Investigating the Recovery of PVDF/TiO2 Photocatalyst for Methylene Blue Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Analytical Techniques
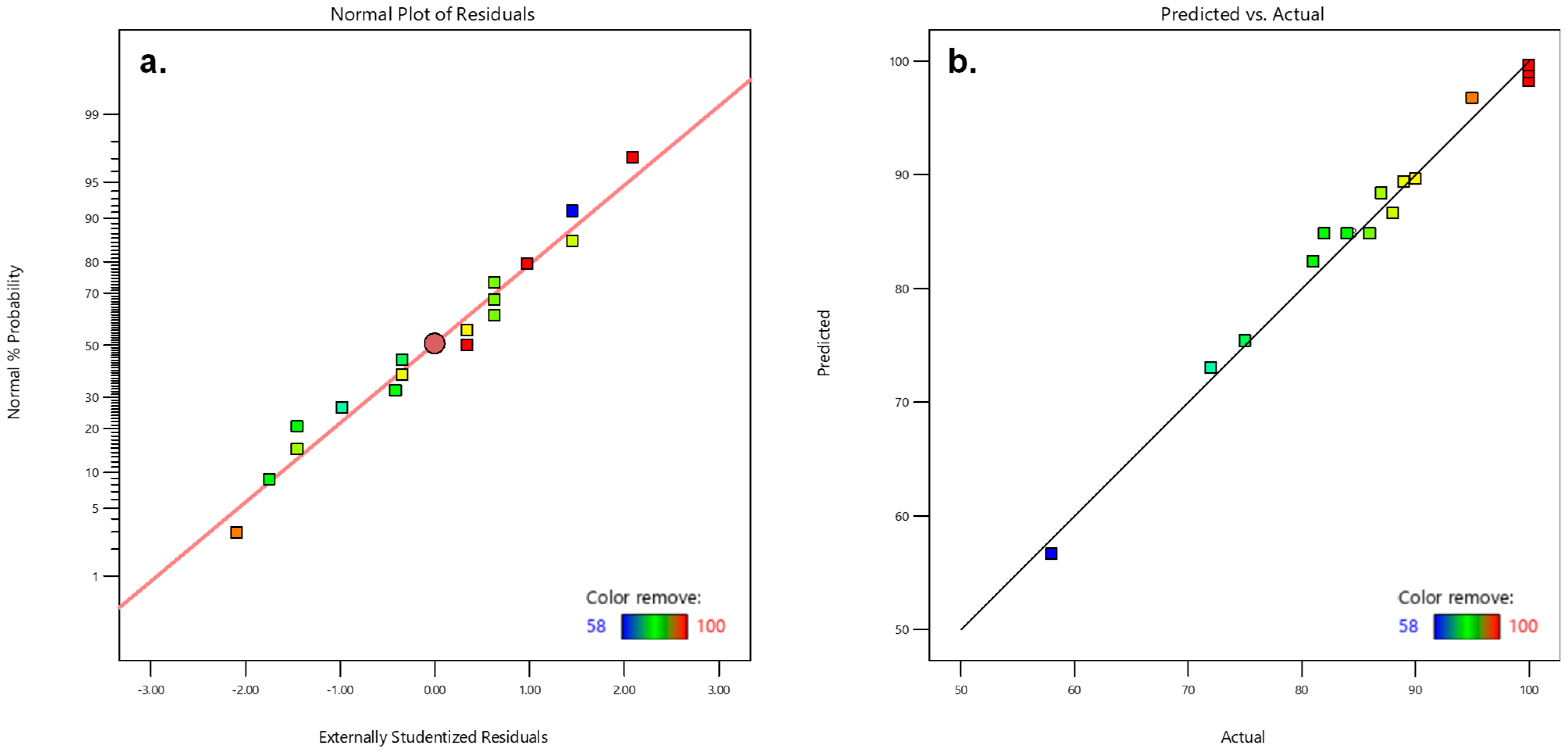
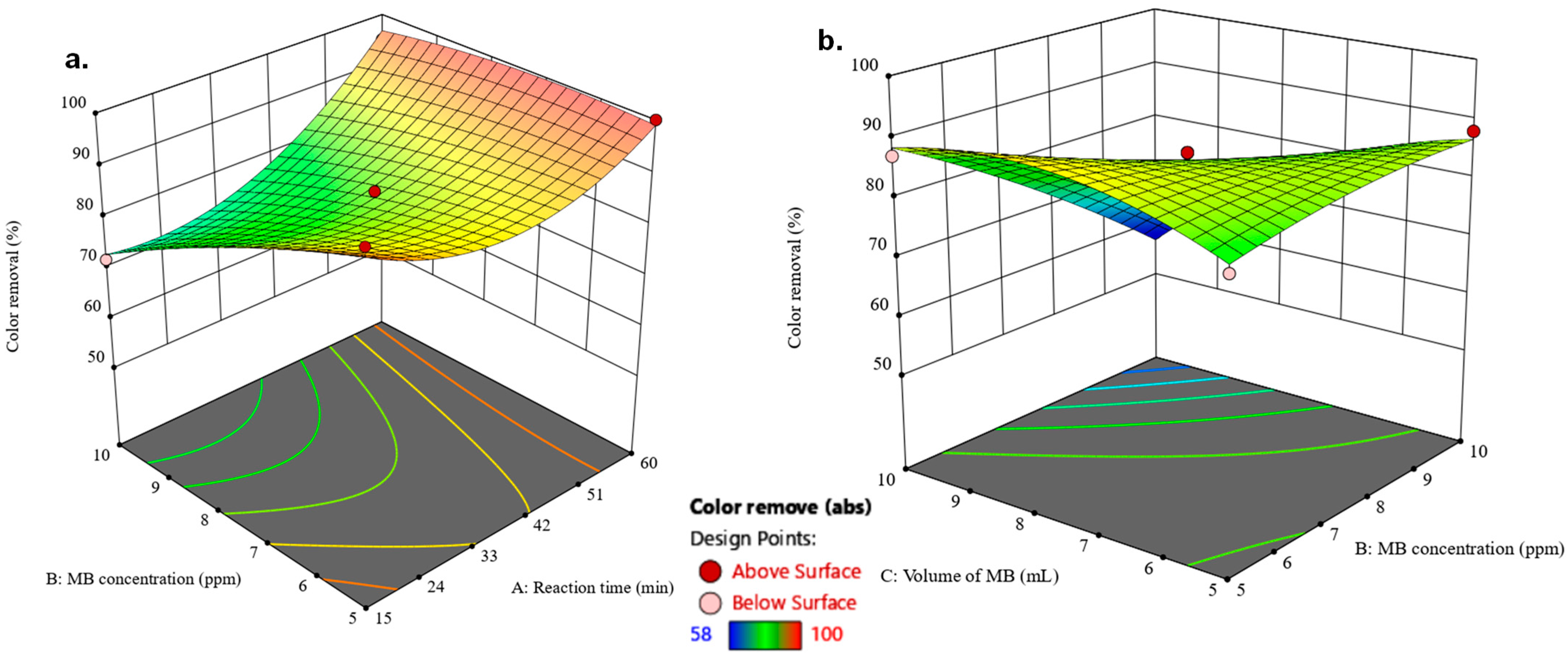
2.3. Box–Behnken Model for Color Removal
3. Results and Discussion
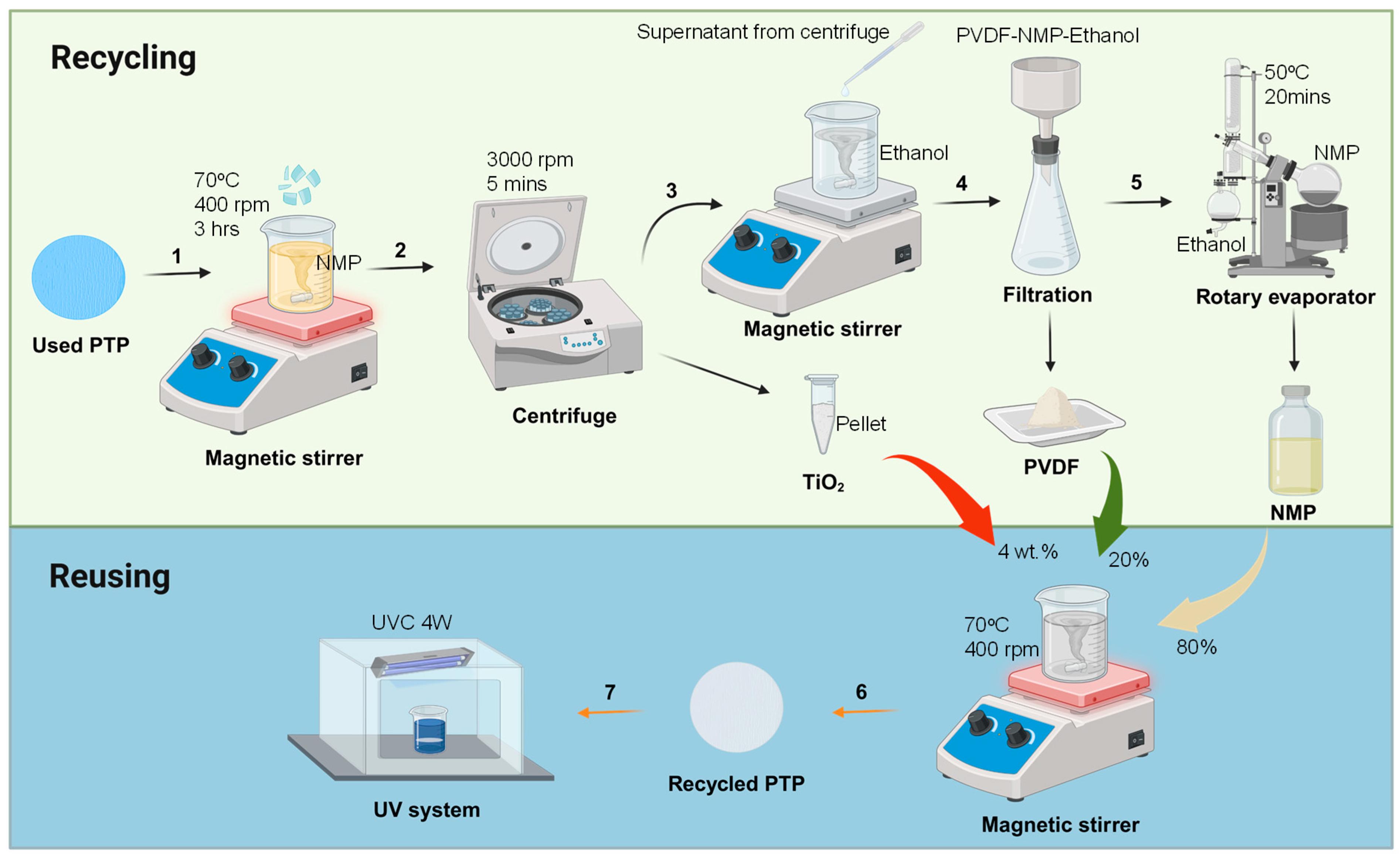
3.1. Formation and Proposed Recovery Method
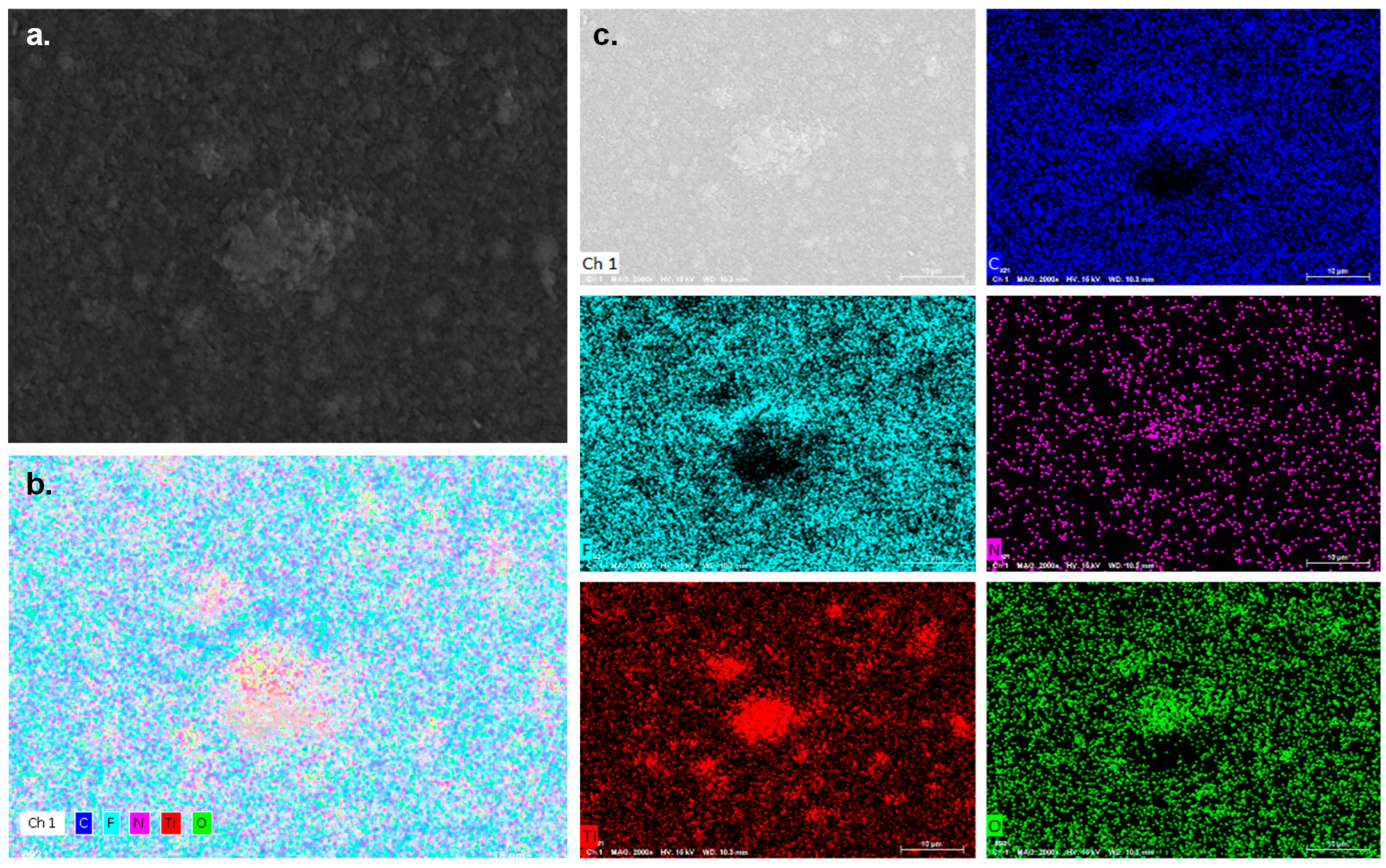
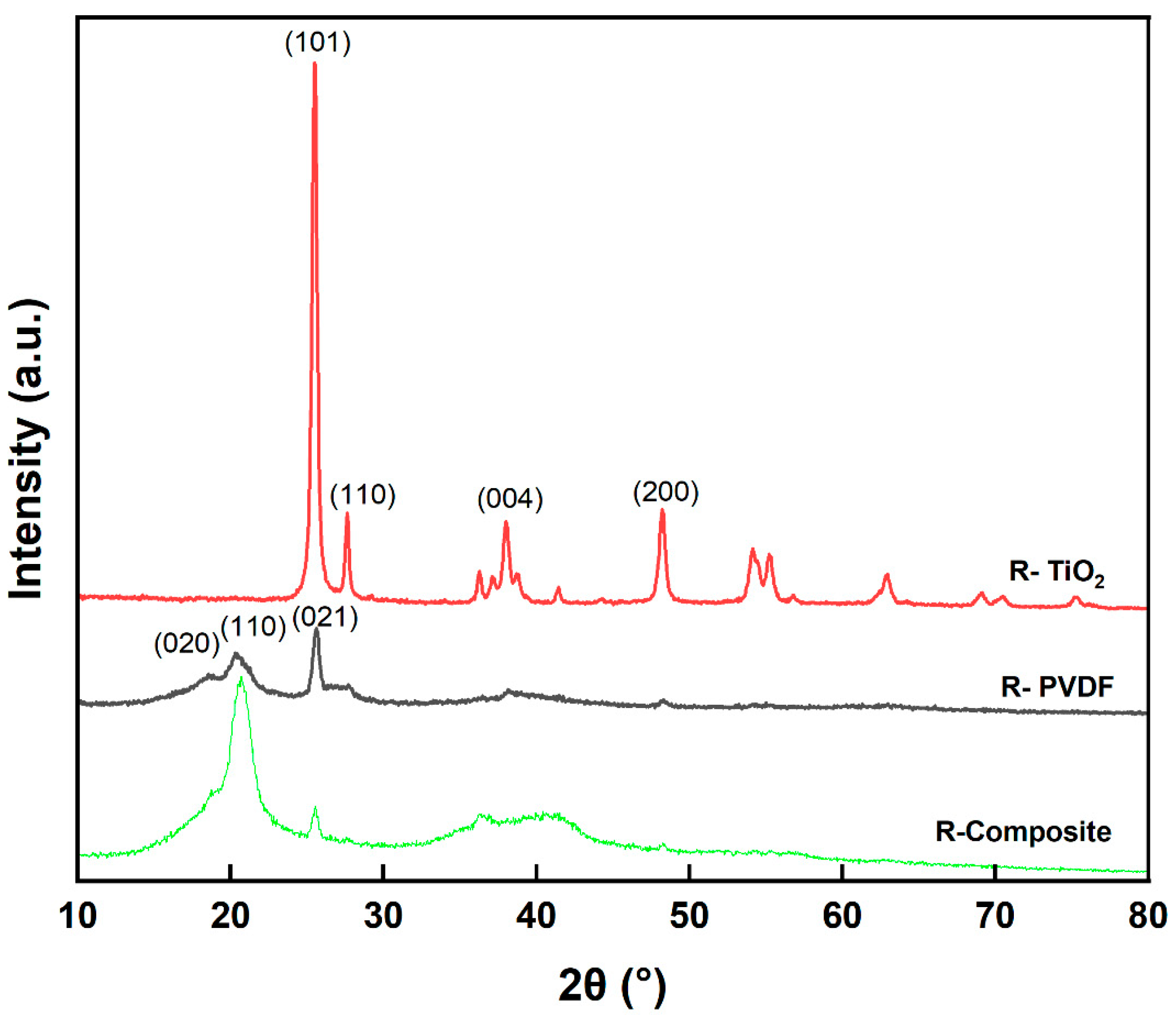
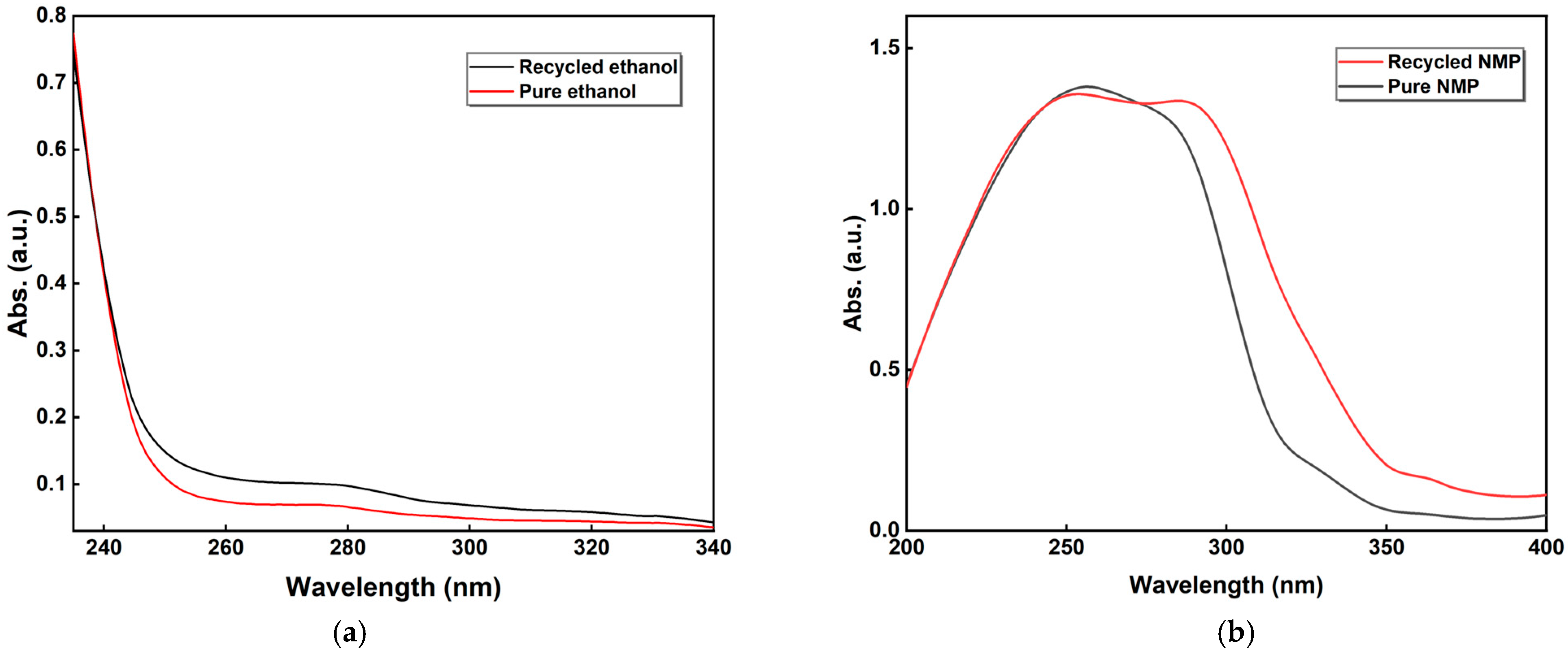
3.2. Recycled Material Characterization
3.3. Recovery Calculation
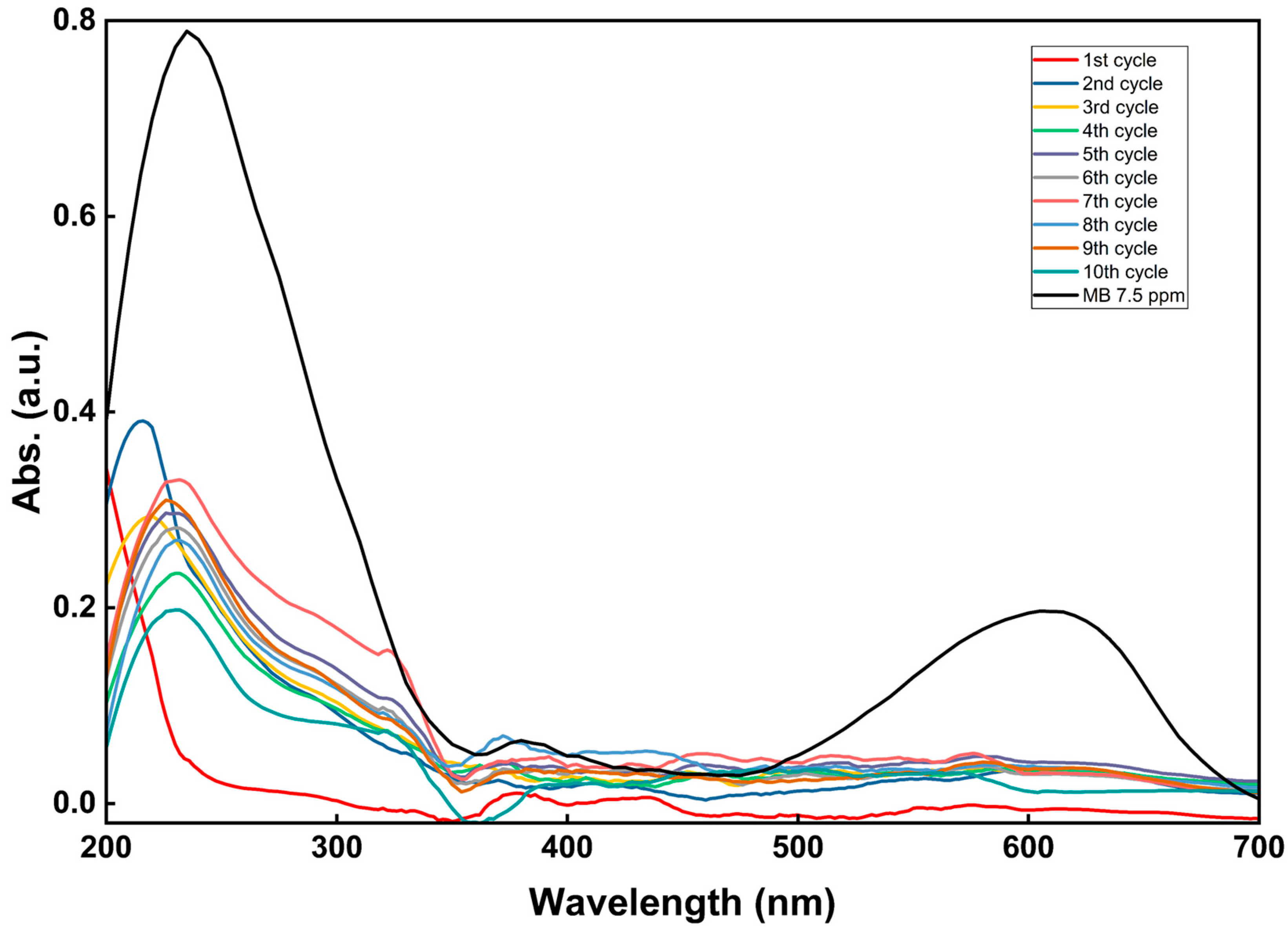
3.4. Reuse Investigation
3.4.1. Methylene Blue Removal Model
3.4.2. Stability Evaluation
3.4.3. Removal Mechanisms
3.5. Summary and Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater Treatment and Reuse: A Review of its Applications and Health Implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Mohadesi, M.; Sanavi Fard, M.; Shokri, A. The application of modified nano-TiO2 photocatalyst for wastewater treatment: A review. Int. J. Environ. Anal. Chem. 2024, 104, 2571–2592. [Google Scholar] [CrossRef]
- Nasir, A.M.; Jaafar, J.; Aziz, F.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F.; Aziz, M. A review on floating nanocomposite photocatalyst: Fabrication and applications for wastewater treatment. J. Water Process Eng. 2020, 36, 101300. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Chinthala, M.; Vo, D.-V.N. Biopolymer-supported TiO2 as a sustainable photocatalyst for wastewater treatment: A review. Environ. Chem. Lett. 2022, 20, 3071–3098. [Google Scholar] [CrossRef]
- Kanakaraju, D.; anak Kutiang, F.D.; Lim, Y.C.; Goh, P.S. Recent progress of Ag/TiO2 photocatalyst for wastewater treatment: Doping, co-doping, and green materials functionalization. Appl. Mater. Today 2022, 27, 101500. [Google Scholar] [CrossRef]
