Abstract
The increasing presence of persistent pollutants in industrial wastewater underscores the shortcomings of conventional treatment methods, prompting the adoption of advanced oxidation processes (AOPs) for sustainable water remediation. This review examines the development of AOPs, focusing on their ability to produce hydroxyl radicals and reactive oxygen species (ROS) to mineralize complex pollutants. Homogeneous systems such as Fenton’s reagent show high degradation efficiency. However, challenges like pH sensitivity, catalyst recovery issues, sludge generation, and energy-intensive operations limit their scalability. Heterogeneous catalysts, such as TiO2-based photocatalysts and Fe3O4 composites, offer improved pH adaptability, visible-light activation, and recyclability. Emerging innovations like ultraviolet light emitting diode (UV-LED)-driven systems, plasma-assisted oxidation, and artificial intelligence (AI)-enhanced hybrid reactors demonstrate progress in energy efficiency and process optimization. Nevertheless, key challenges remain, including secondary byproduct formation, mass transfer constraints, and economic feasibility for large-scale applications. Integrating AOPs with membrane filtration or biological treatments enhances treatment synergy, while advances in materials science and computational modeling refine catalyst design and reaction mechanisms. Addressing barriers in energy use, catalyst durability, and practical adaptability requires multidisciplinary collaboration. This review highlights AOPs as pivotal solutions for water security amid growing environmental pollution, urging targeted research to bridge gaps between laboratory success and real-world implementation.
1. Introduction
The escalating discharge of complex pollutants from industrial activities poses a severe threat to both environmental and human health. Toxic substances such as dyes, phenols, heavy metals, and persistent organic compounds not only contaminate natural water bodies but also disrupt aquatic ecosystems, reduce biodiversity, and compromise the safety of drinking water sources [1]. The emerging complexity of industrial wastewater has necessitated paradigm shifts in water treatment technologies, particularly as conventional methods struggle to address recalcitrant organic pollutants. AOPs have emerged as transformative solutions through their capacity to generate reactive oxygen species (ROS), primarily hydroxyl radicals (-OH), which exhibit unparalleled oxidation potential (E° = 2.8 V) for mineralizing persistent contaminants [2,3]. These processes leverage cutting-edge physicochemical mechanisms to degrade contaminants ranging from endocrine disruptors to a wide range of water pollutants, achieving degradation efficiencies exceeding 100% for a wide range of compounds under optimized conditions [4,5]. However, the field faces critical challenges in balancing treatment efficacy with operational sustainability, driving innovative approaches in catalyst engineering, energy optimization, and hybrid system design. Contemporary wastewater matrices present unprecedented challenges with their complex mixtures of pharmaceuticals, microplastics, and industrial byproducts. Traditional biological treatment methods fail against these non-biodegradable compounds, while physical separation techniques merely transfer pollutants between phases rather than eliminating them. AOPs address this through radical-mediated chain reactions that sequentially break down complex molecules into simpler inorganic compounds [6]. The evolution from first-generation homogeneous systems like Fenton’s reagent to advanced heterogeneous catalysis reflects the progression of this field toward sustainable water treatment. Recent breakthroughs in materials science have yielded novel photocatalysts like TiO2 and hybrid composites that achieve high degradation efficiency under visible light, significantly improving upon conventional UV-dependent systems [7].
Despite their demonstrated efficacy, AOPs are not without challenges. Many of these processes require high energy input, making large-scale implementation economically and environmentally challenging. Additionally, the formation of intermediate byproducts during oxidation can sometimes lead to new toxicological concerns, necessitating further treatment. Scalability remains another major issue, as the efficiency of AOPs observed in laboratory-scale experiments often diminishes in real-world applications due to complex wastewater matrices, mass transfer limitations, and catalyst deactivation [8,9]. Recent innovations in reactor design, hybrid AOPs, and the integration of AI and ML models have shown promising advancements in addressing these challenges. AI and ML techniques have been increasingly used to optimize AOP conditions, predict degradation efficiencies, and improve process control, enhancing the overall sustainability and cost-effectiveness of these treatment methods [10]. Given the urgent need for sustainable and efficient wastewater treatment technologies, AOPs continue to evolve with novel catalytic materials, improved reactor configurations, and synergistic combinations with other treatment methods, such as biological processes and membrane filtration [11,12,13]. The development of energy-efficient AOPs, such as UV-LED-driven oxidation and plasma-based systems, further holds great potential for enhancing the feasibility of these technologies in real-world applications [14]. Addressing key challenges such as energy consumption, catalyst stability, and secondary pollutant formation will be crucial in ensuring that AOPs transition from research settings to full-scale implementation [15].
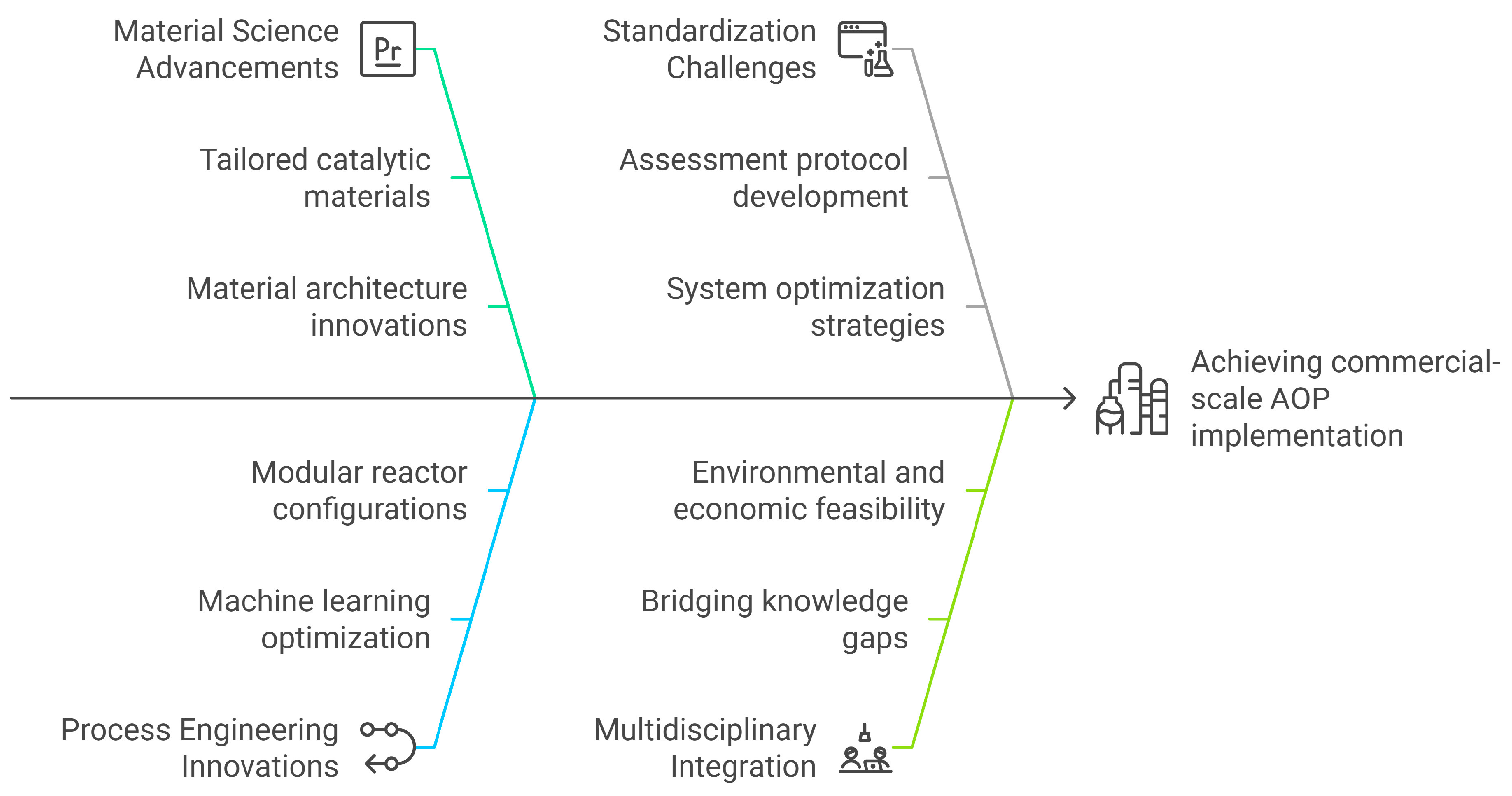
This review systematically examines the transition of AOPs from laboratory-scale developments to industrial-scale viability, elucidating the synergistic interplay between advancements in material science and innovations in process engineering. This work distinctly offers a comprehensive synthesis of recent breakthroughs, including tailored catalytic material architectures, machine learning-assisted process optimization, and modular hybrid reactor configurations, and proposes a strategic framework for scaling these technologies to commercial applications, as illustrated in Figure 1. Furthermore, it critically addresses methodological challenges in establishing standardized assessment protocols and system optimization strategies while presenting actionable solutions to bridge existing knowledge gaps in the field. By integrating multidisciplinary perspectives, this analysis offers a forward-looking paradigm for enhancing the environmental and economic feasibility of AOPs in real-world implementations.
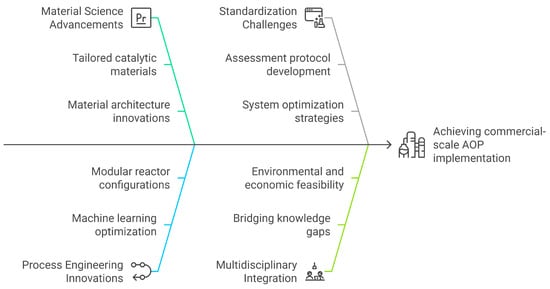
Figure 1.
Overview of the multidisciplinary pathways toward commercial-scale AOP implementation.
2. Contemporary Types of AOPs
AOPs are a suite of chemical treatment technologies designed to degrade persistent organic pollutants, pharmaceuticals, and industrial contaminants in water and wastewater. These processes rely on the generation of ROS, particularly hydroxyl radicals, which non-selectively oxidize complex pollutants into simpler, less harmful compounds. Their versatility in generating ROS through diverse mechanisms such as ozonation, electrochemical reactions, and photocatalysis makes them indispensable for addressing modern environmental challenges. This section explores six prominent AOPs, evaluating their mechanisms, efficiencies, and applications.
2.1. Fenton Oxidation
The Fenton process employs a mixture of ferrous iron (Fe2+) and hydrogen peroxide (H2O2) to generate •OH under acidic conditions (pH 2–4). The reaction follows a cyclic mechanism where Fe3+ is reduced back to Fe2+, sustaining radical production. The advantages of Fenton oxidation include its simplicity, rapid reaction rates, and the use of relatively inexpensive and readily available reagents. Homogeneous Fenton systems are effective for degrading aromatics and pesticides but face drawbacks like iron sludge generation and narrow pH operability. Heterogeneous Fenton variants, using solid catalysts (e.g., iron oxides), mitigate sludge issues and broaden the pH range. Fenton oxidation can have a wide range of applications, but reagent costs and H2O2 instability are still a limitation [16].
2.2. Ozone Oxidation
Ozone (O3) oxidation leverages the potent oxidizing capacity of ozone, either through direct reaction with pollutants or indirect decomposition into hydroxyl radicals. In aqueous solutions, ozone reacts selectively with electron-rich organic compounds, such as phenols and dyes. Under alkaline conditions, O3 decomposes into •OH, enhancing its oxidative scope to include non-polar contaminants. Ozonation is widely employed in municipal water treatment and textile effluent remediation due to its efficacy in disinfection and micropollutant removal. However, limitations include high energy consumption for ozone generation, low solubility in water, and potential formation of toxic byproducts like bromate in bromide-containing waters [17].
2.3. Electrochemical Oxidation
Electrochemical oxidation (EO) utilizes an applied electric current to degrade pollutants at anode surfaces or via electrogenerated oxidants. Direct EO involves electron transfer from organic molecules to the anode, while indirect EO employs mediators like hypochlorite or peroxydisulfate. The choice of electrode material (e.g., boron-doped diamond, platinum) critically influences efficiency and selectivity. EO is prized for its operational flexibility, scalability, and suitability for high-salinity wastewater, such as landfill leachate. Challenges include electrode fouling, high capital costs, and energy demands. Recent advances focus on hybrid systems combining EO with photocatalysis to enhance performance [18].
2.4. Photolysis or Photocatalysis
Photolysis involves pollutant degradation via UV light, which cleaves chemical bonds directly or activates oxidants like H2O2. In photocatalysis, semiconductors (e.g., TiO2, ZnO) absorb UV or visible light, generating electron–hole pairs that drive redox reactions. TiO2-based systems are prominent for mineralizing organic pollutants, with modifications like doping extending their activity to visible light. Solar-driven photocatalysis offers sustainability benefits but suffers from low quantum efficiency and light-scattering losses in turbid waters. Applications span dye degradation, volatile organic compound (VOC) abatement, and self-cleaning surfaces. However, limitations include the need for UV light sources, which can be energy-intensive, and the potential for reduced efficiency in turbid or colored waters that absorb or scatter UV light. In photocatalysis, the recovery and reuse of the catalyst can be challenging, and the process may require long reaction times for the complete degradation of certain pollutants [19].
2.5. Radiation
High-energy radiation, such as gamma rays or electron beams, ionizes water molecules to produce •OH, hydrated electrons (e−aq), and H• radicals. These species collectively degrade pollutants via oxidation, reduction, and radical chain reactions. Radiation AOPs excel in treating mixed contaminants, including endocrine disruptors and perfluorinated compounds, without chemical additives. However, the limitations are significant, including high operational costs due to the need for specialized equipment and safety measures to protect against radiation exposure. Additionally, the public perception and regulatory requirements associated with the use of radiation can pose challenges to the implementation of this technology [20].
2.6. Sonolysis
Sonolysis employs ultrasonic waves (20 kHz–1 MHz) to induce cavitation bubbles in water. Bubble collapse generates localized temperatures (>5000 K) and pressures, thermolyzing water into •OH and H•. The process is particularly effective for hydrophobic and volatile pollutants, which accumulate at the bubble interface. The advantages of sonolysis include its ability to operate under ambient conditions and without the need for chemical additives. However, the process has limitations, such as low energy efficiency, leading to high operational costs and limited scalability for large-scale applications. The effectiveness of sonolysis can also be influenced by factors such as ultrasonic frequency and intensity, as well as the physicochemical properties of the wastewater [21].
