Supercritical Fluid Extraction of Amazonian Oils and Fats: Promising Species, Equipment, Yields, Composition, and Potential Uses
Abstract
1. Introduction
2. Procedure of Search and Selection of Scientific Articles
3. Amazonian Oleaginous Plants
3.1. Palms
3.2. Trees
3.3. Other Plants
4. Conventional Extraction of Amazonian Oils/Fats
| Raw-Material | Extraction Methods | Yield | |
|---|---|---|---|
| Moringa oleifera Lamarck | Mechanical pressing | 11% | [52] |
| Caryocar brasiliense Camb | Hydraulic pressing Pressure: 0.5 ton/cm2 Temperature: 30 °C | 32% | [62] |
| Hydraulic pressing + enzymatic treatment Pressure: 0.5 ton/cm2 Temperature: 30 °C | 39% | ||
| Euterpe edulis Mart. | Cold pressing Temperature: 25 °C Raw-material moisture: 10% | 10% | [41] |
| Hot pressing Temperature: 50 °C Raw-material moisture: 10% | 20% | ||
| Persea americana Mill. | Cold pressing Pressing force: 9 tons Raw-material moisture: 5–6% | 42% | [53] |
| Passiflora edulis | Mechanical pressing | 19.5% | [67] |
| Jatropha curcas | Bielenberg press | 26% | [69] |
| Jatropha curcas | Screw pressing Temperature: 80 °C Raw-material moisture: 4.5% | 16.2% | [70] |
| Parinari pachyphylla Rusby | Hydraulic pressing | - | [54] |
| Euterpe precatoria | Expeller press Temperature: 25–30 °C Particle size: 1 mm | 10% | [31] |
| Mauritia flexuosa | 22% | ||
| Oenocarpus bataua | 28% |
| Raw-Material | Extraction Conditions | Yield | |
|---|---|---|---|
| Jatropha curcas L. | Soxhlet Solvent: hexane Temperature: 70–80 °C Time: 8–10 h | 13.7–54.4% | [51] |
| Moringa oleifera Lamarck | Soxhlet Solvent: hexane Time: 4 h | 36% | [52] |
| Swietenia macrophylla king | Maceration Solvent: petroleum ether Temperature: 60–80 °C | 8.6% | [48] |
| Maceration Solvent: Chloroform Temperature: 60–80 °C | 6.2% | ||
| Maceration Solvent: Methanol Temperature: 60–80 °C | 5.4% | ||
| Psophocarpus tetragonolobus | Soxhlet Solvent: petroleum ether Time: 6 h Particle size: 1 mm | 20% | [49] |
| Hevea brasiliensis | 37.6% | ||
| Terminalia catappa | 52.1% | ||
| Gustavia macarenensis Philipson | Soxhlet Solvent: hexane Time: 4 h Particle size: 1 mm | 53.6% | [50] |
| Euterpe precatoria | Soxhlet Solvent: Petroleum benzene Temperature: 40–60 °C Particle size: 1 mm | 18% | [31] |
| Oenocarpus bataua | 62% | ||
| Mauritia flexuosa | 33% | ||
| Euterpe oleracea | Soxhlet Solvent: hexane Particle size: 0.84–0.42 mm | 35.4% | [39] |
| Annona hypoglauca | 15% | ||
| Oenocarpus bacaba | 43.9% | ||
| Mauritia flexuosa | 23.2% | ||
| Mauritia aculeata | 26% | ||
| Byrsonima crassifolia | 46.5% | ||
| Byrsonima coccolobifolia | 38% | ||
| Bactris gasipaes var. yellow. | 17.1% | ||
| Bactris gasipaes var. red | 27.3% | ||
| Barcella odora | 12% | ||
| Astrocaryum acaule | 33.1% | ||
| Acrocomia aculeata | Soxhlet Solvent: hexane Temperature: 69 °C Time: 8 h Raw-material moisture: 3.6% Particle size: 0.5 mm | 25.6% | [47] |
| Soxhlet Solvent: Dichloromethane Temperature: 40 °C Time: 8 h Raw-material moisture: 3.6% Particle size: 0.5 mm | 26.8% | ||
| Acrocomia aculeata | Soxhlet Solvent: hexane Temperature: 68 °C Time: 8 h Raw-material moisture: 3.3% Particle size: 0.841 mm | 43.6% | [17] |
| Plukenetia volubilis L. | Soxhlet Solvent: hexane Temperature: 60 °C Time: 20 h Raw-material moisture: 2.36% Particle size: 1.51–2.01 mm | 40.6% | [65] |
| Passiflora edulis | Soxhlet Solvent: hexane Time: 4 h | 23.6% | [67] |
| Maceration Solvent: hexane Time: 24 h | 30% | ||
| Maceration Solvent: ethanol Time: 24 h | 19.9% | ||
| Virola surinamensis (Rol. ex Rottb.) Warb. | Soxhlet Solvent: Petroleum ether Time: 4 h Raw-material moisture: 2.5% Particle size: 0.24 mm | 61.4% | [23] |
| Cyphomandra betacea (Cav.) Sendtn. | Soxhlet Solvent: hexane Time: 6 h Raw-material moisture: 4.97% Particle size: 0.68 mm | 24.1% | [71] |
5. SFE-CO2
5.1. Processes Parameters
| Raw-Material | Extraction Conditions | Yield | |
|---|---|---|---|
| Passiflora edulis seeds | Temperature: 60 °C Pressure: 35 MPa Time: 2.5 h Raw-material moisture: 10% | 15.7% | [74] |
| Temperature: 60 °C Pressure: 20 MPa Time: 1.34 h | 21.6% | [67] | |
| Temperature: 60 °C Pressure: 35 MPa Time: 2.5 h Raw-material moisture: 7.8% Flow rate: 30 g/min | 15.7% | [33] | |
| Oenocarpus distichus Mart. pulp | Temperature: 50 °C Pressure: 35 MPa Time: 3 h | 45.2% | [12] |
| Temperature: 60 °C Pressure: 27 MPa Time: 3 h | 46% | ||
| Cyphomandra betacea (Cav.) Sendtn. seeds | Temperature: 51 °C Pressure: 38 MPa Time: 2.5 h S/F: 22.5 Raw-material moisture: 4.5% Particle size: 0.69 mm | 17.4% | [71] |
| Oenocarpus bacaba pulp | Temperature: 60 °C Pressure: 42 MPa Time: 3 h Raw-material moisture: 4.2% | 60.4% | [77] |
