Preparation of Chemically Activated Porous Carbon Derived from Rubber-Seed Shell for CO2 Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of RSS-AC by Two-Step Activation Method
2.2. Preparation of RSS-AC by Three-Step Activation Method
2.3. Characterization of RSS-Derived AC
3. Results
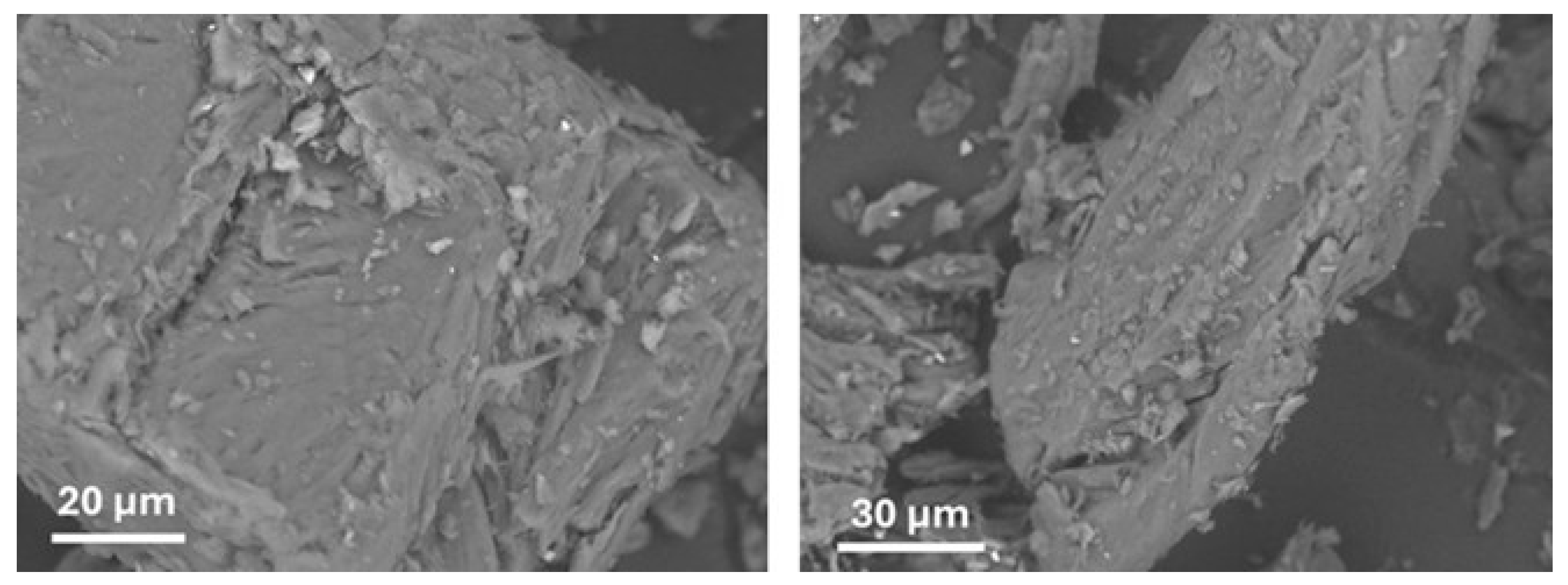
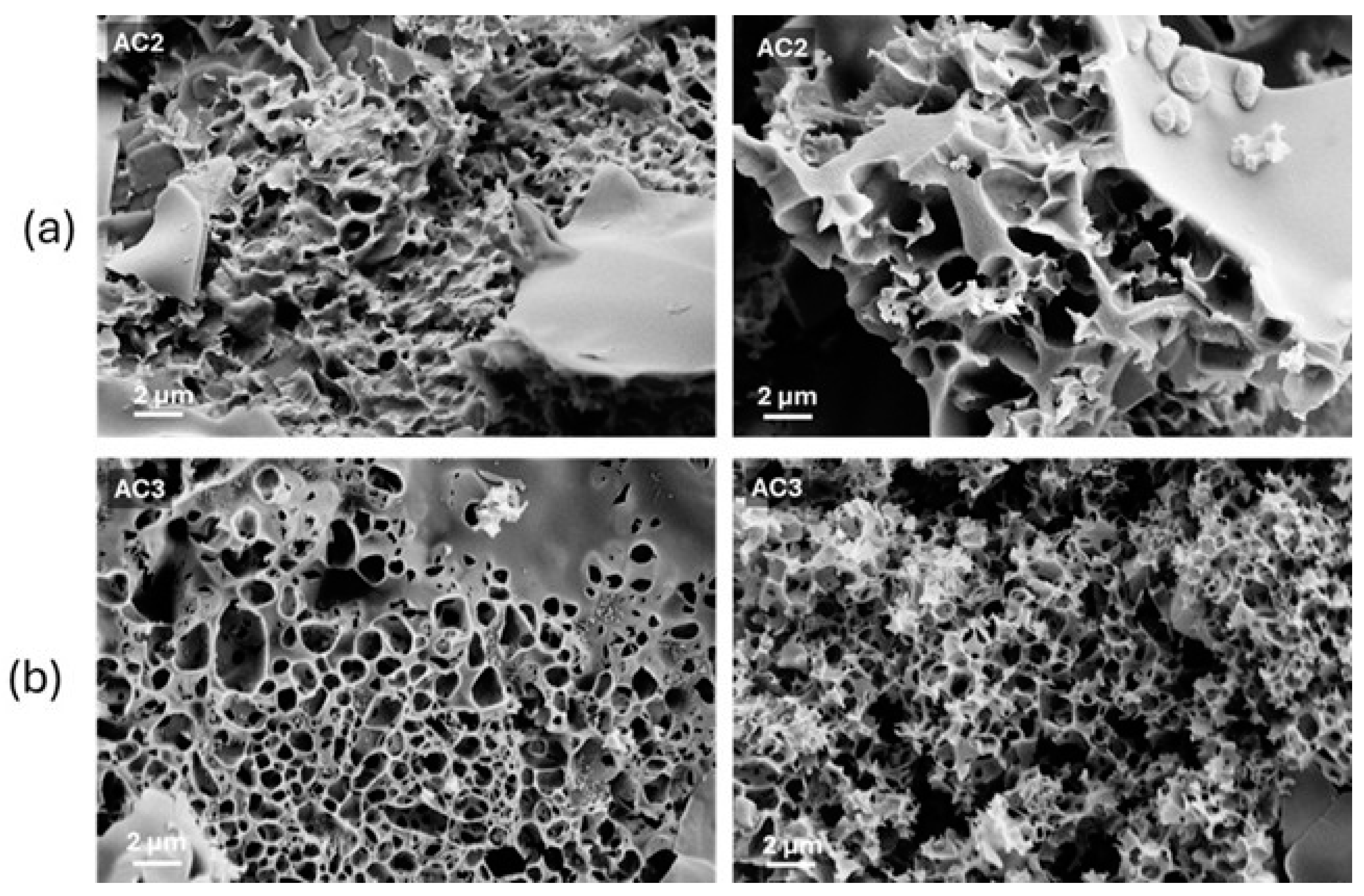
3.1. Structure and Morphology
3.2. Elemental Composition
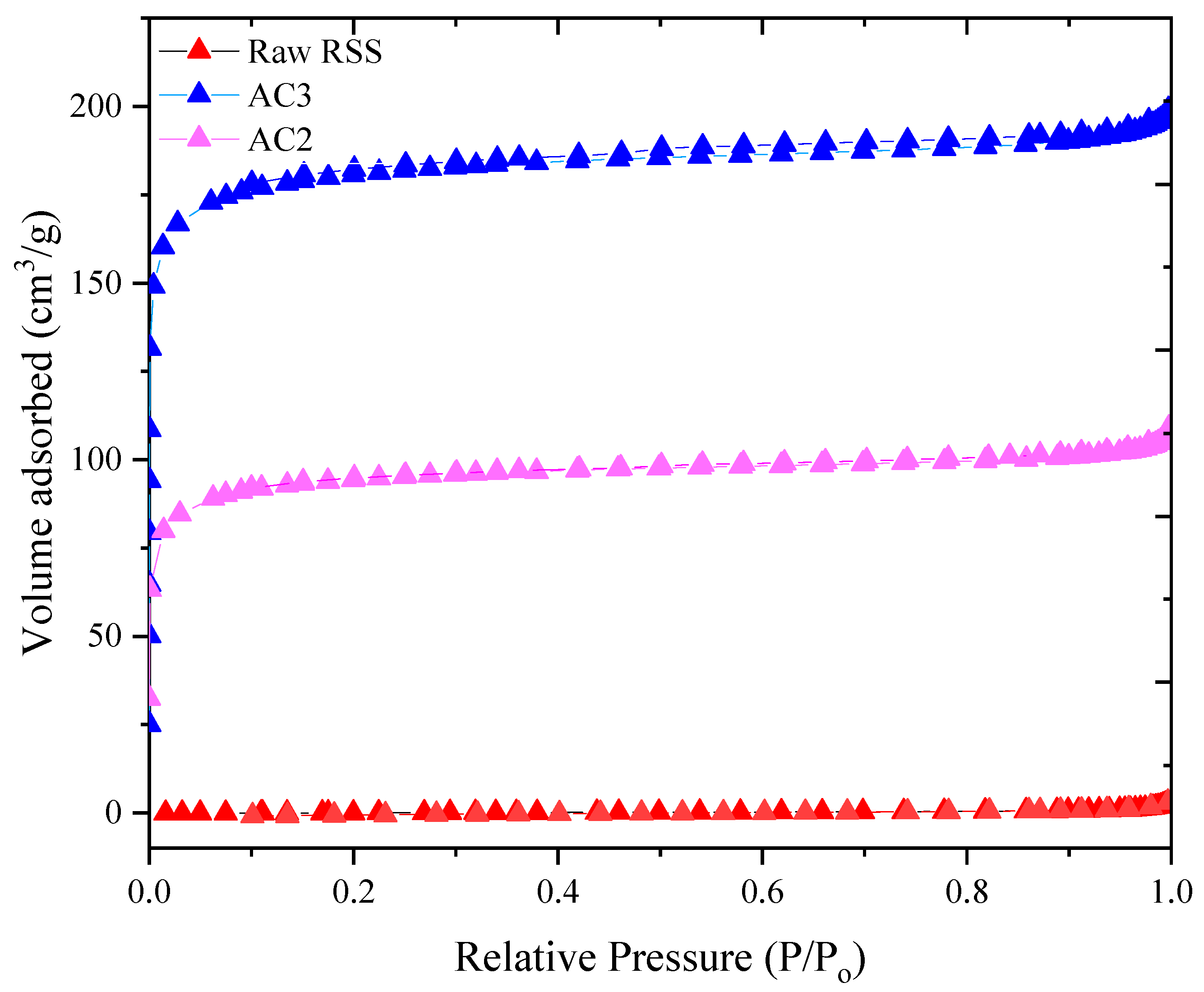
3.3. Surface Area and Porosity
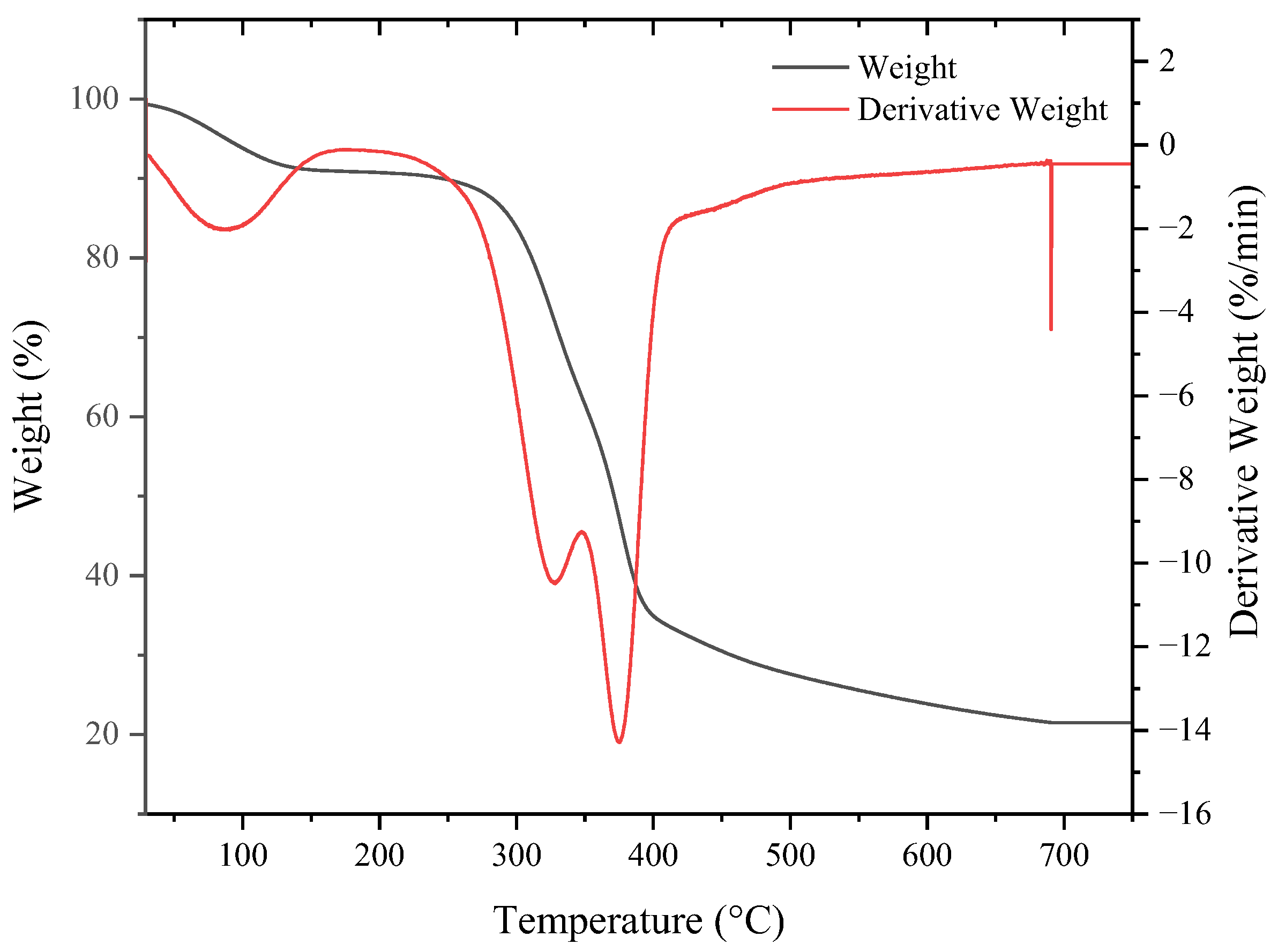
3.4. Thermal Stability
3.5. CO2 Adsorption Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- McDonald, J.D.; Kracko, D.; Doyle-Eisele, M.; Garner, C.E.; Wegerski, C.; Senft, A.; Knipping, E.; Shaw, S.; Rohr, A. Carbon capture and sequestration: An exploration inhalation toxicity assessment of amine-trapping solvents and their degradation products. Environ. Sci. Technol. 2014, 48, 10821–10828. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A. Review of post-combustion carbon dioixde capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Ngu, L.H.; Hashim, S.S. A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenh. Gases Sci. Technol. 2021, 11, 1076–1117. [Google Scholar] [CrossRef]
- Rochelle, G.T. Conventional amine scrubbing for CO2 capture. In Absorption-Based Post-Combustion Capture of Carbon Dioxide; Feron, P.H.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 35–67. [Google Scholar]
- Rochelle, G.T. Air pollution impacts of amine scrubbing for CO2 capture. Carbon Capture Sci. Technol. 2024, 11, 100192. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, F.; He, Y.; Ziao, P.; Webley, P.A. Impact of operating parameters on CO2 capture using carbon monolith by electrical swing adsorption technology (ESA). Chem. Eng. J. 2017, 327, 441–453. [Google Scholar] [CrossRef]