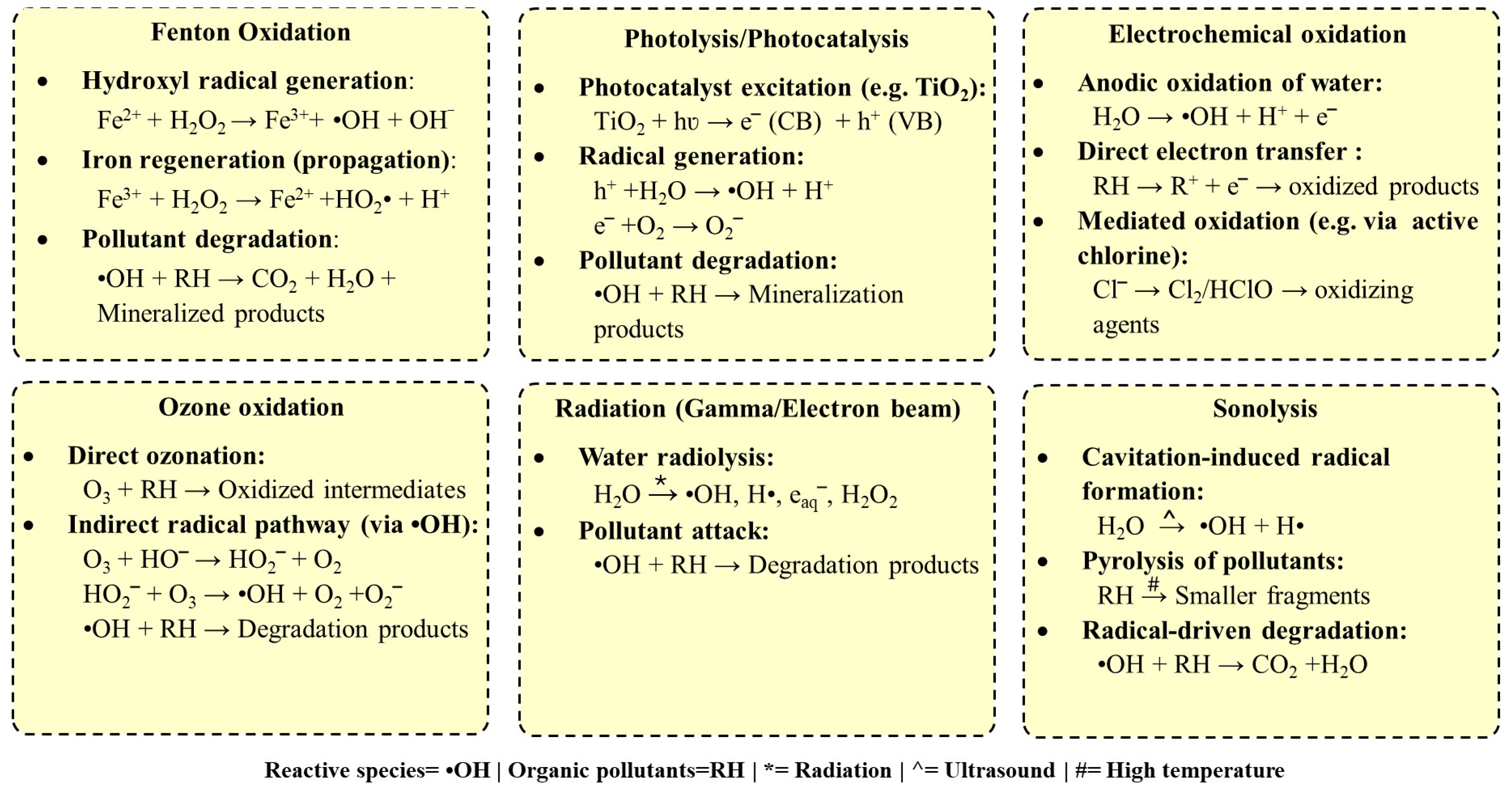
While each type of AOP offers unique advantages in degrading pollutants, they also present specific limitations that must be considered when selecting the appropriate treatment method for a particular application. Figure 2 illustrates the generalized reactions of various AOPs. A comparative analysis of these AOPs, highlighting their unique advantages and limitations, is presented in Table 1, providing insights into their applicability for different treatment scenarios.
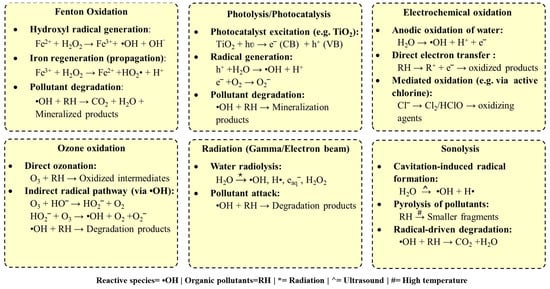
Figure 2.
Reactions involved in various types of advanced oxidation processes.

Table 1.
Unique advantages and limitations of various AOPs.
3. General Limitations in AOPs
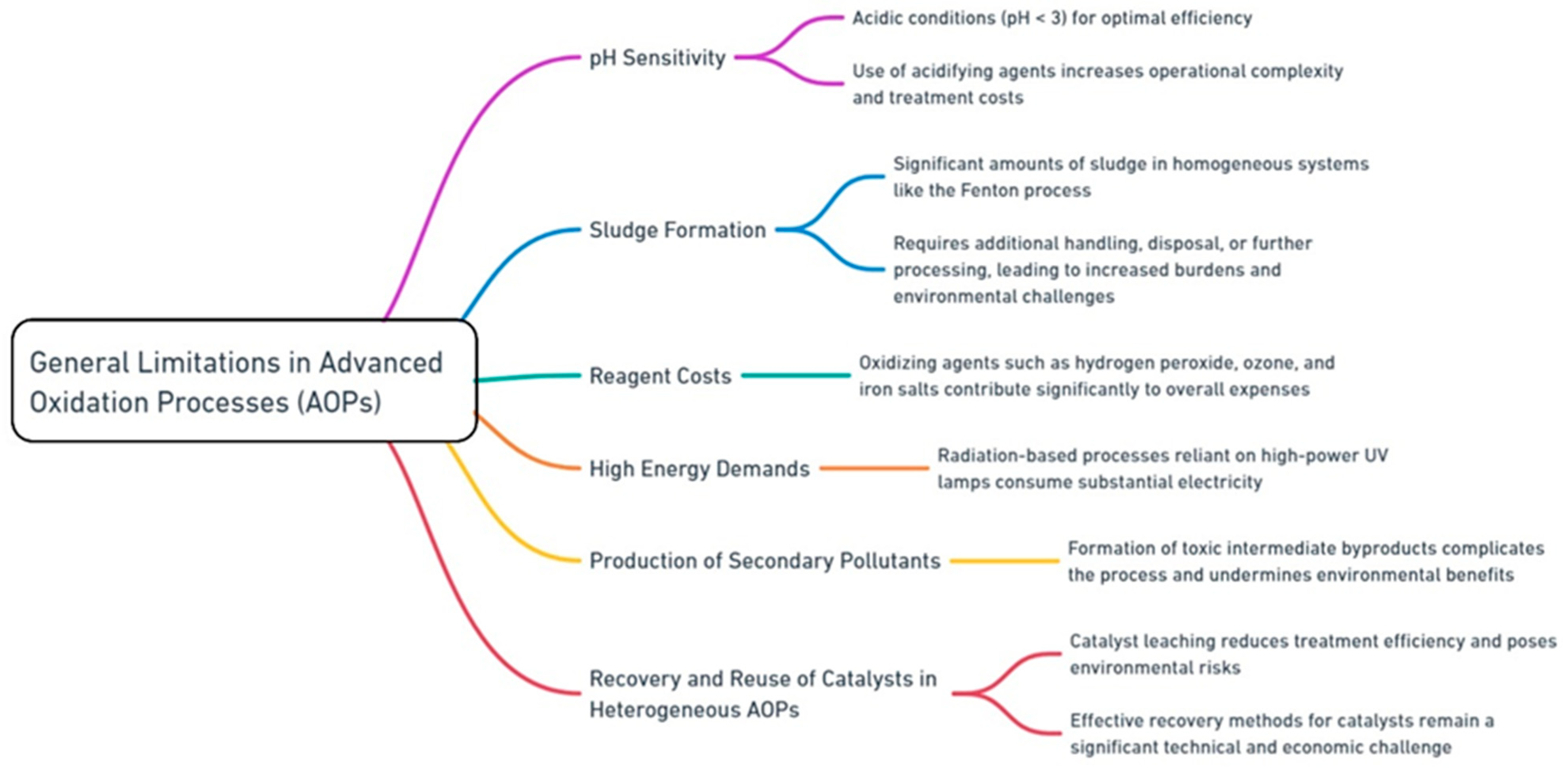
AOPs have gained recognition for their ability to degrade persistent and complex pollutants in wastewater. However, their widespread application is hindered by several inherent challenges. One of the most significant limitations is the pH sensitivity of many AOPs. Processes such as the Fenton and photo-Fenton reactions exhibit optimal efficiency under highly acidic conditions, typically at a pH below 3. This requires the use of acidifying agents, which not only increase operational complexity but also elevate the overall treatment costs [22]. Additionally, sludge formation is a major concern in several AOPs, particularly homogeneous systems like the Fenton process. The substantial amounts of sludge generated during these treatments require additional handling, disposal, or further processing, leading to increased operational burdens and environmental challenges [23]. Reagent costs also represent a critical limitation for many AOPs. Oxidizing agents such as hydrogen peroxide, ozone, and iron salts are essential for driving these processes, yet their recurring consumption contributes significantly to the overall expenses [24]. The high energy demands of certain AOPs further compound this issue. Radiation-based processes such as UV/H2O2 and UV/O3 rely on high-power UV lamps, consuming substantial amounts of electricity, which limits their economic feasibility for large-scale applications [25]. Furthermore, some AOPs inadvertently produce secondary pollutants, including toxic intermediate byproducts, during the degradation of complex organic compounds. The formation of such byproducts necessitates additional treatment steps, complicating the process and undermining its environmental benefits [26]. Another major bottleneck lies in the recovery and reuse of catalysts in heterogeneous AOPs. Catalysts such as TiO2 and Fe3O4 often leach into treated water, reducing the overall treatment efficiency and posing environmental risks. Effective recovery methods for these catalysts remain a significant technical and economic challenge, further limiting the widespread adoption of AOPs [27]. Figure 3 highlights the general limitations associated with conventional AOPs.
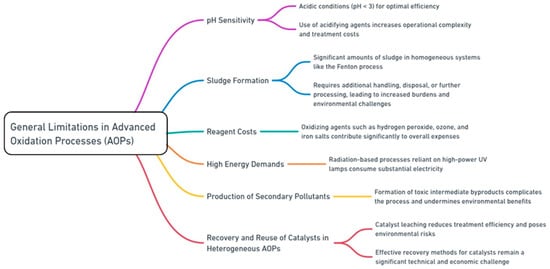
Figure 3.
General limitations and challenges with conventional AOPs.
4. Categoric Limitations of Specific AOPs
While the general limitations of AOPs are significant, specific challenges associated with individual categories of AOPs also hinder their efficiency and scalability. Solar-driven AOPs, such as the solar photo-Fenton process, are recognized for their cost-effectiveness, as they utilize sunlight as a renewable energy source. However, the process still requires acidic conditions to maintain Fe2+ conversion, leading to challenges associated with acid usage and subsequent sludge formation. These limitations reduce its viability for large-scale or real-world applications despite its high efficacy in degrading pollutants like dyes and pharmaceuticals [28]. Similarly, solar photocatalysis, which uses semiconductors like TiO2 to generate reactive species, is constrained by the material’s wide band gap. TiO2 is active primarily under UV-A light, which constitutes only a small portion of the solar spectrum, thereby limiting its energy efficiency. Efforts to expand light absorption to the visible spectrum have yet to achieve widespread implementation [29].
Radiation-driven AOPs, including UV/H2O2 and UV/O3 processes, are highly effective at generating hydroxyl radicals for pollutant degradation. However, these systems are characterized by high energy consumption due to their dependence on UV lamps, which also have limited penetration depth in water. Additionally, these processes can generate secondary byproducts, such as bromates and chlorinated compounds, posing environmental and regulatory concerns [30,31].
Catalytic AOPs also face unique challenges. Traditional Fenton processes, while efficient, are limited by their narrow operational pH range and significant sludge generation. Heterogeneous Fenton processes mitigate some of these issues by employing solid catalysts, but problems such as catalyst stability, leaching, and reduced activity over time remain unresolved [32]. Similarly, catalytic ozonation improves ozone utilization and hydroxyl radical generation but relies on the development of durable and cost-effective catalysts. The lack of such sustainable materials limits the scalability of catalytic ozonation, especially for industrial applications [33].
Emerging AOPs, such as sonolysis and plasma-based processes, have introduced novel mechanisms for pollutant degradation. Sonolysis generates hydroxyl radicals through acoustic cavitation, but the high energy demands of ultrasonic systems have made its large-scale application economically unfeasible [34]. Plasma-based AOPs produce reactive species through electrical discharges, showing promise in treating persistent contaminants like per- and polyfluoroalkyl substances (PFASs). However, these processes face significant limitations in reactor design and energy efficiency, which hinder their widespread adoption [35].
Combined AOPs and integrated systems aim to leverage the synergistic effects of multiple treatment methods. Examples include peroxone (O3/H2O2), sono-photocatalysis, and photo-Fenton coupling, all of which have demonstrated improved degradation efficiencies. However, the optimization of these hybrid systems for diverse wastewater streams is a complex challenge, requiring precise control of operational parameters. Similarly, the integration of AOPs with biological processes has shown potential for cost-effective treatment, yet the compatibility between these two systems often depends on the effective pretreatment of wastewater, which can be resource-intensive [36]. Table 2 outlines the specific limitations associated with different categories of AOPs, highlighting key challenges that affect their efficiency and scalability.

Table 2.
Categoric limitations of AOPs.
5. Advancements Addressing Current Limitations
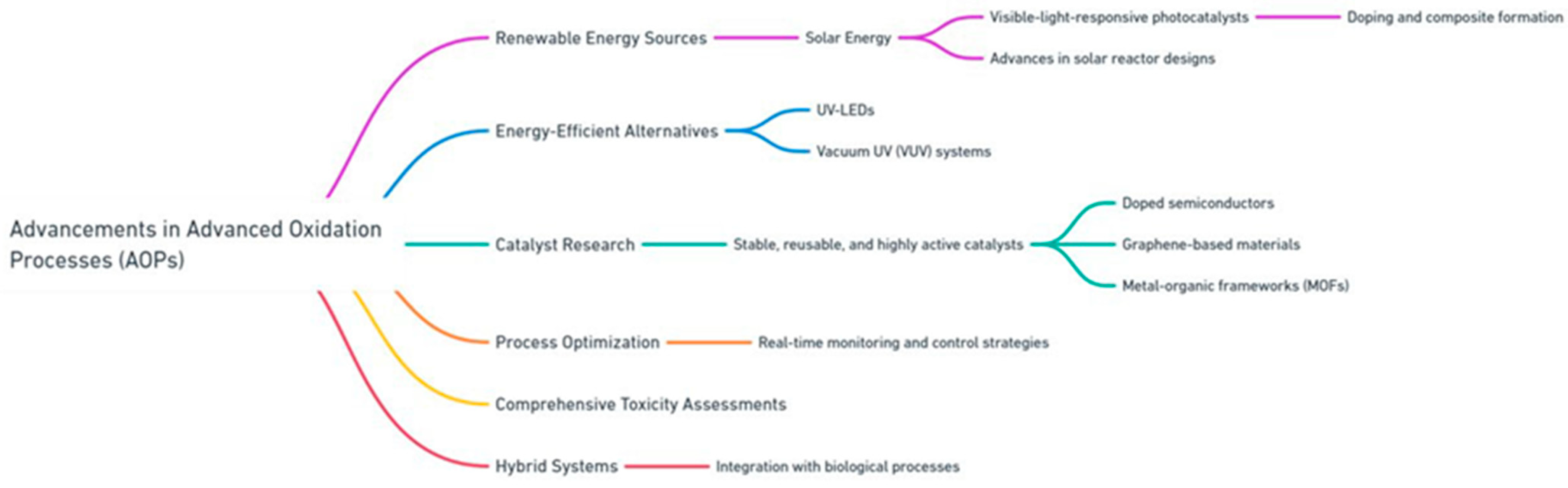
To overcome these challenges, recent advancements in AOP technology have focused on improving cost efficiency, environmental sustainability, and scalability. One promising approach is the utilization of renewable energy sources, particularly solar energy, to drive AOPs. Research into visible-light-responsive photocatalysts, achieved through doping and composite formation, has significantly enhanced the ability of materials like TiO2 to absorb a broader spectrum of sunlight. Advances in solar reactor designs, including both concentrating and non-concentrating systems, have further optimized light distribution and reaction efficiency, making solar-driven AOPs more practical for real-world applications [36,37].
