Abstract
Amazonian oils and fats (AOFs) have promising composition and bioactive properties, and their processing with supercritical fluids presents several opportunities and challenges for the industry. Our review is dedicated to unraveling the advances in the application of supercritical fluid extraction (SFE) to produce these exceptional oils and fats from Amazonian raw materials, such as fruits, pulps, seeds, and industrial by-products. Our review found that Amazonian plant species produce lipid-rich raw materials and also grow naturally in other regions with similar climates. AOFs present high nutritional value and functional properties due to their content of bioactive compounds. The properties of AOFs are attributed to their major and minor components; lipid molecules represent the major fraction and some fat-soluble compounds, such as tocopherols, phytosterols, terpenes and others, constitute the minor fraction. The production of AOFs by SFE requires properly prepared raw materials and process optimization. Several scientific articles report on the production of AOFs by SFE, but application studies in product development are still scarce. The composition and technological properties of AOFs show a wide spectrum of opportunities for the development of nutritional and functional products. Aspects such as the scaling up of the SFE process, agroforestry or agronomic production, and product development require further studies to promote the AOFs industry in a way that contributes to sustainable development, food security, and the quality of life and health of the Amazonian populations.
1. Introduction
Oils and fats present a complex composition that includes fatty acids, acylglycerols, phospholipids, sphingolipids, and sterols [1]; this composition may vary depending on the raw material. Lipids are an important source of energy in the human diet and play important roles in metabolic processes [2]. Currently, the lipids used in the industry for the manufacture of a wide range of products come from industrial crops and exotic tropical species [3]. A wide variety of tropical plant species thrive in the Amazon region [4]. This diversity stems from the presence of unique ecosystems along the banks of the Amazon River in South America [5]. The literature highlights the potential of Amazonian species for the production of oils and fats due to their yield and composition [3]. Some Amazonian raw materials produce fats with a higher content of saturated fatty acids, such as the seeds of Theobroma grandiflorum [6], which presents interesting bioactive compounds for the manufacture of food products for human [7] and animal [8] consumption. Cupuassu (T. grandiflorum) seeds are used to produce products similar to those derived from cocoa [9]; the liquor made from both seeds (cupuassu and cocoa) has a similar lipid content, but the liquor from cupuassu has a lower content of palmitic fatty acid [10]. Other Amazonian raw materials have a high content of oils rich in unsaturated and polyunsaturated fatty acids, such as the fruits of Mauritia flexuosa [11], Oenocarpus distichus [12], seeds of Bertholletia excelsa [13], and others. The composition of fatty acids, secondary metabolites, and physicochemical and thermodynamic properties of AOFs [7] offer a horizon of diverse opportunities for the development of new products, such as cosmetics, functional foods, pharmaceuticals, and biofuels [7,14,15]. On the other hand, some of these raw materials have managed to position themselves in local and international markets as minimally processed products and/or derivatives, contributing to the economy of the countries of the Amazon region [16]. However, the Amazon region offers a greater diversity of species that produce lipid-rich raw materials that are little known to date. In this context, the present review aims to describe the main scientific advances in the application of supercritical fluid technology to produce AOFs, highlighting new Amazonian species as potential for the oils and fats industry.
The processing of raw materials to obtain lipids involves unit operations based on conventional and/or emerging technologies. Conventional technologies include the Soxhlet method [17], the Bligh and Dyer method [18], dynamic maceration [19], and mechanical pressing [20,21]. The quality of lipids for use in products and responsibility to the environment are motivations that have driven scientific research for the development of unit operations using new technologies, such as SFE [22,23], ultrasound [20], microwave [24], and variations of the SFE method [25,26,27,28]. SFE is considered an emerging technology characterized by its extraction selectivity, low operating temperatures, no use of toxic solvents, and the use of GRAS (Generally Recognized as Safe) solvents as polarity modifiers to enhance the extraction of polar molecules [29]. Supercritical carbon dioxide (scCO2) was more effective than the Soxhlet method with petroleum ether to obtain seed oil from Virola surinamensis [23]; scCO2 yielded Acrocomia aculeata kernel oil with a higher content of phytosteroids and tocopherols compared to compressed propane and Soxhlet method with n-hexane [17]. Renealmia petasites Gagnep. seed oil with a higher composition of terpenes was obtained with scCO2 compared to the Soxhlet methods with petroleum ether and maceration with hexane [19]. The yield and phytochemical content (tocopherols, volatile, and semi-volatile) of Dipteryx alata Vogel oil obtained with scCO2 was superior compared to continuous expeller press [21]. In the literature, many scientific publications are available on the application of scCO2 for the extraction of oils and fats from a wide variety of raw materials. The oils and fats of Amazonian origin can be obtained from fruits and seeds, from trees [7,15,30], palms [7,14,31], and other types of plants [7,19,32,33,34].
The present review article discusses the technological advancement of the application of scCO2 in the production of lipid compounds from Amazonian raw materials. The composition of fatty acids and lipid-soluble compounds is addressed, highlighting promising Amazonian species. The potential applications of the main fatty acids and lipid-soluble bioactive compounds are also described. In the following sections of the review, the SFE process for obtaining Amazonian oils and fats is described, highlighting the potential of this method in comparison to conventional methods. The potential of AOFs, especially those obtained by SFE, is highlighted in comparison to conventional methods. The diversity of Amazonian species studied to date using supercritical fluids is shown, highlighting the particularities of each raw material and the way of preparation. The composition of AOFs and their bioactive properties are analyzed and discussed as a basis for the development of the functional products industry and future perspectives.
2. Procedure of Search and Selection of Scientific Articles
The review period was from 2015 to 2024 in the SCOPUS database, using the keywords “Oil extraction by mechanical pressing”, “Supercritical fluid extraction oilseeds”, “SFE tropical oily fruit”, “CO2 extraction oil pulp fruit”, “SFE fatty acid”, “SFE bioactive compounds oilseeds”, “SFE bioactive compounds oily fruit”, “SFE Terpenes oilseeds”, “SFE Phytosterols oilseeds”, “SFE Carotenoids oilseeds”, “SFE fat-soluble vitamins”, “SFE oil: Validation”, “SFE oil: Optimization”, “SFE oilseeds: scale-up criteria”, “SFE oilseeds: Industrial scale”, and “Supercritical fluid extraction oil modeling”. The articles were selected using two criteria: (1) the Amazonian origin of the raw materials used in the studies, and (2) the article should focus on SFE of oils or/and fats, SFE processing, and application of the lipid fraction and/or liposoluble compounds obtained by SFE. The first criterion was pre-selection; only original articles were considered, and the scientific name of the samples used in the studies was used to verify the Amazonian origin in the GBIF [35], Tropicos [36], and KEW [37] databases. Additionally, articles that used samples of species collected in countries that do not belong to the Amazon region were considered, but the species must occur naturally in the Amazon region. Finally, the articles used in the present review were selected using the second criterion to the articles pre-selected with the first criterion.
3. Amazonian Oleaginous Plants
The Amazon region has a great diversity of plant species with potential for the production of oils/fats, which can be obtained from seeds and fruit pulp produced by palms, trees, lianas, cactus, climbing shrubs, and other types of plants (Table 1).

Table 1.
Oleaginous species from the Amazon region.
3.1. Palms
Table 1 shows a total of twenty palm species that were reported to produce fruits, pulp, and seeds with potential for oil/fat production. A greater number of raw materials studied come from the Brazilian Amazon [7,12,14,15,39,41,42,43,44,45], followed by Colombia [31,40], Peru [11,20], and Costa Rica [38]. The processing of Amazonian palm fruits integrates usual unitary operations, such as selection, washing/disinfection, pulping (manual and/or mechanical), dehydration (forced convection drying and/or freeze-drying), and milling [11,12,20,31,38,39,40,42,43,44,45]. Some fruits (O. mapora and O. distichus) require heat treatment in order to soften the pulp and facilitate the separation of the pulp from the peel and seeds [12,20]. The fruits of Amazonian palms are gaining importance in the industry due to their composition, nutritional characteristics, and functional properties; in the most recent studies, the fruits that constitute the samples in scientific research come from the direct collection of the palms in natural environment [11,40,42,43], experimental centers [31,38,45], and germplasm bank [12], and were also acquired from markets [39,44] and processing companies [20,39,41]. At the industry level, palm fruits are processed to obtain oils and fats [7], and research laboratories evaluated the production of biodiesel from O. bacaba oil [15].
3.2. Trees
A total of twenty-six species of fruit-producing trees that have seeds and/or pulps with high lipid content were recently reported in the literature (Table 1). The studies used raw materials (fruits, seeds, and pulp) in natura collected from the field [25,48,50,51,52,53,54,57,59], experimental centers [49,60], agricultural fields [18,53,61], commercial centers [39,55], and processing companies [56]. Another set of the studies used derivatives of Amazonian raw materials from universities [15] and processing companies [6,7,30,58]. Based on the number of studies with Amazonian raw materials (in natura products and derivatives), the country that stood out most was Brazil [6,7,15,18,25,30,39,52,53,55,59,60,61], followed by Indonesia [49], Ecuador [50], Colombia [54], Malaysia [48], Chile [57], China [58], and India [51]. Although countries such as Indonesia, Malaysia, Chile, China, and India do not belong to the Amazon region, scientific studies conducted in those countries involved raw materials from species that grow in those countries [48,49,51,57] and that also grow in the Amazon region. It could also be observed that they used processed raw materials, probably from the Amazonian regions [58].
The processing of tree fruits presents some particularities due to the morphological characteristics of each species. Usually, the processing of Amazonian oils/fats is constituted by unitary operations such as selection, pulping, drying, and milling [25,50,53]. Some species produce fruits with a modified morphology and have no pulp (mesocarp), such as the fruits of Pterodon spp. [55], G. decorticans [57], and others, requiring shelling and dehulling operations, both mechanical unit operations performed manually [18,48,54,57].
3.3. Other Plants
Other Amazonian plants with high lipid potential include various classifications, such as liana, cactus, climbing shrub, scrambling subshrub, and rhizomatous geophyte (Table 1). Table 1 shows seven oilseed-producing species from Brazil [7,19,30], Colombia [33,65], Ecuador [34], and Romania [32]. Some of the species of these groups are widely produced in the agricultural sector in several countries that do not even belong to the Amazon region, such as S. lycopersicum [32], P. edulis [30], H. megalanthus [34], and P. volubilis [65]. According to our review, it was verified that the scientific studies used nuts, seeds, and seed oils, which were acquired from processing companies; this allows us to deduce that there is an interesting level of industrialization for the productive sector.
AOFs have various properties and particularities that differentiate them from oils and fats obtained from conventional oilseeds. Some of these particularities are described below:
The oil obtained from Oenocarpus bacaba, Platonia insignis, Bertholletia excelsa, and Elaeis guineensis has great potential for the production of biodiesel; a negative aspect of these oils is the presence of metals, considered contaminants, which occur naturally or are produced in the catalysis reaction in the process of obtaining biodiesel [15]. The species J. curcas and M. oleifera Lamarck also have the potential for the production of biodiesel [51,52] due to their high content of monounsaturated fatty acids, especially oleic acid, although there are varieties of J. curcas that produce seeds with a higher content of linoleic acid [51]. On the other hand, the oil obtained from the seeds of M. oleifera was subjected to transesterification, obtaining 86% of an ester with technical characteristics of adequate quality for the production of biodiesel [52].
In a recent study, a variety of oil palm (Elaeis oleifera Quepos (CB9204)) has been reported to produce fruits with a higher content of tocotrienols (890 μg/g of oil) and vitamin E (892 μg/g of oil) compared to varieties of E. guineensis; the content of these compounds was evaluated in oil extracted by conventional methods [38]. On the other hand, Acrocomia aculeata is a widely distributed species in the Amazon region; the fruit of this palm has productivity and oil quality like palm oil and can be a substitute for this crop in the Amazon region. Its oil yield from the pulp is about 75%, and from the seeds is 65% [46]. The oils obtained from C. guianensis, O. bataua, and A. speciosa are also rich in vitamins (A and E) and unsaturated fatty acids, which gives them medicinal properties for the treatment of skin diseases, being used empirically in Amazon populations as natural cosmetics [14].
