnace to prevent oxygen inflow, heating to 700 °C at a rate of 10 °C/min, and maintaining the temperature for approximately one hour. The resulting biochar was then allowed to cool naturally to room temperature. The final biochar samples were ground, sieved through a 2 mm sieve, stored in airtight zip-lock bags, and prepared for further analysis. The selected biomasses belong to the lignocellulosic category, with wheat straw and corn cob falling under non-woody waste biomass [26].
2.2. Biochar Characterization
The biochar was characterized using various techniques, including the Brunauer–Emmett–Teller (BET) method and CHNS elemental analysis, as described in detail by Beljin et al. [27]. Additionally, energy-dispersive X-ray spectroscopy (EDS), Fourier-transform infrared spectroscopy (FTIR), and powder X-ray diffraction (pXRD) were carried out for structural and compositional analysis.
- FTIR Analysis. Functional groups present on the biochar surface were identified through Fourier-transform infrared spectroscopy (FTIR) using a “Nicolet Nexus 670” (Thermo Fisher Scientific, Waltham, MA, USA). Samples were prepared by forming KBr-based tablets, and spectra were recorded within the 4000–400 cm−1 range using the diffuse reflection mode at a resolution of 4 cm−1.
- pXRD Analysis. The crystalline structure of the biochar was examined using a Rigaku Miniflex 2 X-ray diffractometer equipped with a Cu Kα radiation source. The pXRD patterns were recorded with a 4°/min resolution, covering a 2θ range of 4–80° (2θ = 10–80°).
- SEM and EDS Analysis. The surface morphology of the biochar samples was analyzed using a Thermo Fisher Scientific Apreo C scanning electron microscope (Waltham, MA, USA). Elemental composition was determined via the integrated energy-dispersive X-ray spectroscopy (EDS) system. The scanning electron microscope was operated at a 40 nA current and 20 kV acceleration voltage for optimal imaging and analysis.
2.3. Chemicals and Methods of Analysis
All PAHs examined in this study (naphthalene, fluorene, fluoranthene, and pyrene, each with a purity greater than 99%) were obtained from Sigma-Aldrich Chemical Company (St. Louis, MI, USA). All chemicals used in the adsorption experiment setup (calcium chloride, CaCl2; sodium azide, NaN3; methanol) were obtained from J.T. Baker. After the adsorption experiments, the supernatants were subjected to liquid–liquid extraction using hexane (J.T. Baker, certified for organic residue analysis) and subsequently analyzed by gas chromatography coupled with mass spectrometry (GC/MS, Agilent 7890A/5975C; Santa Clara, CA, USA). The GC/MS analysis was conducted using an HP-5MS column (J&W Scientific; Santa Clara, CA, USA), employing phenanthrene-d10 as an internal standard. Analytical conditions included pulsed splitless injection with a 50:1 split ratio, an inlet temperature of 300 °C, a column flow rate of 34.5 mL/min, an initial oven temperature of 55 °C held for 1 min, followed by a ramp of 25 °C/min to 300 °C, maintained for 3 min.
2.4. Adsorption Experiment Setup
Adsorption isotherms were performed in triplicate at room temperature (20 ± 2 °C) in 40 mL glass vials sealed with Teflon-lined silicon septa and covered with silver foil. The background solution comprised 0.01 M CaCl2 in deionized water with 100 mg/L NaN3 added as a biocide. Owing to the low solubility of the PAHs, stock solutions (1000 µg/mL) were prepared in methanol (J.T. Baker, for organic residue analysis) prior to spiking the background solution. Initial PAH concentrations ranged from 1 to 1000 µg/L, with the volume of methanol stock solution being kept below 0.1% (v/v), a proportion that was previously shown not to affect the adsorption behavior of hydrophobic organic contaminants. Adsorbent amounts varied between 1 and 50 mg, ensuring a sample-to-solution ratio that achieved 20–80% solute uptake. Headspace in the vials was minimized to reduce losses from evaporation.
The experimental procedure involved sonicating vials containing the background solution and adsorbent for 30 min, followed by spiking with a predetermined volume of PAH stock solution. The mixtures were then continuously shaken at room temperature for 3 h, based on preliminary kinetic studies conducted over 168 h. The adsorption experiments were conducted over 24 h, a duration selected based on preliminary tests indicating that equilibrium was reached within this period. In these preliminary studies (Beljin et al., 2024 [27]), the amount adsorbed plateaued between 20 and 24 h, suggesting that the adsorption equilibrium was approached. Although this is a commonly used contact time in batch adsorption studies, it does not imply rapid kinetics. We acknowledge that the adsorption equilibrium over 24 h represents moderate-to-slow kinetics, which may limit the practical application in high-throughput systems. Nonetheless, the 24 h duration was sufficient to assess the equilibrium behavior under controlled laboratory conditions.
After shaking, the vials were allowed to stand for 24 h before sampling the clear supernatant to determine the equilibrium concentration of PAHs. To determine the initial PAH concentrations and correct for non-adsorption-related losses, two control vials without the adsorbent were treated identically. The recovery rates of PAHs in these controls were within the range expected for the analytical methods used, indicating negligible losses due to volatilization or biodegradation. Solid-phase concentrations were calculated by mass balance between the aqueous and solid phases.
3. Results
3.1. Biochar Characterization
Table 2 summarizes the general characteristics of biochar’s prepared from different feedstock biomasses.

Table 2.
Characterization of the biochars.
Energy-dispersive X-ray spectroscopy (EDS) was employed to determine the elemental composition of the wheat-straw- and corn-cob-biochar samples. The results, expressed in weight percent (wt%), are summarized in Table 3. Carbon was the dominant component in both samples, as expected for biochar, with the wheat-straw biochar showing a slightly higher carbon content (69 wt%) compared to corn-cob biochar (63 wt%). The presence of light elements such as carbon and oxygen were noted, although it should be acknowledged that EDS has a limited sensitivity for low-Z elements, including C, O, and H. This limitation arises due to the low energy of their characteristic X-rays and possible absorption by the detector window. Nonetheless, these results provide a useful semi-quantitative overview of elemental distributions. The data have been rounded to one decimal place to reflect the typical accuracy limits of EDS (±0.1 wt.%). Spectra were collected from both selected points and broader areas of the surface to obtain representative elemental data. However, spatial distribution maps (elemental mapping) were not recorded or saved during these measurements. Further element composition was confirmed by CHNS analysis.

Table 3.
EDS results of investigated biochars.
The given results showed a slightly elevated oxygen content in wheat-straw biochar suggests a presence of oxygen-containing functional groups [28], which may influence the hydrophilicity and adsorption characteristics [29]. Meanwhile, the higher silicon content in corn-cob biochar implies a greater ash content, potentially affecting the biochar’s porosity and stability [30].
Specific surface area (BET analysis), pore analysis, scanning electron microscopy (SEM), and CHNS analysis. The characterization results of the tested biochars, including the BET specific surface area (SSA), elemental composition, and surface morphology, are summarized in Table 4 and Figure 1. The BET surface area of the materials ranged from 61 to 111 m2/g, indicating notable differences in porosity and surface development between the samples. Biochar derived from corn biomass exhibited the highest SSA (111 m2/g), suggesting a highly porous structure rich in surface sites favorable for adsorption processes. Such an extensive surface area enhances the biochar’s capacity to interact with contaminants, particularly when targeting pollutants that require a large number of binding sites for efficient removal. On the other hand, biochar derived from straw presented the lowest SSA (61 m2/g), implying fewer available sites for adsorption. This reduced surface area may limit its effectiveness in applications requiring intensive molecular interactions, especially for larger or more complex contaminants. The observed differences in SSA can be attributed to the structural transformations that occur during pyrolysis, where factors like feedstock composition and temperature influence the development of micro- and mesoporous networks.

