Abstract
The growing demand for sustainable and environmentally friendly cosmetic products has driven innovations using plant residues as raw materials for high-value-added applications. This study focuses on the enzymatic hydrolysis of plant residues to extract bioactive compounds, with the potential for application as functional ingredients in cosmetics. Enzymatic processes are highlighted for their ability to optimize extraction, preserving the bioactivity of the compounds while significantly reducing the environmental footprint compared to conventional resource-intensive methods. This work emphasizes scientific articles that incorporate the principles of the circular economy, promoting the reuse of solid waste and mitigating the need to extract new natural resources. The valorization of waste through advanced biotechnological technologies addresses critical environmental challenges and offers innovative solutions that transform agro-industrial by-products into high-value inputs for the cosmetic industry. The results presented reinforce this approach’s feasibility and positive impact, promoting economic and environmental benefits. This study highlights the transformative role of enzymatic hydrolysis in the transition toward a more sustainable, efficient cosmetics industry integrated with global decarbonization goals.
1. Introduction
The escalating demand for food, driven by population growth, has intensified waste generation at all stages of the agri-food chain, from production to end-user consumption [1]. Approximately one-third of all food produced globally is lost or wasted annually, corresponding to 2.5 billion tons [2]. This scenario raises social and economic concerns and environmental issues due to inadequate disposal methods (landfills and incineration), which are characterized by high energy consumption and substantial greenhouse gas emissions [3].
To address the pressing issues of waste generation and environmental impact, implementing sustainable valorization strategies for agro-industrial and food waste has become imperative. These approaches aim to recover high-value compounds while reducing ecological harm from landfill-dependent disposal systems. Figure 1 illustrates the trend in the number of scientific publications indexed in the ScienceDirect database between 2014 and 2024 based on the keywords “Plant residues waste”, “Plant waste”, and “Plant residue”. This growth reflects heightened scientific and industrial interest in circular-economy solutions, which is driven by regulatory incentives and consumer demand for greener practices.
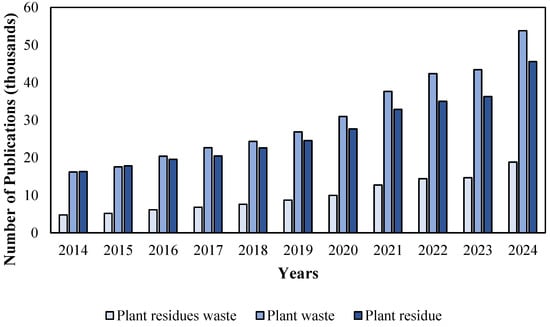
Figure 1.
Number of scientific articles published on plant residues and waste between 2014 and 2024.
Various agro-industrial residues, such as peels, seeds, bagasse, and wastewater from extraction and purification processes, exhibit high potential for the production of bioproducts, including biofertilizers, bioethanol, biolipids, and biodegradable polymers [4,5,6,7]. The soybean industry, for example, seeks to implement integrated systems to fully utilize by-products such as okara, soy whey, and hulls, applying biorefinery technologies and circular-economy principles to achieve zero-waste processing models [8]. Similarly, lignocellulosic residues derived from Camellia oleifera oil extraction have been converted into fermentable sugars through optimized acid pretreatments and enzymatic hydrolysis, balancing process efficiency with resource conservation [9].
Advanced technologies have played a fundamental role in enabling these alternatives, with particular attention to processes such as subcritical hydrolysis, which transforms biomass into sugars without harsh chemical reagents. However, challenges related to solid-to-liquid ratios continue to limit its application on an industrial scale [10]. Furthermore, green solvents, such as deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs), have emerged as promising alternatives for the sustainable extraction of bioactive compounds from agro-industrial residues, offering lower toxicity and environmental impact compared to conventional organic solvents [11].
Nevertheless, despite technological advances and identified opportunities, the implementation of waste valorization strategies still faces challenges related to the variability of waste composition, the presence of antinutritional factors, and technical and economic limitations in integrating these processes into established production chains [12]. In this regard, studies exploring integrated and innovative approaches to the recovery of food and agro-industrial waste are important to support the transition toward more sustainable, resilient, and circular food systems.
The valorization of agro-industrial waste through sustainable and innovative processes becomes an opportunity for bioenergy and biomaterials production and for generating high-value compounds applicable in other industries, such as cosmetics. The cosmetic industry has prioritized safety and sustainability, developing cosmetics and cosmeceuticals formulated with safe, environmentally friendly, and cruelty-free ingredients. This trend responds to the growing demand for products that replace or reduce the use of active ingredients and components that do not meet these criteria [13,14,15].
The application of native proteins in cosmetic formulations is mainly limited by their low water solubility. However, this limitation can be overcome through enzymatic hydrolysis, which enhances the solubility and improves the functional properties of hydrolysates, such as surface hydrophobicity, emulsifying and foaming capacity, substantivity, film-forming ability, improved skin penetration, and increased moisture retention and shine [16,17,18,19,20]. These characteristics make hydrolysates promising ingredients for high-performance cosmetic products.
Protein hydrolysates and their derivatives—polypeptides, oligopeptides, and peptides in particular—are widely used in the cosmetic industry as conditioning agents for hair and skin, owing to their ability to perform various biological functions in skin cells. These compounds can activate signaling pathways and regulate key genetic mechanisms, resulting in beneficial effects on the skin. Furthermore, unlike larger proteins, peptides exhibit superior penetration into the deeper layers of the skin, offering a more efficient and effective delivery system [21,22].
Bioactive peptides are compounds with specific biological functions, including enzyme inhibition, as well as antimicrobial, antioxidant, and anti-inflammatory activities [23,24,25,26]. Enzymatic hydrolysis can also serve as an effective pretreatment to facilitate the recovery of phenolic compounds from agro-industrial residues such as brewer’s spent grain, fruit peels, bran, and other plant by-products [27,28,29,30,31,32]. This pretreatment disrupts the lignocellulosic matrix, releasing phenolic compounds that are often bound to polysaccharides and proteins, thereby increasing their bioavailability and extraction efficiency. Consequently, enzymatic hydrolysis optimizes the recovery of bioactive compounds and contributes to waste valorization by promoting the development of high-value functional ingredients for the cosmetic industry and other applications.
Agro-industrial by-products are emerging as valuable sources of peptides and phenolic compounds, particularly through hydrolysis [33,34]. Protein concentrations in food-industry residues typically range from 5.5% to 42.2% (mean ≈ 20.5%), underscoring their suitability for bioconversion into biopeptides and amino acids [35].