- Ntiamoah, A.; Ling, J.; Xiao, P.; Webley, P.A.; Zhai, Y. CO2 capture by temperature swing adsoprtion: Use of hot CO2-rich gas for regeneration. Ind. Eng. Chem. Res. 2015, 55, 703–713. [Google Scholar] [CrossRef]
- Wiheeb, A.D.; Helwani, Z.; Kim, K.J.; Othman, M.R. Pressure swing adsorption technologies for carbon dioxide capture. Sep. Purif. Rev. 2016, 45, 108–121. [Google Scholar] [CrossRef]
- Cavalcante, C.L. Industrial adsorption separation processes: Fundamentals, modeling and applications. Lat. Am. Appl. Res. 2000, 30, 357–364. [Google Scholar]
- Jiang, Q.; Rentschler, J.; Sethia, G.; Weinman, S.; Perrone, R.; Liu, K. Synthesis of t-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 by adsorption process. Chem. Eng. J. 2013, 230, 380–388. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100564. [Google Scholar] [CrossRef]
- Lee, G.; Ahmed, I.; Jhung, S.H. CO2 adsorption using functionalized metal-organic frameworks under low pressure: Contribution of functional groups, excluding amines, to adsorption. Chem. Eng. J. 2024, 481, 148440. [Google Scholar] [CrossRef]
- Li, L.; Jung, H.S.; Lee, J.W.; Kang, Y.T. Review on applications of metal-organic frameworks for CO2 capture and the performance enhancement mechanisms. Renew. Sustain. Energy Rev. 2022, 162, 112441. [Google Scholar] [CrossRef]
- Zulkifli, Z.I.; Lim, K.L.; Teh, L.P. Metal-Organic Frameworks (MOFs) and their applications in CO2 adsorption and conversion. ChemistrySelect 2022, 7, 20220572. [Google Scholar] [CrossRef]
