Abstract
Ammonia (NH3) plays a vital role in both the agriculture and energy sectors, serving as a precursor for nitrogen fertilizers and as a promising carbon-free fuel and hydrogen carrier. However, the conventional Haber–Bosch process is highly energy-intensive, operating under elevated temperatures and pressures, and contributes significantly to global CO2 emissions. In recent years, nonthermal plasma (NTP)-assisted ammonia synthesis has emerged as a promising alternative that enables ammonia production under mild conditions. With its ability to activate inert N2 molecules through energetic electrons and reactive species, NTP offers a sustainable route with potential integration into renewable energy systems. This review systematically summarizes recent advances in NTP-assisted ammonia synthesis, covering reactor design, catalyst development, plasma–catalyst synergistic mechanisms, and representative reaction pathways. Particular attention is given to the influence of key plasma parameters, such as discharge power, pulse voltage, frequency, gas flow rate, and N2/H2 ratio, on reaction performance and energy efficiency. Additionally, comparative studies on plasma reactor configurations and materials are presented. The integration of NTP systems with green hydrogen sources and strategies to mitigate ammonia decomposition are also discussed. This review provides comprehensive insights and guidance for advancing efficient, low-carbon, and distributed ammonia production technologies.
1. Introduction
Ammonia is a crucial chemical in both the industrial and agricultural sectors, widely used in fertilizers, pharmaceuticals, explosives, synthetic fibers, and various other fields [1,2,3]. It is also utilized in flue gas regulation, where it serves to remove and absorb components such as fly ash, nitrogen oxides (NOx), and carbon dioxide (CO2) [4]. Due to its high hydrogen content and potential for large-scale production, ammonia is regarded as a promising carbon-free industrial fuel. Compared with traditional fossil fuels, ammonia does not release carbon dioxide during combustion, positioning it as an effective alternative energy source for mitigating greenhouse gas emissions. Ammonia not only has a high energy density but is more convenient to store and transport than hydrogen, rendering it an ideal hydrogen carrier. As a fuel, ammonia can be utilized in direct combustion engines or gas turbines, either independently or in combination with other fuels. Moreover, ammonia fuel cells, as an efficient and clean power generation solution, offer robust technical support to the energy industry. Beyond its potential as a fuel, ammonia can also function as a feasible energy storage carrier, enabling the sustainable storage and release of energy.
The industrial production of ammonia primarily depends on the Haber–Bosch process (Figure 1), which synthesizes ammonia by utilizing high-purity nitrogen (N2) and hydrogen (H2) at elevated temperatures (450–650 °C) and pressures (5–20 MPa), with the assistance of iron-based or ruthenium-based catalysts. The following exothermic equilibrium reaction takes place in the synthesis reactor:
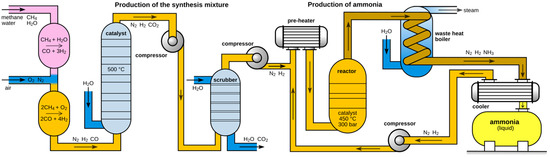
Figure 1.
A flow scheme for the Haber–Bosch process [5].
The majority of the energy consumption in this reaction results from the high temperatures and pressures required for synthesizing ammonia from N2 and H2. Although nitrogen gas constitutes 78% of Earth’s atmosphere and is readily available, the stability of its molecular structure necessitates high temperatures to supply the energy needed to dissociate the N2 bond and drive the reaction.
The Haber–Bosch process is an energy-intensive procedure, accounting for 1–2% of global energy consumption and contributing to 1.44% of global carbon dioxide emissions. With the continued rise in the global population, the demand for fertilizers in agriculture is increasing, and global ammonia production is projected to grow at an annual rate of 1–2%. The high temperature and pressure conditions inherent in the Haber–Bosch process constrain its potential for further optimization. Despite some technological advancements [6,7], the efficiency of this process is approaching its technical bottleneck [8,9]. Therefore, continued reliance on the Haber–Bosch process could impose a greater environmental and economic burden, highlighting the urgent need for the development of green, sustainable, decentralized, and renewable ammonia production technologies under milder conditions [10,11,12].
In this context, this review aims to summarize nonthermal-plasma-assisted ammonia synthesis technologies under low temperature and pressure conditions, specifically discussing their impact on ammonia synthesis from perspectives such as reactor design, catalyst development, plasma parameter optimization, reaction mechanism studies, and alternative hydrogen sources. Additionally, this review explores the auxiliary role of nonthermal plasma in the ammonia synthesis process, with a particular focus on its potential to optimize the plasma catalytic process and enhance ammonia production efficiency.
2. Basic Research on Plasma-Assisted Ammonia Synthesis
2.1. Plasma for Assisting Ammonia Synthesis
Plasma is a distinct state of matter composed of free electrons, ions, and neutral particles, which together are electrically neutral. It is considered the fourth state of matter, alongside solid, liquid, and gas [13,14]. Plasma can be classified into two categories based on its temperature: thermal and nonthermal plasma.
Thermal plasma typically operates at temperatures exceeding tens of thousands of degrees Celsius, with the electron and ion temperatures being nearly identical, thereby achieving thermal equilibrium. The use of thermal plasma invariably leads to substantial energy consumption. In contrast, nonthermal plasma operates at comparatively lower temperatures, generally ranging from room temperature to several thousand degrees Celsius. In this state, the electron temperature (typically exceeding 10,000 K) is significantly higher than both the ion and gas temperatures, and the electrons and ions exist in a nonthermal equilibrium state. High-energy electrons in nonthermal plasma can collide with gas molecules, generating numerous reactive free radicals and excited molecules capable of breaking complex chemical bonds. This lowers the energy barrier for reactions, enabling thermodynamically unfavorable reactions (such as N2 dissociation) to occur under ambient conditions, thereby achieving higher energy efficiency compared to traditional methods [15].
Several types of low-temperature plasma have been investigated for ammonia synthesis, including dielectric barrier discharge (DBD), pulsed discharge, microdischarge, radio frequency discharge, and glow discharge plasmas. Relevant reports on this topic are available in the review by Zhou [16].
2.2. Applications of Plasma-Assisted Ammonia Synthesis
Among the various alternative methods, plasma technology has attracted significant attention due to its potential for ammonia synthesis. Plasma technology can facilitate the dissociation of nitrogen molecules and enable reactions that are challenging to occur under ambient temperature and pressure conditions. NTP systems have been extensively utilized in research for direct ammonia synthesis from N2 and H2. These technologies have been shown to efficiently activate nitrogen molecules to produce ammonia, yielding quantities that are competitive with traditional methods. Additionally, because of their lower energy requirements and potential for integration with renewable energy sources, NTP systems are considered more environmentally friendly [17,18].
An additional significant advantage of utilizing NTP technology for ammonia synthesis lies in its dynamic control capabilities. Plasma can be rapidly activated and deactivated, enabling flexible operation that adapts to fluctuating energy inputs from renewable sources, such as solar or wind energy. This flexibility is essential for small-scale, decentralized ammonia production [19,20].
Despite the considerable potential of NTP, several challenges still need to be addressed. A significant challenge is the typically low ammonia yields associated with plasma processes, which result from competing reactions that may lead to the decomposition of ammonia into nitrogen and hydrogen. This reverse reaction can limit the overall efficiency of ammonia synthesis in plasma systems. Researchers are actively investigating various strategies to mitigate this issue, including the addition of catalysts or the use of specific materials to enhance reactor designs. These enhancements can help stabilize the produced ammonia and prevent the decomposition of vital products [21].
In summary, nonthermal plasma offers a novel and sustainable approach to ammonia synthesis, positioning it as a promising alternative to the energy-intensive Haber–Bosch process. Ongoing research and development are essential for enhancing the efficiency and scalability of NTP-based ammonia synthesis.
3. Nonthermal-Plasma-Assisted Ammonia Synthesis
3.1. Direct Ammonia Synthesis
The application of nonthermal plasma technology in the absence of catalysts has also been extensively studied. This approach capitalizes on the unique properties of nonthermal plasma, which generates high-energy electrons and reactive species at ambient temperatures. Nonthermal plasma species facilitate chemical reactions that are typically thermodynamically unfavorable under conventional conditions. For instance, free radicals, ions, and excited molecules generated by plasma significantly enhance reaction kinetics, thereby promoting the dissociation of the robust N≡N triple bond in nitrogen molecules.
