Spent LiFePO4 to High-Value LiF: Enhanced Mechanical Chlorination Coupled with a Fluorination Reaction Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Pretreatment
2.2. Mechanical Chlorination and Leaching Process
2.3. Fluorination Reaction and Defluoridation Process
2.4. Characterization Methods
3. Results and Discussion
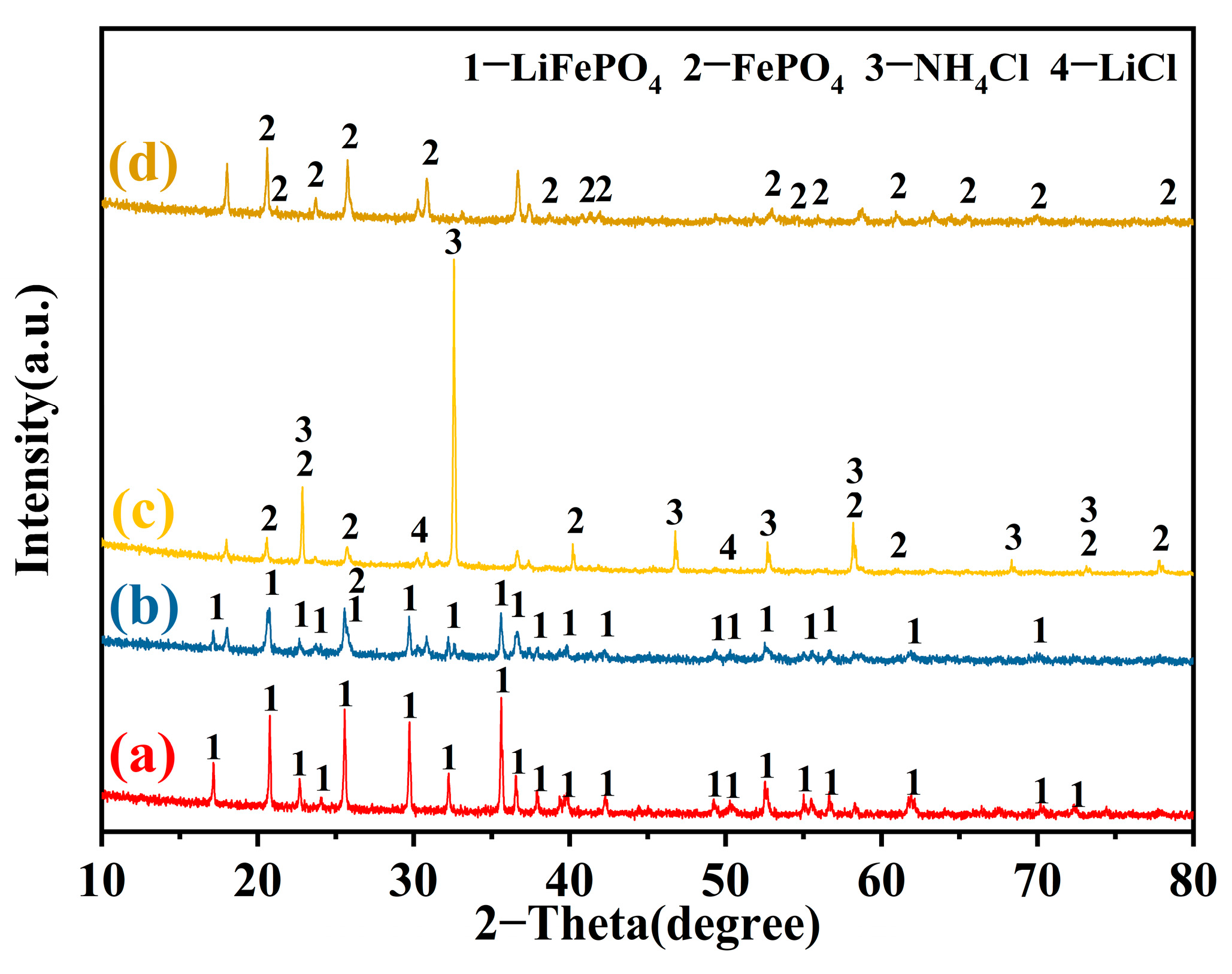
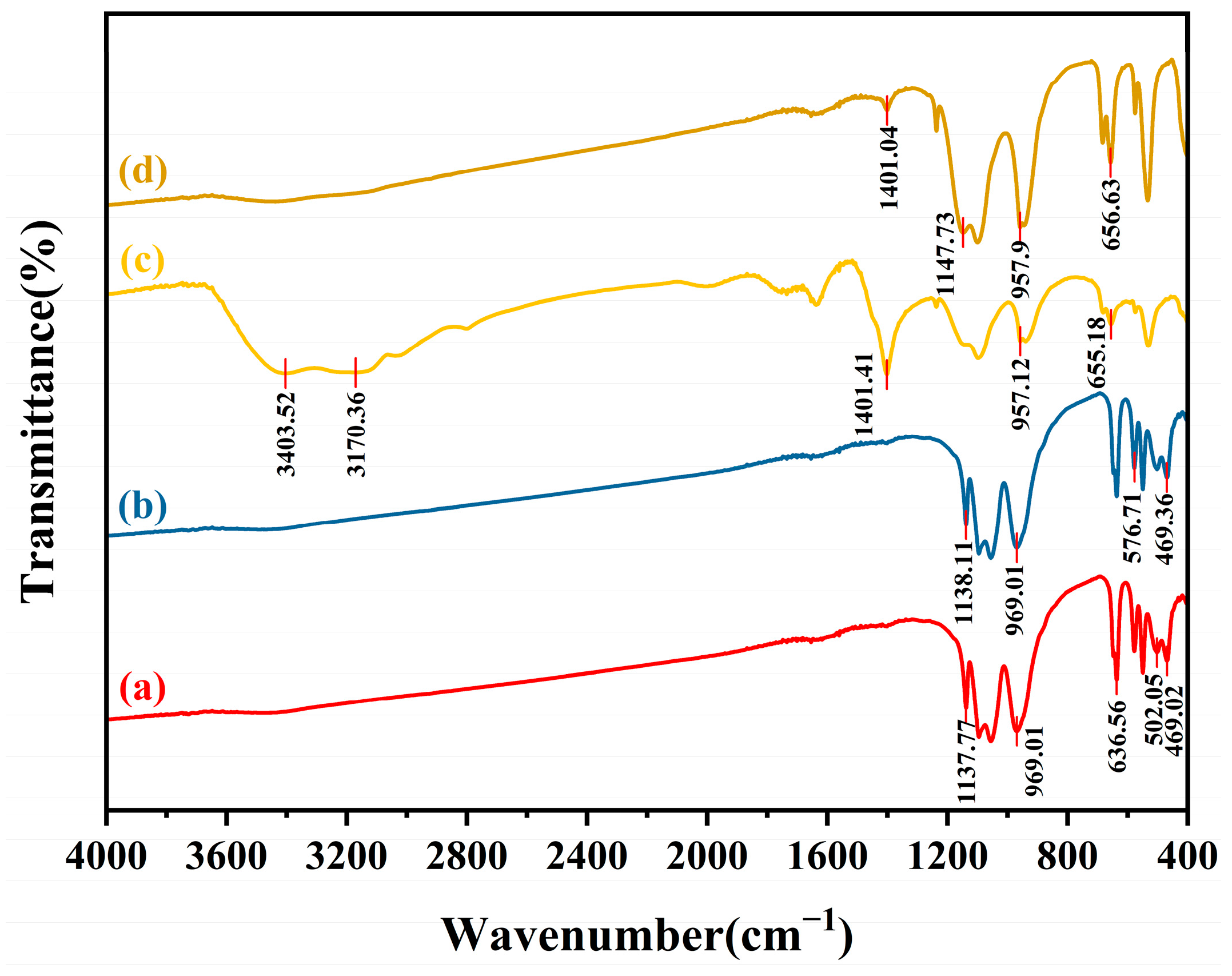
3.1. Optimization of Mechanical Chlorination of LiFePO4
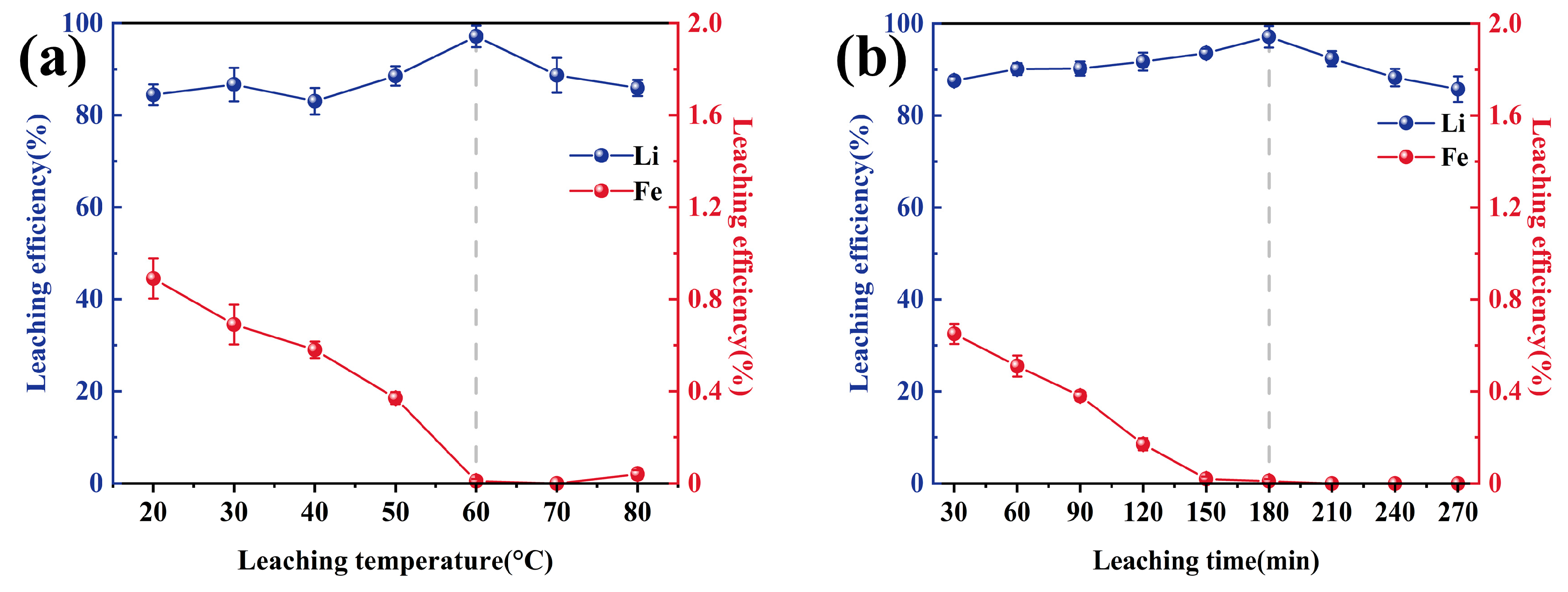
3.2. Water Leaching Separation Effect of Li and Fe
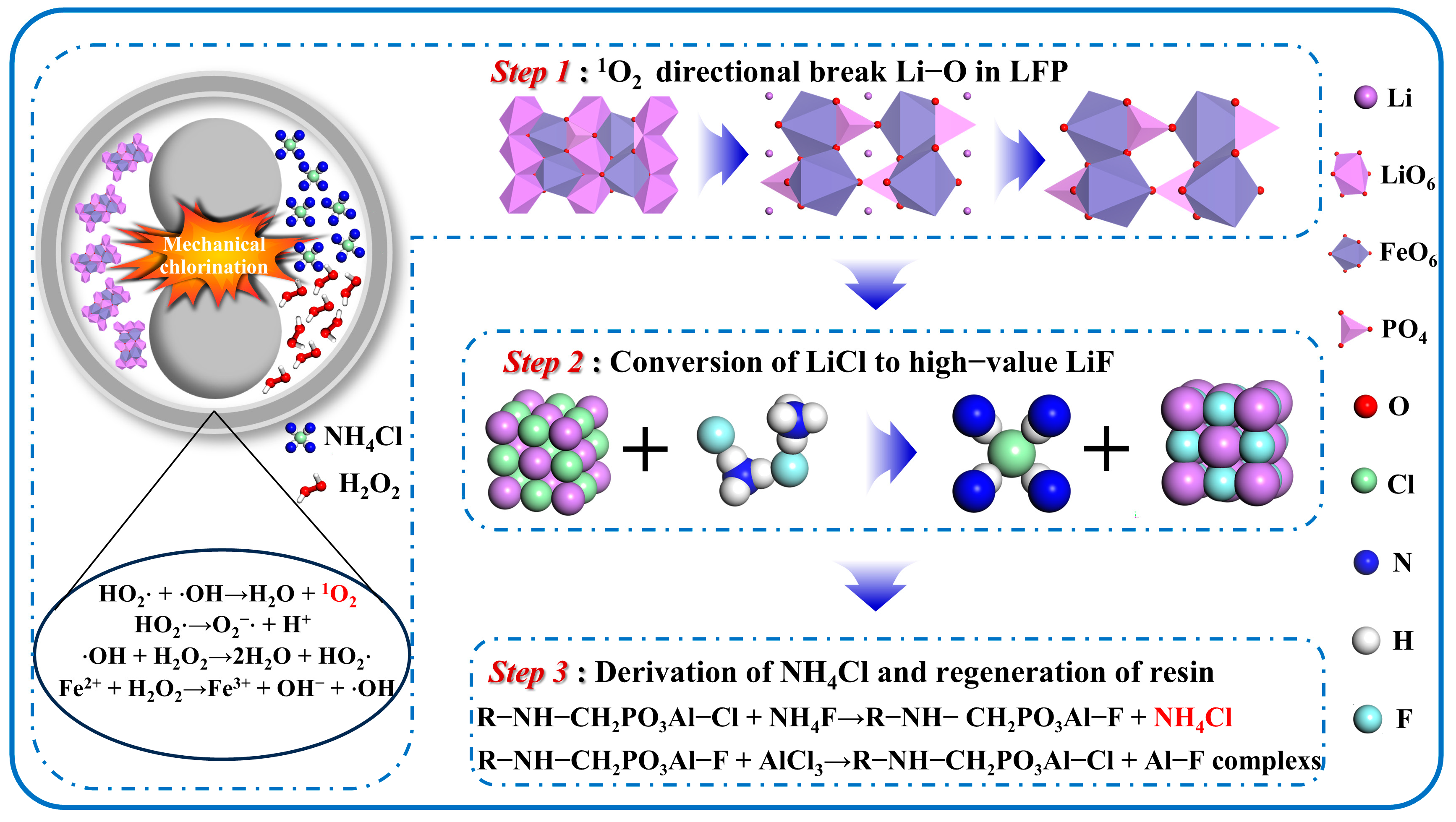
3.3. Mechanism
3.3.1. Mechanism of H2O2-Enhanced Mechanical Chlorination of LFP
3.3.2. Mechanism of Conversion of LiF and Regeneration of NH4Cl
3.4. Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Yang, X.-G.; Liu, T.; Wang, C.-Y. Thermally modulated lithium iron phosphate batteries for mass-market electric vehicles. Nat. Energy 2021, 6, 176–185. [Google Scholar] [CrossRef]
- Feng, J.; Liu, W.; Chen, F. Moving towards a circular economy: A systematic review of barriers to electric vehicle battery recycling. Sustain. Prod. Consum. 2025, 54, 241–260. [Google Scholar] [CrossRef]
- Shentu, H.; Xiang, B.; Cheng, Y.-J.; Dong, T.; Gao, J.; Xia, Y. A fast and efficient method for selective extraction of lithium from spent lithium iron phosphate battery. Environ. Technol. Innov. 2021, 23, 101569. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Ali, H.; Khan, H.A.; Pecht, M.G. Circular economy of Li Batteries: Technologies and trends. J. Energy Storage 2021, 40, 102690. [Google Scholar] [CrossRef]
- Zhao, T.; Li, W.; Traversy, M.; Choi, Y.; Ghahreman, A.; Zhao, Z.; Zhang, C.; Zhao, W.; Song, Y. A review on the recycling of spent lithium iron phosphate batteries. J. Environ. Manag. 2024, 351, 119670. [Google Scholar] [CrossRef]
