Rapid Determination of Thiourea Concentration in Copper Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Standard Solutions
2.3. Spectrophotometric Assay Procedure
2.4. Colorimetric Method Assay Procedure
3. Results and Discussion
3.1. Establishment and Verification of Spectrophotometry for Determining Tu Concentration
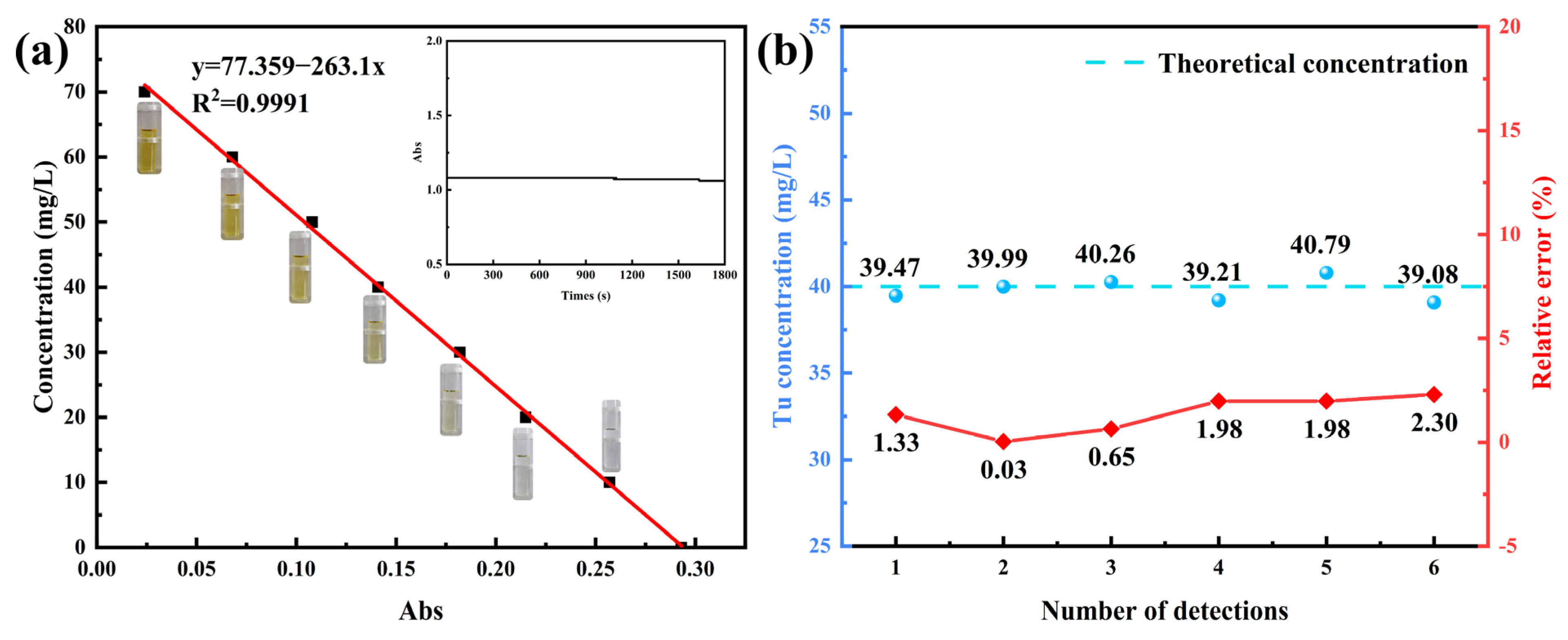
3.1.1. Establishment of Spectrophotometry
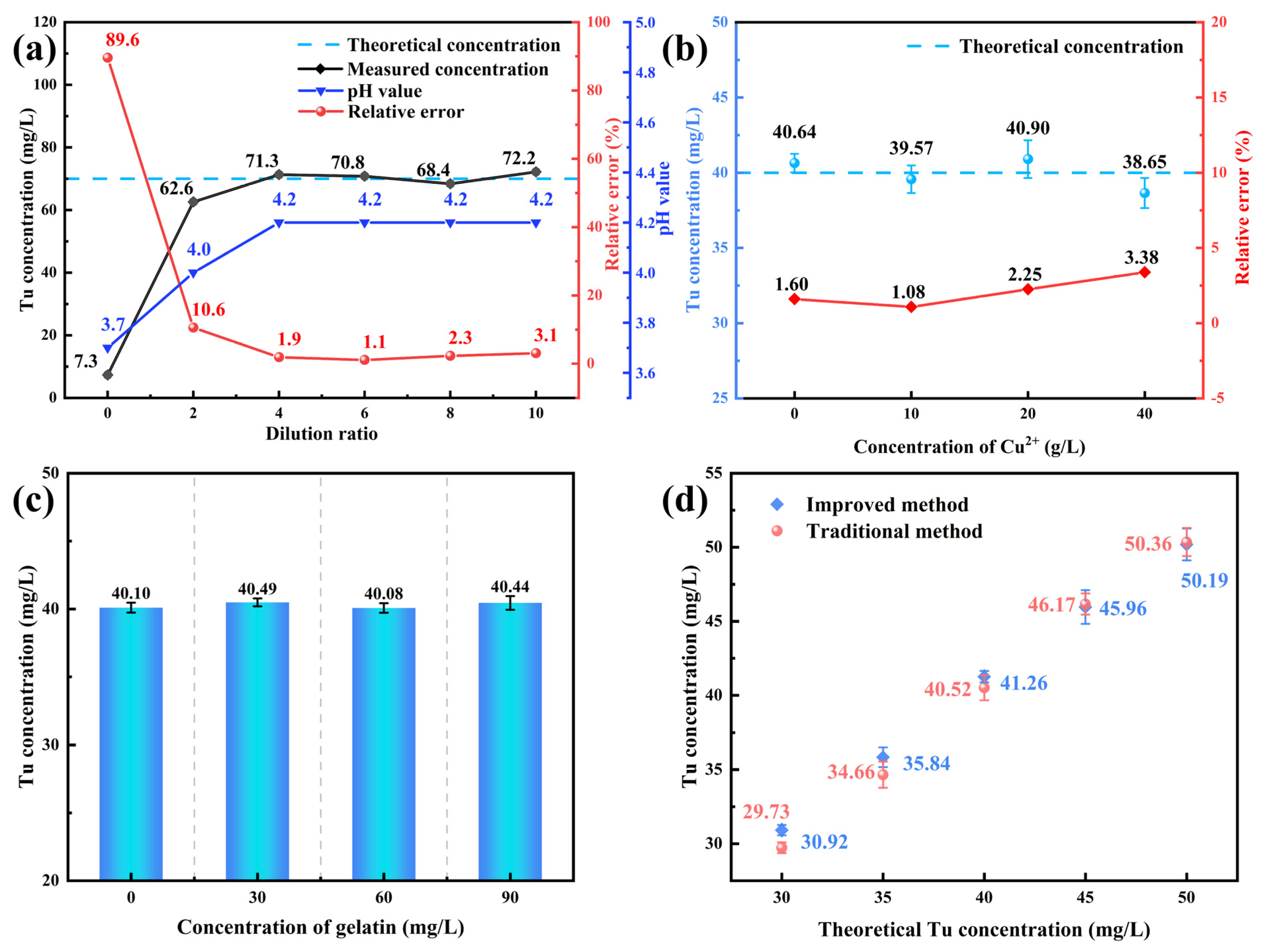
3.1.2. Elimination of Interference Factors in Tu Determination on Spectrophotometry
3.1.3. Accuracy Verification of Tu Concentration Determination by Spectrophotometry
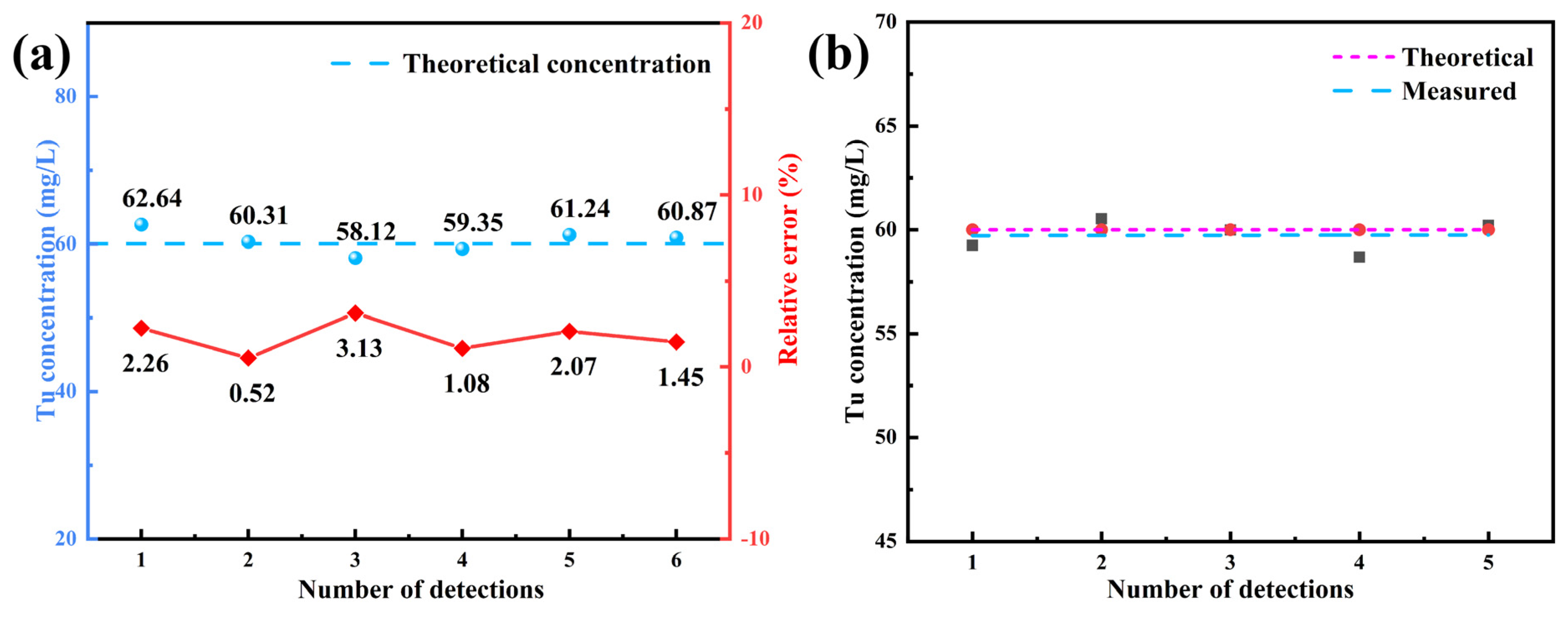
3.1.4. Validation of the Method
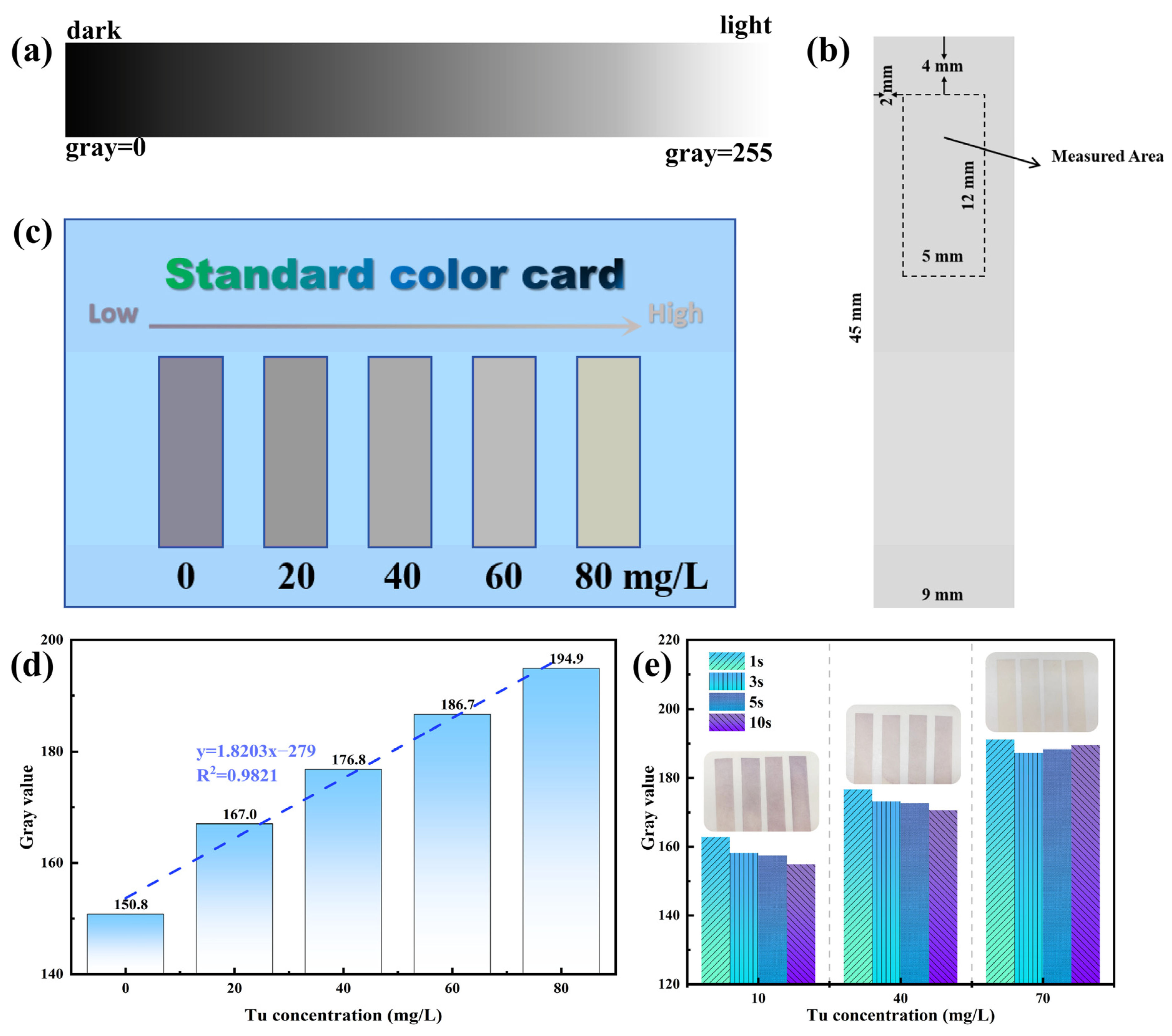
3.2. Establishment and Verification of Colorimetric Method for Determining Tu Concentration
3.2.1. Establishment of Colorimetric Method
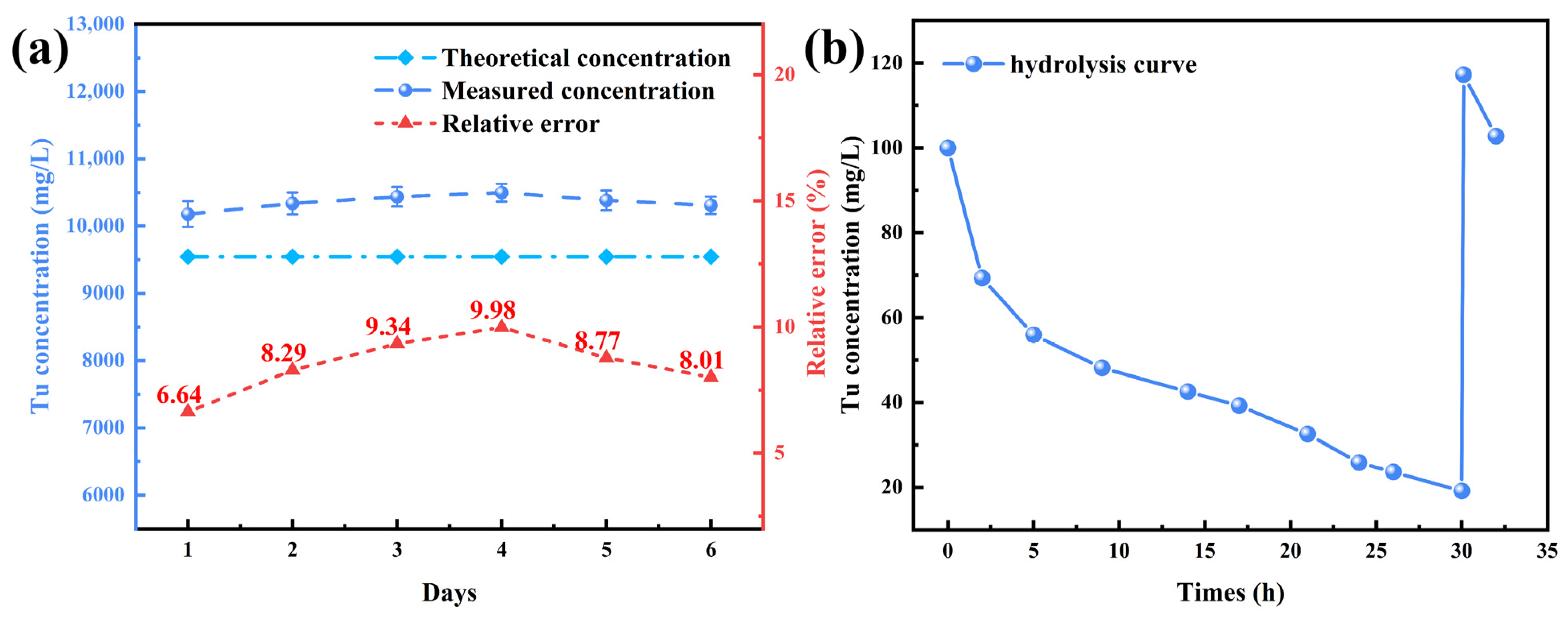
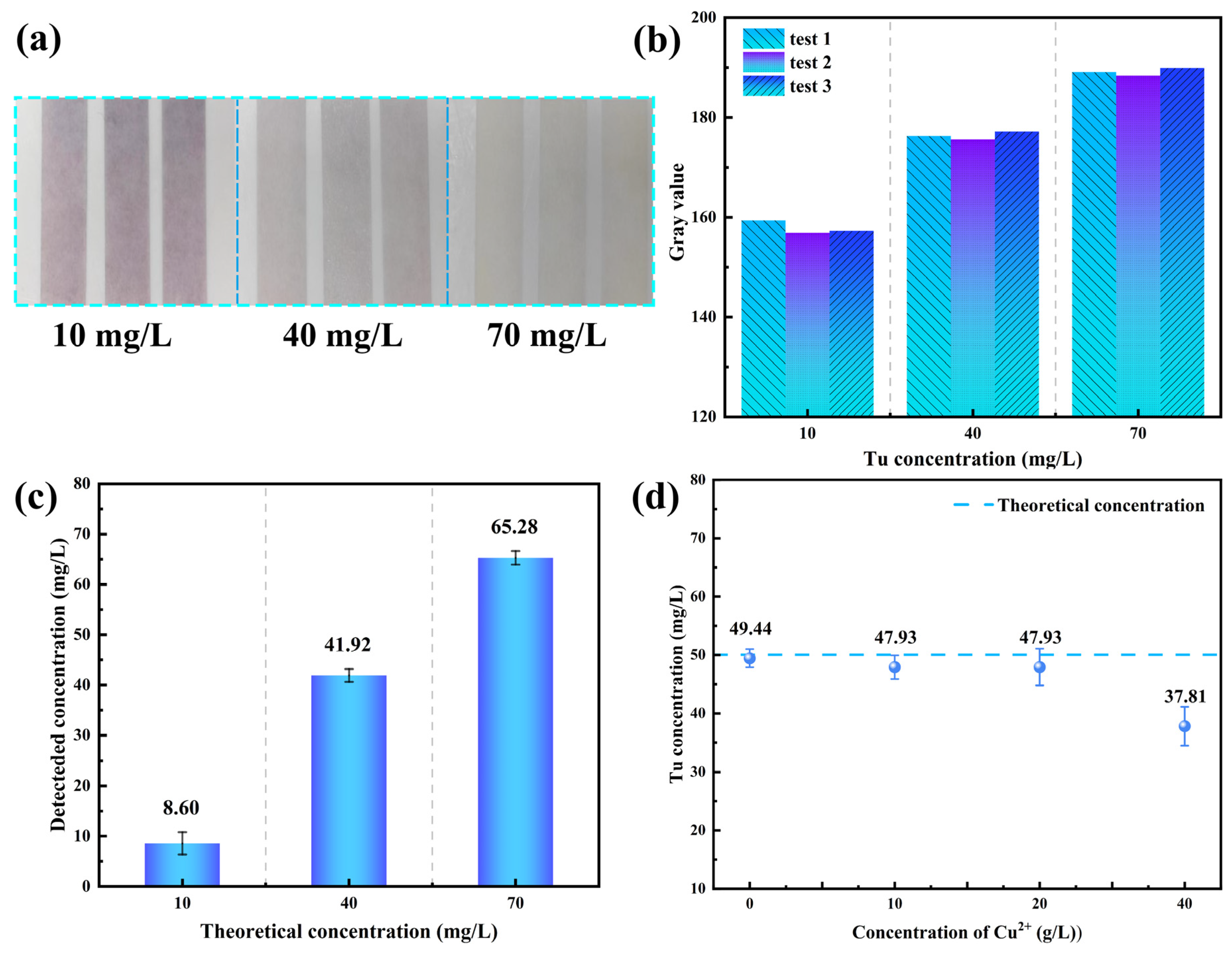
3.2.2. Accuracy Verification and Industrial Application of Colorimetric Method in Electrolyte
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stefanie, K.; Stefan, P. Sector-Level Estimates for Global Future Copper Demand and the Potential for Resource Efficiency. Resour. Conserv. Recycl. 2023, 193, 106941. [Google Scholar]