Uyama et al. [22] investigated ammonia synthesis from nitrogen–hydrogen plasma generated by microwave discharge, observing that ammonia yield increased as pressure decreased, reaching saturation at lower pressures. Under optimal experimental conditions (with a power input of approximately 150 W and a H2/(N2 + H2) ratio of about 0.75), the maximum ammonia yield was achieved. In subsequent studies, microwave and radio-frequency (RF) plasmas were utilized for NTP ammonia synthesis, with results indicating that microwave discharge in the H-band head (along with hydrogen atom lines) was an order of magnitude higher than RF discharge, offering a greater advantage for ammonia synthesis. Nakajima et al. [23] employed atmospheric microwave plasma with nitrogen and hydrogen as model reactions for ammonia synthesis, enhancing ammonia production by injecting hydrogen into the plasma afterglow zone. The introduction of argon to dilute the plasma gas further promoted the formation of active nitrogen species, thereby increasing ammonia yield. Takahashi et al. [24] discovered that at RF powers between 100 and 160 W, the ammonia production rate in H2O/N2 and H2/N2 plasmas ranged from 4 to 7 μmol/s, illustrating the potential of directly synthesizing ammonia from water while bypassing hydrogen production. Anastasopoulou et al. [25] developed a small plasma-assisted ammonia synthesis device based on conventional steam reforming and water electrolysis hydrogen production systems. Chen et al. [26] performed a modeling analysis of ammonia synthesis using Haber–Bosch and plasma methods in Aspen Plus, achieving a conversion rate of 37.9 g⋅kWh−1, which represents the highest yield rate for DBD plasma-based NH3 synthesis processes [27] (with a total power of 23.7 MW). Recent studies have shown that inert porous materials, such as zeolites 5A and 4A [28], significantly enhance ammonia synthesis rates, likely via the in situ adsorption of ammonia by these porous materials [29].
Furthermore, in a single plasma system, the optimal utilization efficiency of active species is often not realized. To address these challenges, catalysts can be incorporated into the plasma, forming a hybrid plasma–catalyst system that combines the high selectivity of catalysts with the low-temperature benefits of plasma.
3.2. Catalytic Ammonia Synthesis
Early research on plasma-assisted ammonia synthesis primarily focused on gas-phase reactions without the use of catalysts. Despite offering a new pathway for ammonia synthesis, relying solely on plasma excitation presents challenges, including low efficiency, poor product selectivity, and substantial energy losses. The reactive species in plasma are diverse, and some may lead to the formation of by-products, potentially affecting the overall reaction rate and ammonia selectivity. To address these challenges, researchers have begun integrating traditional catalysts into the plasma ammonia synthesis process, exploring a novel plasma-catalytic ammonia synthesis method. The use of catalysts in ammonia synthesis marks an innovative shift in conventional chemical processes. Similarly, in plasma-catalytic processes, the inclusion of catalysts can activate inert molecules with strong chemical bonds (e.g., CO2, CH4, and N2).
Consequently, plasma-catalytic ammonia synthesis has emerged as a focal point in recent research. By leveraging the synergistic effects of plasma excitation and catalysts, researchers have gradually mitigated the efficiency challenges associated with exclusive plasma reliance, offering promising solutions for the continued development and industrial application of this technology.
3.2.1. Photocatalysis
Past studies have focused on the reduction of N2 to NH3 using sunlight and semiconductor photocatalysts, garnering significant attention and research. Certain photocatalytic materials have proven practical [30,31,32], offering a more sustainable and cost-effective alternative to the traditional Haber–Bosch process, enabling ammonia production under milder conditions. Figure 2 illustrates a timeline of key research milestones in photocatalytic ammonia synthesis, emphasizing technological breakthroughs and catalyst advancements from 1977 to 2019. This includes notable research directions such as metal-doped catalysts, the introduction of oxygen vacancies, and nitrogen reduction via near-infrared excitation.
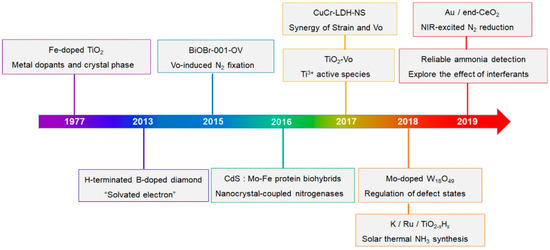
Figure 2.
Key research timelines for photocatalytic ammonia synthesis [33,34,35,36,37,38,39,40,41,42,43,44].
Photocatalytic ammonia synthesis is inspired by natural nitrogen fixation; however, the rate of nitrogen fixation in nature is insufficient to meet the societal demand for ammonia. Strategies for photocatalytic ammonia generation focus primarily on enhancing the active sites of catalysts and introducing transition metals to promote charge separation and weaken the N≡N triple bond [34].
Titanium dioxide (TiO2) is renowned for its stability, nontoxicity, and high photocatalytic activity. Schrauzer et al. [35] demonstrated that TiO2 exhibits excellent performance in ammonia conversion. Additionally, other semiconductor oxides [36,37], metal sulfides [38], and nanomaterials [34] have proven beneficial for ammonia generation in photocatalytic applications. Although some studies, such as those developing photocatalysts with nanostructures, cocatalysts, and surface engineering [30,31], have effectively improved reaction performance, the efficiency of ammonia production using solar energy (with rates around 100 μmol g−1 h−1 at best) still falls short of industrial requirements [33].
The integration of photocatalysis and plasma systems for synergistic reactions has demonstrated significant potential in recent years. Some studies have suggested that incorporating photocatalysis into the plasma nitrogen fixation process can enhance ammonia synthesis. Lamichhane et al. [39] employed ultraviolet light to photocatalyze a titanium dioxide anode, significantly increasing ammonia yield. Research by Sakakura et al. [40] also demonstrated that ultraviolet irradiation of an aqueous phase surface generated more hydrogen atoms, greatly enhancing the NH3 yield.
3.2.2. Electrocatalysis
Electrocatalytic ammonia synthesis is a critical research direction in modern chemistry and energy sectors, particularly in the fields of environmental protection and sustainable energy. Electrocatalytic ammonia synthesis offers several distinct advantages: first, the dissociation of N2 molecules does not require high energy input, making the reaction feasible under relatively simple conditions, which is a significant improvement over traditional methods. Second, electrocatalytic ammonia synthesis relies on electricity, which can be sourced from renewable energy, thereby reducing overall energy consumption. Finally, electrochemical ammonia synthesis offers high flexibility and can support sustainable, distributed on-site ammonia production. Shi et al. [41] developed a unique Cu/Cu2O/Co3O4 electrocatalyst, achieving efficient electrocatalytic nitrate reduction to ammonia (ENRA). The catalyst demonstrated excellent Faradaic efficiencies under both neutral and alkaline conditions, with ammonia production rates reaching 5.7 and 19.8 mg/h/mgcat (19.8 mg of reactant per mg of catalyst per hour), respectively. This study provides new insights into electrocatalytic ammonia synthesis. In remote and developing regions, small-scale, modular ammonia synthesis systems could be deployed.
However, electrocatalytic ammonia synthesis faces several limitations that must be addressed. It demands substantial energy input to break and activate the N≡N triple bond. Furthermore, the low solubility of N2 in water limits the selectivity of electrocatalysts for N2. Researchers have investigated electrocatalysts to overcome this challenge, proposing the introduction of vacancies or atomic doping to enhance catalyst performance [42]. Despite these advancements, efficiency gains have been limited, and environmental concerns persist.
To enhance ammonia synthesis efficiency, numerous researchers have begun investigating the synergistic effects of plasma and electrocatalysts. By employing similar reaction conditions to conventional electrocatalytic ammonia synthesis, plasma-assisted processes can reduce emissions, offering a more sustainable alternative. Plasma-assisted electrocatalytic ammonia synthesis utilizes plasma to convert N2 into more reactive species, which are subsequently efficiently reduced to NH3. Enhancing both yield and efficiency has emerged as a significant challenge. Ren et al. [44] reported a method for plasma-activated N2 oxidation coupled with electrocatalytic NOx reduction for ammonia synthesis. Wu et al. [43] proposed a tandem plasma–electrocatalysis process that integrates a NTP with traditional electrocatalysis for efficient NH3 production directly from air. Their device for synthesizing NH3 from plasma-induced NOx intermediates using a specially customized battery system is shown in Figure 3. The ammonia yield (~1.43 mg NH3 cm−2 h−1) approached 100% Faradaic efficiency. Hollevoet [45] proposed the PNOCRA (plasma nitrogen oxidation and catalytic reduction to ammonia) process, which integrates plasma-assisted nitrogen oxidation with dilute NOx capture technology using diesel engine exhaust aftertreatment systems. The PNOCRA process achieved an energy demand of 4.6 MJ mol−1 NH3, over four times lower than the most advanced plasma ammonia synthesis from N2 and H2 at reasonable yields (>1%). The main challenge in electrocatalytic ammonia synthesis lies in the development of efficient electrocatalysts with low overpotentials, high selectivity, and Faradaic efficiency [43].
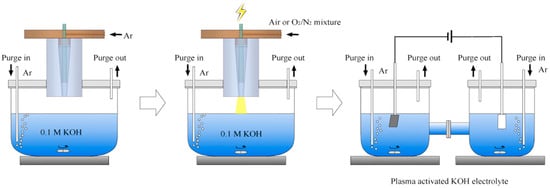
Figure 3.
A model tandem NTP–electrocatalysis system for NH3 synthesis from air [43].
3.2.3. Plasma Catalysis
Nonthermal plasma is primarily used for pretreating catalysts. Additionally, NTP technology can be directly integrated with catalysts during the reaction process, forming a hybrid plasma-catalytic system. This system capitalizes on the high selectivity of the catalyst and the low-temperature advantages of plasma, enabling the efficient and selective conversion of intermediate by-products into desired products. The catalyst incorporated into this system must be compatible with plasma to ensure efficient synergy [46]. Achieving high-efficiency collaboration requires coupling the appropriate catalyst with the correct plasma. The selection of catalyst can lead to substantial differences in the energy efficiency of the final products (Figure 4). Research has been conducted to assess and evaluate the role of catalysts in plasma-assisted ammonia synthesis [47].
