Abstract
The incorporation of essential oils into the oil phase of oil-in-water microemulsions is an emerging strategy for the development of stable water-based topical formulations. The introduction of a suitable polymer to formulate film-forming microemulsions may improve topical administration; however, the effect of formulation variables on film quality attributes has not been studied. In this study, thermodynamically stable microemulsion concentrates consisting of surfactant (Kolliphor® RH40), alone or in combination with cosurfactant Transcutol® at surfactant-to-cosurfactant mass ratio 7:3, cosolvent (propylene glycol), and synthetic oils (medium-chain triglycerides or isopropyl myristate) with tea tree, cinnamon, or thyme essential oil were formulated and diluted with hypromellose solution in a water/isopropanol mixture (1:1 w/w) to produce film-forming microemulsions. The type and concentration of synthetic and essential oils and cosurfactant influenced the dynamics of structural transformations upon dilution as well as the rheological behavior, viscosity, and pH of film-forming microemulsions. Films obtained by casting film-forming microemulsions were opalescent, smooth, flexible, and swellable in artificial sweat and water. The weight and yield of films increase with the synthetic oils present and without cosurfactant added. Optimizing the ratio of essential oil/synthetic oil, the type of synthetic oil, and the inclusion/exclusion of cosurfactant allows for achieving the targeted film attributes for cosmetic and pharmaceutical applications, including wound treatment.
1. Introduction
Essential oils are complex mixtures of volatile low-molecular-weight chemical compounds (e.g., terpenes, terpenoids, phenylpropenes, alcohols, aldehydes, ketones, ethers, esters, and phenols) derived from various herbal materials [1,2,3]. Several hundred essential oils are used in the pharmaceutical and cosmetic industries as fragrances and flavorings due to their pleasant odor or as an alternative to synthetic preservatives and antioxidants, as well as bioactive ingredients with antimicrobial and antioxidant effects [4,5,6,7]. The use of essential oils in pharmaceutical and cosmetic products, especially the development of stable water-based formulations, is a major challenge due to their poor water solubility, high volatility, and susceptibility to oxidation, hydrolysis, and thermal and photochemical degradation [8].
One of the rapidly developing strategies to overcome these shortcomings is essential oil microemulsification (i.e., the incorporation of essential oils into the oil phase of oil-in-water microemulsions) [9,10,11,12]. Oil-in-water microemulsions are thermodynamically stable, transparent, homogeneous dispersions consisting of an oil phase dispersed in the form of droplets typically smaller than 100 nm in a surrounding water phase and stabilized by an interfacial layer of surfactants and cosolvents [13,14]. When essential oil (as a single component or in combination with fatty oils), water, and suitable surfactants and cosolvents are combined in appropriate proportions, an oil-in-water microemulsion is spontaneously formed using simple stirring methods. Therefore, microemulsification of essential oils can offer several advantages, including optical clarity, homogeneity, long-term stability and shelf life of a product, and suitability for cost-effective production at the industrial scale without the need for specialized equipment and high energy input [11,15,16,17,18]. Numerous studies have shown that microemulsions protect essential oils from evaporation and degradation, reduce the risk of skin irritation or allergic reactions, and increase antimicrobial and antioxidant activity, thereby improving efficiency in the care and treatment of various dermatological conditions such as aging, acne, and superficial wounds [12,15,18,19,20,21,22,23,24,25,26,27]. In addition, microemulsions of essential oils can be used as carriers for the topical application of other poorly soluble active substances (e.g., quercetin [28], naringin [10], resveratrol [17], and minoxidil [29]), with the recognized potential for enhanced active substance release and skin permeation. Oil-in-water microemulsions of essential oils with a high aqueous phase content are particularly suitable for more precise delivery and accumulation of active substances in the superficial skin layers [27].
On the downside, microemulsions only form in mixtures of excipients with suitable material attributes (e.g., hydrophilic–lipophilic-balance (HLB) values of surfactants, molecular weight (Mw) of oils) and within the specific concentration ranges [13]. Therefore, the development of microemulsions requires a complex strategy in which several formulation variables, such as the type and concentration of oils, water, surfactants, and cosolvents, the mass ratio of surfactant-to-cosurfactant (Km), and the mass ratio of essential oils/fatty oils are balanced [12,17,30,31]. The study of the phase behavior of multicomponent mixtures is a standard approach to determine the formulation parameters required for the formation of microemulsions, including consideration of the risk of disturbing thermodynamic stability with changing concentrations or introducing new ingredients [13]. However, the influence of essential oils, alone or in combination with fatty oils, on the phase behavior of multicomponent systems has not been systematically investigated so far, which makes the formulation of oil-in-water microemulsions quite difficult.
Another major challenge for research is the low viscosity of oil-in-water microemulsions, which makes it difficult to apply and retain them on the skin. Therefore, there is a growing research interest in the introduction of viscosity-increasing polymers (e.g., hydroxyethyl cellulose [24], SimulgelTM NS [10,17], or hydrogel-forming polymers such as alginate cross-linked with calcium ions [20] and Carbopol® 940 [23,32]) in microemulsions of essential oils. In this way, the advantages of flexibility in terms of consistency, application, and sensorial attributes could be ensured. An innovative approach worthy of investigation could be the incorporation of film-forming polymers into microemulsions of essential oils to obtain film-forming microemulsions that form a thin film at the site of application (in situ). Film-forming microemulsions of essential oils could provide a unique combination of ease and simplicity of application with prolonged retention of the film on the skin. In this way, disadvantages such as stickiness and soiling of the application site and transfer of the formulation to other surfaces can be overcome, and protection of the application site by the film is achieved, which is of particular importance in wound treatment [21,33]. The ability of the polymer to form a thin, flexible, mechanically resistant, swellable, and easily water-washable film could be affected by surfactants, cosolvents, and oils [34,35]; so far, this aspect has not been investigated in film-forming microemulsions with essential oils.
Hypromellose (hydroxypropylmethylcellulose) is partially O-methylated and O-(2-hydroxypropylated) cellulose [36,37]. The Mw of hypromellose varies between 10,000 gmol−1 and 1,500,000 gmol−1 [37] and directly influences the viscosity of the polymer solution [38]. This semi-synthetic polymer dissolves in cold water, as well as in mixtures of water and volatile alcohols (e.g., ethanol and isopropanol), and forms viscous, colloidal solutions. The solutions are stable over a wide pH range (3–11), and their viscosity changes only slightly during long-term storage. Hypromellose is a nonionic amorphous polymer that remains unchanged under the influence of electrolytes and can contribute to increased solubility of poorly soluble substances [38,39]. The increasing importance of hypromellose as a film-forming polymer in drug delivery systems has recently been extensively reviewed by Milinković and Đekić [35].
However, the use of hypromellose as a film-forming polymer in dispersions of essential oils and fatty oils has been little studied. Saingam et al. [40] developed a film-forming formulation for local relief of rheumatic pain with black pepper oil (Piper nigrum L.), methyl salicylate, and menthol dispersed in a solution of hypromellose (1–5%) in a mixture of water, ethanol, and polyethylene glycol 400 or propylene glycol. It was found that the concentrations of the polymer, solvents, and cosolvents had a significant effect on the appearance and pH of the formulations and the drying time after spraying, but the structure and stability of the system and the properties of the films formed were not considered. In addition, Zuniga et al. [41] investigated the appearance, mechanical properties, and water vapor properties of films prepared from an edible oil-in-water emulsion of sunflower oil (1–3% w/w) with hypromellose (2.4–3.5% w/w), with and without the surfactant sodium dodecyl sulfate. The effects of oil and surfactant on the stability and microstructure of hypromellose-based emulsions and the films formed from them were concentration-dependent. The presence of surfactant resulted in a reduction in the average size of oil droplets and improved emulsion stability. However, the introduction of fatty oil in the form of micrometer-sized droplets, with or without surfactant, into the polymer network of hypromellose impaired the mechanical properties of the films and reduced their transparency. As far as we are aware, no study has investigated the use of hypromellose in film-forming oil-in-water microemulsions with essential oils.
The aim of this study was to formulate film-forming oil-in-water microemulsions with the essential oils of cinnamon, thyme, and tea tree using hypromellose as a film-forming polymer. The focus of the research was to elucidate the influence of the material (polymer, surfactants, cosurfactant, cosolvents, and oils) attributes on the mutual interactions and the resulting thermodynamic stability, the phase behavior, and the microstructure of the system, as well as on the topical-application-related quality attributes (appearance, stickiness, flexibility, swelling capacity) of the films formed in situ.
2. Materials and Methods
2.1. Materials
The surfactants were as follows: macrogolglycerol hydroxystearate (Kolliphor® RH40, BASF, Ludwigshafen, Germany), PEG-8 caprylic/capric glycerides (Labrasol®, Gattefosse, Lyon, France), and polysorbate 20 (Fagron, Rotterdam, The Netherlands). The cosurfactant diethylene glycol monoethyl ether (Transcutol®) was a gift from Gattefosse, Lyon, France. The cosolvents (propylene glycol and glycerol) were obtained from Fagron, Rotterdam, The Netherlands. The synthetic oils were medium-chain triglycerides (MCT) (Myritol 318, Fagron, Rotterdam, The Netherlands) and isopropyl myristate (IPM) (Fagron, Rotterdam, The Netherlands). Cinnamon essential oil (Cinnamomum Cassia leaf oil) (Dullberg Konzentra, Hamburg, Germany), thyme essential oil (Thymus vulgaris flower/leaf oil) (Dullberg Konzentra, Hamburg, Germany), and tea tree essential oil (Aetheroleum melaleuca al.) (Kirka Pharma, Belgrade, Serbia) were purchased from local retailers and served as models. The film-forming polymer was hypromellose (nominal viscosity 4000 mpa·s at a concentration of 2%) (Sigma-Aldrich, St. Louis, Missouri, United States). Isopropyl alcohol (Zorka Pharma-Hemija, Šabac, Serbia) was used as a volatile solvent in film-forming microemulsions of essential oils. All excipients were of Ph. Eur. quality. Purified water of Ph. Eur. quality was used throughout the study. Analytical grade sodium chloride, calcium chloride, magnesium sulfate, and potassium dihydrogen phosphate (all from Centrohem, Sremska Mitrovica, Serbia) were used for the preparation of artificial sweat.
2.2. Methods
2.2.1. Formulation, Preparation, and Phase Behavior of Ternary and Pseudo-Ternary Mixtures
To formulate microemulsion concentrates as anhydrous mixtures of oils, surfactants, and cosolvents that mix with water to form an oil-in-water microemulsion, a pool of ternary mixtures of oil, surfactant, and cosolvent and pseudo-ternary mixtures of oil, cosolvent, and a mixture of surfactant and cosurfactant with a defined Km value was prepared. The mixture of surfactant and cosurfactant in the pseudo-ternary systems represented a pseudo-component. Pseudo-ternary mixtures with three Km values (9:1, 7:3, and 1:1) were prepared. In all ternary and pseudo-ternary mixtures, the concentration of surfactant or surfactant/cosurfactant mixture was 70% w/w, the oil content was 10% w/w, and the cosolvent concentration was 20% w/w. The detailed composition of the ternary and pseudo-ternary mixtures prepared is shown in Table 1.

Table 1.