Table 4.
Biochar characterization originating from biomass of corn and biomass of straw.
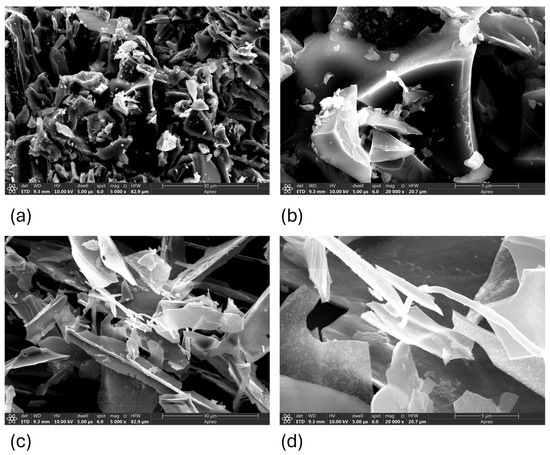
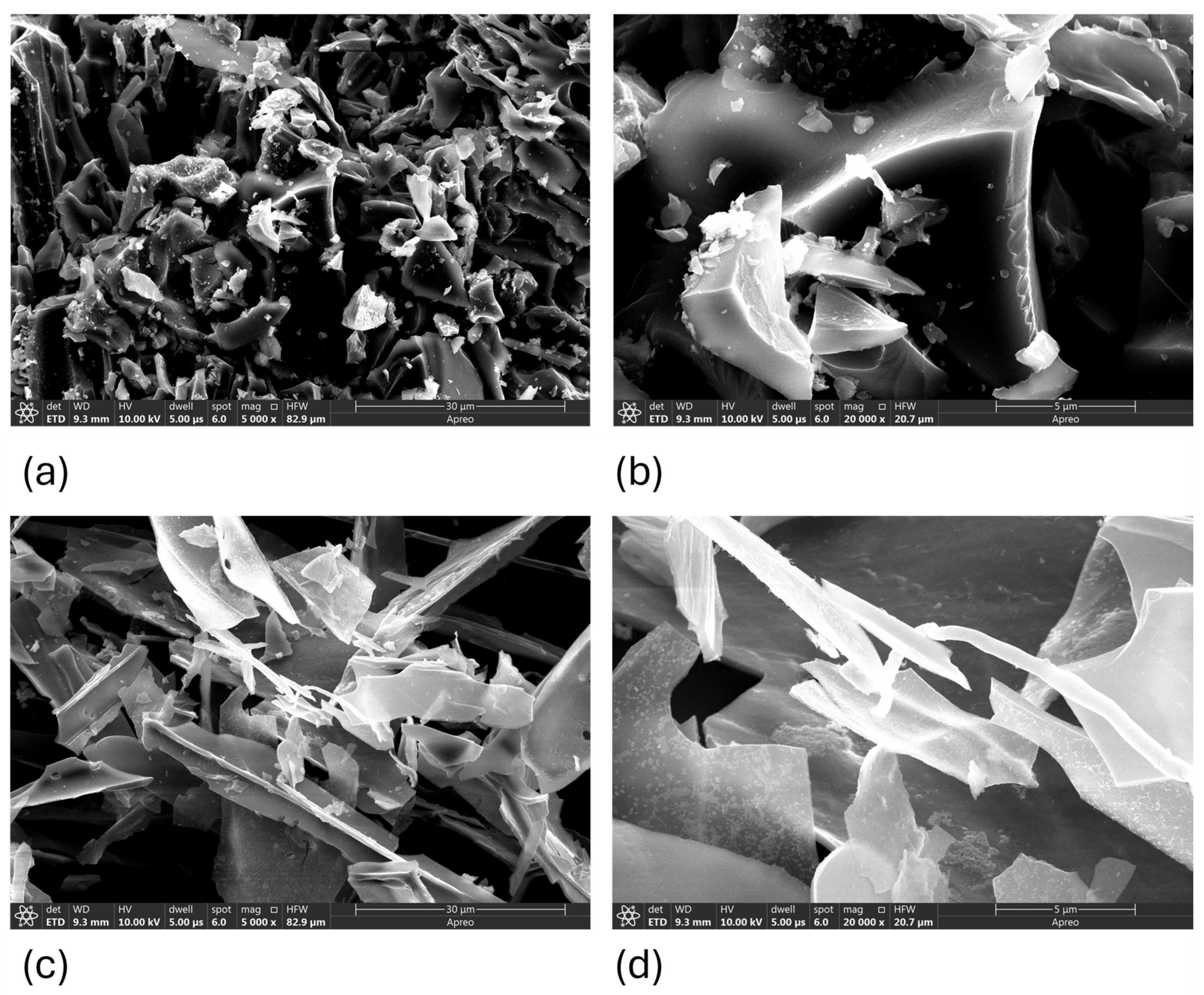
Figure 1.
SEM micrographs of biochar from corn (a,b) and straw (c,d).
Further insights into the internal structure are provided by pore analysis. Corn-derived biochar exhibited a micropore volume of 0.003 cm3/g and mesopore volume of 0.014 cm3/g, reflecting a well-developed porous network that supports the retention of both small and moderately sized molecules. Micropores, due to their large surface-to-volume ratio, are especially effective for trapping gases and small organic molecules, whereas mesopores are better suited for bulkier compounds. Although the straw biochar had a slightly larger average pore radius (28.5 Å), it showed a lower total pore volume (0.022 cm3/g), suggesting that while it may accommodate larger molecules more easily, its overall capacity for adsorbate retention is reduced in comparison to corn biochar (0.028 cm3/g).
The superior adsorption potential of corn biochar is thus linked not only to its larger specific surface area but also to its more favorable pore architecture. These characteristics make it particularly suitable for applications involving a range of contaminants, from small organics to bulkier pollutants such as dyes or pharmaceutical residues. In contrast, straw biochar, with its lower porosity and SSA, may be more appropriate for targeted or less-demanding sorption scenarios.
Elemental analysis further confirmed that carbon is the predominant element in both biochars, with concentrations ranging between 67% and 71%. This high carbon content is typical for thermally treated biomass and reflects the carbonization process that concentrates aromatic structures. Minor amounts of hydrogen, nitrogen, and sulfur were also detected, consistent with the expected decomposition of non-carbon elements during pyrolysis.
Previous studies have highlighted the importance of pyrolysis conditions—particularly temperature—in shaping the physicochemical characteristics of biochar. Research conducted by Zhang et al. [31] indicates that elevated pyrolysis temperatures, around 700 °C, promote the formation of microporous structures and enrich the biochar with oxygen-containing functional groups. These modifications are essential for improving the material’s adsorption capacity, making it more effective in capturing a broad spectrum of organic pollutants from aqueous environments.
Scanning electron microscopy (SEM) was used to investigate the surface morphology of biochars derived from corn cob and wheat straw (Figure 1a,b). Corn-cob biochar exhibited a rougher, more porous surface with visible micro- and mesopores, promoting greater surface availability for adsorption. Wheat-straw biochar presented a smoother, more compact structure, which may limit its adsorption capacity.