- Cheng, J.; Ding, L.; Zhao, J.; Wang, T.; Wang, R.; Chen, C.; Niu, Y. Roles of some additives in continuous electrolytic refining of cathode copper. Electroplat. Finish. 2020, 39, 383–389. [Google Scholar]
- Fang, Y.; Pan, M.; Huang, H.; Shao, Y.; He, Y.; Chen, B.; Guo, Z. Current situation and prospect of additives in copper electrolysis deposition process. Min. Metall. 2021, 30, 61–69. [Google Scholar]
- Quinet, M.; Lallemand, F.; Ricq, L.; Hihn, J.-Y.; Delobelle, P.; Arnould, C.; Mekhalif, Z. Influence of Organic Additives on the Initial Stages of Copper Electrodeposition on Polycrystalline Platinum. Electrochim. Acta 2009, 54, 1529–1536. [Google Scholar] [CrossRef]
- Collet, T.; Wouters, B.; Hallemans, N.; Ramharter, K.; Lataire, J.; Hubin, A. The Time-Varying Effect of Thiourea on the Copper Electroplating Process with Industrial Copper Concentrations. Electrochim. Acta 2023, 437, 141412. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, J.; Zhong, S.; Zhang, P.; Shen, K. Mechanism of Action and Detection Method of Copper Electrowinning Additive. Nonferrous Met. Eng. Res. 2016, 37, 14–16. [Google Scholar]