Among these residues, brewer’s spent grain (BSG) and brewer’s spent hops (BSHs) stand out as rich sources of natural antioxidants. BSG contains 12–25% cellulose, 20–25% hemicellulose, and 12–28% lignin, along with 7.5–13.3% sugars, 15.9–35% proteins, and 6.4–13% lipids. In contrast, BSHs are low in lipids (~1%) but particularly high in proteins and amino acids (40–52%). The total phenolic content in BSG has been reported at 7–10 mg gallic-acid equivalents (GAEs)/g, whereas BSHs range from 10 to 18 mg GAEs/g [36]. Through optimized extraction, BSG can yield 24.84–38.83 µmol of GAEs/g of polyphenols, with ferulic acid as the predominant compound, a molecule not detected in BSHs extracts [37].
Citrus peels (sweet oranges, grapefruits, Ellendale mandarins, and Yen Ben lemons) are also prolific sources of high-value phenolic acids—ferulic, p-coumaric, sinapic, caffeic, vanillic, gallic, and chlorogenic. Sweet orange residues exhibit the highest total phenolic content (1790 mg GAEs/g), followed by grapefruit (1550 mg GAEs/g), mandarin (1211 mg GAEs/g), and lemon (1190 mg GAEs/g) [38]. Upcycling these residues provides a cost-effective source of natural antioxidants and advances circular economy practices by transforming materials that would otherwise be discarded or underutilized into high-value bioactive ingredients (Figure 2).
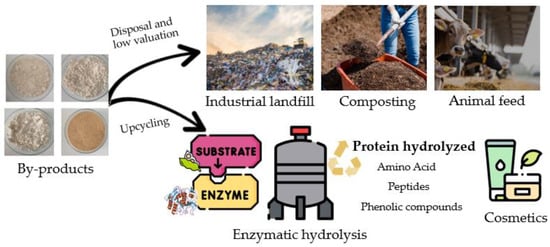
Figure 2.
Circular economy practices for by-products.
Therefore, this article explores the potential for sustainable valorization of plant-based residues through enzymatic hydrolysis, focusing on the extraction of bioactive compounds for application as functional ingredients in cosmetic formulations. The growing demand for sustainable and environmentally friendly cosmetics, combined with the need for industrial practices that promote circularity and waste reduction, underscores the importance of developing innovative processes with minimal environmental impact.
2. Fundamental Principles of Enzymatic Hydrolysis
Enzymatic hydrolysis cleaves large biomolecules into smaller fragments by incorporating water across specific chemical bonds. Unlike chemical hydrolysis, which relies on strong acids or bases, enzymatic processes employ enzymes that operate under mild temperature and pH conditions [39,40]. This selectivity lowers energy requirements and minimizes hazardous by-products, but it also preserves the structural integrity and bioactivity of the released compounds [41,42].
Beyond its environmental advantages, enzymatic hydrolysis aligns with broader sustainability goals due to the renewable and biodegradable nature of enzymes. These biological catalysts naturally integrate into ecological cycles, supporting principles of the circular economy [43]. The valorization of agro-industrial by-products has gained momentum in response to increasing sustainability demands and the scarcity of natural resources.
The application of enzymatic hydrolysis enables the efficient conversion of plant residues into high-value raw materials, thereby facilitating the recycling and reuse of these by-products [44]. This strategy not only mitigates greenhouse gas emissions and reduces the volume of waste destined for landfills but also ensures that the process aligns with global sustainable development objectives [45,46].
Different enzymes, including cellulases, hemicellulases, pectinases, and proteases, target distinct components of plant cell walls and intracellular matrices. These enzymes facilitate the degradation of complex carbohydrates, proteins, and other macromolecules [42,47]. The proteases can be applied individually or in combinations to optimize reaction efficiency and the peptide profile.
Among the most commonly used proteases is Alcalase®, which is a serine endoprotease derived from Bacillus licheniformis and commercially formulated as a liquid preparation containing glycerol, water, and protease extract [48,49]. Bromelain, a cysteine endoprotease extracted from the stem of Ananas comosus, is another widely utilized enzyme known for its broad substrate specificity [50]. Neutrase®, a zinc-dependent metalloprotease from Bacillus amyloliquefaciens, is valued for its neutral activity and broad cleavage profile [51]. Flavourzyme®, produced by Aspergillus oryzae, combines exo- and endoproteolytic activity, including aminopeptidases and carboxypeptidases, resulting in hydrolysates with low bitterness and improved sensory properties [51]. Protamex®, another protease complex derived from Bacillus spp., is known for its versatility in producing hydrolysates with various degrees of hydrolysis and functional profiles [52].
For carbohydrate breakdown, Viscozyme® L (Aspergillus aculeatus) combines arabinase, cellulase, β-glucanase, hemicellulase, and xylanase activities to dismantle lignocellulosic scaffolds, thereby enhancing protein and phenolic release [53,54].
Enzymatic hydrolysis valorizes plant by-products through a multistep mechanism, starting with an initial pretreatment to disrupt lignocellulosic structures and expose cell wall polymers followed by adsorption of hydrolytic enzymes onto substrate surfaces through specific interactions between active sites [55,56]. Cellulases, hemicellulases, and pectinases synergistically cleave β-1,4-glycosidic bonds, ester bonds, and pectic galacturonan, releasing soluble oligosaccharides, monosaccharides, and phenolic glycosides [57]. This hydrolytic release generates phenolic aglycones and bioactive peptides through the rupture of glycosidic and peptide bonds, which significantly increases molecular solubility and bioavailability.
Despite its potential, the integration of enzymatic hydrolysis into large-scale industrial processes presents challenges. These include high operational costs, difficulties in scaling up, and limitations of enzymes in breaking down complex plant cell wall structures [58]. Enzymes require controlled conditions to maintain their activity and stability, which may not be easily replicated in industrial settings, limiting their efficiency and increasing production costs.
To overcome these challenges, process design and technology are needed to establish databases of hydrolysate properties, coupled with comprehensive techno-economic assessments that can inform enzyme selection and operational planning [59]. Additionally, leveraging modern computational tools, such as in silico simulations and bioinformatics, can significantly enhance enzyme and substrate selection, while the incorporation of green technologies like ultrasound, microwave-assisted extraction, and pulsed electric fields offers promising avenues to improve yield and reduce environmental impact [60].
The development of continuous bioreactors, including membrane-based and immobilized enzyme systems, further supports prolonged, stable operations and lower production costs. Equally important is the need to navigate the regulatory landscape to secure market acceptance, a challenge best met through strengthened collaboration between academic researchers and industry stakeholders, ensuring that scientific innovations translate effectively into commercially viable products [59].
3. Valorization of Plant By-Products Through Enzymatic Hydrolysis
The valorization of plant by-products through enzymatic hydrolysis has gained increasing prominence as a sustainable strategy to recover high-value compounds from agro-industrial residues, which are often rich in fibers, proteins, lipids, and bioactive molecules [61]. By-products, including husks and seeds, have demonstrated significant potential for applications in the pharmaceutical, cosmetic, and nutraceutical sectors due to their high content of phenolic compounds, such as polyphenols, oligophenols, and monophenols [62,63,64,65].
