pH-Responsive Gold Nanoparticle/PVP Nanoconjugate for Targeted Delivery and Enhanced Anticancer Activity of Withaferin A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Studies
2.3. Physalis minima-Mediated AuNPs and Withaferin A
2.4. Functionalization of Physalis minima-Mediated AuNPs
2.4.1. Preparation of PVP-Capped Gold Nanoparticles
2.4.2. Folic Acid (FA)-Conjugated AuNPs
2.4.3. Preparation of AuNPs–PVP–Withaferin A NC
2.5. HPLC Analysis of Withaferin A Plant Extract
2.6. Evaluation of Withaferin A Loading Efficiency
2.7. In Vitro Release of Withaferin A
2.8. Cell Culture Maintenance
2.9. MTT Assay (Cell Viability Assay Method)
2.10. Acridine Orange/Ethidium Bromide (AO/EtBr) Dual Staining Technique for the Evaluation of Apoptotic Induction
2.11. Implementation of DAPI Labelling for Analyzing Apoptotic Induction
2.12. Western Blotting
2.13. Data Analysis
3. Results
3.1. HPLC Analysis of Withaferin A from Plant Extract
3.2. XRD and FTIR Analysis
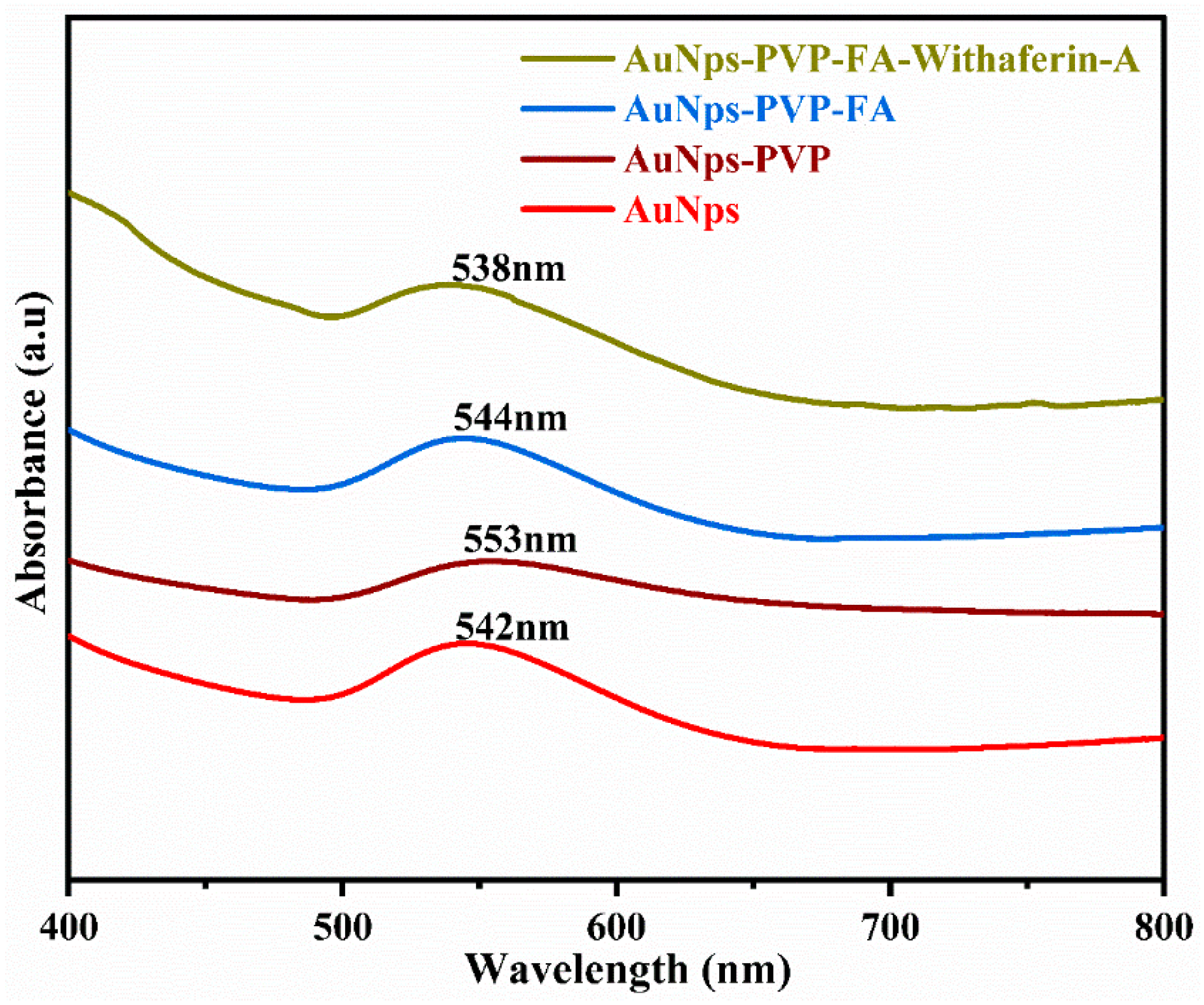
3.3. UV–Visible Spectroscopy Analysis
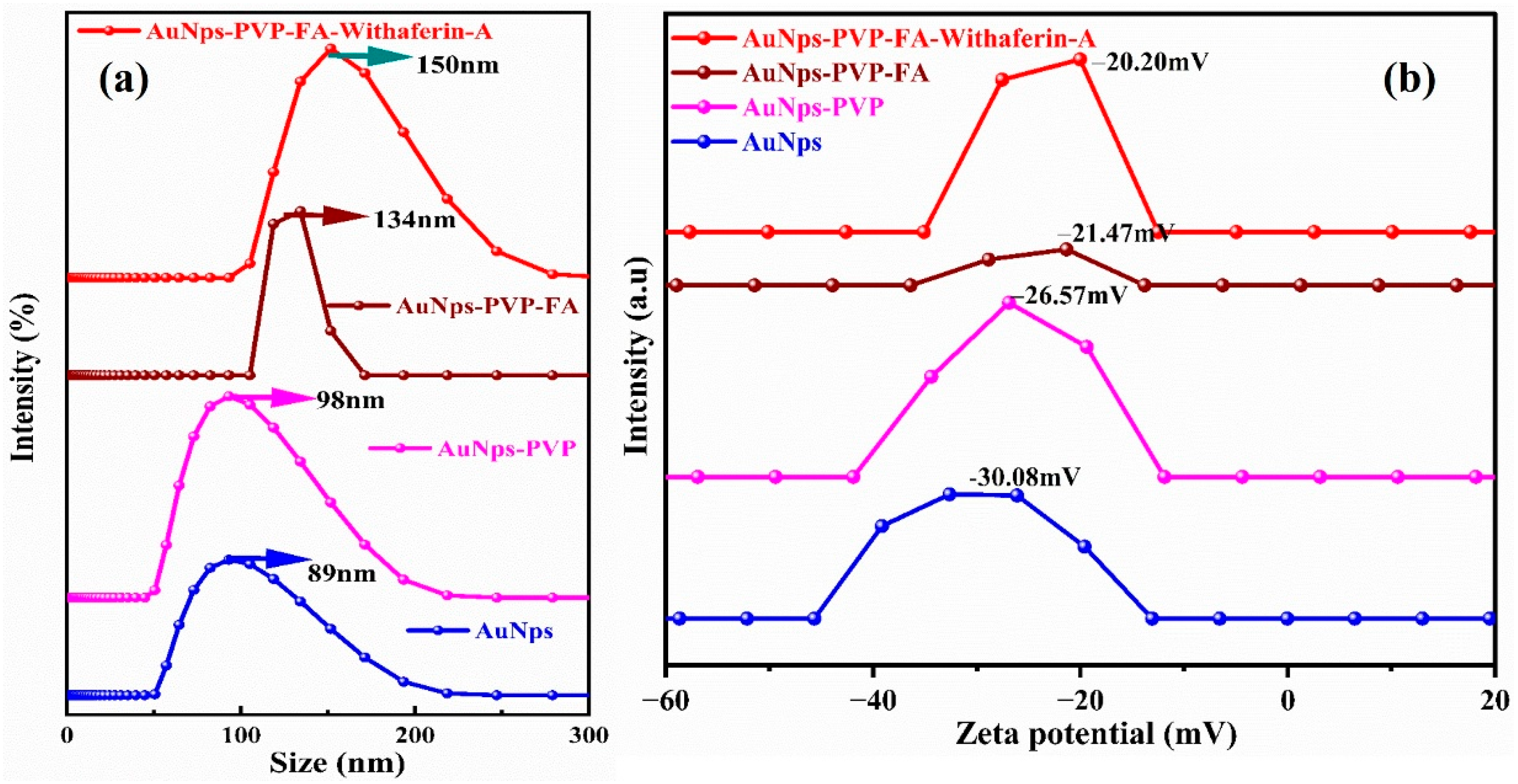
3.4. Hydrodynamic Particle Size and Surface Charge Analysis
3.5. Morphological Analysis of AuNPs–PVP–FA with Withaferin A Drug
3.6. Drug Loading and Drug Release Studies
3.7. Drug Release Study (In Vitro)
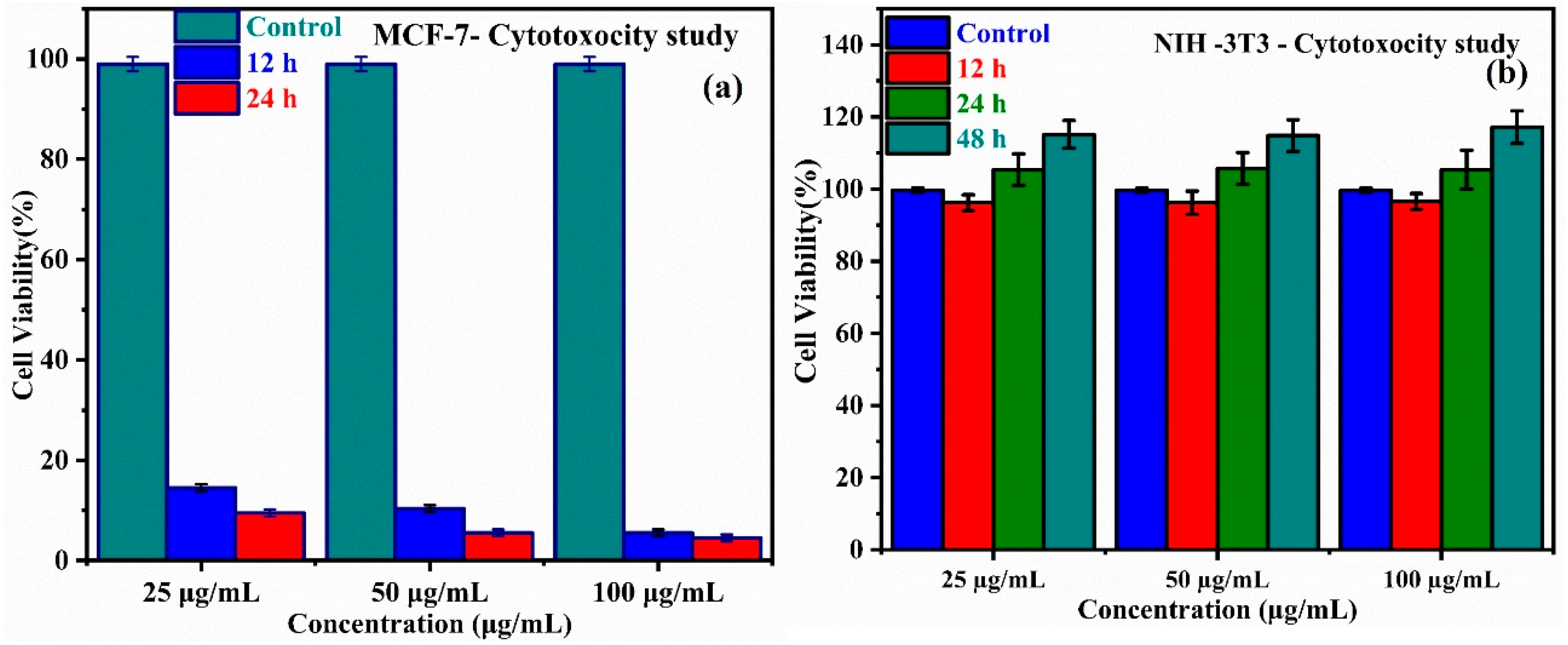
3.8. In Vitro Cytotoxicity and Cell Viability
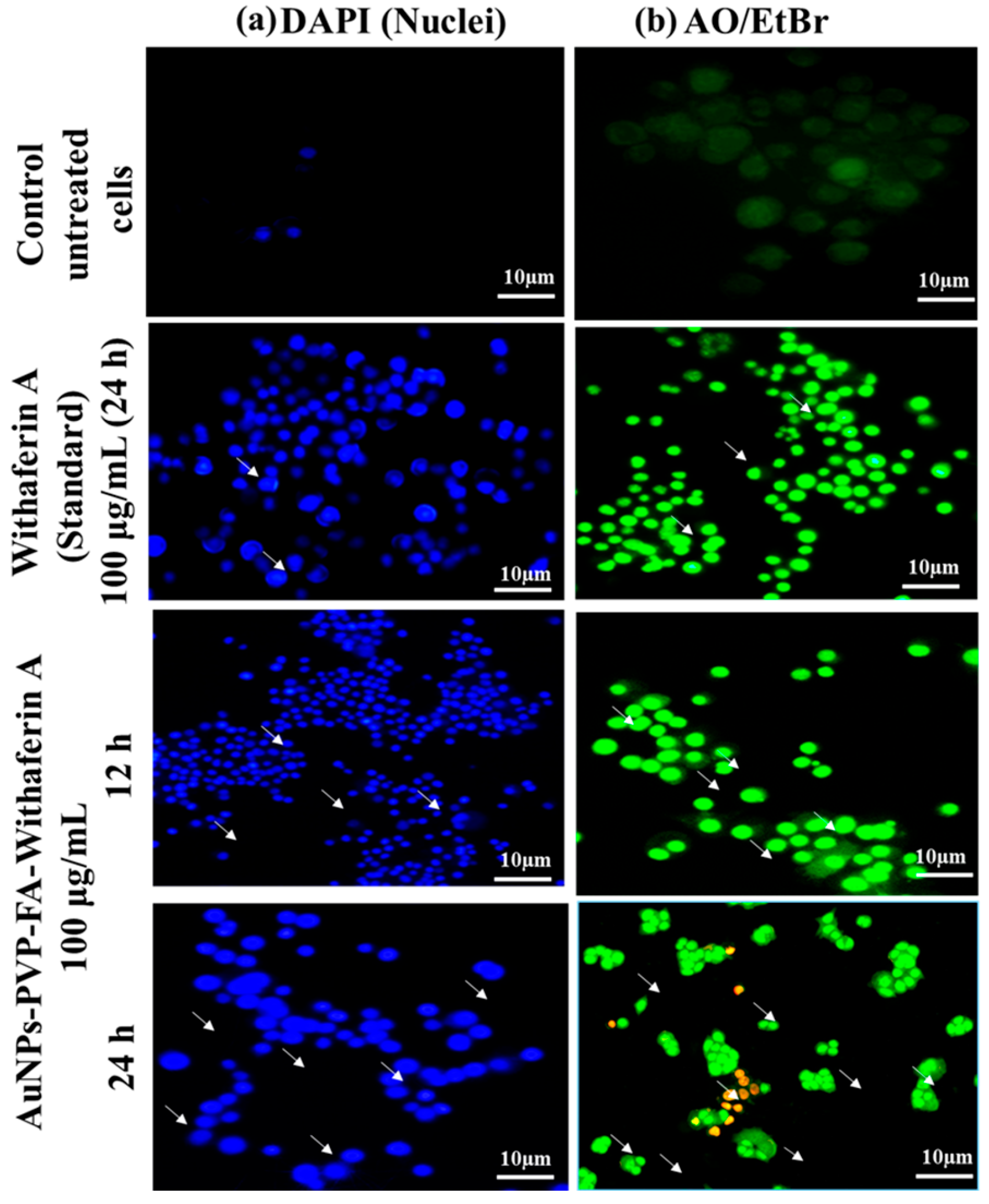
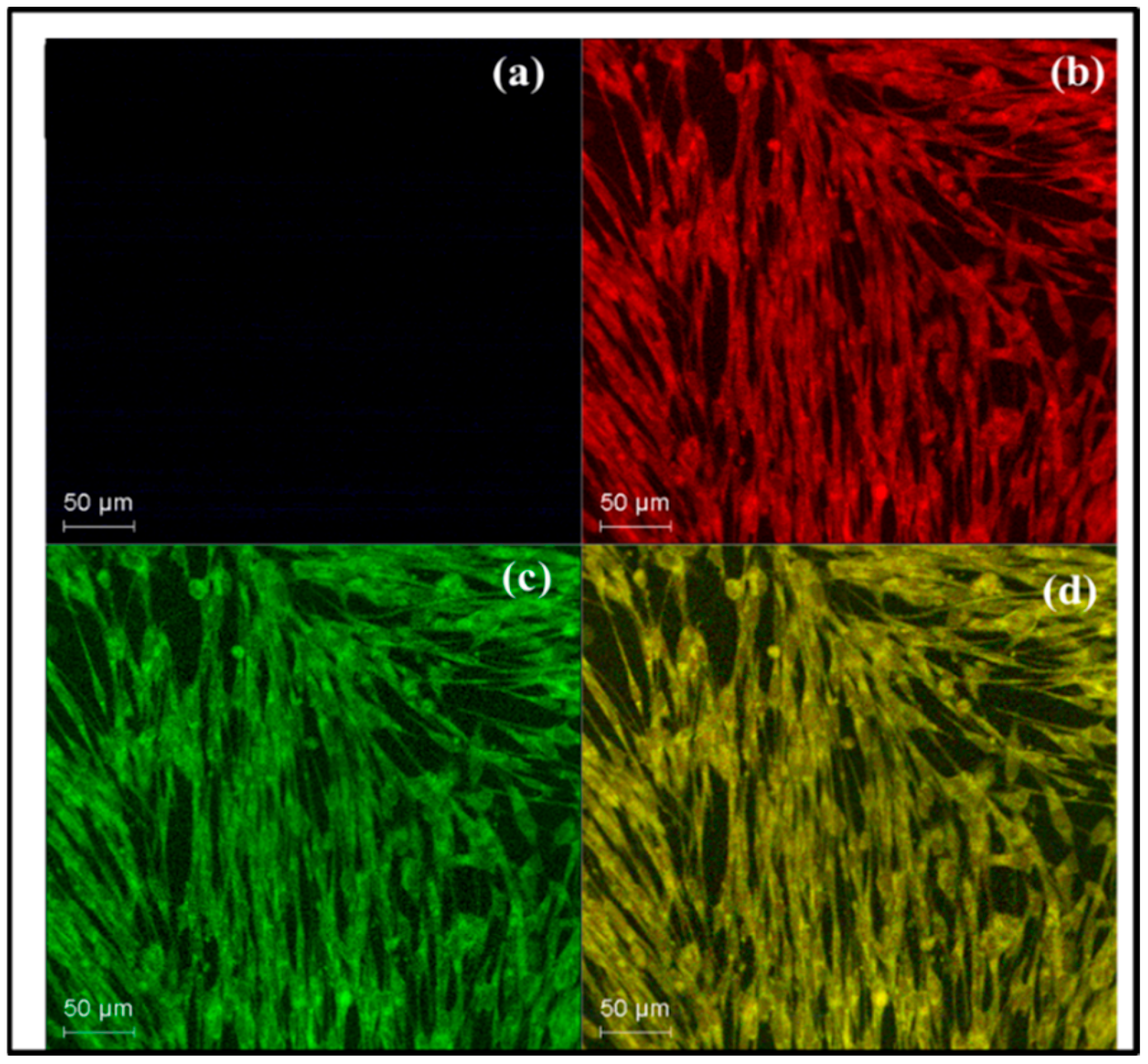
3.9. Morphological Observations of Cell Viability
3.10. Western Blotting Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | gold nanoparticles |
| PVP | polyvinylpyrrolidone |
| FA | folic acid |
| DLE | frug loading efficiency |
| DLS | dynamic light scattering |
| ZETA | Zero Energy Thermonuclear Analysis |
| HPLC | high-performance liquid chromatography |
| FTIR | Fourier-transform infrared spectroscopy |
| NC | nanoconjugate |