| Plukenetia volubilis L. seeds | Temperature: 60 °C Pressure: 50 MPa Time: 3 h Raw-material moisture: 2.4% Particle size: 1.5–2 mm | 23.5% | [65] |
| Temperature: 60 °C Pressure: 45 MPa Time: 4.5 h Raw-material moisture: 3.3% Particle size: 1.64 mm | 26.8% ** | ||
| Lycopersicon esculentum L. seeds | Temperature: 60 °C Pressure: 35 MPa Time: 2 h Particle size: 1 mm | 7.8% | [78] |
| Virola surinamensis seeds | Temperature: 80 °C Pressure: 35 MPa Time: 3 h Raw-material moisture: 2.5% Particle size: 0.24 mm | 64.4% | [23] |
| Acrocomia aculeata kernel | Temperature: 40 °C Pressure: 22 MPa Time: 3.3 h Raw-material moisture: 3.3% Particle size: 0.481 mm | 41.6% | [17] |
| Pterodon spp. fruits | Temperature: 50 °C Pressure: 20 MPa Time: 4.5 h Raw-material moisture: 2.3% Solvent flow rate: 12 g CO2/min Particle size: 0.57 mm | 31.1% | [55] |
| Pentaclethra macroloba seeds | Temperature: 40 °C Pressure: 30 MPa Solvent flow rate: 0.7 kg CO2/h Particle size: 1.19 mm | 42.05% | [56] |
| Temperature: 40 °C Pressure: 30 MPa Time: 120 min Solvent flow rate: 4 mL/min Particle size: 1.19 mm | 23.3% | [79] | |
| Solanum lycopersicum seeds | Temperature: 60.2 °C Pressure: 40 MPa Solvent flow rate: 64.6 g/min Particle size: 1.00 mm | 16.9% | [80] |
| Psidium guava seeds | Temperature: 50 °C Pressure: 45 MPa Time: 60 min Raw-material moisture: 8% Co-solvent: ethanol at 3 mL/min | 10.45% | [81] |
| Temperature: 52 °C Pressure: 35.7 MPa Time: 150 min Solvent flow rate: 30 g CO2/min Raw-material moisture: 8.05% Average particle diameter: 0.490 mm | 8.6% | [82] | |
| Maximiliana maripa pulp | Pure CO2 Temperature: 60 °C Pressure: 20 MPa Time: 170 min Solvent flow rate: 2 cm3/min Raw-material moisture: 11.7% Mean particle diameter: 1.6 × 10−3 m | 3.6% | [42] |
| CO2 + Ethanol Temperature: 60 °C Pressure: 20 MPa Time: 60 min Raw-material moisture: 11.7% Average particle diameter: 1.6 × 10−3 m | 20.4% | ||
| Pterocaulon lorentzii leaves and inflorescences | Temperature: 40 °C Gradual pressure increments: 9 to 30 MPa Time: 100 (leaves) and 110 min (inflorescences) Solvent flow rate: 1 kg/h Raw-material moisture: 5.27 (inflorescences) and 2.08% (leaves) Average thickness of fibers: 0.061 (inflorescences) and 0.148 mm (leaves) | 109.05 (leaves) and 610.03 mg coumarins/g of extract (inflorescences) | [83] |
| Lippia graveolens HBK leaves. | Temperature: 45 °C Pressure: 35 MPa Time: 300 min Solvent flow rate: 30 g CO2/min | 4.1% | [84] |
| Capsicum annuum L. fruits | Temperature: 65 °C Pressure: 35.78 MPa Time: 90 min Particle size: 0.78 mm Raw-material moisture: 4–6% Solvent flow rate: 3 L/min | 4.91% | [75] |
| Genipa americana L. fruits | Temperature: 60 °C Pressure: 30 MPa Mean particle diameter: 0.23 mm Raw-material moisture: 5.1% Solvent flow rate: 2.5 g CO2/min | 4.6% | [85] |
| Bactris gasipaes epicarp | Temperature: 40 °C Pressure: 40 MPa Time: 210 min Raw-material moisture: 7.1% | 12.12% | [40] |
| Geoffroea decorticans almond | Temperature: 60 °C Pressure: 40 MPa Time: 120 min Raw-material moisture: 4.93% Solvent flow rate: 3.62 g CO2/min | 47% | [57] |
| Dipteryx alata Vogel seeds | CO2 + water Temperature: 60 °C Pressure: 20 MPa Time: 240 min Raw-material moisture: 2.30 g/100 g Mean particle diameter: <0.85 mm Solvent flow rate: 3 mL/min | 21.67 g/100 g | [21] |
| CO2 + Alcohol Temperature: 50 °C Pressure: 30 MPa Time: 240 min Raw-material moisture: 2.30 g/100 g Mean particle diameter: <0.85 mm Solvent flow rate: 3 mL/min | 31.06 g/100 g | ||
| CO2 + Alcohol:water Temperature: 60 °C Pressure: 20 MPa Time: 240 min Raw-material moisture: 2.30 g/100 g Mean particle diameter: <0.85 mm Solvent flow rate: 3 mL/min | 21.67 g/100 g | ||
| CO2 Temperature: 45 °C Pressure: 35 MPa Time: 185 min Average particle diameter: 1.8 mm | 22 g/100 g | [25] | |
| Capsicum annuum industrial waste | CO2 Temperature: 60 °C Pressure: 20 MPa Mean particle diameter: 0.48 mm Solvent flow rate: 3 g CO2/min | 9.60% | [86] |
| CO2 + Ethanol Temperature: 40 °C Pressure: 25 MPa Mean particle diameter: 0.48 mm Solvent flow rate: 3 g CO2/min Ratio ethanol/sample: 0.5 mL/g | 10.08% | ||
| Mauritiella armata Mart. pulp | Temperature: 40 °C Pressure: 30 MPa Time: 61 min Mean particle diameter: 2.107 mm Solvent flow rate: 17 g/min | 41.57 g/100 g | [43] |
| Cyperus esculentus L. nut | Temperature: 40 °C Pressure: 28 MPa Time: 90 min | 28.56 g/100 g | [87] |
| Rubus glaucus seeds | Temperature: 60 °C Pressure: 35 MPa Time: 150 min Raw-material moisture: 7.60% Solvent flow rate: 30 g/min | 14.5% | [33] |
| Annona squamosa seeds | Temperature: 45 °C Pressure: 25 MPa Time: 60 min Solvent flow rate: 2.5 mL/min Raw-material moisture: 6.21% Average particle size: 427 nm | 0.29 g/g | [88] |