- Almoneef, M.M.; Jedli, H.; Mbarek, M. Experimental study of CO2 adsorption using activated carbon. Mater. Res. Express 2021, 8, 65602. [Google Scholar] [CrossRef]
- Hiremath, V.; Jadhav, A.H.; Lee, H.; Kwon, S.; Seo, J.G. Highly reversible CO2 capture using amino acid functionalized ionic liquids immobilized on mesoporous silica. Chem. Eng. J. 2016, 287, 602–617. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase change solvents for post-combustion CO2 capture: Principle, advances and challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Ello, A.S.; de Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Development of microporous carbons for CO2 capture by KOH activation of African palm shells. J. CO2 Util. 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Plaza, M.G.; Garcia, S.; Rubiera, F.; Pis, J.J.; Pevida, C. Post-combustion CO2 capture with a commercial activated carbon: Comparison of different regeneration strategies. Chem. Eng. J. 2010, 163, 41–47. [Google Scholar] [CrossRef]
- Ramli, N.; Samat, O.; Ismail, M.K.; Shaupi, N.S.A. Analysis of production factors for the rubber smallholder sector in Malaysia. J. Entrep. Bus. 2021, 9, 83–99. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. A comparison of two methods for producing CO2 capture adsorbents. Energy Procedia 2009, 1, 1107–1113. [Google Scholar] [CrossRef]
- Plaza, M.G.; Gonzalez, A.S.; Pevida, C.; Pis, J.J.; Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Erto, A.; Moreno-Pirajan, J.C. Chemical modification of activated carbon monoliths for CO2 adsorption. J. Therm. Anal. Calorim. 2013, 114, 1039–1047. [Google Scholar] [CrossRef]
