Abstract
This study examines the effect of inoculum (0.5–3 g/L) and substrate concentration (40–200 g/L) on butyric acid and biohydrogen production by batch dark fermentation at 37 °C. Clostridium pasteurianum DSM525 and Cheese Whey Powder (CWP) were used in the experiments. The results showed that biohydrogen and butyric acid production increased with a 1.5 g/L microorganism concentration and 80 g/L CWP. A Cumulative Hydrogen Formation (CHF) of 458 mL, butyric acid (BA) of 1.523 g/L, butyric acid to acetic acid (BA/AA) ratio of 3.07 g BA/g AA, hydrogen production yield (YH2/TS) of 1278.63 mL H2/g TS, and butyric acid production yield (YBA/TS) of 0.37 g BA/g TS at 1.5 g/L inoculum concentration was achieved. The hydrogen (HPR) and butyric acid production rates (BAPR) were similarly the highest at 1.5 g/L. The maximum specific hydrogen (SHPR) and butyric acid rates (SBAPR) were obtained at 2 g/L and 1 g/L organism concentrations, respectively. In variations of substrate concentrations, 651.1 mL of CHF, 1.1 g/L of BA, 3.23 g BA/g AA of BA/AA ratio, 576 mL H2/g TS of YH2/TS, and 27.4 g BA/g TS of YBA/TS were accomplished. HPR and SHPR were the highest at 80 g/L CWP due to no substrate inhibition. The BAPR was at its maximum at 100 g/L, BA accumulation was faster, and the SBAPR was at maximum 40 g/L CWP. The results showed a good adaptation of C. pasteurianum to the butyric acid-derived hydrogen production pathway. This strategy could build a renewable and sustainable process with dual valuable outputs.
1. Introduction
Green fuel and green chemical production achieved via biomass valorization is one of the supreme environmentally friendly approaches for reducing the use of fossil fuels [1]. Currently, due to global warming, the use of fossil fuels is highly discouraged. Lately, there has been a rising awareness of renewable and sustainable bio-fuels and green bio-chemicals as an alternative to fossil fuels and synthetic chemicals [2]. Butyric acid, as a green chemical, is a promising biochemical alternative to artificial chemicals [3,4]. Butyric acid is commonly utilized in the nutrition, medical, and cosmetic industries as a flavor, aroma, and fragrance [5]. Moreover, butyric acid is used in the treatment of cancer, hemoglobinopathies, and gastrointestinal illnesses, as well as in vasoconstrictor medications, because of its anti-cancer effects [6].
Hydrogen is a rich energy power supply established generally through a significant fermentation process, forming acetic acid and butyric acid [7]. 1 mol of glucose produces 2 moles of H2 and 2 moles of butyric acid (Equation (1)) [8]. This study aims to produce butyric acid in an environmentally sustainable manner (green chemical) in alliance with hydrogen. Acetic acid is also an end product in the fermentation media, which provides more hydrogen (theoretically, 4 mols of hydrogen gas) [1]. Many studies in the literature focus on higher hydrogen production potentials [9,10,11,12,13,14,15,16,17,18,19,20]. However, studies concentrating on predominantly produced butyric acid during hydrogen production are rare in the literature. Therefore, the end products are valorized in this study to obtain a valuable biofuel and green chemical from the dark fermentation pathway.
C6H12O6 + 2H2O → 2H2 + 2CO2 + 2CH3COOH (butyric acid)
Butyric acid is one of the organic acids formed by bacterial fermentation of countless feedstocks rich in organic carbon [9]. Organisms such as mixed cultures, C. pasteurianum, Clostridium sensu stricto, Lactobacillus sp., C. tyrobutyricum, C. butyricum, C. thermobutyricum, C. acetobutylicum can be selected for butyric acid formation [10,11,12,13,14,15]. In the present study, C. pasteurianum was used as a dark fermentation culture, while its butyric acid production ability is high [10,12,14,16,17,18]. C. pasteurianum is a Gram-positive, strict anaerobe-forming endospore [16]. These bacteria are known for their high substrate amount acceptance, inhibitor resistance, and sustainability against contrary conditions during hydrogen, butyric acid, and butanol production [17]. C. pasteurianum consumes glycerol to produce butanol as the primary product and butyrate, acetate, lactate, 1,3-propanediol, ethanol, H2, and CO2 as secondary products [17].
Sarma et al. [18] achieved 1.19 mmol/L h specific butyric acid production rate (SPBR), 3.85 mmol/L h specific hydrogen production rate (SHBR), and 0.95 mol H2/mol glycerol hydrogen production yield by fermenting glycerol with C. pasteurianum. The operating environments for these organisms depend on several factors, including temperature (mesophilic or thermophilic), alkalinity, duration period, and process type.
In many recent studies, researchers have sought various types of sustainable feedstocks for the production of valuable products during the fermentation process [19,20]. Starchy and lignocellulosic waste substrates are widely used for hydrogen production. There are several examples in the literature focussing on hydrogen production. The researchers used substrates such as sugarcane vinasse, cheese whey (CW), dairy industry wastewater, glucose, glycerol, food waste, glucose-xylose mixtures, rice straw, lignocellulosic substrates, and pulp and paper industry wastewater [21,22,23,24,25,26,27]. Scientific studies have shown that food waste can be transformed into commercially valuable products (chemicals, biofertilizers, biogas, biohydrogen, bioethanol, and biobutanol) [27,28]. It is important to note that this process is not only environmentally friendly but also economically viable. CW and the dried form of CW named as Cheese Whey Powder (CWP), are lactose-rich wastes, valorized for hydrogen fermentation [29]. Barba [30] concluded that approximately 200 million tons of whey is produced worldwide in 2020. This research will utilize CWP, with the primary objective being to establish the potential of CWP as a sustainable and significant supply of hydrogen and butyric acid production within the perspective of abundant dairy industry wastes.
The crucial, key process parameters in dark fermentation are the pretreatment of mixed culture, type of organism achieving enzymatic activities, pH, fermentation temperature, feed-to-mass ratio, residence time, and substrate pretreatment [31,32]. In addition, feedstock and biomass concentration are very important factors affecting productivity [33]. Low substrate concentrations cause rate limitations, while high ones cause product inhibition. Likewise, low concentrations of microorganisms result in long lag phases with slow processing, and high biomass levels have a detrimental effect by consuming the end products [34]. There are different types of inhibitory factors in dark fermentation, dependent on substrate and biomass concentrations, that may cause low productivity, such as Total Volatile Fatty Acid (TVFA) and hydrogen gas [35]. The TVFA accumulation rate increases with increasing biomass concentrations, which also causes fast pH falls, indicating a detached force of H+ ion transfer by devastating the microbial proton gradient [36]. The accumulation of biohydrogen gas in the headspace of the fermentation media leads to high hydrogen partial pressure where the threshold inhibitory partial pressure is 10−3 atm. This causes a shift in the metabolic pathway to different end products like acetone, butanol, ethanol, and lactic acid or prevention of biomass growth and substrate consumption in the lag phase [35].