| Annona muricata L. seeds | Temperature: 40 °C Pressure: 30 MPa Time: 210 min Solvent flow rate: 0.7 kg/min Raw-material moisture: 4.98% Mean particle size: 0.53 mm | 16.4% | [89] |
| Pachira aquatica Aubl. seeds | Temperature: 60 °C Pressure: 30 MPa Time: 120 min Solvent flow rate: 1.21 kg/h Raw-material moisture: 3.25% Average particle size: 1.19 mm | 51.78% | [90] |
| Bertholletia excelsa cake (by-products) | Temperature: 60 °C Pressure: 40 MPa Time: 180 min Solvent flow rate: 40 g/min Raw-material moisture: 0.25% Particle size: <1 mm | 59.2% | [13] |
| Renealmia petasites Gagnep. seeds | Temperature: 40 °C Pressure: 35 MPa Time: 40 min Solvent flow rate: 1.06 × 10−4 kg CO2/s | 4.15% | [19] |
| Gliricidia sepium seeds | Temperature: 60 °C Pressure: 30 MPa Time: 90 min Solvent flow rate: 2.5 mL/min | 11.79% | [91] |
| Oenocarpus mapora H. Karst fruit | Temperature: 60 °C Pressure: 35 MPa | 7.45% | [20] |
| Temperature: 43.5 °C Pressure: 27.07 MPa Time: 120 min Solvent flow rate: 39 g/min Raw-material moisture: 6% Particle size: 350–500 μm | 30.4 g/100 g | [22] | |
| Mauritia flexuosa L.f. pulp | Temperature: 42 °C Pressure: 20 MPa Time: 60 min Solvent flow rate: 42 g CO2/min | 44.85% | [11] |
| Carica papaya seeds | Temperature: 65 °C Pressure: 25 MPa Time: 240 min Solvent flow rate: 15 g CO2/min Particle size: 0.26 mm Co-solvent flow rate: 10% of CO2 flow rate | 36.67% | [92] |
| Pterodon emarginatus Vogel seeds | Temperature: 40 °C Pressure: 30 MPa Time: 65 min Solvent flow rate:4.5 g CO2/min Average particle size: 0.87 mm | 40 g/100 g | [59] |
| Dipteryx lacunifera Ducke cake | Temperature: 40 °C Pressure: 20 MPa Time: 240 min Solvent flow rate: 1.0 kg/h Raw-material moisture: 4.50 g/100 g | 15.52% | [60] |
| Astrocaryum vulgare pulp | Temperature: 60 °C Pressure: 30 MPa Time: 180 min Solvent flow rate: 8.85 × 10−5 kg/s Average particle size: 1 mm Raw-material moisture: 5% | 36.75% | [44] |
| Caryocar brasiliense Cambess almonds | Temperature: 45 °C Pressure: 25 MPa Time: 110 min Solvent flow rate: 5.0 g/min Average particle size: 3.5 g/min | 27.6% | [61] |
| Hyptis suaveolens (L.) Poit seeds | Temperature: 80 °C Pressure: 45 MPa Time: 193 min Solvent flow rate: 0.88 kg/h Raw-material moisture: 8.94% Particle size: > 75 μm | 62.36% | [93] |
| Passiflora ligularis Juss. (seeds) | Temperature: 40 °C Pressure: 40 MPa Time: 60 min Solvent flow rate: 3.62 g/min Raw-material moisture: 1.57% Mean particle diameter: 0.60 mm | 24.97% | [73] |
| Astrocaryum aculeatum almonds | Temperature: 50 °C Pressure: 25 MPa Solvent flow rate: 1.8 × 10−4 kg/s Raw-material moisture: 3.7% Particle size: 1.19 mm | 34.41 g/100 g | [72] |
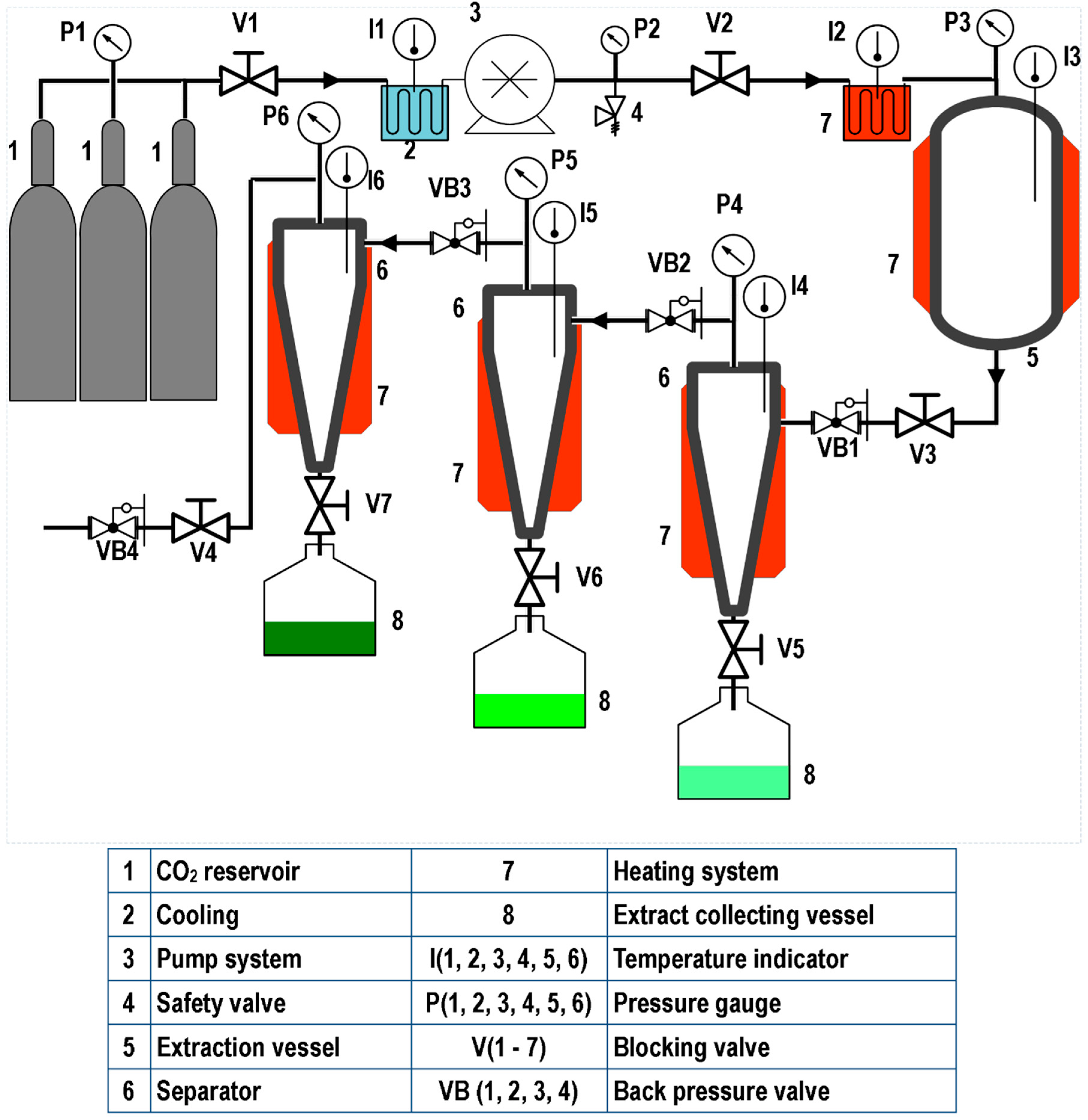
5.2. SFE Equipment
6. Fatty Acids from AOFs
7. Fat-Soluble Bioactive from AOFs
8. Potential Uses and Perspectives of Amazonian Lipids Obtained by SFE
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dupont, J.L. LIPIDS|Chemistry and Classification. In Encyclopedia of Human Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 126–132. [Google Scholar] [CrossRef]
- van Duijn, G. Vegetable Oils and Fats. In Encyclopedia of Food Safety, 2nd ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 1–4, pp. 46–59. [Google Scholar] [CrossRef]
- Talbot, G. Specialty Oils and Fats in Food and Nutrition: Properties, Processing and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Barrufol, M.; Schmid, B.; Bruelheide, H.; Chi, X.; Hector, A.; Ma, K.; Michalski, S.; Tang, Z.; Niklaus, P.A. Biodiversity Promotes Tree Growth during Succession in Subtropical Forest. PLoS ONE 2013, 8, e81246. [Google Scholar] [CrossRef]
- Malhi, Y.; Riutta, T.; Wearn, O.R.; Deere, N.J.; Mitchell, S.L.; Bernard, H.; Majalap, N.; Nilus, R.; Davies, Z.G.; Ewers, R.M.; et al. Logged tropical forests have amplified and diverse ecosystem energetics. Nature 2022, 612, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.V.; Rodrigues, A.M.d.C.; de Oliveira, P.D.; da Silva, D.A.; da Silva, L.H.M. Technological properties of amazonian oils and fats and their applications in the food industry. Food Chem. 2017, 221, 1466–1473. [Google Scholar] [CrossRef]
- Serra, J.L.; da Cruz Rodrigues, A.M.; de Freitas, R.A.; de Almeida Meirelles, A.J.; Darnet, S.H.; da Silva, L.H.M. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Quispe, S.C.; Quispe, H.C.; Rios, E.G.; Viteri, J.E.D.; Chañi-Paucar, L.O.; Berrocal, M.H.M. Efecto de dietas balanceadas con harina de semillas de copoazú (Theobroma grandiflorum) en el crecimiento de Paco (Piaractus brachypomus Cuvier). Livest. Res. Rural. Dev. 2018, 30. Available online: http://www.lrrd.org/lrrd30/1/larr30017.html (accessed on 17 January 2025).