With increasing pyrolysis temperature, both materials exhibited significant degradation of their original fibrous plant structures. This led to the development of fractured surfaces with evident porosity and structural defects. Corn-cob-derived biochar (Figure 1a) revealed a more porous and coarse surface morphology, with well-developed cavities and a relatively uniform distribution of pore structures. In contrast, wheat-straw-derived biochar (Figure 1b) retained some fibrous characteristics and appeared less porous, with surface features that were smoother and more irregular. These morphological differences are relevant for interpreting the adsorption potential. SEM images indicate that the corn-derived biochar possesses a higher surface roughness and more accessible pore volume, consistent with the higher specific surface area (SSA) observed in the BET analysis. The presence of both macro- and mesoporous structures suggests a dual-mode adsorption mechanism—rapid surface uptake via macropores and slower intraparticle diffusion via mesopores. Additionally, SEM allows assessment of the sample homogeneity; corn-cob biochar appears more structurally uniform, while wheat-straw biochar shows localized surface variations. These characteristics have direct implications for potential applications in adsorption and catalysis.
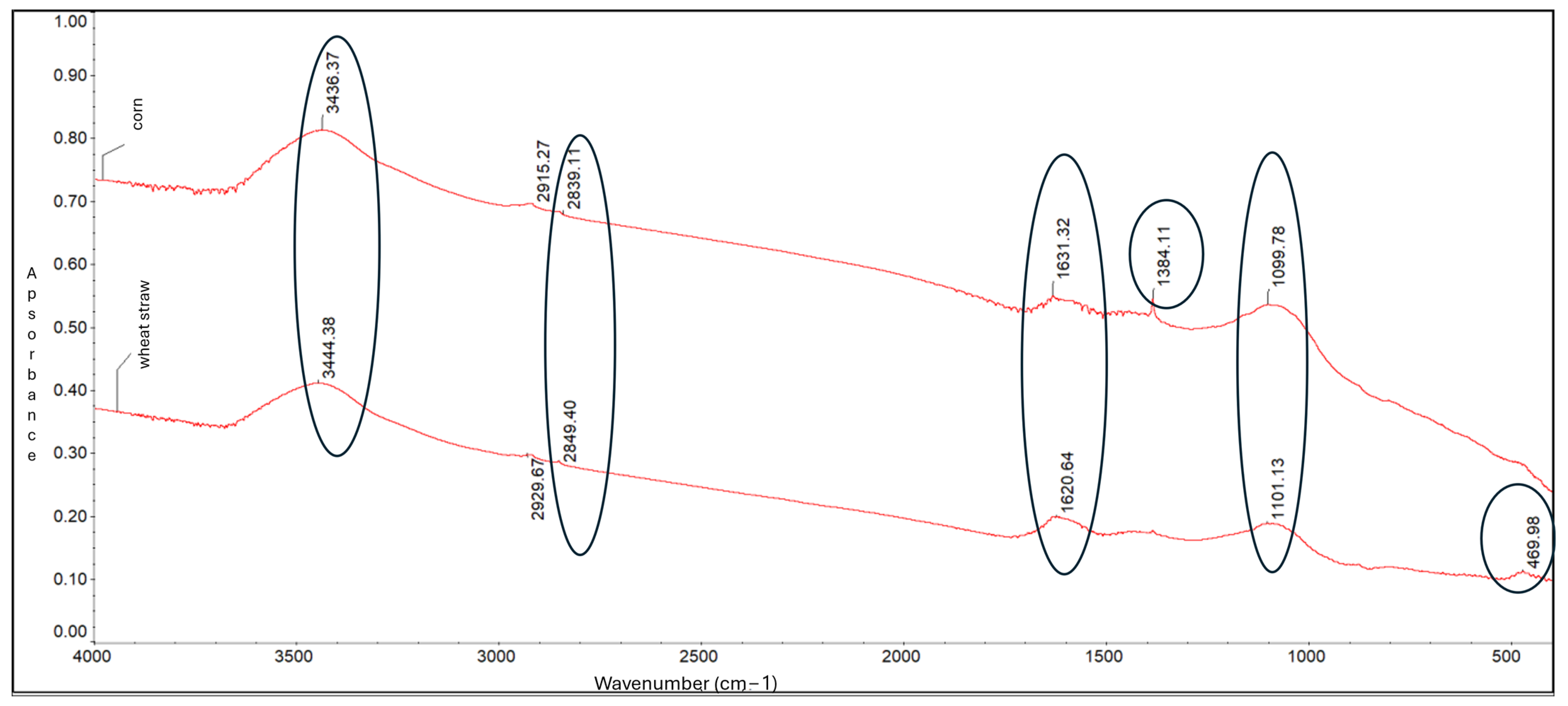
FTIR spectroscopy. FTIR analysis identified key functional groups present on the biochar surfaces (Figure 1). Both biochars displayed absorption bands at around 3436 cm−1, attributed to O-H stretching vibrations (hydroxyl and phenolic groups). Bands near 2839 cm−1 indicated methylene (-CH2-) groups, while peaks around 1631 cm−1 corresponded to C=C stretching vibrations, characteristic of aromatic structures (Figure 2).
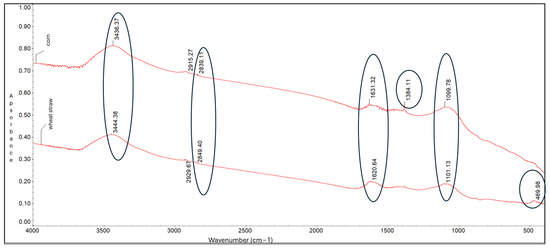
Figure 2.
FTIR spectra of biochar derived from corn-cob and wheat-straw biomass.
Additional bands at 1384 cm−1 and 1099 cm−1 were linked to C-H bending (alkanes/alkyl groups) and C-O-C stretching vibrations (polysaccharides), respectively. A weaker band around 469 cm−1, attributed to O-Si-O stretching, was more pronounced in corn-cob biochar—consistent with its higher silicon content.
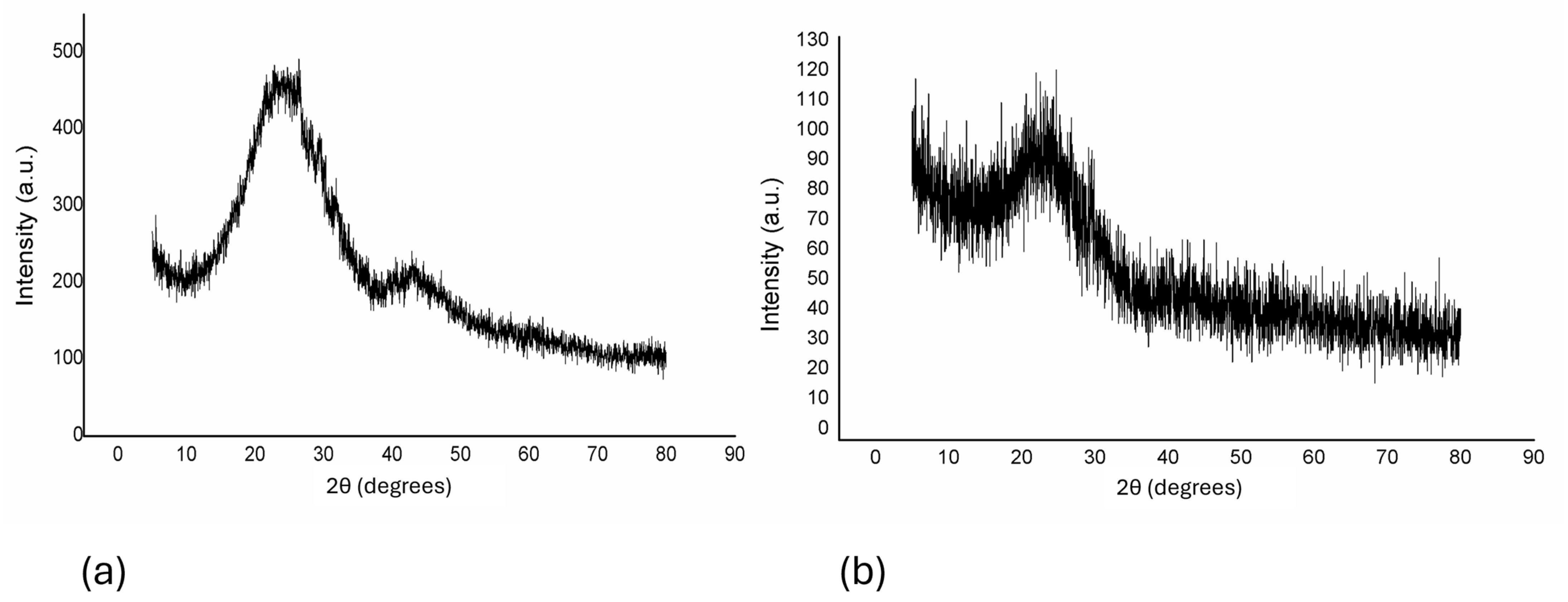
pXRD analysis. The pXRD patterns (Figure 3) indicated that both biochars possess aromatic and carbonized structures, with a prominent diffraction peak around 23° (2θ), characteristic of amorphous carbon (JCPDS card no. 75–1621) [32,33]. The biochars displayed highly graphitic and aromatic structures, reinforcing their potential for adsorption applications.