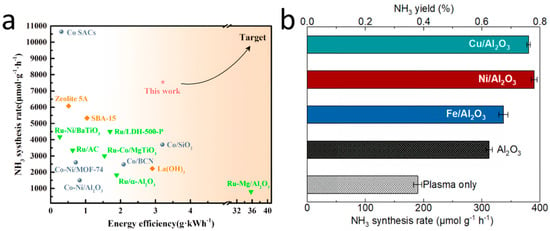
Figure 4.
Comparative analysis of the performance of plasma-assisted ammonia synthesis catalysts: (a) NH3 synthesis rates and yield [48]; (b) comparison of the NH3 synthesis rate and energy efficiency in DBD plasma-catalytic NH3 synthesis packed with different catalysts [47].
In comparison to a standalone plasma system, combining transition metal catalysts (such as nickel (Ni) and ruthenium (Ru)) with plasma has demonstrated substantial improvements in ammonia production rate and energy efficiency. Transition metals on the catalyst surface facilitate the formation of intermediates necessary for ammonia synthesis, such as NH2 species, which are critical for the subsequent formation of NH3 [12]. These metals not only enhance catalytic activity (Figure 5b) but modify plasma discharge characteristics. Moreover, doping transition metals onto the surface of Al2O3 results in a more uniform plasma discharge compared with Al2O3 or plasma alone, and it elevates the average electron energy (Figure 5a) [47].
Ammonia synthesis traditionally relies on supported transition metal catalysts (e.g., Fe, Co, Ni). Research has demonstrated that nickel (Ni)- and ruthenium (Ru)-based catalysts show higher activity than the iron-based catalysts used in the traditional Haber–Bosch process. Among these, Ni-based catalysts are more cost-effective, while Ru-based catalysts exhibit superior catalytic activity under low-temperature and low-pressure conditions [48]. However, Ru exhibits strong reactant adsorption, which accelerates H2 accumulation on the Ru surface, leading to “H2 poisoning” and catalyst deactivation. Gao et al. [48] investigated the role of Ru-Co-based catalysts in enhancing NH3 synthesis performance by optimizing energy efficiency and synthesis rate through carrier selection and reaction condition adjustments. The results revealed that Ru-Co/AC catalysts exhibited the highest energy efficiency and synthesis rate. This further confirmed that catalysts’ support characteristics (such as surface area, pore structure, metal dispersion, and surface basicity) influence plasma catalytic performance.
Low ammonia yields are commonly observed in plasma processes, primarily because of competitive reactions that result in the decomposition of ammonia into nitrogen and hydrogen. This reverse reaction limits the overall efficiency of ammonia production in plasma systems [49,50]. Previous research has primarily focused on developing hybrid plasma processes by adjusting plasma treatment parameters or utilizing different catalysts. Researchers are actively exploring several strategies to mitigate this issue, including the use of specially designed catalytic materials that stabilize the ammonia produced and prevent decomposition during plasma reactions [21,51]. Consequently, the focus of research has shifted from catalyst design to optimizing the interaction between plasma and catalysts. Hua et al. [52] employed RuNb/ST (a Sn0.2Ti0.8O2 support modified with RuO2 and Nb2O5) as the catalyst. By introducing Nb2O5, they enhanced the surface acidity and tuned the electronic structure of Ru, thereby improving the adsorption and conversion performance of ammonia and enhancing the catalyst’s resistance to poisoning. This approach provides valuable insights into efficient nitrogen–hydrogen synergistic reactions under plasma activation, offering strategies to regulate the adsorption of active species and suppress side reactions.
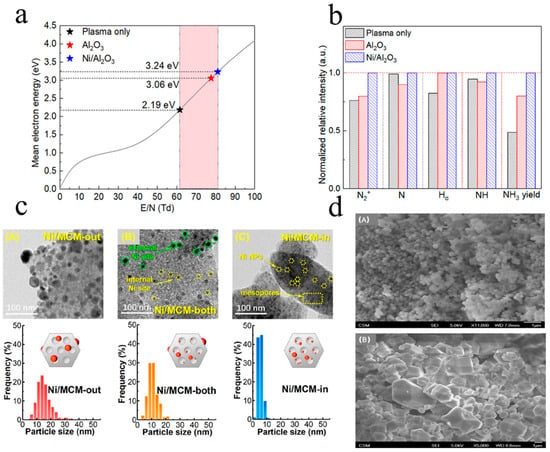
Figure 5.
Catalyst structure and performance: (a) mean electron energy for plasma only, packed with Al2O3 and Ni/Al2O3 [47]; (b) normalized relative intensity of N2+, N, Hα, and NH (SEI of 26.8 kJ L−1) [47]; (c) HRTEM images and PSDs of (A) Ni/MCM-out, (B) Ni/MCM-both, and (C) Ni/MCM-in (all in the reduced state) [53]; (d) representative SEM images of (A) ZIF-67 and (B) ZIF-8 crystals [54].
Studies have demonstrated that the morphology and surface characteristics of catalysts are crucial for their performance. Metal catalysts supported on mesoporous materials (such as Ru/MCM-41 [21] and Ni-Mg/SBA15 [22]) have proven highly effective in promoting plasma-catalyzed NH3 synthesis. For example, Wang et al. [53] developed a custom-supported nickel catalyst using mesoporous materials (MCM-41) (Figure 5c), creating a strategy for plasma–catalyst interaction with a “shielding protection” effect to enhance ammonia generation efficiency. The mesoporous structure prevents the decomposition of ammonia during the reaction process by enabling the generated ammonia to diffuse into the pores, effectively protecting it from decomposition. Microporous crystals have become highly attractive catalytic materials for plasma-assisted ammonia synthesis. For instance, the zeolite imidazole framework (ZIF) [54] can serve as a catalyst, where plasma-induced nitrogen and hydrogen are catalytically converted into ammonia on ZIF-8 and ZIF-67. These materials exhibit high ammonia synthesis rates due to their unique structure (Figure 5d), which enhances the interaction between plasma and reactants. For example, they achieve ammonia synthesis rates of up to 42.16 μmol NH3/min GCAT, outperforming conventional catalysts such as zeolites and oxides.
The energy cost of plasma-catalyzed ammonia synthesis remains higher than the threshold required for industrial viability. Thus, identifying optimized strategies for plasma-assisted ammonia synthesis continues to be a central focus of current research. In this context, Zhang et al. [55] proposed a novel plasma-assisted chemical looping (PACL) strategy for the efficient synthesis of ammonia from N2 and H2O under mild temperature conditions. This approach significantly improved the reaction stability and offers a promising direction for the development of more effective plasma-based ammonia synthesis methods.
A comparison of different ammonia synthesis methods is presented in Table 1.

Table 1.
Comparison of ammonia synthesis methods.
4. Influencing Factors of Nonthermal-Plasma-Catalytic Ammonia Synthesis
4.1. Reactor Structure
The design of reactors is critical to the efficiency of plasma-assisted ammonia synthesis. Various reactor configurations, including dielectric barrier discharge reactors, membrane-filled reactors, and microwave plasma reactors [56], can significantly influence the interaction between plasma and catalysts.
According to several studies [5,27,57,58,59], DBD reactors, which operate under standard temperature and pressure, offer relatively high ammonia synthesis efficiencies. Consequently, DBD is frequently considered an ideal reactor design for plasma-assisted ammonia synthesis.
For instance, Wang et al. [47] developed a temperature-controlled DBD reactor, using water as the electrode, to directly synthesize ammonia from N2 and H2 with transition metal catalysts (M/Al2O3, where M = Fe, Ni, Cu) at near-room temperature. The experimental setup, shown in Figure 6, achieved a maximum ammonia synthesis rate of 471 μmol g−1 h−1 with Ni/Al2O3 as the catalyst and plasma activation. This rate was twice as high as that obtained using plasma alone. The use of water as the electrode was another key feature of this setup, facilitating subsequent optical diagnostics of the reaction via intensified charge-coupled device (ICCD) imaging and optical emission spectroscopy, while maintaining the reaction at room temperature.
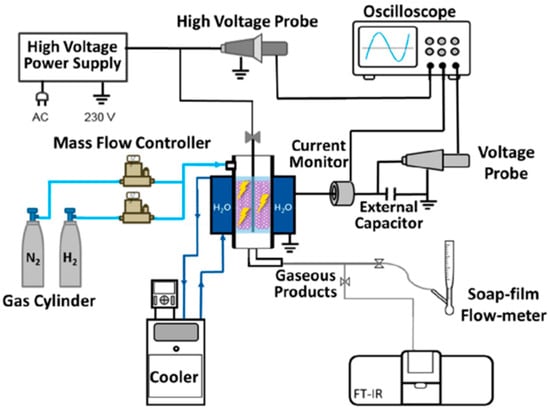
Figure 6.
Schematic diagram of the experimental setup [47].