- Ali, H.M.; Arabpour Roghabadi, F.; Ahmadi, V. Solid-supported photocatalysts for wastewater treatment: Supports contribution in the photocatalysis process. Sol. Energy 2023, 255, 99–125. [Google Scholar] [CrossRef]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Z.; Li, M.; Li, X.; Sun, P.; Zhou, L. Construction of magnetic bifunctional β-cyclodextrin nanocomposites for adsorption and degradation of persistent organic pollutants. Carbohydr. Polym. 2020, 230, 115564. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Kim, D.W.; Hong, S.H.; Park, Y.M.; Park, T.J. Visible light-driven g-C3N4@ZnO heterojunction photocatalyst synthesized via atomic layer deposition with a specially designed rotary reactor. Appl. Surf. Sci. 2019, 487, 206–210. [Google Scholar] [CrossRef]
- Meng, L.; Xu, W.; Zhang, Q.; Yang, T.; Shi, S. Study of nanostructural bismuth oxide films prepared by radio frequency reactive magnetron sputtering. Appl. Surf. Sci. 2019, 472, 165–171. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Wisz, G.; Sibiński, M.; Starowicz, Z.; Głowa, Ł.; Szczerba, M.; Cholewa, M. Performance improvement of TiO2/CuO by increasing oxygen flow rates and substrate temperature using DC reactive magnetron sputtering method. Optik 2020, 206, 164297. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Sheikh Abdul Kadir, S.H.; Kurniawan, T.A.; Jilani, A. Immobilization techniques of a photocatalyst into and onto a polymer membrane for photocatalytic activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef] [PubMed]
- Erusappan, E.; Thiripuranthagan, S.; Radhakrishnan, R.; Durai, M.; Kumaravel, S.; Vembuli, T.; Kaleekkal, N.J. Fabrication of mesoporous TiO2/PVDF photocatalytic membranes for efficient photocatalytic degradation of synthetic dyes. J. Environ. Chem. Eng. 2021, 9, 105776. [Google Scholar] [CrossRef]