Compositions of the ternary and pseudo-ternary mixtures.
A total of 48 mixtures (12 ternary mixtures and 36 pseudo-ternary mixtures) were prepared, which differed in the type of synthetic oil used (MCT or IPM), the surfactant (Kolliphor® RH40, Labrasol® or polysorbate 20), and the cosolvent (glycerol or propylene glycol). In addition, the pseudo-ternary mixtures differed according to the Km value (9:1; 7:3; 1:1). Each mixture was prepared from precisely measured quantities of ingredients, which were mixed with a magnetic stirrer at a speed of 300 rpm until a homogeneous mixture was obtained. The mixtures were stored in glass vials sealed with rubber stoppers at a room temperature that did not exceed 25 °C.
The phase behavior of the prepared ternary and pseudo-ternary mixtures was examined 72 h after preparation and included a visual assessment of their appearance with the aim of identifying those that had the appearance of microemulsion concentrates, meaning that they were clear, homogeneous, single-phase liquids [42]. Immediately prior to observation, all ternary and pseudo-ternary mixtures were centrifuged for 30 min at a speed of 3000 rpm in a laboratory centrifuge type MPW 56/MPW (Med. Instruments, Warsaw, Poland). When the mixtures are shaken, phase separation may occur, so thermodynamically unstable or metastable systems can be detected. For mixtures that were clear, homogeneous, single-phase liquids, the transmittance (%T) was measured using the Evolution 300 UV spectrophotometer (Thermo Scientific, Waltham, MA, USA) at a wavelength of 600 nm to objectively evaluate their transparency by an instrumental test. Purified water was used as a blank. To determine whether these mixtures were anhydrous concentrates from which oil-in-water microemulsions could be formed, the droplet size after dilution with purified water at a mass ratio of 9:1 was determined by the photon correlation spectroscopy (PCS) technique using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, Worcestershire, UK). The instrument is equipped with a He-Ne laser that generates light with a wavelength of 633 nm and a scattered light detector with a detection angle of 90°. A glass cuvette containing the diluted sample was placed in the temperature-controlled chamber of the instrument, and the measurement was performed at 20 ± 0.1 °C in triplicate. The average droplet size (Z–ave) and the polydispersity index (PdI) were calculated using the Dispersion Technology Software (DTS) (v7.01) integrated in the instrument. Those samples that met the criteria in terms of Z-ave (≤100 nm) and PdI (≤0.250), indicating high monodispersity to moderate polydispersity [43], were considered as oil-in-water microemulsions, while the corresponding undiluted mixtures with %T ≥ 90% were classified as microemulsion concentrates and used for further investigation.
The evolution of the system structure during the dilution of microemulsion concentrates with purified water was characterized by conductometric titration [42,44]. The conductivity of the tested samples was measured as a function of a gradually increasing water content using a CDM 230 conductometer (Radiometer, Brønshøj, Copenhagen, Denmark) at a frequency of 94 Hz. After adding each portion of water, the sample was homogeneously mixed on a magnetic stirrer at 300 rpm. The electrode of the conductometer was then immersed in the sample, and the conductivity was recorded after equilibrium was reached. The recorded temperature fluctuated during the titration, within 20 ± 2 °C.
2.2.2. Formulation, Preparation, and Phase Behavior of Microemulsion Concentrates
Considering the composition of the ternary and pseudo-ternary systems, which have characteristics of microemulsion concentrates that can be continuously diluted with water to form water-in-oil microemulsions, the formulation of the corresponding microemulsion concentrates with essential oils was carried out by partially or completely replacing the synthetic oil (MCT or IPM) with essential oil. The microemulsion concentrates with essential oils therefore contained only essential oil at a concentration of 10% w/w or a mixture of essential oil (5% w/w) and MCT (5% w/w) or of essential oil (5% w/w) and IPM (5% w/w). Each essential oil microemulsion concentrate was prepared by accurately measuring the required amounts of the ingredients in a sealed Erlenmeyer flask and mixing with a magnetic stirrer at 300 rpm until a homogeneous mixture was obtained. The prepared concentrates were transferred to glass vials and immediately sealed with rubber stoppers and parafilm to prevent evaporation of the essential oils. The concentrates were stored at a room temperature of no more than 25 °C and protected from direct light (in the laboratory cabinet).
The phase behavior of the microemulsion concentrates with essential oils was investigated after 72 h of preparation by visual assessment of color, clarity, consistency, and homogeneity before and after centrifugation for 30 min at a speed of 3000 rpm (laboratory centrifuge MPW 56/MPW (Med. Instruments, Warsaw, Poland)). The microemulsion concentrates with essential oils were observed against a white light source. In order to exclude metastable systems, another group of samples was observed after 3 months of storage at a room temperature of no more than 25 °C and protected from daylight. The third group of samples was also subjected to stress conditions by exposing them to cyclic temperature changes of 5 ± 3 °C (for 24 h), 23 ± 3 °C (for 24 h), and 40 ± 1 °C (for 24 h) for 10 cycles, also protected from direct exposure to light. After full exposure to the stress conditions and at regular intervals (once a week during the first month and then once a month) during storage under ambient conditions, the appearance of the samples (color, clarity, consistency, homogeneity) and their tendency to physical destabilization (e.g., phase separation, sedimentation) were checked after centrifugation for 30 min at 3000 rpm in a laboratory centrifuge MPW 56/MPW (Med. Instruments, Warsaw, Poland). For mixtures that were clear, homogeneous, single-phase systems, the %T value was measured using an Evolution 300 UV spectrophotometer (Thermo Scientific, Waltham, MA, USA) at a wavelength of 600 nm, and the droplet size was determined after dilution with purified water at a mass ratio of 9:1 using the same PCS analytical method used for testing microemulsion concentrates without essential oils. Conductometric characterization of structure transformation during dilution of microemulsion concentrates with essential oils was performed as described for microemulsion concentrates containing only synthetic oils.
2.2.3. Formulation and Preparation of Film-Forming Microemulsions
Film-forming microemulsions with essential oils were prepared by combining microemulsion concentrates with essential oils and a hypromellose solution in a mixture of isopropanol and water in a 1:1 mass ratio. Each film-forming essential oil microemulsion contained 1 part by weight of the essential oil microemulsion concentrate and 9 parts by weight of hypromellose solution. The hypromellose solution was prepared by dispersing the required amount of the polymer in purified water, which was heated to 80 °C and then stored in a refrigerator (at 5 ± 3 °C) for 24 h to dissolve completely. In addition, the hypromellose solution was stirred with the laboratory propeller mixer RZR 2020 (Heidolph, Schwabach, Germany) at 500 rpm for 1 h at room temperature. Then, the appropriate amount of isopropanol was added to the prepared aqueous hypromellose solution and stirred at 300 rpm to obtain a clear homogeneous polymer solution. The required amount of essential oil microemulsion concentrate was added to the hypromellose solution while stirring at the same speed to produce a homogeneous film-forming essential oil microemulsion. The final concentration of hypromellose in each prepared microemulsion with essential oil was 2.5%, the mass ratio of water and isopropanol was 1:1, and the final concentration of essential oil was 0.5% w/w or 1% w/w.
2.2.4. Physicochemical Characterization of Microemulsion Concentrates and Film-Forming Microemulsions
In order to elucidate the potential interactions between the polymer and the components of the microemulsion, primarily surfactants and cosurfactants, the physicochemical characterization of both the microemulsion concentrates and the film-forming microemulsions with essential oils included rheological and pH measurements. The rheological characterization was performed using a rotational rheometer (RheolabMC120, PaarPhysica, Stuttgart, Germany) with an MK22 cone-and-plate measuring system at a temperature of 20 ± 0.1 °C and a controlled shear rate of 0–200 1/s (ascending curve) and 200–0 1/s (descending curve). All measurements were carried out in triplicate. The data obtained were used to determine the flow type and viscosity. The pH measurement of the microemulsion concentrates and film-forming microemulsions with essential oils was carried out using the potentiometric method with pH meter HI 9321 (Hanna Instruments, Woonsocket, RI, USA) at a temperature of 20 ± 3 °C. The electrode of the pH meter was immersed in the sample, and the pH value after reaching equilibrium was read on the display of the instrument.
2.2.5. Examination of the Properties of Films
The ability of the formulated microemulsions with essential oils to form films on an inert flat surface was evaluated by using the casting method [9]. A precisely measured amount (5.0 g) of the freshly prepared film-forming microemulsion with essential oil was poured onto the bottom of a 9 cm diameter glass Petri dish, evenly distributed with gentle shaking, and allowed to dry for 24 h at a temperature not exceeding 25 °C, protected from direct light and air flow (in the laboratory cabinet). For comparison, the same amount of the solution of hypromellose 2.5% w/w in a mixture of water and isopropanol (1:1) was also cast into a Petri dish and evenly spread before being dried for 24 h under the same conditions as the film-forming microemulsion with essential oils. The dry films were peeled off and stored in hermetic containers for further characterization.
The weight of the dried film (FW) was determined as the difference between the weight of the Petri dish containing the dried film and the weight of the empty Petri dish. The yield in weight percent (Y (% w/w)) was then calculated based on the weight of the dried film (FW) (in grammes) and the weight of the film-forming microemulsion with essential oil (FFMW) poured into the Petri dish (in grammes), using Equation (1):
The appearance (visual), stickiness, flexibility, and swelling of the film in artificial sweat and water were then tested.
The stickiness of the dry films was assessed by gently pressing a cotton pad onto the surface. The presence of cotton fibers on the surface of the film was assessed visually. The stickiness of the film surface can be classified as follows: high (when a dense layer of cotton fibers was present), moderate (the layer of cotton fibers was thin), and low (the cotton fibers were rare or absent) [45].
To evaluate the flexibility of the films, the folding endurance test [46] was used, in which the film was repeatedly folded at an angle of 180° in the middle. The folding endurance value represents the number of times the film can be folded without visible deformation (twisting, stretching), cracking, or detachment. A folding endurance value of 300 or more is considered to have excellent flexibility.
The swelling capacity of dry films was tested in two media: artificial sweat and purified water. The artificial sweat was freshly prepared according to the composition proposed by Shimamura et al. [47]. The swelling ability of the dry films with essential oils was determined as the weight gain of the film at certain time intervals according to the method proposed by Hota et al. [34]. The film sample was cut into a rectangular shape weighing 200 mg and transferred to an Erlenmeyer flask containing 2.00 g of medium. The samples were shaken on a Heidolph Unimax 1010® laboratory shaker at a speed of 150 rpm. After 30 min, 60 min, and 120 min, the medium was separated from the swollen film, and the mass of the separated medium (Md) was measured. For each time point, the weight percentage of the swollen film S (% w/w) was calculated using Equation (2), based on the data obtained for Md:
For each time point, the experiment was repeated three times, and the average ± S.D. was calculated.
