Effects of Supercritical CO2 Immersion Time on CO2/CH4 Gas Seepage Characteristics in Coal
Abstract
1. Introduction
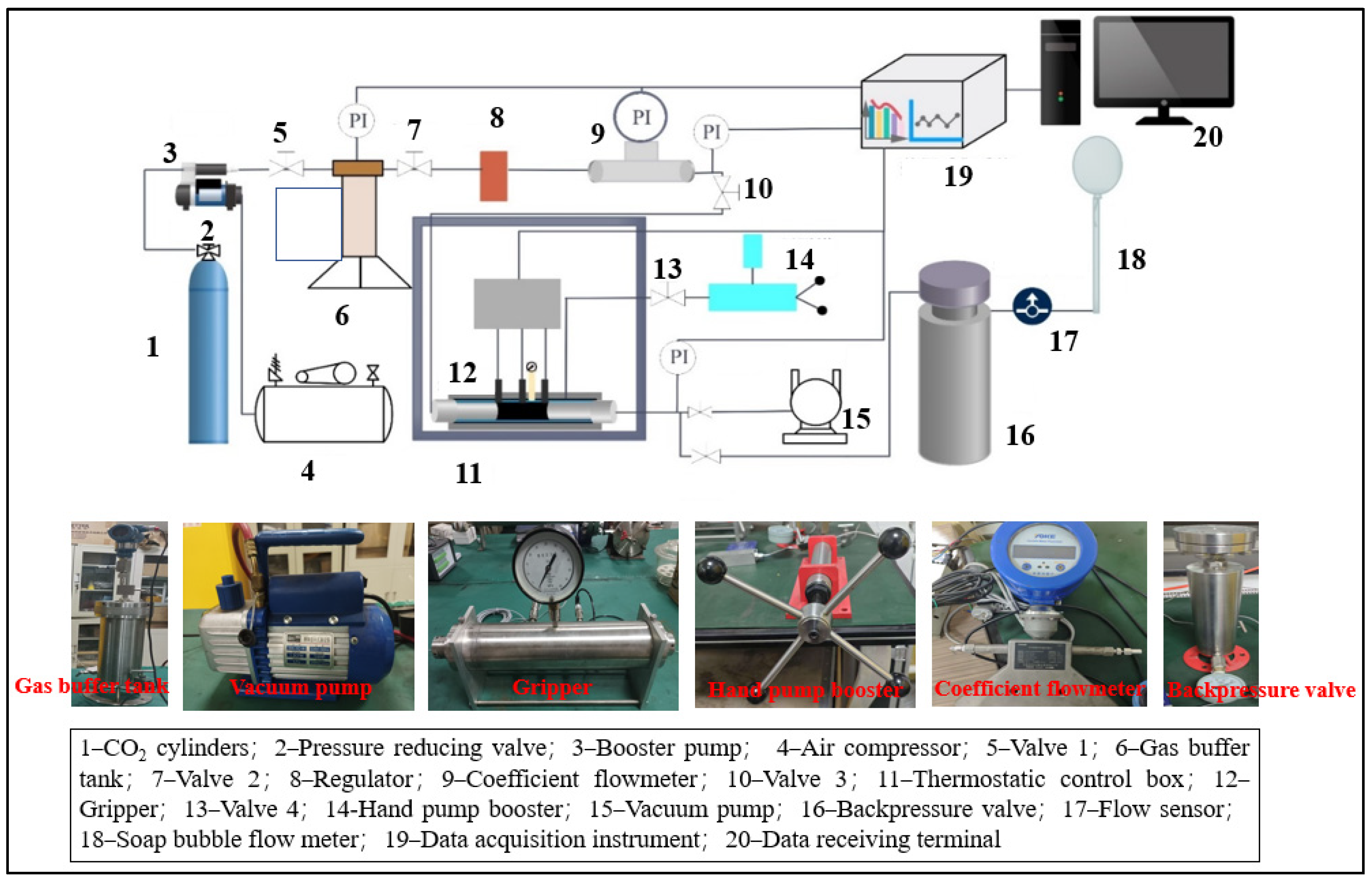
2. Experimental Methods
2.1. Subsection
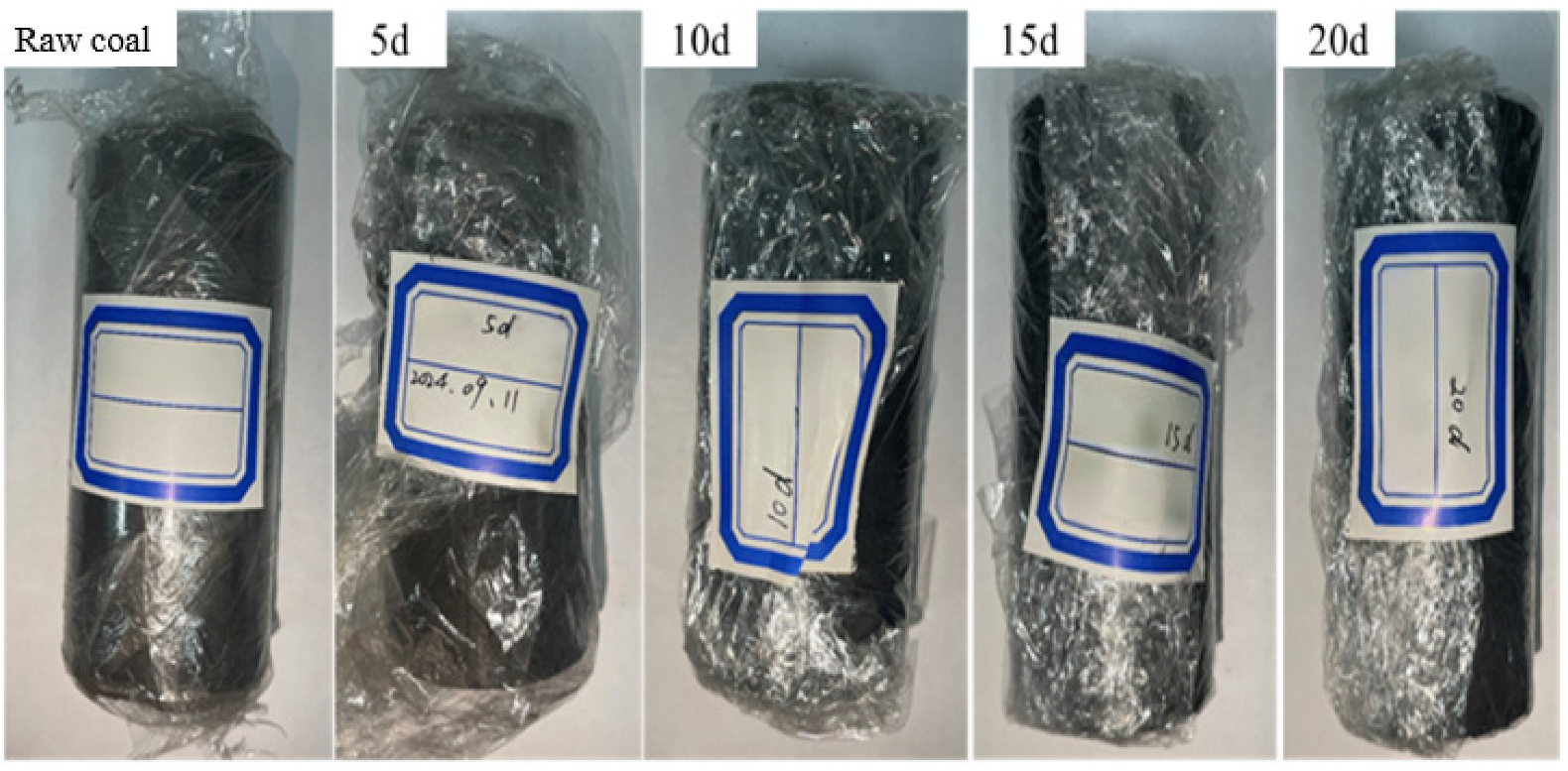
2.1.1. Coal Sample Selection and Pretreatment
2.1.2. Experimental Program
- (1)
- Coal samples in supercritical CO2 medium solution leaching treatment
- (2)
- Permeability experiment of coal samples after immersion under single-component gas (CO2/CH4) conditions.