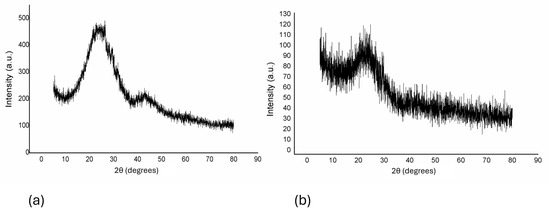
Figure 3.
pXRD diffractogram of materials derived from (a) corn-cob biomass and (b) wheat-straw biomass.
3.2. Adsorption Performance
Preliminary adsorption experiments, focusing on naphthalene, fluorene, fluoranthene, and pyrene, demonstrated that both biochars effectively adsorbed PAHs. The adsorption equilibrium was achieved within 24 h.
The adsorption affinities, expressed as LogKd for a concentration of Ce = 0.05 mg/L (solubility of the given compound in water), for all tested PAHs ranged from 4.35 to 5.69 L/kg. In general, no significant difference in adsorption between corn biochar and straw biochar was found (Table 5). However, some differences in the adsorption capacity and nonlinearity of adsorption isotherms for individual compounds were observed.

Table 5.
Parameters of the Freundlich model.
It is important to note that the Freundlich constant KF, while often referred to as an indicator of adsorption capacity, is not directly comparable across systems unless the exponent n is similar. Because KF is influenced by the value of 1/n, the adsorption capacity Kd was calculated at a fixed concentration Ce = 0.05 mg/L for proper comparison.
For naphthalene, the adsorption capacity was higher for straw biochar at (18,444 mg/g)/(mg/L)n compared to corn biochar at (10,163 mg/g)/(mg/L)n, while the nonlinearity of adsorption was similar, with slightly higher values for straw biochar (n = 0.819 compared to n = 0.732 for corn).
When it came to fluorene, corn biochar had a much higher adsorption capacity at (188,620 mg/g)/(mg/L)n compared to straw biochar at (77,296 mg/g)/(mg/L)n, although the nonlinearity of adsorption was slightly higher for corn biochar (n = 0.839 for corn and n = 0.789 for straw).
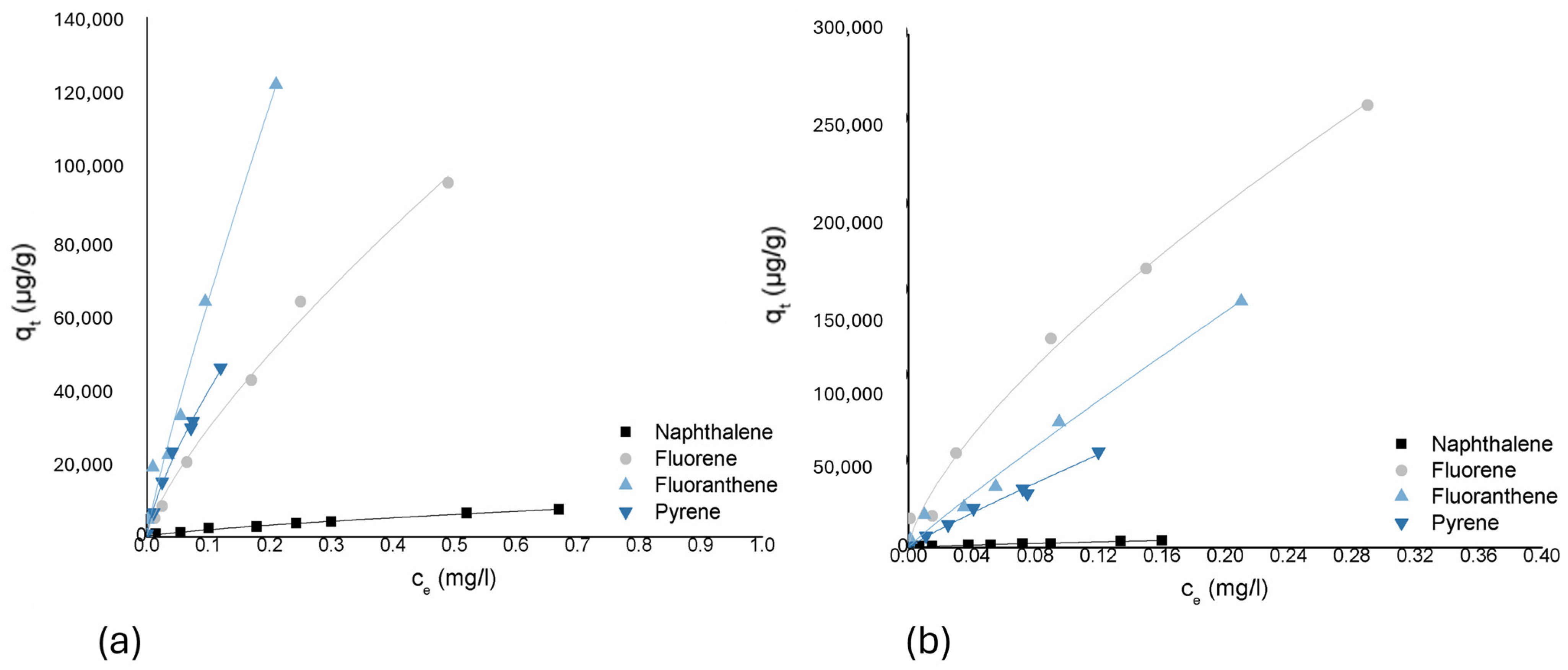
Fluoranthene and pyrene showed less variation between the two biochars. For fluoranthene, both corn biochar and straw biochar obtained high KF values, with the adsorption capacity being slightly higher for straw biochar at (660,116 mg/g)/(mg/L)n compared to corn at (552,874 mg/g)/(mg/L)n, but with very similar nonlinearity of the adsorption isotherms (n = 0.923 for straw and n = 0.897 for corn). For pyrene, straw biochar again showed a higher adsorption capacity at (293,398 mg/g)/(mg/L)n compared to corn at (254,947 mg/g)/(mg/L)n, but the differences in adsorption nonlinearity were less significant (Figure 4).
