Conversion of Levulinic Acid to γ-Valerolactone Using Hydrotalcite-Derived Cu-Ni Bimetallic Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Catalyst
2.2.2. Characterization of Samples
2.2.3. Transfer Hydrogenation Reaction
2.2.4. Reaction Product Analysis
3. Results
3.1. Structural and Morphological Characterization of Cu-Ni2/Al2O3 Catalyst and Its Precursor
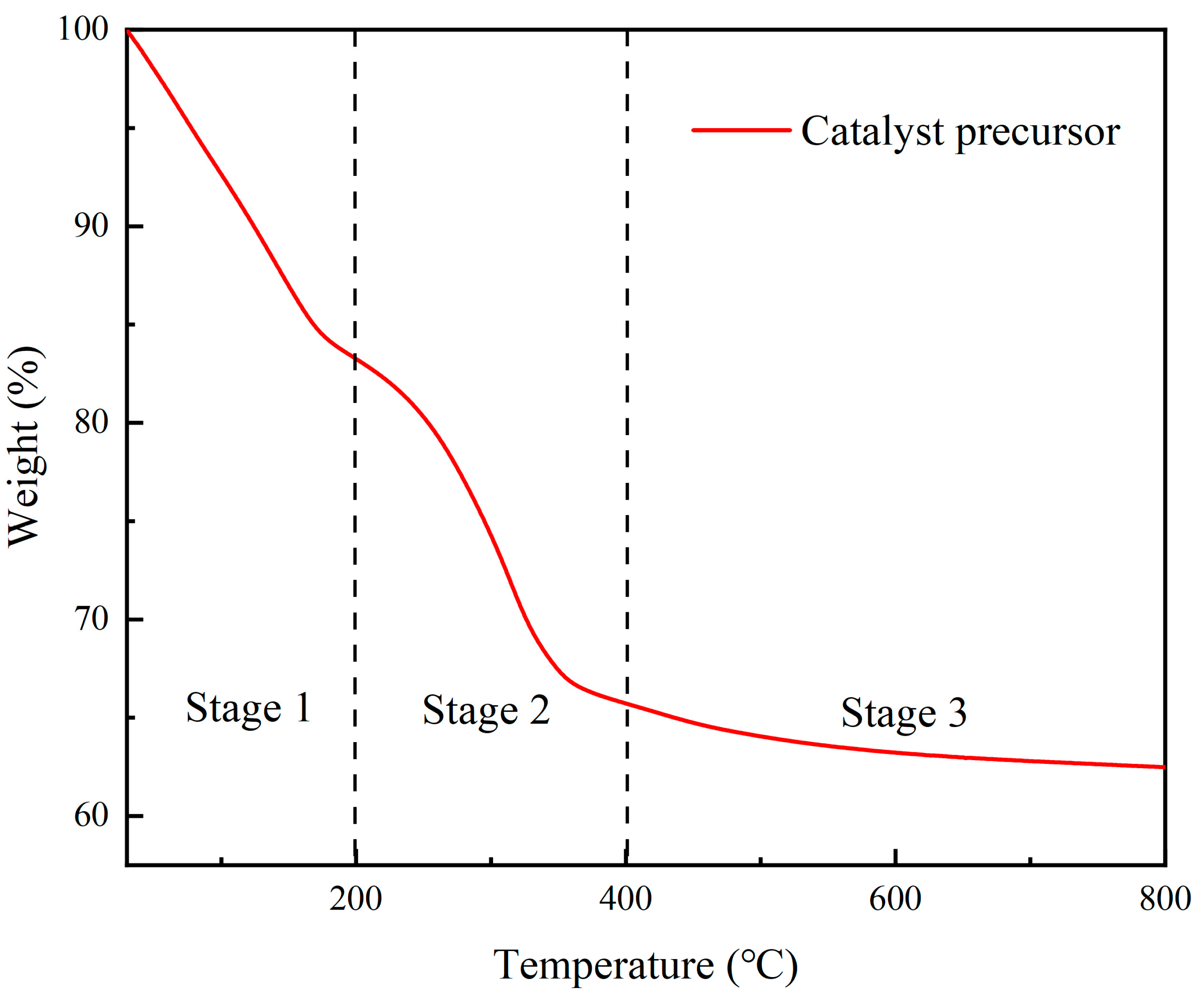
3.1.1. TGA Analysis Results
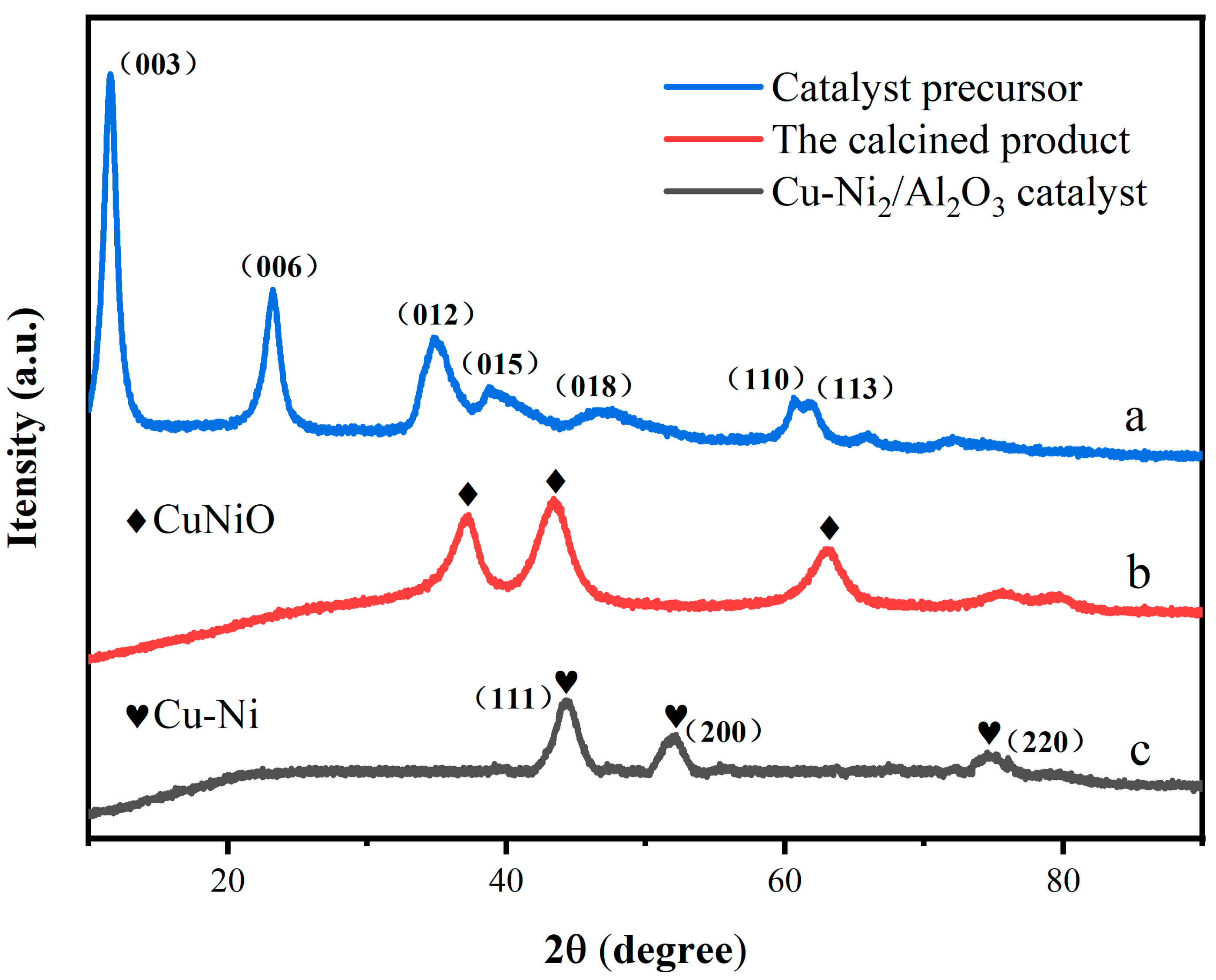
3.1.2. XRD Analysis Results
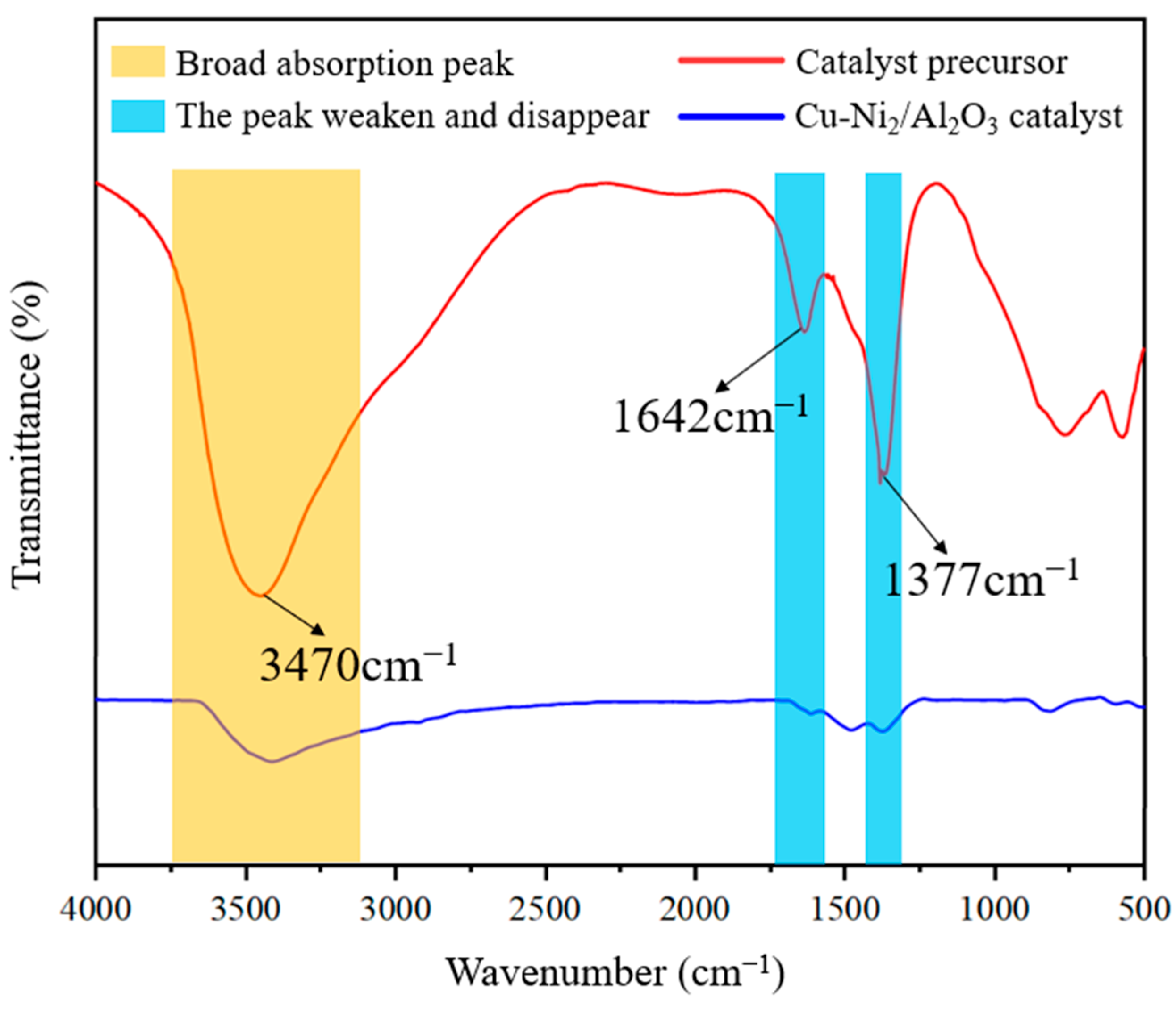
3.1.3. FTIR Analysis Results
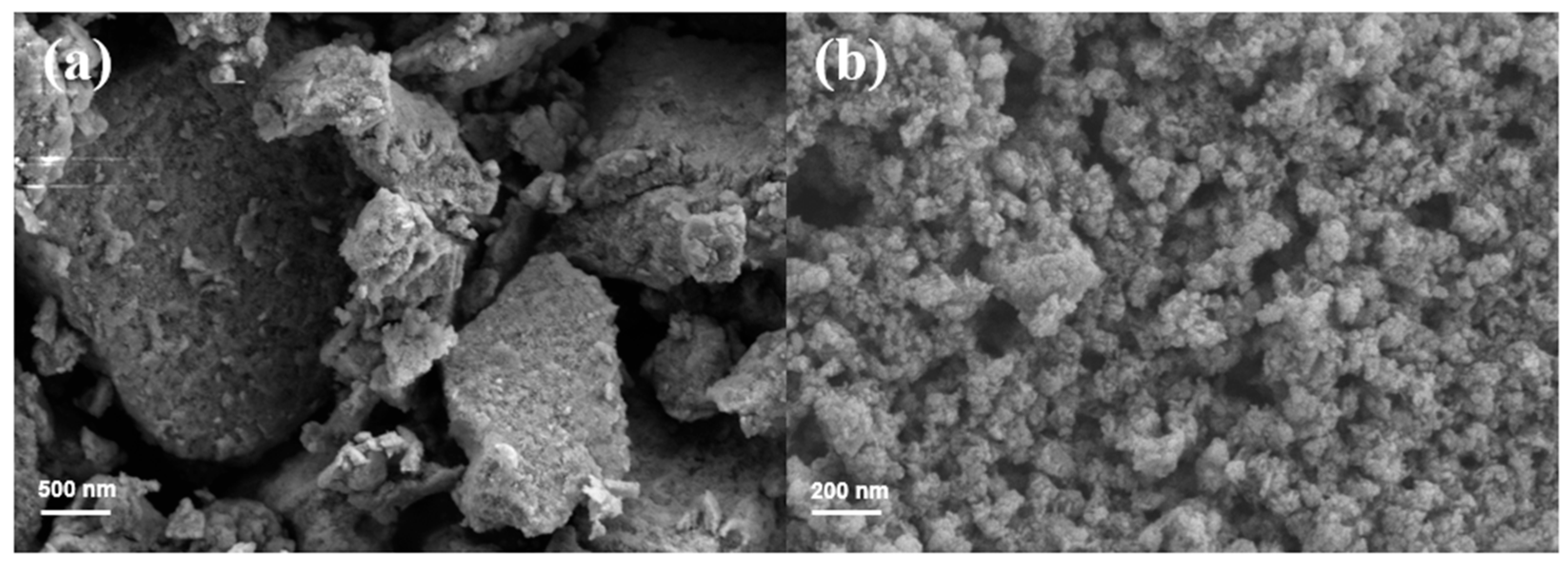
3.1.4. SEM Analysis Results
3.2. Effect of Hydrogen-Donating Solvents on the Catalytic LA-to-GVL Production
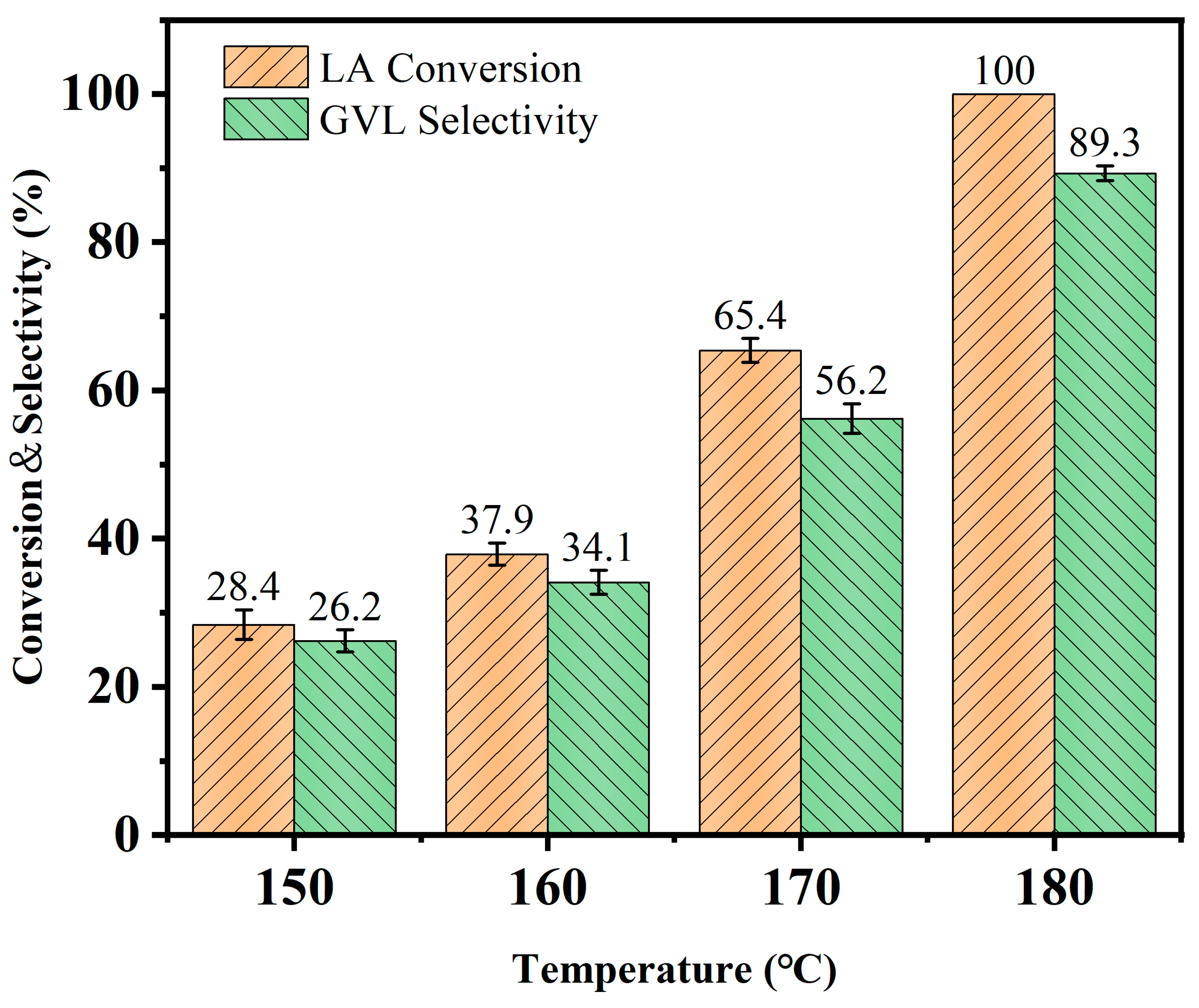
3.3. Effect of Reaction Temperature on Catalytic LA-to-GVL Preparation
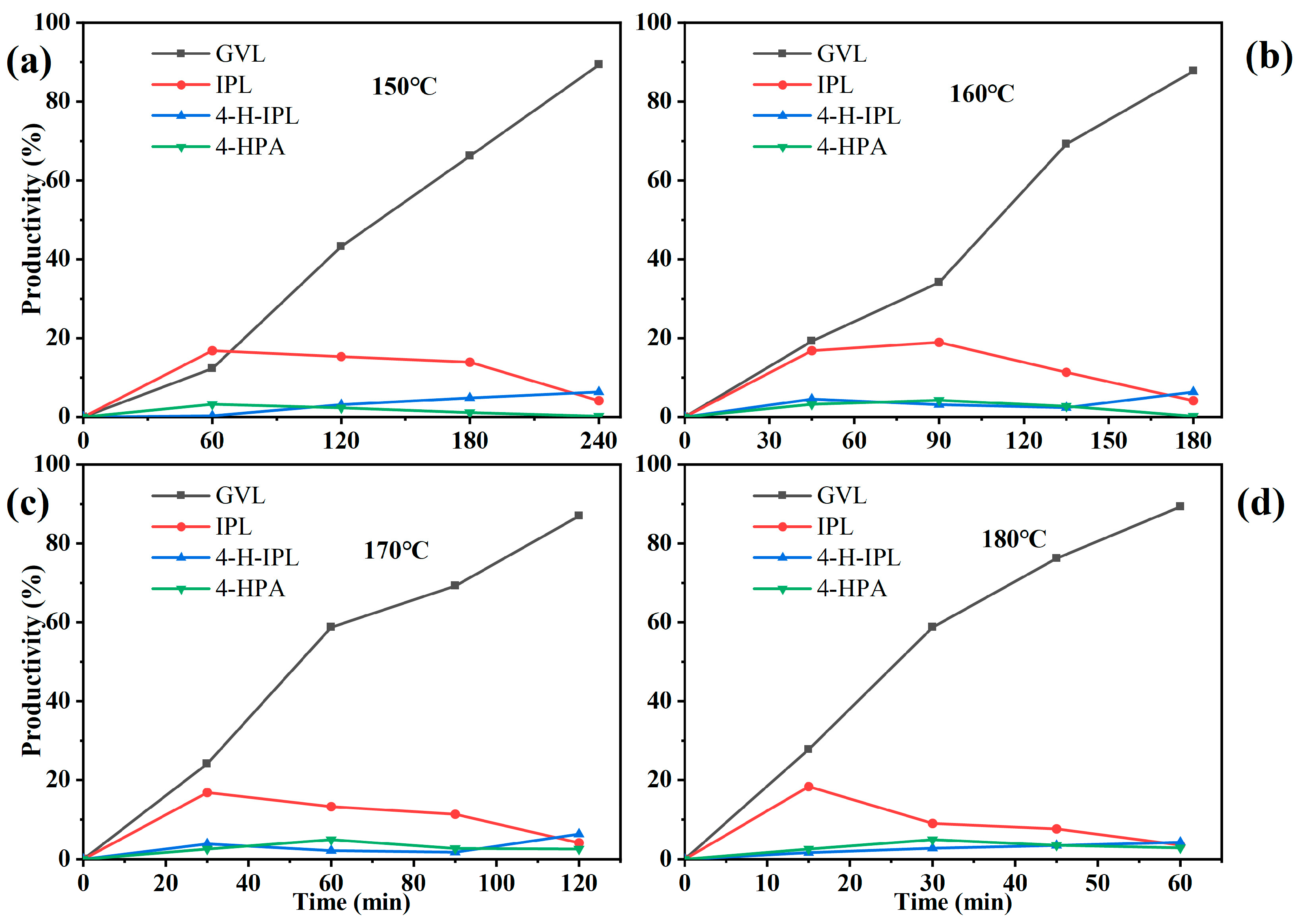
3.4. Effect of Reaction Time on Catalytic LA-to-GVL Preparation
3.5. Recycling Effects of Cu-Ni2/Al2O3 Catalysts
3.6. Cu-Ni2/Al2O3 Catalyst Expansion Reaction
3.7. Reaction Mechanism of Cu-Ni2/Al2O3 Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Halkos, G.; Gkampoura, E.-C. Reviewing Usage, Potentials, and Limitations of Renewable Energy Sources. Energies 2020, 13, 2906. [Google Scholar] [CrossRef]
- Ang, T.-Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Energy Strategy Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Maradin, D. Advantages and Disadvantages of Renewable Energy Sources Utilization. Int. J. Energy Econ. Policy 2021, 11, 176–183. [Google Scholar] [CrossRef]
- Kothari, D.P.; Singal, K.C.; Rakesh, R. Renewable Energy Sources and Emerging Technologies; PHI Learning Pvt. Ltd.: Delhi, India, 2021. [Google Scholar]
