Cryoconservation Modifies Ion Transport Pathways in the Skin Microenvironment: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Solutions
- -
- RS—the Ringer solution (K+ 4.0 mM; Na+ 147.2 mM; Ca2+ 2.2 mM; Mg2+ 2.6 mM; Cl− 160.8 mM), a basic solution with iso-osmotic properties and a pH of 7.4. Mineral compounds (KCl, NaCl, CaCl2, MgCl2) were purchased from Avantor, Zabrze, Poland.
- -
- DMSO—dimethyl sulfoxide 5% (0.704 mol/L) (Sigma-Aldrich, Burlington, NJ, USA) solution, diluted in RS.
- -
- Ami—amiloride hydrochloride hydrate (0.1 mmol/L) 3,5-diamino-6-chloro-2-carboxylic acid (Sigma-Aldrich, Burlington, NJ, USA); used as an inhibitor of the transepithelial transport of sodium ions, diluted in RS.
- -
- Bume—bumetanide (0.1 mmol/L) 3-butylamino-4-phenoxy-5-sulfamoylbenzoic acid (Sigma-Aldrich, Burlington, NJ, USA); used as an inhibitor of the transepithelial transport of chloride ions, diluted in RS.
- -
- AB—a mixture of amiloride (0.1 mmol/L) and bumetanide (0.1 mmol/L) solutions.
2.3. Experimental Procedure
- (1)
- Control (n = 23)—the samples were incubated in RS solution.
- (2)
- Group subjected to deep freezing, divided into 3 subgroups:
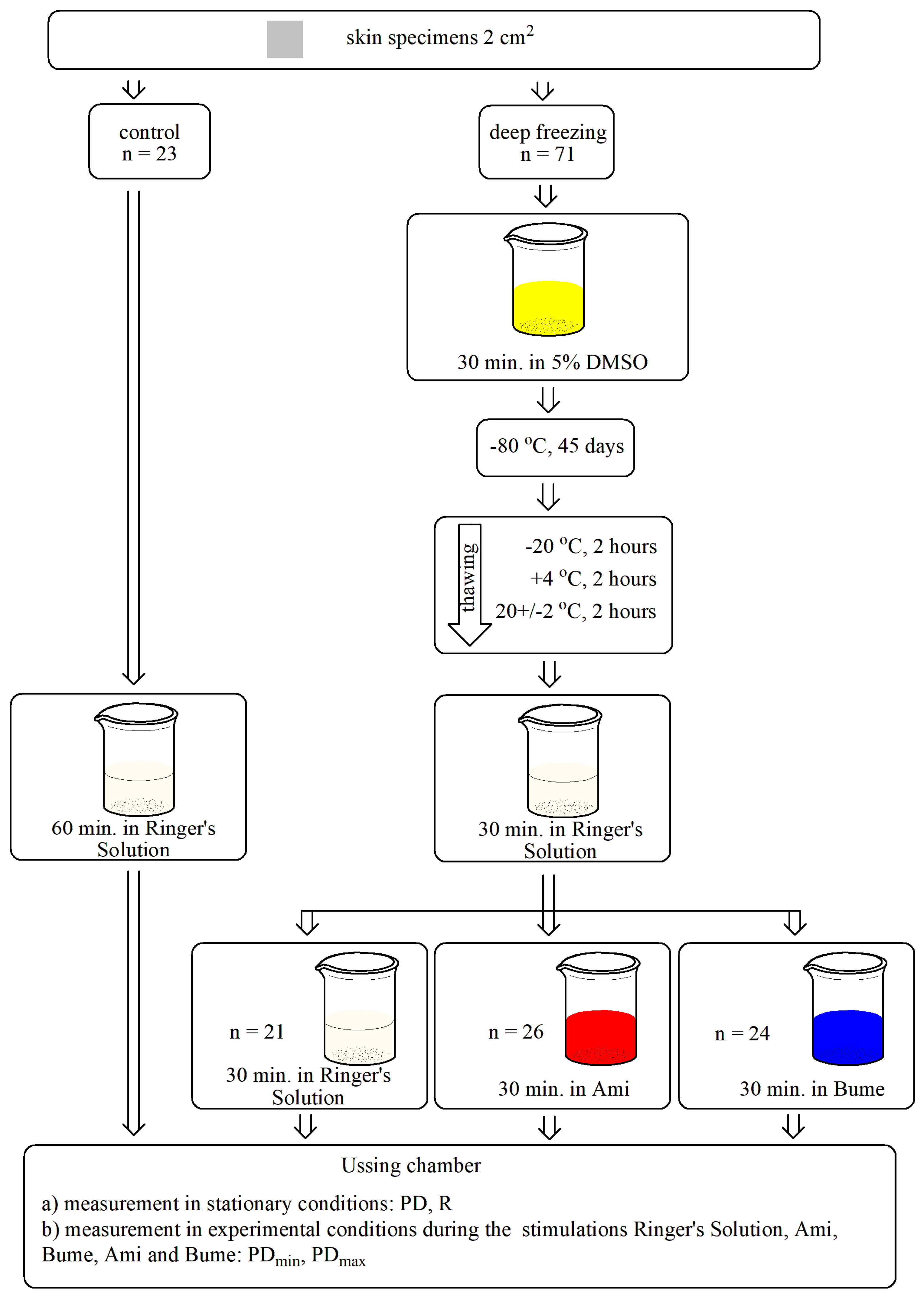
- (1)
- transepithelial electric potential—changes in transepithelial electrical potential measured continuously under stationary conditions (PD, mV),
- (2)
- minimal and maximal transepithelial electrical potential measured during a 15-s stimulation (PDmin, PDmax, mV),
- (3)
- transepithelial electrical resistance (R, Ω × cm2), determined by stimulating the tissue sample with a stimulus current intensity of 10 µA for each side of the tested skin specimen. Subsequently, the corresponding voltage change was measured, and resistance was counted according to Ohm’s law.
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ami | amiloride (0.1 mmol/L) solution |
| AB | mixture of amiloride (0.1 mmol/L) and bumetanide (0.1 mmol/L) solution |
| Bume | bumetanide (0.1 mmol/L) solution |
| DMSO | dimethyl sulfoxide |
| ENaC | epithelial sodium channel |
| PD | transepithelial electric potential measured during stationary conditions (mV) |
| PDmax | maximal transepithelial electric potential measured during 15-s stimulation (mV) |
| PDmin | minimal transepithelial electric potential measured during 15-s stimulation (mV) |
| R | resistance (Ω × cm2) |
| RS | Ringer solution |
References
- Bosco, F.; Governa, M.; Rossati, L.; Vigato, E.; Vassanelli, A.; Aprili, G.; Franchini, M. The use of banked skin in the Burns Centre of Verona. Blood Transfus. 2011, 9, 156–161. [Google Scholar] [CrossRef]
- Cleland, H.; Wasiak, J.; Dobson, H.; Paul, M.; Pratt, G.; Paul, E.; Herson, M.; Akbarzedeh, S. Clinical application and viability of cryopreserved cadaveric skin allografts in severe burn: A retrospective analysis. Burns 2014, 40, 61–66. [Google Scholar] [CrossRef]