- Kang, M.S.; Kim, S.K.; Kim, K.; Kim, J.J. The Influence of Thiourea on Copper Electrodeposition: Adsorbate Identification and Effect on Electrochemical Nucleation. Thin Solid Film. 2008, 516, 3761–3766. [Google Scholar]
- Duca, G.; Lis, A.; Gladchi, V.; Travin, S. Indirect Photolysis of Cysteine and Thiourea in the Aquatic Environment. Inorg. Chim. Acta 2023, 557, 121682. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Y.; Zhu, X.; Zhang, W.; Wang, C.; Jiao, H. Ultrasensitive and Selective Colorimetric Detection of Thiourea Using Silver Nanoprobes. Analyst 2011, 136, 5256–5260. [Google Scholar] [CrossRef]
- Spataru, N.; Spataru, T.; Fujishima, A. Voltammetric Determination of Thiourea at Conductive Diamond Electrodes. Electroanal 2005, 17, 800–805. [Google Scholar] [CrossRef]
- Akeneev, Y.A.; Zakharova, E.A.; Slepchenko, G.B.; Pikula, N.P. Voltammetric Determination of Thiourea in Copper Refinery Electrolytes. J. Anal. Chem. 2005, 60, 514–517. [Google Scholar] [CrossRef]
- Manea, F.; Radovan, C.; Schoonman, J. Amperometric Determination of Thiourea in Alkaline Media on a Copper Oxide–Copper Electrode. J. Appl. Electrochem. 2006, 36, 1075–1081. [Google Scholar]