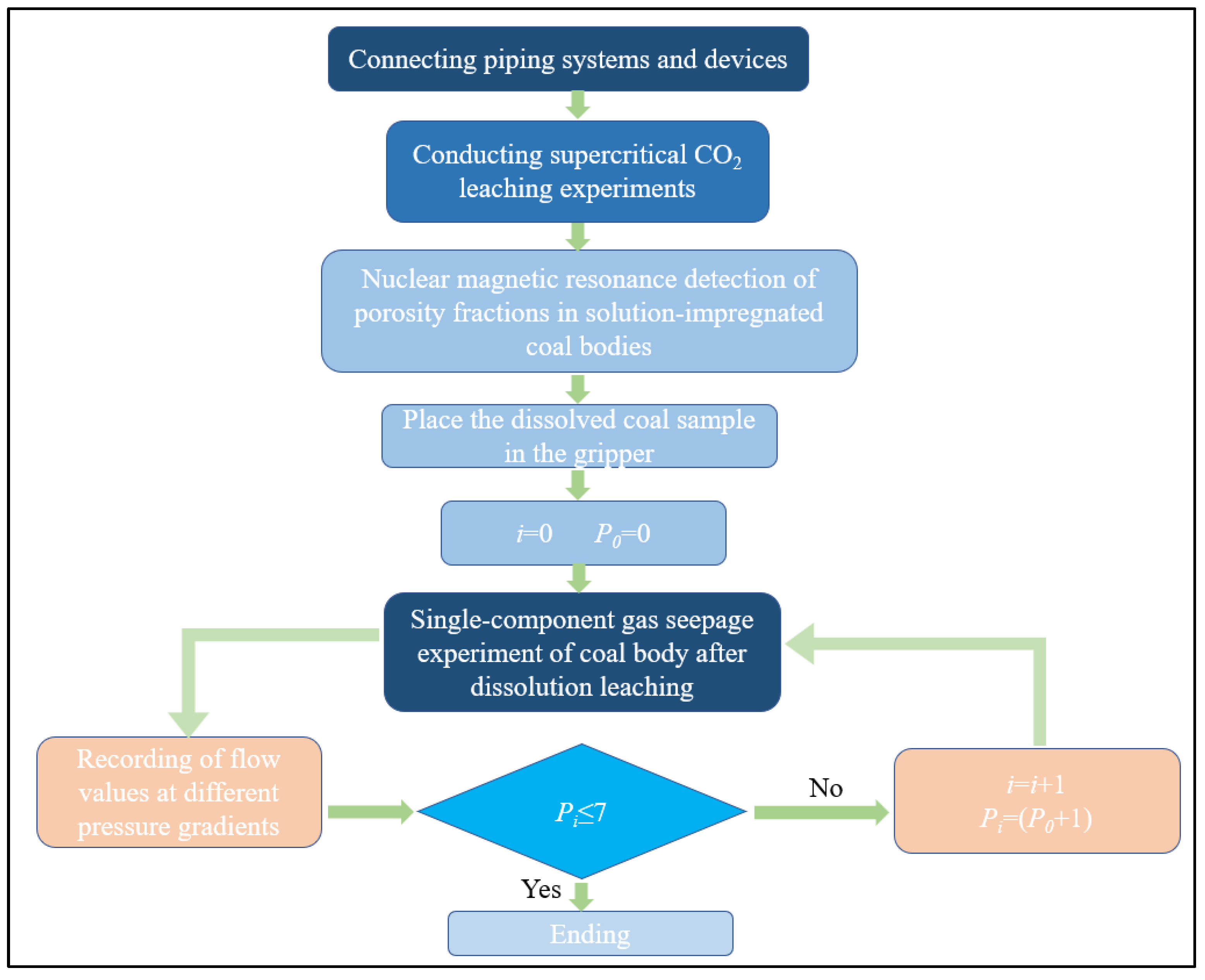
2.2. Experimental Preparation Stage
- (1)
- The coal samples were placed in a high-pressure leaching vessel, where supercritical CO2 was injected for the leaching experiments at different time intervals. After leaching, the coal samples were placed in a vacuum-pressurized water saturation device, where they were evacuated to saturation. The pore structure of the water-saturated coal samples and the pore structure of the centrifuged coal samples were measured using NMR systems and a centrifuge.