| TEM | transmission electron microscopy |
| SEM | scanning electron microscopy |
| UV | ultraviolet |
| SPR | surface plasmon resonance |
| DAPI-40 | 6-diamidino-2-phenylindole |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| Ao/EtBr | acridine orange/ethidium bromide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| IC50 | half-maximum inhibitory concentration |
| CCK8 | Cell Counting Kit 8 |
| FA–Withaferin A AuNPs–PVP NCs | folic acid–Withaferin A–gold nanoparticles–polyvinylpyrrolidone nanoconjugates |
References
- Ortiz-Casas, B.; Galdámez-Martínez, A.; Gutiérrez-Flores, J.; Ibañez, A.B.; Panda, P.K.; Santana, G.; de la Vega, H.A.; Suar, M.; Rodelo, C.G.; Kaushik, A.; et al. Bio-acceptable 0D and 1D ZnO nanostructures for cancer diagnostics and treatment. Mater. Today 2021, 50, 533–569. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Halder, T.; Ghosh, B. Cytological, genetical and phytochemically stable meta-Topolin (mT)-induced mass propagation of underutilized Physalis minima L. for production of Withaferin A. Biocatal. Agric. Biotechnol. 2021, 33, 102012. [Google Scholar] [CrossRef]
- Kaur, G.; Chauhan, K.; Anand, N.; Kaur, S. Evaluation of In Vitro and In Vivo Protective Efficacy of Bauhinia variegata Against Leishmania donovani in Murine Model. Acta Parasitol. 2021, 66, 812–826. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the multifaceted therapeutic potential of Withaferin A and its derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Misra, L.N.; Lal, P.; Kumar, D. Isolation and Characterization of Steroids of Nutraceutical Value in Physalis minima. Prev. Nutr. Food Sci. 2006, 11, 133–139. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Nair, R.M.K.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of Withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. 2020, 27, 26025–26035. [Google Scholar] [CrossRef]