Efforts to address the energy demands of radiation-based AOPs have led to the development of energy-efficient alternatives, such as UV-LEDs and vacuum UV (VUV) systems. These technologies provide higher photon energy while reducing power consumption, offering a more sustainable solution for processes like UV/H2O2 and UV/O3 [38]. Catalyst research has also made significant strides. The development of stable, reusable, and highly active catalysts, including doped semiconductors, graphene-based materials, and metal–organic frameworks (MOFs), has addressed issues of leaching and reduced activity, particularly in heterogeneous systems [8]. Process optimization has emerged as another critical area of innovation. Advances in real-time monitoring and control strategies have enabled the refinement of operational parameters, reducing both energy and reagent consumption while enhancing degradation efficiencies. Furthermore, comprehensive toxicity assessments are increasingly being incorporated into AOP development to ensure the environmental safety of treated water and mitigate the risks associated with secondary pollutants [39]. Hybrid systems have also shown great promise in addressing the limitations of standalone AOPs. By integrating AOPs with biological processes, researchers have demonstrated significant improvements in the treatment of industrial effluents, such as textile wastewater and pulp and paper mill effluents. These integrated systems leverage AOPs as a pretreatment step to break down recalcitrant compounds, enhancing the biodegradability of wastewater for subsequent biological treatment. This approach has proven to be both cost-effective and environmentally sustainable, providing a pathway for large-scale application [40]. Advancements in catalyst innovation, reactor optimization, process integration, and energy efficiency have significantly enhanced the effectiveness and sustainability of advanced oxidation processes. These developments have the potential to address longstanding challenges, paving the way for wastewater treatment technologies that are both practical and environmentally responsible. Figure 4 highlights the key advancements in AOP technologies addressing current limitations.
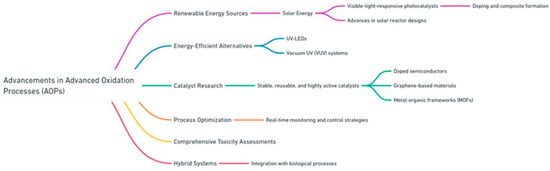
Figure 4.
Key advancements in AOP techniques.
6. Advancement in Reactor Design for AOPs
The efficiency of AOPs hinges on reactor design, which governs mass transfer, light utilization, electrode stability, energy consumption, and scalability. Recent advancements in reactor engineering focus on optimizing hydrodynamic conditions, enhancing interfacial reactions, and integrating hybrid processes. Key challenges include minimizing energy demands, preventing electrode or catalyst deactivation, and scaling laboratory prototypes to industrial applications [41]. Among various AOPs, EO and photocatalytic processes have gained prominence due to their adaptability, eco-friendly nature, and compatibility with renewable energy sources. The following sub-section critically examines innovations in reactor design for these two technologies, leveraging insights from recent studies on EO and photocatalytic systems.
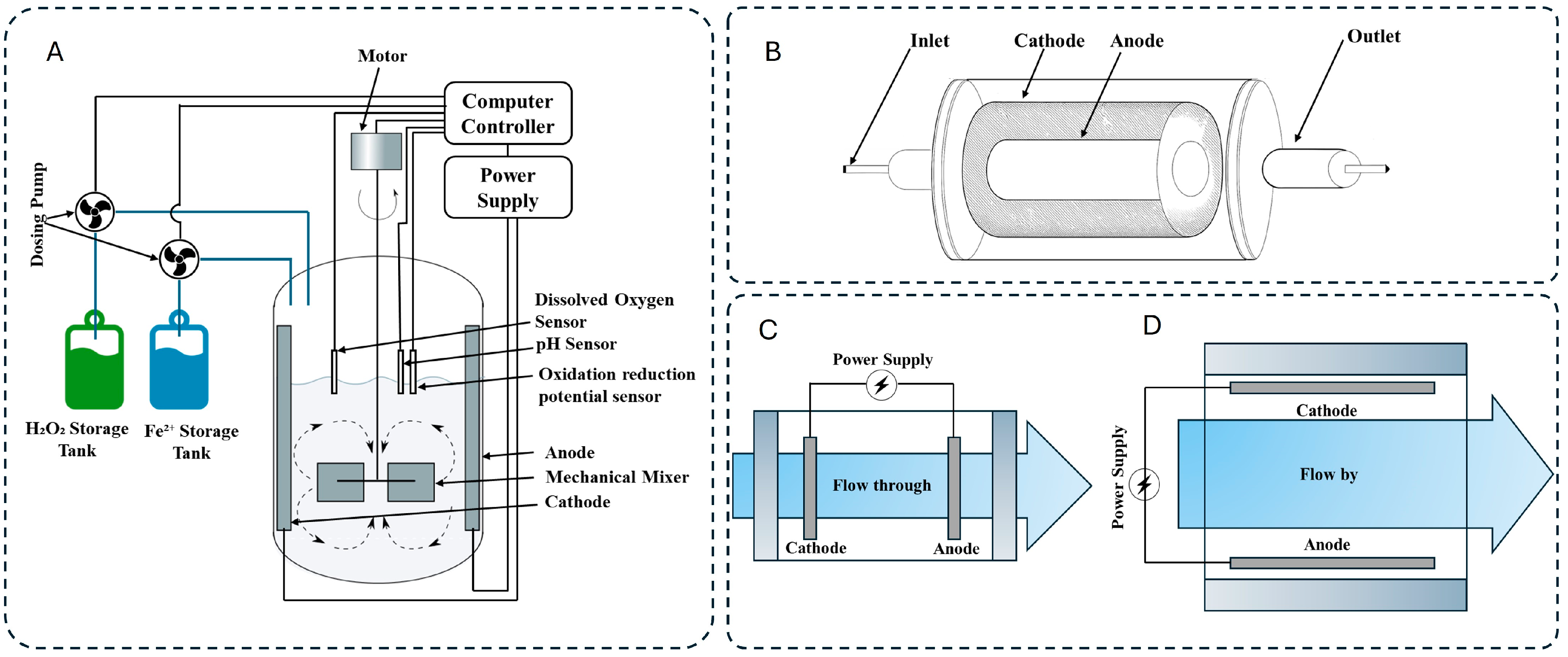
6.1. Configurations and Innovations in Electro-Oxidation Reactors
EO is a versatile wastewater treatment method that relies on electrochemical reactions occurring at the anode and cathode surfaces. Pollutants are degraded either directly, through adsorbed hydroxyl radicals (•OH), or indirectly, via in situ generated oxidants such as hypochlorous acid (HClO), ozone (O3), or hydrogen peroxide (H2O2). The design of EO reactors plays a crucial role in determining current efficiency, mass transfer rates, and electrode longevity [42]. The following sections explore key advancements in EO reactor configurations, highlighting their innovations and limitations.
6.1.1. Plate-Frame (Filter-Press) Reactors
Plate-frame reactors, commonly used in industrial electrolysis, consist of parallel electrodes separated by narrow channels. Their modular design supports scalability and integration with complementary processes like electrocoagulation and electro-Fenton. Several key advancements have enhanced their efficiency. The incorporation of turbulence promoters, such as mesh distributors or rotating electrodes, has disrupted laminar flow, reducing boundary layer thickness and improving mass transfer rates. Wachter et al. demonstrated that these promoters tripled bisphenol S degradation, achieving a 98% removal efficiency. Additionally, the adoption of 3D electrodes, where granular activated carbon (GAC) or metal particles are placed between the anode and cathode, has substantially increased reactive surface area [43]. Ni et al. observed that 3D reactors doubled chemical oxygen demand (COD) removal in Rhodamine B wastewater compared to traditional 2D configurations [44]. Material innovations have further improved EO reactor performance. Boron-doped diamond (BDD) anodes, despite their high cost, have emerged as a superior option due to their exceptional •OH generation and long-term stability. Zhou et al. achieved 87.5% COD removal in landfill leachate using BDD electrodes, which significantly outperformed conventional Ti/RuO2 anodes [45]. However, plate-frame reactors are not without limitations. The presence of dead zones and uneven current distribution can hinder performance. Although rotating electrodes mitigate these issues, they introduce mechanical complexity and higher energy costs, limiting their widespread application.
6.1.2. Tubular Plug-Flow Reactors
Tubular reactors, which utilize concentric or helical electrodes, offer uniform fluid dynamics and efficient heat dissipation. Several innovative strategies have improved their effectiveness. The use of mesh electrodes has been shown to enhance turbulence and reduce back-reflux by 50%, leading to an 80% COD removal rate for Evans blue dye, as demonstrated by Li et al. [46]. Computational fluid dynamics (CFD) simulations have also played a crucial role in optimizing flow patterns and electrode spacing. Wang et al. designed a vertical-flow tubular reactor using CFD, achieving a 200% increase in mass transfer coefficients compared to conventional designs [47]. Another significant innovation in tubular reactors is the development of spiral flow designs. Guo and You introduced a reactor that increased turbulent intensity by 500–700%, effectively reducing energy consumption for methylene blue degradation by half [48]. Despite these advancements, tubular reactors face challenges in scalability due to the uneven current distribution inherent in cylindrical geometries. Consequently, their industrial application remains largely confined to niche areas such as textile wastewater treatment.
6.1.3. Flow-Through vs. Flow-By Modes
Fluid dynamics play a crucial role in EO reactor performance, with two primary operational modes: flow-by and flow-through. In flow-by mode, the fluid moves parallel to the electrodes, but thick boundary layers (~100 µm) can hinder mass transfer. Increasing flow rates to turbulent regimes (e.g., 300 L/h) has been shown to enhance total organic carbon (TOC) removal for dipyrone by 92.5% [49]. However, this approach has limitations, as excessive flow rates can lead to increased energy consumption without proportional gains in treatment efficiency. Flow-through mode, where the fluid moves perpendicularly through porous electrodes, has emerged as a superior alternative. This approach significantly reduces boundary layers to less than 1 µm, improving mass transfer and degradation efficiency. Chen et al. achieved a remarkable 97% tetracycline degradation with an energy consumption of just 0.18 kWh per gram of dissolved organic carbon (DOC), outperforming flow-by systems. Despite these benefits, flow-through reactors require durable, porous electrodes that resist fouling. Recent studies on macroporous TiO2/SnO2-Sb anodes indicate that pore size plays a crucial role in reaction kinetics, with 20 µm pores proving optimal for organic degradation [50,51].
The general configurations of all electro-oxidation reactors explained above are illustrated in Figure 5.
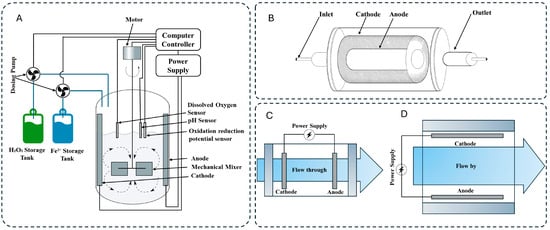
Figure 5.
Configuration of (A) plate-frame, (B) tubular plug-flow, (C) flow-through, (D) flow-by EO reactors.
6.2. Configurations and Innovations in Photocatalytic Reactors
Photocatalytic AOPs utilize semiconductor materials such as TiO2, ZnO, and graphitic carbon nitride (g-C3N4), which, when activated by UV or visible light, generate electron–hole pairs. These pairs subsequently produce reactive oxygen species (·OH and O2− radicals) that facilitate pollutant degradation. While laboratory-scale studies have demonstrated remarkable efficiency, scaling up photocatalytic reactors for industrial applications presents significant challenges. The following sections critically examine various reactor geometries and operational parameters that influence the transition from laboratory experiments to large-scale implementation.
6.2.1. Cylindrical Reactors
Cylindrical reactors, one of the most commonly explored configurations, are typically irradiated using centrally placed lamps, ensuring efficient radial flow distribution. These reactors, when equipped with recirculation mechanisms, demonstrate high degradation efficiencies. For example, Ochoa-Gutierrez et al. achieved 99% methylene blue degradation in a solar-driven reactor using TiO2-coated substrates. However, in slurry-based systems, catalyst opacity significantly hampers light penetration, limiting photocatalytic activity. To mitigate this, immobilized catalysts are often employed, though they reduce the available active surface area. Microreactors with extremely small volumes (e.g., 1 × 10−4 L) have also been investigated, demonstrating rapid benzoylecgonine degradation with complete removal in just two minutes [52]. Despite their efficiency, the scalability of such microreactors remains a major hurdle. Figure 6A presents a schematic illustration of the cylindrical photo reactor.
6.2.2. Flat-Plate Reactors
Rectangular reactors, particularly flat-plate designs, offer flexible lamp positioning, allowing for top, side, or submerged illumination. Their modular nature facilitates scalability while maintaining high photocatalytic performance. Vaiano et al. demonstrated the successful immobilization of ZnO on polystyrene pellets within a 0.2 L reactor, achieving 100% caffeine removal under UV-LED irradiation [53,54]. Similarly, Bahmani et al. designed a larger 5 L flat-plate system utilizing UiO-66(Ti)-Fe3O4-WO3, which led to 91.8% ammonia degradation [55]. However, as reactor size increases, non-uniform light distribution reduces quantum efficiency, necessitating CFD-guided lamp arrangements to optimize light exposure. Figure 6B presents a schematic illustration of the flat-plate photo reactor.
6.2.3. Innovative Photocatalyst Modifications
Advancements in photocatalyst modification have significantly improved light absorption and charge separation. Doping with elements such as nitrogen, sulfur, and tungsten extends the absorption spectrum into the visible range. For instance, Mesgari et al. reported 59.3% methyl orange degradation under visible light using N-doped TiO2 [56]. Additionally, heterojunctions like TiO2/WO3 and g-C3N4/Ag enhance charge separation, increasing photocatalytic efficiency [57,58]. Z-scheme heterostructures, further improve charge transfer, reducing recombination losses [59]. However, these modifications introduce challenges such as dopant leaching, particularly in metal-based systems like photo-Fenton catalysts, where Fe2+ leaching can affect long-term stability [60].
6.2.4. Hybrid Photoreactors
Hybrid photoreactors integrating AOPs with biological treatments offer efficient solutions for degrading recalcitrant contaminants in wastewater. However, the lack of predictive models has hindered optimization. Esfahani et al. developed a first integrated AOP-bio model using Simulink® 9.11, reformulating mass balances to link process variables. Testing various configurations, including photo-Fenton followed by biological treatment and vice versa, the study monitored TOC and substrate concentrations, enabling systematic wastewater treatment design [11]. Additionally, solar-driven AOPs, coupled with energy harvesting (EH) technologies, offer sustainable water purification for off-grid areas and disaster relief. Huo et al. reviewed solar-induced hybrid EHs utilizing piezo-, tribo-, pyro-, and thermo-assisted photocatalysis to enhance AOP efficiency under fluctuating conditions. These systems simultaneously harvest solar, mechanical, and thermal energy, enabling self-powered water treatment. Their implementation could revolutionize decentralized water purification by providing energy-efficient and resilient solutions [61].
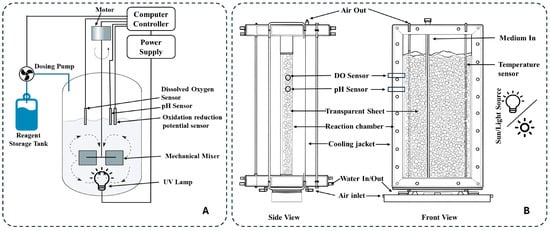
Figure 6.