There are many articles discussing the effects of substrate or organism concentration on enhancing biohydrogen production potential [31,32,33,34,35,36,37,38]. However, few articles have been studied about microorganism and substrate concentration on both improving butyric acid and biohydrogen gas production as a dual (green fuel and green chemical) benefit [38]. The novelty of this study is enhancing hydrogen production on the butyric acid pathway with CWP as substrate. Therefore, in this study, different C. pasteurianum DSM 525 inoculum concentrations (0.5–3 g/L) and CWP concentrations (40–200 g/L) were tried to enhance butyric acid production along with hydrogen gas production at mesophilic dark fermentation conditions.
2. Materials and Methods
2.1. CWP Characterization and Preparation for Fermentation
CWP was obtained from PAKMAYA Baker’s Yeast Company. It was taken from the company in a commercial package and was newly prepared with a one-year expiration date. Table 1 and Table 2 give the characterization of the CWP used in the experiments.

Table 1.
The common characterization parameters of the whey powder used in the experiments.

Table 2.
The characterization of trace elements of the whey powder used in the experiments.
The substrate that contained protein was pretreated before use in fermentation media due to the adverse effects of protein content in CWP. CWP was adjusted to pH 2 with concentrated H2SO4 and then thermally treated at 90 °C for 30 min. After thermal treatment, the protein was discarded via precipitation, and the supernatant was used in the trials [37]. 10 g of CWP after deproteinization has a total sugar concentration of approximately 9.5 ± 0.25 g/L and a lactose concentration of 6.65 ± 0.5 g/L lactose, which was high due to processed CWP for industrial use.
2.2. Culture
C. pasteurianum DSM 525 was obtained in lyophilized form from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ), Braunschweig, Germany. The microorganism was then inoculated into a medium containing MgSO4 (0.25 g/L), K2HPO4 (2.8 g/L), yeast extract (0.6 g/L), KH2PO4 (3.9 g/L), peptone (10 g/L), and L-cysteine (0.1 g/L), lactose (10 g/L) (All chemicals were taken from Merck, Darmstadt, Germany) [1]. Anaerobic conditions were maintained by sparging nitrogen gas (99.99% pure) through the top of the bottle, and the organisms were incubated at an initial pH of 7.2 (WTW, Inolab), at 37 °C, in an incubator, for 2 days. The culture was then inoculated (Nüve) into the fermentation media.
An inoculum was prepared in a 5 L vessel with 2 L working volume to achieve a dense culture for batch biomass concentration experiments. The same procedure was carried out for substrate concentration experiments in a 2 L vessel with a 1 L working volume.
2.3. Batch Dark Fermentation Assay
Different microorganism and substrate concentration trials to investigate butyric acid along with hydrogen gas were conducted in 250 mL serum bottles in batch mode. All the dark fermentation processes were conducted at 37 °C. The experimental medium was prepared using deproteinized CWP as the substrate. The experiments were conducted with working volumes of 200 mL and 150 mL for the effect of microorganism and substrate concentration variation on butyric acid and hydrogen gas production potential, respectively (Isolab-Germany Boro 3.3, 250 mL serum bottles). All experiments were conducted with three individual parallel repetitions at the same conditions. The fermentation period of the experiments was kept as long as eight days to observe the possible changes in end product production.
Firstly, the effects of different concentrations of the organism (Clostridium pasteuranum DSM 525) (0.5-1-1.5-2.2.5-3 g/L) were considered to improve end product formation, with a constant CWP concentration of 45 g/L. A dense culture of inoculum was used to obtain the organism concentrations. It was centrifuged in sterile conditions, and the pellet was diluted with 100 mL of pure water. Following the measurement of the biomass concentration in the dense inoculum (15.2 g/L), it was diluted to the required biomass concentration. Subsequently to the process of inoculation, the biomass concentrations were analyzed to verify the compatibility of the inoculum with the desired parameters. Secondly, different substrate concentrations (40-80-100-150-200 g/L) were tried with the selected organism concentration obtained from the first experiments. An inoculum concentration of 1.5 g/L was used in these experiments. Prior to fermentation, the pH of the medium was adjusted to 7.2 using 10 M NaOH. The bottles were then sterilized via autoclaving at 121 °C for 15 min, and nitrogen gas was passed through the top to ensure anaerobic conditions for 20 min. Finally, the bottles were sealed with stoppers and caps and verified for gas tightness using silicone stoppers. The fermentation medium comprised 2.8 g/L K2HPO4, 3.9 g/L KH2PO4, 0.25 g/L MgSO4, 0.1 g/L L-Cysteine, 0.6 g/L yeast extract, and 1.0 g/L peptone, 0.7 g/L CaCO3, 2.55 g/L NaCl, and 100 mg/L Na2S2O3, 0.0133 g/L NiCl2, 0.013 g/L ZnSO4, 0.0055 g/L FeSO4, 0.0135 g/L CuCl2 (All chemicals were taken from Merck, Darmstadt, Germany) [38]. The pH was manually controlled and adjusted to 6.5 during the fermentation period in all tested bottles. Total sugar (TS) concentration, pH, Volatile Fatty Acid (VFA) concentration, solvent (such as butanol, etc.) concentrations, saccharide concentrations (such as lactose, glucose, galactose, etc.), biomass concentrations, biohydrogen volumes, and total gas volumes were analyzed daily in the fermentation trials.
2.4. Analytical Techniques
All tested bottles were prepared randomized due to the experimental setup, and samples were taken from each bottle on a daily basis [39]. To analyze the liquid portion of the fermentation media, 5 mL samples were centrifuged at 8000 rpm for 15 min. TS concentration, pH, and VFA concentration were analyzed in the clear supernatant. The samples were analyzed in triplicates, and the results were reproducible within a 3% deviation. TS concentration was determined using acid-phenol spectrometry with the Dubois method [40]. Biomass concentrations were determined according to the standard methods by filtering 5 mL samples from fermentation media and the feed through a 0.45 μm millipore filter and drying at 105 °C until constant weight. The same method was used in the SS (suspended solids) analysis. The difference in the suspended solids content of the fermentation media and feed was considered as the biomass content during fermentation [41]. Furthermore, to verify the biomass concentration analysis, cell protein concentrations were detected by using a Modified Lowry protein assay kit (Thermo Scientific, Waltham, MA, USA; data not shown). TP (Total Phosphorus, Merck spectroquant no: 114543, Darmstadt, Germany), TN (Total Nitrogen, Merck, Darmstadt, Germany ), NH4+ (Ammonium, Merck spectroquant no: 114537, Darmstadt, Germany), NO3− (Nitrate, Merck spectroquant no: 109713, Darmstadt, Germany) were analyzed by using the analytical kits and a spectrometer. The characterization parameters given in Table 2 (Li, Be, B, Na, Mg, Al, K, Ca, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Mo, Ag, Cd, Sn, Sb, Ba, Tl, Pb) were analyzed through an Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Agilent 7700x, with HMI [42]. The analysis of organic acid concentrations, saccharide concentrations, and solvent concentrations was conducted using High-Purity Liquid Chromatography (HPLC, Agilent 1100; Santa Clara, CA, USA, Aminex HPX-84 H column). The mobile phase consisted of 5 mM H2SO4 at a flow rate of 0.6 mL/min. Organic acid concentrations were determined with a UV detector at 220 nm. The saccharides and solvents were analyzed with an RID detector at 50 °C. The saccharides were analyzed by HPLC to verify the TS results analyzed by using the Dubois method. Gas-phase analysis was performed by taking daily samples from the fermentation bottles with a gas-tight glass syringe. The hydrogen gas concentration was measured using gas chromatography (Agilent 6890 N-GC, Santa Clara, CA, USA) with a GC column of Alltech, Hayesep D 80/100 (6-inch × 1/8-inch × 0.85 inch). Nitrogen was used as the carrier gas at a flow rate of 30 mL/min. The furnace, injection, detector, and filament temperatures were 35 °C, 120 °C, 120 °C, and 140 °C, respectively. The total gas produced was calculated using the water displacement method with 2% H2SO4 and 10% NaCl [41].