- Hahm, E.; Lee, J.; Singh, S.V. Role of mitogen-activated protein kinases and Mcl-1 in apoptosis induction by Withaferin A in human breast cancer cells. Mol. Carcinog. 2013, 53, 907–916. [Google Scholar] [CrossRef]
- Huang, M.; He, J.-X.; Hu, H.-X.; Zhang, K.; Wang, X.-N.; Zhao, B.-B.; Lou, H.-X.; Ren, D.-M.; Shen, T. Withanolides from the genus Physalis: A review on their phytochemical and pharmacological aspects. J. Pharm. Pharmacol. 2019, 72, 649–669. [Google Scholar] [CrossRef]
- Meng, Q.; Fan, J.; Liu, Z.; Li, X.; Zhang, F.; Zhang, Y.; Sun, Y.; Li, L.; Liu, X.; Hua, E. Cytotoxic Withanolides from the Whole Herb of Physalis angulata L. Molecules 2019, 24, 1608. [Google Scholar] [CrossRef] [PubMed]
- Lavie, D.; Glotter, E.; Shvo, Y. 1371. Constituents of Withania somnifera Dun. Part IV. The structure of Withaferin A. J. Chem. Soc. 1965, 1371–7517. [Google Scholar] [CrossRef]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef]
- Berghe, W.V.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef]
- Hosny, M.; Eltaweil, A.S.; Mostafa, M.; El-Badry, Y.A.; Hussein, E.E.; Omer, A.M.; Fawzy, M. Facile synthesis of gold nanoparticles for anticancer, antioxidant applications, and photocatalytic degradation of toxic organic pollutants. ACS Omega 2022, 7, 3121–3133. [Google Scholar] [CrossRef]
- Sharma, J.R.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, S.; Madiehe, A.M.; Katti, K.; Meyer, M. Anticancer and Drug-Sensitizing Activities of Gold Nanoparticles Synthesized from Cyclopia genistoides (Honey Bush) Extracts. Appl. Sci. 2023, 13, 3973. [Google Scholar] [CrossRef]
- Tagde, P.; Kulkarni, G.T.; Mishra, D.K.; Kesharwani, P. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101613. [Google Scholar] [CrossRef]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.-J.; Park, Y. Folic acid and chitosan-functionalized gold nanorods and triangular silver nanoplates for the delivery of anticancer agents. Int. J. Nanomed. 2022, 17, 1881–1902. [Google Scholar] [CrossRef]