After enzymatic hydrolysis of plant by-products, the resulting bioactive compounds are typically characterized by a combination of physicochemical (protein content, aminogram, degree of hydrolysis, yield, content of phenolic compounds, high-performance liquid chromatography, gas chromatography, solubility, and stability) and biological (antioxidant, antimicrobial, anti-inflammatory activity, and enzyme inhibition) analyses to assess their potential for industrial applications, particularly in cosmetics and pharmaceuticals.
Numerous studies attest to the versatility of enzyme-assisted extractions (EAEs). For instance, [66] reported the effective valorization of chokeberry pomace through EAEs using cellulolytic and xylanolytic enzymes, which significantly enhanced the recovery of water-soluble fractions, monosaccharides, and phenolic compounds while also improving antioxidant properties.
Similarly, El Kantar et al. [67] optimized the release of fermentable sugars and polyphenols from orange peels through enzymatic hydrolysis with Viscozyme® L, both independently and in combination with high-voltage electrical discharges, demonstrating the synergistic effect of physical and enzymatic treatments. In the context of fruit seed valorization, the enzymatic hydrolysis of plum seeds generated bioactive peptides with antioxidant and antihypertensive activities, particularly through the use of Alcalase® [68], while a one-pot protease-based extraction enabled the sustainable recovery of oils and proteins from various fruit seeds and kernels, with oils rich in unsaturated fatty acids and protein hydrolysates showing moderate degrees of hydrolysis [69].
Furthermore, Meini et al. [70] optimized the aqueous enzymatic extraction of phenolic compounds from grape pomace using a combination of pectinase, cellulase, and tannase, achieving a 66% increase in phenolic yield and an 80% enhancement in antioxidant capacity. These studies collectively demonstrate the versatility of enzymatic hydrolysis as a sustainable and efficient approach for the valorization of diverse plant by-products, enabling the recovery of high-value bioactive compounds with potential applications across various industrial sectors.
Additionally, innovative applications include the extraction of natural pigments, such as the brown pigments from Camellia oleifera shells, which exhibited promising stability and bioactivity, including antioxidant and antimicrobial properties, following ultrasound-assisted enzymatic extraction [71]. Polysaccharides with anticancer potential have been obtained from Rosa roxburghii fruit through ultrasound-assisted enzymatic methods, with purified fractions inducing apoptosis in cancer cells via ROS-dependent pathways [72]. Furthermore, aqueous enzymatic extraction (AEE) has proven highly effective for oil recovery from various plant residues, including gardenia fruit [73], passion fruit peels [74], and Idesia polycarpa fruit [75].
These approaches, often optimized through statistical methodologies such as response surface designs, have resulted in higher extraction yields, improved nutritional profiles, and enhanced functional properties of oils, pectins, and other biomolecules when compared to conventional chemical or solvent-based methods. Table 1 summarizes key studies, highlighting improvements in extract quality and extraction efficiency achieved via enzymatic treatments.

Table 1.
Studies on obtaining bioactive compounds through enzymatic hydrolysis.
The hemicellulase-assisted extract exhibited the highest antioxidant capacity in the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay (5075 ± 43 mg Trolox equivalent (TE)/100 g), while the cellulase-assisted extract performed best in the 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay (8138 ± 31 mg TE/100 g dw). Enzyme-assisted extraction facilitated the release of phenolic compounds from the plant matrix, increasing their bioavailability and antioxidant properties [47]. Enzyme-treated chicory extract was able to inhibit the growth of S. aureus and P. aeruginosa after only 7 days of incubation [81]. Cold-pressed oil cake from pumpkin seed (Cucurbita pepo L.) inhibited 50% of the free radicals DPPH and ABTS at a concentration of 13.07 μg/mL and 64.70 μg/mL, respectively [82]. The protein extract derived from rice husks showed antioxidant capacity similar to the standards (25,327 ± 0.44%), such as those used in the cosmetic and food industry: butylated hydroxyanisole (BHA) with DPPH of 22.16%, butylhydroxytoluene (BHT) with DPPH of 31.31 ± 2.4, vitamin C with DPPH of 28.8 ± 1.47, and vitamin E with DPPH of 25.1 ± 3.04. This activity was possibly due to the presence of highly reactive amino acids [22].
4. Cosmetic Applications and Benefits of Bioactive Compounds Derived from Plant By-Products
Hydrolysates derived from soy, rice, and corn proteins have been evaluated by the Cosmetic Ingredient Review Expert Panel and are deemed safe for use in cosmetic products. These compounds play a critical role as skin and hair conditioning agents and are widely utilized due to their hydrating and protective properties. Similarly, hydrolyzed wheat gluten and hydrolyzed wheat protein have been classified as safe for cosmetic use, provided they are formulated with peptides of a molecular weight (MW) not exceeding 3500 Da. This specification ensures safety, efficacy, and a reduced risk of skin sensitization [83,84].
Bioactive peptides obtained through the hydrolysis of protein matrices demonstrate a broad spectrum of biological activities, making them up-and-coming candidates for innovative cosmeceutical product development. Notably, these peptides exhibit antioxidant, antimicrobial, and anti-inflammatory properties and can inhibit enzymes associated with skin aging, such as elastase, collagenase, tyrosinase, and hyaluronidase. By acting on these targets, they contribute directly to maintaining skin integrity and delaying the visible signs of aging [85].
Among bioactive peptides, those derived from the enzymatic hydrolysis of soy protein (Glycine max L.) and its by-products have been extensively studied for their antioxidant, anti-inflammatory, antiatherosclerotic, and anticarcinogenic effects [86,87]. Incorporating these peptides into cosmetic formulations enhances oxidative stress protection and promotes cellular renewal, yielding significant skin health benefits and multifunctional product opportunities.
Table 2 presents studies highlighting the application of enzymatic hydrolysis as a method for extracting bioactive compounds from plant by-products, with a focus on their use as cosmetic ingredients.

Table 2.
Studies on the enzymatic hydrolysis of by-products to extract peptides and bioactive compounds for application in the cosmetics industry.
From a circular economy perspective, agro-industrial residues, such as brewers’ spent grain (BSG), have garnered increasing attention as sustainable sources of bioactive compounds for the cosmetic industry. The enzymatic hydrolysis of BSG enhances the bioavailability of phenolic compounds and peptides, broadening their potential for industrial applications [90].
Peptides derived from BSG exhibit antioxidant, anti-inflammatory, and wound-healing properties, making them valuable ingredients for skin care and protective products [91]. Additionally, hydroxycinnamic acids in BSG, such as ferulic acid and p-coumaric acid, can be recovered and incorporated into cosmetic formulations to protect against premature aging and UV-induced damage [92].