- Ye, L.; Han, Y.; Feng, J.; Lu, X. A review about GVL production from lignocellulose: Focusing on the full components utilization. Ind. Crops Prod. 2020, 144, 112031. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, D.; Zhao, X. Conversion of lignocellulose to biofuels and chemicals via sugar platform: An updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose. Renew. Sustain. Energy Rev. 2021, 146, 111169. [Google Scholar] [CrossRef]
- Liu, H.; Lin, Q.; Li, R.; Chang, M.; Ren, J.J.C.E.P.T. Synthesis of Furan Compounds from Hemicelluloses. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Clean Energy Production Technologies (CEPT); Springer: Singapore, 2022. [Google Scholar]
- Deng, L.; Li, J.; Lai, D.-M.; Fu, Y.; Guo, Q.-X. Catalytic Conversion of Biomass-Derived Carbohydrates into γ-Valerolactone without Using an External H 2 Supply. Angew. Chem. 2009, 121, 6651–6654. [Google Scholar] [CrossRef]
- Liguori, F.; Moreno-Marrodán, C.; Barbaro, P.J.A.C. Environmentally Friendly Synthesis of γ-Valerolactone by Direct Catalytic Conversion of Renewable Sources. ACS Catal. 2015, 5, 1882–1894. [Google Scholar]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tong, X.; Wang, Y.; Jin, G.; Guo, X.J.C.C. Highly active Ir/SiC catalyst for aqueous hydrogenation of levulinic acid to γ-valerolactone. Green Chem. 2020, 139, 105971. [Google Scholar]
- Vaccaro, L.; Anastasiou, I.; Ferlin, F.; Carpisassi, L. Aerobic waste-minimized Pd-catalysed C–H alkenylation in GVL using a tube-in-tube heterogeneous flow reactor. Green Chem. 2021, 23, 6576–6582. [Google Scholar] [CrossRef]
- Siddiqui, N.; Pendem, C.; Goyal, R.; Khatun, R.; Khan, T.S.; Samanta, C.; Chiang, K.; Shah, K.; Ali Haider, M.; Bal, R. Study of γ-valerolactone production from hydrogenation of levulinic acid over nanostructured Pt-hydrotalcite catalysts at low temperature. Fuel 2022, 323, 124272. [Google Scholar] [CrossRef]