- Kumar, J.; Neiber, R.R.; Park, J.; Ali Soomro, R.; Greene, G.W.; Ali Mazari, S.; Young Seo, H.; Hong Lee, J.; Shon, M.; Wook Chang, D.; et al. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: Strategies for highly selective lithium recovery. Chem. Eng. J. 2022, 431, 133993. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Qu, D. Proof-of-Concept study of ion-exchange method for the recycling of LiFePO4 cathode. Waste Manag. 2023, 157, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. A Closed-Loop Process for Selective Metal Recovery from Spent Lithium Iron Phosphate Batteries through Mechanochemical Activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980. [Google Scholar] [CrossRef]
- Zhao, T.; Mahandra, H.; Marthi, R.; Traversy, M.; Choi, Y.; Ghahreman, A. Process Development for Selective Recovery of Lithium from Black Mass of Spent LiFePO4 Batteries. In Proceedings of the 61st Conference of Metallurgists, COM 2022; Springer: Cham, Switzerland, 2023; pp. 601–605. [Google Scholar]
- Yang, Y.; Meng, X.; Cao, H.; Lin, X.; Liu, C.; Sun, Y.; Zhang, Y.; Sun, Z. Selective recovery of lithium from spent lithium iron phosphate batteries: A sustainable process. Green Chem. 2018, 20, 3121–3133. [Google Scholar] [CrossRef]
- Forte, F.; Pietrantonio, M.; Pucciarmati, S.; Puzone, M.; Fontana, D. Lithium iron phosphate batteries recycling: An assessment of current status. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2232–2259. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.; Cheng, L.; Gu, J.; Yang, J.; Yuan, H.; Chen, Y.; Wu, Y. Ultra-fast mechanochemistry reaction process: An environmentally friendly instant recycling method for spent LiFePO4 batteries. Sep. Purif. Technol. 2024, 335, 126174. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, E.; Lin, J.; Sun, S.; Zhang, X.; Chen, R.; Wu, F.; Li, L. Acid-free mechanochemical process to enhance the selective recycling of spent LiFePO4 batteries. J. Hazard. Mater. 2023, 443, 130160. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, N.; Wei, X.; Zhang, S.; Li, F.; Wu, P.; Chen, Y. Efficient separation of aluminum foil from mixed-type spent lithium-ion power batteries. J. Environ. Manag. 