- Franchini, M.; Zanini, D.; Bosinelli, A.; Fiorini, S.; Rizzi, S.; D’Aloja, C.; Vassanelli, A.; Gandini, G.; Aprili, G. Evaluation of cryopreserved donor skin viability: The experience of the regional tissue bank of Verona. Blood Transfus. 2009, 7, 100–105. [Google Scholar] [CrossRef]
- Wood, J.M.; Soldin, M.; Shaw, T.J.; Szarko, M. The biomechanical and histological sequelae of common skin banking methods. J. Biomech. 2014, 47, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- de Oliviera, L.C.C.; Vacari, G.Q.; Duarte, J.M.B. A Method to Freeze Skin Samples for Cryobanks: A Test of Some Cryoprotectants for an Endangered Deer. Biopreserv. Biobank. 2024, 22, 211–216. [Google Scholar] [CrossRef]
- Pianigiani, E.; Tognetti, L.; Ierardi, F.; Mariotti, G.; Rubegni, P.; Cevenini, G.; Perotti, R.; Fimiani, M. Assessment of cryopreserved donor skin viability: The experience of the regional tissue bank of Siena. Cell Tissue Bank. 2016, 17, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Praça, F.S.; Medina, W.S.; Eloy, J.O.; Petrilli, R.; Campos, P.M.; Ascenso, A.; Bentley, M.V. Evaluation of critical parameters for in vitro skin permeation and penetration studies using animal skin models. Eur. J. Pharm. Sci. 2018, 111, 121–132. [Google Scholar] [CrossRef]
- Raj, N.; Voegeli, R.; Rawlings, A.V.; Doppler, S.; Imfeld, D.; Munday, M.R.; Lane, M.E. A fundamental investigation into aspects of the physiology and biochemistry of the stratum corneum in subjects with sensitive skin. Int. J. Cosmet. Sci. 2017, 39, 2–10. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Effect of Frozen Human Epidermis Storage Duration and Cryoprotectant on Barrier Function Using Two Model Compounds. Skin Pharmacol. Physiol. 2016, 29, 31–40. [Google Scholar] [CrossRef]
- Holzer, P.W.; Leonard, D.A.; Shanmugarajah, K.; Moulton, K.N.; Ng, Z.Y.; Cetrulo, C.L.; Sachs, D. A Comparative Examination of the Clinical Outcome and Histological Appearance of Cryopreserved and Fresh Split-Thickness Skin Grafts. J. Burn Care Res. 2017, 38, e55–e61. [Google Scholar] [CrossRef]
- Ghetti, M.; Topouzi, H.; Theocharidis, G.; Papa, V.; Williams, G.; Bondioli, E.; Cenacchi, G.; Connelly, J.T.; Higgins, C.A. Subpopulations of dermal skin fibroblasts secrete distinct extracellular matrix: Implications for using skin substitutes in the clinic. BJD 2018, 179, 381–393. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Zhang, Q.; Gong, H.; Gao, D.; Wang, Y.; Li, B.; Li, X.; Zheng, H.; Wu, Z.; et al. Spatially resolved proteomic map shows that extracellular matrix regulates epidermal growth. Nat. Commun. 2022, 13, 4012. [Google Scholar] [CrossRef]