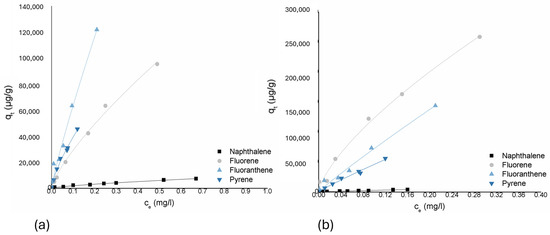
Figure 4.
Freundlich isotherms for compounds from the PAHs group and two biochars: (a) corn-cob based biochar and (b) wheat-straw based biochar.
Although both biochars showed high efficiency in the adsorption of PAHs, certain compounds showed minor differences in the adsorption capacity and nonlinearity, indicating that the physicochemical characteristics of the biochar, as well as its origin, may affect adsorption.
4. Discussion
The findings of this study highlight the efficacy of biochars derived from corn cob and wheat straw as adsorbents for removing chosen PAHs—naphthalene, fluorene, fluoranthene, and pyrene—from aqueous systems. Adsorption affinities, expressed as Log Kd at a concentration of 0.05 mg/L, ranged from 4.35 to 5.69 L/kg, indicating strong binding capacities comparable to those reported for other carbon-based adsorbents like activated carbon and biochar from woody biomass [34,35,36]. The Freundlich isotherm modeling further elucidated the adsorption behavior, revealing high capacities (KF) and nonlinearity coefficients (n) of between 0.732 and 0.923, which suggest a combination of physical adsorption (e.g., pore filling) and chemical interactions (e.g., π-π stacking, hydrogen bonding) with PAH molecules [37,38,39]. These results underscore the potential of agricultural-residue-derived biochars as sustainable alternatives for water remediation, particularly in regions with abundant corn and wheat waste.
A key observation is the differential adsorption performance between the two biochars, driven by their physicochemical properties. Corn-cob biochar exhibited a superior specific surface area (SSA) of 111 m2/g compared to 61 m2/g for wheat-straw biochar, as determined by BET analysis. This higher SSA likely accounts for its significantly greater adsorption capacity for fluorene (KF = 188,620 vs. 77,296 (mg/g)/(mg/L)^n), a moderately hydrophobic PAH (log Kow ≈ 4.18). The SEM analysis complements this finding, showing that corn-cob biochar possesses a rougher surface with a heterogeneous distribution of micro- and mesopores, enhancing the availability of adsorption sites [40]. In contrast, wheat-straw-biochar’s smoother, more compact morphology may restrict pore accessibility, limiting its physical adsorption potential despite a comparable carbon content (67.8% for straw vs. 71% for corn, per CHNS analysis). This structural disparity aligns with previous studies linking porosity and the SSA to the enhanced uptake of organic pollutants [41,42].
Interestingly, wheat-straw biochar outperformed corn-cob biochar for naphthalene (KF = 18,444 vs. 10,163 (mg/g)/(mg/L)^n) and pyrene (KF = 293,398 vs. 254,947 (mg/g)/(mg/L)^n), suggesting that surface chemistry plays a pivotal role beyond mere physical structure. The EDS analysis revealed a higher oxygen content in wheat-straw biochar (69.95% vs. 63.73%), corroborated by FTIR spectra showing pronounced O-H (3436 cm−1) and C-O-C (1099 cm−1) bands. These oxygen-containing functional groups likely facilitate stronger hydrogen bonding or polar interactions with lighter PAHs like naphthalene (log Kow ≈ 3.37), as well as π-π interactions with heavier, more aromatic compounds like pyrene (log Kow ≈ 5.18) [24,43]. Conversely, corn-cob-biochar’s elevated silicon content (11.35% vs. 0.806%) and detectable O-Si-O stretching (469 cm−1) indicate a higher ash fraction, which may stabilize its porous structure but contribute less to chemical adsorption mechanisms [44,45,46].
The pXRD patterns of both biochars, showing a broad peak at 23° (2θ), confirm the presence of amorphous carbon with graphitic domains, a feature critical for π-π stacking with PAHs’ aromatic rings [24,47]. This structural characteristic, combined with the aromatic C=C stretching vibrations (1631 cm−1) observed in FTIR, supports the high adsorption affinities observed across all tested PAHs. However, the minimal difference in Log Kd for fluoranthene (5.87 vs. 5.83) and pyrene (5.66 vs. 5.69) between corn-cob and wheat-straw biochars suggests that PAH hydrophobicity and molecular size may dominate the adsorption behavior for larger compounds, overshadowing biochar-specific traits [48]. This convergence in performance for heavier PAHs could be attributed to their lower solubility and stronger partitioning into the biochar’s hydrophobic carbon matrix, as noted by Beljin et al. [5].
The pyrolysis temperature of 700 °C used in this study likely optimized carbonization and SSA development, consistent with findings that high-temperature pyrolysis enhances biochar stability and porosity [49]. However, wheat-straw-biochar’s lower SSA indicates that feedstock composition—such as the higher lignin in corn cob versus cellulose in wheat straw—may limit physical property enhancement [50]. The rapid adsorption equilibrium within 24 h suggests efficient mass transfer, likely due to the biochars’ porous networks, though the long-term stability of adsorbed PAHs remains a critical gap. Desorption risks, influenced by environmental factors like pH or microbial activity, could compromise biochar efficacy over time. Additionally, the higher ash content in corn-cob biochar (reflected in its silicon and calcium levels) might affect its performance in real-world aqueous systems with competing ions or organic matter, warranting further field-scale studies [51,52,53].
A comparison table for PAH adsorption from water samples using various materials, including biochar from different biomass sources and activated carbon, can significantly enhance the understanding of their effectiveness (Table 6). Activated carbon (AC) demonstrated the highest removal efficiency, achieving up to 98.1% for PAHs in aqueous solutions [54]. Biochar produced from wheat straw and willow showed variable effectiveness, with reductions in PAH concentrations ranging from 37 to 86% depending on the biomass source and application rate [55].
In terms of water treatment, biochar doses of 100 mg/L achieved up to a 78% reduction for benzo(a)pyrene, indicating its potential as a cost-effective alternative [56]. While biochar is less effective than AC, it offers advantages such as a lower cost and environmental impact, making it a viable option in specific contexts [57].
The physicochemical properties of both materials significantly influence their adsorption capacities, necessitating tailored approaches for different water treatment scenarios [58].
The removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous environments remains a critical area of research due to their persistence and toxicity. Adsorption has been widely recognized as an effective method for PAH removal, with various sorbents demonstrating significant potential.
Furthermore, combined materials, such as activated carbon and rice bran, achieved rapid adsorption efficiencies of 92.1% and 91.5%, respectively, within 60 min, while sawdust achieved a slightly lower adsorption (88.5%) after 30 min [59].
Activated carbon derived from apricot stones demonstrated a higher adsorption capacity for naphthalene (29.95 mg/g) compared to coal tar pitch (18.75 mg/g) [60]. In another study, activated carbon produced from wheat straw pyrolyzed at 800 °C for six hours under oxygen-limited conditions achieved a 98.6% removal efficiency of fluoranthene using a dosage of 2 g/L, highlighting the critical role of pyrolysis conditions and material properties in optimizing sorbent performance [61].