- Yin, J.; Roso, M.; Boaretti, C.; Lorenzetti, A.; Martucci, A.; Modesti, M. PVDF-TiO2 core-shell fibrous membranes by microwave-hydrothermal method: Preparation, characterization, and photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 106250. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Liu, X.; Li, Q.; Zheng, Y. A novel PVDF-TiO2@g-C3N4 composite electrospun fiber for efficient photocatalytic degradation of tetracycline under visible light irradiation. Ecotoxicol. Environ. Saf. 2021, 210, 111866. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Song, Y.; Zhang, J.; Qin, S.; Yang, Y.; Li, J.; Cui, Z. In situ growth of TiO2 and its immobilization on PVDF films for the adsorption and photocatalytic degradation of dye. Int. J. Hydrogen Energy 2024, 51, 837–847. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Zhou, Y.; Yang, L.; Zhang, Y.; Wu, Z.; Liu, G.; Zheng, J. Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions. Membranes 2022, 12, 386. [Google Scholar] [CrossRef]
- Tian, C.; Chen, J.; Bai, Z.; Wang, X.; Dai, R.; Wang, Z. Recycling of end-of-life polymeric membranes for water treatment: Closing the loop. J. Membr. Sci. Lett. 2023, 3, 100063. [Google Scholar] [CrossRef]
- Liang, L.; Ma, Y.; Ji, X.; Ma, J.; Zhang, W.; Song, L. The sustainable recycling of polyvinylidene fluoride membrane for tribological application. J. Appl. Polym. Sci. 2023, 140, e54287. [Google Scholar] [CrossRef]
- Patel, R.V.; Raj, G.B.; Chaubey, S.; Yadav, A. Investigation on the feasibility of recycled polyvinylidene difluoride polymer from used membranes for removal of methylene blue: Experimental and DFT studies. Water Sci. Technol. 2022, 86, 194–210. [Google Scholar] [CrossRef]
- Bilici, Z.; Bouchareb, R.; Sacak, T.; Yatmaz, H.C.; Dizge, N. Recycling of TiO2-containing waste and utilization by photocatalytic degradation of a reactive dye solution. Water Sci. Technol. 2020, 83, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-x.; Liu, G.; Li, R.; Meng, Y.; Wu, J. Polyporous PVDF/TiO2 photocatalytic composites for photocatalyst fixation, recycle, and repair. J. Am. Ceram. Soc. 2021, 104, 6290–6298. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Liu, H.; Huang, Q.; Chen, M. Fabrication and properties of recycled poly (vinylidene fluoride) (PVDF) hollow fiber membranes. Desalin. Water Treat. 2017, 87, 82–90. [Google Scholar] [CrossRef]