2021, 298, 113500. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.-S.; Zhang, Z.-Y.; Zhang, C.-C. An environmentally friendly process for selective recovery of lithium and simultaneous synthesis of LiFe5O8 from spent LiFePO4 battery by mechanochemical. J. Clean. Prod. 2023, 396, 136504. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, B.; Xu, Y.; Hu, J.; Deng, W.; Zou, G.; Hou, H.; Yang, Y.; Sun, W.; Hu, Y.; et al. Enabling the sustainable recycling of LiFePO4 from spent lithium-ion batteries. Green Chem. 2022, 24, 2506–2515. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Yao, Y.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. A green and effective room-temperature recycling process of LiFePO4 cathode materials for lithium-ion batteries. Waste Manag. 2019, 85, 437–444. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. Economical recycling process for spent lithium-ion batteries and macro- and micro-scale mechanistic study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]
- Amrute, A.P.; Łodziana, Z.; Schreyer, H.; Weidenthaler, C.; Schüth, F. High-surface-area corundum by mechanochemically induced phase transformation of boehmite. Science 2019, 366, 485–489. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, D.; He, Q.; Yu, J. The feasibility of ofloxacin degradation and electricity generation in photo-assisted microbial fuel cells with LiNbO3/CF photocatalytic cathode. Sep. Purif. Technol. 2020, 250, 117106. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.-S.; Yue, X.-H.; Zhang, Z.-Y. In-situ inducing hydroxyl radicals for the stripping of cathode materials from spent lithium iron phosphate battery. J. Clean. Prod. 2022, 372, 133749. [Google Scholar] [CrossRef]