- Manju, S.; Sreenivasan, K. Enhanced drug loading on magnetic nanoparticles by layer-by-layer assembly using drug conjugates: Blood compatibility evaluation and targeted drug delivery in cancer cells. Langmuir 2011, 27, 14489–14496. [Google Scholar] [CrossRef]
- Gajendiran, M.; Jo, H.; Kim, K.; Balasubramanian, S. Green synthesis of multifunctional PEG-carboxylate π back-bonded gold nanoconjugates for breast cancer treatment. Int. J. Nanomed. 2019, 14, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Al-Ansari, M.M.; Narenkumar, J.; Al-Humaid, L.; Arunkumar, P.; Santhanam, A. Synthesis of gold nanoparticles (AuNPs) with improved anti-diabetic, antioxidant and anti-microbial activity from Physalis minima. J. King Saud Univ. Sci. 2022, 34, 102197. [Google Scholar] [CrossRef]
- Pechyen, C.; Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N. Biogenic synthesis of gold nanoparticles mediated by Spondias dulcis (Anacardiaceae) peel extract and its cytotoxic activity in human breast cancer cell. Toxicol. Rep. 2022, 9, 1092–1098. [Google Scholar] [CrossRef]
- Sangwan, R.S.; Chaurasiya, N.D.; Lal, P.; Misra, L.; Tuli, R.; Sangwan, N.S. Withanolide A is inherently de novo biosynthesized in roots of the medicinal plant Ashwagandha (Withania somnifera). Physiol. Plant. 2008, 133, 278–287. [Google Scholar] [CrossRef]
- Anadozie, S.O.; Adewale, O.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Isitua, C.C.; Davids, H.; Roux, S. One-pot synthesis, characterization and biological activities of gold nanoparticles prepared using aqueous seed extract of Garcinia kola. Process Biochem. 2023, 128, 49–57. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 2016, 27, 4–25. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Gansukh, E.; Baskar, V.; Kai, G. Subcritical water extraction of withanosides and withanolides from ashwagandha (Withania somnifera L.) and their biological activities. Food Chem. Toxicol. 2019, 132, 110659. [Google Scholar] [CrossRef]