- (2)
- Based on the pressure distribution characteristics of the coal reservoir in the study area, the pressure in the experimental conditions was adjusted according to the corresponding strength of the sample using similarity theory. Given that the experimental coal samples were relatively small with lower strength, the confining pressure and gas pressure were proportionally reduced to reflect the field conditions. The coal columns, leached for different time intervals, were placed into a loading device, and the confining pressure was gradually applied up to 8 MPa.
- (3)
- Before the experiment began, a vacuum pump was used to evacuate the coal column inside the loading device. The data acquisition unit was then activated, and the gas inlet valve was opened. Under a constant temperature of 300 K, pure CO2 gas was injected into the loading device at a constant pressure ranging from 1 to 7 MPa. By adjusting the pressure-regulating valve, the injection pressure was gradually increased from 1 MPa to 7 MPa. Simultaneously, the flow rates at the gas inlet and outlet were measured. Once the flow rates stabilized, the injection pressure was increased in steps, and the flow values at different pressure gradients were recorded.
- (4)
- At the end of the experiment, the data acquisition system and the gas cylinder valve were first closed. The pressure relief valve was then opened under ventilated conditions to release the gas pressure from the inlet-end pipeline. Next, the back-pressure valve was adjusted to 0, and the gas pressure accumulated at the outlet was released. Finally, the confining pressure was removed, and the coal column was taken out by opening both ends of the loading device.
- (5)
- The coal samples were replaced with those leached for different durations, and the data were recorded. Steps (3) to (4) were repeated.
- (6)
- The CO2 gas was replaced with CH4 gas, and steps (3) to (4) were repeated.
3. Results
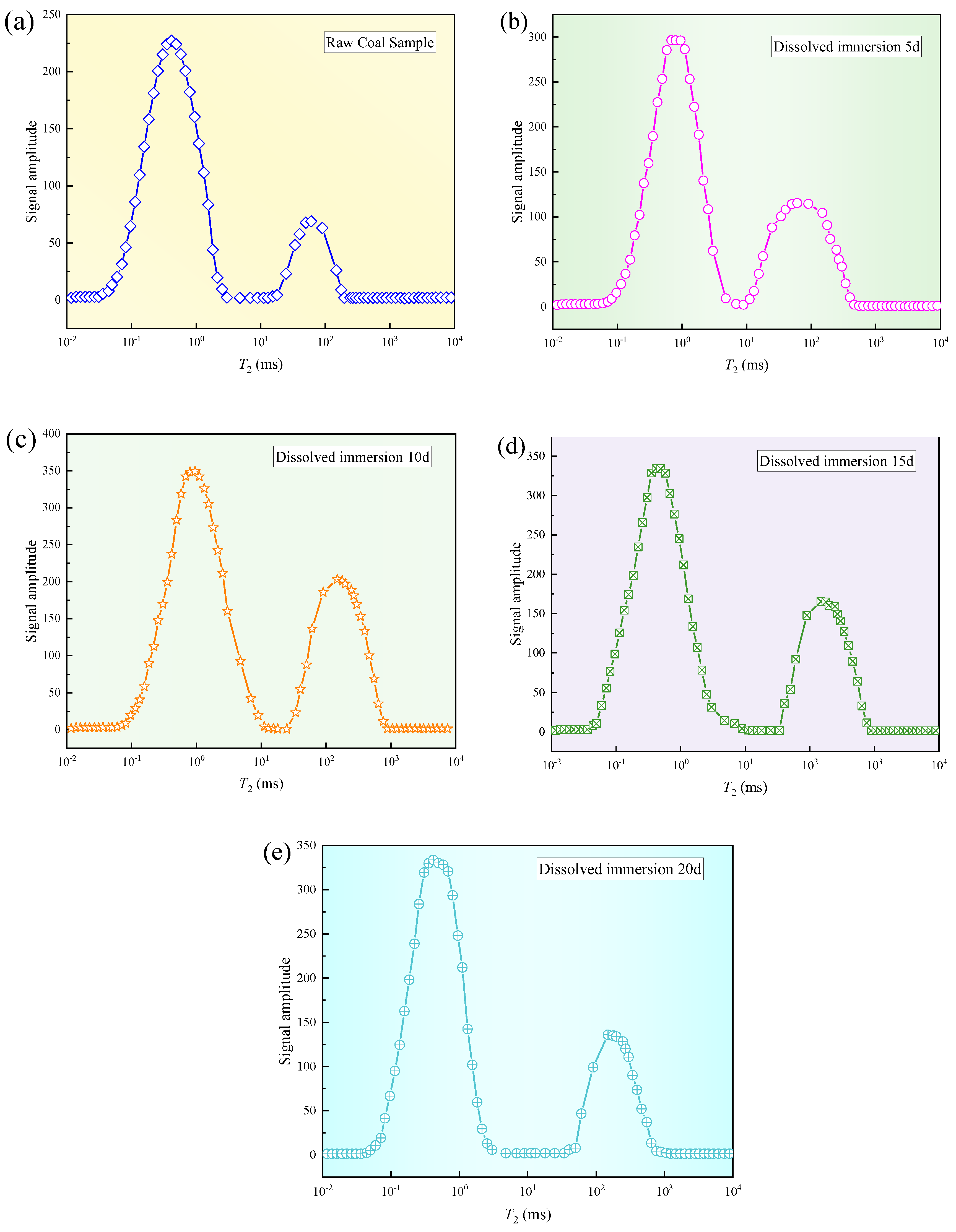
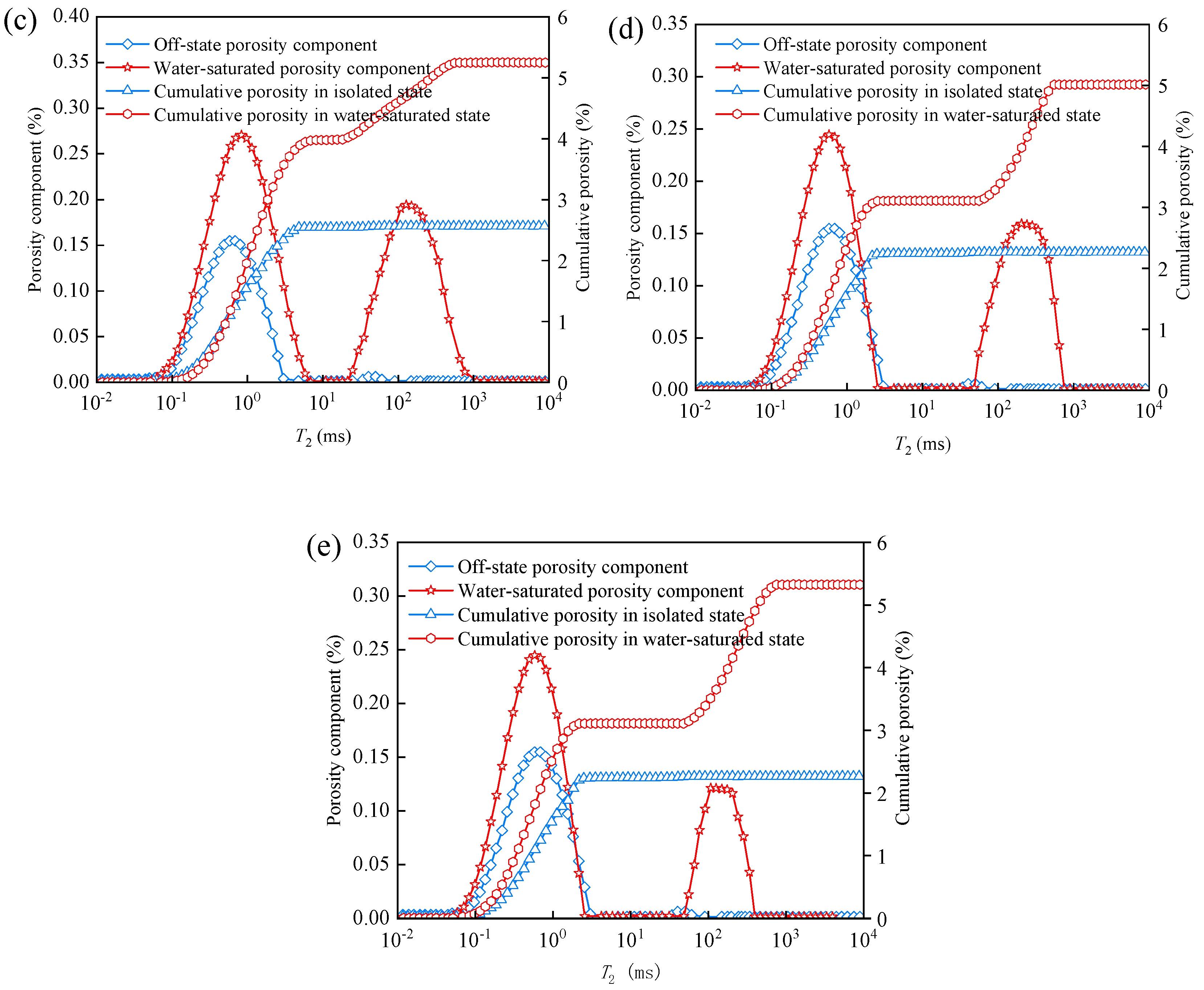
3.1. Pore Distribution in T2 Mapping of Water-Saturated Coal Bodies for Different Dissolution Leaching Time Lengths