3. Results and Discussion
3.1. Phase Behavior of Ternary and Pseudo-Ternary Mixtures and Screening of Microemulsion Concentrates
The excipients used as ingredients of the investigated ternary and pseudo-ternary mixtures were selected in accordance with the available literature following the generally accepted rules that microemulsion concentrates consist of the following: small Mw oils (<20% w/w), hydrophilic surfactants (HLB > 12) (≥20% w/w), and hydrophilic cosolvents (≥20% w/w) [48,49]. The small molecule synthetic oils MCT and IPM were used in the formulated mixtures at a concentration of 10% w/w. MCT contains the glycerol backbone occupied by medium-chain fatty acids, including caprylic acid (C8:0) (Mw = 144.21 gmol−1), capric acid (C10:0) (Mw = 172.26 gmol−1), and lauric acid (C12:0) (Mw = 200.31 gmol−1) [50]. IPM (Mw = 270.451 gmol−1) is the ester of isopropyl alcohol and myristic acid [51]. Hydrophilic nonionic surfactants were as follows: Kolliphor® RH40 (HLB = 14–16), Labrasol® (HLB = 14), and polysorbate 20 (HLB = 16.7). Propylene glycol and glycerol were considered as hydrophilic cosolvents at a fixed concentration of 20% w/w. The synthetic oils, surfactants, and cosolvents considered are well-established pharmaceutical excipients for various applications, including topical dosage forms [37]. To increase the biocompatibility of the formulation by reducing the hydrophilic surfactant content, Transcutol® (HLB = 4.2) was included as a cosurfactant. This excipient is a small molecule (Mw = 134.17 gmol−1) whose use in microemulsions with essential oils for cutaneous application was recently reported in detail [52].
The investigation of the phase behavior of ternary and pseudo-ternary mixtures began with a visual assessment of their appearance after centrifugation, which ensured that the mixtures were in a state of thermodynamic equilibrium. All systems were liquid and had no characteristic color or odor. The observations of the homogeneity and number of phases in each system are summarized in Table S1.
It was found that the formulation parameters considered (Km, type of surfactant, synthetic oil, and cosolvent) had a significant influence on the phase behavior of the mixtures. Mixtures prepared with MCT and glycerol with Labrasol® or Kolliphor® RH40 as surfactant, with and without cosurfactant, were two-phase systems (2Φ) (Table S1); i.e., two separate, adjacent layers were observed that did not mix with each other. For mixtures with the same oil (MCT) and cosolvent (glycerol) in the presence of polysorbate 20 as surfactant, with and without cosurfactant, three clearly separated layers were observed (i.e., they were three-phase systems (3Φ)) (Table S1). When IPM was used as an oil in combination with glycerol, the mixtures with Labrasol® or polysorbate 20, with and without cosurfactant, were 2Φ, while those prepared with Kolliphor® RH40 were 3Φ (Table S1). All mixtures prepared with propylene glycol and the surfactants Labrasol® or polysorbate 20 were 2Φ, in the presence of both oils used (MCT, IPM), with or without cosurfactant. The formation of 2Φ and 3Φ indicates insufficient mutual mixing of the components and partial or complete separation into different layers (two or three, respectively). Such systems do not meet the criteria to be classified as microemulsion concentrates, which are known to be single-phase systems (1Φ). Therefore, the multiphase mixtures were excluded from further consideration.
Only the mixtures with propylene glycol containing Kolliphor® RH40 were homogeneous, single-phase colorless liquids, in the presence of both oils (MCT or IPM), with and without cosurfactant, and as such could be considered as potential microemulsion concentrates. Based on the results obtained, it was found that the type of surfactant and cosolvent had the greatest influence on the phase behavior of the investigated ternary and pseudo-ternary mixtures, while no significant differences in the phase behavior of systems with the same qualitative composition at different Km values were observed. There was also no significant difference found with regard to the influence of MCT and IPM on the formation of homogeneous single-phase systems. Kolliphor® RH40 is produced by reacting 1 mol of hydrogenated castor oil with 40 mol of ethylene oxide and is a mixture of lipophilic glycerol polyethylene glycol hydroxystearates and fatty acid polyglycol esters with hydrophilic components (polyethylene glycols and glycerol ethoxylate) [53]. In contrast to the other two surfactants tested, it is therefore probably able to improve the mixing of the ingredients sufficiently to form a homogeneous system. In addition, propylene glycol is a less polar cosolvent (dielectric constant = 32) and a smaller molecule (Mw = 76.09 gmol−1) than glycerol (dielectric constant = 40.2, Mw = 92.09 gmol−1) [54], so better mixing with synthetic oils and other components of lower polarity can be expected.
To investigate whether the single-phase ternary and pseudo-ternary systems prepared with Kolliphor® RH40 and propylene glycol have the character of microemulsion concentrates, an instrumental analysis of transparency was performed, and Z-ave and PdI were determined after dilution with water. The %T, Z-ave, and PdI values are shown in Table 2.

Table 2.
Transmittance (%T) of single-phase systems comprising Kolliphor® RH40 and propylene glycol and average droplet size (Z-ave) and polydispersity index (PdI) in dispersions obtained by diluting them with water in a mass ratio of 9:1. The labels of the samples incorporate the abbreviation of synthetic oil (MCT or IPM) and “0” for the systems that do not contain cosurfactant or Km value (9:1, 7:3, or 1:1) for the pseudo-ternary systems.
The %T values were below 90% for the system with MCT with cosurfactant at Km 9:1 (MCT-9:1) and Km 1:1 (MCT-1:1) and for the system with IPM with cosurfactant at Km 1:1 (IPM-1:1). The observed values of %T could indicate a limited mutual solubility of the components, which means that these pseudo-ternary mixtures are not isotropic; i.e., they are not homogeneous at the molecular level. Isotropy is another key property of microemulsion concentrates that indicates a homogeneous molecular arrangement in the multicomponent system [55].
The droplet size distribution curves are shown in Figure 1.
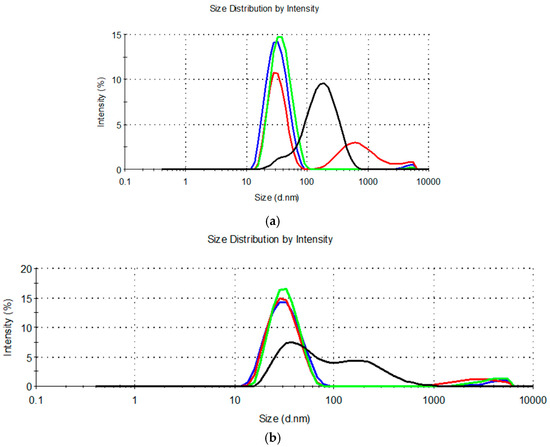
Figure 1.
Droplet size distribution for diluted microemulsion concentrates containing: (a) MCT, propylene glycol, and Kolliphor® RH40 or Kolliphor® RH40 + Transcutol® (MCT-0 (▬), MCT-9:1 (▬), MCT-7:3 (▬) and MCT-1:1 (▬)); (b) IPM, propylene glycol and Kolliphor® RH40 or Kolliphor® RH40 + Transcutol® (IPM-0 (▬), IPM-9:1 (▬), IPM-7:3 (▬) and IPM-1:1 (▬)).
Measurement of droplet size in the diluted MCT-9:1, MCT-1:1, IPM-9:1, and IPM-1:1 systems revealed a high polydispersity (PdI > 0.250) (Table 2) due to a bimodal or multimodal droplet size distribution (Figure 1). This means that dilution of these systems with water does not form thermodynamically stable oil-in-water microemulsions with a narrow distribution of droplet sizes, but kinetically unstable nanodispersions that are likely prone to aggregation or sedimentation of the dispersed droplets [14,56].
The second inherent characteristic of microemulsion concentrates is the ability to absorb a large amount of water and form an oil-in-water microemulsion with a Z-ave of typically 10–100 nm and a narrow unimodal size distribution [13,55,57]. Therefore, the MCT-9:1, MCT-1:1, IPM-9:1, and IPM-1:1 systems did not exhibit the properties of microemulsion concentrates. This result was consistent with the observation of Das et al. [17], that increasing the Transcutol® content in the mixture with the nonionic surfactant polysorbate 80 (HLB = 15) beyond the 1:2 mass ratio leads to a decrease in the area of the microemulsion, which is likely due to the higher cosurfactant/surfactant ratio disrupting the surfactant layer at the interface. Based on our results, the same can be assumed if the Transcutol® content at Km 9:1 was much lower than the surfactant.
Other mixtures classified as single-phase by visual assessment (Table S1) showed %T > 90% (Table 2), confirming the high mutual solubility of the ingredients and isotropy. Furthermore, after dilution of these systems with water, oil droplet dispersions were formed with Z-ave below 100 nm (Table 2) and a narrow unimodal size distribution (PdI < 0.250) (Figure 1), which corresponds to the properties of oil-in-water microemulsions. The %T and droplet size characterization results were in good agreement with each other and allowed for reliably identifying four mixtures that can be classified as microemulsion concentrates: MCT-0, MCT-7:3, IPM-0, and IPM-7:3. Comparison of the Z-ave values of the obtained oil-in-water microemulsions pointed out that the average droplet size was similar between the systems containing different synthetic oils, so the oil type had no significant effect on the droplet size. However, Z-ave was lower in microemulsions from samples that did not contain cosurfactant (MCT-0 and IPM-0), which is due to a higher proportion of surfactant and consequently a higher capacity to reduce the interfacial tension between the oil and water phases and increase the interface curvature required to form smaller droplets of the dispersed phase [14]. In addition, the dielectric constant of the cosurfactant is 14.1 and HLB = 4.2 [52], so Transcutol® as a weakly polar small molecule (Mw = 134.17 gmol−1) can be expected to partially mix with the synthetic oils used and increase the total concentration of the dispersed oil phase, which may also be associated with an increase in the average droplet size.
For the microemulsion concentrates MCT-0, MCT-7:3, IPM-0, and IPM-7:3, the structural transformation upon dilution with water was also investigated, and the concentration range of the aqueous phase at which oil-in-water microemulsions are formed was determined by conductometric titration. The results of the conductometric titrations are shown in Figure 2 as a collection of curves of the system conductivity as a function of the increasing aqueous phase content.
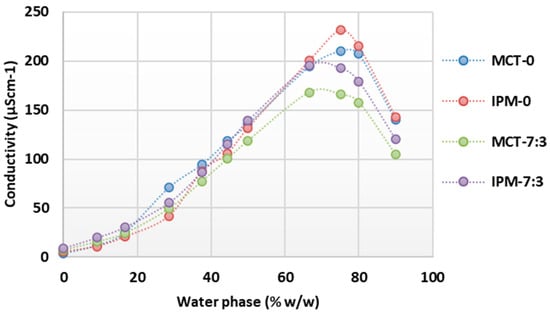
Figure 2.
Conductivity as a function of increasing water phase concentration during dilution of microemulsion concentrates MCT-0, MCT-7:3, IPM-0, and IPM-7:3.
It is known that with the increase in the proportion of the aqueous phase during the dilution of microemulsion concentrates, a continuous structural change from a water-in-oil to an oil-in-water microemulsion occurs without detectable phase separation, which can be registered by a change in electrical conductivity [22,42,44]. When analyzing the conductometric curves obtained (Figure 2), it was found that the conductivity in all systems was <50 µScm−1 at water concentrations of up to 16.67% w/w, which corresponds to the formation of systems with droplets of the aqueous phase dispersed in a continuous oil phase and having relatively limited mobility, as reflected in the low conductivity values. The systems were transparent, and a water-in-oil microemulsion was assumed to form at water concentrations up to 16.67% w/w.