- Phan, H.N.Q.; Leu, H.J.; Nguyen, V.N.D. Unleashing the potential of electrooxidation and PVDF/TiO2 photocatalysis for textile dye wastewater treatment. Int. J. Environ. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Phan, H.N.Q.; Leu, H.-J.; Nguyen, V.N.D. Enhancing pharmaceutical wastewater treatment: Ozone-assisted electrooxidation and precision optimization via response surface methodology. J. Water Process Eng. 2024, 58, 104782. [Google Scholar] [CrossRef]
- Ferreira, N.; Viana, T.; Henriques, B.; Tavares, D.S.; Jacinto, J.; Colónia, J.; Pinto, J.; Pereira, E. Application of response surface methodology and box–behnken design for the optimization of mercury removal by Ulva sp. J. Hazard. Mater. 2023, 445, 130405. [Google Scholar] [CrossRef]
- Narukulla, S.; Bogadi, S.; Tallapaneni, V.; Sanapalli, B.K.R.; Sanju, S.; Khan, A.A.; Abdul, M.; Hasi Rani, B.; Tonmoy, K.M.; Veera Venkata, S.R.K.; et al. Comparative study between the Full Factorial, Box–Behnken, and Central Composite Designs in the optimization of metronidazole immediate release tablet. Microchem. J. 2024, 207, 111875. [Google Scholar] [CrossRef]
- Alturki, S.F.; Suwaed, M.S.; Ghareeb, A.; AlJaberi, F.Y.; Hassan, A.A. Statistical analysis and optimization of mechanical-chemical electro-Fenton for organic contaminant degradation in refinery wastewater. J. Eng. Res. 2024. [Google Scholar] [CrossRef]
- Latif, A.; Maqbool, A.; Zhou, R.; Arsalan, M.; Sun, K.; Si, Y. Optimized degradation of bisphenol A by immobilized laccase from Trametes versicolor using Box-Behnken design (BBD) and artificial neural network (ANN). J. Environ. Chem. Eng. 2022, 10, 107331. [Google Scholar] [CrossRef]