- Rossitto, G.; Mary, S.; Chen, J.Y.; Boder, P.; Chew, K.S.; Neves, K.B.; Alves, R.L.; Montezano, A.C.; Welsh, P.; Petrie, M.C.; et al. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat. Commun. 2020, 11, 4222. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D.; Cohen, S.R.; Fagien, S. Preoperative skin conditioning: Extracellular matrix clearance and skin bed preparation, a new paradigm. Aesthet. Surg. J. 2019, 39, S103–S111. [Google Scholar] [CrossRef] [PubMed]
- Bostan, L.E.; Almqvist, S.; Pullar, C. A pulsed current electric field alters protein expression creating a wound healing phenotype in human skin cells. Regen. Med. 2020, 15, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.; Mycielska, M.; Madeja, Z.; Fraser, S.P.; Korohoda, W. Directional movement of rat prostate cancer cells in direct-current electric field: Involvement of voltage-gated Na+ channel activity. J. Cell Sci. 2001, 114, 2697–2705. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Szewczyk-Golec, K. Analysis of changes in sodium and chloride ion transport in the skin. Sci. Rep. 2020, 10, 18094. [Google Scholar] [CrossRef]
- Kasting, G.B.; Bowman, L.A. Electrical analysis of fresh, excised human skin: A comparison with frozen skin. Pharm. Res. 1990, 7, 1141–1146. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zeitchek, M.; Cooper, G.; Jia, S.; Xie, P.; Quereshi, H.; Zhong, A.; Porterfield, M.; Galiano, R.; et al. Hydration status regulates sodium flux and inflammatory pathways through epithelial sodium channel (ENaC) in the skin. J. Investig. Dermatol. 2015, 135, 796–806. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zhong, A.; Xie, P.; Jia, S.; Xie, Z.; Zeitchek, M.; Niknam-Bienia, S.; Zhao, J.; Porterfield, M.; et al. Sodium channel Nax is a regulator in epithelial sodium homeostasis. Sci. Transl. Med. 2015, 7, 312ra177. [Google Scholar] [CrossRef]
- Yang, H.Y.; Charles, R.P.; Hummler, E.; Baines, D.L.; Isseroff, R.R. The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J. Cell Sci. 2013, 126, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Shuto, T.; Mizunoe, S.; Tomita, A.; Koga, T.; Sato, T.; Takeya, M.; Suico, M.A.; Niibori, A.; Sugahara, T.; et al. CFTR-deficiency renders mice highly susceptible to cutaneous symptoms during mite infestation. Lab. Investig. 2011, 91, 509–518. [Google Scholar] [CrossRef]
- Reddy, M.M.; Quinton, P.M. PKA mediates constitutive activation of CFTR in human sweat duct. J. Membr. Biol. 2009, 231, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sheppard, D.N.; Hug, M.J. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros. 2004, 3 (Suppl. 2), 123–126. [Google Scholar] [CrossRef] [PubMed]
- Baumbauer, K.M.; DeBerry, J.J.; Adelman, P.C.; Miller, R.H.; Hachisuka, J.; Lee, K.H.; Ross, S.; Koerber, R.; Davis, B.; Albers, K. Keratinocytes can modulate and directly initiate nociceptive responses. eLife 2015, 4, e09674. [Google Scholar] [CrossRef]