The equations used to determine the potential for hydrogen and butyric acid production (CHF, yield, rates, specific production rates, etc.) were sourced from Kargi et al. and Karakaya and Ozmihci [38,41]. The results of three individual experiments for each trial were calculated to find the averages.
2.5. Statistical Analysis
IBM SPSS Statistics software v29 (SPSS Inc., Cary, NC, USA) was used to conduct statistical analyses. One-way analysis of variance (ANOVA) followed by Dunnett post hoc multiple comparisons tests for pairwise comparison of means (at p ≤ 0.05) at a confidence level of 95% were conducted on the CHF and butyric acid production results on both experimental setups to determine the significant differences among different fermentation media [39].
3. Results and Discussion
3.1. Effects of Initial Microorganism Concentrations on Butyric Acid and Hydrogen Production Potential
Figure 1a shows the amount of CHF in different initial microorganism concentrations at 37 °C, with time. As seen from the graph, the CHF elevated until 1.5 g/L with increasing initial microorganism concentrations. According to this figure, 1.5 g/L (11% v/v) the initial microorganism concentration gave the highest CHF amount at 458 mL. Further increases in initial organism concentrations gave lower CHF, probably due to hydrogen consumption where solvents may be produced [43,44,45,46]. In the literature, higher concentrations of CHF were achieved with mixed cultures, although they were not aimed at increasing the butyrate production. The literature reveals results similar to those of our study, depicting an inoculum concentration of 10% v/v enhancing hydrogen production [41,43]. Furthermore, biomass to substrate ratio (Xo/So) was calculated from the results where an optimum of Xo/So = 0.035 g biomass/g CWP was found giving the highest CHF.
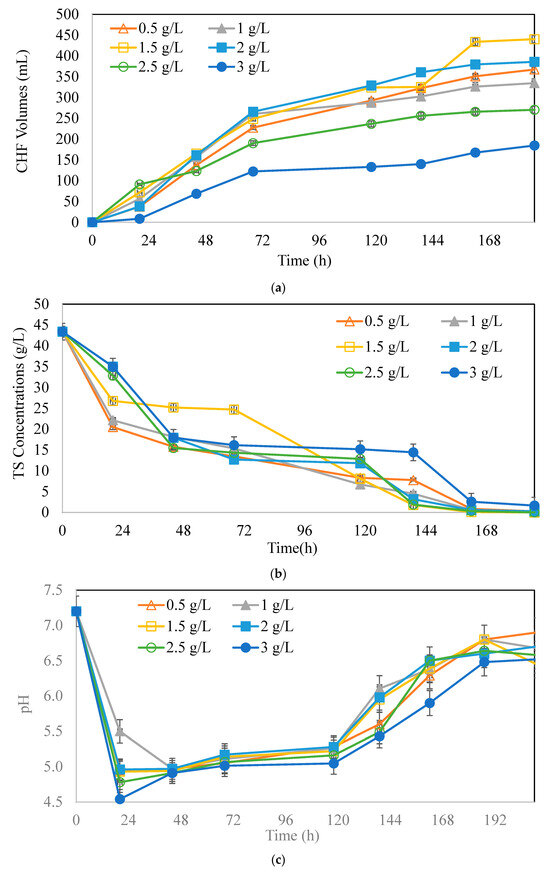
Figure 1.
Variation of (a) CHF volumes (mL), (b) TS concentrations, and (c) pH with time, at different initial microorganism concentrations (CWP:40 g/L);  : 0.5 g/L,
: 0.5 g/L,  : 1 g/L,
: 1 g/L,  : 1.5 g/L,
: 1.5 g/L,  : 2 g/L,
: 2 g/L,  : 2.5 g/L,
: 2.5 g/L,  : 3 g/L.
: 3 g/L.
 : 0.5 g/L,
: 0.5 g/L,  : 1 g/L,
: 1 g/L,  : 1.5 g/L,
: 1.5 g/L,  : 2 g/L,
: 2 g/L,  : 2.5 g/L,
: 2.5 g/L,  : 3 g/L.
: 3 g/L.
Figure 1b indicates the variations of sugar consumption with time. The initial substrate concentration was constant at 45 g/L CWP with 43 ± 2 g/L TS. Within 24 h, the organism consumed the sugar with no lag phase. The sugar in all the tested bottles was consumed completely. Figure 1c plots the variations of pH with time. The initial pH in the experimental setup was 7.2 and dropped to around 4.5–5.5 within 24 h. The lowest pH was observed at an initial microorganism concentration of 3 g/L, while the highest was observed at a concentration of 1 g/L. Between 48 and 144 h, the pH remained around 5 to 5.5. After 144 h, the pH did not decrease despite adjustments. The sharp drop in all bottles showed that organic acid production (accumulation in the fermentation media) started rapidly after a good amount of sugar consumption and increased over time (not shown in graphs), as proven by organic acid amounts. The pH decreased with increasing microorganism concentrations for the first 24 h, indicating good microorganism activity, which resulted in different end-product productions.
In Figure 2a, the production of butyric acid (BA) and Total Volatile Fatty Acid (TVFA) were depicted for different initial microorganism concentrations. The highest BA, at 1.5 g/L, and TVFA production, at 5.9 g/L, was obtained at an initial microorganism concentration of 1.5 g/L. In the literature, pH, as an important parameter, was investigated for butyric acid production. A study revealed that under a controlled pH of 6.5, the expression of the genes butyrate kinase (buk) and hydrogenase (hyd) was enhanced, and the gene acetate kinase (ack) was reduced [47]. The sharp drop in pH in our experimental setup was manually adjusted to 6.5 once a day to achieve expressions of butyrate kinase and hydrogenase at the desired levels [47]. The pH decreased with increasing organic acid accumulation in the fermentation media, proving the success of the organism’s activity. C. pasterianum used in our study was a wild-type organism that showed good adaptation after a period of time. However, it should be taken into account that a modified type of strain may show a faster adaptation to the desired fermentation conditions than a wild type.