Configuration of (A) cylindrical photo reactor and (B) flat-plate photo reactor.
Figure 6.
Configuration of (A) cylindrical photo reactor and (B) flat-plate photo reactor.
7. Recent Studies on Innovative AOPs for Sustainable Wastewater Treatment
Recent advancements in AOPs have demonstrated significant progress in addressing complex wastewater challenges across industries. By leveraging novel catalytic systems, hybrid methods, and optimized integration strategies, researchers are advancing the scalability and adaptability of AOPs for diverse effluents. This section highlights key innovations tailored for textile, pharmaceutical, and emerging contaminant-laden wastewater, alongside pioneering large-scale applications that bridge laboratory research with industrial implementation.
In the context of textile wastewater, sulfate radical-based AOPs (SR-AOPs) have emerged as a promising solution for degrading recalcitrant organic pollutants. Nidheesh et al. demonstrated the efficacy of SR-AOPs in treating dyes and toxic chemicals through the activation of sulfate radicals (SO4•−) via metal catalysis, UV, or ultrasound. Under optimized conditions, the process achieved 96.6% COD removal, showcasing adaptability across a wide pH range and multiple activation pathways [62]. Similarly, Kore et al. combined hydrodynamic cavitation (HC), UV irradiation, and peroxymonosulfate (PMS) to degrade the Reactive Blue 222 dye. This synergistic approach generated SO4•− radicals, achieving 85.66% TOC reduction within 180 min, even in the presence of salts and detergents, highlighting its robustness for complex textile effluents [63].
For pharmaceutical wastewater, hybrid AOP systems have shown remarkable efficiency in removing persistent drug residues. Husein et al. integrated green-synthesized copper nano-adsorbents with AOPs to eliminate non-steroidal anti-inflammatory drugs (NSAIDs) such as diclofenac, naproxen, and ibuprofen. The sequential adsorption and radical-driven degradation achieved removal efficiencies of 91.4%, 86.9%, and 74.4%, respectively [64]. Zhou et al. further advanced catalytic efficiency by developing a single-atom Mn catalyst (SA-Mn-NSC) to activate peroxymonosulfate (PMS) for enrofloxacin degradation, achieving over 99% pollutant removal in just 10 min due to the catalyst’s stability and reactive species generation [65]. Hassani et al. employed UVA-LED-assisted CoFe2O4-reduced graphene oxide (CFO-rGO) nanocomposites to activate peroxymonosulfate, achieving > 99% bisphenol A (BPA) degradation within 30 min through light–catalyst synergy, underscoring rapid mineralization capabilities [66].
The treatment of emerging contaminants such as PFASs, endocrine disruptors, and antibiotics has also seen breakthroughs. Shi et al. combined electrocoagulation (EC) and EO using zinc/stainless-steel and Ti4O7 electrodes, achieving >95% PFAS removal within 60 min of EO treatment [67]. Maher et al. enhanced estrogenic compound (E1, E2) degradation by sequencing EC and EO, which improved hydroxyl radical production and achieved over 50% efficiency compared to standalone EO [68]. Aseman-Bashiz and Sayyaf integrated ozone, peroxydisulfate, and EC to treat ofloxacin, achieving 98% removal under optimized conditions through synergistic radical generation, demonstrating efficacy against antibiotic residues [69].
Large-scale applications further illustrate the industrial viability of AOPs. The TERI Advanced Oxidation Technology (TADOX®), developed by The Energy and Resources Institute, integrates nanotechnology-based AOPs into pre- and post-biological treatment stages. Case studies across pesticide, pharmaceutical, and tannery sectors demonstrated improved biodegradability, toxicity reduction, and >95% COD/BOD removal, enabling water reuse and regulatory compliance [70]. For olive mill wastewater (OMW), Mehdaoui et al. applied a sono-heterogeneous Fenton process with a magnetic catalyst [GTA-(PDA-g-DAC)@Fe3O4], achieving 95.66% COD reduction and 92.75% amino compound removal under optimized conditions [71]. De Carluccio et al. combined electro-Fenton pre-treatment with a moving bed biofilm reactor (MBBR) for OMW co-treatment, achieving 85% phenol removal and 41% COD reduction, offering a cost-effective strategy for small-scale mills. Giraldo-Loaiza et al. designed a three-stage system (ion exchange, bicarbonate-activated peroxide AOP, and biological treatment) for Colombian textile effluents, achieving 95.88% TOC removal through optimized H2O2 and NaHCO3 concentrations, emphasizing the synergy between AOPs and biological processes [72].
These studies collectively underscore the versatility of AOPs in tackling diverse wastewater streams, from targeted contaminant degradation to integrated, large-scale systems. By combining advanced oxidation with complementary technologies, researchers are addressing scalability challenges and paving the way for industrial adoption. Continued innovation in catalyst design, hybrid processes, and system optimization will further enhance the feasibility of AOPs as sustainable, high-efficiency solutions for global water remediation needs.
8. Scope and Types of AI/ML Algorithm Used in AOPs
The application of AI in AOPs for water remediation has emerged as a transformative approach to tackle the growing challenges of water pollution, particularly from persistent organic pollutants and emerging contaminants. AI-driven models can analyze vast datasets generated from experimental studies, allowing for the identification of intricate relationships between input variables (such as current density, pH, and reaction time) and output variables (like pollutant removal efficiency and energy consumption). This capability is particularly crucial in AOPs, where the processes are often nonlinear and influenced by multiple interacting factors. By employing AI techniques, researchers can develop predictive models that not only forecast the performance of AOPs under various conditions but also optimize operational parameters to achieve maximum pollutant degradation while minimizing energy usage [73]. Moreover, the use of AI in AOPs facilitates the implementation of real-time monitoring and control systems, which can dynamically adjust operational parameters based on feedback from the treatment process. This adaptability is essential for addressing the variability in wastewater characteristics and ensuring consistent treatment performance. Additionally, AI can assist in the design of hybrid systems that combine multiple AOPs, further enhancing the overall treatment efficiency [73,74,75]. The subsection below provides a detailed overview of the AI techniques employed in wastewater treatment, focusing on artificial neural networks (ANNs), Support Vector Regression (SVR), Adaptive Neuro-Fuzzy Inference System (ANFIS), metaheuristic algorithms, Extreme Gradient Boosting (XGBoost), and other relevant methods.
8.1. XGBoost
XGBoost is a robust and scalable machine learning model based on gradient boosting, designed for supervised learning tasks such as classification and regression. It builds predictive models by iteratively optimizing an ensemble of weak learners, typically decision trees, to minimize a specified loss function. XGBoost stands out for its speed, accuracy, and ability to handle large-scale datasets efficiently [76]. A distinctive feature of XGBoost is its regularization mechanism, which penalizes model complexity to prevent overfitting. This approach enhances generalization and ensures robust performance on unseen data. The model employs a second-order Taylor approximation to calculate loss and gradient values, enabling faster and more precise updates during training. Additionally, XGBoost supports parallelized tree construction and efficient memory utilization, making it highly suitable for large datasets and real-time applications [77]. In water remediation, XGBoost has shown exceptional utility in optimizing AOPs, accurately modeling the complex relationships between operational parameters and treatment efficacy. This capability allows researchers to fine-tune AOP conditions for maximal degradation of contaminants, minimizing experimental effort and cost. XGBoost’s ability to handle missing data, non-linear relationships, and high-dimensional inputs makes it a versatile tool for solving complex environmental challenges. It also offers extensive hyperparameter tuning options, allowing users to balance trade-offs between bias and variance, further enhancing its adaptability. These strengths position XGBoost as an invaluable tool in AOP-based water remediation, offering precision, scalability, and computational efficiency for optimizing treatment processes [78].
8.2. ANNs
ANNs are computational systems inspired by the structure and functioning of the human brain. Comprising interconnected nodes or neurons organized in layers, these models are capable of learning complex patterns and relationships within data. Their flexibility and capacity to handle non-linear relationships make ANNs highly suitable for applications in wastewater treatment, where such complexities are common [79]. By leveraging their adaptability, ANNs have been utilized in various processes to model and optimize treatment systems effectively. In the domain of wastewater treatment, especially AOPs, ANNs have demonstrated significant potential, particularly in modeling and optimizing electrochemical processes like electrooxidation, electrocoagulation, and electro-Fenton treatments. By analyzing input parameters such as current density, pH, and electrolysis time, ANNs can accurately predict pollutant removal efficiencies, enabling enhanced process control and efficiency [80]. Moreover, these networks have proven effective in predicting system performance with remarkable precision, often achieving correlation coefficients exceeding 0.99. Such accuracy underscores their value as predictive tools for understanding and managing wastewater treatment systems. A noteworthy feature of ANN-based approaches in wastewater research is their reliance on experimental datasets, often with sample sizes of fewer than 150. This highlights the growing reliance on data-driven methodologies to overcome the limitations of traditional modeling techniques [81]. However, while ANNs excel at capturing complex data relationships, their application is not without challenges. One significant issue is overfitting, particularly when the models are trained on small datasets. Overfitting reduces the ability of the system to generalize, making it less effective when applied to unseen data. To address these limitations, techniques such as regularization, cross-validation, and meticulous hyperparameter tuning are critical for developing robust and reliable ANN models [82].
8.3. SVR
SVR, a regression-specific variant of Support Vector Machines (SVMs), is designed to predict continuous outputs by finding a function that deviates minimally from the true target values within a specified margin while maintaining maximum flatness. This unique approach ensures that the model remains robust and generalizable, making SVR particularly effective in applications requiring precision and reliability [83]. In the field of wastewater treatment, SVR has emerged as a powerful tool for modeling and prediction. Comparative studies have often highlighted its superior performance over ANNs, especially in terms of prediction accuracy. For instance, SVR has demonstrated exceptional capability in modeling complex processes, such as the degradation of organic pollutants, capturing intricate relationships between variables with remarkable precision. Its ability to handle high-dimensional data and non-linear relationships makes it an excellent choice for addressing the multifaceted challenges of wastewater treatment systems. A significant advantage of SVR lies in its reduced susceptibility to overfitting compared to ANNs, particularly when working with limited datasets. This characteristic is critical in wastewater treatment applications, where data availability can often be a constraint. Additionally, its capacity to manage high-dimensional inputs further enhances its applicability in scenarios where multiple variables influence treatment efficacy. These strengths position SVR as a reliable and efficient tool for optimizing wastewater treatment processes, offering both accuracy and robustness across a range of challenging conditions [84].
8.4. ANFIS
ANFIS blends the adaptive learning capabilities of ANNs with the reasoning and interpretability of fuzzy logic. This hybrid approach enables the modeling of complex systems characterized by uncertainty and imprecision, making it particularly suitable for applications in wastewater treatment, where variability in data is often significant. In AOP-based wastewater treatment, ANFIS has been effectively utilized to model and control various electrochemical processes. By integrating fuzzy logic, ANFIS captures intricate relationships between input variables and treatment outcomes, providing valuable insights that are often difficult to achieve with conventional modeling techniques. Moreover, ANFIS excels in optimizing operational parameters, such as current density and treatment duration, ensuring that wastewater treatment processes achieve maximum efficiency and effectiveness. This dual functionality of modeling and optimization highlights its versatility as a tool for tackling the complexities of wastewater management. One of the key strengths of ANFIS lies in its ability to handle uncertainty and variability in datasets, a common challenge in wastewater treatment applications. By incorporating fuzzy logic into its framework, ANFIS offers a flexible and robust approach to process optimization, accommodating the inherent imprecision in real-world data. This capability not only enhances the reliability of predictions but also supports more informed decision-making, reinforcing its value in designing and managing advanced wastewater treatment systems [85].
8.5. Metaheuristic Algorithms
Metaheuristic algorithms are powerful optimization techniques designed to explore and exploit complex problem spaces in search of optimal solutions. These algorithms, such as Genetic Algorithms (GAs) and Particle Swarm Optimization (PSO), have gained significant traction in wastewater treatment applications due to their versatility and effectiveness in addressing challenging optimization tasks. By guiding the search process intelligently, metaheuristic algorithms provide robust solutions to problems characterized by numerous variables and conflicting objectives [86,87]. In wastewater treatment, metaheuristic algorithms have been widely applied to optimize operational conditions for various AOPs. For example, they are employed to identify the optimal current density, reaction time, and other parameters required for maximum pollutant removal. This optimization ensures that treatment processes are not only effective but also resource-efficient. Furthermore, metaheuristic algorithms play a critical role in tuning the hyperparameters of artificial intelligence models used in wastewater treatment, enhancing the accuracy and reliability of these models in predicting treatment outcomes and system performance. One of the most significant advantages of metaheuristic algorithms is their ability to handle multi-objective optimization problems. Wastewater treatment often involves balancing multiple performance criteria, such as maximizing pollutant removal efficiency while minimizing energy consumption. Metaheuristic algorithms excel in such scenarios by enabling simultaneous optimization of these conflicting objectives. Their adaptability, combined with their capacity to navigate complex solution spaces, makes metaheuristic algorithms indispensable tools for improving the design, operation, and sustainability of advanced wastewater treatment systems [88].