- Pang, Z.; Sakamoto, T.; Tiwari, V.; Kim, Y.S.; Yang, F.; Dong, X.; Güler, A.D.; Guan, Y.; Caterina, M. Selective keratinocyte stimulation is sufficient to evoke nociception in mice. Pain 2015, 156, 656–665. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Bogusiewicz, J.; Chajdas, D.; Szewczyk-Golec, K.; Lampka, M.; Olszewska-Słonina, D. The immediate influence of deltamethrin on ion transport through rabbit skin: An in vitro study. Pest. Biochem. Physiol. 2018, 148, 144–150. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, X.; Zhang, X.; Liu, K.S.; Zhang, J.; Chen, J.; Yu, M.K.; Tsang, L.; Chung, Y.; Wang, Y.; et al. Dynamically Regulated CFTR Expression and Its Functional Role in Cutaneous Wound Healing. J. Cell. Physiol. 2015, 230, 2049–2058. [Google Scholar] [CrossRef]
- Mrázová, H.; Koller, J.; Kubišová, K.; Fujeríková, G.; Klincová, E.; Babál, P. Comparison of structural changes in skin and amnion tissue grafts for transplantation induced by gamma and electron beam irradiation for sterilization. Cell Tissue Bank. 2016, 17, 255–260. [Google Scholar] [CrossRef]
- da Silva Serra, I.; Husson, Z.; Bartlett, J.D.; Smith, E.S. Characterization of cutaneous and articular sensory neurons. Mol. Pain 2016, 12, 1744806916636387. [Google Scholar] [CrossRef]
- Naaldijk, Y.; Johnson, A.A.; Friedrich-Stöckigt, A.; Stolzing, A. Cryopreservation of dermal fibroblasts and keratinocytes in hydroxyethyl starch-based cryoprotectants. BMC Biotechnol. 2016, 16, 85. [Google Scholar] [CrossRef]
- Pielesz, A.; Gawłowski, A.; Biniaś, D.; Bobiński, R.; Kawecki, M.; Klama-Baryła, A.; Kitala, D.; Łabuś, W.; Glik, J.; Paluch, J. The role of dimethyl sulfoxide (DMSO) in ex-vivo examination of human skin burn injury treatment. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 196, 344–352. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Silva, C.S.; Souza, M.N. Electrical impedance model for evaluation of skin irritation in rabbits and humans. Skin Res. Technol. 2007, 13, 259–267. [Google Scholar] [CrossRef]
- Abdayem, R.; Callejon, S.; Portes, P.; Kirilov, P.; Demarne, F.; Pirot, F.; Jannin, V.; Haftek, M. Modulation of transepithelial electric resistance (TEER) in reconstructed human epidermis by excipients known to permeate intestinal tight junctions. Exp. Dermatol. 2015, 24, 686–691. [Google Scholar] [CrossRef]
- Wester, R.C.; Christoffel, J.; Hartway, T.; Poblete, N.; Maibach, H.I.; Forsell, J. Human cadaver skin viability for in vitro percutaneous absorption: Storage and detrimental effects of heat-separation and freezing. Pharm. Res. 1998, 15, 82–84. [Google Scholar] [CrossRef]