- Kaur, M.; Noonia, A.; Dogra, A.; Singh Thind, P. Optimising the parameters affecting degradation of Cypermethrin in an aqueous solution using TiO2/H2O2 mediated UV photocatalysis: RSM-BBD, kinetics, isotherms and reusability. Int. J. Environ. Anal. Chem. 2023, 103, 1153–1167. [Google Scholar] [CrossRef]
- Asfaha, Y.G.; Zewge, F.; Yohannes, T.; Kebede, S. Application of hybrid electrocoagulation and electrooxidation process for treatment of wastewater from the cotton textile industry. Chemosphere 2022, 302, 134706. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Wang, J.; Lee, Y.J.; Ramkumar, S.S. Visible Light Photocatalytic Functional TiO2/PVDF Nanofibers for Dye Pollutant Degradation. Part. Part. Syst. Charact. 2019, 36, 1900091. [Google Scholar] [CrossRef]
- Vembuli, T.; Thiripuranthagan, S.; Kumaravel, S. Enhanced removal of hazardous organic contaminants with advanced visible light-active F-doped TiO2/rGO/PVDF photocatalytic membranes. J. Alloys Compd. 2024, 1005, 175997. [Google Scholar] [CrossRef]
- Wang, A.; Chen, C.; Liao, L.; Qian, J.; Yuan, F.-G.; Zhang, N. Enhanced β-Phase in Direct Ink Writing PVDF Thin Films by Intercalation of Graphene. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1497–1502. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Membr. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Kulkarni, N.D.; Kumari, P. Development of highly flexible PVDF-TiO2 nanocomposites for piezoelectric nanogenerator applications. Mater. Res. Bull. 2023, 157, 112039. [Google Scholar] [CrossRef]
- Park, A.; Jung, J.-Y.; Kim, S.; Kim, W.; Seo, M.Y.; Kim, S.; Kim, Y.J.; Lee, W.B. Crystallization behavior of polyvinylidene fluoride (PVDF) in NMP/DMF solvents: A molecular dynamics study. RSC Adv. 2023, 13, 12917–12924. [Google Scholar] [CrossRef]
- Haponska, M.; Trojanowska, A.; Nogalska, A.; Jastrzab, R.; Gumi, T.; Tylkowski, B. PVDF Membrane Morphology—Influence of Polymer Molecular Weight and Preparation Temperature. Polymers 2017, 9, 718. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, W.; Zhou, Y.; Chen, Y.; Cao, X.; Yang, Y.; Xu, J.; Jiang, Y. Effect of crystalline phase on the dielectric and energy storage properties of poly(vinylidene fluoride). J. Mater. Sci. Mater. Electron. 2016, 27, 7280–7286. [Google Scholar] [CrossRef]