- Jodan, I.; Wantala, K.; Amini, N.; Shahmoradi, B.; Ghaslani, M.; Lee, S.M.; Yang, J.; Puttaiah, S.H. Fabrication of a Sensitive Electrochemical Sensor Based on Ag Nanoparticles and Alizarin Yellow Polymer: Application to the Detection of an Environmental Pollutant Thiourea. Korean J. Chem. Eng. 2020, 37, 1609–1615. [Google Scholar] [CrossRef]
- Abbasi, S.; Khani, H.; Hosseinzadeh, L.; Safari, Z. Determination of Thiourea in Fruit Juice by a Kinetic Spectrophotometric Method. J. Hazard. Mater. 2010, 174, 257–262. [Google Scholar] [CrossRef]
- Arab Chamjangali, M.; Bagherian, G.; Goudarzi, N.; Mehrjoo-Irani, S. A New and Sensitive Reaction Rate Method for Spectrophotometric Determination of Trace Amounts of Thiourea in Different Water Samples Based on an Induction Period. J. Anal. Sci. Technol. 2015, 6, 10. [Google Scholar] [CrossRef][Green Version]
- Jiang, H.; Wang, B.; Tang, R.; Tan, Y.; Qi, M.; Zhang, X. Inhibition to Dual Enzyme-like Activities of Ag/CeO2 Nanozymes for the Detection of Thiourea. Microchem. J. 2023, 185, 108251. [Google Scholar] [CrossRef]
- Pedre, I.; Méndez DeLeo, L.; Sánchez-Loredo, M.G.; Battaglini, F.; González, G.A. Electrochemical Sensor for Thiourea Focused on Metallurgical Applications of Copper. Sens. Actuators B Chem. 2016, 232, 383–389. [Google Scholar]