The use of AOFs in the formulation of functional foods is a promising alternative for the food industry because they contain fatty acids of various degrees of unsaturation. The degree of unsaturation of fatty acids gives oils and fats different indices of atherogenicity and thrombogenicity, which are important characteristics for the formulation of healthier foods. These characteristics were observed in the fat extracted from T. grandiflorum, and the oil from P. macroloba and P. edulis [6]. Also, the oil obtained from the pulp of C. brasiliense has medicinal and health-promoting potential because it is rich in unsaturated fatty acids, carotenoids, and phenolic compounds [62]. H. megalanthus, known as the dragon fruit, is cultivated in many Asian countries, as well as in several countries that belong to the Amazon region and other countries with a tropical climate; the oil from its seeds is an interesting source of linoleic acid, which represents 69% of the total composition of its fatty acids [34]. The oleic, linoleic, and linolenic acid composition of oil from E. oleracea, A. hypoglauca, M. aculeata, Byrsonima crassifolia, B. coccolobifolia, B. odora, A. acaule, B. gasipaes var. red, and B. gasipaes var. yellow was evaluated by nuclear magnetic resonance, all the oils presented a high content of oleic acid in relation to the other fatty acids, followed by linoleic acid, which was in higher concentration in the oil of three species (A. hypoglauca, B. crassifolia, and B. coccolobifolia), while linolenic acid was found in low concentration in the oil of all species, varying from 0.3 to 2.4% [39].
O. phalerata oil, A. murumuru fat, and P. insiginis fat have a higher content of saturated fatty acids, such as lauric and palmitic acid, a higher proportion of monounsaturated fatty acids was observed in Mauritia vinifera oil and P. filamentosa, and a high content of polyunsaturated fatty acids was observed in the seeds of Passiflora spp.; the presence of unsaturated fatty acids present functional properties of great importance for the prevention of human health [7].
The AOFs, like the oils and fats of commercial oilseeds, have a higher composition of acylglycerides, made up of saturated, unsaturated and/or polyunsaturated fatty acids. Studies have shown that the fatty acid composition of the acylglycerides of oils and fats strongly influences the thermodynamic properties associated with phase transitions, which represent an important technological characteristic of food processing. Accordingly, it has been observed that the oil from the pulp of A. vulgare Mart. (Tucumã) is similar to the oil from the pulp of M. flexuosa Mart., the oil from the seed of H. brasiliensis is similar to the oil from the seed of P. edulis, and the oil from the seed of A. vulgare Mart is similar to the fat of the seeds of V. surinamensis [30].
AOFs contain other lipid or fat-soluble compounds that have biological activity of great interest for medical and/or pharmacological applications; in this group of compounds are fucosterol and β-sitosterol. Both compounds were detected in the ethereal extract obtained from the seeds of S. macrophylla; this species is used in Malaysia for the treatment of diabetes mellitus [48]. S. macrophylla is a species that is widely distributed in the Amazon region. Recently, the antihyperglycemic activity of seed extracts obtained with different organic solvents was studied, which showed a promising effect for the treatment of this condition [48].
The presence of bioactive compounds in AOFs may contribute to their functional properties. For example, in the case of oil obtained from the fruit of G. macarenensis, characterized by its higher fraction of unsaturated acids and the presence of bioactive compounds, the latter confer antioxidant properties [50].
While it is true that the raw materials of Amazonian origin that we present in this review are potential sources of oils rich in bioactive molecules, we should not ignore other macronutrients, such as proteins, for example, the seeds of P. tetragonolobus, H. brasiliensis and T. catappa, have an interesting protein content for the industry [49]. After the extraction of the oil from the seed of these three species, the solid by-product of the extraction process has a higher concentration of protein, which is ideal for incorporation in the formulation of feed rations for ruminants [49].
4. Conventional Extraction of Amazonian Oils/Fats
Table 2 and Table 3 summarize some studies with Amazonian oleaginous raw materials extracted by conventional methods. The conventional methods used for the recovery of Amazonian oils/fats were grouped into mechanical methods (Table 2) and methods using organic solvents (Table 3).
Extraction with organic solvents allows for obtaining a higher yield compared to mechanical methods. This can be attractive for the industry, but studies show that the application of organic solvents affects the physicochemical and compositional characteristics of lipid extracts [31,52,67]. When hexane was used as an extraction solvent to obtain oil from M. oleifera seeds, the oil obtained presented a high acidity index (20.5 mg KOH/g oil) compared to the oil obtained by mechanical pressing (8.8 mg KOH/g oil); this indicates that the oil obtained with hexane is of lower quality than the oil obtained by mechanical pressing, due to the high content of free fatty acids [52]. Using hexane with two extraction methods (Soxhlet and maceration), the maceration method obtained a higher yield (30%) of oil from P. edulis seeds, but the yield decreased (19.9%) when replaced with ethanol; on the other hand, the acidity index of the oil obtained with ethanol was higher than the oil obtained with hexane [67]. On the other hand, when studying the extraction of oil from E. precatoria, M. flexuosa, and O. bataua pulps, it was observed that the acidity index was higher for oils obtained by mechanical pressing compared to the extraction method with organic solvent [31]; this difference in relation to the other studies reported in the literature may be due to the type of mechanical pressing machine used and the type of organic solvent used. Extraction methods using organic solvents present a higher extraction yield than mechanical methods but can generate negative effects on the composition and quality of Amazonian oil that may limit its application in the natural products industry. Conventional methods using organic solvents to obtain oils are highly polluting. Alternatively, other emerging technologies can be applied, such as supercritical fluids [68], which will be described in the following section.

Table 2.
Mechanical extraction of AOFs.
Table 2.
Mechanical extraction of AOFs.
| Raw-Material | Extraction Methods | Yield | |
|---|---|---|---|
| Moringa oleifera Lamarck | Mechanical pressing | 11% | [52] |
| Caryocar brasiliense Camb | Hydraulic pressing Pressure: 0.5 ton/cm2 Temperature: 30 °C | 32% | [62] |
| Hydraulic pressing + enzymatic treatment Pressure: 0.5 ton/cm2 Temperature: 30 °C | 39% | ||
| Euterpe edulis Mart. | Cold pressing Temperature: 25 °C Raw-material moisture: 10% | 10% | [41] |
| Hot pressing Temperature: 50 °C Raw-material moisture: 10% | 20% | ||
| Persea americana Mill. | Cold pressing Pressing force: 9 tons Raw-material moisture: 5–6% | 42% | [53] |
| Passiflora edulis | Mechanical pressing | 19.5% | [67] |
| Jatropha curcas | Bielenberg press | 26% | [69] |
| Jatropha curcas | Screw pressing Temperature: 80 °C Raw-material moisture: 4.5% | 16.2% | [70] |
| Parinari pachyphylla Rusby | Hydraulic pressing | - | [54] |
| Euterpe precatoria | Expeller press Temperature: 25–30 °C Particle size: 1 mm | 10% | [31] |
| Mauritia flexuosa | 22% | ||
| Oenocarpus bataua | 28% |

Table 3.
Extraction with organic solvents of AOFs.
Table 3.
Extraction with organic solvents of AOFs.
| Raw-Material | Extraction Conditions | Yield | |
|---|---|---|---|
| Jatropha curcas L. | Soxhlet Solvent: hexane Temperature: 70–80 °C Time: 8–10 h | 13.7–54.4% | [51] |
| Moringa oleifera Lamarck | Soxhlet Solvent: hexane Time: 4 h | 36% | [52] |
| Swietenia macrophylla king | Maceration Solvent: petroleum ether Temperature: 60–80 °C | 8.6% | [48] |
| Maceration Solvent: Chloroform Temperature: 60–80 °C | 6.2% | ||
| Maceration Solvent: Methanol Temperature: 60–80 °C | 5.4% | ||
| Psophocarpus tetragonolobus | Soxhlet Solvent: petroleum ether Time: 6 h Particle size: 1 mm | 20% | [49] |
| Hevea brasiliensis | 37.6% | ||
| Terminalia catappa | 52.1% | ||
| Gustavia macarenensis Philipson | Soxhlet Solvent: hexane Time: 4 h Particle size: 1 mm | 53.6% | [50] |
| Euterpe precatoria | Soxhlet Solvent: Petroleum benzene Temperature: 40–60 °C Particle size: 1 mm | 18% | [31] |
| Oenocarpus bataua | 62% | ||
| Mauritia flexuosa | 33% | ||
| Euterpe oleracea | Soxhlet Solvent: hexane Particle size: 0.84–0.42 mm | 35.4% | [39] |
| Annona hypoglauca | 15% | ||
| Oenocarpus bacaba | 43.9% | ||
| Mauritia flexuosa | 23.2% | ||
| Mauritia aculeata | 26% | ||
| Byrsonima crassifolia | 46.5% | ||
| Byrsonima coccolobifolia | 38% | ||
| Bactris gasipaes var. yellow. | 17.1% | ||
| Bactris gasipaes var. red | 27.3% | ||
| Barcella odora | 12% | ||
| Astrocaryum acaule | 33.1% | ||
| Acrocomia aculeata | Soxhlet Solvent: hexane Temperature: 69 °C Time: 8 h Raw-material moisture: 3.6% Particle size: 0.5 mm | 25.6% | [47] |
| Soxhlet Solvent: Dichloromethane Temperature: 40 °C Time: 8 h Raw-material moisture: 3.6% Particle size: 0.5 mm | 26.8% | ||
| Acrocomia aculeata | Soxhlet Solvent: hexane Temperature: 68 °C Time: 8 h Raw-material moisture: 3.3% Particle size: 0.841 mm | 43.6% | [17] |
| Plukenetia volubilis L. | Soxhlet Solvent: hexane Temperature: 60 °C Time: 20 h Raw-material moisture: 2.36% Particle size: 1.51–2.01 mm | 40.6% | [65] |
| Passiflora edulis | Soxhlet Solvent: hexane Time: 4 h | 23.6% | [67] |
| Maceration Solvent: hexane Time: 24 h | 30% | ||
| Maceration Solvent: ethanol Time: 24 h | 19.9% | ||
| Virola surinamensis (Rol. ex Rottb.) Warb. | Soxhlet Solvent: Petroleum ether Time: 4 h Raw-material moisture: 2.5% Particle size: 0.24 mm | 61.4% | [23] |
| Cyphomandra betacea (Cav.) Sendtn. | Soxhlet Solvent: hexane Time: 6 h Raw-material moisture: 4.97% Particle size: 0.68 mm | 24.1% | [71] |
5. SFE-CO2
5.1. Processes Parameters
SFE can be considered a unit operation in the production process of extracting oils and fats from different raw materials. The processing of oils and fats with supercritical fluids includes stages such as preparation/conditioning of raw material, preparation of the extraction unit with supercritical fluids prior to extraction, SFE extraction, and cleaning of the extraction unit.
Conditioning of Amazonian raw materials for the SFE process at the laboratory scale involves different unitary operations, such as dehulling, drying, and milling of A. aculeatum seeds [72], pulping to obtain passion fruit seeds and freeze-drying of seeds [73], pulping and freeze-drying of A. vulgare pulp [44]; these operations were implemented for in natura raw materials and depending on the type of source (seeds, pulp, other parts) of oils/fats these unit conditioning operations may vary for SFE extraction. The reader is referred to the articles cited in Table 4 for the unit operations implemented for various in natura feedstocks and some agro-industrial residues.