- Dobrzeniecka, W.; Daca, M.; Nowakowska, B.; Sobiesiak, M.; Szewczyk-Golec, K.; Woźniak, A.; Hołyńska-Iwan, I. The impact of diclofenac gel on the skin ion transport. An in vitro study. Molecules 2023, 28, 1332. [Google Scholar] [CrossRef]
- Dłubała, K.; Wasiek, S.; Pilarska, P.; Szewczyk-Golec, K.; Mila-Kierzenkowska, C.; Łączkowski, K.Z.; Sobiesiak, M.; Gackowski, M.; Tylkowski, B.; Hołyńska-Iwan, I. The Influence of Retinol Ointment on Rabbit Skin (Oryctolagus cuniculus) Ion Transport—An In Vitro Study. Int. J. Mol. Sci. 2024, 25, 9670. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Boggula, V.R.; Vaknine, H.; Sharma, S.; Kleyman, T.; Hanukoglu, A. Expression of epithelial sodium channel (ENaC) and CFTR in the human epidermis and epidermal appendages. Histochem. Cell Biol. 2017, 147, 733–748. [Google Scholar] [CrossRef]
- Sintov, A.C. Cumulative evidence of the low reliability of frozen/thawed pig skin as a model for in vitro percutaneous permeation testing. Eur. J. Pharm. Sci. 2017, 102, 261–263. [Google Scholar] [CrossRef]
- Huhn, K.; Linz, P.; Pemsel, F.; Michalke, B.; Seyferth, S.; Kopp, C.; Chaudri, M.A.; Rothhammer, V.; Dörfler, A.; Uder, M.; et al. Skin sodium is increased in male patients with multiple sclerosis and related animal models. Proc. Natl. Acad. Sci. USA 2021, 118, e2102549118. [Google Scholar] [CrossRef]
| PD (mV) | R (Ω × cm2) | |||||
|---|---|---|---|---|---|---|
| Incubation | Median | Upper Quartile | Lower Quartile | Median | Upper Quartile | Lower Quartile |
| Control (n = 23) | −0.27 | −0.11 | −0.45 | 11,554 | 30,794 | 5874 |
| RS (n = 21) | 0 | 0 | −0.14 | 7955 | 13,267 | 3676 |
| Ami (n = 26) | 0 | 0.48 | −0.23 | 3101 | 6700 | 2202 |
| Bume (n = 26) | −0.12 | −0.29 | 0 | 5642 | 10,379 | 5119 |
| Parameter | Control vs. RS (p) | Control vs. Ami (p) | Control vs. Bume (p) | RS vs. Ami (p) | RS vs. Bume (p) | Ami vs. Bume (p) |
|---|---|---|---|---|---|---|
| R | 0.0487 | <0.001 | 0.1632 | <0.001 | 0.0055 | 0.0107 |
| PD | <0.001 | 0.8331 | 0.2122 | 0.01 | 0.0094 | 0.3433 |
| Incubation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 23) | RS (n = 21) | Ami (n = 26) | Bume (n = 26) | ||||||
| Stimulation | Parameters | PDmin (mV) | PDmax (mV) | PDmin (mV) | PDmax (mV) | PDmin (mV) | PDmax (mV) | PDmin (mV) | PDmax (mV) |
| RS | median | −0.46 | 0.4 | −0.92 | 0.64 | n/o | n/o | ||
| upper quartile | −0.27 | 1.34 | −0.27 | 1.65 | |||||
| lower quartile | −1.07 | 0 | −1.78 | 0.27 | |||||
| Ami | median | −0.37 | 0.34 | −0.76 | 0.64 | −0.34 | 0.58 | −0.46 | 0.29 |
| upper quartile | −0.12 | 1.8 | −0.21 | 0.85 | 0.49 | 2.14 | −0.34 | 0 | |
| lower quartile | −1.28 | −0.36 | −1.59 | 0.43 | −0.92 | 0 | −0.89 | 0.64 | |
| Bume | median | −0.49 | 0.15 | −0.70 | 1.01 | −0.38 | 0.19 | −0.34 | 0.34 |
| upper quartile | 0 | 1.16 | −0.15 | 1.53 | 0 | 0.61 | −0.18 | 1.07 | |
| lower quartile | −1.25 | −0.44 | −1.65 | 0.34 | −1.22 | −0.11 | −0.55 | 0.15 | |