- Pereira, A.L.F.; Abreu, V.K.G.; Rodrigues, S. Cupuassu—Theobroma grandiflorum. In Exotic Fruits; Academic Press: Cambridge, MA, USA, 2018; pp. 159–162. [Google Scholar] [CrossRef]
- de Oliveira, T.B.; Genovese, M.I. Chemical composition of cupuassu (Theobroma grandiflorum) and cocoa (Theobroma cacao) liquors and their effects on streptozotocin-induced diabetic rats. Food Res. Int. 2013, 51, 929–935. [Google Scholar] [CrossRef]
- Best, I.; Cartagena-Gonzales, Z.; Arana-Copa, O.; Olivera-Montenegro, L.; Zabot, G. Production of Oil and Phenolic-Rich Extracts from Mauritia flexuosa L.f. Using Sequential Supercritical and Conventional Solvent Extraction: Experimental and Economic Evaluation. Processes 2022, 10, 459. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; da Silva, M.P.; de Sousa, S.H.B.; Bezerra, P.D.N.; Menezes, E.G.O.; da Silva, N.J.N.; Banna, D.A.D.D.S.; Araújo, M.E.; Carvalho Junior, R.N.D. Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Vasquez, W.V.; Hernández, D.M.; del Hierro, J.N.; Martin, D.; Cano, M.P.; Fornari, T. Supercritical carbon dioxide extraction of oil and minor lipid compounds of cake byproduct from Brazil nut (Bertholletia excelsa) beverage production. J. Supercrit. Fluids 2021, 171, 105188. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Revisiting Amazonian plants for skin care and disease. Cosmetics 2017, 4, 25. [Google Scholar] [CrossRef]
- Alves, B.S.F.; Carvalho, F.I.M.; Cruz, A.S.; Filho, H.A.D.; Dantas, K.G.F. Determination of Ca, Mg, Na, and K in biodiesel of oilseed from northern Brazil. Rev. Virtual Quim. 2018, 10, 542–550. [Google Scholar] [CrossRef]
- Silva, T.C.; Araujo, E.C.G.; da Silva Lins, T.R.; Reis, C.A.; Sanquetta, C.R.; da Rocha, M.P. Non-timber forest products in Brazil: A bibliometric and a state of the art review. Sustainability 2020, 12, 7151. [Google Scholar] [CrossRef]
- Trentini, C.P.; Cuco, R.P.; Cardozo-Filho, L.; da Silva, C. Extraction of macauba kernel oil using supercritical carbon dioxide and compressed propane. Can. J. Chem. Eng. 2019, 97, 785–792. [Google Scholar] [CrossRef]
- Zanqui, A.B.; da Silva, C.M.; Ressutte, J.B.; de Morais, D.R.; Santos, J.M.; Eberlin, M.N.; Cardozo-Filho, L.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Brazil Nut Oil Extraction Using Subcritical n-Propane: Advantages and Chemical Composition. J. Braz. Chem. Soc. 2020, 31, 603–612. [Google Scholar] [CrossRef]
- Santos, L.C.D.; Álvarez-Rivera, G.; Sánchez-Martínez, J.D.; Johner, J.C.F.; Barrales, F.M.; de Oliveira, A.L.; Cifuentes, A.; Ibáñez, E.; Martínez, J. Comparison of different extraction methods of Brazilian “pacová” (Renealmia petasites Gagnep.) oilseeds for the determination of lipid and terpene composition, antioxidant capacity, and inhibitory effect on neurodegenerative enzymes. Food Chem. X 2021, 12, 100140. [Google Scholar] [CrossRef]
- Muñoz, A.M.; Casimiro-Gonzales, S.; Gómez-Coca, R.B.; Moreda, W.; Best, I.; Cajo-Pinche, M.I.; Loja, J.F.; Ibañez, E.; Cifuentes, A.; Ramos-Escudero, F. Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus mapora H. Karst): Evaluating Quality, Polyphenol Content and Antioxidant Activity. Foods 2022, 11, 1518. [Google Scholar] [CrossRef]
- Peixoto, V.O.D.S.; Silva, L.d.O.; Castelo-Branco, V.N.; Torres, A.G. Baru (Dipteryx alata Vogel) Oil Extraction by Supercritical-CO2: Improved Composition by Using Water as Cosolvent. J. Oleo Sci. 2022, 71, 201–213. [Google Scholar] [CrossRef]
- Barriga-Sánchez, M.; Casimiro-Gonzales, S.; Ramos-Escudero, F.; Muñoz, A.M.; Anticona, M. Supercritical CO2 assisted extraction of freeze-dried sinami fruit pulp (Oenocarpus mapora H. karst) oil: An experimental optimization approach. Lwt 2024, 198, 115956. [Google Scholar] [CrossRef]
- Cordeiro, R.M.; Ana, A.P.; Pinto, R.H.H.; da Costa, W.A.; da Silva, S.H.M.; de Souza Pinheiro, W.B.; Arruda, M.S.P.; Carvalho, R.N., Jr. Supercritical CO2 extraction of ucuúba (Virola surinamensis) seed oil: Global yield, kinetic data, fatty acid profile, and antimicrobial activities. Chem. Eng. Commun. 2019, 206, 86–97. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, L.; Kong, F.; Wang, S.; Hong, K.; Lei, F.; He, D. Effects of Extraction Strategies on Yield, Physicochemical and Antioxidant Properties of Pumpkin Seed Oil. Foods 2023, 12, 3351. [Google Scholar] [CrossRef] [PubMed]
- Chañi-Paucar, L.O.; Osorio-Tobón, J.F.; Johner, J.C.F.; Meireles, M.A.A. A comparative and economic study of the extraction of oil from Baru (Dipteryx alata) seeds by supercritical CO2 with and without mechanical pressing. Heliyon 2021, 7, e05971. [Google Scholar] [CrossRef] [PubMed]
- Chañi-Paucar, L.O.; Johner, J.C.F.; Hatami, T.; Meireles, M.A.A. Simultaneous integration of supercritical fluid extraction and mechanical cold pressing for the extraction from Baru seed. J. Supercrit. Fluids 2022, 183, 105553. [Google Scholar] [CrossRef]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Bélliard, T.; Castello, J.; Van Hecke, É.; Lanoisellé, J.L. Grape seed oil extraction: Interest of supercritical fluid extraction and gas-assisted mechanical extraction for enhancing polyphenol co-extraction in oil. Comptes Rendus Chim. 2014, 17, 284–292. [Google Scholar] [CrossRef]
- Santos, P.D.; De Aguiar, A.C.; Viganó, J.; Boeing, J.S.; Visentainer, J.V.; Martínez, J. Supercritical CO2 extraction of cumbaru oil (Dipteryx alata Vogel) assisted by ultrasound: Global yield, kinetics and fatty acid composition. J. Supercrit. Fluids 2016, 107, 75–83. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Pardauil, J.J.R.; de Molfetta, F.A.; Braga, M.; de Souza, L.K.C.; Filho, G.N.R.; Zamian, J.R.; da Costa, C.E.F. Characterization, thermal properties and phase transitions of amazonian vegetable oils. J. Therm. Anal. Calorim. 2017, 127, 1221–1229. [Google Scholar] [CrossRef]
- Jaramillo, J.E.C.C.; Bautista, M.P.C.; Solano, O.A.A.; Achenie, L.E.K.; Barrios, A.F.G. Impact of the mode of extraction on the lipidomic profile of oils obtained from selected Amazonian fruits. Biomolecules 2019, 9, 329. [Google Scholar] [CrossRef]
- Popescu, M.; Iancu, P.; Plesu, V.; Bildea, C.S. Carotenoids Recovery Enhancement by Supercritical CO2 Extraction from Tomato Using Seed Oils as Modifiers. Process 2022, 10, 2656. [Google Scholar] [CrossRef]
- Arturo-Perdomo, D.; Mora, J.P.J.; Ibáñez, E.; Cifuentes, A.; Hurtado-Benavides, A.; Montero, L. Extraction and Characterization of the Polar Lipid Fraction of Blackberry and Passion Fruit Seeds Oils Using Supercritical Fluid Extraction. Food Anal. Methods 2021, 14, 2026–2037. [Google Scholar] [CrossRef]
- Altuna, J.L.; Silva, M.; Álvarez, M.; Quinteros, M.F.; Morales, D.; Carrillo, W. Yellow pitaya (Hylocereus megalanthus) fatty acids composition from Ecuadorian Amazonia. Asian J. Pharm. Clin. Res. 2018, 11, 218–221. [Google Scholar] [CrossRef]
- GBIF, GBIF|Global Biodiversity Information Facility, (n.d.). Available online: https://www.gbif.org/es/ (accessed on 1 July 2024).
- Tropicos.org, Missouri Botanical Garden, (n.d.). Available online: https://www.tropicos.org/home (accessed on 1 July 2024).
- Royal Botanic Gardens Kew, Plants of the World Online, (n.d.). Available online: https://powo.science.kew.org/ (accessed on 15 May 2024).