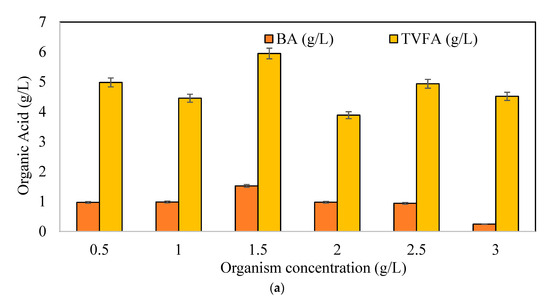
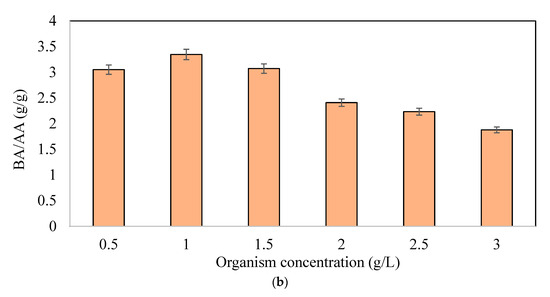
Figure 2.
(a) Total organic acid and maximum butyric acid concentrations, (b) BA/AA (butyric acid/acetic acid) ratios with different initial microorganism concentrations (CWP: 40 g/L).
Figure 2b shows the butyric acid to acetic acid ratios (BA/AA). BA/AA ratios increased up to 1 g/L of microorganism concentration and then decreased with the increasing concentrations. Based on Figure 2b, the initial microorganism concentration of 1 g/L had the highest BA/AA ratio of 3.346 g BA/g AA, followed by a concentration of 1.5 g/L with a ratio of 3.071 g BA/g AA. At concentrations above 1.5 g/L, TVFA concentrations decreased with increasing organism concentrations. Although the TVFA production was expected to increase at high organism concentrations (>1.5 g/L) due to the decrease in pH, no rise in TVFA was observed after some time (differing for each concentration). This may be due to the transition of the pathway to the solventogenic phase, while the microorganism used is not only a butyric acid producer (C. pasteurianum DSM 525), but it is also known as a butanol producer [44,45,46]. Acetone, ethanol, and butanol were also monitored with a point of view determining whether the organism produced solvents under the treated conditions. In all tested bottles, these three by-products were observed (data not shown). Specifically, the acetone concentration increased with increasing organism concentrations. No ethanol was monitored in the fermentation media. At low organism concentrations of up to 1.5 g/L, the butanol concentrations were around 1 ± 0.5 g/L at the end of the fermentation period, produced after 168 h. At a high organism concentration of more than 1.5 g/L, the butanol concentration was approximately 2.5 ± 0.3 g/L at the end of the fermentation time produced earlier than 168 h.
Figure 3a indicates the hydrogen (YH2/TS) and butyric acid production yields (YBA/TS) with different initial organism concentrations. Based on the results, the maximum hydrogen production yield of 1278.63 mL H2/g TS and butyric acid production yield of 0.37 g BA/g TS were achieved at an initial organism concentration of 1.5 g/L. The yield of butyric acid increased with an increase in the initial concentration of microorganisms up to 1.5 g/L. However, with further increases in initial organism concentrations, the yields decreased, and the amount of the yields were close to each other. This behavior shows that organism concentrations of more than 1.5 g/L cause a metabolic shift into different end products. Thus, high organism concentrations are not required to enhance hydrogen and butyric acid production, whereas the optimum biomass level was 1.5 g/L.
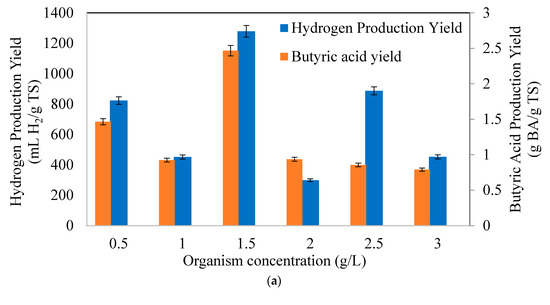
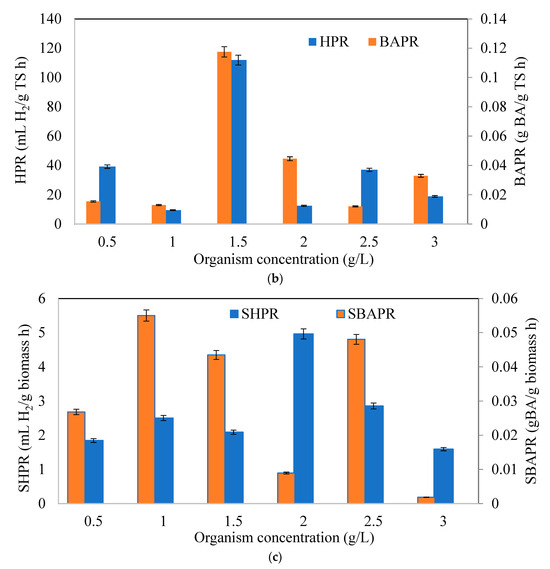
Figure 3.
Variation of (a) hydrogen (YH2/TS) and butyric acid yields (YBA/TS), (b) hydrogen (HPR) and butyric acid production rates (BAPR), (c) specific hydrogen (SHPR) and butyric acid production rates (SBAPR) at different initial microorganism concentrations (CWP: 40 g/L).
Figure 3b signifies the hydrogen production rate (HPR) and butyric acid production rate (BAPR) with initial organism concentrations. The highest HPR and BAPR were obtained at the same initial organism concentration (1.5 g/L), and the values were 111.88 mLH2/g TS h and 0.0176 g BA/g TS h, respectively. Specific hydrogen (SHPR) and specific butyric acid production rates are depicted in Figure 3c. The maximum SHPR (4.96 mL H2/ g biomass h) was found in 2 g/L, while the maximum SBAPR (55 mg BA/ g biomass h) was observed in 1 g/L organism concentration. The SHPR and SBAPR values indicated that the production of hydrogen and butyric acid requires a certain period of time, which is a typical outcome in mild dark fermentation conditions, as evidenced by the corresponding CHF and BA amounts. As seen from the results, it can be concluded that lower organism concentrations than 1.5 g /L adapted faster to lactose than higher concentrations. First the glucose was consumed in the fermentation bottles and then the organisms tried to adapt to consumption of lactose due to diauxic growth, which affected the production potentials of hydrogen and butyric acid [48,49].