As the water content increases further, the conductivity increases sharply, which corresponds to the continuous transformation of the structure into a water-continuous type, where oil phase droplets are dispersed in the water phase. In the presence of the two synthetic oils, the samples with cosurfactant (MCT-7:3 and IPM-7:3) reach a lower maximum conductivity value (<200 µScm−1) at 66.67% w/w of the water phase compared to the samples without cosurfactant, where the maximum conductivity value (>200 µScm−1) was reached at 75% w/w of the water phase.
Considering that, in the systems without cosurfactant, the surfactant content was higher and they had a high HLB value, it is reasonable to expect the presence of dissolved hydrophilic surfactant components in the aqueous phase and the eventual formation of micelles, which act as mobile charge carriers and increase the overall conductivity of the system. This assumption is supported by the observation of Lv et al. [28], that the polyoxyethylene group of nonionic surfactants may have a small negative charge that contributes to the conductivity of oil-in-water microemulsions.
After reaching the maximum conductivity in the tested systems, the conductivity decreased (Figure 2), which is probably due to the decrease in charge carrier concentration by further dilution with water. At a water content of 90% w/w, the conductivity was in the range of 100–150 µScm−1, indicating an oil-in-water structure, which is consistent with the results of the droplet size measurements (Table 2, Figure 1). Similar observations for a continuous structural transformation in microemulsions containing polysorbate 80 and propylene glycol were published by Sutthisawatkul et al. [27], including a sharp increase in conductivity with a maximum at 70% water content, followed by a decrease in conductivity with a further increase in water content to 90%.
The systems with IPM showed higher values of maximum conductivity (192.9 µScm−1 for IPM-0; 231.8 µScm−1 for IPM-7:3) compared to the corresponding systems with MCT (maximum conductivity 165.8 µScm−1 for MCT-0 and 210.5 µScm−1 for MCT-7:3). This can be attributed to the significantly lower Mw and viscosity of IPM (4.5 mPa·s) compared to MCT (25–33 mPa·s) [58,59]. The lower viscosity of IPM is probably favorable to facilitate the continuous transformation of the structure from oil-continuous to water-continuous and probably requires less surfactant to stabilize the interface, thereby increasing the overall free charge carrier content in the aqueous phase. Furthermore, as a synthetic ester oil, IPM likely has many times higher conductivity than MCT. Spohner [60] showed that the conductivity of methyl oleate is 39.80 nS/m, while the conductivity of MCT type V is 4.62 nS/m.
In summary, it can be said that the presence/absence of cosurfactant and the type of synthetic oil have an influence on the dynamics of the structural transformations in the microemulsion concentrates investigated. The process of structure transformation of the microemulsion concentrate into an oil-in-water microemulsion was slightly facilitated by the absence of a cosurfactant and by the use of a low-viscosity synthetic oil (IPM). This view is supported by the result that the values of Z-ave were lower in diluted systems without cosurfactant (Table 2). In addition, it was shown that the microemulsion concentrates MCT-0, MCT-7:3, IPM-0, and IPM-7:3 can be continuously diluted with water while remaining homogeneous and transparent and during which a transformation of the structure into an oil-in-water microemulsion occurs, which is considered to be a property of crucial importance for the preparation of film-forming microemulsions by dispersing the concentrate in a polymer solution.
3.2. Phase Behavior of Microemulsion Concentrates with Essential Oils
According to the composition of the microemulsion concentrates MCT-0, MCT-7:3, IPM-0, and IPM-7:3, a total of 18 microemulsion concentrates with essential oils of cinnamon (“C” group concentrates), thyme (“T” group concentrates), and tea tree (“M” group concentrates) were prepared by replacing the synthetic oil in whole or in part with essential oil. The names and compositions of the microemulsion concentrates with each essential oil are listed in Table 3.

Table 3.
Composition of microemulsion concentrates with essential oil of cinnamon (group labeled with the prefix “C”), thyme (group labeled with the prefix “T”), or tea tree (group labeled with the prefix “M”). The prefixes are added to the labels of the corresponding microemulsion concentrates without essential oils. C-0, T-0, and M-0 are the labels of the systems without cosurfactant, where the synthetic oil is completely replaced by essential oil.
For the formulation of microemulsion concentrates with essential oils, it had to be investigated whether the synthetic oil could be partially or completely replaced by essential oil without affecting the phase behavior and thermodynamic stability of the system, as well as the ability to form an oil-in-water microemulsion by dilution with water. The phase behavior of the microemulsion concentrates with essential oils was investigated using the same tests that were performed for microemulsion concentrates with synthetic oils. A visual assessment of the appearance after centrifugation showed that all systems with essential oils were single-phase, homogeneous, clear liquids with an odor characteristic of the essential oil they contained. The systems of the “M” group with tea tree essential oil were colorless, those with thyme essential oil (“T” group) were light yellow, and the color of the systems with cinnamon essential oil (“C” group) was yellow (Figure 3).

Figure 3.
Visual appearance of the microemulsion concentrates with tea tree essential oil (the “M” group samples, top row), thyme essential oil (the “T” group samples, middle row), and cinnamon essential oil (the “C” group samples, bottom row) (the photos were taken in daylight by the Samsung Galaxy A50 triple camera 25 MP PDAF f/1.7 snapper + 8 MP fixed-focus, f/2.2 ultra-wide + 5 MP, fixed-focus, f/2.2 depth sensor (Samsung, Seoul, Republic of Korea)).
The yellow color of the “C” group systems came from the incorporated cinnamon essential oil, which is golden yellow. Also, the light yellow color of the “T” group came from thyme essential oil. Tea tree essential oil is almost colorless, so its presence did not affect the color of the “M” group systems. Within the “C” and “T” groups, the color was more intense in systems T-0, T-7:3, C-0, and C-7:3 (Figure 3), which had the highest concentrations of essential oil in their compositions (i.e., synthetic oil was completely replaced by essential oil).
Table 4 shows the %T values of the concentrates with essential oils of thyme, cinnamon, and tea tree, as well as values of Z-ave and PdI, in dispersions obtained by diluting them 10-fold by water.

Table 4.
Transmittance (%T) of microemulsion concentrates with essential oils and average droplet size (Z-ave) and polydispersity index (PdI) in dispersions obtained by diluting them with water (in a mass ratio of 9:1).
The transmittance of all microemulsion concentrates with essential oils was >90%. The transparency and high %T values indicated that the ingredients were homogeneously mixed and well soluble in each other, forming isotropic single-phase systems. The %T values of the systems in the “C” group ranged from 91.89% to 98.01%. The lowest transmittance values in this group were found in the systems C-0 and C-7:3, where the synthetic oil was completely replaced by cinnamon essential oil. In the microemulsion concentrates with thyme and tea tree essential oils, all %T values were above 95%. In the “T” group, the %T values ranged from 96.03% to 99.36%, and in the “M” group, from 98.25% to 98.90%. The lowest transmittance in the “T” group was recorded for the T-0 and T-7:3 systems, in which the colorless synthetic oil was completely replaced by the light yellow thyme essential oil.
The difference in %T range between the groups is related to the differences in color and refractive index of the essential oils. Cinnamon essential oil has the highest refractive index (1.53 to 1.54) [61], followed by thyme essential oil (1.502) [62] and tea tree essential oil with the lowest (1.478) [63]. A wider range of %T values was observed in the “C” group, which could be influenced by the more intense color of the present cinnamon essential oil and the highest value of its refractive index. Accordingly, the lowest variation of %T values was observed in the “M” group containing tea tree essential oil, which is almost colorless and has the lowest refractive index, so its presence did not significantly affect the optical properties of the “M” group systems.
The values of Z-ave and PdI (Table 4) showed that all tested systems with essential oils had the characteristics of microemulsion concentrates from which, after dilution in water, oil-in-water microemulsions were formed with droplets with a hydrodynamic diameter of 13.07 nm to 27.20 nm and a unimodal narrow distribution (PdI < 0.250) (Figure 4).
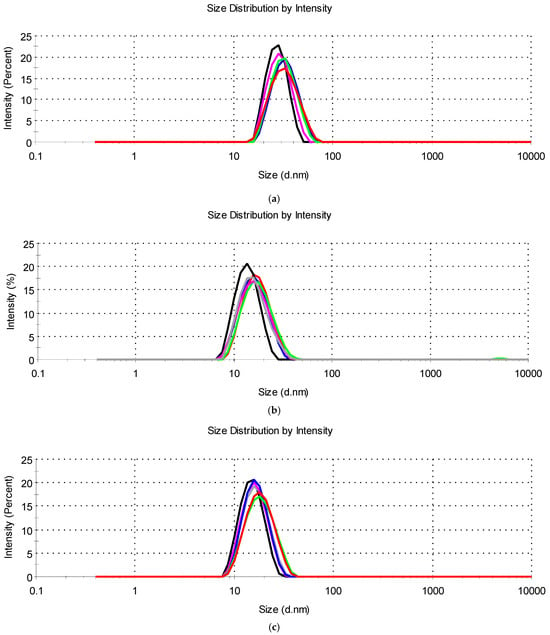
Figure 4.
Droplet size distribution for diluted microemulsion concentrates containing: (a) tea tree essential oil (M-MCT-0 (▬), M-IPM-0 (▬), M-0 (▬), M-MCT-7:3 (▬), M-IPM-7:3 (▬), and M-7:3 (▬)); (b) cinnamon essential oil (C-MCT-0 (▬), C-IPM-0 (▬), C-0 (▬), C-MCT-7:3 (▬), C-IPM-7:3 (▬), and C-7:3 (▬)); (c) thyme essential oil (T-MCT-0 (▬), T-IPM-0 (▬), T-0 (▬), T-MCT-7:3 (▬), T-IPM-7:3 (▬), and T-7:3 (▬)).
Similarly, Catanzano et al. [20] observed the droplets of tea tree essential oil of 21.95 nm (PdI = 0.232) in an optimized microemulsion based on polysorbate 80 and ethanol. Lv et al. [28] also showed a droplet size of peppermint oil, clove oil, and rosemary oil of less than 20 nm in diluted oil-in-water microemulsions with Kolliphor® EL (HLB 12–14) + 1,2-propanediol, while Lee et al. [10] reported the droplet size of peppermint oil, lavender oil, and eucalyptus oil microemulsions between 26.75 and 38.92 nm using polysorbate 80 with ethanol or Transcutol®. A small droplet size of ~18.98 nm was reported for microemulsified lemongrass leaf essential oil and mango seed kernel extract in combination with soybean oil and coconut oil using Kolliphor® RH40 and macrogol 400 [23]. Sieniawska et al. [26] obtained droplets with a size of 10 to 20 nm by microemulsification of a mixture of essential oil and soybean oil in a water/propylene glycol mixture using polysorbate 80.