Based on our experience, preparing SFE equipment for laboratory and pilot-scale extraction is quite similar. In the first instance, we must point out that the SFE equipment requires auxiliary equipment for its operation, such as an air compressor to activate the CO2 compression pump and a cooling bath to cool the head of the CO2 compression pump. First, the two auxiliary units must be turned on. The air compression pump must reach 6 bar pressure, and the cooling bath should be −2 °C, adjustable as needed. Commercial pilot plants and industrial SFE equipment are usually equipped with electric CO2 compression pumps. On the other hand, the extraction vessel is loaded with the previously conditioned raw material; depending on the configuration and dimensions of the vessel, the extraction bed can be filled completely or partially, and the empty space of the vessel can be filled with an inert material (such as glass beads), which allows a better economy of the solvent. Once the extraction vessel is ready, the operating conditions of temperature and pressure are conditioned. With the conditioning of pressure and temperature, the extraction of the components without CO2 flow starts, commonly called static extraction and can have different durations (5 min [43], 30 min [12], 50 min [19], among others). After the static extraction, the dynamic extraction begins; in this stage, a constant extraction flow is established. Depending on the supercritical conditions and the oil and fat content of the raw material, the SFE process time may vary until complete extraction.
After the SFE extraction, the equipment must undergo cleaning for further extraction operation. Usually, the cleaning consists of passing a solvent (ethanol, water and a mixture of both) through the piping system and valves of the SFE equipment. Some oily extracts may have components that are difficult to clean with the mentioned solvents; in that situation, it is possible to use cleaners recommended for the pharmaceutical industry, such as alkaline cleaners. However, conditions of use must be established, such as concentration, temperature, and volume of cleaner to be used depending on the raw material under extraction.
In general, the factors that can influence the yield and composition of oils and fats in the SFE process are particle size, CO2 flux, type of polarity modifier (cosolvent), and cosolvent concentration, pressure, and temperature (Table 4). Thermodynamic parameters (temperature and pressure) influenced the oil extraction yield of P. edulis seeds [74], the fatty acid composition of O. distichus oil [12], the microbial activity of V. surinamensis seed oils [23], and the antioxidant activity of B. gasipaes oils [40]. When evaluating the effect of pressure, temperature, and mass flow on P. volubilis oil extraction, it was observed that they affected the yield, quality indexes, and oil composition [65]. The use of ethanol as a polarity modifier in the supercritical extraction of M. maripa oil increased the extraction yield and composition [42]. In C. annuum oil extraction, the parameters of temperature, pressure, extraction time, and particle size influenced the extraction yield [75]. The yield and composition of D. alata almond oil were influenced by the factors of pressure, temperature, cosolvent type, and cosolvent concentration [21]. On the other hand, scale-up studies to obtain AOFs by SFE are limited; the most recent study reports a new scale-up method based on a dimensionless number called Gama (Ga) [76]. This new method allowed the reproduction of similar oil extraction yields from A. vulgare pulp for small and large scale [76]. Another successful scale-up experience was carried out with P. volubilis seeds, the scaling technique was to keep constant the ratio between CO2 flow (g/min) and raw material mass (g) [65]. These scale-up experiments are promising, but further studies are needed for other Amazonian raw materials, and other aspects such as stability, shelf life, and bioactive properties of AOFs should also be evaluated.

Table 4.
SFE of AOFs.
Table 4.
SFE of AOFs.
| Raw-Material | Extraction Conditions | Yield | |
|---|---|---|---|
| Passiflora edulis seeds | Temperature: 60 °C Pressure: 35 MPa Time: 2.5 h Raw-material moisture: 10% | 15.7% | [74] |
| Temperature: 60 °C Pressure: 20 MPa Time: 1.34 h | 21.6% | [67] | |
| Temperature: 60 °C Pressure: 35 MPa Time: 2.5 h Raw-material moisture: 7.8% Flow rate: 30 g/min | 15.7% | [33] | |
| Oenocarpus distichus Mart. pulp | Temperature: 50 °C Pressure: 35 MPa Time: 3 h | 45.2% | [12] |
| Temperature: 60 °C Pressure: 27 MPa Time: 3 h | 46% | ||
| Cyphomandra betacea (Cav.) Sendtn. seeds | Temperature: 51 °C Pressure: 38 MPa Time: 2.5 h S/F: 22.5 Raw-material moisture: 4.5% Particle size: 0.69 mm | 17.4% | [71] |
| Oenocarpus bacaba pulp | Temperature: 60 °C Pressure: 42 MPa Time: 3 h Raw-material moisture: 4.2% | 60.4% | [77] |
| Plukenetia volubilis L. seeds | Temperature: 60 °C Pressure: 50 MPa Time: 3 h Raw-material moisture: 2.4% Particle size: 1.5–2 mm | 23.5% | [65] |
| Temperature: 60 °C Pressure: 45 MPa Time: 4.5 h Raw-material moisture: 3.3% Particle size: 1.64 mm | 26.8% ** | ||
| Lycopersicon esculentum L. seeds | Temperature: 60 °C Pressure: 35 MPa Time: 2 h Particle size: 1 mm | 7.8% | [78] |
| Virola surinamensis seeds | Temperature: 80 °C Pressure: 35 MPa Time: 3 h Raw-material moisture: 2.5% Particle size: 0.24 mm | 64.4% | [23] |
| Acrocomia aculeata kernel | Temperature: 40 °C Pressure: 22 MPa Time: 3.3 h Raw-material moisture: 3.3% Particle size: 0.481 mm | 41.6% | [17] |
| Pterodon spp. fruits | Temperature: 50 °C Pressure: 20 MPa Time: 4.5 h Raw-material moisture: 2.3% Solvent flow rate: 12 g CO2/min Particle size: 0.57 mm | 31.1% | [55] |
| Pentaclethra macroloba seeds | Temperature: 40 °C Pressure: 30 MPa Solvent flow rate: 0.7 kg CO2/h Particle size: 1.19 mm | 42.05% | [56] |
| Temperature: 40 °C Pressure: 30 MPa Time: 120 min Solvent flow rate: 4 mL/min Particle size: 1.19 mm | 23.3% | [79] | |
| Solanum lycopersicum seeds | Temperature: 60.2 °C Pressure: 40 MPa Solvent flow rate: 64.6 g/min Particle size: 1.00 mm | 16.9% | [80] |
| Psidium guava seeds | Temperature: 50 °C Pressure: 45 MPa Time: 60 min Raw-material moisture: 8% Co-solvent: ethanol at 3 mL/min | 10.45% | [81] |
| Temperature: 52 °C Pressure: 35.7 MPa Time: 150 min Solvent flow rate: 30 g CO2/min Raw-material moisture: 8.05% Average particle diameter: 0.490 mm | 8.6% | [82] | |
| Maximiliana maripa pulp | Pure CO2 Temperature: 60 °C Pressure: 20 MPa Time: 170 min Solvent flow rate: 2 cm3/min Raw-material moisture: 11.7% Mean particle diameter: 1.6 × 10−3 m | 3.6% | [42] |
| CO2 + Ethanol Temperature: 60 °C Pressure: 20 MPa Time: 60 min Raw-material moisture: 11.7% Average particle diameter: 1.6 × 10−3 m | 20.4% | ||
| Pterocaulon lorentzii leaves and inflorescences | Temperature: 40 °C Gradual pressure increments: 9 to 30 MPa Time: 100 (leaves) and 110 min (inflorescences) Solvent flow rate: 1 kg/h Raw-material moisture: 5.27 (inflorescences) and 2.08% (leaves) Average thickness of fibers: 0.061 (inflorescences) and 0.148 mm (leaves) | 109.05 (leaves) and 610.03 mg coumarins/g of extract (inflorescences) | [83] |
| Lippia graveolens HBK leaves. | Temperature: 45 °C Pressure: 35 MPa Time: 300 min Solvent flow rate: 30 g CO2/min | 4.1% | [84] |
| Capsicum annuum L. fruits | Temperature: 65 °C Pressure: 35.78 MPa Time: 90 min Particle size: 0.78 mm Raw-material moisture: 4–6% Solvent flow rate: 3 L/min | 4.91% | [75] |
| Genipa americana L. fruits | Temperature: 60 °C Pressure: 30 MPa Mean particle diameter: 0.23 mm Raw-material moisture: 5.1% Solvent flow rate: 2.5 g CO2/min | 4.6% | [85] |
| Bactris gasipaes epicarp | Temperature: 40 °C Pressure: 40 MPa Time: 210 min Raw-material moisture: 7.1% | 12.12% | [40] |
| Geoffroea decorticans almond | Temperature: 60 °C Pressure: 40 MPa Time: 120 min Raw-material moisture: 4.93% Solvent flow rate: 3.62 g CO2/min | 47% | [57] |
| Dipteryx alata Vogel seeds | CO2 + water Temperature: 60 °C Pressure: 20 MPa Time: 240 min Raw-material moisture: 2.30 g/100 g Mean particle diameter: <0.85 mm Solvent flow rate: 3 mL/min | 21.67 g/100 g | [21] |
| CO2 + Alcohol Temperature: 50 °C Pressure: 30 MPa Time: 240 min Raw-material moisture: 2.30 g/100 g Mean particle diameter: <0.85 mm Solvent flow rate: 3 mL/min | 31.06 g/100 g | ||
| CO2 + Alcohol:water Temperature: 60 °C Pressure: 20 MPa Time: 240 min Raw-material moisture: 2.30 g/100 g Mean particle diameter: <0.85 mm Solvent flow rate: 3 mL/min | 21.67 g/100 g | ||
| CO2 Temperature: 45 °C Pressure: 35 MPa Time: 185 min Average particle diameter: 1.8 mm | 22 g/100 g | [25] | |
| Capsicum annuum industrial waste | CO2 Temperature: 60 °C Pressure: 20 MPa Mean particle diameter: 0.48 mm Solvent flow rate: 3 g CO2/min | 9.60% | [86] |
| CO2 + Ethanol Temperature: 40 °C Pressure: 25 MPa Mean particle diameter: 0.48 mm Solvent flow rate: 3 g CO2/min Ratio ethanol/sample: 0.5 mL/g | 10.08% | ||
| Mauritiella armata Mart. pulp | Temperature: 40 °C Pressure: 30 MPa Time: 61 min Mean particle diameter: 2.107 mm Solvent flow rate: 17 g/min | 41.57 g/100 g | [43] |
| Cyperus esculentus L. nut | Temperature: 40 °C Pressure: 28 MPa Time: 90 min | 28.56 g/100 g | [87] |
| Rubus glaucus seeds | Temperature: 60 °C Pressure: 35 MPa Time: 150 min Raw-material moisture: 7.60% Solvent flow rate: 30 g/min | 14.5% | [33] |
| Annona squamosa seeds | Temperature: 45 °C Pressure: 25 MPa Time: 60 min Solvent flow rate: 2.5 mL/min Raw-material moisture: 6.21% Average particle size: 427 nm | 0.29 g/g | [88] |
| Annona muricata L. seeds | Temperature: 40 °C Pressure: 30 MPa Time: 210 min Solvent flow rate: 0.7 kg/min Raw-material moisture: 4.98% Mean particle size: 0.53 mm | 16.4% | [89] |
| Pachira aquatica Aubl. seeds | Temperature: 60 °C Pressure: 30 MPa Time: 120 min Solvent flow rate: 1.21 kg/h Raw-material moisture: 3.25% Average particle size: 1.19 mm | 51.78% | [90] |
| Bertholletia excelsa cake (by-products) | Temperature: 60 °C Pressure: 40 MPa Time: 180 min Solvent flow rate: 40 g/min Raw-material moisture: 0.25% Particle size: <1 mm | 59.2% | [13] |
| Renealmia petasites Gagnep. seeds | Temperature: 40 °C Pressure: 35 MPa Time: 40 min Solvent flow rate: 1.06 × 10−4 kg CO2/s | 4.15% | [19] |
| Gliricidia sepium seeds | Temperature: 60 °C Pressure: 30 MPa Time: 90 min Solvent flow rate: 2.5 mL/min | 11.79% | [91] |
| Oenocarpus mapora H. Karst fruit | Temperature: 60 °C Pressure: 35 MPa | 7.45% | [20] |
| Temperature: 43.5 °C Pressure: 27.07 MPa Time: 120 min Solvent flow rate: 39 g/min Raw-material moisture: 6% Particle size: 350–500 μm | 30.4 g/100 g | [22] | |
| Mauritia flexuosa L.f. pulp | Temperature: 42 °C Pressure: 20 MPa Time: 60 min Solvent flow rate: 42 g CO2/min | 44.85% | [11] |
| Carica papaya seeds | Temperature: 65 °C Pressure: 25 MPa Time: 240 min Solvent flow rate: 15 g CO2/min Particle size: 0.26 mm Co-solvent flow rate: 10% of CO2 flow rate | 36.67% | [92] |
| Pterodon emarginatus Vogel seeds | Temperature: 40 °C Pressure: 30 MPa Time: 65 min Solvent flow rate:4.5 g CO2/min Average particle size: 0.87 mm | 40 g/100 g | [59] |
| Dipteryx lacunifera Ducke cake | Temperature: 40 °C Pressure: 20 MPa Time: 240 min Solvent flow rate: 1.0 kg/h Raw-material moisture: 4.50 g/100 g | 15.52% | [60] |
| Astrocaryum vulgare pulp | Temperature: 60 °C Pressure: 30 MPa Time: 180 min Solvent flow rate: 8.85 × 10−5 kg/s Average particle size: 1 mm Raw-material moisture: 5% | 36.75% | [44] |
| Caryocar brasiliense Cambess almonds | Temperature: 45 °C Pressure: 25 MPa Time: 110 min Solvent flow rate: 5.0 g/min Average particle size: 3.5 g/min | 27.6% | [61] |
| Hyptis suaveolens (L.) Poit seeds | Temperature: 80 °C Pressure: 45 MPa Time: 193 min Solvent flow rate: 0.88 kg/h Raw-material moisture: 8.94% Particle size: > 75 μm | 62.36% | [93] |
| Passiflora ligularis Juss. (seeds) | Temperature: 40 °C Pressure: 40 MPa Time: 60 min Solvent flow rate: 3.62 g/min Raw-material moisture: 1.57% Mean particle diameter: 0.60 mm | 24.97% | [73] |
| Astrocaryum aculeatum almonds | Temperature: 50 °C Pressure: 25 MPa Solvent flow rate: 1.8 × 10−4 kg/s Raw-material moisture: 3.7% Particle size: 1.19 mm | 34.41 g/100 g | [72] |