- Sha, Y.; Lou, J.; Bai, S.; Wu, D.; Liu, B.; Ling, Y. Facile preparation of nitrogen-doped porous carbon from waste tobacco by a simple pre-treatment process and their application in electrochemical capacitor and CO2 capture. Mater. Res. Bull. 2015, 64, 327–332. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Rashidi, A.; Ghoreyshi, A.A. Evaluation of CO2 adosprtion with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. Chem. Eng. J. 2014, 254, 503–513. [Google Scholar] [CrossRef]
- Boujibar, O.; Souikny, A.; Ghamouss, F.; Achak, O.; Dahbi, M.; Chafik, T. CO2 capture using N-containing nanoporous activated carbon obtained from argan fruit shells. J. Environ. Chem. Eng. 2018, 6, 1995–2002. [Google Scholar] [CrossRef]
- Linares-Solano, A.; Lillo-Rodenas, M.A.; Marco-Lozar, J.P.; Kunowsky, M.; Romero-Anaya, A.J. NaOH and KOH for preparing activated carbons in energy and environmental applications. Int. J. Energy Environ. Econ. 2012, 20, 59–91. [Google Scholar]
- Okman, I.; Karagoz, S.; Tay, T.; Erdem, M. Activated carbons from grape seeds by chemical activation with potassium carbonate and potassium hydroxide. Appl. Surf. Sci. 2014, 293, 138–142. [Google Scholar] [CrossRef]
- Jin, X.-J.; Yu, Z.-M.; Wu, Y. Preparation of activated carbon from lignin obtained by straw pulping by KOH and K2CO3 chemical activation. Cellul. Chem. Technol. 2012, 46, 79–85. [Google Scholar]
- Deng, H.; Li, G.; Yang, H.; Tang, J.; Tang, J. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 2010, 163, 373–381. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Juan-Juan, J.; Cazorla-Amorós, D.; Linares-Solano, A. About reactions occuring during chemical activation with hydroxides. Carbon 2004, 42, 1371–1375. [Google Scholar] [CrossRef]
- McKee, D.W. Mechanisms of the alkali metal catalysed gasification of carbon. Fuel 1983, 62, 170–175. [Google Scholar] [CrossRef]