- Yang, C.; Wang, P.; Li, J.; Wang, Q.; Xu, P.; You, S.; Zheng, Q.; Zhang, G. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe(III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chem. Eng. J. 2021, 417, 129340. [Google Scholar] [CrossRef]
- Mohamat, R.; Bakar, S.A.; Mohamed, A.; Muqoyyanah; Othman, M.H.D.; Kamal, S.N.E.A.M.; Mamat, M.H.; Ahmad, M.K.; Remakrishna, S. Incorporation of Different Polymeric Additives for Polyvinylidene Fluoride Membrane Fabrication and Its Performance on Methylene Blue Rejection and Antifouling Improvement. J. Polym. Environ. 2023, 31, 3466–3479. [Google Scholar] [CrossRef]
- Polisetti, V.; Ray, P. Nano SiO and TiO embedded polyacrylonitrile/polyvinylidene fluoride ultrafiltration membranes: Improvement in flux and antifouling properties. J. Appl. Polym. Sci. 2021, 138, 49606. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, L.; Igwegbe, C.A.; Rahdar, S.; Banach, A.M. Application of response surface methodology in the degradation of Reactive Blue 19 using H2O2/MgO nanoparticles advanced oxidation process. Int. J. Ind. Chem. 2018, 9, 241–253. [Google Scholar] [CrossRef]
- Asaithambi, P.; Aziz, A.R.A.; Daud, W.M.A.B.W. Integrated ozone—Electrocoagulation process for the removal of pollutant from industrial effluent: Optimization through response surface methodology. Chem. Eng. Process. Process Intensif. 2016, 105, 92–102. [Google Scholar] [CrossRef]
- Sari, Y.; Gareso, P.L.; Armynah, B.; Tahir, D. A review of TiO2 photocatalyst for organic degradation and sustainable hydrogen energy production. Int. J. Hydrogen Energy 2024, 55, 984–996. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, P.; Panda, A.B.; Shahi, V.K. Photocatalytic TiO2 incorporated PVDF-co-HFP UV-cleaning mixed matrix membranes for effective removal of dyes from synthetic wastewater system via membrane distillation. J. Environ. Chem. Eng. 2021, 9, 105904. [Google Scholar] [CrossRef]