- Duan, M.; Huang, J.; Yong, F. Extraction—Spectrophotometry determination of thiourea in copper electrolyte. Electroplat. Pollut. Control 1998, 26–27. [Google Scholar]
- Collet, T.; Wouters, B.; Eeltink, S.; Schmidt, P.; Ramharter, K.; Hubin, A. An Ex Situ and Operando Analysis of Thiourea Consumption and Activity during a Simulated Copper Electrorefining Process. Electroanal. Chem. 2022, 920, 116581. [Google Scholar] [CrossRef]
- Rethmeier, J.; Neumann, G.; Stumpf, C.; Rabenstein, A.; Vogt, C. Determination of Low Thiourea Concentrations in Industrial Process Water and Natural Samples Using Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. A 2001, 934, 129–134. [Google Scholar] [CrossRef]
- Fabbri, L.; Giurlani, W.; Mencherini, G.; De Luca, A.; Passaponti, M.; Piciollo, E.; Fontanesi, C.; Caneschi, A.; Innocenti, M. Optimisation of Thiourea Concentration in a Decorative Copper Plating Acid Bath Based on Methanesulfonic Electrolyte. Coatings 2022, 12, 376. [Google Scholar] [CrossRef]
- Jin, S.; Ghali, E. Effect of Thiourea on the Copper Cathode Polarization Behavior in Acidic Copper Sulfate at 65 °C. Metall. Mater. Trans. B 2001, 32, 887–893. [Google Scholar]