3.2. Changes in Porosity Fractions and Cumulative Porosity of Water-Saturated and Centrifuged Coal for Different Dissolution Leaching Durations
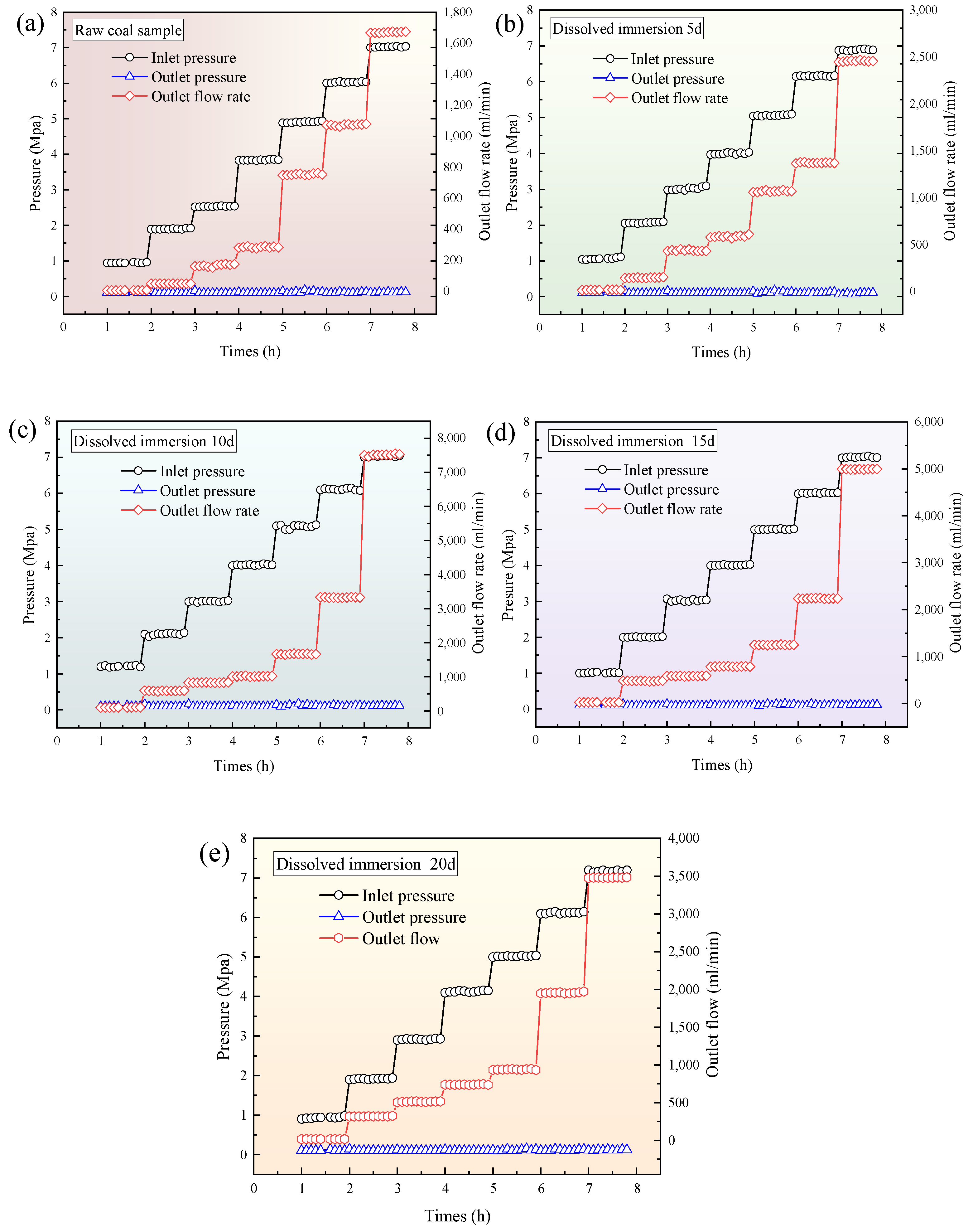
3.3. Time-Varying Characteristics of Gas Seepage in Coal Samples with Different Leaching Times
- (1)
- The higher dynamic viscosity of CO2: Under the same pressure conditions, CO2 has a higher dynamic viscosity than CH4, which leads to greater flow resistance along the flow path [25].
- (2)
- CO2’s tendency to enter micronanopores: CO2 is more likely to enter micronano-sized pores due to its molecular properties, and it has a higher specific surface area compared with CH4. This allows CO2 to adsorb onto the surface of coal, increasing local resistance.
- (3)
- Transition of CO2 into a liquid-like state: As the injection pressure increases, CO2 undergoes a physical transition toward a liquid-like state, while CH4 remains in a gaseous state. The interactions between CO2 and the coal matrix create more resistance than those between CH4 and the coal. Thus, after impregnation, the permeability of CH4 in the coal is stronger than that of CO2.