- Ling, C.; Liu, X.; Li, H.; Wang, X.; Gu, H.; Wei, K.; Li, M.; Shi, Y.; Ben, H.; Zhan, G.J.A.C. Atomic-layered Cu5 nanoclusters on FeS2 with dual catalytic sites for efficient and selective H2O2 activation. Angew. Chem. 2022, 134, e202200670. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Fu, W.; Tian, S.; Yang, Y.; Yang, K.; Xu, X.; Zhang, X. Oriented generation of singlet oxygen in H2O2 activation for water decontamination: Regulation of oxygen vacancies over α-MnO2 nanocatalysts. Environ. Sci. Nano 2023, 10, 1428–1440. [Google Scholar] [CrossRef]
- Wen, X.; Miao, J.; Mandler, D.; Long, M. Rotating ring-disk electrode method to evaluate performance of electrocatalysts in hydrogen peroxide activation via rapid detection of hydroxyl radicals. Chem. Eng. J. 2023, 454, 140312. [Google Scholar] [CrossRef]
- Couch, K.; Leresche, F.; Farmer, C.; McKay, G.; Rosario-Ortiz, F.L. Assessing the source of the photochemical formation of hydroxylating species from dissolved organic matter using model sensitizers. Environ. Sci. Process. Impacts 2022, 24, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Yang, Y.; Chen, J.; Wu, Y.; Ma, Z.; Wu, D. Synergistic activation of persulfate by manganese sulfide and Fe2+: Roles of Fe and enhancement mechanisms. J. Environ. Chem. Eng. 2023, 11, 111454. [Google Scholar] [CrossRef]
- Li, B.; Chen, B.; Wei, Z. Challenging established norms: The unanticipated role of alcohols in UV/PDS radical quenching. J. Hazard. Mater. 2024, 478, 135502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical-Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Cai, L.; Yao, Q.; Du, X.; Tao, X.; Zou, M.; Zhou, J.; Dang, Z.; Lu, G. Identification of Superoxide Contribution through the Quenching Method and Model System. ACS EST Eng. 2024, 4, 2145–2154. [Google Scholar] [CrossRef]
- Cai, L.; Yao, Q.; Du, X.; Zhong, J.; Lu, H.; Tao, X.; Zhou, J.; Dang, Z.; Lu, G. Validation of quenching effectiveness and pollutant degradation ability of singlet oxygen through model reaction system. J. Hazard. Mater. 2023, 460, 132488. [Google Scholar] [CrossRef]