The determined Z-ave in all microemulsions with essential oils of cinnamon, thyme, or tea tree (Table 4) was lower compared to systems of the corresponding composition without essential oils (Table 2). Furthermore, in all samples with essential oils, the droplet size distribution was narrower compared to microemulsion concentrates without essential oils. The presence of essential oils probably facilitated the microemulsification of the hydrophobic ingredients and the formation of oil-in-water microemulsions with small droplets. Similar considerations regarding the effect of essential oils on reducing droplet size and narrowing the size distribution in oil-in-water microemulsions have been published in recent studies [11,18] in which the low-molecular-weight amphiphilic components of essential oils (e.g., phenols, terpenes) were assumed to behave as cosurfactants, potentially penetrating the surfactant interfacial film, reducing interfacial tension and increasing its curvature, thus reducing droplet size. The main constituents of cinnamon, thyme, and tea tree oils are cinnamaldehyde (Mw 132.16 gmol−1), terpinen-4-ol (Mw 154.25 gmol−1), and thymol (Mw 150.22 gmol−1), respectively [11].
Based on the results of the droplet size characterization, a positive influence of these low-molecular-weight constituents of essential oils on the flexibility of the surfactant film at the oil-water interface could be expected. This is confirmed by the observation of Das et al. [17] that synthetic MCT-type oil decreases the microemulsion area as its proportion in the mixture with tea tree essential oil increases. An additional aspect to consider is the replacement of the synthetic oil with essential oil, which reduces the proportion of hydrophobic ingredients that contribute to the reduction in droplet size. Accordingly, in groups “C”, “T”, and “M”, we observed that the Z-ave of the microemulsions in which the synthetic oil was completely replaced by essential oil were consistently smaller than the microemulsions containing a mixture of synthetic and essential oil (Table 4). No significant difference in droplet size was observed between the corresponding systems containing MCT and IPM.
In the presence of all three essential oils, larger droplet sizes were observed in the systems with the cosurfactant (Table 4), which is most likely due to the lower proportion of the surfactant and is consistent with the results for microemulsion concentrates without essential oils (Table 2). A similar observation in the gradual increase of the droplet size in oil-in-water microemulsions containing peppermint oil, clove oil, or rosemary oil with the decrease in surfactant mixture/oil ratio was reported by Lv et al. [28].
Conductometric titrations of microemulsion concentrates with essential oils confirmed the continuous transformation of the structure with an increase in the content of the water phase up to the oil-in-water microemulsions (Figure 5). A significant influence of the cosurfactant on reduction of the conductivity maximum was also observed; consistently smaller values were obtained when the cosurfactant was present in all groups (“M”, “C” and “T”). However, differences between the conductivity maxima of systems containing different synthetic oils (MCT or IPM) were not observed and are probably not noticeable, as half of the synthetic oil was replaced by essential oil. Accordingly, the conductivity maximum was always higher in the systems containing only the essential oil (M-0, M-7:3, C-0, C-7:3, T.0, and T-7:3). A similarity was observed between the conductometric curves and the maxima of the microemulsion concentrates with cinnamon and thyme essential oil, while the maximum conductivity values were higher on the curves of the microemulsion concentrates with tea tree essential oil.
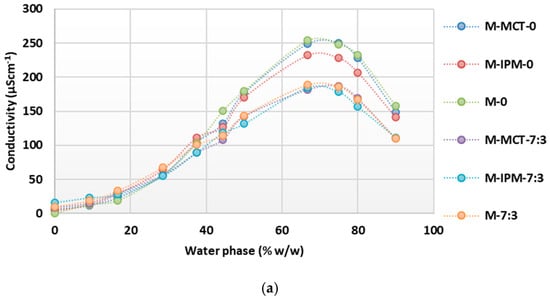
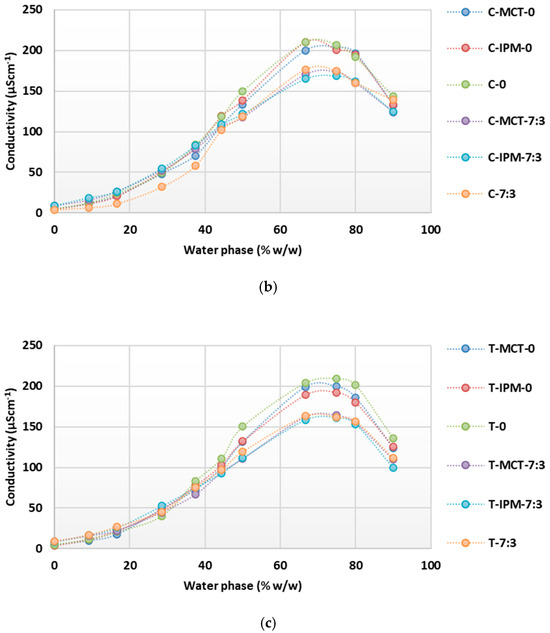
Figure 5.
Conductivity as a function of increasing water phase concentration during dilution of microemulsion concentrates with: (a) tea tree essential oil; (b) cinnamon essential oil; (c) thyme essential oil.
Of the three essential oils compared, the largest average droplet size was observed in systems containing tea tree essential oil, while it was significantly smaller in the presence of thyme and cinnamon essential oils, which were also very similar to each other. Only systems with cinnamon and thyme essential oils were selected for further study, as the droplet size and structural transformation were very similar when diluted with water, so the significance of this aspect for the quality attributes of the film-forming microemulsions and films could be excluded. In addition, a smaller oil droplet size is desirable to enable stable incorporation of the hydrophobic droplets into the matrix of the hydrophilic film-forming polymer [41].
3.3. Physicochemical Characteristics of Microemulsion Concentrates and Film-Forming Microemulsions with Essential Oils
The microemulsion concentrates containing the essential oils of cinnamon and thyme were used for the formulation of film-forming microemulsions by dispersing the microemulsion concentrate of the essential oil in a solution of hypromellose in a mixture of water/isopropanol in a mass ratio of 1:1. The concentration of hypromellose was determined on the bases of preliminary tests on the ability of this polymer to form a film from a solution in a mixture of equal amounts of water and isopropanol. A solution containing 2.5% w/w hypromellose formed a thin transparent colorless film on an inert flat surface.
All 12 film-forming microemulsions with essential oils were colorless, transparent, homogeneous, viscous liquids, with a faint odor of the essential oil they contained. The homogeneity and clarity of the film-forming microemulsions indicated a satisfactory compatibility between the polymer solution and the dispersed hydrophobic and amphiphilic components of the microemulsion concentrates. In addition, rheological characterization was performed and the pH of the microemulsion concentrates and the corresponding film-forming microemulsions with essential oils was measured to elucidate the structure of the systems formed by dispersing the microemulsion concentrate in the hypromellose solution in a water/isopropanol mixture.
3.3.1. Flow Behavior and Viscosity of Microemulsion Concentrates and Film-Forming Microemulsions with Essential Oils
The flow curves of microemulsion concentrates and film-forming microemulsions with essential oils of cinnamon and thyme are shown in Figure 6.
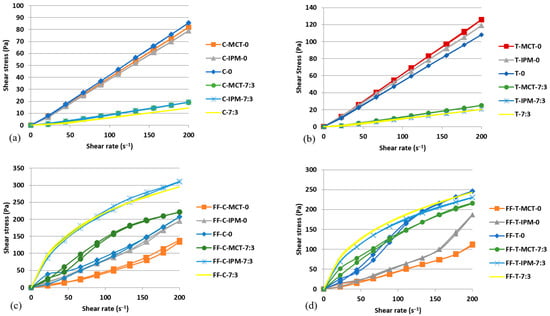
Figure 6.
Flow curves of: (a) microemulsion concentrates with cinnamon essential oil, (b) microemulsion concentrates with thyme essential oil, (c) film-forming microemulsions with cinnamon essential oil, (d) film-forming microemulsions with thyme essential oil.
The obtained flow curves for microemulsion concentrates with essential oils (Figure 6a,b) were fitted using a mathematical model describing Newtonian flow. All obtained values of the correlation coefficient for the Newtonian flow type were >0.95, which proves that the investigated microemulsion concentrates with essential oils are Newtonian systems, which means that the shear rate and shear stress are linearly correlated and that the viscosity is unchanged in the investigated shear rate range; i.e., has an absolute value [64]. The Newtonian flow behavior of the microemulsion concentrates supported the view that they were homogeneous and isotropic molecular dispersions (i.e., solutions). The values of absolute viscosity (η) of microemulsion concentrates with essential oils are listed in Table 5.

Table 5.
Absolute viscosity (η) of microemulsion concentrates (MC) with cinnamon (“C” group) and thyme (“T” group) essential oils and maximum apparent viscosity (ηapp @ 22.2 s−1) and minimal apparent viscosity (ηapp @ 200 s−1) of corresponding film-forming microemulsions (FFM) with essential oils (labeled with the prefix “FF”).
The η value of each microemulsion concentrate with cinnamon essential oil was lower compared to the values of the corresponding microemulsion concentrate with thyme essential oil. When comparing pairs of microemulsion concentrates differing only by the type of essential oil, the influence of other formulation variables can be excluded so the observed differences in absolute viscosity depend only on the type of essential oil. Furthermore, it was found that in the presence of both essential oils, the addition of the cosurfactant lowered the absolute viscosity of the microemulsion concentrates compared to the corresponding samples without cosurfactant (Table 5). The decrease in concentrate viscosity due to the addition of the cosurfactant can be associated with a simultaneous decrease in the concentration of the surfactant Kolliphor® RH 40. Kolliphor® RH 40 has, as stated, the consistency of a paste at 20 °C [53], while the cosurfactant Transcutol® is a low-viscosity liquid [52]. No clear relationship was found between the η values of the microemulsion concentrates, which differed only in terms of the oil phase (essential oil alone or a mixture of essential and synthetic oils).
Figure 6c,d show the flow curves of film-forming microemulsions with cinnamon and thyme essential oils. All film-forming microemulsions were non-Newtonian systems in which the viscosity changes as a function of the shear rate. Significant differences were observed in the flow curves of film-forming microemulsions with cosurfactant compared to those without cosurfactant. The flow curves of the system with cosurfactant corresponded to the shear-thinning materials, in which the gradient of shear stress decreases with increasing shear rate and the viscosity also decreases with increasing shear rate [64]. Table 5 shows that the ηapp values of the film-forming microemulsions with cosurfactant at the maximum shear rate (200 s−1) were lower than the ηapp values at the minimum shear rate (22.2 s−1).
In contrast, the flow curves of film-forming microemulsions without cosurfactant indicated the rheological behavior of a shear-thickening material; i.e., both the shear stress and the viscosity of the system increase at higher shear rates [64]. In these systems, the ηapp values at the maximum shear rate (200 s−1) were higher than the ηapp values at the minimum shear rate (22.2 s−1) (Table 5).
The ηapp values of all film-forming microemulsions with cosurfactant were higher than those of the corresponding systems without cosurfactant at the same shear rate. The concentration of the surfactant Kolliphor® RH40, which contains molecules with long polyoxyethylene tails, is reduced in film-forming microemulsions with cosurfactant. Based on the previous characterization of oil-in-water microemulsions obtained by diluting the concentrates with water, it was assumed that the surfactant is predominantly distributed at the interface, so it may be present in the continuous aqueous phase in a limited concentration. Under these conditions, there is a possibility of the formation of premicellar aggregates of the long polyoxyethylene tail components of the surfactant Kolliphor® RH 40 along the polymer chains of hypromellose, which is consistent with the observations of Mondal et al. [65]. As hypothesized, surfactants with long tails in molecule form aggregate at concentrations far below their critical micelle concentration (cmc) and form a complex with polar groups of nonionic cellulose derivative-type polymers via hydrophilic, hydrophobic, and electrostatic intermolecular bonds. The formed premicellar aggregates with strongly hydrogen-bonded water act as a junction that further promotes polymer crosslinking and increases the viscosity of the solution [65]. The observed shear-thinning behavior of film-forming microemulsions with cosurfactant is simply a consequence of the breaking of established bonds between polymer chains under the influence of increasing shear rate.