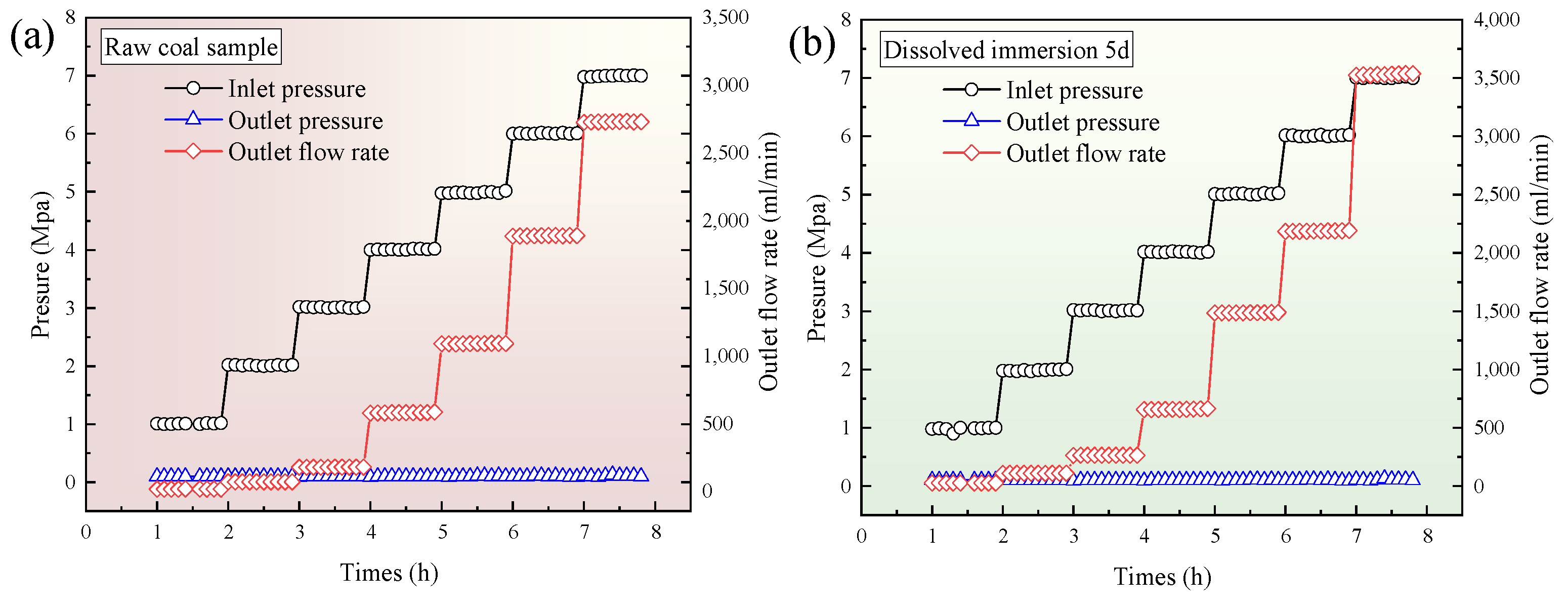
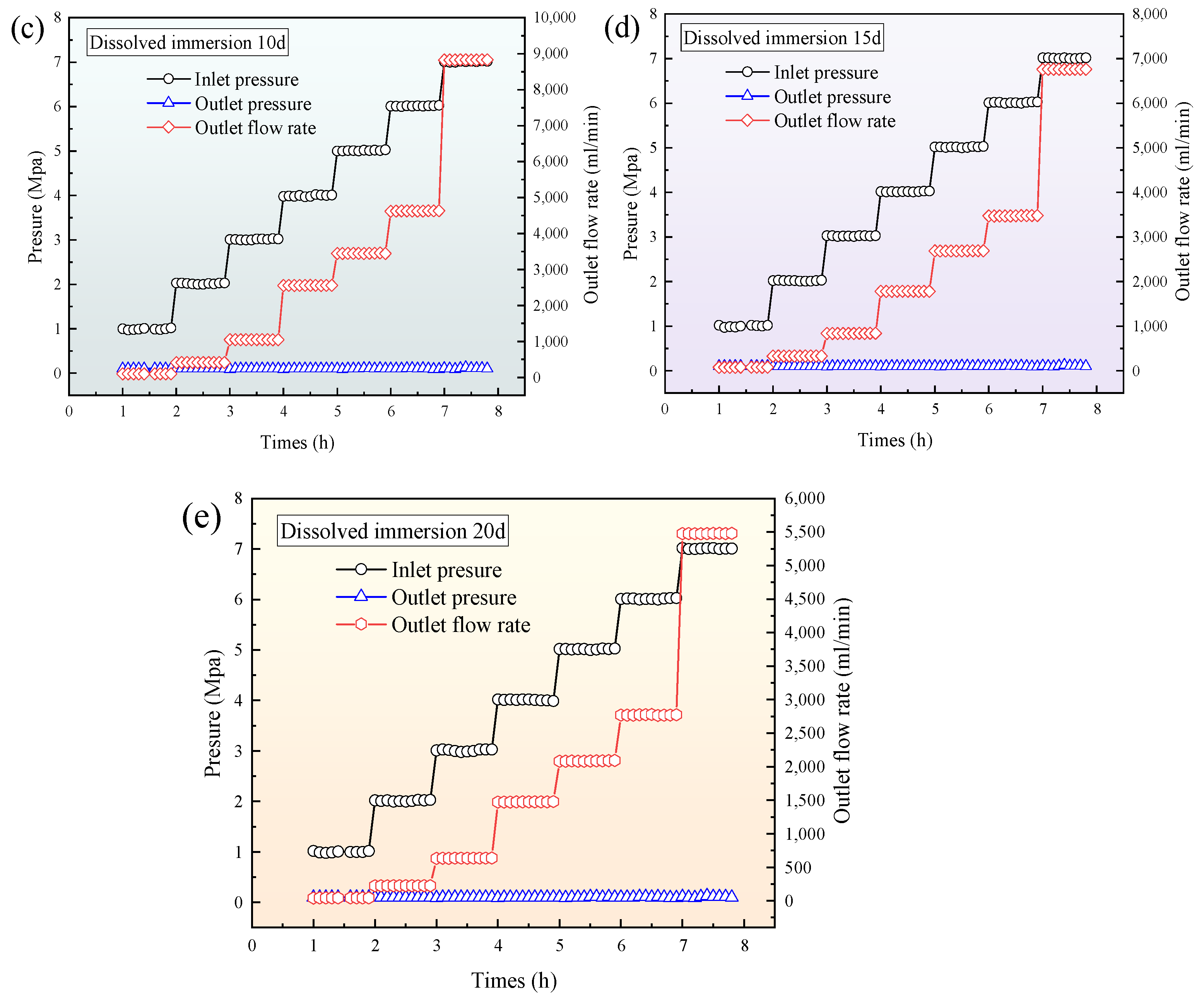
3.4. Relationship Between Outlet Flow Rate and Inlet Pressure of Single-Component CO2 and CH4 Gases with Different Leaching Times
3.5. Relationship Between Seepage Pressure Gradient and Permeability of Single-Component CO2 and CH4 Gases with Different Leaching Times
4. Conclusions
- (1)
- The evolution of the coal′s pore structure with the supercritical CO2 immersion time showed a staged development characteristic. The original coal sample had a porosity of 2.06%, and after immersion for 5 d, 10 d, 15 d, and 20 d, the porosities increased by 2.78%, 3.26%, 3.22%, and 2.86%, respectively. This evolution process revealed a three-stage variation pattern of the coal porosity: “increase–stabilization–decline”. Based on this, it is suggested that in practical engineering applications, the treatment time should be controlled at around 10 d to achieve the optimal permeability enhancement effect.