- Mai, N.X.D.; Bae, J.; Kim, I.T.; Park, S.H.; Lee, G.-W.; Kim, J.H.; Lee, D.; Son, H.B.; Lee, Y.-C.; Hur, J. A recyclable, recoverable, and reformable hydrogel-based smart photocatalyst. Environ. Sci. Nano 2017, 4, 955–966. [Google Scholar] [CrossRef]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K.; Kuvarega, A.T. Fabrication of magnetic recoverable Fe3O4/TiO2 heterostructure for photocatalytic degradation of rhodamine B dye. Inorg. Chem. Commun. 2022, 145, 109966. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Lu, W.; Lu, Y.; Hu, H. Easily recyclable photocatalyst Bi2WO6/MOF/PVDF composite film for efficient degradation of aqueous refractory organic pollutants under visible-light irradiation. J. Mater. Sci. 2019, 54, 6238–6257. [Google Scholar] [CrossRef]




| Variable | Input Parameter | Factorial Level | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| A | Reaction time (min) | 15 | 37.5 | 60 |
| B | Methylene blue concentration (ppm) | 5 | 7.5 | 10 |
| C | Volume of methylene blue (mL) | 5 | 7.5 | 10 |
| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|
| Linear | 0.0817 | 0.0025 | 0.4140 | 0.0128 |
| 2FI | 0.0513 | 0.0054 | 0.6374 | −0.1251 |
| Quadratic | 0.0002 | 0.3051 | 0.9640 | 0.8482 |
| Cubic | 0.3051 | - | 0.9722 | - |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Coefficient of Variance% | R2 |
|---|---|---|---|---|---|---|---|
| Model | 1815.42 | 9 | 201.71 | 48.61 | <0.0001 | 2.37 | 0.9843 |
| A | 300.13 | 1 | 300.13 | 72.32 | <0.0001 | - | - |
| B | 378.12 | 1 | 378.12 | 91.11 | <0.0001 | - | - |
| C | 288.00 | 1 | 288.00 | 69.40 | <0.0001 | - | - |
| AB | 132.25 | 1 | 132.25 | 31.87 | 0.0008 | - | - |
| AC | 4.00 | 1 | 4.00 | 0.9639 | 0.3589 | - | - |
| BC | 324.00 | 1 | 324.00 | 78.07 | <0.0001 | - | - |
| A2 | 302.42 | 1 | 302.42 | 72.87 | <0.0001 | - | - |