This reactor design demonstrates the potential for efficient ammonia synthesis under mild conditions and opens avenues for future developments in plasma-assisted catalytic processes.
The plasma catalytic process involves both gas-phase and surface reactions. Two typical reaction modes exist. The first mode involves placing the catalyst in the discharge region, where it directly interacts with the plasma, a process known as in-plasma catalysis (IPC, Figure 7a). The second mode places the catalyst downstream of the plasma, where the plasma and catalyst are physically separated, referred to as postplasma catalysis (PPC, Figure 7b). Both approaches have been discussed in relevant studies [60]. In-plasma catalysis (IPC) enables efficient utilization of reactive plasma species, significantly enhancing reaction rates and reducing the required reaction temperature. However, IPC often suffers from limited catalyst stability, elevated energy consumption, and potential degradation due to the high-energy plasma environment. In contrast, postplasma catalysis (PPC) effectively shields the catalyst from direct plasma exposure and allows for energy cost reduction through thermal energy recovery. Nevertheless, its reaction rate is constrained by the limited transfer of reactive species from the plasma phase to the catalyst surface. Further optimization of both strategies is required to overcome their respective limitations.
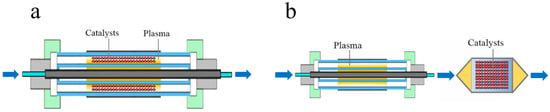
Figure 7.
Two typical reaction modes of plasma catalysis: (a) in-plasma catalysis, IPC; (b) postplasma catalysis, PPC [14].
Studies related to power supply have shown that pulse power sources are more effective than AC power sources. The use of pulse power facilitates the generation of nonequilibrium plasma, which can achieve higher reaction activity with lower energy input, thereby enhancing reaction efficiency [61,62].
The selection of materials for the reactor, including ferroelectric materials [51] or specific coatings [63], may also influence plasma discharge characteristics and overall reaction efficiency. Research has demonstrated that incorporating metal nanoparticles into the reactor may alter the electric field and enhance plasma activity, though the specific effects can vary based on the materials used and their configuration.
4.2. Reaction Mechanisms
We have learned that plasma generates high-energy species that promote the rupture of the strong N≡N bond in nitrogen molecules. In addition to reactor structural design, the integration of advanced diagnostic techniques remains crucial for understanding the underlying reaction mechanisms. Techniques such as optical emission spectroscopy and intensified charge-coupled device (ICCD) imaging enable researchers to monitor the reaction process of plasma-assisted ammonia synthesis in real time, providing insights into the involved reaction mechanisms, thus optimizing the process.
In plasma–catalyst systems, in situ surface detection of reactions has been performed by researchers [64,65] to better understand the potential mechanisms of plasma–catalyst interactions. In catalyst development, studies [47,48,66] have demonstrated the reaction mechanisms of various transition metal catalysts, including ruthenium (Ru), copper (Cu), and nickel (Ni), in plasma-assisted ammonia synthesis. Ruthenium catalysts exhibit excellent performance in ammonia synthesis due to their high nitrogen dissociation ability and low reaction barriers. However, their strong hydrogen adsorption results in “hydrogen poisoning”, which suppresses the reaction. Consequently, ruthenium is often combined with other metals, such as cobalt (Co) or nickel (Ni), to enhance resistance to hydrogen poisoning and improve catalytic stability. Copper catalysts exhibit lower nitrogen dissociation activity than ruthenium; however, under plasma conditions, they synergize with active species in the plasma (e.g., nitrogen and hydrogen radicals), enhancing ammonia synthesis efficiency, particularly at low temperatures. Nickel catalysts, because of their excellent hydrogenation activity, promote the generation of hydrogen atoms under low-temperature conditions and react with nitrogen to form ammonia, avoiding hydrogen poisoning. All three catalysts exhibit enhanced catalytic activity and reaction selectivity through synergistic interactions with the plasma. However, the surface properties of the catalyst, metal dispersion, acidity/basicity, and interactions between the catalyst and plasma remain key factors in determining catalytic efficiency.
Herrera [67] employed a packed-bed dielectric barrier discharge reactor to evaluate plasma reactions with various metal catalysts (Fe/Al2O3, Ni/Al2O3, Co/Al2O3). The study found that, despite the presence of various catalysts, the macroscopic electrical properties of the plasma (e.g., capacitance, filamentary current, and vibration temperature) did not exhibit significant changes. However, the ammonia synthesis rate was found to vary. Ultimately, the study concluded that the catalytic effect on ammonia synthesis was enhanced by the interaction between the catalyst and the plasma products. Rouwenhorst et al. [68] systematically investigated the performance of various metal catalysts, including Ru, Co, and Pt, under DBD plasma conditions. Their results suggest that the rate-determining step in plasma-assisted ammonia synthesis involves the reaction between nitrogen radicals generated in the plasma phase and hydrogen atoms adsorbed on the catalyst surface. This mechanistic insight offers valuable guidance for the rational design of more efficient catalysts for plasma-driven ammonia synthesis.
Research on supported metal catalysts [69] suggests that the plasma-catalyzed ammonia synthesis process can be explained by four potential reaction mechanisms (Figure 8):
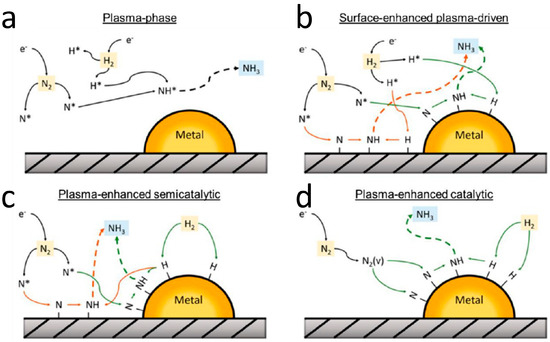
Figure 8.
Possible pathways for catalytic plasma in ammonia synthesis [59,69]: (a) plasma phase; (b) surface-enhanced plasma-driven reaction; (c) plasma-enhanced semicatalytic reaction; (d) plasma-enhanced catalytic reaction.
- Plasma phase: In this pathway, nitrogen radicals (N•) and hydrogen radicals (H•) generated in the plasma environment directly combine to form ammonia (NH3) without the involvement of a catalyst. In this process, the plasma is highly active, and the concentration of radicals is abundant. The reaction rate is primarily determined by the concentration of radicals and their collision frequency.
- Surface-enhanced plasma-driven reaction: Nitrogen radicals (N•) and hydrogen radicals (H•) first adsorb onto the catalyst surface, which has a high electron density and active sites (such as metal active sites). This adsorption enhances the reactivity of the radicals and facilitates ammonia formation.
- Plasma-enhanced semicatalytic reaction: When the catalyst has high activity for hydrogen dissociation but insufficient activity for nitrogen dissociation, nitrogen radicals (N•) adsorb onto the catalyst surface (including both the support and active metal). These radicals then combine with hydrogen atoms (H) dissociated from the catalyst surface to form ammonia (NH3). In this reaction, only the dissociation of hydrogen is catalyzed, while the dissociation of nitrogen is not.
- Plasma-enhanced catalytic reaction: This is the ideal synergistic reaction between plasma and catalyst. In this scenario, the catalyst has high activity for both nitrogen and hydrogen dissociation. The plasma excites the nitrogen molecules to a vibrational state (N2(v)), which then dissociates on the active sites of the catalyst surface. Hydrogen also dissociates on the catalyst surface, and the subsequently adsorbed intermediates combine to form ammonia (NH3).
From a microscopic perspective, the reaction process is initiated by the activation of reactants through plasma, which subsequently dissociate into active species. These active species then recombine to form intermediate products, ultimately leading to the formation of NH3. Specifically, nitrogen atoms (N) and hydrogen atoms (H) combine to form NH* radicals, which are gradually hydrogenated to eventually form NH3.
The intermediate product NH* is regarded as a key species in ammonia synthesis. It has been found that multiple pathways exist for the formation of NH* radicals. Initially, NH radicals can be formed when nitrogen atoms (N) and hydrogen atoms (H) dissociate from N2 and H2, respectively, and directly combine [70,71] (Equation (2)). Subsequent studies [72] have reported that the reaction between N2+ and H radicals can also lead to the production of NH* radicals (Equation (3)). As research advanced, Gómez et al. [73] pointed out that N* and H2 could also react to form NH* radicals (Equation (4)).
NH2* is also considered a precursor in ammonia synthesis [5,74]. It was demonstrated by Wang et al. [47] that weak acid sites and metal sites play important roles in increasing the concentration of NH2* radicals, which can further hydrogenate to form NH3 (as shown in Equations (5) and (6)). The (s) notation indicates that the free radicals are adsorbed onto the solid surface, facilitating the reaction by enhancing contact between the reactants and the catalyst surface. However, it was confirmed by Shah et al. [19] that NH2* is not a necessary intermediate in the reaction, as NH* can directly combine with H2 to form NH3, as shown in Equation (7).
It is well-known that the ammonia synthesis reaction is reversible, meaning that, in addition to ammonia formation, ammonia decomposition reactions also take place during the process. Ammonia decomposition into intermediates such as H, NH*, and NH2* results in a decrease in the energy efficiency of ammonia production, limiting the overall rate of ammonia synthesis. Therefore, understanding how to control the reversible reactions to drive them in the optimal direction is crucial for achieving efficient reactions.