In contrast, the phenomenon of premicellar aggregate formation does not occur at surfactant concentrations above cmc, so it was probably not expected in film-forming microemulsions without cosurfactant, in which a higher concentration of Kolliphor® RH40 surfactant was present (Table 5). Therefore, the polymer network in these systems was more relaxed and corresponded to an overall lower apparent viscosity compared to the systems with the cosurfactant. However, in systems without cosurfactant, exposure to an increasing shear rate can induce phenomena such as the formation of nanodroplet clusters and shear-induced interactions between chains of different polymer molecules, leading to a relatively strong association, recognizable as an increase in viscosity (i.e., shear-thickening) [66]. The practical advantage of shear-thinning rheological behavior associated with the topical application of film-forming microemulsions is reflected in the reduction in viscosity that facilitates spreading, and, after the cessation of shear force, viscosity recovers and ensures retention at the application site. In contrast, when applying shear-thickening film-forming microemulsions, the viscosity increases slightly, which makes spreading more difficult, and there is a risk of oil droplet clusters forming in the film during drying, which may impair its quality attributes.
Film-forming microemulsions differing only in the type of essential oil had comparable apparent viscosity, especially at the maximum shear rate (Table 5). In contrast to the microemulsion concentrates, the concentration of the essential oil in the film-forming microemulsions was ten times lower, so the specific influence of the essential oil components on the viscosity was not substantial.
3.3.2. pH of Microemulsion Concentrates and Film-Forming Microemulsions with Essential Oils
The results of the pH measurements of the microemulsion concentrates and the film-forming microemulsions with the essential oils of cinnamon and thyme are summarized in Table 6.

Table 6.
pH of microemulsion concentrates (MC) with cinnamon (“C” group) and thyme (“T” group) essential oils and of corresponding film-forming microemulsions (FFM) with essential oils (labeled with the prefix “FF”).
The pH values of the microemulsion concentrates with thyme essential oil were always higher compared to those of the corresponding concentrate with cinnamon essential oil, which is likely a consequence of the different compositions of the essential oils. The pH values of the microemulsion concentrates with cinnamon essential oil containing the cosurfactant were in the range of 3.82 to 4.00, while the values of the concentrates without cosurfactant were between 4.38 and 4.72. For the microemulsion concentrates with thyme essential oil, the pH value of the systems without cosurfactant was between 5.56 and 5.57 and thus higher than the pH value of the systems with cosurfactant (4.26–4.32). From the results obtained, it can be concluded that the presence of cosurfactant significantly lowers the pH of the microemulsion concentrates in the systems formulated with both essential oils.
The pH values of the film-forming microemulsions with cinnamon essential oil were between 4.87 and 5.73; the range for the samples without cosurfactant was from 5.26 to 5.73, and with cosurfactant from 4.87 to 5.04. The pH values of the film-forming microemulsions with thyme essential oil without cosurfactant (from 6.60 to 6.81) were also higher than in the systems of the same group with cosurfactant (from 5.43 to 5.57). A significant influence of the cosurfactant is confirmed in connection with the lowering of the pH value of the film-forming microemulsions with essential oils. In agreement with the findings of Punitha et al. [67], hypromellose at lower pH values in aqueous solutions exhibits coiling of the polymer molecules, which is why polymer interactions with water are favored, while at higher pH values the polymer molecules are in the extended conformation and can entangle to form a gel. The overall relatively low pH values of the film-forming microemulsions ensure the consistency of thickened liquids without gel formation.
The liquid consistency is suitable for easy spreading on the skin, especially if it is damaged, which ensures good acceptance by patients. Film-forming microemulsions with essential oils had a smooth skin feel. In addition, the pH values of the film-forming microemulsions with essential oils were found to be slightly acidic, in the range of 4.87–6.81 (Table 6), which is highly compatible with the pH of the human skin surface (4–6), including wounds (e.g., the pH of diabetic wounds is 6.5–8), and avoids the risk of skin irritation [68,69].
3.4. Characteristics of Films Obtained from Film-Forming Microemulsions with Essential Oils
Figure 7 shows the appearance of dry films obtained from film-forming microemulsions with cinnamon and thyme essential oils.
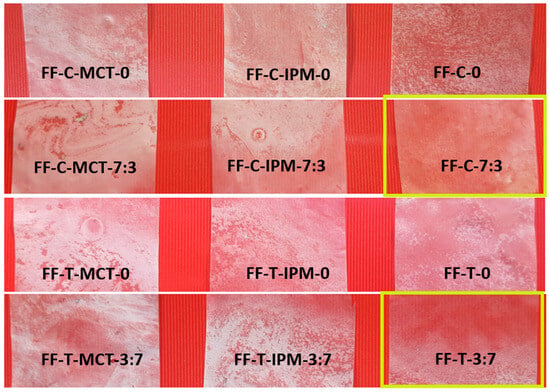
Figure 7.
Appearance of dry films obtained from film-forming microemulsions with cinnamon and thyme essential oils (the photos were taken in daylight by the Samsung Galaxy A50 triple camera 25 MP PDAF f/1.7 snapper + 8 MP fixed-focus, f/2.2 ultra-wide + 5 MP, fixed-focus, f/2.2 depth sensor (Samsung, Seoul, Republic of Korea)). The films FF-C-7:3 and FF-T-7:3 with the best appearance (homogeneous and least opalescent) are marked with a yellow rectangle.
In contrast to the film formed from a hypromellose solution in a water/isopropanol 1:1 mixture, which was colorless and transparent, the dry films formed from microemulsions with essential oils were opalescent, and, in some of them, inhomogeneities in appearance were observed, which could be the result of drying of the surfactant/polymer aggregates with entrapped oil droplet clusters and fine air bubbles being incorporated during the preparation of the microemulsion. The films had a smooth surface and were easy to peel from the glass surface. They did not tend to tear during peeling. The films formed from microemulsions without synthetic oils and with the cosurfactant (FF-C-7:3 and FF-T-7:3) had the best esthetic properties and were the least opalescent.
Films containing synthetic oil (MCT or IPM) in addition to essential oil were more opalescent. In agreement with the results of the rheological analysis, coalescence of oil droplets could occur in the film-forming microemulsions without cosurfactant under agitation. The risk of formation of clusters of micrometer-sized fatty oil droplets in hypromellose-based films has also been reported, affecting their optical properties during drying [41]. There also appears to be a risk of cluster formation when drying films with incorporated clusters of nanodroplets. The clusters of oil nanodroplets formed during drying are immobilized in the hypromellose polymer matrix and refract light to a greater extent, reducing the transparency of films without cosurfactant compared to films obtained from film-forming microemulsions with cosurfactant, where the risk of coalescence was probably not substantial.
The results of the dry film weight (FW) and the calculated yield values (Y (% w/w)) are shown in Table 7.

Table 7.
Film weight (FW) and calculated yield (Y (% w/w)) (the films are labeled with the same labels of the corresponding film-forming microemulsions with essential oils from which they were obtained).
Based on the composition of the film-forming microemulsions with essential oils, the theoretical yield was 12.5% w/w, which corresponds to the sum of the concentration of the microemulsion concentrate (10% w/w) and the hypromellose (2.5% w/w). The experimentally determined results of FW and Y (% w/w) show the amount of ingredients of the formulation remaining in the film after evaporation of the volatile solvents (water and isopropanol). The components of the essential oils are also volatile molecules, so the decrease in yield compared to the theoretical yield indicates the extent of evaporation of the essential oils themselves.
The yield of films with cinnamon essential oil was in the range of 8.60–11.00% w/w, while that of films with thyme essential oil was 7.60–10.60% w/w. In relation to the theoretical yield, these values were lower, indicating a decrease in mass due to the evaporation of volatile components of the essential oils during drying. The films with thyme essential oil had a lower mass and yield compared to the corresponding films with cinnamon essential oil, probably due to the higher volatility of the components and the lower density of thyme essential oil (0.917 g/mL) [62] compared to cinnamon essential oil (1.03 g/mL) [70].
In both groups, the films without cosurfactant had a higher weight and yield. This could be a consequence of the reduction of free surface area due to the coalescence of the oil droplets in the clusters and the resulting reduction in the degree of evaporation of the essential oil. When the cosurfactant is present, the droplets of the oil phase coalesce less or not at all, so they have a larger total surface area over which the volatiles can evaporate. The result that the films containing essential oil in combination with low-volatile synthetic oils (FF-C-MCT, FF-C-IPM, FF-T-MCT, and FF-T-IPM) without cosurfactant had the highest mass and yield due to the lower evaporation losses (Table 7) supports the previous assumptions. In contrast, films prepared without synthetic oils and with the cosurfactant (FF-C-7:3 and FF-T-7:3) had the best appearance but lower weight and yield, probably due to the higher evaporation of the essential oils. Optimizing the ratio of essential oil/synthetic oil, the type of synthetic oil and the inclusion/exclusion of the cosurfactant could make it possible to achieve the targeted film attributes, such as appearance and evaporation rate of the essential oil. Although the presence of synthetic oil might have affected the appearance of the film, it could limit the evaporation rate of the essential oil and provide additional benefits to the skin, such as hydrophobicity of the film and emollient effect. On the other hand, Almasi et al. [9] indicated differences in mechanical properties and a higher release rate and better antimicrobial activity of thyme essential oil from films with smaller nanodroplets in a sodium-alginate matrix obtained from a microemulsion compared to films for which a nanoemulsion with larger oil droplets was used.
Cotton fibers did not stick to the dry films with essential oils when light pressure was applied. Therefore, the films formed in situ do not stick to clothing or transfer to contact surfaces after drying. In addition, the rapid evaporation of isopropanol is useful to provide a cooling effect at the application site and reduce the risk of irritation to the compromised skin barrier. However, it is important to emphasize that the cotton pad test is only a rough estimate and is not precise or sensitive enough to detect differences in the stickiness of the tested films depending on different formulation parameters.
The dry films with essential oils of cinnamon and thyme were not brittle. Their flexibility was evaluated to assess the endurance of bending or stretching on the skin. After performing the folding endurance test, all films were found to have satisfactory flexibility (more than 300 folds) to meet patient compliance under conditions corresponding to actual use. There was no change in film relief, stretching, or cracking. The films with essential oils were even more stretchable compared to the hypromellose film. The non-volatile components of the film, such as propylene glycol, Kolliphor® RH40, and Transcutol®, exhibited good plasticizing properties, as they improve the mobility of the polymer chains, probably by partially breaking interpolymeric hydrogen bonds, thus enabling high folding endurance [35,52].
The determination of the swelling capacity of essential oil films in artificial sweat and water was performed with the aim of assessing the capacity of films formed in situ to absorb sweat and exudate, as well as the ability to wet and swell in water, which correlates with water solubility and ease of rinsing from the application site [21]. The results of the determination of the weight percentage of the swollen film S (% w/w) in artificial sweat and water are shown in Figure 8.