3.2. Effects of Substrate Concentrations on Butyric Acid and Hydrogen Production Potential
A range of 40–200 g/L CWP concentrations were used to investigate the effects of substrate concentration on hydrogen and butyric acid production. The trials were performed using the same methods as previous experiments. The organism concentration (1.5 g/L) improving hydrogen and butyric acid production in the first experiments was used in the second one. Figure 4a–c represent variations of CHF, TS concentration, and pH with time, respectively. As shown in Figure 4a, 80 g/L CWP gave the highest CHF, which was 651 mL. The second highest CHF, which was close to 80 g/L CWP, was obtained with 100 g/L CWP at 612 mL. The lowest CHF volume was 137 mL (200 g/L CWP).
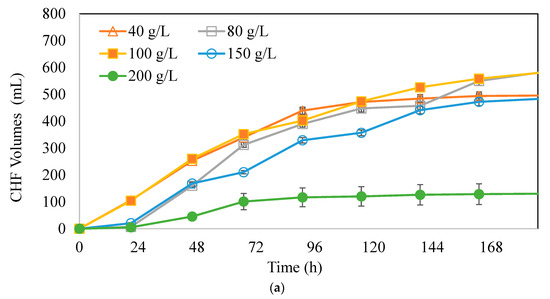
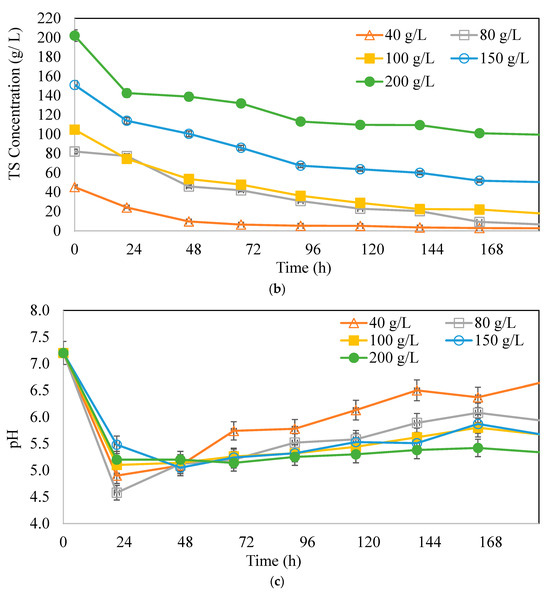
Figure 4.
Variation of (a) CHF volumes (mL), (b) TS concentration, and (c) pH with time at different CWP concentrations (Xo: 1.5 g/L),  : 40 g/L,
: 40 g/L,  : 80 g/L,
: 80 g/L,  : 100 g/L,
: 100 g/L,  : 150 g/L,
: 150 g/L,  : 200 g/L.
: 200 g/L.
 : 40 g/L,
: 40 g/L,  : 80 g/L,
: 80 g/L,  : 100 g/L,
: 100 g/L,  : 150 g/L,
: 150 g/L,  : 200 g/L.
: 200 g/L.
Figure 4b represents the consumption of different substrate concentrations. It can be observed that at CWP concentrations of 40 g/L, 80 g/L, and 100 g/L CWP, the TS were nearly consumed. In contrast, at CWP concentrations of 150 g/L and 200 g/L CWP, 25% and 44% of TS remained in the media at the end of the fermentation period. At substrate concentrations above 100 g/L, it was observed that the substrate could not be utilized, and CHF could not be produced at a desired level, indicating that substrate and organic acid inhibitions were present at concentrations above 100 g/L.
The results showed an optimum initial biomass-to-substrate ratio of Xo/So = 0.13 g biomass/g CWP, which gives the highest CHF. This result differed from the effects of biomass concentration on butyric acid and hydrogen production potential experiments because the lowest feedstock amount of the substrate concentration experiments was used. The Xo/So ratio of 0.13 g biomass/g CWP proves that the optimum biomass concentration can tolerate higher initial substrate concentrations. Argun et al. [34] also found a biomass-to-substrate ratio of 0.13 g biomass/g wheat powder enhancing hydrogen gas production potential, which was comparable with our results. Figure 4c, the variation of pH with time, depicts that, in all tested bottles, the pH dropped below 5.5 within 24 h due to sugar consumption and organic acid accumulation. The pH was manually adjusted with NaOH periodically to 6.5 to avoid the shift to solventogenic pathway. After 24 h, the pH varied between 5 and 6.5 in different trials.
Figure 5a,b represent butyric acid (BA), total Volatile Fatty Acid (TVFA), and the ratio of BA/AA with different substrate concentrations. In Figure 5a, 1.17 g/L and 1.10 g/L BA was produced at 150 g/L and 80 g/L CWP, respectively. The BA concentrations were close to each other in all experimental media. This probably was due to the fact that the microorganism concentration of 1.5 g/L cannot provide more butyric acid production, and the pathway shifts to different end products (not shown in graphs). In order to prove this phenomenon, solvents as by-products were also examined in the experimental setup. The solvent concentration observed in these experiments was very close to the ones obtained in a variation of biomass concentration experiments up to 80 g/L CWP concentration. The TVFA concentrations increased with increasing substrate concentrations up to 150 g/L CWP. The highest TVFA production was 5.02 g/L at 150 g/L CWP, followed by 200 g/L CWP at 4.17 g/L. When compared with the CHF results, high CWP concentrations gave lower CHF, indicating possible TVFA inhibition. Furthermore, at CWP concentrations over 80 g/L, the butanol concentration was constant around 1.5 ± 0.2 g/L, and the acetone concentration increased with increasing CWP concentrations up to 4.5 ± 0.2 g/L at the end of the fermentation period. The lower butyric acid production also affected productivity at higher sugar concentrations, probably mirroring the osmotic stress. Nutrients such as salts and sugar in high amounts are known to cause osmotic pressure on the microorganisms used. The medium that was used did not have high salts causing osmotic pressure; however, it did have high lactose concentrations (100, 150, and 200 g/L CWP). This may affect the reduction in organic acid production. It is acknowledged that osmotic shock, triggered by inoculating cells into a solution with a high concentration of sugar, can alter the phospholipid structure of the cell membranes or even kill cells [50].
Figure 5.
(a) Total organic acid and maximum butyric acid concentrations, (b) BA/AA (butyric acid/acetic acid) ratios at different CWP concentrations (Xo: 1.5 g/L).
In Figure 5b, the BA/AA ratio elevated from 2.11 (40 g/L CWP) to 3.23 g BA/g AA at 80 g/L CWP. BA/AA ratios at further increases in substrate concentration decreased and did not contribute to CHF. Different studies in the literature demonstrated higher substrate concentrations enhancing hydrogen production potentials [36]. However, there were no data revealing BA/AA ratio improvements except in our previous study, giving a BA/AA ratio of 2.6 g BA/AA, with a hydrogen production yield of 3.7 mmol H2/mmol lactose [38].