In recent years, efforts have been made by researchers to explore the relevant reaction mechanisms from new perspectives in order to achieve more efficient synthesis outcomes. For example, quantum cascade laser dual comb spectroscopy was employed by Sadiekp et al. [75] (with the experimental setup shown in Figure 9a) to conduct high-resolution spectroscopic analysis of the NH3 formation process in nonthermal plasmas. It was discovered that the vibrational temperature and rotational temperature of NH3 differed under various vibrational modes. The formation of NH3 is dependent on vibrationally excited N2, providing new theoretical support for plasma-activated ammonia synthesis.
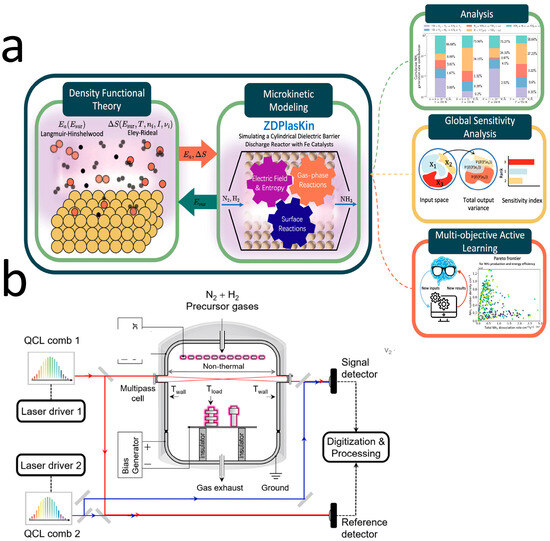
Figure 9.
Exploring new perspectives on reaction mechanisms: (a) DFT–microkinetic model [50]; (b) schematic of the QCL-DCS setup [75].
Shao et al. [50], by combining density functional theory (DFT) with microkinetic modeling (Figure 9b), demonstrated that the adsorption energy of reactants and the reaction pathways could be significantly altered, thereby impacting ammonia synthesis efficiency. Furthermore, by optimizing process parameters, ammonia yield can be improved while energy losses are reduced.
4.3. Parameter Optimization
The incorporation of plasma has enabled more efficient ammonia production, and the regulation of plasma parameters has attracted the attention of researchers, as the optimization of these parameters is crucial for both the efficiency and selectivity of the reaction.
Firstly, the applied power voltage must ensure that the external electric field between the electrodes attains the required breakdown strength. According to Paschen’s law, plasma can form under specific electrode gaps and gas pressures when the voltage attains the breakdown voltage. The application of voltage directly increases the energy input to the plasma, thereby promoting the excitation and dissociation of N2 and H2 [76]. High-energy electrons collide with nitrogen molecules, causing N2 molecules to dissociate into nitrogen atoms (N) and nitrogen molecular ions (N2+), while hydrogen gas dissociates into hydrogen atoms (H). These active intermediates are crucial for ammonia synthesis. However, excessively high pulse voltages may induce unwanted side reactions, such as ammonia decomposition. Therefore, the pulse voltage must be carefully regulated to ensure the effective dissociation of nitrogen and hydrogen while avoiding energy waste.
Increasing the pulse frequency raises the electron density and the number of active species, as the energy input during discharge increases. A higher pulse frequency results in a greater charge in the discharge channel, thereby enhancing discharge activity [77]. In NTP catalytic ammonia synthesis over a Ru/γ-Al2O3 catalyst, increasing the discharge frequency from 8.2 kHz to 11 kHz caused a drop in output voltage and power, which, in turn, led to a decrease in the ammonia synthesis rate and energy efficiency [78].
Optimizing the pulse width is crucial for balancing energy input and utilization efficiency. The pulse width refers to the discharge duration of the highest voltage in a single pulse. Extending the discharge duration of a single pulse can continuously excite active particles. Longer pulse widths allow for more energy to be injected [76], increasing the production of active particles and promoting further reactions between nitrogen and hydrogen to form ammonia. However, when the pulse width exceeds the optimal value, the energy input increases, but ammonia synthesis efficiency decreases, and it may even result in ammonia decomposition [77].
In addition to electrical parameters, some researchers have observed significant effects by introducing noble gases, such as argon and helium, into the plasma reaction process. Noble gases enhance the plasma’s electron density and the number of excited-state particles [77,79], effectively improving the dissociation capability of reactant molecules. The introduction of noble gases promotes the dissociation of N2 and enhances the formation of N2+ and H2+, thereby improving ammonia synthesis efficiency [80]. However, it is important to note that excessive addition of noble gases incurs a cost in both production rate and energy consumption, which in turn affects ammonia yield. Therefore, the concentration of noble gases must be precisely controlled to achieve the optimal reaction outcome.
The N2/H2 molar ratio and gas flow rate also significantly influence the reaction. In traditional thermal catalytic ammonia synthesis, a stoichiometric N2/H2 ratio (1:3) is typically used. However, under NTP conditions, studies [81,82] have shown that a nitrogen-rich environment positively affects ammonia synthesis. This environment improves the effective utilization of nitrogen sources in the reaction, thereby enhancing ammonia synthesis efficiency. This is because, during discharge, the dissociation energy of hydrogen is lower than that of nitrogen, and increasing hydrogen content reduces the likelihood of N2 activation. Increasing the gas flow rate reduces the residence time of reactive species within the plasma system [83]. Maintaining a lower gas flow rate, while appropriately increasing the flow rate, allows more reactant gases to enter the system, increasing particle collisions and favoring ammonia production. However, research [84] has shown that excessively high gas flow rates lead to fewer collisions between reactant molecules and high-energy electrons or other active species, resulting in a decrease in ammonia concentration as flow rate increases.
Figure 10 illustrates the effects of various plasma parameters, such as applied voltage, gas flow rate, and gas volume ratio, on the experimental outcomes.
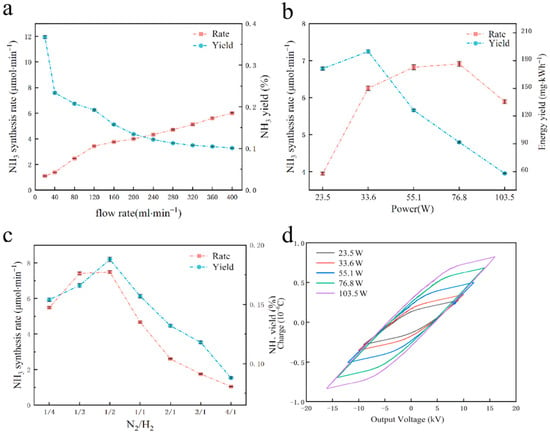
Figure 10.
The effects of various plasma parameters [78]: (a) total flow rate; (b) discharge power; (c) N2 and H2 gas volume ratio; (d) different discharge powers of Lissajous figure.
Recent research has made considerable progress in optimizing plasma parameters for ammonia synthesis. Notable studies include the work by Li et al. [85], who employed a fluidized bed reactor for plasma-catalyzed ammonia synthesis. They compared the effects of catalyst particle motion in the fluidized bed reactor to that in a fixed-bed reactor, finding that the fluidized bed reactor exhibited higher efficiency. This comparison provided valuable insights into optimizing plasma-catalyzed ammonia synthesis reactors. Similarly, Ramírez et al. [73] designed a novel ferroelectric-filled bed reactor and explored the effects of operating frequency, ferroelectric particle size, and electrode spacing on reactor performance.
In summary, plasma parameters, including pulse voltage, pulse width, pulse frequency, noble gas addition, gas flow rate, and feed gas ratios, all significantly influence the ammonia synthesis mechanism. Optimizing these parameters can significantly improve the ammonia synthesis rate, reduce energy consumption, and enhance reaction selectivity and stability. Precise control of these parameters is essential for achieving efficient ammonia synthesis.
4.4. Feedstock Sources
The sources of hydrogen and nitrogen for ammonia synthesis are critical factors in the plasma-assisted ammonia production process. Atmospheric air is typically used for nitrogen, obtained through an air separation unit (ASU) [4]; however, nitrogen fixation efficiency may be influenced by impurities or specific conditions in the plasma reactor. Hydrogen is generally derived from high-purity hydrogen, which has the advantage of not containing other impurity atoms. However, industrial hydrogen is primarily derived from fossil fuels, resulting in increased energy consumption and carbon dioxide emissions. Steam methane reforming (SMR) is currently the most widely used technology for hydrogen production in ammonia synthesis. The hydrogen produced through SMR serves as the primary feedstock for ammonia synthesis in the Haber–Bosch process. Relevant studies [86,87,88] have concentrated on enhancing hydrogen production efficiency; however, challenges remain regarding energy consumption and environmental impact. Researchers are investigating more environmentally friendly methods for hydrogen production. Consequently, the production of green hydrogen, aligned with the low-carbon concept, is anticipated to become a key area of focus.
In addition to industrial hydrogen, hydrogen can also be derived from biomass and water. The pathways of materials involved in the reaction are illustrated in Figure 11.