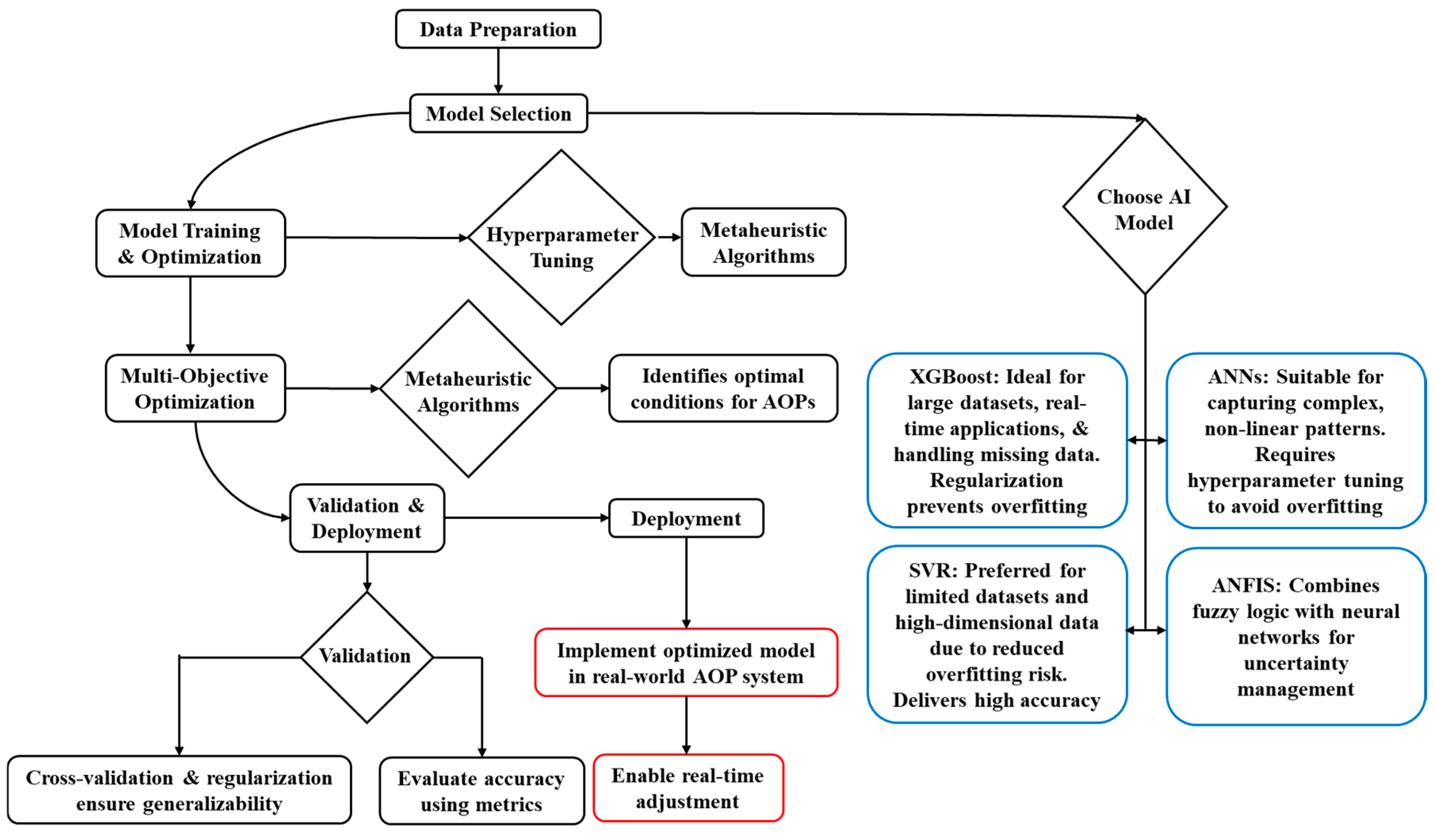
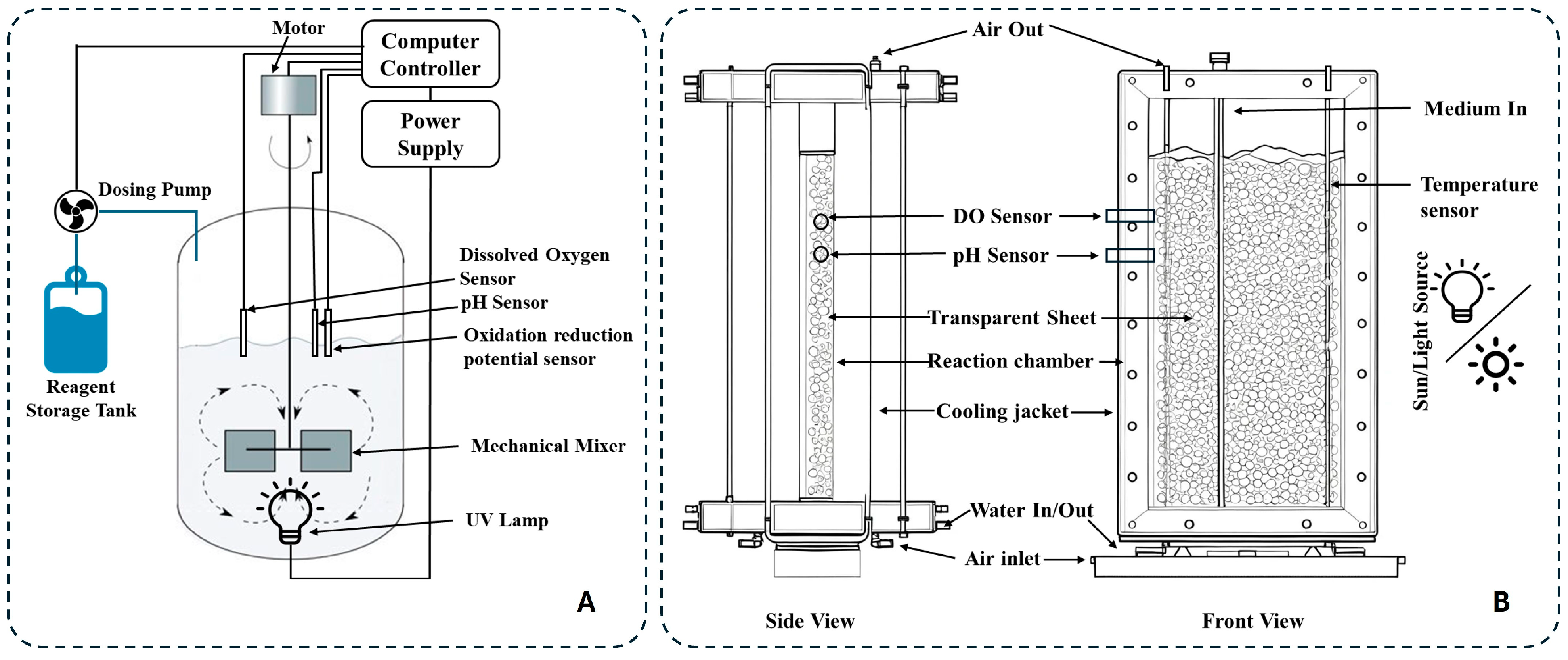
The selection of AI/ML methods for optimizing AOPs depends on specific operational and data-driven requirements, such as the scale and quality of datasets, the complexity of relationships between variables (e.g., non-linear or high-dimensional interactions), and the need to mitigate overfitting risks in limited data scenarios. Additionally, the ability to balance multi-objective trade-offs, such as maximizing pollutant removal efficiency while minimizing energy consumption, influences the choice of models, with metaheuristic algorithms often complementing AI techniques to address these challenges. Figure 7 illustrates the integrated AI-driven AOP optimization workflow, aligning methodological choices with practical implementation stages for enhanced treatment efficacy.
Figure 7.
AI-driven AOP optimization workflows.
8.6. Recent Advancements in AOP Using AI/ML
The integration of advanced oxidation processes (AOPs) with machine learning (ML) has seen substantial progress, revolutionizing the optimization and efficiency of wastewater treatment technologies. A notable study employed the XGBoost model to predict reaction rate constants (k) for the EO process targeting organic pollutant degradation. Using a dataset of 315 data points encapsulating pollutant characteristics and reaction conditions, the model achieved impressive performance with an R2 of 0.84 and an RMSE of 0.79 during external validation. Predictions closely matched experimental results, maintaining relative errors below 5%. These findings underscored the transformative potential of ML-based approaches in optimizing the EO process, enhancing its efficacy for water purification [89]. Further advancements explored catalytic advanced oxidation processes (CAOPs) such as catalytic wet air oxidation (CWAO) and electrochemical oxidation (EO) using artificial neural networks (ANNs) for performance modeling. ANNs demonstrated superior accuracy in analyzing operational parameters like temperature, pH, and catalyst dosage compared to traditional methods such as multiple linear regression (MLR) and RSM. With R2 values reaching 0.9984 and minimal mean square errors (MSEs), these models delivered highly precise predictions. The integration of Shapley Additive Explanations (SHAP) provided additional interpretability by revealing the influence of specific features on model predictions. This development emphasized the potential of interpretable AI techniques in advancing AOP optimization for environmental applications [90]. A focus on UV/H2O2 and ozonation processes for pharmaceutical contaminant degradation demonstrated AI’s efficacy in optimizing treatment performance. ANNs were utilized to predict the impact of variables such as pH, initial concentrations, and hydrogen peroxide dosage on degradation efficiency. The UV/H2O2 process achieved 88% and 80% degradation efficiencies for sulfamethoxazole (SMX) and metronidazole (MNZ), respectively, while ozonation achieved 98% and 79%. ANN models showed high predictive accuracy, with R2 values up to 0.98, reaffirming the capability of AI in enhancing AOP performance for pharmaceutical wastewater treatment [91]. In the development of novel AOP technologies, biochar-catalyzed peroxymonosulfate (BC-PMS) emerged as a promising method for degrading emerging contaminants (ECs). XGBoost models were applied to predict reaction rate constants (k) based on contaminant characteristics, reaction conditions, and biochar properties. The model demonstrated robust predictive accuracy with an R2 of 0.996 for training and 0.841 for testing datasets. A hierarchical ML pipeline facilitated contaminant-oriented optimization strategies, bridging ML model development with practical environmental applications. This advancement highlighted a significant step toward creating effective tools for optimizing AOP efficiency in wastewater treatment [92]. Catalytic wet air oxidation (CWAO), catalytic wet peroxide oxidation (CWPO), and wet electrocatalytic oxidation (WEO) further showcased the potential of ANNs in optimizing TOC removal efficiencies. Compared to RSM, ANN models exhibited superior predictive performance. For instance, in the CWAO process, ANNs achieved an MSE of 6.10, significantly outperforming RSM’s 11.89. Similarly, enhanced TOC and chemical oxygen demand (COD) removal efficiencies were predicted for CWPO and WEO processes, demonstrating the effectiveness of AI in optimizing operational parameters across various AOPs [93]. An integrated AOP approach for dye wastewater treatment combined a solar-triggered AOP reactor with a pilot-scale pre-treatment unit. The system employed ZnO/ZnO-GO NanoMat as a photocatalyst, along with processes like coagulation, flocculation, activated sand filtration, polyacrylonitrile (PAN) fiber filtration, and activated carbon filtration (ACF). ML models, including MLR and ANNs, were used to predict water quality parameters. While MLR provided straightforward predictions, ANNs excelled in capturing intricate relationships, achieving high predictive accuracy (R2adj > 0.9). This combined system effectively remediated textile effluents, achieving a zero-liquid discharge (ZLD) system, demonstrating the sustainability and efficiency of integrating AOPs with ML for wastewater treatment [94]. The advancements mentioned in this section are summarized in Table 3, highlighting the AI/ML methods used and their applications in optimizing AOP systems.

Table 3.
Summary of recent advancements in AOP systems integrated with AI/ML methods.
8.7. Regulatory and Environmental Impact of AI in AOP Systems (Authors View)
The integration of AI into AOPs for wastewater treatment presents transformative potential but raises regulatory and environmental considerations. Regulatory frameworks must adapt to address AI’s role in optimizing chemical dosing, energy use, and real-time pollutant monitoring. Compliance with water quality standards requires transparency in AI decision-making to ensure accountability, particularly when handling hazardous contaminants. Environmental benefits include enhanced treatment efficiency, reduced chemical consumption, and lower energy footprints, minimizing secondary pollution risks. However, AI systems depend on energy-intensive data processing, potentially offsetting sustainability gains if powered by non-renewable sources. Additionally, the disposal of AI-linked electronic waste and risks of algorithmic bias in contaminant detection warrant scrutiny. Policymakers must balance innovation with safeguards, promoting standardized protocols for AI validation and lifecycle assessments to ensure net-positive environmental outcomes. Collaboration among regulators, technologists, and environmental scientists will be key to harmonizing AI-driven AOP advancements with ecological and regulatory integrity.
9. Factors Influencing Scalability of AOP-Based Treatment Techniques for Industrial Upscale
The scalability of AOPs for industrial applications depends on a series of interconnected factors, which include radical production efficiency, reactor design, operational costs, and the transportability of ROS. The following subsection explores these factors, supported by examples and insights from the existing literature.
9.1. Half-Life of Radicals and Transport Considerations
Long-term stability tests for catalysts in AOPs for wastewater treatment are critical to ensure that catalysts remain effective over extended operation periods despite exposure to harsh conditions. These tests evaluate parameters such as activity loss, structural changes, and catalyst deactivation mechanisms (e.g., surface fouling, leaching of active sites, and changes in crystalline phases) over time. Such studies not only help in predicting catalyst lifespan and cost-effectiveness but also provide insights into optimizing reactor design and operational conditions for industrial applications [95]. Most of the AOPs depend on the ROS for wastewater treatment; hence, the half-life of ROS significantly impacts scalability. Short-lived radicals like •OH and SO4•− need to be produced in situ, as their transportation from production sites to application points is impractical due to rapid degradation [96,97]. Conversely, long-lived oxidants like hydrogen peroxide, ozone, and hypochlorous acid (HClO) are more amenable to transport and storage, making them suitable for batch reactor operations where production and application are separated spatially and temporally [98,99]. Table 4 summarizes the half-life, production pathways, and portability considerations of various ROS. The requirement for in situ production of short-lived radicals also presents challenges in reactor design, necessitating systems that integrate production, application, and disposal seamlessly. This limitation drives the need for continuous-flow reactors equipped with advanced catalysts and efficient mixing strategies.

Table 4.
Commonly used ROS used in various AOPs.
9.2. Material Innovations for Radical Delivery
Recent advancements in material science have enabled significant progress in the delivery and immobilization of radicals in AOPs, enhancing their scalability for industrial applications. Hybrid catalytic oxidation systems, particularly those utilizing catalytic ceramic membranes (CCMs), have demonstrated promising potential for synergistic degradation of organic micropollutants (MPs) while improving water filtration. These systems employed metal oxide (MeOx)-functionalized CCMs to immobilize active catalysts within porous structures, ensuring controlled and efficient generation of ROS. Advanced fabrication techniques, such as ultrasonic-assisted impregnation of TiO2 on mesoporous dendritic silica (MDS) or calcination of metal–phenolic coordination polymers to form CuO nanocages, were shown to significantly enhance catalytic activity by increasing surface area, reducing electron–hole recombination, and facilitating efficient pollutant diffusion [100,101]. Mesoporous CuO nanocages, characterized by their high specific surface area and accessible active sites, exhibited exceptional performance in peroxymonosulfate (PMS)-based oxidation, achieving high degradation rates of bisphenol A (BPA) across a wide pH range [101]. Similarly, TiO2/MDS composites displayed remarkable efficiency in activating persulfate for the degradation of micropollutants like carbamazepine by leveraging the proximity of reactants to active sites and minimizing charge carrier recombination [100]. These systems demonstrated resilience to the presence of competing organic constituents and inorganic ions, highlighting their potential for real-world wastewater treatment applications.
Innovative approaches incorporating MOFs further advanced AOPs by addressing challenges related to scalability and catalyst immobilization. Functionalized supports, such as wood aerogels, were utilized to immobilize MOFs like MIL-100(Fe), enabling their application in sulfate radical-based AOPs (SR-AOPs). These composites featured open porous structures that enhanced accessibility to active sites and facilitated the generation of ROS, such as sulfate radicals (SO4•−) and singlet oxygen (¹O2). The MIL-100(Fe)/wood aerogel systems achieved efficient degradation of antibiotics, including tetracycline, in wastewater while maintaining high mineralization rates and catalytic performance across a broad pH range. Furthermore, these systems demonstrated the feasibility of continuous operation in fixed-bed reactors, showcasing their potential for industrial-scale applications in SR-AOPs by overcoming the limitations of traditional powdered MOFs [102,103]. These material innovations underscored the critical role of hybrid systems and advanced supports in addressing scalability challenges, including high synthesis costs, material instability, and operational continuity.
9.3. Conversion Efficiency and Reactor Design
One of the most critical factors influencing the scalability of AOPs is the conversion efficiency of parent materials into ROS. The percentage conversion of these materials into radicals or oxidizers plays a pivotal role in determining the feasibility of scaling up AOPs for industrial applications. The conversion efficiency is typically measured as the percentage of parent materials transformed into radicals or oxidizers, and it varies significantly depending on the type of AOP and the reactor design used. For instance, hydroxyl radicals (•OH) are often generated from H2O2 in both batch and continuous-flow reactors, with conversion efficiencies ranging from 5% to 20%. The conversion efficiency, as reported in the literature, can be optimized using novel catalysts and oxidizers, with some efficiencies reaching up to 100%. Such high efficiencies are often achieved in controlled laboratory environments, but translating these results into industrial settings requires innovative continuous-flow reactor designs that ensure consistent mixing and energy efficiency. Section 6 delves deeper into the reactor and design-related aspects of various AOP-based wastewater treatment techniques. Apart from reactor design, the conversion efficiency is significantly influenced by the initial concentration of the parent material, chemical/physical mechanisms involved, and operational conditions like pH and temperature [15]. For example, the activation of persulfates can be achieved through various methods, including UV radiation, ultrasound, heating, and the use of MOFs or carbon-based materials. Each of these methods has its own set of advantages and limitations, and their effectiveness can vary depending on the specific application. For instance, Ike et al. explored the role of silver, iron, and copper-based activation of persulfates, as well as metal-free activation using nanocarbon materials. They found that waste heat was the most sustainable option for pollutant degradation, highlighting the importance of selecting the appropriate activation method for scalable AOP applications [104].