Figure 6a indicates hydrogen (YH2/TS) and butyric acid production yields (YBA/TS), 6b hydrogen (HPR) and butyric acid (BAPR) production rates, and 6c specific hydrogen (SHPR) and butyric acid (SBAPR) production rates at different CWP concentrations. The hydrogen production yields declined with rising substrate concentrations. The BA production yield and rate increased up to 80 g/L and 100 g/L CWP amounts and then decreased with additional substrate concentrations, respectively. The highest hydrogen yield was achieved with 40 g/L CWP (1163.3 mL H2/g TS). The maximum butyric acid yield was 0.365 g BA /g TS h at 80 g/L CWP, and the BAPR was 0.005 g BA/g TS h at 100 g/L CWP. The highest SHPR and SBAPR were observed at a substrate concentration of 40 g/L as 175 mL H2/g biomass h and 630 mg BA/ g biomass h, respectively.
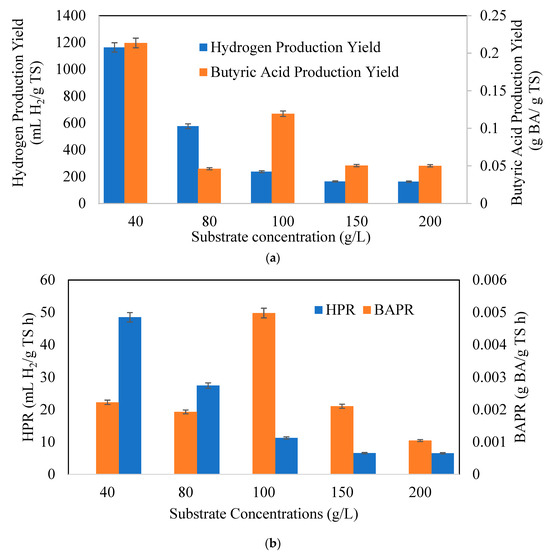
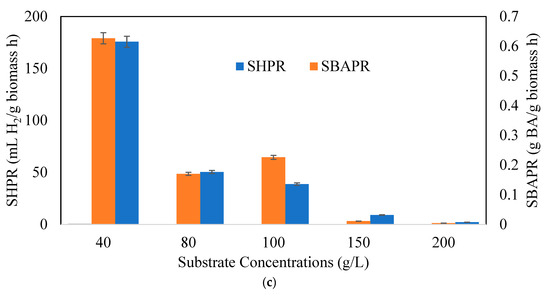
Figure 6.
Variation of (a) hydrogen and butyric acid yield, (b) Hydrogen (HPR) and Butyric Acid Production Rate (BAPR), and (c) Specific Hydrogen (SHPR) and Butyric Acid Production Rate (SBAPR), at different CWP concentrations (Xo: 1.5 g/L).
From an overall point of view, besides the rates of butyric acid production, butyric acid yield, BA/AA ratio, and CHF, the optimal substrate concentration was found to be 80 g/L in our study. In hydrogen production yields and rates, 40 g/L was better than the other concentrations due to the absence of substrate inhibition in the fermentation media. However, in butyrate production, except at specific rates, higher substrate concentrations provided better yields and rates. This benchtop experiment showed that CWP used as a lactose-based feedstock can enhance hydrogen and butyric acid production as a dual outcome, even though C. pasterianum can utilize lactose well after adaptation.
In the literature, there are studies using higher butyric acid concentrations and productivity with different microorganisms, such as one considered a novel strain, C. tyrobutyricum NRRL 67062, with production rates of 15.62 g/L butyric acid and 0.50 g/L h butyric acid productivity from 60 g/L glucose. They revealed that this strain is tolerant to high glucose concentrations (250 g/L) and high butyric acid concentrations (58 g/L) [47]. Furthermore, genetically modified strains are able to produce 32.5 g/L (C. acetobutylicum strain HCBEKW) butyric acid [50]. However, the above studies did not monitor hydrogen as an output in the fermentation media. Studies monitoring hydrogen besides those using butyric acid with CW as substrate found lower butyric acid concentrations. For example, Dessi et al. [51] produced 2.5 g/L butyric acid and 2.0 L H2/ L d biohydrogen rate in an up-flow anaerobic sludge blanket system. Other studies using CW to produce hydrogen obtained percentages of butyric acid in the fermentation end product composition ranging between 2 and 16% [8,20,25], which were lower than in this study.
Most of the butyric acid refining studies are laboratory-scaled ones. Further investigations need to be performed in order to achieve economic recovery because of their costs and low productivity. Purification costs are high to obtain a pure product. Low feedstock and processing costs can be obtained by using CW to achieve an economically feasible process. The favorable recovery technique is still distillation, which requires high energy because of butyric acid’s boiling point (163.5 °C). New trends use membranes, solvent extraction, and adsorbents for purification, which all have their unique, challenging parameters [51,52]. A techno-economic analysis of a small-scale biorefinery process for producing butyric acid was conducted, utilizing wheat straw as a substrate and Clostridium tyrobutyricum as an inoculum. The cost of this process was determined to be 2.97 USD per kilogram of butyric acid [53]. In light of this study, future investigations may be needed to alter these limitations, such as the low productivity and the low tolerance of the organism to high product concentrations, with simultaneous separation of gas and liquid products, the addition of adsorbents, and fed-batch or continuous operating systems, to enhance butyric acid production along with hydrogen. The addition of adsorbents may alter organic acid inhibition and may help control pH easily.
4. Conclusions
In the experiments performed, the highest butyric acid concentration and CHF were observed with 1.5 g/L inoculum concentrations and 80 g/L CWP concentrations. Further increases than 1.5 g/L biomass and 80 g/L CWP gave lower CHFs and organic acids, probably due to the shift in the metabolic pathway to other end products, such as butanol.
The results of this study were comparable with those from the literature. They showed high butyric acid ratios along with good hydrogen production capacities, which have the potential for producing commercial products in the form of green fuels and chemicals. This double harvest may contribute to constructing a renewable and sustainable economy.