- Lu, S. Discussion on the function and mechanism of additives in copper electrolysis. Nonferrous Met. Extr. Metall. 1984, 41–46. [Google Scholar]
- Bolzán, A.E.; Wakenge, I.B.; Piatti, R.C.V.; Salvarezza, R.C.; Arvia, A.J. The Behaviour of Copper Anodes in Aqueous Thiourea-Containing Sulphuric Acid Solutions. Open Circuit Potentials and Electrochemical Kinetics. Electroanal. Chem. 2001, 501, 241–252. [Google Scholar] [CrossRef]
- Thakur, D.; Dubey, N.P.; Singh, R. A Review on Spike and Recovery Method in Analytical Method Development and Validation. Crit. Rev. Anal. Chem. 2024, 54, 2053–2071. [Google Scholar] [CrossRef]
- Raposo, F.; Ibelli-Bianco, C. Performance Parameters for Analytical Method Validation: Controversies and Discrepancies among Numerous Guidelines. TrAC Trends Anal. Chem. 2020, 129, 115913. [Google Scholar]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; Flávio da S Petruci, J.; Batista, A.D. Novel Approaches for Colorimetric Measurements in Analytical Chemistry—A Review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar]
- Ren, D.; Mei, J.; Bao, J.; Wei, F.; Xu, G.; Yang, J.; Sun, Y.; Hu, Q.; Cen, Y. A Novel Profuse Color Card for Convenient Visual Determination of Iodide in Human Urine Based on Catalytic Oxidation Reaction. J. Pharm. Biomed. 2020, 191, 113580. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, W.; Zhao, X.; Zhang, J.; Chen, W.; Jiang, X. Mixing-to-Answer Iodide Sensing with Commercial Chemicals. Anal. Chem. 2018, 90, 8276–8282. [Google Scholar]
- Cui, K.; Lv, M.; Wang, R.; Han, L.; Liu, K.; Wang, Y. Experimental study on reaction conditions of iodine and starch. Sci. Technol. Inf. 2007, 29–31. [Google Scholar]

| Add Scalars (μg) | Total Measured (μg) | Spiked Recovery Rate (%) | Average Spiked Recovery Rate (%) |
|---|---|---|---|
| +0 | 19.48 | 100.00 | 102.19 |
| +10 | 29.74 | 102.60 | |
| +20 | 40.26 | 103.90 | |
| +30 | 50.26 | 102.60 | |
| +40 | 61.31 | 104.58 | |
| +50 | 69.20 | 99.44 |
| Samples | Theoretical Concentration (mg/L) | Colorimetric Method (mg/L) | Spectrophotometric Method (mg/L) |
|---|---|---|---|
| 1 | 10.0 | 0–20 | 11.1 |
| 2 | 35.0 | 30–40 | 34.1 |
| 3 | 60.0 | 50–70 | 60.2 |
| Methods | Reproducibility (%) | Linear Range (mg/L) | Detection Speed (min) | Ref. |
|---|---|---|---|---|
| Voltammetry | 20 | 1.0–30.0 | <60 | [11] |