- Irías-Mata, A.; Stuetz, W.; Sus, N.; Hammann, S.; Gralla, K.; Cordero-Solano, A.; Vetter, W.; Frank, J. Tocopherols, Tocomonoenols, and Tocotrienols in Oils of Costa Rican Palm Fruits: A Comparison between Six Varieties and Chemical versus Mechanical Extraction. J. Agric. Food Chem. 2017, 65, 7476–7482. [Google Scholar] [CrossRef]
- Santos, R.C.; Chagas, E.A.; de Melo Filho, A.A.; Takahashi, J.A.; Montero, I.F.; Santos, G.F.D.; Chagas, P.C.; de Melo, C.G.R. Chemical characterization of oils and fats from amazonian fruits by 1H NMR. Chem. Eng. Trans. 2018, 64, 235–240. [Google Scholar] [CrossRef]
- Reyes-Giraldo, A.F.; Gutierrez-Montero, D.J.; Rojano, B.A.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A. SEQUENTIAL EXTRACTION PROCESS OF OIL AND ANTIOXIDANT COMPOUNDS FROM CHONTADURO EPICARP. J. Supercrit. Fluids 2020, 166, 105022. [Google Scholar] [CrossRef]
- Da Cunha, A.L.A.; Freitas, S.P.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Chemical composition and oxidative stability of jussara Euterpe edulis M.) oil extracted by cold and hot mechanical pressing. Grasas y Aceites 2017, 68, e218. [Google Scholar] [CrossRef]
- Barbi, R.C.T.; de Souza, A.R.C.; de Melo, A.M.; Teixeira, G.L.; Corazza, M.L.; Ribani, R.H. Fatty acid profile and lipid quality of Maximiliana maripa oil obtained by supercritical CO2 and pressurized ethanol. J. Supercrit. Fluids 2020, 165, 104979. [Google Scholar] [CrossRef]
- de Souza, F.G.; Náthia-Neves, G.; de Araújo, F.F.; Audibert, F.L.D.; Delafiori, J.; Neri-Numa, I.A.; Catharino, R.R.; de Alencar, S.M.; de Almeida Meireles, M.A.; Pastore, G.M. Evaluation of antioxidant capacity, fatty acid profile, and bioactive compounds from buritirana (Mauritiella armata Mart.) oil: A little-explored native Brazilian fruit. Food Res. Int. 2021, 142, 110260. [Google Scholar] [CrossRef]
- dos Santos, O.V.; Pinaffi-Langley, A.C.d.C.; Ferreira, M.C.R.; de Souza, A.L.G.; Carvalho-Junior, R.N.; Teixeira-Costa, B.E. Effect of extraction type on the fatty acids profile and physicochemical properties of biolipids from Astrocaryum vulgare pulp: Supercritical CO2 versus n-hexane extractions. Int. J. Food Sci. Technol. 2023, 58, 3982–3995. [Google Scholar] [CrossRef]
- Sorita, G.D.; Favaro, S.P.; Rodrigues, D.d.S.; Junior, W.P.d.S.; Leal, W.G.d.O.; Ambrosi, A.; Di Luccio, M. Aqueous enzymatic extraction of macauba (Acrocomia aculeata) pulp oil: A green and sustainable approach for high-quality oil production. Food Res. Int. 2024, 182, 114160. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.A.; Berton, L.H.C.; Diaz, B.G.; Ferrari, R.A. Macauba: A promising tropical palm for the production of vegetable oil. OCL–Oilseeds Fats Crop Lipids 2018, 25, D108. [Google Scholar] [CrossRef]
- Trentini, C.P.; Santos, K.A.; da Silva, E.A.; Garcia, V.A.D.S.; Cardozo-Filho, L.; da Silva, C. Oil extraction from macauba pulp using compressed propane. J. Supercrit. Fluids 2017, 126, 72–78. [Google Scholar] [CrossRef]
- Hashim, M.A.; Yam, M.F.; Hor, S.Y.; Lim, C.P.; Asmawi, M.Z.; Sadikun, A. Anti-hyperglycaemic activity of Swietenia macrophylla king (Meliaceae) seed extracts in normoglycaemic rats undergoing glucose tolerance tests. Chin. Med. 2013, 8, 11. [Google Scholar] [CrossRef]
- Jayanegara, A.; Harahap, R.P.; Rozi, R.F.; Nahrowi. Effects of lipid extraction on nutritive composition of winged bean (Psophocarpus tetragonolobus), rubber seed (Hevea brasiliensis), and tropical almond (Terminalia catappa). Vet. World 2018, 11, 446–451. [Google Scholar] [CrossRef]
- Mera, J.J.R.; Abreu-Naranjo, R.; Alvarez-Suarez, J.M.; Viafara, D. Chemical characterization, fatty acid profile and antioxidant activity of Gustavia macarenensis fruit mesocarp and its oil from the amazonian region of Ecuador as an unconventional source of vegetable oil. Grasas y Aceites 2019, 70, e298. [Google Scholar] [CrossRef]
- Kumar, R.; Das, N. Seed oil of Jatropha curcas L. germplasm: Analysis of oil quality and fatty acid composition. Ind. Crops Prod. 2018, 124, 663–668. [Google Scholar] [CrossRef]
- Gomes, F.S.P.; De Sobral, A.D.; Da Silva, A.M.R.B.; Da Rocha, M.A.G. Moringa oleifera: A promising agricultural crop and of social inclusion for Brazil and semi-Arid regions for the production of energetic biomass (biodiesel and briquettes). OCL–Oilseeds Fats. Crop. Lipids 2018, 25, D106. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonça, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef]
- Lafont-Mendoza, J.J.; Espinosa-Fuentes, E.A.; Severiche-Sierra, C.A.; Jaimes-Morales, J.; Marrugo-Ligardo, Y.A. Proximal analysis of the residual cake obtained with extraction by pressing seeds of Perehuetano. ARPN J. Eng. Appl. Sci. 2018, 13, 8069–8072. [Google Scholar]
- Zaniol, F.; Calisto, J.F.F.; Cozzer, G.; Ferro, D.M.; Dias, J.L.; Rodrigues, L.G.G.; Mazzutti, S.; Rezende, R.S.; Simões, D.A.; Ferreira, S.R.S.; et al. Comparative larvicidal effect of Pterodon spp. extracts obtained by different extraction methods. J. Supercrit. Fluids 2020, 166, 104993. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Maciel, L.G.; Mazzutti, S.; Gonçalves, C.B.; Ferreira, S.R.S.; Block, J.M. Composition, thermal behavior and antioxidant activity of pracaxi (Pentaclethra macroloba) seed oil obtained by supercritical CO2. Biocatal. Agric. Biotechnol. 2020, 24, 101521. [Google Scholar] [CrossRef]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenéz, D.; Muñoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161, 104824. [Google Scholar] [CrossRef]
- Obaidat, R.; Aleih, H.; Mashaqbeh, H.; Altaani, B.; Alsmadi, M.M.; Alnaief, M. Development and Evaluation of Cocoa Butter Taste Masked Ibuprofen Using Supercritical Carbon Dioxide. AAPS PharmSciTech 2021, 22, 106. [Google Scholar] [CrossRef]
- Chañi-Paucar, L.O.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Technical and economic evaluation of supercritical CO2 extraction of oil from sucupira branca seeds. J. Supercrit. Fluids 2022, 181, 105494. [Google Scholar] [CrossRef]
- Polmann, G.; Teixeira, G.L.; Santos, P.H.; Rivera, G.Á.; Ibañez, E.; Cifuentes, A.; Ferreira, S.R.S.; Block, J.M. Chemical characterization of gurguéia nut (Dipteryx lacunifera Ducke) and press cake oil obtained by hydraulic pressing and supercritical extraction. Biomass Convers. Biorefinery. 2023, 14, 19065–19080. [Google Scholar] [CrossRef]