Author Contributions
G.K. performed all the experimental studies and prepared the draft of the paper under the supervision of S.Ö. S.Ö. edited the draft and corrected the paper for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Research Funds of the Dokuz Eylül University by a grant number of FBA-2023-2970.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
All the experiments were carried out in Dokuz Eylül University, Environmental Engineering Department, Bioprocess Laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ammonium | NH4+ | Biomass To Substrate Ratio | Xo/So |
| Butyric Acid To Acetic Acid Ratio | BA/AA | Butyric Acid Production Rate | BAPR |
| Cheese Whey | CW | Cheese Whey Powder | CWP |
| Cumulative Hydrogen Formation | CHF | Hydrogen Production Yield | YH2/TS |
| Hydrogen Production Rate | HPR | High-Purity Liquid Chromatography | HPLC |
| Inductively Coupled Plasma-Mass Spectrometry | ICP-MS | IBM SPSS Statistics Software V29 | SPSS |
| Initial Biomass Concentration | Xo | Initial Substrate Concentration | So |
| Gas Chromatography | GC | Nitrate | NO3− |
| Refractive Index Detection | RID | Suspended Solids | SS |
| Specific Hydrogen Rate | SHPR | Specific Butyric Acid Production Rate | SPBR |
| Total Nitrogen | TN | Total Sugar | TS |
| Total Phosphorus | TP | Total Volatile Fatty Acid | TVFA |
| Ultraviolet | UV | Volatile Fatty Acid | VFA |
References
- Özmıhçı, S.; Hacıoğlu, İ.; Altındağ, E.E. Impacts of mycotoxin on biohydrogen production from waste dry fruits. J. Mater. Cycles Waste Manag. 2022, 24, 1736–1746. [Google Scholar] [CrossRef]
- Câmara-Salim, I.; González-García, S.; Feijoo, G.; Moreira, M.T. Screening the environmental sustainability of microbial production of butyric acid produced from lignocellulosic waste streams. Ind. Crops Prod. 2021, 162, 113280. [Google Scholar] [CrossRef]
- Hussain, A.; Lee, J.; Xiong, Z.; Wang, Y.; Lee, H.S. Butyrate production and purification by combining dry fermentation of food waste with a microbial fuel cell. J. Environ. Manag. 2021, 300, 113827. [Google Scholar] [CrossRef]
- Sauer, M.; Marx, H. Co-cultures and synthetic microbial communities for green chemical production. Curr. Opin. Green Sustain. Chem. 2023, 42, 100842. [Google Scholar] [CrossRef]
- Liu, S.; Duncan, S.; Qureshi, N.; Rich, J. Fermentative production of butyric acid from paper mill sludge hydrolysates using Clostridium tyrobutyricum NRRL B-67062/RPT 4213. Biocatal. Agric. Biotechnol. 2018, 14, 48–51. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. Butyric acid dominant volatile fatty acids production: Bio-Augmentation of mixed culture fermentation by Clostridium butyricum. J. Environ. Chem. Eng. 2020, 8, 104496. [Google Scholar] [CrossRef]
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the potential of bio-hydrogen production by fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Rossi, A.; Zonfa, T.; De Gioannis, G.; Muntoni, A. Continuous fermentative hydrogen production from cheese whey—New insights into process stability. Int. J. Hydrogen Energy 2022, 47, 21044–21059. [Google Scholar] [CrossRef]
- Bevilacqua, R.; Regueira, A.; Mauricio-Iglesias, M.; Lema, J.M.; Carballa, M. Understanding the effect of trace elements supplementation on volatile fatty acids production from proteins. J. Environ. Chem. Eng. 2021, 9, 105934. [Google Scholar] [CrossRef]
- Khanna, S.; Goyal, A.; Moholkar, V.S. Production of n-butanol from biodiesel derived crude glycerol using Clostridium pasteurianum immobilized on Amberlite. Fuel 2013, 112, 557–561. [Google Scholar] [CrossRef]
- Jang, Y.S.; Woo, H.M.; Im, J.A.; Kim, I.H.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum for enhanced production of butyric acid. Appl. Microbiol. Biotechnol. 2013, 97, 9355–9363. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.Y.; Lee, K.M.; Youn, S.H.; Lee, S.M.; Woo, H.M.; Oh, M.K.; Um, Y. Butyric acid production from softwood hydrolysate by acetate-consuming Clostridium sp. S1 with high butyric acid yield and selectivity. Bioresour. Technol. 2016, 218, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Malkmes, M.J.; Zhu, L.; Huang, H.; Jiang, L. Metal-organic frameworks coupling simultaneous saccharication and fermentation for enhanced butyric acid production from rice straw under visible light by Clostridium tyrobutyricum CtΔack::cat1. Bioresour. Technol. 2021, 332, 125117. [Google Scholar] [CrossRef]
- Richmond, C.; Han, B.; Ezeji, T.C. Stimulatory Effects of Calcium Carbonate on Butanol Production by Solventogenic Clostridium Species. Cont. J. Microbiol. 2011, 5, 18–28. [Google Scholar]
- Wu, H.; Wang, C.; Chen, P.; He, A.Y.; Xing, F.X.; Kong, X.P.; Jiang, M. Effects of pH and ferrous iron on the coproduction of butanol and hydrogen by Clostridium beijerinckii IB4. Int. J. Hydrogen Energy 2017, 42, 6547–6555. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Wang, S.; Liu, F. Stimulation of ferrihydrite nanorods on fermentative hydrogen production by Clostridium pasteurianum. Bioresour. Technol. 2019, 283, 308–315. [Google Scholar] [CrossRef]
- Moon, C.; Hwan Lee, C.; Sang, B.I.; Um, Y. Optimization of medium compositions favoring butanol and 1,3-propanediol production from glycerol by Clostridium pasteurianum. Bioresour. Technol. 2011, 102, 10561–10568. [Google Scholar] [CrossRef]
- Sarma, S.; Anand, A.; Dubey, V.K.; Moholkar, V.S. Metabolic flux network analysis of hydrogen production from crude glycerol by Clostridium pasteurianum. Bioresour. Technol. 2017, 242, 169–177. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Carvalho, A.K.F.; Souza, C.R.F.; De Castro, H.F.; Bachmann, L.; Said, S.; Oliveira, W.P. Immobilized enzyme-driven value enhancement of lignocellulosic-based agricultural byproducts: Application in aroma synthesis. J. Clean. Prod. 2021, 284, 124728. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Mota, V.T.; de Oliveira, V.M.; Zaiat, M. Hydrogen and organic acid production from dark fermentation of cheese whey without buffers under mesophilic condition. J. Environ. Manag. 2022, 304, 114253. [Google Scholar] [CrossRef]
- Fu, H.; Yang, S.T.; Wang, M.; Wang, J.; Tang, I.C. Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing xylose catabolism genes for glucose and xylose co-utilization. Bioresour. Technol. 2017, 234, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; dos Santos, G.M.; Delforno, T.P.; de Souza Moraes, B.; da Silva, A.J. Biochemical butyrate production via dark fermentation as an energetically efficient alternative management approach for vinasse in sugarcane biorefineries. Renew. Energy 2020, 158, 3–12. [Google Scholar] [CrossRef]
- Kao, W.C.; Lin, D.S.; Cheng, C.L.; Chen, B.Y.; Lin, C.Y.; Chang, J.S. Enhancing butanol production with Clostridium pasteurianum CH4 using sequential glucose-glycerol addition and simultaneous dual-substrate cultivation strategies. Bioresour. Technol. 2013, 135, 324–330. [Google Scholar] [CrossRef]