- Borhan, A.; Taha, M.F.; Hamzah, A.A. Characterization of activated carbon from wood sawdust prepared via chemical activation using potassium hydroxide. Adv. Mater. Res. 2014, 832, 132–137. [Google Scholar] [CrossRef]
- Singh, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rashidi, N.A.; SuzanaYusup; Borhan, A. Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Procedia Eng. 2016, 148, 630–637. [Google Scholar] [CrossRef]
- Zelenka, T. Adsorption and desorption of nitrogen at 77 K on micro- and meso- porous materials: Study of transport kinetics. Microporous Mesoporous Mater. 2016, 227, 202–209. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.-b.; Peng, J.-h.; Li, N.; Zhu, Z.-y. Preparation of high surface area activated carbons from tobacco stems with K2CO3 activation using microwave radiation. Ind. Crops Prod. 2008, 27, 341–347. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Huang, C.-M.; Ming-Yau, H.; Chen, H. Preparation of high-surface area PAN-based activated carbon by solution blowing process for CO2 adsorption. Sep. Purif. Technol. 2011, 82, 19–27. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 2013, 389, 230–235. [Google Scholar] [CrossRef]
- Gottipati, R.; Mishra, S. A kinetic study on pyrolysis and combustion characteristics of oil cakes: Effect of cellulose and lignin content. J. Fuel Chem. Technol. 2011, 39, 265–270. [Google Scholar] [CrossRef]
- de Oliveira, G.F.; de Andrade, R.C.; Trindade, M.A.G.; Andrade, H.M.C.; de Carvalho, C.T. Thermogravimetric and spectroscopic study (TG-DTA/FT-IR) of activated carbon from the renewable biomass source babassu. Quim. Nova 2017, 40, 284–292. [Google Scholar] [CrossRef]