- Mateus, L.S.; Dutra, J.M.; Favareto, R.; da Silva, E.A.; Pinto, L.F.; da Silva, C.; Cardozo-Filho, L. Optimization Studies and Compositional Oil Analysis of Pequi (Caryocar brasiliense Cambess) Almonds by Supercritical CO2 Extraction. Molecules 2023, 28, 1030. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Moraes, I.C.F.; de Rosso, V.V.; Rodrigues, C.E.d.C.; de Souza, A.C. Effect of the application of an enzymatic pretreatment on bioactive compounds of Caryocar brasiliense Camb pulp oil. J. Food Process. Preserv. 2018, 42, e13828. [Google Scholar] [CrossRef]
- Zoccali, M.; Giuffrida, D.; Salafia, F.; Rigano, F.; Dugo, P.; Casale, M.; Mondello, L. Apocarotenoids profiling in different Capsicum species. Food Chem. 2021, 334, 127595. [Google Scholar] [CrossRef]
- Pires, F.B.; Dolwitsch, C.B.; Ugalde, G.A.; Menezes, B.B.; Fontana, M.E.Z.; Rieffeld, R.C.; Sagrillo, M.R.; Essi, L.; Mazutti, M.A.; da Rosa, M.B.; et al. Chemical study, antioxidant activity, and genotoxicity and cytotoxicity evaluation of Ruellia angustiflora. Nat. Prod. Res. 2021, 35, 5317–5322. [Google Scholar] [CrossRef]
- Triana-Maldonado, D.M.; Torijano-Gutiérrez, S.A.; Giraldo-Estrada, C. Supercritical CO2 extraction of oil and omega-3 concentrate from Sacha inchi (Plukenetia volubilis L.) from Antioquia, Colombia. Grasas y Aceites 2017, 68, e172. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Delvar, A.; de Caro, P.; Caro, Y.; Sing, A.S.C.; Thomas, R.; Raynaud, C. Semi-Siccative Oils and Bioactive Fractions Isolated from Reunion Island Fruit Co-Product: Two Case Studies. Eur. J. Lipid Sci. Technol. 2019, 121, 1800391. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies 2023, 16, 5852. [Google Scholar] [CrossRef]
- Aboubakar, X.; Goudoum, A.; Bébé, Y.; Mbofung, C.M.F. Optimization of Jatropha curcas pure vegetable oil production parameters for cooking energy. S. Afr. J. Chem. Eng. 2017, 24, 196–212. [Google Scholar] [CrossRef]
- Romuli, S.; Karaj, S.; Latif, S.; Müller, J. Performance of mechanical co-extraction of Jatropha curcas L. kernels with rapeseed, maize or soybean with regard to oil recovery, press capacity and product quality. Ind. Crops Prod. 2017, 104, 81–90. [Google Scholar] [CrossRef]
- Achicanoy, D.D.; Benavides, A.H.; Martínez-Correa, H.A. Study of supercritical CO2 extraction of tamarillo (Cyphomandra betacea) seed oil containing high added value compounds. Electrophoresis 2018, 39, 1917–1925. [Google Scholar] [CrossRef]
- Carvalho, L.M.S.; Oliveira, A.M.B.; Grimaldi, R.; de Souza, P.T.; Batista, E.A.C.; Martínez, J. Supercritical fluid and pressurized liquid extraction of spent tucumã-do-Amazonas (Astrocaryum aculeatum) almonds. J. Supercrit. Fluids 2024, 209, 106238. [Google Scholar] [CrossRef]
- Vardanega, R.; Fuentes, F.S.; Palma, J.; Bugueño-Muñoz, W.; Cerezal-Mezquita, P.; Ruiz-Domínguez, M.C. Valorization of granadilla waste (Passiflora ligularis, Juss.) by sequential green extraction processes based on pressurized fluids to obtain bioactive compounds. J. Supercrit. Fluids 2023, 194, 105833. [Google Scholar] [CrossRef]
- Pantoja-Chamorro, A.L.; Hurtado-Benavides, A.M.; Martinez-Correa, H.A. Caracterización de aceite de semillas de maracuyá (Passiflora edulis Sims.) procedentes de residuos agroindustriales obtenido con CO2 supercrítico. Acta Agron. 2017, 66, 178–185. [Google Scholar]
- Shah, N.A.; Prasad, R.V.; Patel, B.B. Optimization of Supercritical Fluid Extraction of Paprika (cv. Reshampatti) Oil, Capsaicin and Pigments. Flavour. Fragr. J. 2020, 35, 469–477. [Google Scholar] [CrossRef]
- Menezes, G.E.O.; Barbosa, J.R.; Pires, C.S.F.; Ferreira, M.C.R.; De Souza e Silva, A.P.; Siqueira, L.M.M.; Junior, R.N.D.C. Development of a new scale-up equation to obtain Tucumã-of-Pará (Astrocaryum vulgare Mart.) oil rich in carotenoids using supercritical CO2 as solvent. J. Supercrit. Fluids 2022, 181, 105481. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Sena, C.; Santos, O.V.; Da Costa, W.A.; Rodrigues, A.M.C.; Carvalho, R.N. Extraction of bacaba (Oenocarpus bacaba) oil with supercritical CO2: Global yield isotherms, fatty acid composition, functional quality, oxidative stability, spectroscopic profile and antioxidant activity. Grasas Y Aceites 2018, 69, 246. [Google Scholar] [CrossRef]
- Durante, M.; Montefusco, A.; Marrese, P.P.; Soccio, M.; Pastore, D.; Piro, G.; Mita, G.; Lenucci, M.S. Seeds of pomegranate, tomato and grapes: An underestimated source of natural bioactive molecules and antioxidants from agri-food by-products. J. Food Compos. Anal. 2017, 63, 65–72. [Google Scholar] [CrossRef]
- Mohammadnezhad, P.; Valdés, A.; Barrientos, R.E.; Ibáñez, E.; Block, J.M.; Cifuentes, A. A Comprehensive Study on the Chemical Characterization and Neuroprotective Evaluation of Pracaxi Nuts Extracts Obtained by a Sustainable Approach. Foods 2023, 12, 3879. [Google Scholar] [CrossRef] [PubMed]
- Solaberrieta, I.; Mellinas, A.C.; Espagnol, J.; Hamzaoui, M.; Jiménez, A.; Garrigós, M.C. Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques. Foods 2022, 11, 2408. [Google Scholar] [CrossRef]
- Kapoor, S.; Gandhi, N.; Tyagi, S.K.; Kaur, A.; Mahajan, B.V.C. Extraction and characterization of guava seed oil: A novel industrial byproduct. LWT 2020, 132, 109882. [Google Scholar] [CrossRef]
- Narváez-Cuenca, C.E.; Inampues-Charfuelan, M.L.; Hurtado-Benavides, A.M.; Parada-Alfonso, F.; Vincken, J.P. The phenolic compounds, tocopherols, and phytosterols in the edible oil of guava (Psidium guava) seeds obtained by supercritical CO2 extraction. J. Food Compos. Anal. 2020, 89, 103467. [Google Scholar] [CrossRef]
- Medeiros-Neves, B.; Diel, K.A.P.; Eifler-Lima, V.L.; Teixeira, H.F.; Cassel, E.; Vargas, R.M.F.; Von Poser, G.L. Influence of the supercritical CO2 extraction in the stability of the coumarins of Pterocaulon lorentzii (Asteraceae). J. CO2 Util. 2020, 39, 101165. [Google Scholar] [CrossRef]