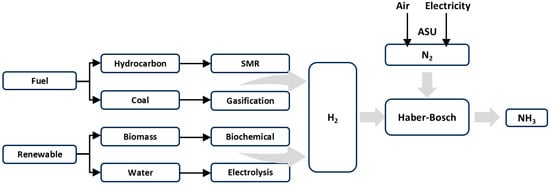
Figure 11.
Reaction material source pathway.
Although the types of final by-products and the mechanism of plasma-assisted ammonia synthesis may vary with different feedstocks, ammonia synthesis inevitably involves two processes: the conversion of feedstock into reactive species in the gas-phase plasma, followed by the interaction of these reactive species to produce NH3 and other by-products.
According to research by Zeng et al. [15], hydrogen produced from renewable energy sources such as solar, wind, and hydropower can significantly reduce dependence on fossil fuels and facilitate the direct synthesis of zero-carbon emission chemicals. In reaction systems where water (H2O) serves as the hydrogen source, its decomposition generates hydrogen atoms (H) and hydroxyl radicals (OH). Between these, hydroxyl radicals (OH) play a crucial role in the ammonia synthesis process within the N2 + H2 reaction system. On the one hand, OH radicals can react with nitrogen atoms (N) to form nitrogen oxides, such as NO, which are among the main by-products of the reaction system. On the other hand, two OH radicals can combine to form hydrogen peroxide (H2O2). Research [16] shows that H2O2 is one of the products in the N2 + H2O plasma reaction system, where it plays a key role throughout the nitrogen fixation process. Gomez et al. [89] studied electrochemical ammonia synthesis near atmospheric pressure from both technoeconomic and environmental perspectives, comparing it with the Haber–Bosch process. The results indicated that water consumption for the reaction is substantial.
Although the hydrogen sources mentioned above are more environmentally friendly, they currently face challenges related to high energy consumption and cost. However, with advancements in energy technology, the efficiency and economics of various hydrogen production methods are steadily improving. Therefore, in future ammonia synthesis processes, the use of industrial hydrogen may become a factor that reduces competitiveness.
5. Conclusions and Outlook
Nonthermal plasma-assisted ammonia synthesis has emerged as a promising strategy for green NH3 production because of its ability to operate under ambient conditions, efficiently activate inert molecules, respond rapidly to system variations, and integrate well with renewable energy sources. This review presents a comprehensive overview of recent advances in the field, including progress in reactor design, plasma parameter optimization, catalyst development, and plasma–catalyst synergistic mechanisms. In particular, four representative reaction pathways are systematically categorized and analyzed.
Experimental studies have demonstrated that adjusting key parameters—such as discharge power, applied voltage, frequency, gas flow rate, and N2/H2 feed ratio—along with appropriate discharge modes and reactor configurations can significantly enhance ammonia yield and energy efficiency. The spatial distribution of the electric field, energy input characteristics, and interfacial transport dynamics are identified as critical factors influencing reaction performance.
Further research is required to deepen the understanding of multiscale reaction mechanisms by integrating in situ diagnostics with theoretical modeling, and to expand coupling strategies with renewable feedstocks such as air, water, and biomass. Meanwhile, the lack of standardized data reporting remains a notable limitation. Many studies fail to include standard deviations, error margins, or the number of replicates when presenting ammonia yield or energy efficiency and often omit crucial information on instrumental accuracy, experimental uncertainty, and calculation assumptions. To improve transparency and scientific rigor, future experiments should clearly state the number of repeated trials, incorporate error bars in graphical data, and provide detailed descriptions of measurement procedures and data processing methods. These practices are essential to enhance reproducibility and comparability and, ultimately, to support the standardization and upscaling of NTP-assisted ammonia synthesis technologies.
Author Contributions
Investigation, writing—original draft, X.Y.; formal analysis, D.W.; data curation, L.W.; investigation, X.Z.; methodology, D.Y.; resources, investigation, Z.L.; project administration, supervision, writing—review and editing, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Baima Lake Laboratory Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LBMHZ24E060003, LBMHZ25E060001), the Science and Technology Program Projects of Zhejiang Provincial Administration for Market Regulation (ZD2024006), and Zhejiang Provincial Natural Science Foundation of China (Grant No. LQN25E060007).
Data Availability Statement
The data are available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cesaro, Z.; Ives, M.; Nayak-Luke, R.; Mason, M.; Bañares-Alcántara, R. Ammonia to power: Forecasting the levelized cost of electricity from green ammonia in large-scale power plants. Appl. Energy 2021, 282, 116009. [Google Scholar] [CrossRef]
- Fasihi, M.; Weiss, R.; Savolainen, J.; Breyer, C. Global potential of green ammonia based on hybrid PV-wind power plants. Appl. Energy 2021, 294, 116170. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, S.; Cheng, J. Study on coal water slurries prepared from coal chemical wastewater and their industrial application. Appl. Energy 2020, 268, 114976. [Google Scholar] [CrossRef]
- Chisalita, D.-A.; Petrescu, L.; Cormos, C.-C. Environmental evaluation of european ammonia production considering various hydrogen supply chains. Renew. Sustain. Energy Rev. 2020, 130, 109964. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma catalytic ammonia synthesis: State of the art and future directions. J. Phys. D Appl. Phys. 2019, 52, 483001. [Google Scholar] [CrossRef]
- Osorio-Tejada, J.; Tran, N.N.; Hessel, V. Techno-environmental assessment of small-scale Haber-Bosch and plasma-assisted ammonia supply chains. Sci. Total Env. 2022, 826, 154162. [Google Scholar] [CrossRef]
- Sugiyama, K.; Akazawa, K.; Oshima, M.; Miura, H.; Matsuda, T.; Nomura, O. Ammonia synthesis by means of plasma over MgO catalyst. Plasma Chem. Plasma Process. 1986, 6, 179–193. [Google Scholar] [CrossRef]
- Li, S.-J.; Lai, L.; Mei, P.; Li, Y.; Cheng, L.; Ren, Z.-H.; Liu, Y. Equilibrium and dynamic surface properties of cationic/anionic surfactant mixtures based on bisquaternary ammonium salt. J. Mol. Liq. 2018, 254, 248–254. [Google Scholar] [CrossRef]
- Pfromm, P.H. Towards sustainable agriculture: Fossil-free ammonia. J. Renew. Sustain. Energy 2017, 9, 034702. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Herrera, F.A.; Kim, J.; Rumbach, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 2018, 1, 269–275. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef]
- Marnellos, G.; Stoukides, M. Ammonia synthesis at atmospheric pressure. Science 1998, 282, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Rani, D.A.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Dong, F. Activation and characterization of environmental catalysts in plasma-catalysis: Status and challenges. J. Hazard. Mater. 2022, 427, 128150. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, S.; Hu, X.; Zhang, C.; Ostrikov, K.K.; Shao, T. Recent advances in plasma-enabled ammonia synthesis: State-of-the-art, challenges, and outlook. Faraday Discuss. 2023, 243, 473–491. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, R.; Zhou, R.; Liu, B.; Zhang, T.; Xian, Y.; Cullen, P.J.; Lu, X.; Ostrikov, K. Sustainable ammonia production by non-thermal plasmas: Status, mechanisms, and opportunities. Chem. Eng. J. 2021, 421, 129544. [Google Scholar] [CrossRef]
- Liu, W.; Xia, M.; Zhao, C.; Chong, B.; Chen, J.; Li, H.; Ou, H.; Yang, G. Efficient ammonia synthesis from the air using tandem non-thermal plasma and electrocatalysis at ambient conditions. Nat. Commun. 2024, 15, 3524. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, H.; Lv, J.; Zhang, M.; Wan, J.; Gerrits, N.; Wu, A.; Lan, B.; Wang, W.; Wang, S.; et al. Enhanced NH(3) Synthesis from Air in a Plasma Tandem-Electrocatalysis System Using Plasma-Engraved N-Doped Defective MoS(2). JACS Au 2023, 3, 1328–1336. [Google Scholar] [CrossRef]