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Ghani, N.A. CO2 adsorption performance on surface-functionalized activated carbon impregnated with pyrrolidinium-based ionic liquid. Processes 2022, 10, 2372. [Google Scholar] [CrossRef]
- Gopalan, J.; Raman, A.A.A.; Buthiyappan, A. Green adsorbents for CO2 adsorption: MgO impregnated palm kernel shell-based activated carbon. Int. J. Environ. Sci. Technol. 2024, 21, 6773–6788. [Google Scholar] [CrossRef]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption. Processes 2019, 11, 855. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2020, 260, 116382. [Google Scholar] [CrossRef]
- Yue, L.; Xia, Q.; Wang, L.; Wang, L.; DaCosta, H.; Yang, J.; Hu, X. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell. J. Colloid Interface Sci. 2018, 511, 259–267. [Google Scholar] [CrossRef]
- Aroua, M.K.; Daud, W.M.A.W.; Yin, C.Y.; Adinata, D. Adsorption capacities of carbon dioxide, oxygen, nitrogen and methane on carbon molecular basket derived from polyethyleneimine impregnation on microporous palm shell activated carbon. Sep. Purif. Technol. 2008, 62, 609–613. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Modeling CO2 adsorption on amine-functionalized mesoporous silica: 1. A semi-empirical equilibrium model. Chem. Eng. J. 2010, 161, 173–181. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Al-Mammori, A.; Hajari, A.; Rownaghi, A.A.; Razaei, F. Formulation of Aminosilica Adsorbents into 3D-Printed Monoliths and Evaluation of Their CO2 Capture Performance. ACS Appl. Mater. Interfaces 2017, 9, 7489–7498. [Google Scholar] [CrossRef]