Phenolic acids are particularly effective in preventing photoaging due to their high antioxidant capacity [93]. Furthermore, they exhibit depigmenting effects by inhibiting tyrosinase activity, thereby aiding in the reduction of hyperpigmentation [94]. These compounds also demonstrate antibacterial and anti-inflammatory properties, making them effective in wound healing and treating dermatological conditions such as acne, seborrheic dermatitis, and atopic dermatitis [95].
The extraction of polyphenols for the development of sustainable and high-performance cosmetics, exploiting their antioxidant, anti-inflammatory, and UV protection properties, requires enzymes specialized in degrading the cell wall, breaking down structural barriers, and releasing intracellular bioactives. The success of these extractions depends both on the appropriate choice of enzyme (alone or in customized enzyme cocktails) and on the fine-tuning of reaction conditions—pH, temperature, and ionic strength—to maximize catalytic activity and release efficiency of target compounds [96].
Enzymatic hydrolysis is a highly effective strategy for extracting bioactive compounds from grape pomace, including resveratrol, a potent antioxidant and anti-inflammatory agent widely used in cosmetic and pharmaceutical industries for its proven efficacy in preventing premature aging and reducing inflammation [64,65,67]. This process also facilitates the extraction of additional bioactive compounds from wine by-products, such as those with antiaging, anti-inflammatory, and wound-healing properties, thereby expanding the potential application of these agro-industrial residues in high-value cosmetic formulations [97,98].
Upcycling can be a promising business alternative for valorizing plant residues by transforming waste into profit. Innovative companies and startups are investing in the potential of ingredients derived from plant residues. TransferTech from Brazil, for instance, uses enzymatic technology to produce protein hydrolysates. Similarly, Upgrain employs the concept of upcycling to convert plant residues into sustainable raw materials. This process demonstrates how the strategic utilization of by-products can yield highly functional ingredients for biocosmetic applications, thereby bridging environmental sustainability with technological innovation. In parallel, the Brazilian startup Nun Tecnologia Sustentável has developed an exclusive extract from the secondary processing of olives.
Furthermore, waste valorization is also a central strategy for the Brazilian startup Cacaus Biocosméticos. By utilizing cocoa residues to formulate products, the company harnesses potent antioxidant properties and imparts anti-inflammatory and antimicrobial activities to its formulations. This approach significantly enhances the value of food-industry by-products and broadens the opportunities for developing effective and sustainable biocosmetic products.
5. Economic and Environmental Benefits of By-Products Recovery
Globalization, population growth, and the need for large-scale food production have led to the adoption of a linear agricultural model focused on immediate economic outcomes, often at the expense of environmental health. In contrast to traditional agriculture, which adhered to circular sustainability models by reintegrating waste into the production cycle, modern agriculture accumulates tons of waste in landfills. This practice squanders resources and hinders the recovery of bioactive compounds, such as polyphenols, flavonoids, tannins, and essential oils that possess significant antioxidant and anti-inflammatory properties and could be transformed into high-value-added products [97,98].
Despite their richness in bioactive compounds, plant by-products are commonly utilized in animal feed production. While this approach is considered a sustainable alternative, adding value to generated waste and reducing environmental impacts, it fails to exploit its potential fully. Valuable components that could be allocated to higher-value applications, such as human nutrition, nutraceutical products, and cosmetics, are lost. Consequently, recovering these ingredients through the valorization of agro-industrial by-products emerges as an innovative and promising strategy [99,100,101].
Utilizing agro-industrial waste for the extraction of bioactive compounds in cosmetics offers a new approach that transforms low-value by-products into high-value ingredients, reducing raw material costs and adding revenue streams for both agricultural and industrial sectors [102,103]. In addition, by repurposing waste, companies can lower disposal expenses and mitigate the risks associated with environmental regulations [104,105], minimizing the ecological footprint of agro-industrial operations by reducing landfill burden and associated greenhouse gas emissions while promoting sustainable resource management [106].
Waste reduction and the valorization of by-products are essential strategies for strengthening sustainability in the industry. With increasing environmental awareness, several sectors have rethought the destination of their waste. In agribusiness, for example, plant residues represent a significant portion of by-products, and their high volume can cause serious environmental impacts. The accumulation of organic waste in landfills brings significant environmental challenges. The slow degradation of these wastes occupies large volumes and releases greenhouse gases, such as methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O), that contribute to global warming [107,108]. In the specific case of protein waste, it is estimated that between 15 and 750 kg of carbon dioxide are emitted per kilogram of wasted edible protein [109,110].
The proper disposal and the reuse of waste are essential for the economy and environmental preservation. The circular economy aims to add value to waste by diverting it from methods such as environmental disposal, incineration, or landfill. This concept introduces a new perspective on the reuse and valorization of agro-industrial waste, reducing the need for additional natural resources [111].
In this way, transforming this waste into new products presents a promising opportunity to mitigate environmental damage and generate economic value. An effective strategy is the recovery of bioactive compounds from plant waste, which can be incorporated into the food, cosmetics, and pharmaceutical industries [112,113].
By converting plant waste into high-value-added ingredients, companies not only open up new sources of revenue but also reduce the costs related to the final disposal of waste. This practice reduces dependence on the extraction of new raw materials, contributing to the preservation of ecosystems, water, and energy resources. Thus, a business model based on the circular economy drives innovation and competitiveness, creating a virtuous cycle of sustainable production [114].
6. Conclusions
Plant residues and by-products contain a wide diversity of bioactive molecules with significant potential for application in the cosmetic industry. These compounds, including antioxidants, polyphenols, vitamins, amino acids, and peptides, provide functional benefits to cosmetic formulations, such as moisturizing, anti-inflammatory, and antiaging effects.
The upcycling of residual materials that would otherwise be discarded or undervalued adds economic value to these by-products and represents an important step toward strengthening the circular economy. This process significantly reduces the demand for virgin raw materials, thereby minimizing the environmental impacts associated with the extraction of new natural resources. Moreover, it contributes to the mitigation of greenhouse gas emissions and, consequently, enhances the sustainability of the sector.