- Maumela, M.; Marx, S.; Meijboom, R. Heterogeneous Ru Catalysts as the Emerging Potential Superior Catalysts in the Selective Hydrogenation of Bio-Derived Levulinic Acid to γ-Valerolactone: Effect of Particle Size, Solvent, and Support on Activity, Stability, and Selectivity. Catalysts 2021, 11, 292. [Google Scholar] [CrossRef]
- Nandi, S.; Saha, A.; Patel, P.; Khan, N.-u.H.; Kureshy, R.I.; Panda, A.B. Hydrogenation of Furfural with Nickel Nanoparticles Stabilized on Nitrogen-Rich Carbon Core–Shell and Its Transformations for the Synthesis of γ-Valerolactone in Aqueous Conditions. ACS Appl. Mater. Interfaces 2018, 10, 24480–24490. [Google Scholar] [CrossRef]
- Yuan, J.; Li, S.-S.; Yu, L.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Copper-based catalysts for the efficient conversion of carbohydrate biomass into γ-valerolactone in the absence of externally added hydrogen. Energy Environ. Sci. 2013, 6, 3308–3313. [Google Scholar] [CrossRef]
- Murugesan, K.; Alshammari, A.S.; Sohail, M.; Jagadeesh, R.V. Levulinic Acid Derived Reusable Cobalt-Nanoparticles-Catalyzed Sustainable Synthesis of γ-Valerolactone. ACS Sustain. Chem. Eng. 2019, 7, 14756–14764. [Google Scholar] [CrossRef]
- Yu, N.; Lu, H.; Yang, W.; Zheng, Y.; Hu, Q.; Liu, Y.; Wu, K.; Liang, B. Transfer hydrogenation of levulinic acid to γ-valerolactone over acid site-modified CuNi alloy. Biomass Convers. Biorefin. 2024, 14, 8271–8282. [Google Scholar] [CrossRef]
- Bai, J.; Cheng, C.; Liu, Y.; Wang, C.; Liao, Y.; Chen, L.; Ma, L. Selective hydrogenation of levulinic acid to γ-valerolactone on Ni-based catalysts. Mol. Catal. 2021, 516, 112000. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, J.; Wang, Y.; Guo, H.; Qi, X. Bimetallic Ni-Zn@OMC catalyst for selective hydrogenation of levulinic acid to γ-valerolactone in water. Fuel Process. Technol. 