** Pilot scale.
5.2. SFE Equipment
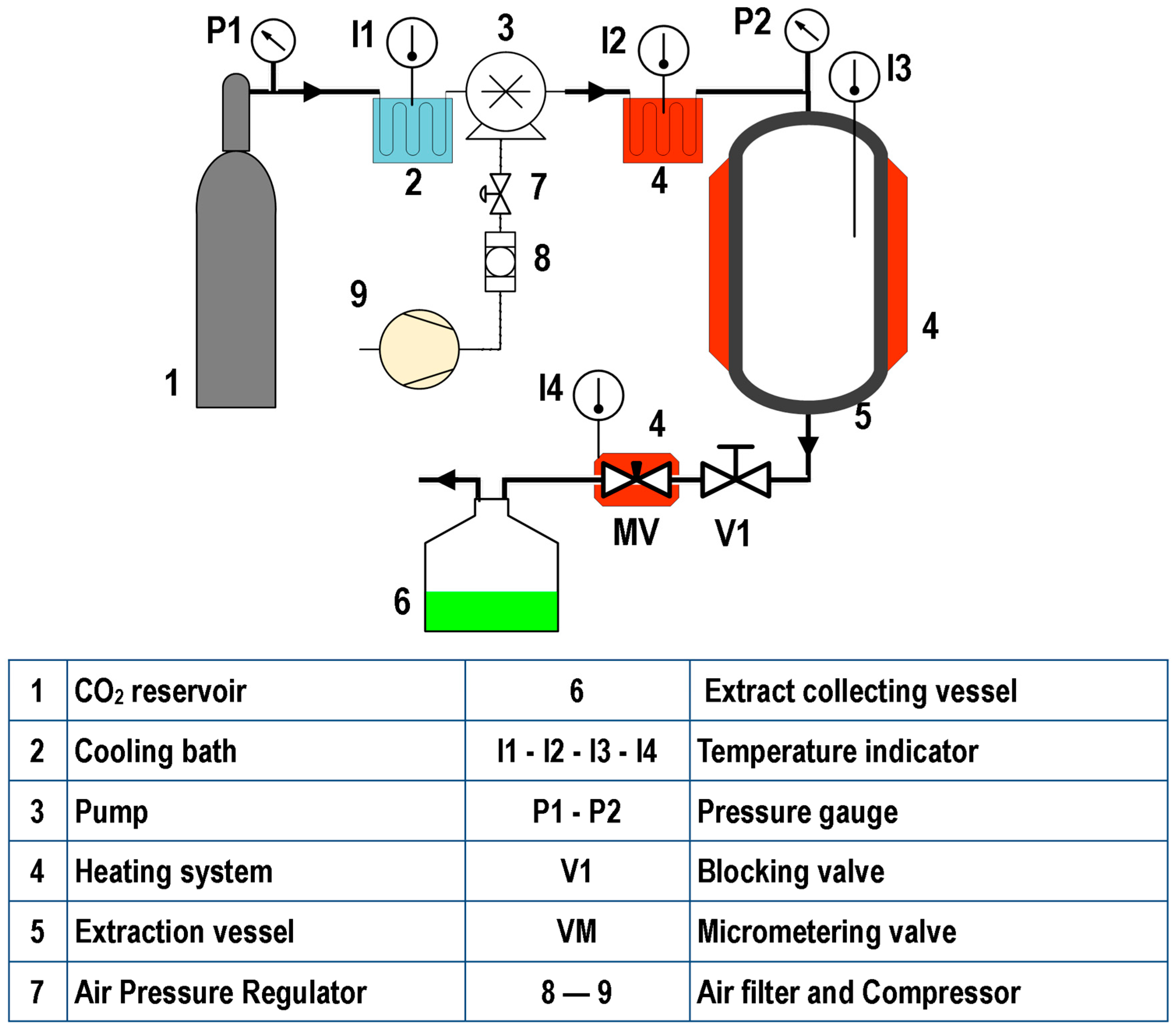
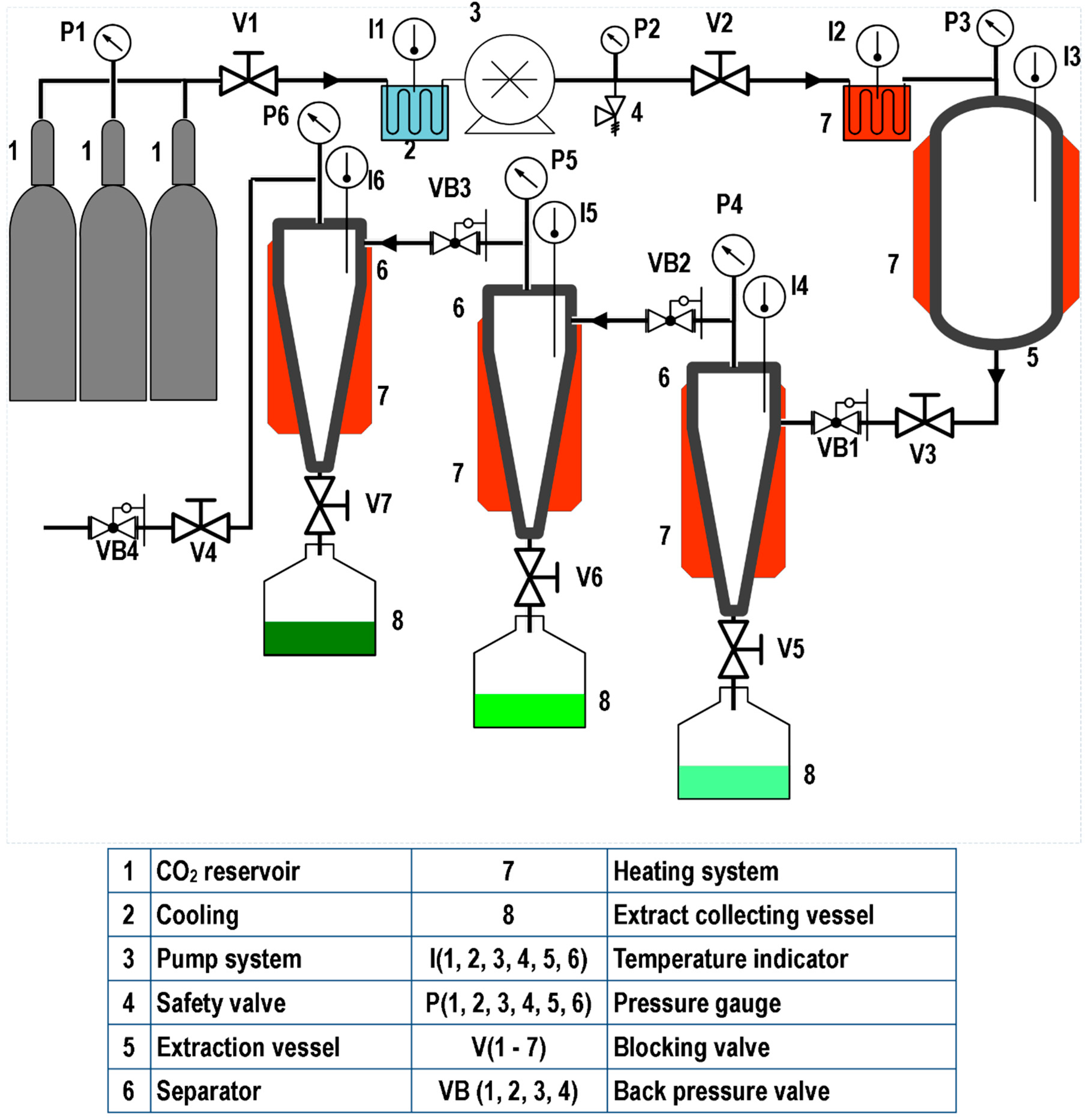
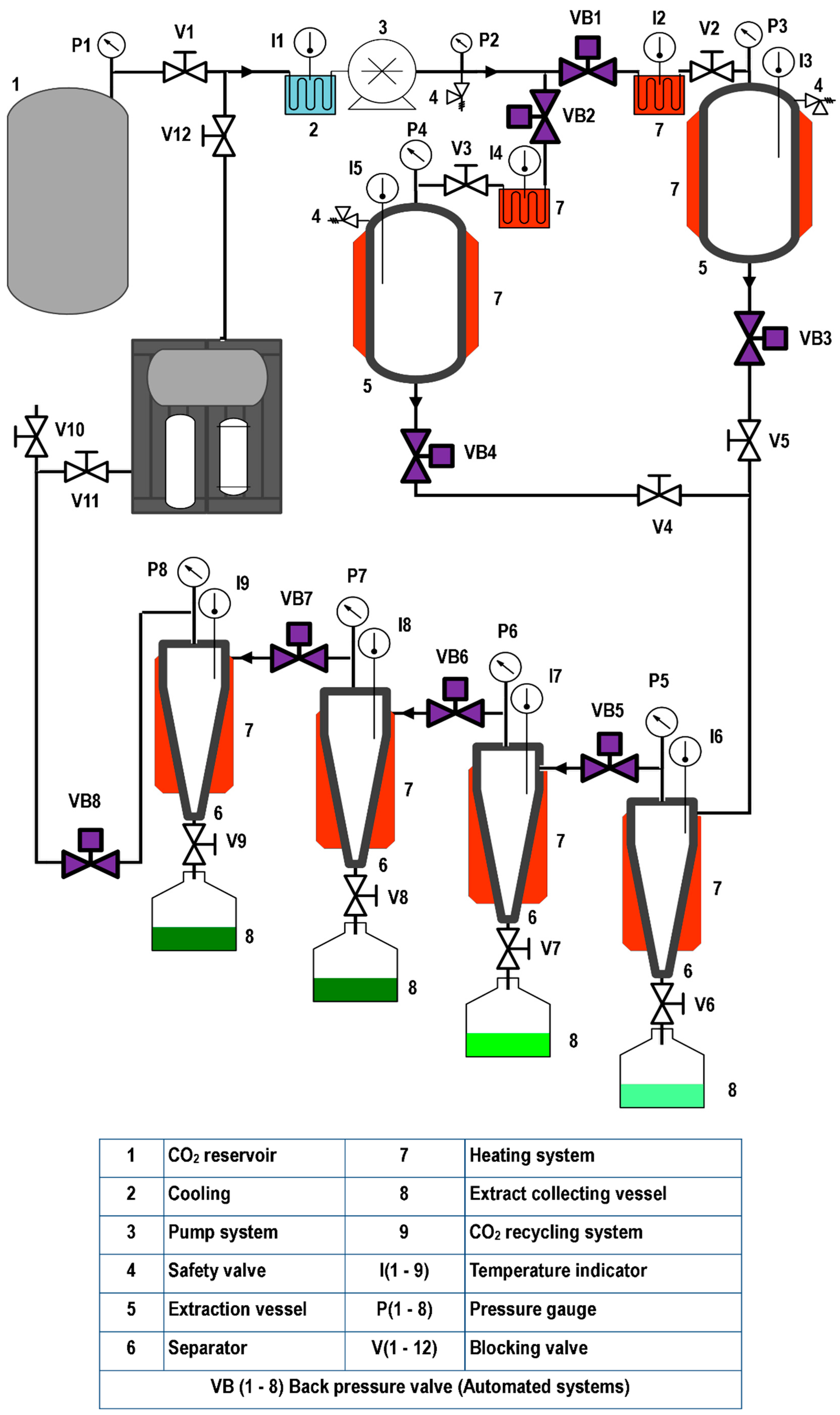
Figure 1, Figure 2 and Figure 3 illustrate representative diagrams of laboratory, pilot, and industrial-scale SFE equipment. Usually, laboratory-scale SFE equipment is used for studies to determine the dynamic extraction behavior and to determine thermodynamic conditions of extraction of bioactive components from raw materials. The scale-up of the SFE process involves some modifications in the configuration of the SFE equipment, mainly due to the volume of CO2 consumption; in laboratory scale equipment, the extract is collected at the end of the extraction line immediately after depressurization during dynamic extraction. This is different in pilot plant (Figure 2) and industrial scale (Figure 3) equipment, requiring separation vessels located after the extraction vessel in order to progressively reduce the solvent pressure to a pressure that allows its reuse through a recirculation system; in pilot scale equipment, the recirculation system may or may not be included (Figure 2). In the literature consulted, studies are described that used pilot plant and industrial-scale SFE equipment for the extraction of oils/fats from Amazonian raw materials. In the extraction of oil from P. edulis seeds, a pilot scale equipment with a 0.5 L vessel, incorporated with two 0.5 L separation vessels, was used to obtain 15.7% of oil rich in linoleic acid [33]. A different configuration of pilot SFE equipment was used in the extraction of oil from O. mapora pulp; the equipment consisted of a 1 L capacity vessel [22]. In the extraction of oleoresins from L. graveolens, equipment consisting of an extraction vessel, a separator and a CO2 recirculation system was used; according to the results of the study, this technology proved to be efficient [84]. Another pilot SFE equipment configuration was used for the recovery of oil from B. excelsa cake from beverage production; the equipment consisted of a 273 mL vessel with two 0.5 L separators [13]. A semi-industrial unit with a 4.2 L extraction vessel was used for the extraction of oil from tomato waste [66]. In another work, a unit with a 12 L extraction vessel and two 6 L separators was used to obtain omega-3-rich P. volubilis oil [65].
Figure 1.
Laboratory SFE equipment.
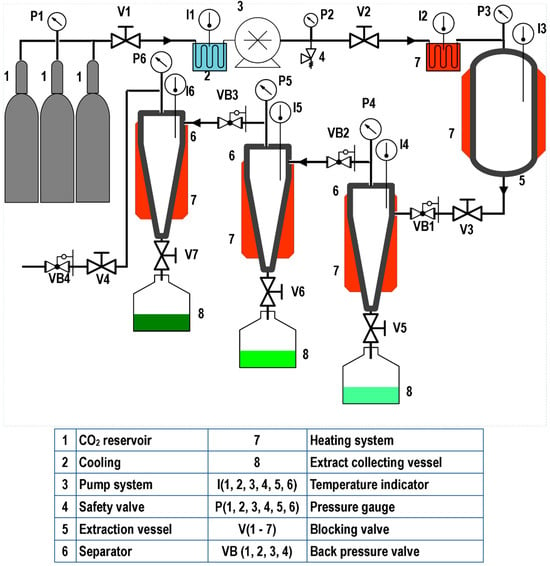
Figure 2.
Pilot SFE equipment.
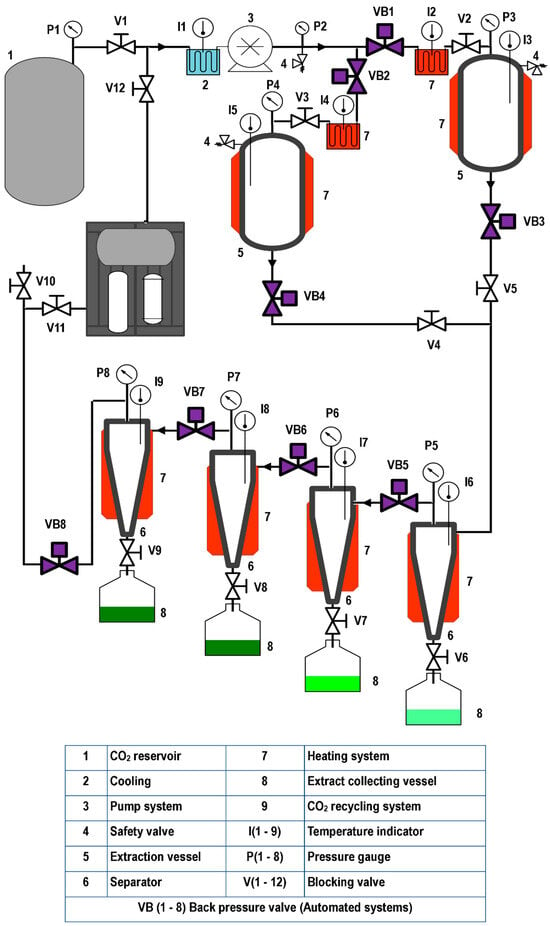
Figure 3.
Industrial SFE equipment.
6. Fatty Acids from AOFs
Table 5 shows the main fatty acids found in oils and fats extracted from some Amazonian raw materials using SFE. Note that some of these species are not exclusive to the Amazon region and may be found elsewhere. Recent research was conducted using pulp, seeds, fruits, leaves, and by-products (cakes) from the oil and fat industry. Some oils extracted via supercritical fluids from seeds, such as P. edulis [67] and C. betacea [71], are notable for their high content of polyunsaturated fatty acids, particularly linoleic and linolenic acids. On the other hand, oils from other raw materials presented a high content of monounsaturated fatty acids, with oleic acid standing out, which was observed in oils from the pulp of E. oleracea [94], O. distichus [12], O. bacaba [77], and seeds of M. oleifera [95], among others. Several studies have shown the importance of unsaturated fatty acids in human nutrition [96,97]. Unsaturated fatty acids, linoleic (18:2n6) and α-linolenic (18:3n3), are essential fatty acids since the human body cannot synthesize and must be obtained from dietary sources; these are precursors of the long-chain polyunsaturated fatty acids (PUFAs) of the omega-6 and omega-3 family [98,99]. PUFAs are necessary to maintain cell membranes, brain functions, and the transmission of nerve impulses under normal conditions, among other functions [99,100]. Other lipid extracts obtained by supercritical fluids presented a high content of saturated fatty acids, such as the one obtained from seeds of V. surinamensis [23] and pulp of O. distichus [12]. An interesting cis-11-Eicosenoic acid (C20:1) content was observed in extracts obtained from M. oleifera leaves, this fatty acid having antimicrobial properties against antibiotic-resistant Staphylococcus aureus [95]. The oil from the seeds of V. surinamensis also showed antimicrobial activity against S. aureus [23]. The variability of the composition and potential bioactive properties of the fatty acids of AOFs obtained by SFE reveals an opportunity for the development of functional products and the need for further research efforts for new applications.

Table 5.
Main fatty acids of AOFs that are obtained by SFE-CO2 from some Amazonian species.
7. Fat-Soluble Bioactive from AOFs