- Shah, J.; Wang, W.; Bogaerts, A.; Carreon, M.L. Ammonia Synthesis by Radio Frequency Plasma Catalysis: Revealing the Underlying Mechanisms. ACS Appl. Energy Mater. 2018, 1, 4824–4839. [Google Scholar] [CrossRef]
- Mindong, B.; Zhitao, Z.; Xiyao, B.; Mindi, B.; Wang, N. Plasma synthesis of ammonia with a microgap dielectric barrier discharge at ambient pressure. IEEE Trans. Plasma Sci. 2003, 31, 1285–1291. [Google Scholar] [CrossRef]
- Tzaguy, A.; Masip-Sanchez, A.; Avram, L.; Sole-Daura, A.; Lopez, X.; Poblet, J.M.; Neumann, R. Electrocatalytic Reduction of Dinitrogen to Ammonia with Water as Proton and Electron Donor Catalyzed by a Combination of a Tri-ironoxotungstate and an Alkali Metal Cation. J. Am. Chem. Soc. 2023, 145, 19912–19924. [Google Scholar] [CrossRef] [PubMed]
- Uyama, H.; Matsumoto, O. Synthesis of ammonia in high-frequency discharges. II. Synthesis of ammonia in a microwave discharge under various conditions. Plasma Chem. Plasma Process. 1989, 9, 421–432. [Google Scholar] [CrossRef]
- Nakajima, J.; Sekiguchi, H. Synthesis of ammonia using microwave discharge at atmospheric pressure. Thin Solid Films 2008, 516, 4446–4451. [Google Scholar] [CrossRef]
- Takahashi, J.; Sasaki, K. Production rates and destruction frequencies of ammonia in inductively coupled H2O/N2 and H2/N2 plasmas. Contrib. Plasma Phys. 2023, 64, e202300167. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Keijzer, R.; Patil, B.; Lang, J.; van Rooij, G.; Hessel, V. Environmental impact assessment of plasma-assisted and conventional ammonia synthesis routes. J. Ind. Ecol. 2020, 24, 1171–1185. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Li, Q.; Yang, G.; Liu, L.; Li, M.; Li, K.; Wang, F. Technical and economic analysis of renewable energy systems with hydrogen-ammonia energy storage: A comparison of different ammonia synthesis methods. J. Energy Storage 2025, 113, 115549. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Engelmann, Y.; van ‘t Veer, K.; Postma, R.S.; Bogaerts, A.; Lefferts, L. Plasma-driven catalysis: Green ammonia synthesis with intermittent electricity. Green. Chem. 2020, 22, 6258–6287. [Google Scholar] [CrossRef]
- Shah, J.R.; Gorky, F.; Lucero, J.; Carreon, M.A.; Carreon, M.L. Ammonia synthesis via atmospheric plasma catalysis: Zeolite 5A, a case of study. Ind. Eng. Chem. Res. 2020, 59, 5167–5176. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Mani, S.; Lefferts, L. Improving the Energy Yield of Plasma-Based Ammonia Synthesis with In Situ Adsorption. ACS Sustain. Chem. Eng. 2022, 10, 1994–2000. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. Engl. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, N.; Wu, Z.; Xie, X. Artificial nitrogen fixation over bismuth-based photocatalysts: Fundamentals and future perspectives. J. Mater. Chem. A 2020, 8, 4978–4995. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Waterhouse, G.I.N.; Zhang, T. Photocatalytic ammonia synthesis: Recent progress and future. EnergyChem 2019, 1, 100013. [Google Scholar] [CrossRef]
- Vu, M.-H.; Sakar, M.; Do, T.-O. Insights into the Recent Progress and Advanced Materials for Photocatalytic Nitrogen Fixation for Ammonia (NH3) Production. Catalysts 2018, 8, 621. [Google Scholar] [CrossRef]
- Schrauzer, G.; Guth, T. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J. Am. Chem. Soc. 2002, 99, 7189–7193. [Google Scholar] [CrossRef]
- Lashgari, M.; Zeinalkhani, P. Photocatalytic N2 conversion to ammonia using efficient nanostructured solar-energy-materials in aqueous media: A novel hydrogenation strategy and basic understanding of the phenomenon. Appl. Catal. A Gen. 2017, 529, 91–97. [Google Scholar] [CrossRef]
- Endoh, E.; Leland, J.K.; Bard, A.J. Heterogeneous photoreduction of nitrogen to ammonia on tungsten oxide. J. Phys. Chem. 1986, 90, 6223–6226. [Google Scholar] [CrossRef]
- Miyama, H.; Fujii, N.; Nagae, Y. Heterogeneous photocatalytic synthesis of ammonia from water and nitrogen. Chem. Phys. Lett. 1980, 74, 523–524. [Google Scholar] [CrossRef]
- Lamichhane, P.; Adhikari, B.C.; Nguyen, L.N.; Paneru, R.; Ghimire, B.; Mumtaz, S.; Lim, J.S.; Hong, Y.J.; Choi, E.H. Sustainable nitrogen fixation from synergistic effect of photo-electrochemical water splitting and atmospheric pressure N2 plasma. Plasma Sources Sci. Technol. 2020, 29, 045026. [Google Scholar] [CrossRef]
- Sakakura, T.; Uemura, S.; Hino, M.; Kiyomatsu, S.; Takatsuji, Y.; Yamasaki, R.; Morimoto, M.; Haruyama, T. Excitation of H2O at the plasma/water interface by UV irradiation for the elevation of ammonia production. Green. Chem. 2018, 20, 627–633. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, L.; Xiong, L.; Wang, X.; Yu, Y.; Yang, M. Synergistic Cu/Cu2O/Co3O4 catalyst with crystalline-amorphous interfaces for efficient electrochemical nitrate reduction to ammonia. Chem. Eng. J. 2025, 507, 160393. [Google Scholar] [CrossRef]
- Chen, A.; Xia, B.Y. Ambient dinitrogen electrocatalytic reduction for ammonia synthesis. J. Mater. Chem. A 2019, 7, 23416–23431. [Google Scholar] [CrossRef]
- Wu, A.; Yang, J.; Xu, B.; Wu, X.-Y.; Wang, Y.; Lv, X.; Ma, Y.; Xu, A.; Zheng, J.; Tan, Q.; et al. Direct ammonia synthesis from the air via gliding arc plasma integrated with single atom electrocatalysis. Appl. Catal. B Environ. 2021, 299, 120667. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, C.; Wang, L.; Tan, X.; Wang, Z.; Wei, Q.; Zhang, Y.; Qiu, J. Microscopic-Level Insights into the Mechanism of Enhanced NH(3) Synthesis in Plasma-Enabled Cascade N(2) Oxidation-Electroreduction System. J. Am. Chem. Soc. 2022, 144, 10193–10200. [Google Scholar] [CrossRef]
- Hollevoet, L.; Jardali, F.; Gorbanev, Y.; Creel, J.; Bogaerts, A.; Martens, J.A. Towards Green Ammonia Synthesis through Plasma-Driven Nitrogen Oxidation and Catalytic Reduction. Angew. Chem. Int. Ed. Engl. 2020, 59, 23825–23829. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Craven, M.; Yu, X.; Ding, J.; Bryant, P.; Huang, J.; Tu, X. Plasma-Enhanced Catalytic Synthesis of Ammonia over a Ni/Al(2)O(3) Catalyst at Near-Room Temperature: Insights into the Importance of the Catalyst Surface on the Reaction Mechanism. ACS Catal. 2019, 9, 10780–10793. [Google Scholar] [CrossRef]
- Gao, B.; Cao, G.; Hu, D.; Guo, L.; Ba, Z.; Li, C.; Zhao, J.; Fang, Y. Insight into the effect of support properties on DBD plasma-catalytic NH3 synthesis over Ru-Co bimetallic catalysts. Fuel 2025, 38, 1338022. [Google Scholar] [CrossRef]
- Kamarinopoulou, N.S.; Wittreich, G.R.; Vlachos, D.G. Direct HCN synthesis via plasma-assisted conversion of methane and nitrogen. Sci. Adv. 2024, 10, eadl4246. [Google Scholar] [CrossRef]
- Shao, K.; Mesbah, A. A Study on the Role of Electric Field in Low-Temperature Plasma Catalytic Ammonia Synthesis via Integrated Density Functional Theory and Microkinetic Modeling. JACS Au 2024, 4, 525–544. [Google Scholar] [CrossRef]
- Navascues, P.; Garrido-Garcia, J.; Cotrino, J.; Gonzalez-Elipe, A.R.; Gomez-Ramirez, A. Incorporation of a Metal Catalyst for the Ammonia Synthesis in a Ferroelectric Packed-Bed Plasma Reactor: Does It Really Matter? ACS Sustain. Chem. Eng. 2023, 11, 3621–3632. [Google Scholar] [CrossRef]
- Hua, Z.; Song, H.; Zhou, C.; Xin, Q.; Zhou, F.; Fan, W.; Liu, S.; Zhang, X.; Zheng, C.; Yang, Y.; et al. A promising catalyst for catalytic oxidation of chlorobenzene and slipped ammonia in SCR exhaust gas: Investigating the simultaneous removal mechanism. Chem. Eng. J. 2023, 473, 145106. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Xu, S.; Zhao, S.; Chen, G.; Weidenkaff, A.; Hardacre, C.; Fan, X.; Huang, J.; Tu, X. Shielding Protection by Mesoporous Catalysts for Improving Plasma-Catalytic Ambient Ammonia Synthesis. J. Am. Chem. Soc. 2022, 144, 12020–12031. [Google Scholar] [CrossRef]
- Gorky, F.; Lucero, J.M.; Crawford, J.M.; Blake, B.; Carreon, M.A.; Carreon, M.L. Plasma-Induced Catalytic Conversion of Nitrogen and Hydrogen to Ammonia over Zeolitic Imidazolate Frameworks ZIF-8 and ZIF-67. ACS Appl. Mater. Interfaces 2021, 13, 21338–21348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, H.; Long, Y.; Cao, A.; Wang, K.; Gao, L.; Zhao, Z.; Zhang, H.; Zhang, H.; Yan, J. Efficient ammonia synthesis from N2 and H2O over γ-Al2O3 at mild temperatures via atmospheric plasma-assisted chemical looping. Appl. Catal. B Environ. Energy 2025, 371, 125206. [Google Scholar] [CrossRef]