Author Contributions
Conceptualization, B.M.S.P., C.E.D.O. and J.L.B.; validation, B.M.S.P., C.E.D.O. and J.L.B.; formal analysis, B.M.S.P., C.E.D.O. and J.L.B.; investigation, B.M.S.P., C.E.D.O. and J.L.B.; resources, L.D.V. and D.E.F.; data curation, L.D.V. and R.M.D.; writing—original draft preparation, B.M.S.P., C.E.D.O. and J.L.B.; writing—review and editing, B.M.S.P., C.E.D.O., J.L.B., D.E.F., L.D.V., R.M.D. and M.V.T.; supervision, R.M.D. and M.V.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This material is based upon work supported by Universidade Regional Integrada do Alto Uruguai e das Missões (URI), Federal University of Santa Maria (UFSM), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Grant Number: 24/2551-0002182-5.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Author Diana Exenberger Finkler was employed by the company Marina Tecnologia Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmad, T.; Esposito, F.; Cirillo, T. Valorization of Agro-Food by-Products: Advancing Sustainability and Sustainable Development Goals 2030 through Functional Compounds Recovery. Food Biosci. 2024, 62, 105194. [Google Scholar] [CrossRef]
- Torabi, P.; Hamdami, N.; Soltanizadeh, N.; Farhadian, O.; Le-Bail, A. Restaurant Food Waste Valorization by Microwave-Assisted Hydrolysis: Optimization, Typological and Biochemical Analysis. Clean. Mater. 2024, 13, 100269. [Google Scholar] [CrossRef]
- An, L.; Zhang, X.; Lu, J.; Wan, J.; Liu, Y. Valorization of Food Waste to Biofertilizer and Carbon Source for Denitrification with Assistance of Plant Ash and Biochar toward Zero Solid Discharge. Bioresour. Technol. 2025, 420, 132119. [Google Scholar] [CrossRef] [PubMed]
- Flores-Maltos, D.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Teixeira, J.A.; Aguilar, C.N. Biotechnological Production and Application of Fructooligosaccharides. Crit. Rev. Biotechnol. 2016, 36, 259–267. [Google Scholar] [CrossRef]
- Ahmad, A.; Othman, I.; Tardy, B.L.; Hasan, S.W.; Banat, F. Enhanced Lactic Acid Production with Indigenous Microbiota from Date Pulp Waste and Keratin Protein Hydrolysate from Chicken Feather Waste. Bioresour. Technol. Rep. 2022, 18, 101089. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols Be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Benvenuti, J.; Fisch, A.; Dos Santos, J.H.Z.; Gutterres, M. Silica-Based Adsorbent Material with Grape Bagasse Encapsulated by the Sol-Gel Method for the Adsorption of Basic Blue 41 Dye. J. Environ. Chem. Eng. 2019, 7, 103342. [Google Scholar] [CrossRef]
- Karim, A.; Osse, E.F.; Khalloufi, S. Innovative Strategies for Valorization of Byproducts from Soybean Industry: A Review on Status, Challenges, and Sustainable Approaches towards Zero-Waste Processing Systems. Heliyon 2025, 11, e42118. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; Wang, M.; Liao, Y.; Li, Z.; Khan, M.R.; Qin, Z. Enhancing the Valorization Efficiency of Camellia Oil Extraction Wastes through Sequential Green Acid Pretreatment and Solid-State Fermentation-Based Enzymatic Hydrolysis. Ind. Crops Prod. 2024, 217, 118893. [Google Scholar] [CrossRef]
- Morales-Gutiérrez, G.; Marulanda-Cardona, V. Process Model and Comparative Life Cycle Assessment (LCA) of a Biorefinery Concept Based on Fractionated Subcritical Water Hydrolysis for Sugar Cane Trash Valorization. Biomass Bioenergy 2025, 196, 107740. [Google Scholar] [CrossRef]
- Jaganmohanrao, L. Valorization of Onion Wastes and By-Products Using Deep Eutectic Solvents as Alternate Green Technology Solvents for Isolation of Bioactive Phytochemicals. Food Res. Int. 2025, 206, 115980. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Joye, I.J. Improving Agricultural Sustainability—A Review of Strategies to Valorize Tomato Plant Residues (TPR). Waste Manag. 2024, 190, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetics Industry: A Review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Silva, I.G.S.; Almeida, F.C.G.; Rufino, R.D.; Sarubbo, L.A. Plant-Derived Biosurfactants: Extraction, Characteristics and Properties for Application in Cosmetics. Biocatal. Agric. Biotechnol. 2021, 34, 102036. [Google Scholar] [CrossRef]
- Ketemepi, H.K.; Awang, M.A.B.; Seelan, J.S.S.; Mohd Noor, N.Q.I. Extraction Process and Applications of Mushroom-Derived Protein Hydrolysate: A Comprehensive Review. Future Foods 2024, 9, 100359. [Google Scholar] [CrossRef]
- Nasri, R.; Younes, I.; Jridi, M.; Trigui, M.; Bougatef, A.; Nedjar-Arroume, N.; Dhulster, P.; Nasri, M.; Karra-Châabouni, M. ACE Inhibitory and Antioxidative Activities of Goby (Zosterissessor Ophiocephalus) Fish Protein Hydrolysates: Effect on Meat Lipid Oxidation. Food Res. Int. 2013, 54, 552–561. [Google Scholar] [CrossRef]
- Melgosa, R.; Trigueros, E.; Sanz, M.T.; Cardeira, M.; Rodrigues, L.; Fernández, N.; Matias, A.A.; Bronze, M.R.; Marques, M.; Paiva, A.; et al. Supercritical CO2 and Subcritical Water Technologies for the Production of Bioactive Extracts from Sardine (Sardina Pilchardus) Waste. J. Supercrit. Fluids 2020, 164, 104943. [Google Scholar] [CrossRef]
- Elmalimadi, M.B.; Jovanović, J.R.; Stefanović, A.B.; Tanasković, S.J.; Djurović, S.B.; Bugarski, B.M.; Knežević-Jugović, Z.D. Controlled Enzymatic Hydrolysis for Improved Exploitation of the Antioxidant Potential of Wheat Gluten. Ind. Crops Prod. 2017, 109, 548–557. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Lin, D.; Sun, L.C.; Chen, Y.L.; Liu, G.M.; Miao, S.; Cao, M.J. Peptide/Protein Hydrolysate and Their Derivatives: Their Role as Emulsifying Agents for Enhancement of Physical and Oxidative Stability of Emulsions. Trends Food Sci. Technol. 2022, 129, 11–24. [Google Scholar] [CrossRef]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Ben Mlouka, M.A.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F. Antioxidant, Antityrosinase and Antibiofilm Activities of Synthesized Peptides Derived from Vicia Faba Protein Hydrolysate: A Powerful Agents in Cosmetic Application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Vargas-Escobar, P.; Flórez-Acosta, O.; Corrales-García, L.L. Renewing the Potential of Rice Crop Residues as Value-Added Products in the Cosmetics Industry. Heliyon 2024, 10, e28402. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Suarez, M.; Muguerza, B.; Bravo, F.I. Long-Term Administration of Protein Hydrolysate from Chicken Feet Induces Antihypertensive Effect and Confers Vasoprotective Pattern in Diet-Induced Hypertensive Rats. J. Funct. Foods 2019, 55, 28–35. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Leduc, A.; Hervy, M.; Rangama, J.; Delépée, R.; Fournier, V.; Henry, J. Shrimp By-Product Hydrolysate Induces Intestinal Myotropic Activity in European Seabass (Dicentrarchus Labrax). Aquaculture 2018, 497, 380–388. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Hayes, M.; Reig, M.; Toldrá, F. Effect of Cooking and in Vitro Digestion on the Antioxidant Activity of Dry-Cured Ham by-Products. Food Res. Int. 2017, 97, 296–306. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of Pomegranate Peel Polyphenols Encapsulated in Orange Juice Industry By-Product and Their Incorporation in Cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Yagi, S.; Zengin, G.; Uba, A.I.; Maciejewska-Turska, M.; Sieniawska, E.; Świątek, Ł.; Rajtar, B.; Bahşi, M.; Guler, O.; Dall’Acqua, S.; et al. Exploring Chemical Composition, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Glaucium Acutidentatum Hausskn. & Bornm. from Turkey Flora: A Novel Source of Bioactive Agents to Design Functional Applications. Antioxidants 2024, 13, 643. [Google Scholar] [CrossRef]
- Maia, P.D.D.S.; dos Santos Baião, D.; da Silva, V.P.F.; de Araújo Calado, V.M.; Queiroz, C.; Pedrosa, C.; Valente-Mesquita, V.L.; Pierucci, A.P.T.R. Highly Stable Microparticles of Cashew Apple (Anacardium Occidentale L.) Juice with Maltodextrin and Chemically Modified Starch. Food Bioprocess Technol. 2019, 12, 2107–2119. [Google Scholar] [CrossRef]
- Bittencourt, L.L.D.A.; Silva, K.A.; de Sousa, V.P.; Fontes-Sant’Ana, G.C.; Rocha-Leão, M.H. Blueberry Residue Encapsulation by Ionotropic Gelation. Plant Foods Hum. Nutr. 2018, 73, 278–286. [Google Scholar] [CrossRef]
- Dag, D.; Kilercioglu, M.; Oztop, M.H. Physical and Chemical Characteristics of Encapsulated Goldenberry (Physalis Peruviana L.) Juice Powder. LWT Food Sci. Technol. 2017, 83, 86–94. [Google Scholar] [CrossRef]
- da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus Ulmifolius Schott Fruits: A Detailed Study of Its Nutritional, Chemical and Bioactive Properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Samel, A.; Wojciechowski, K. Lupine Protein Enzymatic Hydrolysates Obtained in a Simultaneous Extraction-Hydrolysis Process as Foaming Agents. LWT 2024, 208, 116713. [Google Scholar] [CrossRef]
- Mamoudou, H.; Mune Mune, M.A. Investigating Bambara Bean (Vigna Subterranea (Verdc.) L.) Protein and Hydrolysates: A Comprehensive Analysis of Biological and Biochemical Properties. Appl. Food Res. 2024, 4, 100489. [Google Scholar] [CrossRef]
- Dhiman, S.; Thakur, B.; Kaur, S.; Ahuja, M.; Gantayat, S.; Sarkar, S. Closing the Loop: Technological Innovations in Food Waste Valorisation for Global Sustainability; Springer International Publishing: Cham, Switzerland, 2025; ISBN 4362102501073. [Google Scholar]
- Bravi, E.; De Francesco, G.; Sileoni, V.; Perretti, G.; Galgano, F.; Marconi, O. Brewing By-Product Upcycling Potential: Nutritionally Valuable Compounds and Antioxidant Activity Evaluation. Antioxidants 2021, 10, 165. [Google Scholar] [CrossRef]
- Codina-Torrella, I.; Rodero, L.; Almajano, M.P. Brewing By-Products as a Source of Natural Antioxidants for Food Preservation. Antioxidants 2021, 10, 1512. [Google Scholar] [CrossRef]
- Bisht, B.; Gururani, P.; Aman, J.; Vlaskin, M.S.; Anna I, K.; Irina A, A.; Joshi, S.; Kumar, S.; Kumar, V. A Review on Holistic Approaches for Fruits and Vegetables Biowastes Valorization. Mater. Today Proc. 2023, 73, 54–63. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Health Applications of Soy Protein Hydrolysates. Int. J. Pept. Res. Ther. 2020, 26, 2333–2343. [Google Scholar] [CrossRef]
- Habinshuti, I.; Nsengumuremyi, D.; Muhoza, B.; Ebenezer, F.; Yinka Aregbe, A.; Antoine Ndisanze, M. Recent and Novel Processing Technologies Coupled with Enzymatic Hydrolysis to Enhance the Production of Antioxidant Peptides from Food Proteins: A Review. Food Chem. 2023, 423, 136313. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá, F. Advanced Enzymatic Hydrolysis of Food Proteins for the Production of Bioactive Peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Singh, R.; Jain, R.; Soni, P.; Santos-Villalobos, S.D.L.; Chattaraj, S.; Roy, D.; Mitra, D.; Gaur, A. Graphing the Green Route: Enzymatic Hydrolysis in Sustainable Decomposition. Curr. Res. Microb. Sci. 2024, 7, 100281. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-Assisted Supercritical Fluid Extraction: An Integral Approach to Extract Bioactive Compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Ghinea, C.; Ungureanu-Comăniță, E.D.; Țâbuleac, R.M.; Oprea, P.S.; Coșbuc, E.D.; Gavrilescu, M. Cost-Benefit Analysis of Enzymatic Hydrolysis Alternatives for Food Waste Management. Foods 2025, 14, 488. [Google Scholar] [CrossRef]
- Gupta, N.; Mahur, B.K.; Izrayeel, A.M.D.; Ahuja, A.; Rastogi, V.K. Biomass Conversion of Agricultural Waste Residues for Different Applications: A Comprehensive Review. Environ. Sci. Pollut. Res. 2022, 29, 73622–73647. [Google Scholar] [CrossRef]
- Stanek-Wandzel, N.; Krzyszowska, A.; Zarębska, M.; Gębura, K.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Tomaka, M. Evaluation of Cellulase, Pectinase, and Hemicellulase Effectiveness in Extraction of Phenolic Compounds from Grape Pomace. Int. J. Mol. Sci. 2024, 25, 13538. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Sun-Waterhouse, D.; Ivan Neil Waterhouse, G.; Zhao, M.; Zhang, J.; Wang, F.; Su, G. Two-Stage Selective Enzymatic Hydrolysis Generates Protein Hydrolysates Rich in Asn-Pro and Ala-His for Enhancing Taste Attributes of Soy Sauce. Food Chem. 2021, 345, 128803. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Castañeda-Valbuena, D.; Berenguer-Murcia, Á.; Kamli, M.R.; Tavano, O.; Fernandez-Lafuente, R. Immobilization of Papain: A Review. Int. J. Biol. Macromol. 2021, 188, 94–113. [Google Scholar] [CrossRef]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef]