AOFs are notable for their rich content of bioactive secondary metabolites (Table 6). Table 6 shows some minority compounds present in oil obtained by the SFE process from species occurring in the Amazon region; tocopherols, triterpenes, and phytosterols were identified. Tocopherols were identified in the seed oil of P. edulis [67], A. aculeata [17], and C. betacea [71], with P. edulis oil standing out for its γ-tocopherol content [67]. Tocopherols are characterized by being a natural form of vitamin E, with anti-inflammatory action and antioxidant properties [101]. The squalene triterpene was identified in the species P. edulis [74] and C. betacea [71]. Squalene is biosynthetic precursor for phytosterol and cholesterol [102]. This compound is naturally found in oils such as olive, palm, wheat germ, amaranth, and rice bran oil; however, shark liver oil remains the richest source [102]. Squalene has antioxidant properties, acts on skin hydration, has a preventive effect on breast cancer, is antitumor and cardioprotective, and decreases serum cholesterol levels [103]. Phytosterols were identified in the species P. edulis [67,74], A. aculeata [17], and C. betacea [71], with β-sitosterol having the highest concentration present in P. edulis seed oil [67]. Phytosterols are compounds found in Amazonian oils and fats (Table 6). Phytosterol supplementation can contribute to the treatment of hypercholesterolemia in children and adolescents [104]. The bioactive components of AOFs tend to be lost during refining in industrialization [105]; in that context, it is important to develop adequate extraction and transformation processes to develop quality products with functional properties and commercial value [106]. In this section, we focus on the compounds reported in Table 6, but in the literature consulted, other minority components are reported in oils and fats from Amazonian raw materials obtained by SFE.

Table 6.
Bioactive compounds identified in oils and fats extracted by SFE.
8. Potential Uses and Perspectives of Amazonian Lipids Obtained by SFE
Recent studies on AOFs highlight their considerable potential for health benefits and broad industrial applications, including in the food, pharmaceutical, and cosmetic sectors [7,106]. The thermophysical, chemical, and nutritional properties of fats/oils obtained from Amazonian species, such as T. grandiflorum, A. murumuru, A. vulgare, V. surinamensis, C. guianensis, P. macroloba, O. bacaba, B. excelsa, and O. bataua, are interesting for the development of food products [6,107,108]. Recent research cited in this review shows that the application of supercritical fluids using carbon dioxide as an extraction solvent has proven to be a good eco-friendly alternative to produce Amazonian lipids of high biological value. Although these scientific studies show the advantages of the SFE process compared to conventional techniques, there are still limited scientific studies on the application of AOFs obtained by SFE for product development. But due to their composition of lipid and lipid-soluble compounds, Amazonian oils extracted by supercritical fluids present diverse properties, such as antioxidant capacity [19,20,43,56,81,87,90], capacity to inhibit neurodegenerative enzymes [19], oxidative stability [87], nutraceutical [22,44,81], antibacterial [84], and allelopathic activity [94], which would allow them to be used as ingredients or additives in a wide range of products of interest to the food, pharmaceutical, cosmetic, and other industries. On the other hand, an interesting alternative that requires further research is the use of AOFs for their enrichment with liposoluble components, with the objective of developing functional products. For example, rice oil can be used as a cosolvent for extracting lycopene from papaya by SFE [109], and other applications can be made with conventional oils and fats. Recent research shows a promising horizon for the development of products with raw materials rich in lipids of Amazonian origin for markets specialized in natural products. From a technological perspective, the implementation of an AOFs industry would face the challenge of scaling up the SFE process. Table 4 in Section 5 shows the parameters of the SFE process at non-commercial scales. Process times can be observed in some cases to be considerably long, mainly due to the high content of the lipid fraction in the raw material, making the SFE process considerably extended. In this regard, some innovations have been reported in the literature, such as SFEAP [110], GAME [111], and simultaneous extraction with cold pressing and supercritical fluids [26]. Although these innovations are promising, scaling up to a commercial level is still pending. On the other hand, the complexity of the composition of bioactive molecules in the lipid fraction of Amazonian raw materials could become a challenge for the manufacture of products with specific functional properties. The prospects for industrialization of AOFs obtained by SFE are interesting but challenging for the scientific and industrial community; some aspects that need to be addressed are the supply and preparation of raw materials due to the variability of their physical, chemical, and perishability characteristics of each Amazonian species. On the other hand, it is necessary to address the development of technological packages that allow the processing of AOFs to maintain the functional properties from the raw material to the manufactured product.