- Lin, Q.; Su, K.; Huang, Y.; He, Y.; Zhang, J.; Yang, X.; Xu, H. Molecular Crystal Structure Simulations and Structure-Magnetic Properties of LiFePO4 Composite Particles Optimized by La. Molecules 2024, 29, 3933. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Fan, E.; Zhang, X.; Li, Z.; Dai, Y.; Chen, R.; Wu, F.; Li, L.J.A.E.M. Sustainable upcycling of spent lithium-ion batteries cathode materials: Stabilization by in situ Li/Mn disorder. Adv. Energy Mater. 2022, 12, 2201174. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Peng, C.; Zhao, Z.; Xiao, J.; Zhao, Y.; Feng, W. Achieving burst Li+ channels via quasi-two-dimensional fluorinated metal-organic framework modulating functionalized interface. Nat. Commun. 2025, 16, 1885. [Google Scholar] [CrossRef]
- Song, J.-I.; Choi, Y.-S. Unveiling the potential of lithium fluoride phosphate (Li2MPO4F, M = Fe, V, Mn) for the next generation of lithium-ion batteries: A comparative study based on first principles and molecular dynamic simulations. J. Power Sources 2025, 626, 235765. [Google Scholar] [CrossRef]
- He, T.; Chen, L.; Su, Y.; Lu, Y.; Bao, L.; Chen, G.; Zhang, Q.; Chen, S.; Wu, F. The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-Rich cathode materials. J. Power Sources 2019, 441, 227195. [Google Scholar] [CrossRef]
- Jin, C.; Xiang, A.; Wang, Z.; He, Q.; Li, B.; Zhang, X.; Xiang, Y.; Zhai, P.; Gong, Y. Anion-Reduction-Catalysis Induced LiF-Rich SEI Construction for High-Performance Lithium-Metal Batteries. Adv. Energy Mater. 2025, 15, 2402811. [Google Scholar] [CrossRef]
- Wang, T.; Duan, J.; Zhang, B.; Luo, W.; Ji, X.; Xu, H.; Huang, Y.; Huang, L.; Song, Z.; Wen, J.; et al. A self-regulated gradient interphase for dendrite-free solid-state Li batteries. Energy Environ. Sci. 2022, 15, 1325–1333. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Wan, H.; Li, A.-M.; Liu, Y.; Liou, S.-C.; Zhang, K.; Ren, Y.; Jayawardana, C.; Lucht, B.L.; et al. Revitalizing interphase in all-solid-state Li metal batteries by electrophile reduction. Nat. Mater. 2025, 24, 414–423. [Google Scholar] [CrossRef]