Although biochar generally achieved lower overall PAH removal efficiencies (approximately 15.4%), it significantly enhanced microbial diversity and the abundance of PAH-degrading microorganisms by 43.7%. This suggests that biochar may play a dual role in remediation by both adsorbing contaminants and improving bioremediation potential [20]. In the context of produced water treatment, oil-palm-leaf-waste-derived activated carbon achieved naphthalene removal efficiencies of 92.48% from aqueous solutions and 70.5% from actual produced water samples [62].
These findings confirm that adsorption processes, particularly using activated carbon and biochar, offer an efficient and adaptable strategy for the removal of PAHs from contaminated water systems and can be integrated into broader environmental treatment frameworks.
The comparison between previous studies and the present research highlights important similarities and distinctions in the adsorption performances of biochars and activated carbon for PAH removal from aqueous systems. Previous findings have consistently reported the superior efficacy of activated carbon, achieving up to 98.1% removal of PAHs even at low dosages, whereas biochars, depending on their feedstock, showed moderate efficiencies ranging from 44–86%. Similarly, in this study, biochars derived from corn cob and wheat straw demonstrated strong adsorption capacities, with Log Kd values ranging from 4.35 to 5.69 L/kg and high Freundlich coefficients (KF), indicating performance levels comparable to traditional carbon-based sorbents. However, while prior research has emphasized the dominant role of surface area and porosity, the current study further underscores the critical influence of surface chemistry, particularly oxygen-containing functional groups, in enhancing PAH adsorption through mechanisms such as hydrogen bonding and π-π interactions.

Table 6.
Removal efficiency of different sorbent materials for PAHs in water.
Table 6.
Removal efficiency of different sorbent materials for PAHs in water.
| Paper Scope and Conclusions | Used Material/Obtained Results | Reference |
|---|---|---|
| Impact of biochar on sorption of PAHs in water. The application of biochar led to a decrease in the concentration of the examined PAHs in water. Enhancing the contact duration and increasing the amount of biochar enhances the efficiency of PAH elimination. | 100 mg/L biochar reduced benzo(a)pyrene by 78%. Benzo(g,h,i)perylene and indeno(1,2,3-cd)pyrene decreased by 81%. | [56] |
| Adsorption of PAH from aqueous solutions on different sorbents. The highest efficiency of PAH removal (98.1%) was observed for activated carbon. The sorption processes can be used in aqueous-solution treatment procedures. | Sum of PAH removal: quartz sand bed (75.5%) mineral sorbent bed (58.2%) activated-carbon bed (98.1%) quartz sand/mineral sorbent bed (45.4%) quartz sand/activated-carbon bed (79.4%) quartz sand/mineral sorbent/ activated-carbon bed (69.7%) | [54] |
| Using natural adsorbents to reduce polycyclic aromatic hydrocarbons contamination of oily wastewater. The adsorption process in all investigated systems was favorable, and rice bran and sawdust are suitable alternatives in comparison with activated carbon, economically. | Activated carbon and rice bran had the highest adsorption of PAHs (92.1% and 91.5% respectively) within 60 min. Sawdust had a slightly lower adsorption of PAHs (88.5%) after 30 min. | [59] |
| The activated-carbon adsorption of PAHs. It focuses specifically on the effectiveness of activated-carbon adsorption, particularly wheat straw pyrolyzed under specific conditions, achieving 98.6% removal of fluoranthene. | The best method for PAH removal is AC adsorption using wheat straw pyrolyzed with limiting oxygen conditions at 800 °C for 6 h with 2 g/L dosages. This method achieved a 98.6% removal efficiency of fluoranthene. | [61] |
| Removal of naphthalene from produced water using oil-palm-leaf-waste activated carbon. | 92.48% removal efficiency of naphthalene in aquatic solution. 70.5% removal efficiency from produced water sample. | [62] |
| Valorizing biochar from Dunaliella salina biomass for naphthalene removal from aqueous rural environment. | Dunaliella salina biochar achieved removal efficiencies between 27% and 63%, depending on the naphthalene concentrations, which ranged from 25 to 150 mg/L. | [63] |
Moreover, earlier studies highlighted that biochar not only served as a moderate adsorbent but also improved microbial diversity, suggesting a dual role in bioremediation. Although this aspect was not a primary focus of the present study, the differences observed between the corn-cob and wheat-straw biochars—especially regarding surface area, oxygen content, and ash composition—imply that tailored biochar properties could influence not just adsorption but also biological activity in environmental applications. Importantly, while activated carbon generally outperformed biochar in previous reports, the corn-cob and wheat-straw biochars tested here achieved strong adsorption affinities, particularly for larger PAHs like fluoranthene and pyrene, supporting their potential as sustainable, low-cost alternatives in regions rich in agricultural residues.
In conclusion, both corn-cob and wheat-straw biochars demonstrate robust PAH removal capabilities, with corn-cob biochar excelling in physical adsorption due to its higher SSA and porosity, and wheat-straw biochar leveraging surface chemistry for specific PAHs. These differences highlight the importance of feedstock selection and pyrolysis optimization in tailoring biochar for targeted contaminants. While cost effective and sustainable, their practical application requires addressing the stability, scalability, and interactions with complex water matrices, positioning them as promising alternatives to conventional adsorbents like activated carbon.
5. Conclusions
This study demonstrates that biochars derived from corn cob and wheat straw are highly effective adsorbents for removing polycyclic aromatic hydrocarbons (PAHs)—naphthalene, fluorene, fluoranthene, and pyrene—from aqueous systems, offering a sustainable solution for water remediation. Corn-cob biochar, with its superior specific surface area (111 m2/g) and porous structure, excels in physical adsorption, particularly for fluorene, while wheat-straw biochar, enriched with oxygen-containing functional groups, shows an enhanced chemical affinity for naphthalene and pyrene. Freundlich isotherm modeling revealed strong adsorption affinities (Log Kd = 4.35–5.69 L/kg) and nonlinear behavior (n = 0.732–0.923), indicating a mix of pore filling and chemical interactions like π-π stacking and hydrogen bonding. The pyrolysis temperature of 700 °C optimized carbonization and porosity, though feedstock composition influenced outcomes, with corn cob’s lignin content boosting physical properties and wheat straw’s cellulose favoring surface chemistry. While both biochars rival conventional adsorbents like activated carbon, their practical application hinges on addressing their long-term stability and performance in complex water matrices.
Considering their distinct properties, corn-cob biochar may be more suitable for aquatic systems where physical adsorption dominates and minimal release of dissolved organic matter is critical, due to its stable porous structure, high aromaticity, and lower tendency to leach potentially harmful compounds. In contrast, wheat-straw biochar, while effective in chemical adsorption, may exhibit higher leaching of oxygenated compounds, which could affect aquatic biota. Preliminary ecotoxicological assessments suggest that corn-cob biochar poses lower acute toxicity to aquatic organisms such as Daphnia magna and Pseudokirchneriella subcapitata, likely due to its chemical stability and lower mobility of residual organics. Therefore, for its safe and effective use in real-world water treatment applications, corn-cob biochar currently offers a more robust and environmentally compatible option. Nonetheless, both biochars hold great potential, and further long-term studies under realistic environmental conditions are recommended to confirm their ecological safety and functional durability.