9.4. Cost and Economic Considerations
The cost of scaling up AOPs is a critical factor that influences their adoption in industrial applications. The total cost of AOPs includes not only the cost of chemicals and energy but also the cost of the design life of the AOP plant [105]. Mousset et al. introduced a laboratory-scale cost comparison of five AOPs using the accumulated oxygen-equivalent chemical-oxidation dose (AOCD) as a standardization parameter. They found that electro-Fenton incurred the lowest cost for the mineralization of phenol, followed by ozonation and Fenton oxidation. However, these cost comparisons did not include the operation and maintenance costs of equipment, which can significantly impact the overall cost of AOPs in industrial applications [23].
From the perspective of future industrial implementation, classical hydroxyl radical-based AOPs are estimated to be two orders of magnitude cheaper than sulfate radical-based AOPs, primarily due to the lower cost of oxidants. In terms of cost efficiency among chemical-based activation methods, the combination of hydrogen peroxide and ozone (H2O2/O3) stands out as the most economical, with an estimated treatment cost of approximately USD 0.01 per liter of wastewater treated, achieving degradation efficiencies higher than 95% for several VOCs. By contrast, ozonation processes can incur operational costs ranging from USD 0.02 to USD 0.06 per liter, while hydrogen peroxide alone remains within a narrower range of USD 0.01 to USD 0.02 per liter [106].
Furthermore, life cycle costing (LCC) and life cycle assessment (LCA) are more comprehensive approaches for estimating the long-term costs of AOPs in industrial applications. These approaches consider the social, environmental, and economic impacts of AOPs, as well as the greenhouse gas emissions associated with their operation. For example, Mahbub and Sharma used the LCC approach to predict the cost of civil and water infrastructures, as well as the equivalent CO2 (eCO2) emissions, for sourcing freshwater from various sources for fish bathing in a large aquaculture industry in Australia. The study highlighted the importance of considering the total energy embodied in producing the AOP operational infrastructure, as well as the continuous operational costs associated with reagent use, sludge management, and electrode surface maintenance [107].
10. Challenges and Approaches for Standardized Evaluation and Optimization of AOPs
One of the critical challenges in evaluating AOPs is the inconsistency in experimental approaches, which stems from the absence of standardized methodologies. Studies often employ different experimental setups, reactor designs, and operating conditions, making direct comparisons across systems inherently difficult. This lack of uniformity undermines efforts to establish reliable benchmarks for AOP performance, impeding progress in identifying optimal designs and operating conditions. Addressing these inconsistencies through standardized protocols could significantly enhance the comparability and reproducibility of AOP studies [95]. The use of electrical energy per order (EEO) as a metric has gained popularity for assessing energy efficiency, but its limitations are evident. While EEO is a convenient tool for preliminary evaluations, it falls short in accounting for the complex interplay of factors that influence reactor performance, such as the specific contaminant, water matrix, and reactor design. As a result, EEO alone is insufficient for guiding reactor design or scale-up. Variations in energy consumption across different AOP systems further highlight the need for more comprehensive evaluation metrics that incorporate these variables. This limitation suggests that EEO should be complemented with additional parameters for a more holistic assessment of energy efficiency [108,109]. Scaling up AOPs from laboratory to pilot or full-scale operations presents yet another significant challenge. Laboratory-scale data often fail to capture the complexities encountered in larger systems, where factors such as fluid dynamics, mixing, mass transfer, and light distribution differ substantially. These scale-dependent variables can markedly affect energy efficiency and overall system performance. To bridge this gap, the adoption of theoretical approaches and predictive modeling has been recommended as a means to extrapolate laboratory findings more reliably to larger-scale operations. These tools hold promise for improving the scalability of AOP technologies while maintaining efficiency [110].
Economic considerations also pose significant challenges to the evaluation of AOP systems. A complete cost analysis involves not only energy consumption but also factors such as chemical costs, maintenance requirements, capital investments, and the management of byproducts. However, the lack of comprehensive cost data in the literature hinders meaningful comparisons of different AOPs. Integrating detailed economic analyses into AOP studies would provide a more accurate picture of their feasibility for large-scale implementation and highlight cost-effective pathways for process optimization [111,112]. We broadly categorize AOPs into ozone-based, radiation-driven, catalytic, and other systems, each with unique energy and cost-efficiency characteristics. Ozone-based and UV-based AOPs are considered among the most energy-efficient systems currently deployed at full scale. In contrast, catalytic AOPs, while promising, rely heavily on the properties of the catalyst, including its stability, reusability, and the optimization of mass transfer. Challenges such as catalyst separation and recovery further underscore the need for a holistic process engineering approach to evaluate their efficiency. Other AOPs, such as electrochemical, ultrasound, and plasma-based systems, generally exhibit lower energy efficiency than ozone- and UV-based systems. Their performance varies significantly depending on reactor design and operating conditions, and the lack of standardized experimental setups makes meaningful comparisons difficult [110].
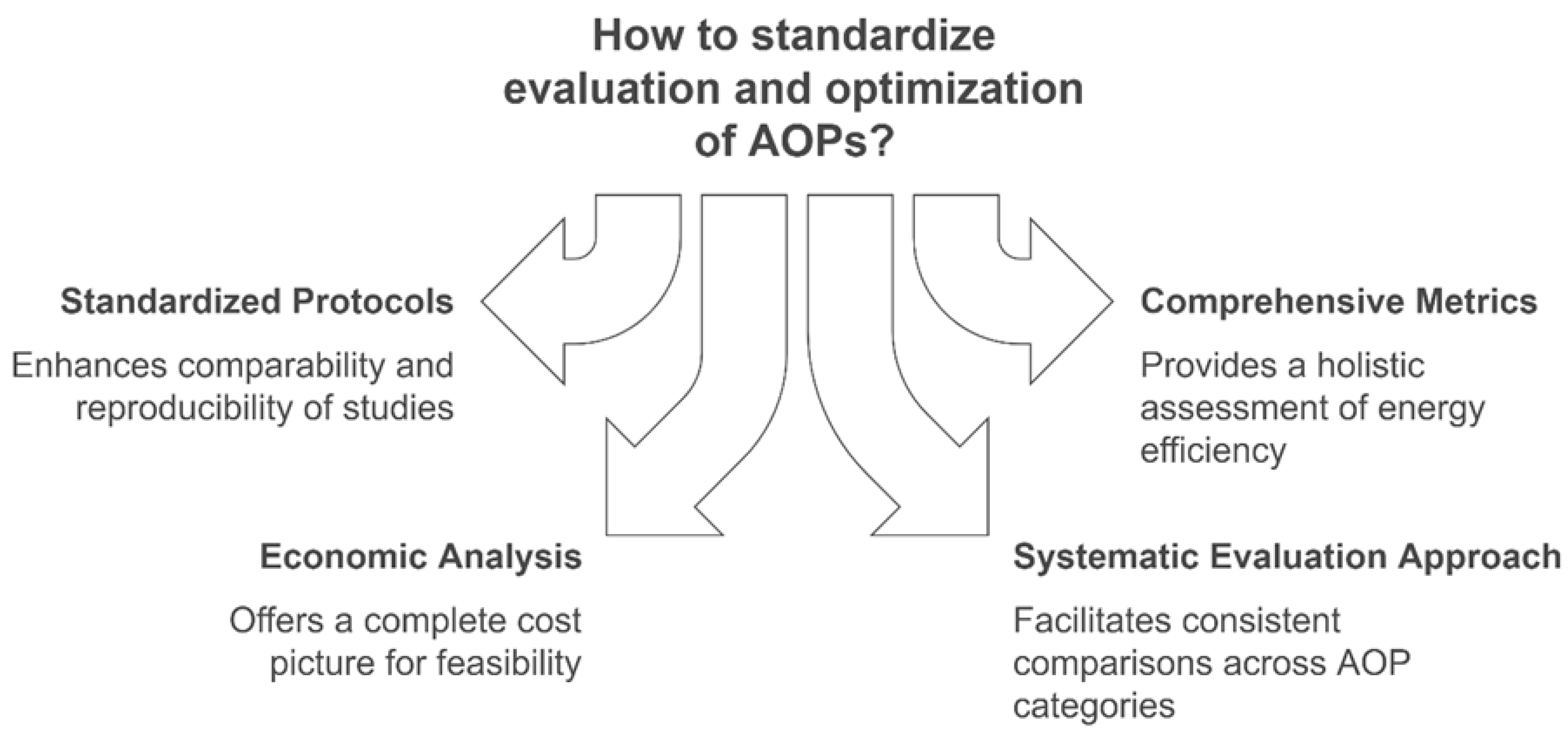
To address these challenges, Hübner et al. propose a systematic two-phase evaluation approach for emerging AOPs. In the first phase, which focuses on basic research and proof-of-concept technology readiness levels (TRL) 1–3, the emphasis is on assessing the feasibility of the AOP, identifying major reactive species, and conducting preliminary cost estimations. The second phase, centered on process development and cost comparison (TRL 3–5), involves optimizing the AOP in a real water matrix, benchmarking its performance and cost against established processes, and conducting a detailed cost–benefit analysis. This systematic approach, coupled with the use of metrics like EEO, albeit with its acknowledged limitations, can provide a structured framework for evaluating AOPs. By addressing key challenges related to energy efficiency, scalability, and cost, this methodology could enable more consistent and meaningful comparisons across AOP categories, facilitating the identification of optimal systems for specific applications [95]. Figure 8 highlights various approaches for the standardized evaluation and optimization of AOPs, emphasizing process efficiency, performance benchmarking, and scalability for real-world applications.
Figure 8.
Approaches for standardized evaluation and optimization of AOPs.
11. Future Perspective
The future of AOPs in wastewater treatment lies in overcoming persistent challenges through interdisciplinary innovation fostering scalability, efficiency, and sustainability. Material science advancements will play a pivotal role in enhancing catalytic performance, with a focus on cost-effective and durable material composites and bio-inspired heterojunctions that mitigate catalyst leaching and improve solar utilization. The development of highly porous materials having a huge surface area to volume ratio presents promising pathways for stabilizing ROS and optimizing mass transfer, while scalable fabrication methods like ultrasonic-assisted impregnation and green chemistry approaches could lower costs and enhance industrial viability. Integrating hybrid systems will be essential for improving treatment efficiency and energy utilization, with EO and photocatalysis combinations, photo-Fenton flow reactors, and catalytic ozonation-membrane filtration hybrids showing potential for higher radical yields and improved pollutant degradation. Moreover, the fusion of AOPs with biological treatment can enhance biodegradability, making treatment processes more cost-effective for complex wastewater streams. The incorporation of computational modeling and AI into AOPs is set to revolutionize reactor design and process optimization, with CFD refining hydrodynamic conditions and AI models enabling real-time monitoring and predictive process control. AI-driven adjustments to UV-LED intensity in photoreactors, along with fouling pattern predictions in electro-catalytic membranes, can enhance operational adaptability, while the implementation of digital twins could bridge lab-scale research to full-scale deployment.
Scaling up AOPs will require innovative reactor designs that mitigate fouling and optimize energy recovery, such as 3D-printed reactors with turbulence promoters or spiral-flow tubular configurations that enhance mass transfer. The integration of renewable energy sources, like hydrogen production in EO reactors, could offset operational costs, while modular containerized systems tailored for specific pollutants, such as PFAS and pharmaceuticals, offer decentralized treatment solutions. Establishing standardized evaluation protocols for energy efficiency, lifecycle cost assessment, and environmental impact analysis will further support industrial adoption, with a structured TRL framework facilitating the transition from proof-of-concept to process benchmarking. Future AOPs must also align with circular economy principles by promoting catalyst recycling, utilizing waste-derived materials, and integrating resource recovery systems. Strategies such as regenerating Fe2+ in Fenton systems and adopting eco-friendly fabrication techniques will help minimize environmental impact while enhancing cost-effectiveness. By shifting wastewater treatment plants towards resource recovery hubs, future research and technological advancements can ensure that AOPs contribute to a more sustainable and resilient environmental management framework.
12. Conclusions
The evolving landscape of AOPs in wastewater treatment underscores a transformative journey marked by both breakthroughs and persistent challenges. While AOPs have demonstrated remarkable efficacy in degrading recalcitrant pollutants, their industrial scalability remains hindered by inherent limitations such as pH dependency, energy-intensive operations, secondary pollutant formation, and high reagent costs [113]. Recent advancements, however, offer promising pathways to address these barriers. Innovations in reactor design, such as 3D electrodes, turbulence promoters, and hybrid configurations, have significantly enhanced mass transfer and energy efficiency, bridging the gap between laboratory-scale success and real-world applicability [114]. The integration of renewable energy sources, particularly solar-driven systems, coupled with visible-light-responsive photocatalysts, exemplifies strides toward sustainable and economically viable solutions [115]. Material science breakthroughs, including MOFs and CCMs, have improved catalyst stability and ROS generation, reducing leaching and enabling continuous operation in fixed-bed reactors [116,117]. These developments highlight the critical role of interdisciplinary collaboration in refining AOP technologies.
AI techniques, particularly ANNs, SVR, ANFIS, and metaheuristic algorithms, have shown significant potential in enhancing the modeling, optimization, and performance prediction of wastewater treatment processes. While challenges such as data limitations and model overfitting exist, ongoing research and advancements in AI methodologies are expected to further improve the effectiveness and reliability of these techniques in addressing the pressing challenges of water pollution and wastewater management [88,118]. Despite these advancements, scalability challenges persist, particularly in reconciling short-lived radical lifetimes with reactor design constraints and economic feasibility [15]. Standardized evaluation frameworks, encompassing energy metrics like EEO and comprehensive lifecycle assessments, are imperative to ensure consistent benchmarking and facilitate industrial adoption [119]. Future research must prioritize hybrid systems that synergize AOPs with biological treatments, leveraging pretreatment strategies to enhance biodegradability and reduce costs. Furthermore, aligning AOP development with circular economy principles through catalyst recycling, waste-derived material utilization, and resource recovery will be pivotal in minimizing environmental footprints and fostering sustainability. As the field progresses, a holistic approach integrating computational modeling, material innovation, and renewable energy integration will drive the next generation of AOPs toward scalable, efficient, and environmentally responsible wastewater treatment solutions.
Author Contributions
S.S.: Writing—review and editing, Writing—original draft, Data curation, Conceptualization. S.P.: Writing—review and editing, Supervision, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Indo-EU Horizon 2020 project (BT/IN/EU-WR/60/SP/2018) funded by the Department of Biotechnology, New Delhi.
Data Availability Statement
Data from our study remain undeposited in any public repository as all pertinent facts and figures utilized herein are comprehensively included with appropriate references.
Acknowledgments
The authors would like to acknowledge the Department of Biosciences and Bioengineering, IIT Guwahati for providing the infrastructural facility.
Conflicts of Interest
The authors declare that they do not possess any known financial conflicts of interest or personal associations that may have impacted the research presented.
References
- Oriji, A.Y.; Isaac, G.O.; Ojo, R.F. Emerging Contaminants in Drinking Water. Emerg. Contam. Food Food Prod. 2024, 44–60. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003438236-5 (accessed on 20 March 2025).