- Lim, H.; Jun, S.; Song, Y.B.; Baeck, K.H.; Bae, H.; Lee, G.; Kim, J.; Jung, Y.S. Rationally Designed Conversion-Type Lithium Metal Protective Layer for All-Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2024, 14, 2303762. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, L.; Hu, X.; Yang, M.; Liu, H.; Gan, C.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Trace Fluorinated Carbon Dots Driven Li-Garnet Solid-State Batteries. Angew. Chem. Int. Ed. 2024, 63, e202410016. [Google Scholar] [CrossRef]
- Deng, Z.; Xu, A.; Wei, X.; Zhang, X.; Pan, B. Formation of Aluminum–Fluoride Complexes Compromises Defluoridation Efficiency of Aluminum-Based Coagulation. Environ. Sci. Technol. 2024, 58, 14918–14928. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, P.; Qian, C.; Chen, Y.; Li, H. Removal of Fluoride Ions from Strongly Acidic Wastewater Using a Chelating Resin Containing Aluminum. Water Air Soil Pollut. 2023, 234, 285. [Google Scholar] [CrossRef]
- Yan, T.; Zhong, S.; Zhou, M.; Guo, X.; Hu, J.; Wang, F.; Zeng, F.; Zuo, S. High-efficiency method for recycling lithium from spent LiFePO4 cathode. Nanotechnol. Rev. 2020, 9, 1586–1593. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, J.; Wang, D.; Jing, Q.; Chen, Y.; Wang, C. Facile and efficient recovery of lithium from spent LiFePO4 batteries via air oxidation–water leaching at room temperature. Green Chem. 2022, 24, 152–162. [Google Scholar] [CrossRef]
- Liu, K.; Wang, M.; Zhang, Q.; Xu, Z.; Labianca, C.; Komárek, M.; Gao, B.; Tsang, D.C.W. A perspective on the recovery mechanisms of spent lithium iron phosphate cathode materials in different oxidation environments. J. Hazard. Mater. 2023, 445, 130502. [Google Scholar] [CrossRef]
- Liu, K.; Liu, L.; Tan, Q.; Li, J. Selective extraction of lithium from a spent lithium iron phosphate battery by mechanochemical solid-phase oxidation. Green Chem. 2021, 23, 1344–1352. [Google Scholar] [CrossRef]