- Tang, Z.; Han, Z.; Yang, G.; Yang, H. Polyethylenimine loaded nanoporous carbon with ultra-large pore volume for CO2 capture. Appl. Surf. Sci. 2013, 277, 47–52. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Wang, Q.; Tao, M.; He, Y.; Shi, Y. Carbon Dioxide Capture with Polyethylenimine-Functionalized Industrial-Grade Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2014, 53, 17468–17475. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. Polyethylenimine Functionalized As-Synthesized KIT-6 Adsorbent for Highly CO2/N2 Selective Separation. Energy Fuels 2016, 30, 9635–9644. [Google Scholar] [CrossRef]
- Jiao, J.; Cao, J.; Xia, Y.; Zhao, L. Improvement of adsorbent materials for CO2 capture by amine functionalized mesoporous silica with worm-hole framework structure. Chem. Eng. J. 2016, 306, 9–16. [Google Scholar] [CrossRef]
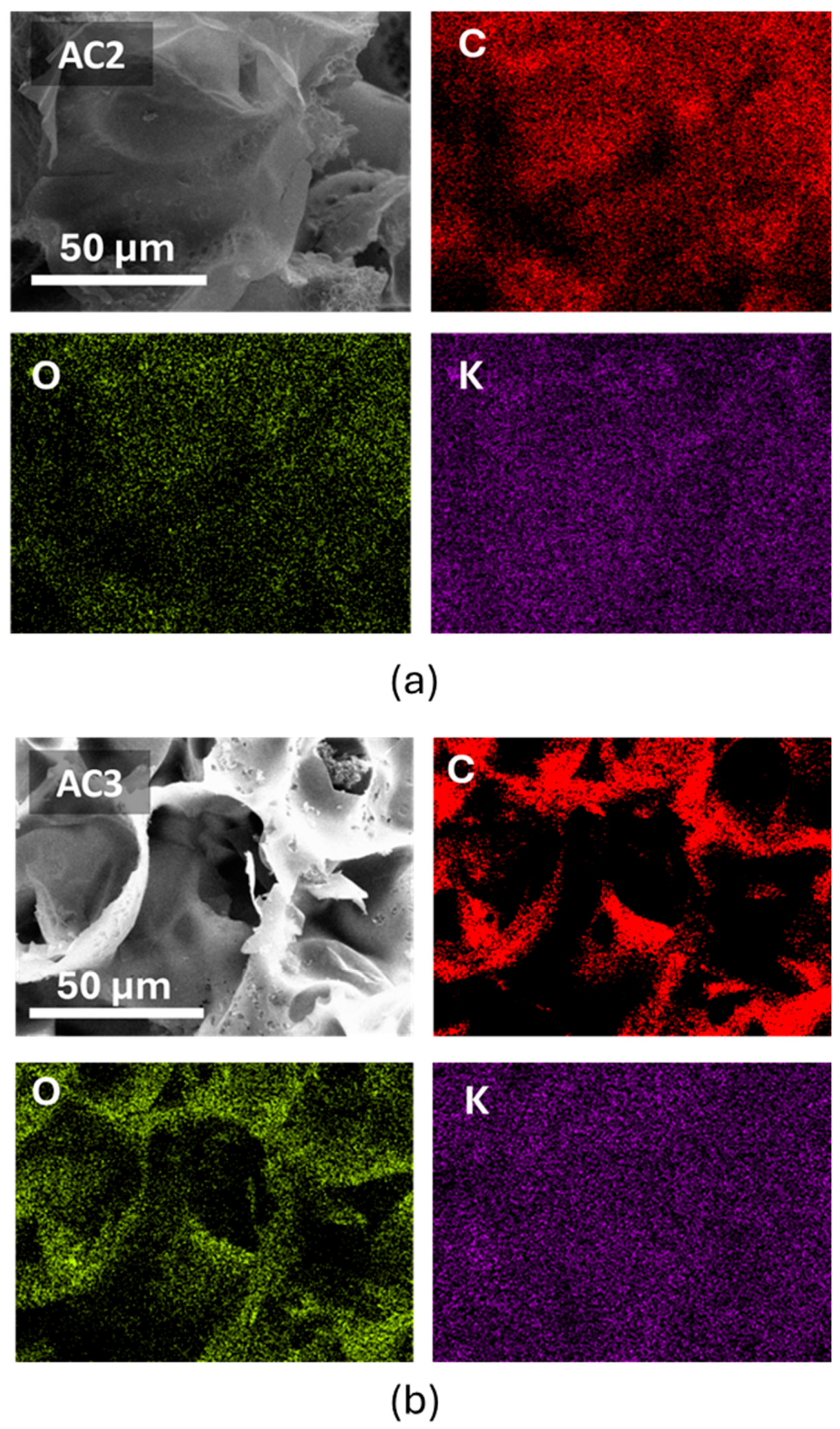


| Elements | Untreated RSS | AC2 | AC3 |
|---|---|---|---|
| Units | wt.% | wt.% | wt.% |
| Carbon | 48.40 | 77.72 | 77.14 |
| Nitrogen | 0.74 | 0.38 | 0.65 |
| Hydrogen | 6.86 | 2.94 | 2.74 |
| Sulfur | 1.22 | 0.60 | 0.26 |
| Oxygen * | 42.78 | 18.36 | 19.21 |
| Sample | BET Surface Area | t-Plot Microporous Surface Area | External Surface Area | Total Pore Volume | Average Pore Diameter | Percentage Micropores |
|---|---|---|---|---|---|---|
| SBET | Smic | Sext | Vt | D | - | |
| Units | m2/g | m2/g | m2/g | cm3/g | Nm | % |
| RSS | 1.14 | - | 0.24 | 0.003 | 18.30 | - |
| AC2 | 474.7 | 409.6 | 65.1 | 0.27 | 6.78 | 86.3 |
| AC3 | 683.4 | 579.2 | 104.3 | 0.37 | 2.16 | 84.7 |
| Adsorbent | Activating Agent | CO2 Partial Pressure | Temperature | CO2 Adsorption Capacity | Reference |
|---|---|---|---|---|---|
| Units | bar | °C | mg/g | ||
| AC2 | K2CO3 | 1 | 25 | 55.74 | This work |
| AC3 | K2CO3 | 1 | 25 | 60.06 | This work |