- (2)
- After the supercritical CO2 soaking, during the single-component gas permeability experiment, the outlet flow rates of both CO2 and CH4 showed an initial increase followed by a decrease as the soaking time progressed. Both gases reached their maximum outlet flow at 10 d of soaking. Compared with the raw coal sample, the outlet flow rates of CO2 and CH4 at 10 d increased by 4.49 times and 3.23 times, respectively. After soaking, the CH4 permeability in the coal body was stronger than that of CO2.
- (3)
- As the pressure gradient increased, the permeability of the CO2 gas for different soaking times showed a characteristic of initially decreasing and then increasing. In contrast, the permeability of the CH4 gas showed a nonlinear increasing trend. When the supercritical CO2 soaking time reached 10 d, due to the extraction and leaching effects of supercritical CO2, the adsorption space within the coal body’s pores transformed into a flow space, resulting in the best permeability for both CO2 and CH4 gases during this stage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Tang, H.; Wu, Y.; Wang, K.; Xu, C. Gas seepage in underground coal seams: Application of the equivalent scale of coal matrix-fracture structures in coal permeability measurements. Fuel 2021, 288, 119641. [Google Scholar] [CrossRef]
- Qiu, L.; Dang, J.; Zhang, J.; Wang, M.; Liu, Q.; Si, L.; Jiang, Z.; Khan, M. Investigating nonlinear resistivity characteristics and mechanisms of coal during various loading stages. J. Appl. Geophys. 2025, 238, 105705. [Google Scholar] [CrossRef]
- Song, D.; Liu, Q.; Qiu, L.; Zhang, J.; Majid, K.; Peng, Y.; Zhao, Y.; Wang, M.; Guo, M.; Hong, T. Experimental study on resistivity evolution law and precursory signals in the damage process of gas-bearing coal. Fuel 2024, 362, 130798. [Google Scholar]
- Li, R.; Wen, H.; Fan, S.; Wang, H.; Cheng, X.; Mi, W.; Liu, B.; Liu, M. Migration characteristics of constant elements in the process of coal dissolution by liquid CO2. Energy 2024, 295, 131006. [Google Scholar] [CrossRef]
- Pang, M.; Pan, H.; Ji, B.; Zhang, H.; Zhang, T. Experimental investigation of flow regime transition characteristics of fractured coal bodies around gas extraction boreholes. Energy 2023, 270, 126758. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, B.; Xue, S.; Chen, Z.; Li, H. Coalbed methane emissions and drainage methods in underground mining for mining safety and environmental benefits: A review. Process Saf. Environ. Prot. 2019, 127, 103–124. [Google Scholar] [CrossRef]
- Wen, H.; Yan, L.; Jin, Y.; Wang, Z.; Guo, J.; Deng, J. Coalbed methane concentration prediction and early-warning in fully mechanized mining face based on deep learning. Energy 2023, 264, 126208. [Google Scholar] [CrossRef]
- Wen, H.; Cheng, B.; Zhang, D.; Jia, B.; Liu, X. Improving coalbed methane recovery in fragmented soft coal seams with horizontal cased well cavitation. Sci. Rep. 2024, 14, 29942. [Google Scholar] [CrossRef]
- Cai, J.; Yang, S.; Hu, X.; Song, W.; Song, Y.; Xu, Q.; Zhang, S. Risk assessment of dynamic disasters induced by gas injection displacement in coal seams. Process Saf. Environ. Prot. 2019, 128, 41–49. [Google Scholar] [CrossRef]
- Sun, Y.; Zuo, L.; Li, X.; Liu, X. Enhancing shale gas recovery by carbon dioxide injection: A method of carbon capture, utilization and storage (CCUS). Process Saf. Environ. Prot. 2023, 179, 484–492. [Google Scholar] [CrossRef]
- Fang, H.; Sang, S.; Liu, S.; Liu, S. Experimental simulation of replacing and displacing CH4 by injecting supercritical CO2 and its geological significance. Int. J. Greenh. Gas Control 2019, 81, 115–125. [Google Scholar] [CrossRef]
- Shen, W.; Ma, T.; Zuo, L.; Yang, X.; Cai, J. Advances and prospects of supercritical CO2 for shale gas extraction and geological sequestration in gas shale reservoirs. Energy Fuels 2024, 38, 789–805. [Google Scholar] [CrossRef]
- Lan, Y.; Yang, Z.; Wang, P.; Yan, Y.; Zhang, L.; Ran, J. A review of microscopic seepage mechanism for shale gas extracted by supercritical CO2 flooding. Fuel 2019, 238, 412–424. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, J.; Yang, Y.; Wu, D.; Zhai, W.; Miao, F. Experimental study on coal seam permeability enhancement and CO2 permeability caused by supercritical CO2. Front. Earth Sci. 2023, 10, 1062580. [Google Scholar] [CrossRef]
- Jiang, R.; Yu, H. Interaction between sequestered supercritical CO2 and minerals in deep coal seams. Int. J. Coal Geol. 2019, 202, 1–13. [Google Scholar] [CrossRef]