9. Conclusions
The Amazon region has favorable soil and climatic conditions for the development of a great diversity of plant species that produce fruits, seeds, pulps, and other parts of the plant, which have a lipid composition and fat-soluble compounds that are promising for various sectors of modern industry. The diversity of Amazonian raw materials represents a challenge for SFE processing due to their physical–mechanical and compositional characteristics, requiring the development of procedures for their conditioning, which involve moisture conditioning, particle size reduction, and shelling of fruits and seeds, among others. The composition of AOFs represents an opportunity for the natural products industry due to their content of polyunsaturated fatty acids and liposoluble secondary metabolites that confer diverse bioactive properties reported in the current scientific literature. The SFE processing of AOFs has been explored at laboratory and pilot scales, requiring commercial scale studies and/or studies on the feasibility and economic viability; in that sense, there may be some limitations due to the seasonality of these raw materials and production areas. The production of AOFs using supercritical fluid technologies is advancing; its potential for various applications in pharmaceuticals, functional foods, and cosmetics is supported due to its composition of bioactive phytochemicals, which makes it stand out from conventional oils and fats. In this context, further efforts in research, development, and innovation are needed for the industrialization of AOFs.
Author Contributions
Conceptualization, L.O.C.-P. and M.A.A.M.; methodology, L.O.C.-P., J.C.M.S. and L.A.P.-C.; writing—original draft preparation, L.O.C.-P., J.P.S., C.L.S.S.M., P.C.-R. and J.C.J.F.; writing—review and editing, L.O.C.-P. and M.A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico Grant BPQ-309825-2020-2.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dupont, J.L. LIPIDS|Chemistry and Classification. In Encyclopedia of Human Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 126–132. [Google Scholar] [CrossRef]
- van Duijn, G. Vegetable Oils and Fats. In Encyclopedia of Food Safety, 2nd ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 1–4, pp. 46–59. [Google Scholar] [CrossRef]
- Talbot, G. Specialty Oils and Fats in Food and Nutrition: Properties, Processing and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Barrufol, M.; Schmid, B.; Bruelheide, H.; Chi, X.; Hector, A.; Ma, K.; Michalski, S.; Tang, Z.; Niklaus, P.A. Biodiversity Promotes Tree Growth during Succession in Subtropical Forest. PLoS ONE 2013, 8, e81246. [Google Scholar] [CrossRef]
- Malhi, Y.; Riutta, T.; Wearn, O.R.; Deere, N.J.; Mitchell, S.L.; Bernard, H.; Majalap, N.; Nilus, R.; Davies, Z.G.; Ewers, R.M.; et al. Logged tropical forests have amplified and diverse ecosystem energetics. Nature 2022, 612, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.V.; Rodrigues, A.M.d.C.; de Oliveira, P.D.; da Silva, D.A.; da Silva, L.H.M. Technological properties of amazonian oils and fats and their applications in the food industry. Food Chem. 2017, 221, 1466–1473. [Google Scholar] [CrossRef]
- Serra, J.L.; da Cruz Rodrigues, A.M.; de Freitas, R.A.; de Almeida Meirelles, A.J.; Darnet, S.H.; da Silva, L.H.M. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Quispe, S.C.; Quispe, H.C.; Rios, E.G.; Viteri, J.E.D.; Chañi-Paucar, L.O.; Berrocal, M.H.M. Efecto de dietas balanceadas con harina de semillas de copoazú (Theobroma grandiflorum) en el crecimiento de Paco (Piaractus brachypomus Cuvier). Livest. Res. Rural. Dev. 2018, 30. Available online: http://www.lrrd.org/lrrd30/1/larr30017.html (accessed on 17 January 2025).
- Pereira, A.L.F.; Abreu, V.K.G.; Rodrigues, S. Cupuassu—Theobroma grandiflorum. In Exotic Fruits; Academic Press: Cambridge, MA, USA, 2018; pp. 159–162. [Google Scholar] [CrossRef]
- de Oliveira, T.B.; Genovese, M.I. Chemical composition of cupuassu (Theobroma grandiflorum) and cocoa (Theobroma cacao) liquors and their effects on streptozotocin-induced diabetic rats. Food Res. Int. 2013, 51, 929–935. [Google Scholar] [CrossRef]
- Best, I.; Cartagena-Gonzales, Z.; Arana-Copa, O.; Olivera-Montenegro, L.; Zabot, G. Production of Oil and Phenolic-Rich Extracts from Mauritia flexuosa L.f. Using Sequential Supercritical and Conventional Solvent Extraction: Experimental and Economic Evaluation. Processes 2022, 10, 459. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; da Silva, M.P.; de Sousa, S.H.B.; Bezerra, P.D.N.; Menezes, E.G.O.; da Silva, N.J.N.; Banna, D.A.D.D.S.; Araújo, M.E.; Carvalho Junior, R.N.D. Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Vasquez, W.V.; Hernández, D.M.; del Hierro, J.N.; Martin, D.; Cano, M.P.; Fornari, T. Supercritical carbon dioxide extraction of oil and minor lipid compounds of cake byproduct from Brazil nut (Bertholletia excelsa) beverage production. J. Supercrit. Fluids 2021, 171, 105188. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Revisiting Amazonian plants for skin care and disease. Cosmetics 2017, 4, 25. [Google Scholar] [CrossRef]
- Alves, B.S.F.; Carvalho, F.I.M.; Cruz, A.S.; Filho, H.A.D.; Dantas, K.G.F. Determination of Ca, Mg, Na, and K in biodiesel of oilseed from northern Brazil. Rev. Virtual Quim. 2018, 10, 542–550. [Google Scholar] [CrossRef]
- Silva, T.C.; Araujo, E.C.G.; da Silva Lins, T.R.; Reis, C.A.; Sanquetta, C.R.; da Rocha, M.P. Non-timber forest products in Brazil: A bibliometric and a state of the art review. Sustainability 2020, 12, 7151. [Google Scholar] [CrossRef]
- Trentini, C.P.; Cuco, R.P.; Cardozo-Filho, L.; da Silva, C. Extraction of macauba kernel oil using supercritical carbon dioxide and compressed propane. Can. J. Chem. Eng. 2019, 97, 785–792. [Google Scholar] [CrossRef]
- Zanqui, A.B.; da Silva, C.M.; Ressutte, J.B.; de Morais, D.R.; Santos, J.M.; Eberlin, M.N.; Cardozo-Filho, L.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Brazil Nut Oil Extraction Using Subcritical n-Propane: Advantages and Chemical Composition. J. Braz. Chem. Soc. 2020, 31, 603–612. [Google Scholar] [CrossRef]
- Santos, L.C.D.; Álvarez-Rivera, G.; Sánchez-Martínez, J.D.; Johner, J.C.F.; Barrales, F.M.; de Oliveira, A.L.; Cifuentes, A.; Ibáñez, E.; Martínez, J. Comparison of different extraction methods of Brazilian “pacová” (Renealmia petasites Gagnep.) oilseeds for the determination of lipid and terpene composition, antioxidant capacity, and inhibitory effect on neurodegenerative enzymes. Food Chem. X 2021, 12, 100140. [Google Scholar] [CrossRef]
- Muñoz, A.M.; Casimiro-Gonzales, S.; Gómez-Coca, R.B.; Moreda, W.; Best, I.; Cajo-Pinche, M.I.; Loja, J.F.; Ibañez, E.; Cifuentes, A.; Ramos-Escudero, F. Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus mapora H. Karst): Evaluating Quality, Polyphenol Content and Antioxidant Activity. Foods 2022, 11, 1518. [Google Scholar] [CrossRef]
- Peixoto, V.O.D.S.; Silva, L.d.O.; Castelo-Branco, V.N.; Torres, A.G. Baru (Dipteryx alata Vogel) Oil Extraction by Supercritical-CO2: Improved Composition by Using Water as Cosolvent. J. Oleo Sci. 2022, 71, 201–213. [Google Scholar] [CrossRef]
- Barriga-Sánchez, M.; Casimiro-Gonzales, S.; Ramos-Escudero, F.; Muñoz, A.M.; Anticona, M. Supercritical CO2 assisted extraction of freeze-dried sinami fruit pulp (Oenocarpus mapora H. karst) oil: An experimental optimization approach. Lwt 2024, 198, 115956. [Google Scholar] [CrossRef]
- Cordeiro, R.M.; Ana, A.P.; Pinto, R.H.H.; da Costa, W.A.; da Silva, S.H.M.; de Souza Pinheiro, W.B.; Arruda, M.S.P.; Carvalho, R.N., Jr. Supercritical CO2 extraction of ucuúba (Virola surinamensis) seed oil: Global yield, kinetic data, fatty acid profile, and antimicrobial activities. Chem. Eng. Commun. 2019, 206, 86–97. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, L.; Kong, F.; Wang, S.; Hong, K.; Lei, F.; He, D. Effects of Extraction Strategies on Yield, Physicochemical and Antioxidant Properties of Pumpkin Seed Oil. Foods 2023, 12, 3351. [Google Scholar] [CrossRef] [PubMed]
- Chañi-Paucar, L.O.; Osorio-Tobón, J.F.; Johner, J.C.F.; Meireles, M.A.A. A comparative and economic study of the extraction of oil from Baru (Dipteryx alata) seeds by supercritical CO2 with and without mechanical pressing. Heliyon 2021, 7, e05971. [Google Scholar] [CrossRef] [PubMed]
- Chañi-Paucar, L.O.; Johner, J.C.F.; Hatami, T.; Meireles, M.A.A. Simultaneous integration of supercritical fluid extraction and mechanical cold pressing for the extraction from Baru seed. J. Supercrit. Fluids 2022, 183, 105553. [Google Scholar] [CrossRef]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Bélliard, T.; Castello, J.; Van Hecke, É.; Lanoisellé, J.L. Grape seed oil extraction: Interest of supercritical fluid extraction and gas-assisted mechanical extraction for enhancing polyphenol co-extraction in oil. Comptes Rendus Chim. 2014, 17, 284–292. [Google Scholar] [CrossRef]
- Santos, P.D.; De Aguiar, A.C.; Viganó, J.; Boeing, J.S.; Visentainer, J.V.; Martínez, J. Supercritical CO2 extraction of cumbaru oil (Dipteryx alata Vogel) assisted by ultrasound: Global yield, kinetics and fatty acid composition. J. Supercrit. Fluids 2016, 107, 75–83. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Pardauil, J.J.R.; de Molfetta, F.A.; Braga, M.; de Souza, L.K.C.; Filho, G.N.R.; Zamian, J.R.; da Costa, C.E.F. Characterization, thermal properties and phase transitions of amazonian vegetable oils. J. Therm. Anal. Calorim. 2017, 127, 1221–1229. [Google Scholar] [CrossRef]
- Jaramillo, J.E.C.C.; Bautista, M.P.C.; Solano, O.A.A.; Achenie, L.E.K.; Barrios, A.F.G. Impact of the mode of extraction on the lipidomic profile of oils obtained from selected Amazonian fruits. Biomolecules 2019, 9, 329. [Google Scholar] [CrossRef]
- Popescu, M.; Iancu, P.; Plesu, V.; Bildea, C.S. Carotenoids Recovery Enhancement by Supercritical CO2 Extraction from Tomato Using Seed Oils as Modifiers. Process 2022, 10, 2656. [Google Scholar] [CrossRef]
- Arturo-Perdomo, D.; Mora, J.P.J.; Ibáñez, E.; Cifuentes, A.; Hurtado-Benavides, A.; Montero, L. Extraction and Characterization of the Polar Lipid Fraction of Blackberry and Passion Fruit Seeds Oils Using Supercritical Fluid Extraction. Food Anal. Methods 2021, 14, 2026–2037. [Google Scholar] [CrossRef]
- Altuna, J.L.; Silva, M.; Álvarez, M.; Quinteros, M.F.; Morales, D.; Carrillo, W. Yellow pitaya (Hylocereus megalanthus) fatty acids composition from Ecuadorian Amazonia. Asian J. Pharm. Clin. Res. 2018, 11, 218–221. [Google Scholar] [CrossRef]
- GBIF, GBIF|Global Biodiversity Information Facility, (n.d.). Available online: https://www.gbif.org/es/ (accessed on 1 July 2024).