| AB | median | −0.19 | 0.21 | −0.76 | 1.01 | −0.37 | 0.21 | −0.44 | 0.41 |
| upper quartile | 0.82 | 0.98 | −0.27 | 1.46 | 0 | 0.76 | −0.21 | 1.71 | |
| lower quartile | −0.95 | −0.34 | −1.83 | 0.24 | −1.68 | −0.12 | −0.89 | 0.18 | |
| Stimulation (15 s) | Control (p) | RS Incubation (p) | Ami Incubation (p) | Bume Incubation (p) |
|---|---|---|---|---|
| Bume | ||||
| PD vs. PDmax | <0.001 | <0.001 | 0.0014 | <0.001 |
| PD vs. PDmin | <0.001 | <0.001 | <0.001 | <0.001 |
| PDmax vs. PDmin | <0.001 | <0.001 | <0.001 | <0.001 |
| Ami | ||||
| PD vs. PDmax | <0.001 | <0.001 | <0.001 | <0.001 |
| PD vs. PDmin | <0.001 | <0.001 | <0.001 | 0.0199 |
| PDmax vs. PDmin | <0.001 | <0.001 | 0.001 | <0.001 |
| AB | ||||
| PD vs. PDmax | <0.001 | <0.001 | <0.001 | <0.001 |
| PD vs. PDmin | <0.001 | <0.001 | <0.001 | 0.0028 |
| PDmax vs. PDmin | <0.001 | <0.001 | <0.001 | <0.001 |
| Stimulation | Control vs. RS (p) | Control vs. Ami (p) | Control vs. Bume (p) | RS vs. Ami (p) | RS vs. Bume (p) | Ami vs. Bume (p) |
|---|---|---|---|---|---|---|
| Bume | ||||||
| PDmin | 0.3717 | 0.1393 | 0.0404 | 0.7898 | 0.2136 | 0.8388 |
| PDmax | 0.0539 | 0.0031 | 0.1455 | 0.7332 | 0.1518 | 0.2635 |
| Ami | ||||||
| PDmin | 0.1451 | 0.1902 | 0.2221 | 0.7484 | 0.4828 | 0.6666 |
| PDmax | 0.3592 | 0.0005 | 0.0653 | 0.2924 | 0.7635 | 0.0952 |
| AB | ||||||
| PDmin | 0.1729 | 0.4931 | 0.3351 | 0.5815 | 0.9542 | 0.8474 |
| PDmax | 0.1551 | 0.0888 | 0.6631 | 0.7713 | 0.1302 | 0.1841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hołyńska-Iwan, I.; Wróblewski, M.; Kałużna, L.; Dziaman, T.; Czuczejko, J.; Zavyalova, O.; Olszewska-Słonina, D.; Szewczyk-Golec, K. Cryoconservation Modifies Ion Transport Pathways in the Skin Microenvironment: An In Vitro Study. Processes 2025, 13, 1493. https://doi.org/10.3390/pr13051493
Hołyńska-Iwan I, Wróblewski M, Kałużna L, Dziaman T, Czuczejko J, Zavyalova O, Olszewska-Słonina D, Szewczyk-Golec K. Cryoconservation Modifies Ion Transport Pathways in the Skin Microenvironment: An In Vitro Study. Processes. 2025; 13(5):1493. https://doi.org/10.3390/pr13051493
Chicago/Turabian StyleHołyńska-Iwan, Iga, Marcin Wróblewski, Lucyna Kałużna, Tomasz Dziaman, Jolanta Czuczejko, Olga Zavyalova, Dorota Olszewska-Słonina, and Karolina Szewczyk-Golec. 2025. "Cryoconservation Modifies Ion Transport Pathways in the Skin Microenvironment: An In Vitro Study" Processes 13, no. 5: 1493. https://doi.org/10.3390/pr13051493
APA StyleHołyńska-Iwan, I., Wróblewski, M., Kałużna, L., Dziaman, T., Czuczejko, J., Zavyalova, O., Olszewska-Słonina, D., & Szewczyk-Golec, K. (2025). Cryoconservation Modifies Ion Transport Pathways in the Skin Microenvironment: An In Vitro Study. Processes, 13(5), 1493. https://doi.org/10.3390/pr13051493