- Binupriya, A.R.; Sathishkumar, M.; Vijayaraghavan, K.; Yun, S. Bioreduction of trivalent aurum to nano-crystalline gold particles by active and inactive cells and cell-free extract of Aspergillus oryzae var. viridis. J. Hazard. Mater. 2009, 177, 539–545. [Google Scholar] [CrossRef]
- Hira, I.; Kumari, R.; Saini, A.K.; Gullilat, H.; Saini, V.; Kumar, A.; Saini, R. Apoptotic cell death induction through pectin, guar gum and zinc oxide nanocomposite in A549 lung adenocarcinomas. Biointerface Res. Appl. Chem. 2021, 12, 1856–1869. [Google Scholar] [CrossRef]
- Mathew, M.S.; Vinod, K.; Jayaram, P.S.; Jayasree, R.S.; Joseph, K. Improved bioavailability of curcumin in gliadin-protected gold quantum cluster for targeted delivery. ACS Omega 2019, 4, 14169–14178. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sreenivasan, K. Conjugating curcumin to water soluble polymer stabilized gold nanoparticles via pH responsive succinate linker. J. Mater. Chem. B 2015, 3, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.M.; Al-Dahmash, N.D.; Ranjitsingh, A.J.A. Synthesis of silver nanoparticles using gum Arabic: Evaluation of its inhibitory action on Streptococcus mutans causing dental caries and endocarditis. J. Infect. Public Health 2021, 14, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green synthesis of gold nanoparticles from Scutellaria barbata and its anticancer activity in pancreatic cancer cell (PANC-1). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1617–1627. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Biogenic proficient synthesis of (Au-NPs) via aqueous extract of Red Dragon Pulp and seed oil: Characterization, antioxidant, cytotoxic properties, anti-diabetic anti-inflammatory, anti-Alzheimer and their anti-proliferative potential against cancer cell lines. Saudi J. Biol. Sci. 2022, 29, 2836–2855. [Google Scholar] [CrossRef]