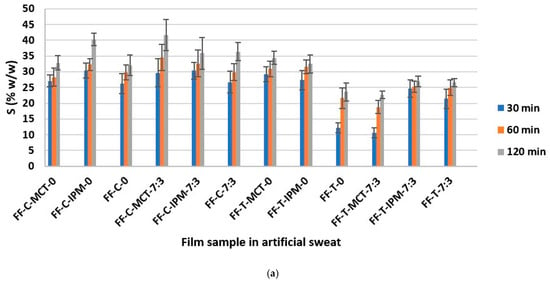
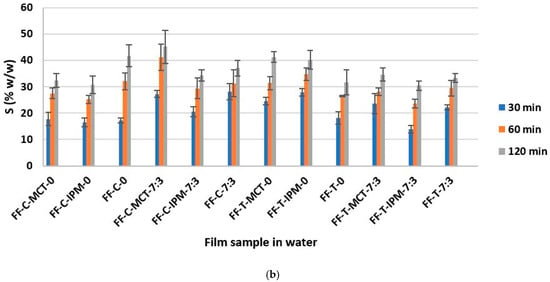
Figure 8.
The weight fraction of the swollen film S (% w/w) as a function of time in: (a) artificial sweat and (b) water.
The extreme S (% w/w) values were calculated taking into account the weight of the examined dry film sample tested: S = 9.09% w/w (the film does not swell) and S = 100% w/w (the film has absorbed the entire amount of medium). The results obtained were between the extreme values indicated, and for each film, an increase in the value of S (% w/w) as a function of time was observed in both media. The overall values ranged from about 10% w/w to 45% w/w in both media.
It can be assumed that the results obtained were influenced by the properties of the hypromellose, which swells on contact with the medium, as well as by the surfactant (Kolliphor® RH40) and the cosurfactant (Transcutol®), which improve the wetting of the film, while the influence of the other formulation parameters varied (concentration and type of essential oil and synthetic oil), could not be assessed, and is probably negligible due to the low content in the film. Our observations are confirmed by the conclusions of the study by Hota et al. [34], according to which surfactants, including Kolliphor® RH40 and Transcutol®, strongly influence the water absorption capacity of hypromellose films.
In addition, smaller differences in S (% w/w) values were observed between different time points in artificial sweat, while they were larger in water (Figure 8). Sodium chloride as a component of artificial sweat can increase the surface activity of anionic surfactants [71]. A similar explanation for the faster wetting and swelling of the investigated films and the smaller differences between the time points in this medium compared to purified water can be proposed, when the small negative charge of the polyoxyethylene group of nonionic surfactant Kolliphor® RH40 is taken into account [28]. From the collected results (Figure 8), the FF-C-MCT-7:3 film can be recognized with the highest swelling capacity in both media, which can be considered promising in terms of sweat and exudate absorption and water washout from the application site.
4. Conclusions
Investigation of the phase behavior of ternary and pseudo-ternary mixtures made it possible to identify thermodynamically stable isotropic microemulsion concentrates with synthetic oils (MCT or IPM) only in the presence of the nonionic surfactant Kolliphor® RH40 (HLB = 14–16) and the cosolvent propylene glycol, without the cosurfactant Transcutol® (HLB = 4.2) or at Km 7:3. The dynamics of the structural transformations when these concentrates were diluted with water were influenced by the Mw of the synthetic oil and the presence/absence of the cosurfactant. The concentrates with Transcutol® resulted in oil-in-water microemulsions with a higher average droplet size. By replacing all or part of the synthetic oil with tea tree, cinnamon or thyme essential oil, microemulsion concentrates suitable for dilution with water were obtained without compromising thermodynamic stability to the point of forming highly diluted oil-in-water microemulsions. Moreover, the introduction of each essential oil facilitated the microemulsification process and reduced the average droplet size. No significant differences were found between the effects of cinnamon and thyme essential oils on Z-ave and PdI, while tea tree oil reduced Z-ave to a lesser extent. Film-forming microemulsions with essential oils of cinnamon and thyme in the presence of a cosurfactant were shear-thinning systems with apparent viscosity, which decreases under the influence of agitation. In these systems, the surfactant-polymer network limits the coalescence of the oil droplets during film drying, which contributes to a higher volatility of the essential oil. In contrast, in the film-forming microemulsions without cosurfactant, the hypromellose molecules do not form extensive mutual bonds, and, under the influence of shear force, the apparent viscosity increases (i.e., shear-thickening), indicating the clustering oil droplets. In addition, the cluster formation of oil droplets in a dry film that eventually limited the volatility of the essential oil was evident. By optimizing the concentration of the cosurfactant and the type and concentration of the synthetic oil, the quality attributes of the films formed in situ, such as weight, appearance, and yield, can be tailored. Film-forming oil-in-water microemulsions with essential oils of cinnamon and thyme can be considered promising formulations for topical application, including wound treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13040990/s1, Table S1: Number of phases (single-phase system (1Φ); two-phase system (2Φ); three-phase system (3Φ)) observed by visual inspection of ternary and pseudo-ternary mixtures as a function of the examined formulation variables (Km and type of surfactant, synthetic oil (MCT or IPM) and co-solvent (propylene glycol or glycerol)).
Author Contributions
Conceptualization, L.Đ.; Methodology, L.Đ.; Investigation, L.Đ., A.Ć. and S.M.; Data curation, L.Đ., A.Ć. and S.M.; Visualization, L.Đ., A.Ć. and S.M; Resources, L.Đ.; Writing—original draft preparation, L.Đ., A.Ć. and S.M.; Writing—review and editing, J.M.B., J.F. and L.P.; Supervision, L.Đ.; Funding Acquisition, L.Đ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation, Republic of Serbia through Grant Agreements with University of Belgrade—Faculty of Pharmacy No 451-03-136/2025-03/200161 and No 451-03-137/2025-03/200161 as well as with Faculty of Technology Novi Sad, University of Novi Sad No 451-03-136/2025-03/200134 and No 451-03-137/2025-03/200134.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Jelena Čizmić, Biljana Milošević, and Gabrijela Davidović, undergraduate students at the University of Belgrade—Faculty of Pharmacy at the time of the study, for their kind assistance in the preparation of the investigated formulations.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| IPM | Isopropyl myristate |
| %T | Transmittance |
| 1Φ | Single-phase system |
| 2Φ | Two-phase system |
| 3Φ | Three-phase system |
| cmc | Critical micellar concentration |
| FFM | Film-forming microemulsion |
| FFMw | Weight of the film-forming microemulsion with essential oil |
| Fw | Weight of the dried film |
| HLB | Hydrophilic-lipophilic-balance |
| Km | Surfactant-to-cosurfactant mass ratio |
| MC | Microemulsion concentrate |
| MCT | Medium-chain triglycerides |
| Md | Mass of the separated medium |
| Mw | Molecular weight |
| PCS | Photon correlation spectroscopy |
| PdI | Polydispersity index |
| S (% w/w) | Weight percentage of the swollen film |
| S.D. | Standard deviation |
| Y (% w/w) | Yield |
| Z-ave | Average droplet size |
| η | Absolute viscosity |
| ηapp | Apparent viscosity |
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, I.; Zarafu, I. Contribution of Essential Oils to the Fight against Microbial Biofilms—A Review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of Essential Oils and Their Volatile Aroma Components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Sieniawska, E.; Trifan, A.; Greige-Gerges, H. Special Issue: Isolation and Utilization of Essential Oils: As Antimicrobials and Boosters of Antimicrobial Drug Activity. Processes 2022, 10, 309. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Almasi, L.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and Characterization of Antimicrobial Biopolymer Films Containing Essential Oil-Loaded Microemulsions or Nanoemulsions. Food Hydrocoll. 2021, 117, 106733. [Google Scholar] [CrossRef]
- Lee, S.H.; Chow, P.S.; Yagnik, C.K. Developing Eco-Friendly Skin Care Formulations with Microemulsions of Essential Oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]
- Thakur, D.; Kaur, G.; Puri, A.; Nanda, R. Therapeutic Potential of Essential Oil-Based Microemulsions: Reviewing State-of-the-Art. Curr. Drug Deliv. 2021, 18, 1218–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Y.-F.; Wei, Z.-F.; Jiang, J.-J.; Peng, J.-Q.; He, Q.-T.; Xu, W.-Y.; Liu, H.-M. Microemulsion of Cinnamon Essential Oil Formulated with Tea Polyphenols, Gallic Acid, and Tween 80: Antimicrobial Properties, Stability and Mechanism of Action. Microorganisms 2023, 11, 2. [Google Scholar] [CrossRef]
- Djekic, L. Computer-Aided Formulation Development of Microemulsion Drug Delivery Systems. In Computer-Aided Applications in Pharmaceutical Technology, 2nd ed.; Djuris, J., Ed.; Woodhead Publishing: Sawston, UK, 2024; pp. 41–59. ISBN 978-0-443-18655-4. [Google Scholar]
- Mcclements, D. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Leelapornpisid, P.; Phongpradist, R.; Viernstein, H.; Mueller, M. Development of Microemulsion Delivery System of Essential oil from Zingiber cassumunar Roxb. Rhizome for Improvement of Stability and Anti-Inflammatory Activity. AAPS PharmSciTech 2017, 18, 1332–1342. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery Systems for Cosmetics—From Manufacturing to the Skin of Natural Antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.H.; Chow, P.S.; Macbeath, C. Microemulsion Composed of Combination of Skin Beneficial Oils as Vehicle: Development of Resveratrol-Loaded Microemulsion Based Formulations for Skin Care Applications. Colloids Surf. B Biointerfaces 2020, 194, 111161. [Google Scholar] [CrossRef]
- Manzoor, A.; Asif, M.; Khalid, S.H.; Ullah Khan, I.; Asghar, S. Nanosizing of Lavender, Basil, and Clove Essential Oils into Microemulsions for Enhanced Antioxidant Potential and Antibacterial and Antibiofilm Activities. ACS Omega 2023, 8, 40600–40612. [Google Scholar] [CrossRef]
- Bisht, A.; Hemrajani, C.; Rathore, C.; Dhiman, T.; Rolta, R.; Upadhyay, N.; Nidhi, P.; Gupta, G.; Dua, K.; Chellappan, D.K.; et al. Hydrogel Composite Containing Azelaic Acid and Tea Tree Essential Oil as a Therapeutic Strategy for Propionibacterium and Testosterone-Induced Acne. Drug Deliv. Transl. Res. 2022, 12, 2501–2517. [Google Scholar] [CrossRef]
- Catanzano, O.; Straccia, M.C.; Miro, A.; Ungaro, F.; Romano, I.; Mazzarella, G.; Santagata, G.; Quaglia, F.; Laurienzo, P.; Malinconico, M. Spray-by-Spray in Situ Cross-Linking Alginate Hydrogels Delivering a Tea Tree Oil Microemulsion. Eur. J. Pharm. Sci. 2015, 66, 20–28. [Google Scholar] [CrossRef]