- Niu, Q.; Cao, L.; Sang, S.; Zhou, X.; Wang, W.; Yuan, W.; Ji, Z.; Wang, H.; Nie, Y. Study on the anisotropic permeability in different rank coals under influences of supercritical CO2 adsorption and effective stress and its enlightenment for CO2 enhance coalbed methane recovery. Fuel 2020, 262, 116515. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Zoback, M.D. Permeability evolution of fractures in shale in the presence of supercritical CO2. J. Geophys. Res. Solid Earth 2021, 126, e2021JB022266. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Su, E.; Cheng, Y. Experimental investigation on the effects of supercritical carbon dioxide on coal permeability: Implication for CO2 injection method. Energy Fuels 2018, 33, 503–512. [Google Scholar] [CrossRef]
- Long, H.; Lin, H.-F.; Yan, M.; Bai, Y.; Tong, X.; Kong, X.-G.; Li, S.-G. Adsorption and diffusion characteristics of CH4, CO2, and N2 in micropores and mesopores of bituminous coal: Molecular dynamics. Fuel 2021, 292, 120268. [Google Scholar] [CrossRef]
- Long, H.; Lin, H.; Yan, M.; Chang, P.; Li, S.G.; Bai, Y. Molecular simulation of the competitive adsorption characteristics of CH4, CO2, N2, and multicomponent gases in coal. Powder Technol. 2021, 385, 348–356. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Petrophysical properties and fluids transportation in gas shale: A NMR relaxation spectrum analysis method. J. China Coal Soc. 2018, 43, 181–189. [Google Scholar]
- Zhai, C.; Sun, Y.; Fan, Y.; Yang, P.; Ge, X.; Wu, F.; Xu, J.; Yu, X.; Liu, T.; Zhao, Y. Application and prospect of low-field nuclear magnetic resonance technology in accurate characterization of coal pore structure. J. China Coal Soc. 2022, 47, 828–848. [Google Scholar]
- Li, R.; Wen, H.; Wang, H.; Fan, S.; Mi, W.; Liu, B.; Cheng, X.; Liu, M. Experimental simulation of water-coal interactions during liquid CO2 injection into coal beds. Energy 2024, 295, 130986. [Google Scholar] [CrossRef]
- Wang, H.; Wen, H.; Li, Z.; Liang, R.; Wang, F.; Fan, S.; Li, R. Experimental study on microstructural modification of coal by liquid CO2 extraction. Fuel 2023, 348, 128631. [Google Scholar] [CrossRef]
- Si, L.; Zhang, H.; Wei, J.; Li, B.; Han, H. Modeling and experiment for effective diffusion coefficient of gas in water-saturated coal. Fuel 2021, 284, 118887. [Google Scholar] [CrossRef]
- Wang, L.; Su, X.; Zhao, W.; Xia, D.; Wang, Q. Enhancement of biomethane production from coal by supercritical CO2 extraction. J. CO2 Util. 2023, 74, 102545. [Google Scholar] [CrossRef]
- Nie, B.; Lu, H.; Li, X.; Li, L.; Lv, C.Q. Experimental study on the characteristic of coal deformation during gas adsorption and desorption process. J. China Coal Soc. 2015, 40, 754–759. [Google Scholar]
- Liu, Y.B.; Cao, S.G.; Li, Y.; Wang, J.; Guo, P.; Xu, J.; Bai, Y.J. Experimental study of swelling deformation effect of coal induced by gas adsorption. Chin. J. Rock Mech. Eng. 2010, 29, 2484–2491. [Google Scholar]
- Li, Y.; Yang, Z.; Ju, X.; Zhou, A. Adsorption and diffusion behavior of CH4 and CO2 in closed and open pores from Zhaozhuang coal. Energy Fuels 2022, 36, 2582–2590. [Google Scholar] [CrossRef]




| Designation | Industrial Analysis/% | |||
|---|---|---|---|---|
| (Mad) | (Vad) | (Aad) | (FCad) | |
| Benxi Group 8# coal sample | 0.74 | 12.64 | 26.00 | 60.62 |
| Serial Number | Drying Time (h) | Temperature (°C) | Pressure (MPa) | Dissolution Time (d) | Note |
|---|---|---|---|---|---|
| 1 | 8.0 | 65.0 | 8.0 | Raw coal sample | Insoluble leaching treatment Supercritical CO2 dissolution leaching treatment |
| 2 | 8.0 | 65.0 | 8.0 | 5.0 | |
| 3 | 8.0 | 65.0 | 8.0 | 10.0 | |
| 4 | 8.0 | 65.0 | 8.0 | 15.0 | |
| 5 | 8.0 | 65.0 | 8.0 | 20.0 |
| Name of Experiment | Seepage Gas | Injection Pressure (MPa) | Name of Experiment | Seepage Gas | Injection Pressure (MPa) |
|---|---|---|---|---|---|
| CO2 seepage experiment | CO2 | 1.0 | CH4 seepage experiment | CH4 | 1.0 |
| 2.0 | 2.0 | ||||
| 3.0 | 3.0 | ||||
| 4.0 | 4.0 | ||||
| 5.0 | 5.0 | ||||
| 6.0 | 6.0 | ||||
| 7.0 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Liu, W.; Li, T.; Fan, S.; Li, R.; Li, L. Effects of Supercritical CO2 Immersion Time on CO2/CH4 Gas Seepage Characteristics in Coal. Processes 2025, 13, 1419. https://doi.org/10.3390/pr13051419
Wang N, Liu W, Li T, Fan S, Li R, Li L. Effects of Supercritical CO2 Immersion Time on CO2/CH4 Gas Seepage Characteristics in Coal. Processes. 2025; 13(5):1419. https://doi.org/10.3390/pr13051419
Chicago/Turabian StyleWang, Ning, Wengang Liu, Tuanjie Li, Shixing Fan, Rijun Li, and Lin Li. 2025. "Effects of Supercritical CO2 Immersion Time on CO2/CH4 Gas Seepage Characteristics in Coal" Processes 13, no. 5: 1419. https://doi.org/10.3390/pr13051419
APA StyleWang, N., Liu, W., Li, T., Fan, S., Li, R., & Li, L. (2025). Effects of Supercritical CO2 Immersion Time on CO2/CH4 Gas Seepage Characteristics in Coal. Processes, 13(5), 1419. https://doi.org/10.3390/pr13051419