- Hejazi, M.; Arshadi, S.; Amini, M.; Baradaran, B.; Shahbazi-Derakhshi, P.; Sameti, P.; Soleymani, J.; Mokhtarzadeh, A.; Tavangar, S.M. Hyaluronic acid-functionalized gold nanoparticles as a cancer diagnostic probe for targeted bioimaging applications. Microchem. J. 2023, 193, 108953. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2017, 46, 861–871. [Google Scholar] [CrossRef]
- Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Baadhe, R.R. Catalytic and eco-toxicity investigations of bio-fabricated monometallic nanoparticles along with their anti-bacterial, anti-inflammatory, anti-diabetic, anti-oxidative and anti-cancer potentials. Colloids Interface Sci. Commun. 2020, 38, 100302. [Google Scholar] [CrossRef]
- Nosrati, H.; Seidi, F.; Hosseinmirzaei, A.; Mousazadeh, N.; Mohammadi, A.; Ghaffarlou, M.; Danafar, H.; Conde, J.; Sharafi, A. Prodrug polymeric nanoconjugates encapsulating gold nanoparticles for enhanced X-ray radiation therapy in breast cancer. Adv. Healthc. Mater. 2022, 11, 2270014. [Google Scholar] [CrossRef]
- Uzma, M.; Sunayana, N.; Raghavendra, V.B.; Madhu, C.S.; Shanmuganathan, R.; Brindhadevi, K. Biogenic synthesis of gold nanoparticles using Commiphora wightii and their cytotoxic effects on breast cancer cell line (MCF-7). Process Biochem. 2020, 92, 269–276. [Google Scholar] [CrossRef]
- Lara-Cruz, C.; Jiménez-Salazar, J.E.; Arteaga, M.; Arredondo, M.; Ramón-Gallegos, E.; Batina, N.; Damián-Matsumura, P. Gold nanoparticle uptake is enhanced by estradiol in MCF-7 breast cancer cells. Int. J. Nanomed. 2019, 14, 2705–2718. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.S.; Usman, F.; Ashfaq, M.; Khalil, R.; Ul-Haq, Z.; Mushtaq, A.; Qaiser, R.; Iqbal, J. Preparation and characterization of anticancer niosomal withaferin–A formulation for improved delivery to cancer cells: In vitro, in vivo, and in silico evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101863. [Google Scholar] [CrossRef]