- Vogelsang-O’dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Pulse Proteins as a Tool to Improve Techno-Functional Properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the Degree of Hydrolysis on Functional Properties and Antioxidant Activity of Enzymatic Soybean Protein Hydrolysates. Molecules 2022, 27, 6110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Angelov, A.; Übelacker, M.; Baudrexl, M.; Ludwig, C.; Rühmann, B.; Sieber, V.; Liebl, W. Proteomic Analysis of Viscozyme L and Its Major Enzyme Components for Pectic Substrate Degradation. Int. J. Biol. Macromol. 2024, 266, 131309. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.N.; Heisel, I.; Satzger, A.; Vizsolyi, E.C.; Oster, S.D.J.; Agarwal, S.; Laforsch, C.; Löder, M.G.J. Tackling the Challenge of Extracting Microplastics from Soils: A Protocol to Purify Soil Samples for Spectroscopic Analysis. Environ. Toxicol. Chem. 2022, 41, 844–857. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.S.; Lee, D.J. Enzymes and Enzymatic Mechanisms in Enzymatic Degradation of Lignocellulosic Biomass: A Mini-Review. Bioresour. Technol. 2023, 367, 128252. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Jiang, Y.; Zheng, W.; Wang, S.; Sang, S.; Li, H. Comprehensively Understanding Enzymatic Hydrolysis of Lignocellulose and Cellulase–Lignocellulose Adsorption by Analyzing Substrates’ Physicochemical Properties. Bioenergy Res. 2020, 13, 1108–1120. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Ramos, C.L.; Kuddus, M.; Rodriguez-Couto, S.; Srivastava, N.; Ramteke, P.W.; Mishra, P.K.; Molina, G. Enzymatic Potential for the Valorization of Agro-Industrial by-Products. Biotechnol. Lett. 2020, 42, 1799–1827. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Tarchi, I.; Loudiyi, M.; Boukria, O.; Cahyana, Y.; Ozogul, F.; Khwaldia, K. Transforming Plant-Based Waste and by-Products into Valuable Products Using Various “Food Industry 4.0” Enabling Technologies: A Literature Review. Sci. Total Environ. 2024, 955, 176872. [Google Scholar] [CrossRef]
- Gasparre, N.; Rosell, C.M.; Boukid, F. Enzymatic Hydrolysis of Plant Proteins: Tailoring Characteristics, Enhancing Functionality, and Expanding Applications in the Food Industry. Food Bioprocess Technol. 2024, 18, 3272–3287. [Google Scholar] [CrossRef]
- Mora, L.; Reig, M.; Toldrá, F. Bioactive Peptides Generated from Meat Industry By-Products. Food Res. Int. 2014, 65, 344–349. [Google Scholar] [CrossRef]
- dos Santos, K.I.P.; Benjamim, J.K.F.; da Costa, K.A.D.; dos Reis, A.S.; de Souza Pinheiro, W.B.; Santos, A.S. Metabolomics Techniques Applied in the Investigation of Phenolic Acids from the Agro-Industrial by-Product of Carapa Guianensis Aubl. Arab. J. Chem. 2021, 14, 103421. [Google Scholar] [CrossRef]
- Liu, T.W.; Hsiao, S.W.; Lin, C.T.; Hsiao, G.; Lee, C.K. Anti-Aging Constituents from Pinus Morrisonicola Leaves. Molecules 2023, 28, 5063. [Google Scholar] [CrossRef] [PubMed]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The Importance of Antioxidants and Place in Today’s Scientific and Technological Studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, F.L.; Ward, E.; Meng, X.; Matharu, A.S. Microwave-Assisted Defibrillation of Microalgae. Molecules 2021, 26, 4972. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Kitrytė, V.; Kraujalienė, V.; Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Chokeberry Pomace Valorization into Food Ingredients by Enzyme-Assisted Extraction: Process Optimization and Product Characterization. Food Bioprod. Process. 2017, 105, 36–50. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High Voltage Electrical Discharges Combined with Enzymatic Hydrolysis for Extraction of Polyphenols and Fermentable Sugars from Orange Peels. Food Res. Int. 2018, 107, 755–762. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Plum (Prunus Domestica L.) by-Product as a New and Cheap Source of Bioactive Peptides: Extraction Method and Peptides Characterization. J. Funct. Foods 2014, 11, 428–437. [Google Scholar] [CrossRef]
- Lolli, V.; Viscusi, P.; Bonzanini, F.; Conte, A.; Fuso, A.; Larocca, S.; Leni, G.; Caligiani, A. Oil and Protein Extraction from Fruit Seed and Kernel By-Products Using a One Pot Enzymatic-Assisted Mild Extraction. Food Chem. X 2023, 19, 100819. [Google Scholar] [CrossRef]
- Meini, M.R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Cheng, G.; Zhu, J.; Si, J.; Wu, T.; Chen, J.; Xu, X.; Feng, S.; Chen, T.; Ding, C.; Zhou, L. Optimization of Ultrasound-Assisted Enzymatic Extraction, Chemical Constituents, Biological Activities, and Stability of Camellia Oleifera Fruit Shell Brown Pigments. LWT 2024, 207, 116625. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, M.; Zhang, M.; Hao, L.; Wu, C. Ultrasound-Assisted Enzymatic Extraction, Structural Characterization, and Anticancer Activity of Polysaccharides from Rosa Roxburghii Tratt Fruit. Int. J. Biol. Macromol. 2024, 259, 127926. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, Y.; Xie, T.; Tang, G.; Song, G.; Li, L.; Yuan, T.; Zheng, F.; Gong, J. Ultrasound-Assisted Aqueous Enzymatic Extraction of Gardenia Fruits (Gardenia Jasminoides Ellis) Oil: Optimization and Quality Evaluation. Ind. Crops Prod. 2023, 191, 116021. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Zapata Zapata, A.D. Enzymatic Extraction of Pectin from Passion Fruit Peel (Passiflora Edulis f. Flavicarpa) at Laboratory and Bench Scale. LWT 2017, 80, 280–285. [Google Scholar] [CrossRef]
- Hou, K.; Yang, X.; Bao, M.; Chen, F.; Tian, H.; Yang, L. Composition, Characteristics and Antioxidant Activities of Fruit Oils from Idesia Polycarpa Using Homogenate-Circulating Ultrasound-Assisted Aqueous Enzymatic Extraction. Ind. Crops Prod. 2018, 117, 205–215. [Google Scholar] [CrossRef]
- Leite, P.; Belo, I.; Salgado, J.M. Enhancing Antioxidants Extraction from Agro-Industrial By-Products by Enzymatic Treatment. Foods 2022, 11, 3715. [Google Scholar] [CrossRef]
- Teixeira, A.J.; Menegat, F.D.; Weschenfelder, L.M.; Oro, C.E.D.; Astolfi, V.; Valduga, E.; Zeni, J.; Backes, G.T.; Cansian, R.L. Enzymatic Hydrolysis of Lignocellulosic Residues and Bromatological Characterization for Animal Feed. Cienc. Rural 2023, 53, e20210720. [Google Scholar] [CrossRef]