| B2 | 9.79 | 1 | 9.79 | 2.36 | 0.1684 | - | - |
| C2 | 96.00 | 1 | 96.00 | 23.13 | 0.0019 | - | - |
| Residual | 29.05 | 7 | 4.15 | - | - | - | - |
| Lack of fit | 16.25 | 3 | 5.42 | 1.69 | 0.3051 | - | - |
| Pure error | 12.80 | 4 | 3.20 | - | - | - | - |
| Cor total | 1844.47 | 16 | - | - | - | - | - |
| Photocatalyst | Target Pollutant | Recycling Method | Recovered Components | Performance of Recycled Product | Reference |
|---|---|---|---|---|---|
| PVDF/TiO2 | Methylene blue | Phase inversion | PVDF, TiO2, and solvents (separately) | >92% over 10 cycles | This work |
| PVDF/TiO2 | Rhodamine B | Solvent addition | PVDF + TiO2 (simultaneously) | >90% over 10 cycles | [23] |
| Agarose-based TiO2 | Methylene blue | Multiple-step purification | TiO2 only | Not reported | [51] |
| Fe3O4/TiO2 | Rhodamine B | Magnetic separation | Fe3O4 only | Not reported | [52] |
| Bi2WO6/MIL-53(Al)/PVDF | Rhodamine B | H2O2 oxidation cleaning | Whole composite film | ~80% over 15 cycles | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.N.D.; Leu, H.-J.; Phan, H.N.Q.; Nguyen, T.-T.; Ngo, D.H.M. Investigating the Recovery of PVDF/TiO2 Photocatalyst for Methylene Blue Degradation. Processes 2025, 13, 1392. https://doi.org/10.3390/pr13051392
Nguyen VND, Leu H-J, Phan HNQ, Nguyen T-T, Ngo DHM. Investigating the Recovery of PVDF/TiO2 Photocatalyst for Methylene Blue Degradation. Processes. 2025; 13(5):1392. https://doi.org/10.3390/pr13051392
Chicago/Turabian StyleNguyen, Vi N. D., Hoang-Jyh Leu, Huy N. Q. Phan, Tan-Trung Nguyen, and Dat H. M. Ngo. 2025. "Investigating the Recovery of PVDF/TiO2 Photocatalyst for Methylene Blue Degradation" Processes 13, no. 5: 1392. https://doi.org/10.3390/pr13051392
APA StyleNguyen, V. N. D., Leu, H.-J., Phan, H. N. Q., Nguyen, T.-T., & Ngo, D. H. M. (2025). Investigating the Recovery of PVDF/TiO2 Photocatalyst for Methylene Blue Degradation. Processes, 13(5), 1392. https://doi.org/10.3390/pr13051392