- Tropicos.org, Missouri Botanical Garden, (n.d.). Available online: https://www.tropicos.org/home (accessed on 1 July 2024).
- Royal Botanic Gardens Kew, Plants of the World Online, (n.d.). Available online: https://powo.science.kew.org/ (accessed on 15 May 2024).
- Irías-Mata, A.; Stuetz, W.; Sus, N.; Hammann, S.; Gralla, K.; Cordero-Solano, A.; Vetter, W.; Frank, J. Tocopherols, Tocomonoenols, and Tocotrienols in Oils of Costa Rican Palm Fruits: A Comparison between Six Varieties and Chemical versus Mechanical Extraction. J. Agric. Food Chem. 2017, 65, 7476–7482. [Google Scholar] [CrossRef]
- Santos, R.C.; Chagas, E.A.; de Melo Filho, A.A.; Takahashi, J.A.; Montero, I.F.; Santos, G.F.D.; Chagas, P.C.; de Melo, C.G.R. Chemical characterization of oils and fats from amazonian fruits by 1H NMR. Chem. Eng. Trans. 2018, 64, 235–240. [Google Scholar] [CrossRef]
- Reyes-Giraldo, A.F.; Gutierrez-Montero, D.J.; Rojano, B.A.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A. SEQUENTIAL EXTRACTION PROCESS OF OIL AND ANTIOXIDANT COMPOUNDS FROM CHONTADURO EPICARP. J. Supercrit. Fluids 2020, 166, 105022. [Google Scholar] [CrossRef]
- Da Cunha, A.L.A.; Freitas, S.P.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Chemical composition and oxidative stability of jussara Euterpe edulis M.) oil extracted by cold and hot mechanical pressing. Grasas y Aceites 2017, 68, e218. [Google Scholar] [CrossRef]
- Barbi, R.C.T.; de Souza, A.R.C.; de Melo, A.M.; Teixeira, G.L.; Corazza, M.L.; Ribani, R.H. Fatty acid profile and lipid quality of Maximiliana maripa oil obtained by supercritical CO2 and pressurized ethanol. J. Supercrit. Fluids 2020, 165, 104979. [Google Scholar] [CrossRef]
- de Souza, F.G.; Náthia-Neves, G.; de Araújo, F.F.; Audibert, F.L.D.; Delafiori, J.; Neri-Numa, I.A.; Catharino, R.R.; de Alencar, S.M.; de Almeida Meireles, M.A.; Pastore, G.M. Evaluation of antioxidant capacity, fatty acid profile, and bioactive compounds from buritirana (Mauritiella armata Mart.) oil: A little-explored native Brazilian fruit. Food Res. Int. 2021, 142, 110260. [Google Scholar] [CrossRef]
- dos Santos, O.V.; Pinaffi-Langley, A.C.d.C.; Ferreira, M.C.R.; de Souza, A.L.G.; Carvalho-Junior, R.N.; Teixeira-Costa, B.E. Effect of extraction type on the fatty acids profile and physicochemical properties of biolipids from Astrocaryum vulgare pulp: Supercritical CO2 versus n-hexane extractions. Int. J. Food Sci. Technol. 2023, 58, 3982–3995. [Google Scholar] [CrossRef]
- Sorita, G.D.; Favaro, S.P.; Rodrigues, D.d.S.; Junior, W.P.d.S.; Leal, W.G.d.O.; Ambrosi, A.; Di Luccio, M. Aqueous enzymatic extraction of macauba (Acrocomia aculeata) pulp oil: A green and sustainable approach for high-quality oil production. Food Res. Int. 2024, 182, 114160. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.A.; Berton, L.H.C.; Diaz, B.G.; Ferrari, R.A. Macauba: A promising tropical palm for the production of vegetable oil. OCL–Oilseeds Fats Crop Lipids 2018, 25, D108. [Google Scholar] [CrossRef]
- Trentini, C.P.; Santos, K.A.; da Silva, E.A.; Garcia, V.A.D.S.; Cardozo-Filho, L.; da Silva, C. Oil extraction from macauba pulp using compressed propane. J. Supercrit. Fluids 2017, 126, 72–78. [Google Scholar] [CrossRef]
- Hashim, M.A.; Yam, M.F.; Hor, S.Y.; Lim, C.P.; Asmawi, M.Z.; Sadikun, A. Anti-hyperglycaemic activity of Swietenia macrophylla king (Meliaceae) seed extracts in normoglycaemic rats undergoing glucose tolerance tests. Chin. Med. 2013, 8, 11. [Google Scholar] [CrossRef]
- Jayanegara, A.; Harahap, R.P.; Rozi, R.F.; Nahrowi. Effects of lipid extraction on nutritive composition of winged bean (Psophocarpus tetragonolobus), rubber seed (Hevea brasiliensis), and tropical almond (Terminalia catappa). Vet. World 2018, 11, 446–451. [Google Scholar] [CrossRef]
- Mera, J.J.R.; Abreu-Naranjo, R.; Alvarez-Suarez, J.M.; Viafara, D. Chemical characterization, fatty acid profile and antioxidant activity of Gustavia macarenensis fruit mesocarp and its oil from the amazonian region of Ecuador as an unconventional source of vegetable oil. Grasas y Aceites 2019, 70, e298. [Google Scholar] [CrossRef]
- Kumar, R.; Das, N. Seed oil of Jatropha curcas L. germplasm: Analysis of oil quality and fatty acid composition. Ind. Crops Prod. 2018, 124, 663–668. [Google Scholar] [CrossRef]
- Gomes, F.S.P.; De Sobral, A.D.; Da Silva, A.M.R.B.; Da Rocha, M.A.G. Moringa oleifera: A promising agricultural crop and of social inclusion for Brazil and semi-Arid regions for the production of energetic biomass (biodiesel and briquettes). OCL–Oilseeds Fats. Crop. Lipids 2018, 25, D106. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonça, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef]
- Lafont-Mendoza, J.J.; Espinosa-Fuentes, E.A.; Severiche-Sierra, C.A.; Jaimes-Morales, J.; Marrugo-Ligardo, Y.A. Proximal analysis of the residual cake obtained with extraction by pressing seeds of Perehuetano. ARPN J. Eng. Appl. Sci. 2018, 13, 8069–8072. [Google Scholar]
- Zaniol, F.; Calisto, J.F.F.; Cozzer, G.; Ferro, D.M.; Dias, J.L.; Rodrigues, L.G.G.; Mazzutti, S.; Rezende, R.S.; Simões, D.A.; Ferreira, S.R.S.; et al. Comparative larvicidal effect of Pterodon spp. extracts obtained by different extraction methods. J. Supercrit. Fluids 2020, 166, 104993. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Maciel, L.G.; Mazzutti, S.; Gonçalves, C.B.; Ferreira, S.R.S.; Block, J.M. Composition, thermal behavior and antioxidant activity of pracaxi (Pentaclethra macroloba) seed oil obtained by supercritical CO2. Biocatal. Agric. Biotechnol. 2020, 24, 101521. [Google Scholar] [CrossRef]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenéz, D.; Muñoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161, 104824. [Google Scholar] [CrossRef]
- Obaidat, R.; Aleih, H.; Mashaqbeh, H.; Altaani, B.; Alsmadi, M.M.; Alnaief, M. Development and Evaluation of Cocoa Butter Taste Masked Ibuprofen Using Supercritical Carbon Dioxide. AAPS PharmSciTech 2021, 22, 106. [Google Scholar] [CrossRef]
- Chañi-Paucar, L.O.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Technical and economic evaluation of supercritical CO2 extraction of oil from sucupira branca seeds. J. Supercrit. Fluids 2022, 181, 105494. [Google Scholar] [CrossRef]
- Polmann, G.; Teixeira, G.L.; Santos, P.H.; Rivera, G.Á.; Ibañez, E.; Cifuentes, A.; Ferreira, S.R.S.; Block, J.M. Chemical characterization of gurguéia nut (Dipteryx lacunifera Ducke) and press cake oil obtained by hydraulic pressing and supercritical extraction. Biomass Convers. Biorefinery. 2023, 14, 19065–19080. [Google Scholar] [CrossRef]
- Mateus, L.S.; Dutra, J.M.; Favareto, R.; da Silva, E.A.; Pinto, L.F.; da Silva, C.; Cardozo-Filho, L. Optimization Studies and Compositional Oil Analysis of Pequi (Caryocar brasiliense Cambess) Almonds by Supercritical CO2 Extraction. Molecules 2023, 28, 1030. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Moraes, I.C.F.; de Rosso, V.V.; Rodrigues, C.E.d.C.; de Souza, A.C. Effect of the application of an enzymatic pretreatment on bioactive compounds of Caryocar brasiliense Camb pulp oil. J. Food Process. Preserv. 2018, 42, e13828. [Google Scholar] [CrossRef]
- Zoccali, M.; Giuffrida, D.; Salafia, F.; Rigano, F.; Dugo, P.; Casale, M.; Mondello, L. Apocarotenoids profiling in different Capsicum species. Food Chem. 2021, 334, 127595. [Google Scholar] [CrossRef]
- Pires, F.B.; Dolwitsch, C.B.; Ugalde, G.A.; Menezes, B.B.; Fontana, M.E.Z.; Rieffeld, R.C.; Sagrillo, M.R.; Essi, L.; Mazutti, M.A.; da Rosa, M.B.; et al. Chemical study, antioxidant activity, and genotoxicity and cytotoxicity evaluation of Ruellia angustiflora. Nat. Prod. Res. 2021, 35, 5317–5322. [Google Scholar] [CrossRef]
- Triana-Maldonado, D.M.; Torijano-Gutiérrez, S.A.; Giraldo-Estrada, C. Supercritical CO2 extraction of oil and omega-3 concentrate from Sacha inchi (Plukenetia volubilis L.) from Antioquia, Colombia. Grasas y Aceites 2017, 68, e172. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Delvar, A.; de Caro, P.; Caro, Y.; Sing, A.S.C.; Thomas, R.; Raynaud, C. Semi-Siccative Oils and Bioactive Fractions Isolated from Reunion Island Fruit Co-Product: Two Case Studies. Eur. J. Lipid Sci. Technol. 2019, 121, 1800391. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies 2023, 16, 5852. [Google Scholar] [CrossRef]
- Aboubakar, X.; Goudoum, A.; Bébé, Y.; Mbofung, C.M.F. Optimization of Jatropha curcas pure vegetable oil production parameters for cooking energy. S. Afr. J. Chem. Eng. 2017, 24, 196–212. [Google Scholar] [CrossRef]
- Romuli, S.; Karaj, S.; Latif, S.; Müller, J. Performance of mechanical co-extraction of Jatropha curcas L. kernels with rapeseed, maize or soybean with regard to oil recovery, press capacity and product quality. Ind. Crops Prod. 2017, 104, 81–90. [Google Scholar] [CrossRef]
- Achicanoy, D.D.; Benavides, A.H.; Martínez-Correa, H.A. Study of supercritical CO2 extraction of tamarillo (Cyphomandra betacea) seed oil containing high added value compounds. Electrophoresis 2018, 39, 1917–1925. [Google Scholar] [CrossRef]
- Carvalho, L.M.S.; Oliveira, A.M.B.; Grimaldi, R.; de Souza, P.T.; Batista, E.A.C.; Martínez, J. Supercritical fluid and pressurized liquid extraction of spent tucumã-do-Amazonas (Astrocaryum aculeatum) almonds. J. Supercrit. Fluids 2024, 209, 106238. [Google Scholar] [CrossRef]