- Javier, O.E.; Alejandro, G.-R.M.; Elizabeth, C.-L.; Guadalupe, P.-F.J.; Emmanuel, P.-E.; Carlos, M.-S.J.; Daniel, M.-C. In Vitro Multi-Bioactive Potential of Enzymatic Hydrolysis of a Non-Toxic Jatropha Curcas Cake Protein Isolate. Molecules 2024, 29, 3088. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, S.; Liu, H.; Wu, J.; Zhang, J.; Huang, M.; Sun, B.; Sun, X. From Baijiu By-Product to Bioactive Goldmine: The Health-Promoting Potential of Jiuzao Extracts. LWT 2025, 223, 117706. [Google Scholar] [CrossRef]
- Trinh, L.T.P.; Choi, Y.-S.; Bae, H.-J. Production of Phenolic Compounds and Biosugars from Flower Resources via Several Extraction Processes. Ind. Crops Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Cankar, K.; Nohynek, L.; Suomalainen, M.; van Arkel, J.; Siika-Aho, M.; Twarogowska, A.; Van Droogenbroeck, B.; Oksman-Caldentey, K.-M. Enzyme-Treated Chicory for Cosmetics: Application Assessment and Techno-Economic Analysis. AMB Express 2022, 12, 152. [Google Scholar] [CrossRef]
- Dyankova, S.; Doneva, M.; Terziyska, M.; Metodieva, P.; Nacheva, I. Optimization of the Process for Obtaining Antioxidant Protein Hydrolysates from Pumpkin Seed Oil Cake Using Response Surface Methodology. Appl. Sci. 2024, 14, 1967. [Google Scholar] [CrossRef]
- Burnett, C.L.; Boyer, I.J.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Plant-Derived Proteins and Peptides as Used in Cosmetics. Int. J. Toxicol. 2022, 41, 5S–20S. [Google Scholar] [CrossRef]
- Burnett, C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Hydrolyzed Wheat Protein and Hydrolyzed Wheat Gluten as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 55S–66S. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential Role of Natural Bioactive Peptides for Development of Cosmeceutical Skin Products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional Significance of Bioactive Peptides Derived from Soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine Max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef]
- Štambuk, P.; Tomašković, D.; Tomaz, I.; Maslov, L.; Stupić, D.; Karoglan Kontić, J. Application of Pectinases for Recovery of Grape Seeds Phenolics. 3 Biotech 2016, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Tedeschi, T.; Prandi, B.; Michelini, E.; Calabretta, M.M.; Babini, E.; Graen-Heedfeld, J.; Bretz, K.; Raddadi, N.; Gianotti, A.; et al. Looking for Peptides from Rice Starch Processing By-Product: Bioreactor Production, Anti-Tyrosinase and Anti-Inflammatory Activity, and in Silico Putative Taste Assessment. Front. Plant Sci. 2022, 13, 929918. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Villain-Gambier, M.; Trébouet, D. By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy. Sustainability 2024, 16, 3472. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. Phenolic Acids Used in the Cosmetics Industry as Natural Antioxidants. Eur. J. Med. Technol. 2019, 4, 24–32. [Google Scholar]
- Almendinger, M.; Rohn, S.; Pleissner, D. Malt and Beer-Related by-Products as Potential Antioxidant Skin-Lightening Agents for Cosmetics. Sustain. Chem. Pharm. 2020, 17, 100282. [Google Scholar] [CrossRef]
- Gomez-Molina, M.; Albaladejo-Marico, L.; Yepes-Molina, L.; Nicolas-Espinosa, J.; Navarro-León, E.; Garcia-Ibañez, P.; Carvajal, M. Exploring Phenolic Compounds in Crop By-Products for Cosmetic Efficacy. Int. J. Mol. Sci. 2024, 25, 5884. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Rafinska, K.; Walczak-Skierska, J.; Buszewski, B. The Influence of Plant Material Enzymatic Hydrolysis and Extraction Conditions on the Polyphenolic Profiles and Antioxidant Activity of Extracts: A Green and Efficient Approach. Molecules 2020, 25, 2074. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Sodhi, G.K.; Kaur, G.; George, N.; Walia, H.K.; Sillu, D.; Rath, S.K.; Saxena, S.; Rios-Solis, L.; Dwibedi, V. Waste to Wealth: Microbial-Based Valorization of Grape Pomace for Nutraceutical, Cosmetic, and Therapeutic Applications to Promote Circular Economy. Process Saf. Environ. Prot. 2024, 188, 1464–1478. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture Waste Valorisation as a Source of Antioxidant Phenolic Compounds within a Circular and Sustainable Bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Tropea, A. Food Waste Valorization. Fermentation 2022, 8, 168. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Kumar, R.; Kumar, A. Environmental Waste Management Strategies and Vermi Transformation for Sustainable Development. Environ. Chall. 2023, 13, 100747. [Google Scholar] [CrossRef]
- Valisakkagari, H.; Chaturvedi, C.; Rupasinghe, H.P.V. Green Extraction of Phytochemicals from Fresh Vegetable Waste and Their Potential Application as Cosmeceuticals for Skin Health. Processes 2024, 12, 742. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef]
- Čolović, D.; Rakita, S.; Banjac, V.; Đuragić, O.; Čabarkapa, I. Plant Food By-Products as Feed: Characteristics, Possibilities, Environmental Benefits, and Negative Sides. Food Rev. Int. 2019, 35, 363–389. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-Industrial by-Products: Valuable Sources of Bioactive Compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Sari, T.P.; Sirohi, R.; Krishania, M.; Bhoj, S.; Samtiya, M.; Duggal, M.; Kumar, D.; Badgujar, P.C. Critical Overview of Biorefinery Approaches for Valorization of Protein Rich Tree Nut Oil Industry By-Product. Bioresour. Technol. 2022, 362, 127775. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bagnani, M.; Soon, W.L.; Mezzenga, R. Turning Food Protein Waste into Sustainable Technologies. Chem. Rev. 2023, 123, 2112–2154. [Google Scholar] [CrossRef]
- Di Domenico Ziero, H.; Ampese, L.C.; Sganzerla, W.G.; Torres-Mayanga, P.C.; Timko, M.T.; Mussatto, S.I.; Forster-Carneiro, T. Subcritical Water Hydrolysis of Poultry Feathers for Amino Acids Production. J. Supercrit. Fluids 2022, 181, 105492. [Google Scholar] [CrossRef]
- Barral-martinez, M.; Fraga-corral, M.; Garcia-perez, P.; Simal-gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of High Added-Value Components from Food Wastes: Conventional, Emerging Technologies and Commercialized Applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Ora, A.; Häkkinen, S.T.; Ritala, A.; Räisänen, R.; Kallioinen-Mänttäri, M.; Melin, K. Innovative Extraction Technologies of Bioactive Compounds from Plant By-Products for Textile Colorants and Antimicrobial Agents. Biomass Convers. Biorefin. 2023, 14, 24973–25002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).