- Zhang, C.; Liu, L.; Pan, Y.; Qin, R.; Wang, W.; Zhou, M.; Zhang, Y. Detection Methodologies and Mechanisms of Reactive Oxygen Species Generated in Fenton/Fenton-like Processes. Sep. Purif. Technol. 2025, 355, 129578. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A Comprehensive Review on Reactive Oxygen Species (ROS) in Advanced Oxidation Processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef] [PubMed]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of Organic Pollutants from Wastewater by Advanced Oxidation Processes and Its Combination with Membrane Processes. Chem. Eng. Process.-Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Vieira, W.T.; De Farias, M.B.; Spaolonzi, M.P.; Da Silva, M.G.C.; Vieira, M.G.A. Latest Advanced Oxidative Processes Applied for the Removal of Endocrine Disruptors from Aqueous Media—A Critical Report. J. Environ. Chem. Eng. 2021, 9, 105748. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, Z.; Duan, X.; Long, M.; Spinney, R.; Dionysiou, D.D.; Xiao, R.; Alvarez, P.J.J. Merits and Limitations of Radical vs. Nonradical Pathways in Persulfate-Based Advanced Oxidation Processes. Environ. Sci. Technol. 2023, 57, 12153–12179. [Google Scholar] [CrossRef]
- Zhang, X.; Kamali, M.; Uleners, T.; Symus, J.; Zhang, S.; Liu, Z.; Costa, M.E.V.; Appels, L.; Cabooter, D.; Dewil, R. UV/TiO2/Periodate System for the Degradation of Organic Pollutants—Kinetics, Mechanisms and Toxicity Study. Chem. Eng. J. 2022, 449, 137680. [Google Scholar] [CrossRef]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced Oxidation Process for the Treatment of Industrial Wastewater: A Review on Strategies, Mechanisms, Bottlenecks and Prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A Detailed Review on Advanced Oxidation Process in Treatment of Wastewater: Mechanism, Challenges and Future Outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Cüce, H.; Özçelik, D. Application of Machine Learning (ML) and Artificial Intelligence (AI)-Based Tools for Modelling and Enhancing Sustainable Optimization of the Classical/Photo-Fenton Processes for the Landfill Leachate Treatment. Sustainability 2022, 14, 11261. [Google Scholar] [CrossRef]
- Esfahani, K.N.; Pérez-Moya, M.; Graells, M. A Hybrid Model Coupling Advanced Oxidation Processes (AOP) and Conventional Bio-Processes for the Removal of Recalcitrant Contaminants in Wastewaters. Comput. Aided Chem. Eng. 2021, 50, 883–889. [Google Scholar] [CrossRef]
- Yu, C.; Xiong, Z.; Zhou, H.; Zhou, P.; Zhang, H.; Huang, R.; Yao, G.; Lai, B. Marriage of Membrane Filtration and Sulfate Radical-Advanced Oxidation Processes (SR-AOPs) for Water Purification: Current Developments, Challenges and Prospects. Chem. Eng. J. 2022, 433, 133802. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Waclawek, S.; Černík, M.; Dionysiou, D.D. The Development and Challenges of Oxidative Abatement for Contaminants of Emerging Concern. In A New Paradigm for Environmental Chemistry and Toxicology; Springer: Singapore, 2020; pp. 131–152. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of Advanced Oxidation Processes (AOPs) in Industrial Applications: A Review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-Examining Fenton and Fenton-like Reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Niu, Q.; Wu, S.; Lin, Y.; Biswas, J.K.; Yang, C. Hydroxyl Radicals in Ozone-Based Advanced Oxidation of Organic Contaminants: A Review. Environ. Chem. Lett. 2024, 22, 3059–3106. [Google Scholar] [CrossRef]
- Najafinejad, M.S.; Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D. Application of Electrochemical Oxidation for Water and Wastewater Treatment: An Overview. Molecules 2023, 28, 4208. [Google Scholar] [CrossRef]
- Singh, G.; Ubhi, M.K.; Jeet, K.; Singla, C.; Kaur, M. A Review on Impacting Parameters for Photocatalytic Degradation of Organic Effluents by Ferrites and Their Nanocomposites. Processes 2023, 11, 1727. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A.; Swiatla-Wojcik, D.; Katsumura, Y.; Wach, R.A. Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review. Appl. Sci. 2021, 11, 12032. [Google Scholar] [CrossRef]
- de Andrade, F.V.; Augusti, R.; de Lima, G.M. Ultrasound for the Remediation of Contaminated Waters with Persistent Organic Pollutants: A Short Review. Ultrason. Sonochem. 2021, 78, 105719. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M. A Critical Review of the Application of Chelating Agents to Enable Fenton and Fenton-like Reactions at High PH Values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef]
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost Comparison of Advanced Oxidation Processes for Wastewater Treatment Using Accumulated Oxygen-Equivalent Criteria. Water Res. 2021, 200, 117234. [Google Scholar] [CrossRef] [PubMed]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Fernandes, J.R.; Peres, J.A.; Tavares, P.B.; Lucas, M.S. Wireless UV-A LEDs-Driven AOP in the Treatment of Agro-Industrial Wastewaters. Environ. Res. 2021, 200, 111430. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced Oxidation Process: A Sustainable Technology for Treating Refractory Organic Compounds Present in Industrial Wastewater. Environ. Sci. Pollut. Res. 2022, 30, 25477–25505. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Makhatova, A.; Zarikas, V.; Arkhangelsky, E.; Poulopoulos, S.G. Photo-Fenton-like Treatment of Municipal Wastewater. Catalysts 2021, 11, 1206. [Google Scholar] [CrossRef]
- El-Saeid, M.H.; Alotaibi, M.O.; Alshabanat, M.; Alharbi, K.; Altowyan, A.S.; Al-Anazy, M. Photo-Catalytic Remediation of Pesticides in Wastewater Using UV/TiO2. Water 2021, 13, 3080. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, Z.; Wang, S.; Shi, X.; Guo, B.; Gao, J.; Wang, D.; Zong, W. Effect of Bromide on Molecular Transformation of Dissolved Effluent Organic Matter during Ozonation, UV/H2O2, UV/Persulfate, and UV/Chlorine Treatments. Sci. Total Environ. 2022, 811, 152328. [Google Scholar] [CrossRef]
- Ghavi, A.; Bagherian, G.; Rezaei-Vahidian, H. Degradation of Paraquat Herbicide Using Hybrid AOP Process: Statistical Optimization, Kinetic Study, and Estimation of Electrical Energy Consumption. Environ. Sci. Eur. 2021, 33, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Lu, J.; Zhou, J.; Chen, Q. Catalytic Activity and Mechanism of Typical Iron-Based Catalysts for Fenton-like Oxidation. Chemosphere 2023, 311, 136972. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic Ozonation for Water and Wastewater Treatment: Recent Advances and Perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaiey, N.A. Trends in Sonochemical Treatment of Oily Wastewater. Pet. Chem. 2024, 64, 1039–1047. [Google Scholar] [CrossRef]
- Radjenovic, J.; Duinslaeger, N.; Avval, S.S.; Chaplin, B.P. Facing the Challenge of Poly- And Perfluoroalkyl Substances in Water: Is Electrochemical Oxidation the Answer? Environ. Sci. Technol. 2020, 54, 14815–14829. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Pandey, S.; Mandari, K.K.; Kim, J.; Kang, M.; Fosso-Kankeu, E. Recent Advancement in Visible-Light-Responsive Photocatalysts in Heterogeneous Photocatalytic Water Treatment Technology. In Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; Fosso-Kankeu, E., Pandey, S., Sinha Ray, S., Eds.; John Wiley & Sons, Ltd: Beverly, CA, USA, 2020; pp. 167–196. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Geng, J.; Song, R.; Liu, X.; Fu, C.; Li, S. Current Advances in UV-Based Advanced Oxidation Processes for the Abatement of Fluoroquinolone Antibiotics in Wastewater. Chin. Chem. Lett. 2024, 36, 110138. [Google Scholar] [CrossRef]
- Gupta, P.; Bhardwaj, G.; Dubey, S.; Tayal, T.; Sengupta, A.; Narad, P. AI-Enabled Process Optimization for Sustainable Wastewater Treatment Solutions. In The AI Cleanse: Transforming Wastewater Treatment Through Artificial Intelligence: Harnessing Data-Driven Solutions; Garg, M.C., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 141–164. [Google Scholar] [CrossRef]
- Dhamorikar, R.S.; Lade, V.G.; Kewalramani, P.V.; Bindwal, A.B. Review on Integrated Advanced Oxidation Processes for Water and Wastewater Treatment. J. Ind. Eng. Chem. 2024, 138, 104–122. [Google Scholar] [CrossRef]
- Dong, G.; Chen, B.; Liu, B.; Hounjet, L.J.; Cao, Y.; Stoyanov, S.R.; Yang, M.; Zhang, B. Advanced Oxidation Processes in Microreactors for Water and Wastewater Treatment: Development, Challenges, and Opportunities. Water Res. 2022, 211, 118047. [Google Scholar] [CrossRef]
- Long, F.; Ghani, D.; Huang, R.; Zhao, C. Versatile Electrode Materials Applied in the Electrochemical Advanced Oxidation Processes for Wastewater Treatment: A Systematic Review. Sep. Purif. Technol. 2025, 354, 128725. [Google Scholar] [CrossRef]
- Wachter, N.; Bocchi, N.; Rocha-Filho, R.C. Use of a Turbulence Promoter in an Electrochemical Filter-Press Reactor: Consolidated Evidence of Significant Enhancement of Organics Mass Transport and Degradation Rates. Sep. Purif. Technol. 2021, 276, 119292. [Google Scholar] [CrossRef]
- Ni, J.; Shi, H.; Xu, Y.; Wang, Q. A Comparison of the Mechanism of TOC and COD Degradation in Rhodamine B Wastewater by a Recycling-Flow Two- and Three-Dimensional Electro-Reactor System. Water 2020, 12, 1853. [Google Scholar] [CrossRef]
- Zhou, B.; Yu, Z.; Wei, Q.; Long, H.Y.; Xie, Y.; Wang, Y. Electrochemical Oxidation of Biological Pretreated and Membrane Separated Landfill Leachate Concentrates on Boron Doped Diamond Anode. Appl. Surf. Sci. 2016, 377, 406–415. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Chen, F.; Wang, J. Three-Dimensional CFD Simulation of the Tubular Electrochemical Reactor with Meshed Plate Electrodes. J. Electrochem. Soc. 2014, 161, E81–E86. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Zhou, M.; Li, X.; Yu, J. Characterization of Hydrodynamics and Mass Transfer in Two Types of Tubular Electrochemical Reactors. Electrochim. Acta 2015, 173, 698–704. [Google Scholar] [CrossRef]
- Guo, X.; You, S. Characterization of Hydrodynamics and Electrochemical Treatment of Dye Wastewater in Two Types of Tubular Electrochemical Reactors. IOP Conf. Ser. Earth Environ. Sci. 2017, 81, 012008. [Google Scholar] [CrossRef]
- Reis, R.M.H.; Baio, J.A.F.; Migliorini, F.L.; Rocha, R.D.S.; Baldan, M.R.; Ferreira, N.G.; Lanza, M.R.D.V. Degradation of Dipyrone in an Electrochemical Flow-by Reactor Using Anodes of Boron-Doped Diamond (BDD) Supported on Titanium. J. Electroanal. Chem. 2013, 690, 89–95. [Google Scholar] [CrossRef]
- Chen, M.; Wang, C.; Zhao, X.; Wang, Y.; Zhang, W.; Chen, Z.; Meng, X.; Luo, J.; Crittenden, J. Development of a Highly Efficient Electrochemical Flow-through Anode Based on Inner in-Site Enhanced TiO2-Nanotubes Array. Environ. Int. 2020, 140, 105813. [Google Scholar] [CrossRef]
- Zeng, W.; Liang, H.; Zhang, H.; Luo, X.; Lin, D.; Li, G. Efficient Electrochemical Oxidation of Sulfamethoxazole by a Novel Reduced TiO2 Nanotube Arrays-Based Flow-through Electrocatalytic Membrane. Sep. Purif. Technol. 2022, 289, 120720. [Google Scholar] [CrossRef]
- Ochoa-Gutiérrez, K.S.; Tabares-Aguilar, E.; Mueses, M.Á.; Machuca-Martínez, F.; Li Puma, G. A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for Solar Photocatalytic Degradation of Water Contaminants. Chem. Eng. J. 2018, 341, 628–638. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic Reactors and It’s Comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Sacco, O. UV-LEDs Floating-Bed Photoreactor for the Removal of Caffeine and Paracetamol Using ZnO Supported on Polystyrene Pellets. Chem. Eng. J. 2018, 350, 703–713. [Google Scholar] [CrossRef]
- Bahmani, M.; Dashtian, K.; Mowla, D.; Esmaeilzadeh, F.; Ghaedi, M. UiO-66(Ti)-Fe3O4-WO3 Photocatalyst for Efficient Ammonia Degradation from Wastewater into Continuous Flow-Loop Thin Film Slurry Flat-Plate Photoreactor. J. Hazard. Mater. 2020, 393, 122360. [Google Scholar] [CrossRef] [PubMed]
- Mesgari, Z.; Saien, J. Pollutant Degradation over Dye Sensitized Nitrogen Doped Titania Substances in Different Configurations of Visible Light Helical Flow Photoreactor. Sep. Purif. Technol. 2017, 185, 129–139. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of Heterostructured G-C3N4/Ag/TiO2 Microspheres with Enhanced Photocatalysis Performance under Visible-Light Irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Ye, L.; Wang, D.; Chen, S. Fabrication and Enhanced Photoelectrochemical Performance of MoS2/S-Doped g-C3N4 Heterojunction Film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef]
- Moon, H.S.; Hsiao, K.C.; Wu, M.C.; Yun, Y.; Hsu, Y.J.; Yong, K. Spatial Separation of Cocatalysts on Z-Scheme Organic/Inorganic Heterostructure Hollow Spheres for Enhanced Photocatalytic H2 Evolution and In-Depth Analysis of the Charge-Transfer Mechanism. Adv. Mater. 2023, 35, 2200172. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F.; et al. Fe-Based Metal Organic Frameworks (Fe-MOFs) for Organic Pollutants Removal via Photo-Fenton: A Review. Chem. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Huo, Z.Y.; Lee, D.M.; Kim, Y.J.; Kim, S.W. Solar-Induced Hybrid Energy Harvesters for Advanced Oxidation Water Treatment. iScience 2021, 24, 102808. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Divyapriya, G.; Ezzahra Titchou, F.; Hamdani, M. Treatment of Textile Wastewater by Sulfate Radical Based Advanced Oxidation Processes. Sep. Purif. Technol. 2022, 293, 121115. [Google Scholar] [CrossRef]
- Kore, V.S.; Manjare, S.D.; Taralkar, S.V. Intensified Degradation of Reactive Blue 222 (RB222) Textile Dye by a Hybrid AOP System of Hydrodynamic Cavitation Coupled with Inline UV and PMS Oxidant. J. Water Process Eng. 2023, 56, 104472. [Google Scholar] [CrossRef]
- Husein, D.Z.; Hassanien, R.; Al-Hakkani, M.F. Green-Synthesized Copper Nano-Adsorbent for the Removal of Pharmaceutical Pollutants from Real Wastewater Samples. Heliyon 2019, 5, e02339. [Google Scholar] [CrossRef]