Author Contributions
Writing—original draft, Writing—review and editing, Methodology, Investigation, J.B.; Conceptualization, Writing—original draft, M.K.I.; Validation, Writing—review and editing, J.A.; Data curation, Formal analysis, Funding acquisition, Project administration, M.V.; Visualization, Writing—review and editing, S.M.; Data curation, Formal analysis, Research, Project administration, Funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Provincial Secretariat for Higher Education and Scientific Research (Project No. 003076058 2024 09418 003 000 000 001/2).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the financial support of the Provincial Secretariat for Higher Education and Scientific Research (Project No. 003076058 2024 09418 003 000 000 001/2). This article is based upon work from COST Action CA20101 Plastics monitoring detection remediation recovery—PRIORITY, supported by COST (European Cooperation in Science and Technology, www.cost.eu (accessed on 31 March 2025)).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Montano, L.; Baldini, G.M.; Piscopo, M.; Liguori, G.; Lombardi, R.; Ricciardi, M.; Esposito, G.; Pinto, G.; Fontanarosa, C.; Spinelli, M.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in the Environment: Occupational Exposure, Health Risks and Fertility Implications. Toxics 2025, 13, 151. [Google Scholar] [CrossRef]
- Venkatraman, G.; Giribabu, N.; Mohan, P.S.; Muttiah, B.; Govindarajan, V.K.; Alagiri, M.; Abdul Rahman, P.S.; Karsani, S.A. Environmental impact and human health effects of polycyclic aromatic hydrocarbons and remedial strategies: A detailed review. Chemosphere 2024, 351, 141227. [Google Scholar] [CrossRef] [PubMed]
- Maletić, S.P.; Beljin, J.M.; Rončević, S.D.; Grgić, M.G.; Dalmacija, B.D. State of the art and future challenges for polycyclic aromatic hydrocarbons is sediments: Sources, fate, bioavailability and remediation techniques. J. Hazard. Mater. 2019, 365, 467–482. [Google Scholar] [CrossRef]
- Beljin, J.; Đukanović, N.; Anojčić, J.; Simetić, T.; Apostolović, T.; Mutić, S.; Maletić, S. Biochar in the Remediation of Organic Pollutants in Water: A Review of Polycyclic Aromatic Hydrocarbon and Pesticide Removal. Nanomaterials 2025, 15, 26. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Olugbenga, O.S.; Adeleye, P.G.; Oladipupo, S.B.; Adeleye, A.T.; John, K.I. Biomass-derived biochar in wastewater treatment- a circular economy approach. Waste Manag. Bull. 2023, 1, 1–14. [Google Scholar] [CrossRef]
- Yan, S.; Yu, W.; Yang, T.; Li, Q.; Guo, J. The Adsorption of Corn Stalk Biochar for Pb and Cd: Preparation, Characterization, and Batch Adsorption Study. Separations 2022, 9, 22. [Google Scholar] [CrossRef]
- Majumder, S.; Sharma, P.; Singh, S.P.; Nadda, A.K.; Sahoo, P.K.; Xia, C.; Sharma, S.; Ganguly, R.; Lam, S.S.; Kim, K.H. Engineered biochar for the effective sorption and remediation of emerging pollutants in the environment. J. Environ. Chem. Eng. 2023, 11, 109590. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Z.; Yao, S.; Li, Z.; Zhang, Z.; Ji, L.; Jing, H. Activated biochar derived from Enteromorpha with high specific surface area for efficient removal of phenanthrene: Experiments, mechanism and DFT calculations. Environ. Pollut. 2024, 340 Pt 2, 122709. [Google Scholar] [CrossRef]
- Qu, J.; Meng, Q.; Peng, W.; Shi, J.; Dong, Z.; Li, Z.; Hu, Q.; Zhang, Q.; Wang, L.; Ma, S.; et al. Application of functionalized biochar for adsorption of organic pollutants from environmental media: Synthesis strategies, removal mechanisms and outlook. J. Clean. Prod. 2023, 423, 138690. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Zhang, X.; Show, P.L. Research advances on production and application of algal biochar in environmental remediation. Environ. Pollut. 2024, 348, 123860. [Google Scholar] [CrossRef] [PubMed]
- Fabian, P.S.; Lee, D.H.; Shin, S.W.; Kang, J.H. Assessment of pyrene adsorption on biochars prepared from green infrastructure plants: Toward a closed-loop recycling in managing toxic stormwater pollutants. J. Water Process Eng. 2022, 48, 102929. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Valipour, M.; Ali, I.; Usman, M.; Iqbal, R.; Zulfiqar, U.; Rizwan, M.; Mahmood, S.; Ullah, A.; et al. Recent trends and economic significance of modified/functionalized biochars for remediation of environmental pollutants. Sci. Rep. 2024, 14, 217. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Li, J.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.; Wang, S.; Song, H.; et al. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon Res. 2022, 1, 4. [Google Scholar] [CrossRef]
- Xin, Z.; Tong, J.; Wang, J.; Ruan, C.; Lyu, J.; Shi, J. Research progress on activated persulfate by biochar: Soil and water environment remediation, mechanism exploration and simulation calculation. Chem. Eng. J. 2024, 493, 152718. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, X.; Ren, Y.; Zhu, X.; Si, S.; Kou, B.; Zhang, Z.; Wang, J.; Shen, B. Remediation of polycyclic aromatic hydrocarbons polluted soil by biochar loaded humic acid activating persulfate: Performance, process and mechanisms. Bioresour. Technol. 2024, 399, 130633. [Google Scholar] [CrossRef]
- Alhothali, A.; Haneef, T.; Mustafa, M.R.U.; Moria, K.M.; Rashid, U.; Rasool, K.; Bamasag, O.O. Optimization of Micro-Pollutants’ Removal from Wastewater Using Agricultural Waste-Derived Sustainable Adsorbent. Int. J. Environ. Res. Public Health 2021, 18, 11506. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Guo, M.; Shang, X.; Ma, Y.; Zhang, K.; Zhang, L.; Zhou, Y.; Gong, Z.; Miao, R. Biochars assisted phytoremediation of polycyclic aromatic hydrocarbons contaminated agricultural soil: Dynamic responses of functional genes and microbial community. Environ. Pollut. 2024, 345, 123476. [Google Scholar] [CrossRef]
- Li, D.; Su, P.; Tang, M.; Zhang, G. Biochar alters the persistence of PAHs in soils by affecting soil physicochemical properties and microbial diversity: A meta-analysis. Ecotoxicol. Environ. Saf. 2023, 266, 115589. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Buss, W.; Hilber, I.; Graham, M.C.; Mašek, O. Composition of PAHs in Biochar and Implications for Biochar Production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, L.; Moghaddam, T.B.; Chen, M.; Wu, S.; Yuan, X. Adsorption mechanism of polycyclic aromatic hydrocarbons using wood waste-derived biochar. J. Hazard. Mater. 2022, 425, 128003. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.; Waseem, M.; Leta, M.K. Review of Techniques for the Removal of Polycyclic Aromatic Hydrocarbons from Produced Water. Environments 2023, 10, 40. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, R.; Kaur, H. A systematic review of lignocellulosic biomass for remediation of environmental pollutants. Appl. Surf. Sci. Adv. 2024, 19, 100547. [Google Scholar] [CrossRef]
- Beljin, J.; Kragulj Isakovski, M.; Simetić, T.; Đukanović, N.; Molnar Jazić, J.; Maletić, S.; Vujić, M. Exploring the Adsorption Behavior of Organic UV Filter on Carbon-Based Materials as Potential Carriers of Organic Contaminants in the Aquatic Environment. Appl. Sci. 2024, 14, 9424. [Google Scholar] [CrossRef]