| Thiourea sensor | — | 1.0–10.0 | — | [17] |
| Extraction–spectrophotometry | 0.62 | 0.0–4.0 | >30 | [18] |
| Ion chromatography | — | 1.0–2.5 | — | [19] |
| Liquid chromatography | — | 2.0–304.5 | — | [20] |
| Spectrophotometry | 2.35 | 0.0–70.0 | ≈20 | This work |
| Colorimetric method | — | 0.0–80.0 | <10 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Yuan, X.; Li, Y.; Zhang, Z.; Xu, Y.; Zhou, W.; Wang, Y. Rapid Determination of Thiourea Concentration in Copper Electrolyte. Processes 2025, 13, 1092. https://doi.org/10.3390/pr13041092
Chen L, Yuan X, Li Y, Zhang Z, Xu Y, Zhou W, Wang Y. Rapid Determination of Thiourea Concentration in Copper Electrolyte. Processes. 2025; 13(4):1092. https://doi.org/10.3390/pr13041092
Chicago/Turabian StyleChen, Liqing, Xiaofeng Yuan, Yulong Li, Zhenqian Zhang, Yangtao Xu, Wenqian Zhou, and Yi Wang. 2025. "Rapid Determination of Thiourea Concentration in Copper Electrolyte" Processes 13, no. 4: 1092. https://doi.org/10.3390/pr13041092
APA StyleChen, L., Yuan, X., Li, Y., Zhang, Z., Xu, Y., Zhou, W., & Wang, Y. (2025). Rapid Determination of Thiourea Concentration in Copper Electrolyte. Processes, 13(4), 1092. https://doi.org/10.3390/pr13041092