- Calva-Cruz, O.d.J.; Badillo-Larios, N.S.; De León-Rodríguez, A.; Espitia-Rangel, E.; González-García, R.; Turrubiartes-Martinez, E.A.; Castro-Gallardo, A.; de la Rosa, A.P.B. Lippia graveolens HBK oleoresins, extracted by supercritical fluids, showed bactericidal activity against multidrug resistance Enterococcus faecalis and Staphylococcus aureus strains. Drug Dev. Ind. Pharm. 2021, 47, 1546–1555. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Vardanega, R.; Hatami, T.; Meireles, M.A.A. Process integration for recovering high added-value products from Genipa americana L.: Process optimization and economic evaluation. J. Supercrit. Fluids 2020, 164, 104897. [Google Scholar] [CrossRef]
- Soldan, A.C.F.; Arvelos, S.; Watanabe, É.O.; Hori, C.E. Supercritical fluid extraction of oleoresin from Capsicum annuum industrial waste. J. Clean. Prod. 2021, 297, 126593. [Google Scholar] [CrossRef]
- Guo, T.; Wan, C.; Huang, F.; Wei, C. Evaluation of quality properties and antioxidant activities of tiger nut (Cyperus esculentus L.) oil produced by mechanical expression or/with critical fluid extraction. LWT 2021, 141, 110915. [Google Scholar] [CrossRef]
- Panadare, D.; Dialani, G.; Rathod, V. Extraction of volatile and non-volatile components from custard apple seed powder using supercritical CO2 extraction system and its inventory analysis. Process Biochem. 2021, 100, 224–230. [Google Scholar] [CrossRef]
- Mesquita, P.C.; Rodrigues, L.G.G.; Mazzutti, S.; da Silva, M.; Vitali, L.; Lanza, M. Intensified green-based extraction process as a circular economy approach to recover bioactive compounds from soursop seeds (Annona muricata L.). Food Chem. X 2021, 12, 100164. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Maciel, L.G.; Mazzutti, S.; Barbi, R.C.T.; Ribani, R.H.; Ferreira, S.R.S.; Block, J.M. Sequential green extractions based on supercritical carbon dioxide and pressurized ethanol for the recovery of lipids and phenolics from Pachira aquatica seeds. J. Clean. Prod. 2021, 306, 127223. [Google Scholar] [CrossRef]
- Macawile, M.C.; Auresenia, J. Comparison of biodiesel yield from seed oils extracted by ultrasound-assisted chemical solvent and supercritical CO2 methods. Int. J. Appl. Sci. Eng. Comp. 2022, 19, 2021279. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Statistical modeling of supercritical extraction of hemp (Cannabis sativa) and papaya (Carica papaya) seed oils through artificial neural network and central composite design. Soft Comput. 2022, 26, 2307–2324. [Google Scholar] [CrossRef]
- Díaz-Cervantes, M.D.; Ramos-Ramírez, E.G.; Gimeno-Seco, M.; Salazar-Montoya, J.A. Supercritical CO2 Extraction of oil from Chan (Hyptis suaveolens (L.) Poit) Seeds and its Physicochemical Characterization, Spectroscopy and Nutritional Analysis. Food Anal. Methods 2023, 16, 918–932. [Google Scholar] [CrossRef]
- De Cássia Rodrigues Batista, C.; De Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; Da Silva Souza Filho, A.P.; Machado, N.T.; Carvalho, R.N. Supercritical CO2 extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2015, 107, 364–369. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.; Al-Nasser, A. Role of Poultry Research in Increasing Consumption of PUFA in Humans. In Nutrition in Health and Disease–Our Challenges Now and Forthcoming Time; IntechOpen: London, UK, 2019; p. 13. [Google Scholar] [CrossRef]
- Martin, C.A.; De Almeida, V.V.; Ruiz, M.R.; Visentainer, J.E.L.; Matshushita, M.; De Souza, N.E.; Visentainer, J.V. Ácidos graxos poliinsaturados ômega-3 e ômega-6: Importância e ocorrência em alimentos. Rev. Nutr. 2006, 19, 761–770. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Introduction to Essential Fatty Acids. In Nutraceutical Fatty Acids from Oleaginous Microalgae: A Human Health Perspective; Wiley: Hoboken, NJ, USA, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Popa, O.; Bəbeanu, N.E.; Popa, I.; Niţə, S.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. Biomed. Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef] [PubMed]
- Spanova, M.; Daum, G. Squalene–biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Mantovani, L.M.; Pugliese, C. Phytosterol supplementation in the treatment of dyslipidemia in children and adolescents: A systematic review. Rev. Paul. Pediatr. 2020, 39, e2019389. [Google Scholar] [CrossRef]
- Chong, W.T.; Lee, Y.Y.; Tang, T.K.; Phuah, E.T. Minor Components in Edible Oil. In Recent Advances in Edible Fats and Oils Technology; Springer: Singapore, 2022; pp. 141–187. [Google Scholar] [CrossRef]
- Ibiapina, A.; Gualberto, L.D.S.; Dias, B.B.; Freitas, B.C.B.; Martins, G.A.D.S.; Filho, A.A.M. Essential and fixed oils from Amazonian fruits: Proprieties and applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 8842–8854. [Google Scholar] [CrossRef]
- Jaramillo-Vivanco, T.; Balslev, H.; Montúfar, R.; Cámara, R.M.; Giampieri, F.; Battino, M.; Cámara, M.; Alvarez-Suarez, J.M. Three Amazonian palms as underestimated and little-known sources of nutrients, bioactive compounds and edible insects. Food Chem. 2022, 372, 131273. [Google Scholar] [CrossRef]
- da Silva, D.A.; da Cruz Rodrigues, A.M.; Santos, A.O.D.; Salvador-Reyes, R.; da Silva, L.H.M. Physicochemical and technological properties of pracaxi oil, cupuassu fat and palm stearin blends enzymatically interesterified for food applications. LWT 2023, 184, 114961. [Google Scholar] [CrossRef]
- Dhakane-Lad, J.; Kar, A.; Patel, A.S. SC-CO2 extraction of lycopene from red papaya using rice bran oil as a co-solvent lessens its degradation during storage. Sep. Sci. Technol. 2023, 58, 2357–2368. [Google Scholar] [CrossRef]
- Johner, J.C.F.; Hatami, T.; Meireles, M.A.A. Developing a supercritical fluid extraction method assisted by cold pressing for extraction of pequi (Caryocar brasiliense). J. Supercrit. Fluids 2018, 137, 34–39. [Google Scholar] [CrossRef]
- Willems, P.; Kuipers, N.J.M.; de Haan, A.B. Gas assisted mechanical expression of oilseeds: Influence of process parameters on oil yield. J. Supercrit. Fluids 2008, 45, 298–305. [Google Scholar] [CrossRef]


| Species | Part of Plant | Oil/Fat | |
|---|---|---|---|
| Palm | |||
| Oenocarpus bacaba | Fruit | Oil | [15] |
| Elaeis oleifera | Fruit | Oil | [38] |
| Oenocarpus bataua Mart. | Fruit | Oil | [7,14,31] |
| Attalea speciosa Mart. (Synonym: Orbignya phalerata) | Seed | Oil | [7,14] |
| Euterpe oleracea | Fruit | Oil | [39] |
| Mauritia aculeata | Fruit | Oil | [39] |
| Barcella odora | Pulp | Oil | [39] |
| Astrocaryum acaule | Pulp | Oil | [39] |