2023, 240, 107559. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Li, C.; Wang, Z.; Evans, D.G.; Duan, X. Synthesis of Cu-Containing Layered Double Hydroxides with a Narrow Crystallite-Size Distribution. Clays Clay Miner. 2003, 51, 566–569. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Guo, Y.; Chen, L. Effective Upgrade of Levulinic Acid into γ-Valerolactone over an Inexpensive and Magnetic Catalyst Derived from Hydrotalcite Precursor. ACS Sustain. Chem. Eng. 2015, 3, 1708–1714. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.-W. Rational design of Ni-based catalysts derived from hydrotalcite for selective hydrogenation of 5-hydroxymethylfurfural. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- Xu, M.; Yao, S.; Rao, D.; Niu, Y.; Liu, N.; Peng, M.; Zhai, P.; Man, Y.; Zheng, L.; Wang, B.; et al. Insights into Interfacial Synergistic Catalysis over Ni@TiO2–x Catalyst toward Water–Gas Shift Reaction. J. Am. Chem. Soc. 2018, 140, 11241–11251. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, F.; Wang, L.; Luo, F. Highly efficient transfer hydrodeoxygenation of vanillin over Sn4+-induced highly dispersed Cu-based catalyst. Appl. Surf. Sci. 2019, 480, 548–556. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Obregón, I.; Gandarias, I.; Ocio, A.; García-García, I.; Alvarez de Eulate, N.; Arias, P.L. Structure-activity relationships of Ni-Cu/Al2O3 catalysts for γ-valerolactone conversion to 2-methyltetrahydrofuran. Appl. Catal. B Environ. 2017, 210, 328–341. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Li, J.; Sun, P.; Long, X.; Li, F. Efficient and versatile CuNi alloy nanocatalysts for the highly selective hydrogenation of furfural. Appl. Catal. B Environ. 2017, 203, 227–236. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, L.; Fan, G.; Zheng, L.; Yang, L.; Li, F. NiCu Nanoparticles for Catalytic Hydrogenation of Biomass-Derived Carbonyl Compounds. ACS Appl. Nano Mater. 2020, 3, 9226–9237. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J. Selective Transfer Hydrogenation of Biomass-Based Furfural and 5-Hydroxymethylfurfural over Hydrotalcite-Derived Copper Catalysts Using Methanol as a Hydrogen Donor. ACS Sustain. Chem. Eng. 2017, 5, 5982–5993. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Ye, L.; Zhang, M.; Xiong, J.; Zhang, R.; Li, X.; Ji, N.; Lu, X. Layered Double Hydroxide-Derived Bimetallic Ni−Cu Catalysts Prompted the Efficient Conversion of γ-Valerolactone to 2-Methyltetrahydrofuran. ChemCatChem 2022, 14, e202101441. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, X.; Wan, G.; Yulin, T.; Shi, S.; Xu, X.; Wang, G. Novel hierarchical CuNiAl LDH nanotubes with excellent peroxidase-like activity for wide-range detection of glucose. Dalton Trans. 2021, 50, 95–102. [Google Scholar] [CrossRef]
- Ziegenheim, S.; Varga, G.; Szabados, M.; Sipos, P.; Pálinkó, I. Cu(II)Cr(III)-LDH: Synthesis, characterization, intercalation properties and a catalytic application. Chem. Pap. 2018, 72, 897–902. [Google Scholar] [CrossRef]
- Ravuru, S.S.; Jana, A.; De, S. Synthesis of NiAl- layered double hydroxide with nitrate intercalation: Application in cyanide removal from steel industry effluent. J. Hazard Mater. 2019, 373, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Lobinsky, A.A.; Tolstoy, V.P. Synthesis of CoAl-LDH nanosheets and N-doped graphene nanocomposite via Successive Ionic Layer Deposition method and study of their electrocatalytic properties for hydrogen evolution in alkaline media. J. Solid State Chem. 2019, 270, 156–161. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Jiang, L.; Wang, X.; Yang, D.; Wei, Q.; Wang, Y.; Wang, R.; Liu, Y.; Yang, Y. Improved electrochemical performances by Ni-catecholate-based metal organic framework grown on NiCoAl-layered double hydroxide/multi-wall carbon nanotubes as cathode catalyst in microbial fuel cells. Bioresour. Technol. 2021, 337, 125430. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, X.; Yang, D.; Wang, X.; Zhang, Y.; Du, Y.; Wang, Y.; Wei, Q.; Wang, R.; et al. Enhanced bioelectrochemical performance of microbial fuel cell with titanium dioxide-attached dual metal organic frameworks grown on zinc aluminum—Layered double hydroxide as cathode catalyst. Bioresour. Technol. 2022, 351, 126989. [Google Scholar] [CrossRef]
- Shafaghat, H.; Lee, I.-G.; Jae, J.; Jung, S.C.; Park, Y.K.J.C.E.J. Pd/C catalyzed transfer hydrogenation of pyrolysis oil using 2-propanol as hydrogen source. Chem. Eng. J. 2019, 377, 119986. [Google Scholar]
- Liu, M.; Li, S.; Fan, G.; Yang, L.; Li, F. Hierarchical Flower-like Bimetallic NiCu catalysts for Catalytic Transfer Hydrogenation of Ethyl Levulinate into γ-Valerolactone. Ind. Eng. Chem. Res. 2019, 58, 10317–10327. [Google Scholar] [CrossRef]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al Ordering in Layered Double Hydroxides Revealed by Multinuclear NMR Spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, D.; Gao, R.; Huang, L.; Shi, L.; Zhang, J. Design of modular catalysts derived from NiMgAl-LDH@m-SiO2 with dual confinement effects for dry reforming of methane. Chem. Commun. 2013, 49, 6770–6772. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, B.; Zhou, H.; Wu, L.; Meng, Q.; Liu, Z.; Han, B. Porous Zirconium–Phytic Acid Hybrid: A Highly Efficient Catalyst for Meerwein–Ponndorf–Verley Reductions. Angew. Chem. (Int. Ed. Engl.) 2015, 127, 9399–9403. [Google Scholar] [CrossRef]