- Brown, S.; Ahmat Ibrahim, S.; Robinson, B.R.; Caiola, A.; Tiwari, S.; Wang, Y.; Bhattacharyya, D.; Che, F.; Hu, J. Ambient Carbon-Neutral Ammonia Generation via a Cyclic Microwave Plasma Process. ACS Appl. Mater. Interfaces 2023, 15, 23255–23264. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zheng, J.; Du, M.; Wu, X.; Song, J.; Cheng, C.; Li, T.; Yang, W. Non-thermal plasma-assisted ammonia production: A review. Energy Convers. Manag. 2023, 293, 117482. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Lefferts, L. Feasibility Study of Plasma-Catalytic Ammonia Synthesis for Energy Storage Applications. Catalysts 2020, 10, 999. [Google Scholar] [CrossRef]
- Hong, J.; Prawer, S.; Murphy, A.B. Plasma Catalysis as an Alternative Route for Ammonia Production: Status, Mechanisms, and Prospects for Progress. ACS Sustain. Chem. Eng. 2018, 6, 15–31. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Fedirchyk, I.; Bogaerts, A. Plasma catalysis in ammonia production and decomposition: Use it, or lose it? Curr. Opin. Green. Sustain. Chem. 2024, 47, 100916. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Addy, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Schiappacasse, C.; Zhang, Y.; Chen, D.; Hatzenbeller, R.; et al. Atmospheric Plasma-Assisted Ammonia Synthesis Enhanced via Synergistic Catalytic Absorption. ACS Sustain. Chem. Eng. 2018, 7, 100–104. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Atmospheric-pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Process. Polym. 2016, 14, 1600157. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, N.; Lin, Y.; Mao, X.; Zhong, H.; Chang, Z.; Shneider, M.N.; Ju, Y. Enhancements of electric field and afterglow of non-equilibrium plasma by Pb(Zr(x)Ti(1-x))O(3) ferroelectric electrode. Nat. Commun. 2024, 15, 3092. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Q.; Zhao, X.; Lin, H.; Qin, W. Kinetic investigation of plasma catalytic synthesis of ammonia: Insights into the role of excited states and plasma-enhanced surface chemistry. Plasma Sources Sci. Technol. 2022, 31, 094009. [Google Scholar] [CrossRef]
- Barboun, P.M.; Otor, H.O.; Ma, H.; Goswami, A.; Schneider, W.F.; Hicks, J.C. Plasma-Catalyst Reactivity Control of Surface Nitrogen Species through Plasma-Temperature-Programmed Hydrogenation to Ammonia. ACS Sustain. Chem. Eng. 2022, 10, 15741–15748. [Google Scholar] [CrossRef]
- Barboun, P.; Mehta, P.; Herrera, F.A.; Go, D.B.; Schneider, W.F.; Hicks, J.C. Distinguishing Plasma Contributions to Catalyst Performance in Plasma-Assisted Ammonia Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 8621–8630. [Google Scholar] [CrossRef]
- Herrera, F.A.; Brown, G.H.; Barboun, P.; Turan, N.; Mehta, P.; Schneider, W.F.; Hicks, J.C.; Go, D.B. The impact of transition metal catalysts on macroscopic dielectric barrier discharge (DBD) characteristics in an ammonia synthesis plasma catalysis reactor. J. Phys. D Appl. Phys. 2019, 52, 224002. [Google Scholar] [CrossRef]
- Rouwenhorstm, K.H.R.; Lefferts, L. Plasma-catalytic Ammonia Synthesis via Eley-Rideal Reactions: A Kinetic Analysis. ChemCatChem 2023, 15, e202300078. [Google Scholar] [CrossRef]
- Lim, K.H.; Yue, Y.; Bella; Gao, X.; Zhang, T.; Hu, F.; Das, S.; Kawi, S. Sustainable hydrogen and ammonia technologies with nonthermal plasma catalysis: Mechanistic insights and technoeconomic analysis. ACS Sustain. Chem. Eng. 2023, 11, 4903–4933. [Google Scholar] [CrossRef]
- Ben Yaala, M.; Saeedi, A.; Scherrer, D.F.; Moser, L.; Steiner, R.; Zutter, M.; Oberkofler, M.; De Temmerman, G.; Marot, L.; Meyer, E. Plasma-assisted catalytic formation of ammonia in N(2)-H(2) plasma on a tungsten surface. Phys. Chem. Chem. Phys. 2019, 21, 16623–16633. [Google Scholar] [CrossRef]
- Carreon, M.; Shah, J.; Gorky, F.; Psarras, P.; Seong, B. Ammonia yield enhancement by hydrogen sink effect during plasma catalysis. ChemCatChem 2019, 12, 1200–1211. [Google Scholar]
- Gómez-Ramírez, A.; Montoro-Damas, A.M.; Cotrino, J.; Lambert, R.M.; González-Elipe, A.R. About the enhancement of chemical yield during the atmospheric plasma synthesis of ammonia in a ferroelectric packed bed reactor. Plasma Process. Polym. 2017, 14, 1600081. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; Cotrino, J.; Lambert, R.; González-Elipe, A. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 2015, 24, 065011. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Nitrides as ammonia synthesis catalysts and as potential nitrogen transfer reagents. Appl. Petrochem. Res. 2014, 4, 3–10. [Google Scholar] [CrossRef]
- Sadiek, I.; Fleisher, A.J.; Hayden, J.; Huang, X.; Hugi, A.; Engeln, R.; Lang, N.; van Helden, J.H. Dual-comb spectroscopy of ammonia formation in non-thermal plasmas. Commun. Chem. 2024, 7, 110. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, J.; Chen, S.; Chen, Y.; Chen, H.; Fan, X. Ammonia synthesis by nonthermal plasma catalysis: A review on recent research progress. J. Phys. D Appl. Phys. 2024, 57, 323001. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, H.; Wang, H.; Lu, K.; Yang, D. Effectiveness of Noble Gas Addition for Plasma Synthesis of Ammonia in a Dielectric Barrier Discharge Reactor. Appl. Sci. 2024, 14, 3001. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Zhou, W.; Lu, W.; Chu, D.; Fang, S. Experimental Investigation of DBD Parameters and Ru/γ-Al2O3 Catalyst on Plasma-Assisted Ammonia Synthesis. IEEE Trans. Plasma Sci. 2023, 51, 3538–3545. [Google Scholar] [CrossRef]
- Li, S.; van Raak, T.; Gallucci, F. Investigating the operation parameters for ammonia synthesis in dielectric barrier discharge reactors. J. Phys. D Appl. Phys. 2020, 53, 014008. [Google Scholar] [CrossRef]
- Hosseini, H. Dielectric barrier discharge plasma catalysis as an alternative approach for the synthesis of ammonia: A review. RSC Adv. 2023, 13, 28211–28223. [Google Scholar] [CrossRef]
- Peng, P.; Li, Y.; Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Atmospheric Pressure Ammonia Synthesis Using Non-thermal Plasma Assisted Catalysis. Plasma Chem. Plasma Process. 2016, 36, 1201–1210. [Google Scholar] [CrossRef]
- Xie, Q.; Zhuge, S.; Song, X.; Lu, M.; Yu, F.; Ruan, R.; Nie, Y. Non-thermal atmospheric plasma synthesis of ammonia in a DBD reactor packed with various catalysts. J. Phys. D Appl. Phys. 2020, 53, 064002. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Zhou, C.; Du, J.; Gan, Y.; Chen, G.; Tu, X. Plasma-catalytic ammonia synthesis over BaTiO3 supported metal catalysts: Process optimization using response surface methodology. Vacuum 2022, 203, 111205. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, Y.; Zeng, Y.; Tu, X. Plasma synthesis of ammonia in a tangled wire dielectric barrier discharge reactor: Effect of electrode materials. J. Energy Inst. 2021, 99, 137–144. [Google Scholar] [CrossRef]
- Li, K.; Chen, S.; Li, M.; Liu, L.; Li, Y.; Yang, G.; Wang, F. Plasma Assisted Ammonia Synthesis in a Fluidized-Bed DBD reactor: Effect of Catalyst Particle Movement. In Proceedings of the 2024 IEEE International Conference on Plasma Science (ICOPS), Beijing, China, 16–20 June 2024; p. 1. [Google Scholar]
- Wang, S.; Shen, Z.; Osatiashtiani, A.; Nabavi, S.A.; Clough, P.T. Ni-based bimetallic catalysts for hydrogen production via (sorption-enhanced) steam methane reforming. Chem. Eng. J. 2024, 486, 150170. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Guo, X.; Ma, S. Calcium-based pellets for continuous hydrogen production by sorption-enhanced steam methane reforming. Int. J. Hydrogen Energy 2024, 49, 897–909. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Lian, H.-Y.; Liu, J.-L.; Li, X.-S. Plasma catalytic steam methane reforming for distributed hydrogen production. Catal. Today 2019, 337, 69–75. [Google Scholar] [CrossRef]
- Gomez, J.R.; Baca, J.; Garzon, F. Techno-economic analysis and life cycle assessment for electrochemical ammonia production using proton conducting membrane. Int. J. Hydrogen Energy 2020, 45, 721–737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).