- Vardanega, R.; Fuentes, F.S.; Palma, J.; Bugueño-Muñoz, W.; Cerezal-Mezquita, P.; Ruiz-Domínguez, M.C. Valorization of granadilla waste (Passiflora ligularis, Juss.) by sequential green extraction processes based on pressurized fluids to obtain bioactive compounds. J. Supercrit. Fluids 2023, 194, 105833. [Google Scholar] [CrossRef]
- Pantoja-Chamorro, A.L.; Hurtado-Benavides, A.M.; Martinez-Correa, H.A. Caracterización de aceite de semillas de maracuyá (Passiflora edulis Sims.) procedentes de residuos agroindustriales obtenido con CO2 supercrítico. Acta Agron. 2017, 66, 178–185. [Google Scholar]
- Shah, N.A.; Prasad, R.V.; Patel, B.B. Optimization of Supercritical Fluid Extraction of Paprika (cv. Reshampatti) Oil, Capsaicin and Pigments. Flavour. Fragr. J. 2020, 35, 469–477. [Google Scholar] [CrossRef]
- Menezes, G.E.O.; Barbosa, J.R.; Pires, C.S.F.; Ferreira, M.C.R.; De Souza e Silva, A.P.; Siqueira, L.M.M.; Junior, R.N.D.C. Development of a new scale-up equation to obtain Tucumã-of-Pará (Astrocaryum vulgare Mart.) oil rich in carotenoids using supercritical CO2 as solvent. J. Supercrit. Fluids 2022, 181, 105481. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Sena, C.; Santos, O.V.; Da Costa, W.A.; Rodrigues, A.M.C.; Carvalho, R.N. Extraction of bacaba (Oenocarpus bacaba) oil with supercritical CO2: Global yield isotherms, fatty acid composition, functional quality, oxidative stability, spectroscopic profile and antioxidant activity. Grasas Y Aceites 2018, 69, 246. [Google Scholar] [CrossRef]
- Durante, M.; Montefusco, A.; Marrese, P.P.; Soccio, M.; Pastore, D.; Piro, G.; Mita, G.; Lenucci, M.S. Seeds of pomegranate, tomato and grapes: An underestimated source of natural bioactive molecules and antioxidants from agri-food by-products. J. Food Compos. Anal. 2017, 63, 65–72. [Google Scholar] [CrossRef]
- Mohammadnezhad, P.; Valdés, A.; Barrientos, R.E.; Ibáñez, E.; Block, J.M.; Cifuentes, A. A Comprehensive Study on the Chemical Characterization and Neuroprotective Evaluation of Pracaxi Nuts Extracts Obtained by a Sustainable Approach. Foods 2023, 12, 3879. [Google Scholar] [CrossRef] [PubMed]
- Solaberrieta, I.; Mellinas, A.C.; Espagnol, J.; Hamzaoui, M.; Jiménez, A.; Garrigós, M.C. Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques. Foods 2022, 11, 2408. [Google Scholar] [CrossRef]
- Kapoor, S.; Gandhi, N.; Tyagi, S.K.; Kaur, A.; Mahajan, B.V.C. Extraction and characterization of guava seed oil: A novel industrial byproduct. LWT 2020, 132, 109882. [Google Scholar] [CrossRef]
- Narváez-Cuenca, C.E.; Inampues-Charfuelan, M.L.; Hurtado-Benavides, A.M.; Parada-Alfonso, F.; Vincken, J.P. The phenolic compounds, tocopherols, and phytosterols in the edible oil of guava (Psidium guava) seeds obtained by supercritical CO2 extraction. J. Food Compos. Anal. 2020, 89, 103467. [Google Scholar] [CrossRef]
- Medeiros-Neves, B.; Diel, K.A.P.; Eifler-Lima, V.L.; Teixeira, H.F.; Cassel, E.; Vargas, R.M.F.; Von Poser, G.L. Influence of the supercritical CO2 extraction in the stability of the coumarins of Pterocaulon lorentzii (Asteraceae). J. CO2 Util. 2020, 39, 101165. [Google Scholar] [CrossRef]
- Calva-Cruz, O.d.J.; Badillo-Larios, N.S.; De León-Rodríguez, A.; Espitia-Rangel, E.; González-García, R.; Turrubiartes-Martinez, E.A.; Castro-Gallardo, A.; de la Rosa, A.P.B. Lippia graveolens HBK oleoresins, extracted by supercritical fluids, showed bactericidal activity against multidrug resistance Enterococcus faecalis and Staphylococcus aureus strains. Drug Dev. Ind. Pharm. 2021, 47, 1546–1555. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Vardanega, R.; Hatami, T.; Meireles, M.A.A. Process integration for recovering high added-value products from Genipa americana L.: Process optimization and economic evaluation. J. Supercrit. Fluids 2020, 164, 104897. [Google Scholar] [CrossRef]
- Soldan, A.C.F.; Arvelos, S.; Watanabe, É.O.; Hori, C.E. Supercritical fluid extraction of oleoresin from Capsicum annuum industrial waste. J. Clean. Prod. 2021, 297, 126593. [Google Scholar] [CrossRef]
- Guo, T.; Wan, C.; Huang, F.; Wei, C. Evaluation of quality properties and antioxidant activities of tiger nut (Cyperus esculentus L.) oil produced by mechanical expression or/with critical fluid extraction. LWT 2021, 141, 110915. [Google Scholar] [CrossRef]
- Panadare, D.; Dialani, G.; Rathod, V. Extraction of volatile and non-volatile components from custard apple seed powder using supercritical CO2 extraction system and its inventory analysis. Process Biochem. 2021, 100, 224–230. [Google Scholar] [CrossRef]
- Mesquita, P.C.; Rodrigues, L.G.G.; Mazzutti, S.; da Silva, M.; Vitali, L.; Lanza, M. Intensified green-based extraction process as a circular economy approach to recover bioactive compounds from soursop seeds (Annona muricata L.). Food Chem. X 2021, 12, 100164. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Maciel, L.G.; Mazzutti, S.; Barbi, R.C.T.; Ribani, R.H.; Ferreira, S.R.S.; Block, J.M. Sequential green extractions based on supercritical carbon dioxide and pressurized ethanol for the recovery of lipids and phenolics from Pachira aquatica seeds. J. Clean. Prod. 2021, 306, 127223. [Google Scholar] [CrossRef]
- Macawile, M.C.; Auresenia, J. Comparison of biodiesel yield from seed oils extracted by ultrasound-assisted chemical solvent and supercritical CO2 methods. Int. J. Appl. Sci. Eng. Comp. 2022, 19, 2021279. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Statistical modeling of supercritical extraction of hemp (Cannabis sativa) and papaya (Carica papaya) seed oils through artificial neural network and central composite design. Soft Comput. 2022, 26, 2307–2324. [Google Scholar] [CrossRef]
- Díaz-Cervantes, M.D.; Ramos-Ramírez, E.G.; Gimeno-Seco, M.; Salazar-Montoya, J.A. Supercritical CO2 Extraction of oil from Chan (Hyptis suaveolens (L.) Poit) Seeds and its Physicochemical Characterization, Spectroscopy and Nutritional Analysis. Food Anal. Methods 2023, 16, 918–932. [Google Scholar] [CrossRef]
- De Cássia Rodrigues Batista, C.; De Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; Da Silva Souza Filho, A.P.; Machado, N.T.; Carvalho, R.N. Supercritical CO2 extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2015, 107, 364–369. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.; Al-Nasser, A. Role of Poultry Research in Increasing Consumption of PUFA in Humans. In Nutrition in Health and Disease–Our Challenges Now and Forthcoming Time; IntechOpen: London, UK, 2019; p. 13. [Google Scholar] [CrossRef]
- Martin, C.A.; De Almeida, V.V.; Ruiz, M.R.; Visentainer, J.E.L.; Matshushita, M.; De Souza, N.E.; Visentainer, J.V. Ácidos graxos poliinsaturados ômega-3 e ômega-6: Importância e ocorrência em alimentos. Rev. Nutr. 2006, 19, 761–770. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Introduction to Essential Fatty Acids. In Nutraceutical Fatty Acids from Oleaginous Microalgae: A Human Health Perspective; Wiley: Hoboken, NJ, USA, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Popa, O.; Bəbeanu, N.E.; Popa, I.; Niţə, S.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. Biomed. Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef] [PubMed]
- Spanova, M.; Daum, G. Squalene–biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Mantovani, L.M.; Pugliese, C. Phytosterol supplementation in the treatment of dyslipidemia in children and adolescents: A systematic review. Rev. Paul. Pediatr. 2020, 39, e2019389. [Google Scholar] [CrossRef]
- Chong, W.T.; Lee, Y.Y.; Tang, T.K.; Phuah, E.T. Minor Components in Edible Oil. In Recent Advances in Edible Fats and Oils Technology; Springer: Singapore, 2022; pp. 141–187. [Google Scholar] [CrossRef]
- Ibiapina, A.; Gualberto, L.D.S.; Dias, B.B.; Freitas, B.C.B.; Martins, G.A.D.S.; Filho, A.A.M. Essential and fixed oils from Amazonian fruits: Proprieties and applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 8842–8854. [Google Scholar] [CrossRef]
- Jaramillo-Vivanco, T.; Balslev, H.; Montúfar, R.; Cámara, R.M.; Giampieri, F.; Battino, M.; Cámara, M.; Alvarez-Suarez, J.M. Three Amazonian palms as underestimated and little-known sources of nutrients, bioactive compounds and edible insects. Food Chem. 2022, 372, 131273. [Google Scholar] [CrossRef]
- da Silva, D.A.; da Cruz Rodrigues, A.M.; Santos, A.O.D.; Salvador-Reyes, R.; da Silva, L.H.M. Physicochemical and technological properties of pracaxi oil, cupuassu fat and palm stearin blends enzymatically interesterified for food applications. LWT 2023, 184, 114961. [Google Scholar] [CrossRef]
- Dhakane-Lad, J.; Kar, A.; Patel, A.S. SC-CO2 extraction of lycopene from red papaya using rice bran oil as a co-solvent lessens its degradation during storage. Sep. Sci. Technol. 2023, 58, 2357–2368. [Google Scholar] [CrossRef]
- Johner, J.C.F.; Hatami, T.; Meireles, M.A.A. Developing a supercritical fluid extraction method assisted by cold pressing for extraction of pequi (Caryocar brasiliense). J. Supercrit. Fluids 2018, 137, 34–39. [Google Scholar] [CrossRef]
- Willems, P.; Kuipers, N.J.M.; de Haan, A.B. Gas assisted mechanical expression of oilseeds: Influence of process parameters on oil yield. J. Supercrit. Fluids 2008, 45, 298–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).