| Palm kernel shell | MgO | 1 | 25 | 20.10 | [51] |
| Rubber seed shell | KOH | 1 | 25 | 54.16 | [52] |
| Sargassum | KOH | 1 | 25 | 22.80 | [53] |
| Rice husk | ZnCl2 | 1 | 25 | 58.52 | [29] |
| Coconut shell | CO2 | 1 | 25 | 73.04 | [54] |
| Adsorbents | CO2 Partial Pressure | Temperature | CO2 Adsorption Capacity | Reference |
|---|---|---|---|---|
| Units | bar | °C | mg/g | |
| AC2 | 1 | 25 | 55.74 | This work |
| AC3 | 1 | 25 | 60.06 | This work |
| Palm kernel shell | 1 | 25 | 33.40 | [55] |
| Ordered mesoporous silica | 1 | 25 | 27.00 | [21] |
| PE-MCM-41 | 1 | 25 | 26.40 | [56] |
| Silica monoliths | 0.1 | 25 | 49.70 | [57] |
| Nanoporous carbon | 1 | 78 | 48.00 | [58] |
| Carbon nanotubes | 1 | 73 | 11.30 | [59] |
| KIT-6 | 1 | 73 | 74.00 | [60] |
| SBA-15 | 1 | 25 | 23.40 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, S.S.; Borhan, A.; Faheem, M. Preparation of Chemically Activated Porous Carbon Derived from Rubber-Seed Shell for CO2 Adsorption. Processes 2025, 13, 1181. https://doi.org/10.3390/pr13041181
Fatima SS, Borhan A, Faheem M. Preparation of Chemically Activated Porous Carbon Derived from Rubber-Seed Shell for CO2 Adsorption. Processes. 2025; 13(4):1181. https://doi.org/10.3390/pr13041181
Chicago/Turabian StyleFatima, Syeda Saba, Azry Borhan, and Muhammad Faheem. 2025. "Preparation of Chemically Activated Porous Carbon Derived from Rubber-Seed Shell for CO2 Adsorption" Processes 13, no. 4: 1181. https://doi.org/10.3390/pr13041181
APA StyleFatima, S. S., Borhan, A., & Faheem, M. (2025). Preparation of Chemically Activated Porous Carbon Derived from Rubber-Seed Shell for CO2 Adsorption. Processes, 13(4), 1181. https://doi.org/10.3390/pr13041181