- Zhou, C.; Liang, Y.; Xia, W.; Almatrafi, E.; Song, B.; Wang, Z.; Zeng, Y.; Yang, Y.; Shang, Y.; Wang, C.; et al. Single Atom Mn Anchored on N-Doped Porous Carbon Derived from Spirulina for Catalyzed Peroxymonosulfate to Degradation of Emerging Organic Pollutants. J. Hazard. Mater. 2023, 441, 129871. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.Y.A.; Ghanbari, F. Insights into the Synergistic Role of Photocatalytic Activation of Peroxymonosulfate by UVA-LED Irradiation over CoFe2O4-RGO Nanocomposite towards Effective Bisphenol A Degradation: Performance, Mineralization, and Activation Mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Shi, H.; Chiang, S.Y.; Wang, Y.; Wang, Y.; Liang, S.; Zhou, J.; Fontanez, R.; Gao, S.; Huang, Q. An Electrocoagulation and Electrooxidation Treatment Train to Remove and Degrade Per- and Polyfluoroalkyl Substances in Aqueous Solution. Sci. Total Environ. 2021, 788, 147723. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.K.; O’Malley, K.N.; Dollhopf, M.E.; Mayer, B.K.; McNamara, P.J. Removal of Estrogenic Compounds from Water via Energy Efficient Sequential Electrocoagulation-Electrooxidation. Environ. Eng. Sci. 2020, 37, 99–108. [Google Scholar] [CrossRef]
- Aseman-Bashiz, E.; Sayyaf, H. Synthesis of Nano-FeS2 and Its Application as an Effective Activator of Ozone and Peroxydisulfate in the Electrochemical Process for Ofloxacin Degradation: A Comparative Study. Chemosphere 2021, 274, 129772. [Google Scholar] [CrossRef]
- Bahadur, N.; Bhargava, N. TERI Advanced Oxidation Technology (TADOX®) to Treat Industrial Wastewater with Integration at Pre- and Post-Biological Stage: Case Studies from India. Water Pract. Technol. 2022, 17, 1692–1705. [Google Scholar] [CrossRef]
- Mehdaoui, R.; Agren, S.; El Haskouri, J.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. An Optimized Sono-Heterogeneous Fenton Degradation of Olive-Oil Mill Wastewater Organic Matter by New Magnetic Glutarlaldehyde-Crosslinked Developed Cellulose. Environ. Sci. Pollut. Res. 2023, 30, 20450–20468. [Google Scholar] [CrossRef]
- Giraldo-Loaiza, C.; Salazar-Loaiza, A.M.; Sandoval-Barrera, M.A.; Macías-Quiroga, I.F.; Ocampo-Serna, D.M.; Sanabria-González, N.R. Integration of Ion Exchange—AOP—Biological System for the Treatment of Real Textile Wastewater. ChemEngineering 2024, 8, 76. [Google Scholar] [CrossRef]
- Senthil Rathi, B.; Senthil Kumar, P.; Sanjay, S.; Prem Kumar, M.; Rangasamy, G. Artificial Intelligence Integration in Conventional Wastewater Treatment Techniques: Techno-Economic Evaluation, Recent Progress and Its Future Direction. Int. J. Environ. Sci. Technol. 2024, 22, 633–658. [Google Scholar] [CrossRef]
- Joy, V.M.; Feroz, S.; Dutta, S. Artificial Intelligence-Based Multiobjective Optimization of Reverse Osmosis Desalination Pretreatment Using a Hybrid ZnO-Immobilized/Photo-Fenton Process. J. Chemom. 2022, 36, e3434. [Google Scholar] [CrossRef]
- Behera, S.K.; Karthika, S.; Mahanty, B.; Meher, S.K.; Zafar, M.; Baskaran, D.; Rajamanickam, R.; Das, R.; Pakshirajan, K.; Bilyaminu, A.M.; et al. Application of Artificial Intelligence Tools in Wastewater and Waste Gas Treatment Systems: Recent Advances and Prospects. J. Environ. Manag. 2024, 370, 122386. [Google Scholar] [CrossRef]
- Dong, J.; Chen, Y.; Yao, B.; Zhang, X.; Zeng, N. A Neural Network Boosting Regression Model Based on XGBoost. Appl. Soft Comput. 2022, 125, 109067. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, Y.; Shang, Y. Research and Application of XGBoost in Imbalanced Data. Int. J. Distrib. Sens. Netw. 2022, 18, 15501329221. [Google Scholar] [CrossRef]
- Liu, S.; Long, Z.; Liu, H.; Zhang, J.; Zhang, G.; Liang, J. Machine Learning Predict the Degradation Efficiency of Aqueous Refractory Organic Pollutants by Ultrasound-Based Advanced Oxidation Processes. J. Water Process Eng. 2024, 66, 106022. [Google Scholar] [CrossRef]
- Abdolrasol, M.G.M.; Suhail Hussain, S.M.; Ustun, T.S.; Sarker, M.R.; Hannan, M.A.; Mohamed, R.; Ali, J.A.; Mekhilef, S.; Milad, A. Artificial Neural Networks Based Optimization Techniques: A Review. Electronics 2021, 10, 2689. [Google Scholar] [CrossRef]
- Huang, R.; Ma, C.; Ma, J.; Huangfu, X.; He, Q. Machine Learning in Natural and Engineered Water Systems. Water Res. 2021, 205, 117666. [Google Scholar] [CrossRef]
- Warren-Vega, W.M.; Montes-Pena, K.D.; Romero-Cano, L.A.; Zarate-Guzman, A.I. Development of an Artificial Neural Network (ANN) for the Prediction of a Pilot Scale Mobile Wastewater Treatment Plant Performance. J. Environ. Manag. 2024, 366, 121612. [Google Scholar] [CrossRef]
- Rather, I.H.; Kumar, S.; Gandomi, A.H. Breaking the Data Barrier: A Review of Deep Learning Techniques for Democratizing AI with Small Datasets. Artif. Intell. Rev. 2024, 57, 1–61. [Google Scholar] [CrossRef]
- Tran, N.K.; Kühle, L.C.; Klau, G.W. A Critical Review of Multi-Output Support Vector Regression. Pattern Recognit. Lett. 2024, 178, 69–75. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Achak, M.; Chennouk, H.; Dewil, R.; Barka, N. The State of Art on the Prediction of Efficiency and Modeling of the Processes of Pollutants Removal Based on Machine Learning. Sci. Total Environ. 2022, 807, 150554. [Google Scholar] [CrossRef]
- Xu, W.L.; Wang, Y.J.; Wang, Y.T.; Li, J.G.; Zeng, Y.N.; Guo, H.W.; Liu, H.; Dong, K.L.; Zhang, L.Y. Application and Innovation of Artificial Intelligence Models in Wastewater Treatment. J. Contam. Hydrol. 2024, 267, 104426. [Google Scholar] [CrossRef] [PubMed]
- Ismail, U.M.; Bani-Melhem, K.; Khan, M.F.; Elnakar, H. Optimizing Membrane Bioreactor Performance in Wastewater Treatment Using Machine Learning and Meta-Heuristic Techniques. Results Eng. 2025, 25, 104626. [Google Scholar] [CrossRef]
- Acosta-Angulo, B.; Diaz-Angulo, J.; Lara-Ramos, J.; Torres-Palma, R.; Martínez-Pachón, D.; Moncayo-Lasso, A.; Machuca-Martínez, F. Analysis of the Applications of Particle Swarm Optimization and Genetic Algorithms on Reaction Kinetics: A Prospective Study for Advanced Oxidation Processes. ChemElectroChem 2022, 9, e202200229. [Google Scholar] [CrossRef]
- Shirkoohi, M.G.; Tyagi, R.D.; Vanrolleghem, P.A.; Drogui, P. Artificial Intelligence Techniques in Electrochemical Processes for Water and Wastewater Treatment: A Review. J. Environ. Health Sci. Eng. 2022, 20, 1089–1109. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Tong, H.; Sun, B.; Liu, Y.; Ren, N.; You, S. Machine Learning Models for Inverse Design of the Electrochemical Oxidation Process for Water Purification. Environ. Sci. Technol. 2023, 57, 17990–18000. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, J.; Shao, X.; Jiang, Y.; Yu, J.; Deng, S.; Li, P. Interpretable Artificial Intelligence for Advanced Oxidation Systems: Principle, Operations and Performance. Process Saf. Environ. Prot. 2023, 180, 242–259. [Google Scholar] [CrossRef]
- Serna-Carrizales, J.C.; Zárate-Guzmán, A.I.; Flores-Ramírez, R.; Díaz de León-Martínez, L.; Aguilar-Aguilar, A.; Warren- Vega, W.M.; Bailón-García, E.; Ocampo-Pérez, R. Application of Artificial Intelligence for the Optimization of Advanced Oxidation Processes to Improve the Water Quality Polluted with Pharmaceutical Compounds. Chemosphere 2024, 351, 141216. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; He, Z.; Zhang, S.; Wang, K.; Ren, N.; Ho, S.H. Discovery of an End-to-End Pattern for Contaminant-Oriented Advanced Oxidation Processes Catalyzed by Biochar with Explainable Machine Learning. Environ. Sci. Technol. 2024, 58, 16867–16876. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Wei, H.; Sun, C. Application of Artificial Intelligence for Predicting Reaction Results in Advanced Oxidation Processes. Environ. Technol. Innov. 2021, 23, 101550. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Singh, K.; Choudhary, A.; Brighu, U.; Singh, S.K.; Bhattacharya, S. Combined Advanced Oxidation Dye-Wastewater Treatment Plant: Design and Development with Data-Driven Predictive Performance Modeling. NPJ Clean. Water 2024, 7, 15. [Google Scholar] [CrossRef]
- Hübner, U.; Spahr, S.; Lutze, H.; Wieland, A.; Rüting, S.; Gernjak, W.; Wenk, J. Advanced Oxidation Processes for Water and Wastewater Treatment—Guidance for Systematic Future Research. Heliyon 2024, 10, e30402. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, P.; Smallridge, A.; Irtassam, A.; Yeager, T. Scalable Production of Hydroxyl Radicals (.OH) via Homogeneous Photolysis of Hydrogen Peroxide Using a Continuous-Flow Photoreactor. Chem. Eng. J. 2022, 427, 131762. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Sharma, V.K. Oxidative Transformations of Environmental Pharmaceuticals by Cl2, ClO2, O3, and Fe(VI): Kinetics Assessment. Chemosphere 2008, 73, 1379–1386. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Amaeze, N.; Richardson, K.; Mackay, W.; Rateb, M.E.; Yaseen, M. Stabilisation of Ozone in Water for Microbial Disinfection. Environments 2022, 9, 45. [Google Scholar] [CrossRef]
- Ntelane, T.S.; Feleni, U.; Mthombeni, N.H.; Kuvarega, A.T. Sulfate Radical-Based Advanced Oxidation Process (SR-AOP) on Titania Supported Mesoporous Dendritic Silica (TiO2/MDS) for the Degradation of Carbamazepine and Other Water Pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130276. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Yan, W.; Yang, S.; Wu, K.; Wang, G.; Jin, P.; Wei, J. Peroxymonosulfate Activation by Mesoporous CuO Nanocage for Organic Pollutants Degradation via a Singlet Oxygen-Dominated Pathway. J. Environ. Chem. Eng. 2021, 9, 106757. [Google Scholar] [CrossRef]
- Peng, H.; Tong, J.; Huang, J.; Yang, Z.; Xiong, W.; Yao, Y.; Xiang, Y.; Xu, Z. In-Situ Immobilization of MIL-100(Fe) on the Microchannels in Wood Aerogel: Efficient Persulfate Activation toward Antibiotic Removal. Sep. Purif. Technol. 2023, 321, 124195. [Google Scholar] [CrossRef]
- Wang, J.S.; Yi, X.H.; Xu, X.; Ji, H.; Alanazi, A.M.; Wang, C.C.; Zhao, C.; Kaneti, Y.V.; Wang, P.; Liu, W.; et al. Eliminating Tetracycline Antibiotics Matrix via Photoactivated Sulfate Radical-Based Advanced Oxidation Process over the Immobilized MIL-88A: Batch and Continuous Experiments. Chem. Eng. J. 2022, 431, 133213. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical Review of the Science and Sustainability of Persulphate Advanced Oxidation Processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced Oxidation Processes: Performance, Advantages, and Scale-up of Emerging Technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, S.; Yu, X.; Kamali, M.; Appels, L.; Dewil, R. Generation of Oxidative Radicals by Advanced Oxidation Processes (AOPs) in Wastewater Treatment: A Mechanistic, Environmental and Economic Review. Rev. Environ. Sci. Bio/Technol. 2023, 22, 205–248. [Google Scholar] [CrossRef]
- Mahbub, P.; Sharma, A. Investigation of Alternative Water Sources for Fish Farming Using Life Cycle Costing Approach: A Case Study in North West Tasmania. J. Hydrol. (Amst.) 2019, 579, 124215. [Google Scholar] [CrossRef]
- Keen, O.; Bolton, J.; Litter, M.; Bircher, K.; Oppenländer, T. Standard Reporting of Electrical Energy per Order (EEO) for UV/H2O2 Reactors (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 1487–1499. [Google Scholar] [CrossRef]
- Prisciandaro, M.; Innocenzi, V. Advanced Wastewater Oxidation Processes and Their Role in Water Reuse for a Circular Economy. In Water Management and Circular Economy; Zamparas, M.G., Kyriakopoulos, G.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 81–102. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Barndõk, H.; Hermosilla, D.; Negro, C.; Blanco, Á. Comparison and Predesign Cost Assessment of Different Advanced Oxidation Processes for the Treatment of 1,4-Dioxane-Containing Wastewater from the Chemical Industry. ACS Sustain. Chem. Eng. 2018, 6, 5888–5894. [Google Scholar] [CrossRef]
- Fabregat, V.; Pagán, J.M. Technical–Economic Feasibility of a New Method of Adsorbent Materials and Advanced Oxidation Techniques to Remove Emerging Pollutants in Treated Wastewater. Water 2024, 16, 814. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Sable, S.; Gamal El-Din, M. Advanced Oxidation Processes for the Degradation of Dissolved Organics in Produced Water: A Review of Process Performance, Degradation Kinetics and Pathway. Chem. Eng. J. 2022, 429, 132492. [Google Scholar] [CrossRef]
- Cornejo, O.M.; Murrieta, M.F.; Aguilar, Z.G.; Rodríguez, J.F.; Márquez, A.A.; León, M.I.; Nava, J.L. Recent Advances in Electrochemical Flow Reactors Used in Advanced Oxidation Processes: A Critical Review. Chem. Eng. J. 2024, 496, 153935. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahu, J.N.; Poonia, A.K.; Ghosh, P. Current Perspective of Nano-Engineered Metal Oxide Based Photocatalysts in Advanced Oxidation Processes for Degradation of Organic Pollutants in Wastewater. Chem. Eng. Res. Des. 2023, 190, 667–686. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanroman, A. Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Appl. Sci. 2022, 12, 8240. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zhang, Y.; Guo, G.; Yang, J.; Yuan, S.; Van der Bruggen, B. Efficient Activity of CoCu@SiC Catalytic Ceramic Membrane via H2 Reduction Treatment for Pollutants Degradation. Chem. Eng. J. 2024, 496, 154017. [Google Scholar] [CrossRef]
- Nagpal, M.; Siddique, M.A.; Sharma, K.; Sharma, N.; Mittal, A. Optimizing Wastewater Treatment through Artificial Intelligence: Recent Advances and Future Prospects. Water Sci. Technol. 2024, 90, 731–757. [Google Scholar] [CrossRef] [PubMed]
- Clímaco Cunha, I.L.; Machado, P.G.; de Oliveira Ribeiro, C.; Kulay, L. Bibliometric Analysis of Advanced Oxidation Processes Studies with a Focus on Life Cycle Assessment and Costs. Environ. Sci. Pollut. Res. 2024, 31, 22319–22338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).