- Dubey, S.; Agarwal, M.; Gupta, A.B. Experimental investigation of Al-F species formation and transformation during coagulation for fluoride removal using alum and PACl. J. Mol. Liq. 2018, 266, 349–360. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, F.; Wang, D. Speciation, stability, and coagulation mechanisms of hydroxyl aluminum clusters formed by PACl and alum: A critical review. Adv. Colloid Interface Sci. 2015, 226, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; George, S. Removal of Fluoride from Drinking Water Using Novel Adsorbent Magnesia-Hydroxyapatite. Water Air Soil Pollut. 2015, 226, 241. [Google Scholar] [CrossRef]





| Elements | Li | Fe | P | Al |
|---|---|---|---|---|
| content (wt%) | 4.12 | 33.89 | 17.48 | 0.19 |
| Elements | Li | F | Fe | Al | Mg | Cu |
|---|---|---|---|---|---|---|
| LiF | 26.62 | 72.88 | 0.05 | 0.03 | 0.02 | 0.01 |
| Li2CO3 | 18.66 | 0.00 | 0.03 | 0.02 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Zhu, N.; Li, F.; Zhang, P.; Wu, P.; Hu, Y. Spent LiFePO4 to High-Value LiF: Enhanced Mechanical Chlorination Coupled with a Fluorination Reaction Mechanism. Processes 2025, 13, 1478. https://doi.org/10.3390/pr13051478
Liang C, Zhu N, Li F, Zhang P, Wu P, Hu Y. Spent LiFePO4 to High-Value LiF: Enhanced Mechanical Chlorination Coupled with a Fluorination Reaction Mechanism. Processes. 2025; 13(5):1478. https://doi.org/10.3390/pr13051478
Chicago/Turabian StyleLiang, Chao, Nengwu Zhu, Fei Li, Pengfei Zhang, Pingxiao Wu, and Yaxi Hu. 2025. "Spent LiFePO4 to High-Value LiF: Enhanced Mechanical Chlorination Coupled with a Fluorination Reaction Mechanism" Processes 13, no. 5: 1478. https://doi.org/10.3390/pr13051478
APA StyleLiang, C., Zhu, N., Li, F., Zhang, P., Wu, P., & Hu, Y. (2025). Spent LiFePO4 to High-Value LiF: Enhanced Mechanical Chlorination Coupled with a Fluorination Reaction Mechanism. Processes, 13(5), 1478. https://doi.org/10.3390/pr13051478