- Kim, J.; Han, J. Bio-based process for the catalytic production of ethyl levulinate from cellulose. Appl. Energy 2021, 300, 117430. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.-M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.-S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Córdova-Pérez, G.E.; Cortez-Elizalde, J.; Silahua-Pavón, A.A.; Cervantes-Uribe, A.; Arévalo-Pérez, J.C.; Cordero-Garcia, A.; de los Monteros, A.E.E.; Espinosa-González, C.G.; Godavarthi, S.; Ortiz-Chi, F.; et al. γ-Valerolactone Production from Levulinic Acid Hydrogenation Using Ni Supported Nanoparticles: Influence of Tungsten Loading and pH of Synthesis. Nanomaterials 2022, 12, 2017. [Google Scholar] [CrossRef]
- Popova, M.; Boycheva, S.; Dimitrov, I.; Dimitrov, M.; Kovacheva, D.; Karashanova, D.; Velinov, N.; Atanasova, G.; Szegedi, A. The Formation of γ-Valerolactone from Renewable Levulinic Acid over Ni-Cu Fly Ash Zeolite Catalysts. Molecules 2024, 29, 5753. [Google Scholar] [CrossRef]
- Hijazi, A.; Khalaf, N.; Kwapinski, W.; Leahy, J. Catalytic valorisation of biomass levulinic acid into gamma valerolactone using formic acid as a H 2 donor: A critical review. RSC Adv. 2022, 12, 13673–13694. [Google Scholar] [CrossRef]

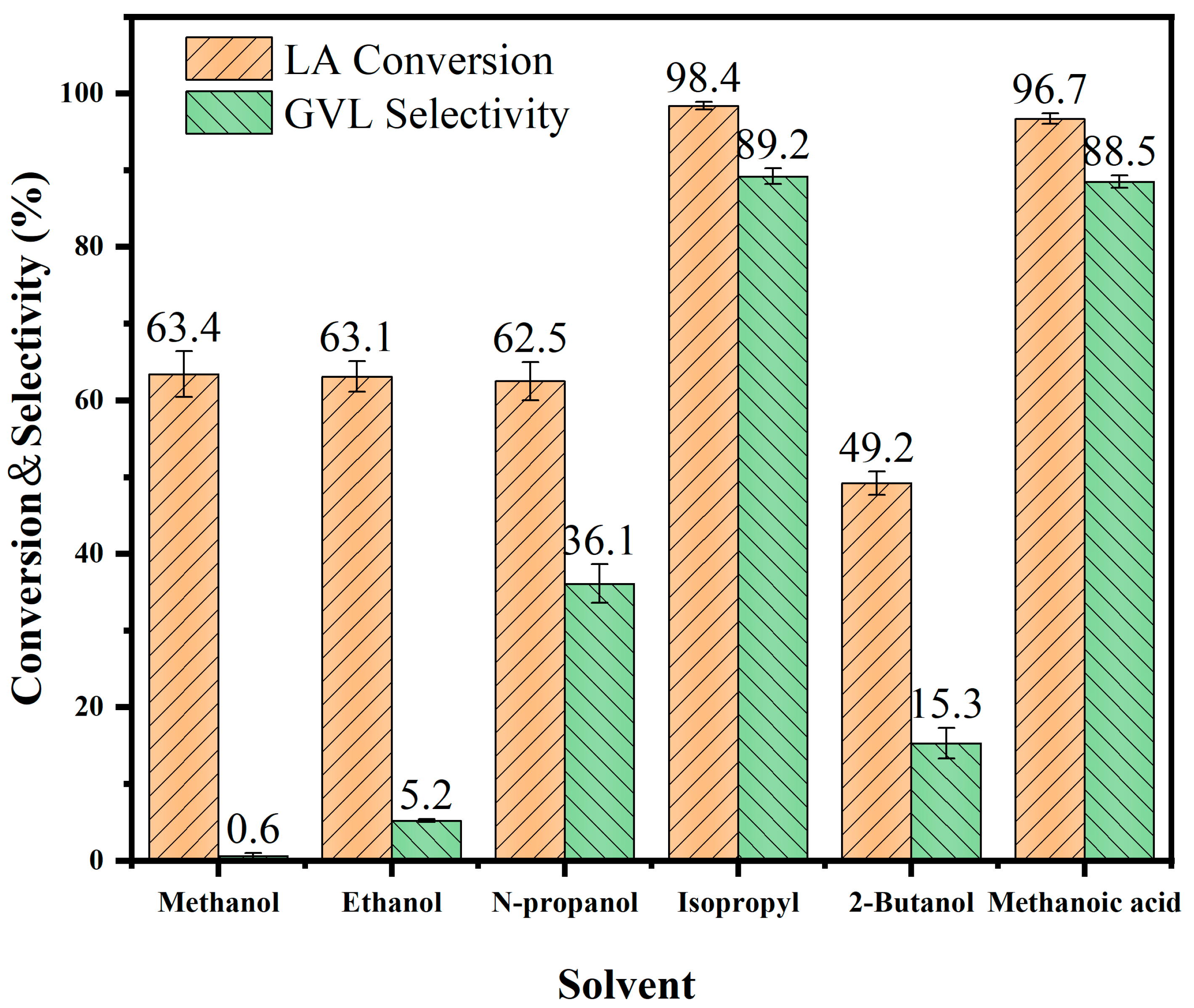



| Solvent | Chemical Formula | Structural Formula | LA Conv. (%) | GVL Sele. (%) |
|---|---|---|---|---|
| Methanol | CH3OH |  | 63.4 | 0.6 |
| Ethanol | C2H5OH | 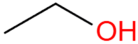 | 63.1 | 5.2 |
| N-propanol | C3H7OH | 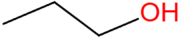 | 62.5 | 36.1 |
| Isopropyl | (CH3)2CHOH | 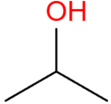 | 98.4 | 89.2 |
| 2-Butanol | C4H9OH | 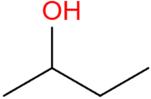 | 49.2 | 15.3 |