- Sokołowski, A.; Boguszewska-Czubara, A.; Kobyłecki, R.; Zarzycki, R.; Kończak, M.; Oleszczuk, P.; Gao, Y.; Czech, B. Increased oxygen content in biochar lowered bioavailability of polycyclic aromatic hydrocarbons-related toxicity to various organisms. Bioresour. Technol. 2024, 407, 131110. [Google Scholar] [CrossRef]
- Tomczyk, A.; Vitková, J.; Botková, N.; Siryk, O.; Kondracki, B.; Szewczuk-Karpisz, K. Ammonia hydroxide and citric acid modified wheat straw-biochars: Preparation, characterization, and environmental applications. Chemosphere 2024, 356, 141916. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.H.; Amirahmadi, E. How do different feedstocks and pyrolysis conditions effectively change biochar modification scenarios? A critical analysis of engineered biochars under H2O2 oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Zhang, P.; Chang, F.; Huo, L.; Yao, Z.; Luo, J. Impacts of Biochar Pyrolysis Temperature, Particle Size, and Application Rate on Water Retention of Loess in the Semiarid Region. Water 2025, 17, 69. [Google Scholar] [CrossRef]
- Sema, A.I.; Khatri, J.; Dhar, B.B.; Kulsi, G.; Bhattacharyya, J. On The Utility of Teakwood Biochar for Iron Contaminants Removal from Water. ES Mater. Manuf. 2023, 21, 871. [Google Scholar] [CrossRef]
- Kajbaf, F.; Derikvand, E.; Babarsad, S.M.; Purmohammadi, M.H.; Kharazi, G.H. Lotus-leaf biochar modified with metal oxide nanoparticles: Synthesise, characterisation and application to the photocatalytic removal of Cr6+, Cr3+ and Co2+ ions. Int. J. Environ. Anal. Chem. 2021, 103, 5851–5867. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Rashad, E.; Saleh, H.N.; Eltaweil, A.S.; Saleh, M.E.; Sillanpaa, M.; Mostafa, A.R. Pinewood sawdust biochar as an effective biosorbent for PAHs removal from wastewater. Biomass Conv. Bioref. 2023, 13, 13443–13459. [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Simultaneous removal of multiple polycyclic aromatic hydrocarbons (PAHs) from urban stormwater using low-cost agricultural/industrial byproducts as sorbents. Chemosphere 2021, 274, 129812. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, Y.-K. Applications of Modified Biochar-Based Materials for the Removal of Environment Pollutants: A Mini Review. Sustainability 2020, 12, 6112. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Zhao, M.; Rong, H.; Xu, Y. The environmental characteristics and applications of biochar. Environ. Sci. Pollut. Res. 2018, 25, 21525–21534. [Google Scholar] [CrossRef]
- Varkolu, M.; Gundekari, S.; Omvesh; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Trivedi, Y.; Sharma, M.; Mishra, R.K.; Sharma, A.; Joshi, J.; Gupta, A.B.; Achintya, B.; Shah, K.; Vuppaladadiyamd, A.K. Biochar potential for pollutant removal during wastewater treatment: A comprehensive review of separation mechanisms, technological integration, and process analysis. Desalination 2025, 600, 118509. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Tan, X.F.; Zhu, S.S.; Wang, R.P.; Chen, Y.D.; Show, P.L.; Zhang, F.F.; Ho, S.H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, W.; Fang, S.; Xu, Z.; Weng, H.; Zhang, X. The Influence of Pyrolysis Temperature and Feedstocks on the Characteristics of Biochar-Derived Dissolved Organic Matter: A Systematic Assessment. Clean Technol. 2024, 6, 1314–1325. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Sun, K.; Yang, Y.; Fang, L.; Xing, B. Effect of Fe and Al ions on the production of biochar from agricultural biomass: Properties, stability and adsorption efficiency of biochar. Renew. Sustain. Energy Rev. 2021, 145, 111133. [Google Scholar] [CrossRef]
- Yao, C.; Wang, B.; Hassan, M.; Xu, H.; Wang, H. Removal mechanisms of polycyclic aromatic hydrocarbons in biochar and its effects on plant growth. J. Environ. Sci. 2025. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Maroušek, J.; Minofar, B.; Maroušková, A.; Strunecký, O.; Gavurová, B. Environmental and economic advantages of production and application of digestate biochar. Environ. Technol. Innov. 2023, 30, 103109. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Smol, M.; Wlodarczyk-Makula, M.; Wloka, D. Adsorption of polycyclic aromatic hydrocarbons (pahs) from aqueous solutions on different sorbents, Civil and environmental engineering reports. Civ. Environ. Eng. Rep. 2014, 13, 87–96. [Google Scholar] [CrossRef][Green Version]
- Kołtowski, M.; Hilber, I.; Bucheli, T.D.; Oleszczuk, P. Effect of activated carbon and biochars on the bioavailability of polycyclic aromatic hydrocarbons in different industrially contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 11058–11068. [Google Scholar] [CrossRef] [PubMed]
- Rosińska, A. Impact of biochar on sorption of polycyclic aromatic hydrocarbons in water. Desalination Water Treat. 2023, 305, 103–113. [Google Scholar] [CrossRef]
- Bentley, M.J.; Kennedy, A.K.; Summers, R.S. Optimizing biochar adsorption relative to activated carbon in water treatment. In Sustainable Biochar for Water and Wastewater Treatment; Mohan, D., Pittman, C.U., Mlsna, T.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 737–773. ISBN 9780128222256. [Google Scholar] [CrossRef]
- Pathak, S.; Sakhiya, A.K.; Anand, A.; Pant, K.K.; Kaushal, P. A state-of-the-art review of various adsorption media employed for the removal of toxic Polycyclic aromatic hydrocarbons (PAHs): An approach towards a cleaner environment. J. Water Process Eng. 2022, 47, 102674. [Google Scholar] [CrossRef]
- Farahani, M.; Behbahaninia, A. Using natural adsorbents to reduce polycyclic aromatic hydrocarbons contamination of oily wastewater. J. Biodivers. Environ. Sci. (JBES) 2014, 5, 274–281. [Google Scholar]
- Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Velasco, L.; Ania, C.O. Characterization and application of activated carbon from biomass and coal wastes for naphthalene removal. Bulg. Chem. Commun. 2011, 43, 552–557. [Google Scholar]
- Razak, N.A.A.; Hanafi, M.H.M.; Razak, N.H.; Ibrahim, A.; Omar, A.A. The Activated Carbon Adsorption of Polycyclic Aromatic Hydrocarbons: The Best Evidence Review. In Proceedings of the 7th International Conference and Exhibition on Sustainable Energy and Advanced Materials (ICE-SEAM 2021), Melaka, Malaysia; Abdollah, M.F.B., Amiruddin, H., Phuman Singh, A.S., Abdul Munir, F., Ibrahim, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Ul Mustafa, M.R.; Khurshid, H.; Ho, Y.C.; Isa, M.H. Removal of Naphthalene from Produced Water Using Oil Palm Leaves Waste Activated Carbon. In Proceedings of the International Conference on Emerging Smart Cities (ICESC2022); Mohammed, B.S., Min, T.H., Sutanto, M.H., Joewono, T.B., As’ad, S., Eds.; Springer Nature: Singapore, 2022. [Google Scholar] [CrossRef]
- Nama, M.; Satasiya, G.; Sahoo, T.P.; Moradeeya, P.G.; Sadukha, S.; Singhal, K.; Saravaia, H.T.; Dineshkumar, R.; Anil Kumar, M. Thermo-chemical behaviour of Dunaliella salina biomass and valorising their biochar for naphthalene removal from aqueous rural environment. Chemosphere 2024, 353, 141639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).