| Bactris gasipaes | Pulp | Oil | [39,40] |
| Astrocaryum murumuru | Seed | Fat | [7] |
| Mauritia vinifera | Fruit | Oil | [7] |
| Euterpe edulis M. | Pulp | Oil | [41] |
| Euterpe precatoria | Fruit | Oil | [31] |
| Oenocarpus distichus Mart. | Fruit | Oil | [12] |
| Maximiliana maripa | Fruit | Oil | [42] |
| Mauritiella armata Mart. | Fruit | Oil | [43] |
| Oenocarpus mapora H. Karst | Pulp | Oil | [20,22] |
| Mauritia flexuosa L.f. | Fruit | Oil | [11,30,31,39] |
| Astrocaryum vulgare | Pulp/Kernel | Oil | [30,44] |
| Acrocomia aculeata | Pulp | Oil | [45,46,47] |
| Tree | |||
| Platonia insignis | Fruit | Oil | [15] |
| Virola surinamensis | Seed | Fat | [7,30] |
| Carapa guianensis Aublet | Seed | Oil | [14] |
| Annona hypoglauca | Seed | Oil | [39] |
| Byrsonima crassifolia | Seed | Oil | [39] |
| Byrsonima coccolobifolia | Seed | Oil | [39] |
| Platonia insignis | Seed | Fat | [7] |
| Theobroma grandiflorum | Seed | Fat | [7] |
| Pentaclethra filamentosa | Seed | Oil | [7] |
| Copaifera langsdorffii | Stem | Oil | [7] |
| Carapa guianensis | Seed | Oil | [7] |
| Theobroma grandiflorum | Seed | Fat | [6] |
| Swietenia macrophylla King | Seed | Extract | [48] |
| Hevea brasiliensis | Seed | Oil | [30,49] |
| Terminalia catappa | Seed | Oil | [30,49] |
| Gustavia macarenensis | Fruit | Oil | [50] |
| Jatropha curcas L. | Seed | Oil | [51] |
| Moringa oleifera Lamarck | Seed | Oil | [52] |
| Persea americana Mill. | Pulp | Oil | [53] |
| Parinari pachyphylla Rusby | Seed | Oil | [54] |
| Pterodon spp. | Fruit | Oil | [55] |
| Pentaclethra macroloba | Seed | Oil | [6,56] |
| Geoffroea decorticans | Seed | Oil | [57] |
| Dipteryx alata Vogel | Seed | Oil | [21,25] |
| Theobroma cacao L. | Seed | Fat | [58] |
| Bertholletia excelsa | Seed | Oil | [7,13,15,18] |
| Pterodon emarginatus Vogel | Seed | Oil | [59] |
| Dipteryx lacunifera Ducke | Seed | Oil | [60] |
| Caryocar brasiliense Cambess. | Seed | Oil | [61,62] |
| Subshrub or shrub | |||
| Capsicum baccatum | Fruit | Apocarotenoids | [63] |
| Capsicum chinense | Fruit | Apocarotenoids | [63] |
| Ruellia angustiflora | Leaves | SFE extract rich in fat-soluble compounds | [64] |
| Liana | |||
| Passiflora edulis | Fruit | Oil | [30] |
| Passiflora spp. | Seed | Oil | [7] |
| Cactus | |||
| Hylocereus megalanthus | Seed | Oil | [34] |
| Climbing shrub | |||
| Plukenetia volubilis L. | Seed | Oil | [65] |
| Rubus glaucus | Seed | Oil | [33] |
| Scrambling subshrub | |||
| Solanum lycopersicum | Seed/peel | Oleoresin/Oil | [32,66] |
| Perennial or rhizomatous geophyte | |||
| Renealmia petasites Gagnep. | Seed | Oil | [19] |
| Raw Material | Fatty Acids * | Content (%) | |
|---|---|---|---|
| P. edulis seeds | Linoleic C18:2 Oleic C18:1n9 Palmitic C16:0 | 70.9 16.6 9.4 | [67] |
| E. oleracea pulp | Oleic C18:1n9 Palmitic C16:0 Linoleic C18:2n6 Palmitoleic C16:1 | 52.73 23.47 15.54 5.49 | [94] |
| V. surinamensis seeds | Myristic C14:0 Lauric C12:0 Palmitic C16:0 Pentadecanoic C15:0 Decanoic C10:0 | 71.66 23.48 1.86 1.44 1.03 | [23] |
| O. distichus pulp | Oleic C18:1n9 Palmitic C16:0 Linoleic C18:2n6 Stearic C18:0 | 66.22 17.62 12.10 2.39 | [12] |
| O. bacaba pulp | Oleic C18:1n9 Palmitic C16:0 Linoleic C18:2n-6 Stearic C18:0 | 60.52 22.05 13.37 2.68 | [77] |
| M. oleifera leaf | cis-11-Eicosenoic C20:1 Palmitic C16:0 Linoleic C18:2n6 Oleic C18:1n9 Stearic C18:0 cis-5,8,11,14,17- Eicosapentaenoic C20:5n-3 Myristic C14:0 | 54.44 23.65 6.84 5.92 3.93 1.99 1.13 | [95] |
| M. oleifera seed | Oleic C18:1n9 Palmitic C16:0 Stearic C18:0 Arachidic C20:0 Linolenic C18:3n3 Palmitoleic C16:1 | 74.50 7.92 7.10 4.28 2.50 1.49 | [95] |
| C. betacea seeds | Linoleic C18:2n6 Oleic C18:1n9 Palmitic C16:0 Stearic C18:0 α-Linolenic C18:3 | 66.67 17.94 10.41 2.84 1.92 | [71] |
| Raw-Material | Compounds Type | Compounds | Content (mg/100g) | |
|---|---|---|---|---|
| P. edulis seed | Tocopherol | α-tocopherol γ-tocopherol δ-tocopherol | 2.5 11.3 3.9 | [67] |
| Phytosterol | Campesterol Estigmasterol β-sitosterol Δ5 avenasterol Δ7 stigmasterol Δ7 avenasterol Methylene cycloartenol Citrostadienol | 38.7 98.6 157.5 18.5 7.1 9.4 8.9 9.6 | ||
| A. aculeata seed | Tocopherol | α-tocopherol γ-tocopherol δ-tocopherol | 1.33 1.81 5.08 | [17] |
| P. edulis seed | Terpene Phytosterol | Squalene Campesterol Estigmasterol β-sitosterol Lanosterol | 11.13 ** 0.78 ** 2.43 ** 2.58 ** 0.58 ** | [74] |
| A. aculeata seed | Phytosterol | Campesterol β-sitosterol | 1.71 22.99 | [17] |
| C. betacea seed | Terpene Tocopherol Phytosterol | Squalene γ-tocopherol Dihydrolanosterol β-sitosterol Cycloartenol | 6.82 ¥ 2.10 ¥ 0.64 ¥ 3.01 ¥ 2.40 ¥ | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chañi-Paucar, L.O.; Maceda Santivañez, J.C.; Paucarchuco Soto, J.; Portal-Cahuana, L.A.; Solis Malaga, C.L.S.; Chagua-Rodríguez, P.; Johner Flores, J.C.; Meireles, M.A.A. Supercritical Fluid Extraction of Amazonian Oils and Fats: Promising Species, Equipment, Yields, Composition, and Potential Uses. Processes 2025, 13, 948. https://doi.org/10.3390/pr13040948
Chañi-Paucar LO, Maceda Santivañez JC, Paucarchuco Soto J, Portal-Cahuana LA, Solis Malaga CLS, Chagua-Rodríguez P, Johner Flores JC, Meireles MAA. Supercritical Fluid Extraction of Amazonian Oils and Fats: Promising Species, Equipment, Yields, Composition, and Potential Uses. Processes. 2025; 13(4):948. https://doi.org/10.3390/pr13040948
Chicago/Turabian StyleChañi-Paucar, Larry Oscar, Julio Cesar Maceda Santivañez, Joselin Paucarchuco Soto, Leif Armando Portal-Cahuana, Carmen Liz Sandra Solis Malaga, Perfecto Chagua-Rodríguez, Julio Cezar Johner Flores, and Maria Angela A. Meireles. 2025. "Supercritical Fluid Extraction of Amazonian Oils and Fats: Promising Species, Equipment, Yields, Composition, and Potential Uses" Processes 13, no. 4: 948. https://doi.org/10.3390/pr13040948
APA StyleChañi-Paucar, L. O., Maceda Santivañez, J. C., Paucarchuco Soto, J., Portal-Cahuana, L. A., Solis Malaga, C. L. S., Chagua-Rodríguez, P., Johner Flores, J. C., & Meireles, M. A. A. (2025). Supercritical Fluid Extraction of Amazonian Oils and Fats: Promising Species, Equipment, Yields, Composition, and Potential Uses. Processes, 13(4), 948. https://doi.org/10.3390/pr13040948