| Methanoic acid | CH2O2 | 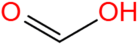 | 96.7 | 88.5 |
| Entry | Substrates | Products | Time (h) | Temp. (℃) | Conv. (%) | Sele. (%) |
|---|---|---|---|---|---|---|
| 1 | 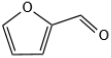 | 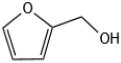 | 1 | 120 | 99 | 96 |
| 2 | 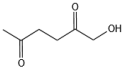 |  | 1 | 180 | 100 | 89 |
| 3 | 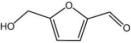 | 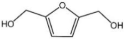 | 1 | 120 | 94 | 96 |
| 4 |  | 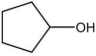 | 4 | 120 | 89 | 99 |
| 5 |  | 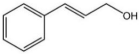 | 2 | 120 | 99 | 99 |
| 6 |  |  | 2 | 110 | 99 | 96 |
| 7 |  | 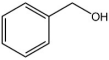 | 2 | 140 | 96 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Chen, G.; Zheng, K.; Li, S.; Xu, J.; Wang, F.; Liu, X.; Weng, R. Conversion of Levulinic Acid to γ-Valerolactone Using Hydrotalcite-Derived Cu-Ni Bimetallic Catalyst. Processes 2025, 13, 1110. https://doi.org/10.3390/pr13041110
Zhao S, Chen G, Zheng K, Li S, Xu J, Wang F, Liu X, Weng R. Conversion of Levulinic Acid to γ-Valerolactone Using Hydrotalcite-Derived Cu-Ni Bimetallic Catalyst. Processes. 2025; 13(4):1110. https://doi.org/10.3390/pr13041110
Chicago/Turabian StyleZhao, Shikang, Guohong Chen, Kaiqi Zheng, Shaojie Li, Jiaqi Xu, Fanan Wang, Xueping Liu, and Rengui Weng. 2025. "Conversion of Levulinic Acid to γ-Valerolactone Using Hydrotalcite-Derived Cu-Ni Bimetallic Catalyst" Processes 13, no. 4: 1110. https://doi.org/10.3390/pr13041110
APA StyleZhao, S., Chen, G., Zheng, K., Li, S., Xu, J., Wang, F., Liu, X., & Weng, R. (2025). Conversion of Levulinic Acid to γ-Valerolactone Using Hydrotalcite-Derived Cu-Ni Bimetallic Catalyst. Processes, 13(4), 1110. https://doi.org/10.3390/pr13041110