- Zhao, Z.-T.; Yang, S.-S.; Luo, G.; Sun, H.-J.; Liu, B.-F.; Cao, G.-L.; Bao, M.-Y.; Pang, J.-W.; Ren, N.-Q.; Ding, J. Biohydrogen fermentation from pretreated biomass in lignocellulose biorefinery: Effects of inhibitory byproducts and recent progress in mitigation strategies. Biotechnology Advances 2025, 79, 10850. [Google Scholar] [CrossRef]
- Perna, V.; Castelló, E.; Wenzel, J.; Zampol, C.; Fontes Lima, D.M.; Borzacconi, L.; Varesche, M.B.; Zaiat, M.; Etchebehere, C. Hydrogen production in an upflow anaerobic packed bed reactor used to treat cheese whey. Int. J. Hydrogen Energy 2013, 38, 54–62. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Kushwaha, D.; Gupta, V.K.; Manikanta, A.; Ramteke, P.W.; Mishra, P.K. Efficient dark fermentative hydrogen production from enzyme hydrolyzed rice straw by Clostridium pasteurianum (MTCC116). Bioresour. Technol. 2017, 238, 552–558. [Google Scholar] [CrossRef]
- Youcai, Z.; Tao, Z. Pretreatment and aged refuse dosage on biohydrogen production from food waste. In Biohydrogen Production and Hybrid Process Development: Energy and Resource Recovery from Food Waste; Elsiever: Amsterdam, The Netherlands, 2021; pp. 149–238. [Google Scholar] [CrossRef]
- Dahiya, S.; Mohan, S.V. Selective control of volatile fatty acids production from food waste by regulating biosystem buffering: A comprehensive study. Chem. Eng. J. 2019, 357, 787–801. [Google Scholar] [CrossRef]
- Lovato, G.; Albanez, R.; Stracieri, L.; Ruggero, L.S.; Ratusznei, S.M.; Rodrigues, J.A.D. Hydrogen production by co-digesting cheese whey and glycerin in an AnSBBR: Temperature effect. Biochem. Eng. J. 2018, 138, 81–90. [Google Scholar] [CrossRef]
- Barba, F.J. An integrated approach for the valorization of cheese whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Tarelho, L.A.d.C.; Capela, M.I. Parametric optimization of the dark fermentation process for enhanced biohydrogen production from the organic fraction of municipal solid waste using Taguchi method. Int. J. Hydrogen Energy 2021, 46, 21372–21382. [Google Scholar] [CrossRef]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef]
- Moussa, R.N.; Moussa, N.; Dionisi, D. Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance. Processes 2022, 10, 156. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Batch dark fermentation of powdered wheat starch to hydrogen gas: Effects of the initial substrate and biomass concentrations. Int. J. Hydrogen Energy 2008, 33, 6109–6115. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Y.; Wang, J. Recent advance in inhibition of dark fermentative hydrogen production. Int. J. Hydrogen Energy 2021, 46, 5053–5073. [Google Scholar] [CrossRef]
- Mıynat, M.E.; Argun, H. Prevention of substrate and product inhibitions by using a dilution strategy during dark fermentative hydrogen production from molasses. Int. J. Hydrogen Energy 2020, 45, 34695–34706. [Google Scholar] [CrossRef]
- Kargi, F.; Ozmihci, S. Utilization of cheese whey powder (CWP) for ethanol fermentations: Effects of operating parameters. Enzym. Microb. Technol. 2006, 38, 711–718. [Google Scholar] [CrossRef]
- Karakaya, G.; Özmıhçı, S. Statistical optimization of biohydrogen fermentation from a perspective of butyrate production. Int. J. Hydrogen Energy 2024, 67, 807–817. [Google Scholar] [CrossRef]
- Casler, M.D.; Vermerris, W.; Dixon, R.A. Replication concepts for bioenergy research experiments. Bioenergy Res. 2015, 8, 1–16. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Kargi, F.; Eren, N.S.; Ozmihci, S. Effect of initial bacteria concentration on hydrogen gas production from cheese whey powder solution by thermophilic dark fermentation. Biotechnol. Prog. 2012, 28, 931–936. [Google Scholar] [CrossRef]
- Kara, M.; Odabsi, M.; Dumanoglu, Y.; Falay, E.O.; Tuygun, G.T.; Altiok, H.; Bayram, A.; Tolunay, D.; Elbir, T. Investigation of atmospheric pollution by biomonitoring of major and trace elements in an industrial region. J. Environ. Sci. Eng. Technol. 2019, 7, 16–25. [Google Scholar] [CrossRef]
- Kriswantoro, J.A.; Chu, C.Y. Biohydrogen production kinetics from cacao pod husk hydrolysate in dark fermentations: Effect of pretreatment, substrate concentration, and inoculum. J. Clean. Prod. 2024, 434, 140407. [Google Scholar] [CrossRef]
- Gallardo, R.; Alves, M.; Rodrigues, L.R. Modulation of crude glycerol fermentation by Clostridium pasteurianum DSM 525 towards the production of butanol. Biomass Bioenergy 2014, 71, 134–143. [Google Scholar] [CrossRef]
- Gallazzi, A.; Branska, B.; Marinelli, F.; Patakova, P. Continuous production of n-butanol by Clostridium pasteurianum DSM 525 using suspended and surface-immobilized cells. J. Biotechnol. 2015, 216, 29–35. [Google Scholar] [CrossRef]
- Sarchami, T.; Johnson, E.; Rehmann, L. Optimization of fermentation condition favoring butanol production from glycerol by Clostridium pasteurianum DSM 525. Bioresour. Technol. 2016, 208, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.C.; Bertolucci, J.; da Silva, T.M.; dos Passos, V.F.; de Gouvêa, P.F.; Dinamarco, T.M.; Reginatto, V. Butyric acid as a sole product from xylose fermentation by a non-solventogenic Clostridium beijerinckii strain under controlled pH and nutritional conditions. Bioresour. Technol. Rep. 2020, 10, 100426. [Google Scholar] [CrossRef]
- Kremling, A.; Bettenbrock, K.; Laube, B.; Jahreis, K.; Lengeler, J.W.; Gilles, E.D. The Organization of Metabolic Reaction Networks: III. Application for Diauxic Growth on Glucose and Lactose. Metab. Eng. 2001, 3, 362–379. [Google Scholar] [CrossRef]
- Yu, Y.; Tangney, M.; Aass, H.C.; Mitchell, W.J. Analysis of the Mechanism and Regulation of Lactose Transport and Metabolism in Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 2007, 73, 1842–1850. [Google Scholar]
- Qureshi, N.; Liu, S.; Saha, B.C. Butyric Acid Production by Fermentation: Employing Potential of the Novel Clostridium tyrobutyricum Strain NRRL 67062. Fermentation 2022, 8, 491. [Google Scholar] [CrossRef]
- Dessì, P.; Asunis, F.; Ravishankar, H.; Cocco, F.G.; De Gioannis, G.; Muntoni, A.; Lens, P.N.L. Fermentative hydrogen production from cheese whey with in-line, concentration gradient-driven butyric acid extraction. Int. J. Hydrogen Energy 2020, 45, 24453–24466. [Google Scholar] [CrossRef]
- Kelbert, M.; Machado, T.O.; Araújo PH, H.; Sayer, C.; de Oliveira, D.; Maziero, P.; Simons, K.E.; Carciofi, B.A.M. Perspectives on biotechnological production of butyric acid from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2024, 202, 114717. [Google Scholar] [CrossRef]
- Suazo, A.; Tapia, F.; Aroca, G.; Quintero, J. Techno-Economic and Life Cycle Assessment of a Small-Scale Integrated Biorefinery for Butyric-Acid Production in Chile. Fermentation 2024, 10, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).