- Gheorghita, D.; Grosu, E.; Robu, A.; Ditu, L.M.; Deleanu, I.M.; Gradisteanu Pircalabioru, G.; Raiciu, A.-D.; Bita, A.-I.; Antoniac, A.; Antoniac, V.I. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials 2022, 15, 6923. [Google Scholar] [CrossRef]
- Leanpolchareanchai, J.; Teeranachaideekul, V. Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals 2023, 16, 999. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.T.; Nguyen, T.T.D.; Vo, D.L.; Than, D.T.M.; Tien, G.P.; Pham, D.T. Microemulsion-Based Topical Hydrogels Containing Lemongrass Leaf Essential Oil (Cymbopogon Citratus (DC.) Stapf) and Mango Seed Kernel Extract (Mangifera Indica Linn) for Acne Treatment: Preparation and in-Vitro Evaluations. PLoS ONE 2024, 19, e0312841. [Google Scholar] [CrossRef]
- Pansang, S.; Maphanta, S.; Tuntijarukorn, P.; Viyoch, J. Skin Irritation Test of a Microemulsion Containing Essential Oil Isolated from Ocimum Basilicum. ScienceAsia 2010, 36, 355–358. [Google Scholar] [CrossRef]
- Prommaban, A.; Chaiyana, W. Microemulsion of Essential Oils from Citrus Peels and Leaves with Anti-Aging, Whitening, and Irritation Reducing Capacity. J. Drug Deliv. Sci. Technol. 2022, 69, 103188. [Google Scholar] [CrossRef]
- Sieniawska, E.; Wota, M.; Mroczek, T. Microemulsions of Essential Oils—An Improvement of Solubility or Something More? Facta Univ. Ser. Phys. Chem. Technol. 2018, 16, 47. [Google Scholar]
- Sutthisawatkul, P.; Piyanaetitham, P.; Chareonviriyaphap, T.; Leepasert, T.; Taengphan, W.; Karpkird, T. Microemulsion Containing Guava Leaves Essential Oil: Enhanced Anti-Inflammatory, Anti-Oxidation, Anti-Tyrosinase Activities and Skin Permeation. J. Drug Deliv. Sci. Technol. 2024, 95, 105536. [Google Scholar] [CrossRef]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 2017, 18, 3097–3104. [Google Scholar] [CrossRef]
- Sakr, F.M.; Gado, A.M.; Mohammed, H.R.; Adam, A.N.I. Preparation and Evaluation of a Multimodal Minoxidil Microemulsion versus Minoxidil Alone in the Treatment of Androgenic Alopecia of Mixed Etiology: A Pilot Study. Drug Des. Devel. Ther. 2013, 7, 413–423. [Google Scholar] [CrossRef]
- Khalid, S.; Kok-Khiang, P. Identification of Phases of Various Oil, Surfactant/Co-Surfactants and Water System by Ternary Phase Diagram. Acta Pol. Pharm. 2014, 71, 301–309. [Google Scholar]
- Chen, J.; Ma, X.; Yao, G.; Zhang, W.; Zhao, Y. Microemulsion-Based Anthocyanin Systems: Effect of Surfactants, Cosurfactants, and Its Stability. Int. J. Food Prop. 2018, 21, 1152–1165. [Google Scholar] [CrossRef]
- Gull, A.; Ahmed, S.; Ahmad, F.J.; Nagaich, U.; Chandra, A. Hydrogel Thickened Microemulsion; a Local Cargo for the Co- Delivery of Cinnamaldehyde and Berberine to Treat Acne Vulgaris. J. Drug Deliv. Sci. Technol. 2020, 58, 101835. [Google Scholar] [CrossRef]
- Blanco, G.E.O.D.; De Souza, C.W.O.; Bernardo, M.P.; Zenke, M.; Mattoso, L.H.C.; Moreira, F.K.V. Antimicrobially Active Gelatin/[Mg-Al-CO3]-LDH Composite Films Based on Clove Essential Oil for Skin Wound Healing. Mater. Today Commun. 2021, 27, 102169. [Google Scholar] [CrossRef]
- Hota, S.S.; Nandi, S.; Mallick, S. Effect of Surfactant on Hydration and Stress-Strain of Hypromellose Film Formation. Rev. Cuba. Farm. 2022, 55, e786. [Google Scholar]
- Milinković, S.; Djekić, L. Hypromellose-Based Films and Film-Forming Systems for Topical Application: Current Status and Perspective in Drug Delivery. Arch. Pharm. 2024, 74, 709–734. [Google Scholar] [CrossRef]
- European Pharmacopeia, 11th ed.; Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM), Council of Europe: Strasbourg, France, 2022.
- Sheskey, P.J.; Cook, W.G.; Cable, C.G. Handbook of Pharmaceutical Excipients, 9th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2020. [Google Scholar]
- Tundisi, L.L.; Mostaço, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl Methylcellulose: Physicochemical Properties and Ocular Drug Delivery Formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A Traditional Pharmaceutical Excipient with Modern Applications in Oral and Oromucosal Drug Delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Saingam, W.; Chankana, N.; Madaka, F.; Sueree, L.; Homchuam, S. Formulation Development of Topical Film Formimg Spray from Piper nigrum L. Thai J. Pharm. Sci. 2018, 42, 219–222. [Google Scholar]
- Zúñiga, R.N.; Skurtys, O.; Osorio, F.; Aguilera, J.M.; Pedreschi, F. Physical Properties of Emulsion-Based Hydroxypropyl Methylcellulose Films: Effect of Their Microstructure. Carbohydr. Polym. 2012, 90, 1147–1158. [Google Scholar] [CrossRef]
- Djekic, L.; Cirkovic, V.; Heleta, M.; Krajisnik, D.; Primorac, M. Water-Dilutable Biocompatible Microemulsion Systems: Design and Characterisation. Tenside Surfactants Deterg. 2013, 50, 409–413. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Ohgita, T.; Demizu, Y.; Saito, H.; Izutsu, K.; Sakai-Kato, K. Current Status and Challenges of Analytical Methods for Evaluation of Size and Surface Modification of Nanoparticle-Based Drug Formulations. AAPS PharmSciTech 2022, 23, 150. [Google Scholar] [CrossRef]
- Djekic, L.; Primorac, M. The Influence of Cosurfactants and Oils on the Formation of Pharmaceutical Microemulsions Based on PEG-8 Caprylic/Capric Glycerides. Int. J. Pharm. 2008, 352, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kathe, K.; Kathpalia, H. Film Forming Systems for Topical and Transdermal Drug Delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin Films as an Emerging Platform for Drug Delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Shimamura, T.; Tairabune, T.; Kogo, T.; Ueda, H.; Numajiri, S.; Kobayashi, D.; Morimoto, Y. Investigation of the Release Test Method for the Topical Application of Pharmaceutical Preparations: Release Test of Cataplasm Including Nonsteroidal Anti-Inflammatory Drugs Using Artificial Sweat. Chem. Pharm. Bull. 2004, 52, 167–171. [Google Scholar] [CrossRef]
- Djekic, L.; Jankovic, J.; Čalija, B.; Primorac, M. Development of Semisolid Self-Microemulsifying Drug Delivery Systems (SMEDDSs) Filled in Hard Capsules for Oral Delivery of Aciclovir. Int. J. Pharm. 2017, 528, 372–380. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of Poorly Water-Soluble Drugs for Oral Administration: Physicochemical and Physiological Issues and the Lipid Formulation Classification System. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of Medium-Chain Fatty Acids: A Concise Review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- PubChem/Isopropyl Myristate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isopropyl-Myristate (accessed on 24 February 2025).
- Musakhanian, J.; Osborne, D.W.; Rodier, J.-D. Skin Penetration and Permeation Properties of Transcutol® in Complex Formulations. AAPS PharmSciTech 2024, 25, 201. [Google Scholar] [CrossRef]
- Kolliphor® RH 40 Technical Information. Available online: https://pharma.basf.com/technicalinformation/30555082/kolliphor-rh-40 (accessed on 24 February 2025).
- Alexandridis, P.; Ivanova, R.; Lindman, B. Effect of Glycols on the Self-Assembly of Amphiphilic Block Copolymers in Water. 2. Glycol Location in the Microstructure. Langmuir 2000, 16, 3676–3689. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J.H. Formulation of Lipid-Based Delivery Systems for Oral Administration: Materials, Methods and Strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Janković, J.; Djekic, L.; Dobričić, V.; Primorac, M. Evaluation of Critical Formulation Parameters in Design and Differentiation of Self-Microemulsifying Drug Delivery Systems (SMEDDSs) for Oral Delivery of Aciclovir. Int. J. Pharm. 2016, 497, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Primorac, M. Microemulsions and Nanoemulsions as Carriers for Delivery of NSAIDs. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs: Formulation Challenges and Potential Benefits; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–94. ISBN 978-0-12-804017-1. [Google Scholar]
- Spectrum/MCT. Available online: https://www.spectrumchemical.com/medium-chain-triglycerides-nf-m4200 (accessed on 24 February 2025).
- HallstarBeauty IPM. Available online: https://www.hallstarbeauty.com/product/hallstar-ipm-nf/ (accessed on 24 February 2025).
- Spohner, M. Study of Dielectric Properties of Mineral Oils and Natural Oils and Methyl Esters of Natural Oils. In Proceedings of the 2017 IEEE 19th International Conference on Dielectric Liquids (ICDL), Manchester, UK, 25–29 June 2017. [Google Scholar]
- PubChem/Cinnamon Oil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cinnamon%20Oil#section=Decomposition (accessed on 24 February 2025).
- ChemicalBook/Thyme Oil. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB6739355.htm (accessed on 24 February 2025).
- ChemicalBook/Tea Tree Oil. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3125672.htm (accessed on 24 February 2025).
- Bao, K.; Lavrov, A.; Nilsen, H.M. Numerical Modeling of Non-Newtonian Fluid Flow in Fractures and Porous Media. Comput. Geosci. 2017, 21, 1313–1324. [Google Scholar] [CrossRef]
- Mondal, S.; Pyne, P.; Patra, A.; Mitra, R.K.; Ghosh, S. Effect of Surfactant Tail Length on the Hydroxypropyl Cellulose-Mediated Premicellar Aggregation of Sodium n-Alkyl Sulfate Surfactants. Langmuir 2021, 37, 6168–6177. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Huang, W.; Wan, H.; Huang, H. Development of Shear Thickening Material. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012024. [Google Scholar] [CrossRef]
- Punitha, S.; Uvarani, R.; Panneerselvam, A. Effect of pH in Aqueous (Hydroxy Propyl Methyl Cellulose) Polymer Solution. Results Mater. 2020, 7, 100120. [Google Scholar] [CrossRef]
- Buraczewska, I.; Lodén, M. Treatment of Surfactant-Damaged Skin in Humans with Creams of Different pH Values. Pharmacology 2005, 73, 1–7. [Google Scholar] [CrossRef]
- Umar, A.K.; Butarbutar, M.; Sriwidodo, S.; Wathoni, N. Film-Forming Sprays for Topical Drug Delivery. Drug Des. Devel. Ther. 2020, 14, 2909–2925. [Google Scholar] [CrossRef]
- ChemicalBook/Cinnamon Oil. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB9437582.htm (accessed on 24 February 2025).
- Ozdemir, O.; Karakashev, S.I.; Nguyen, A.V.; Miller, J.D. Adsorption and Surface Tension Analysis of Concentrated Alkali Halide Brine Solutions. Miner. Eng. 2009, 22, 263–271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).