- Amale, F.R.; Ferdowsian, S.; Hajrasouliha, S.; Kazempoor, R.; Mirzaie, A.; Dakkali, M.S.; Akbarzadeh, I.; Meybodi, S.M.; Mirghafouri, M. Gold nanoparticles loaded into niosomes: A novel approach for enhanced antitumor activity against human ovarian cancer. Adv. Powder Technol. 2021, 32, 4711–4722. [Google Scholar] [CrossRef]
- Ayyanaar, S.; Kesavan, M.P. One-pot biogenic synthesis of gold nanoparticles@saponins niosomes: Sustainable nanomedicine for antibacterial, anti-inflammatory and anticancer therapeutics. Colloids Surf. A Physicochem. Eng. Aspects 2023, 676, 132229. [Google Scholar] [CrossRef]
- Ke, Y.; Aboody, M.S.A.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef]
- Velmathi, G.; Sekar, V.; Kavitha, N.S.; Albeshr, M.F.; Santhanam, A. Biosynthesis of gold nanoparticles by the extremophile bacterium Deinococcus radiodurans and an evaluation of its application in drug delivery. Process Biochem. 2024, 145, 250–260. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Yazdi, M.E.T.; Meshkat, Z.; Toosi, M.S.; Hosseini, S.M. Plant-derived synthesis and characterization of gold nanoparticles: Investigation of its antioxidant and anticancer activity against human testicular embryonic carcinoma stem cells. Process Biochem. 2021, 111, 167–177. [Google Scholar] [CrossRef]
- Selim, M.E.; Hendi, A.A. Gold nanoparticles induce apoptosis in MCF-7 human breast cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 1617–1620. [Google Scholar] [CrossRef]
- Muthukumar, T.; Sudhakumari, N.; Sambandam, B.; Aravinthan, A.; Sastry, T.P.; Kim, J.-H. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 2016, 51, 384–391. [Google Scholar] [CrossRef]






| Drug Concentration (mg/mL) | pH 5 (%) | pH 6 (%) | pH 7 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:1 | 1:2 | 1:4 | 1:1 | 1:2 | 1:4 | 1:1 | 1:2 | 1:4 | |
| 100 | 73.52 | 64.76 | 69.23 | 62.02 | 65.51 | 68.11 | 71.25 | 69.96 | 72.02 |
| 200 | 79.45 | 69.32 | 71.52 | 69.11 | 71.45 | 71.65 | 72.54 | 71.23 | 74.53 |
| 300 | 85.14 | 75.11 | 76.59 | 75.08 | 72.89 | 74.23 | 76.89 | 74.12 | 78.98 |
| 400 | 90.39 | 81.03 | 84.96 | 79.32 | 79.83 | 78.59 | 78.68 | 78.58 | 81.45 |
| 500 | 94.86 | 82.49 | 88.32 | 81.23 | 84.78 | 81.98 | 85.78 | 81.09 | 83.85 |
| Kinetics Release Models’ R2 Values | pH 5 | pH 6 | pH 7 |
|---|---|---|---|
| Zero-order | 0.9561 | 0.9849 | 0.9449 |
| First-order | 0.9657 | 0.9497 | 0.9211 |
| Hickson cross well | 0.9812 | 0.9371 | 0.8852 |
| Kors Myer–peppers | 0.9906 | 0.9897 | 0.9234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, V.; Santhanam, A.; Arunkumar, P. pH-Responsive Gold Nanoparticle/PVP Nanoconjugate for Targeted Delivery and Enhanced Anticancer Activity of Withaferin A. Processes 2025, 13, 1290. https://doi.org/10.3390/pr13051290
Sekar V, Santhanam A, Arunkumar P. pH-Responsive Gold Nanoparticle/PVP Nanoconjugate for Targeted Delivery and Enhanced Anticancer Activity of Withaferin A. Processes. 2025; 13(5):1290. https://doi.org/10.3390/pr13051290
Chicago/Turabian StyleSekar, Velmurugan, Amutha Santhanam, and Paulraj Arunkumar. 2025. "pH-Responsive Gold Nanoparticle/PVP Nanoconjugate for Targeted Delivery and Enhanced Anticancer Activity of Withaferin A" Processes 13, no. 5: 1290. https://doi.org/10.3390/pr13051290
APA StyleSekar, V., Santhanam, A., & Arunkumar, P. (2025). pH-Responsive Gold Nanoparticle/PVP Nanoconjugate for Targeted Delivery and Enhanced Anticancer Activity of Withaferin A. Processes, 13(5), 1290. https://doi.org/10.3390/pr13051290







