Effects of Fluidized Bed Coating with Carboxymethyl Cellulose and Pectin on the Physicochemical Properties of Fermented Black Bean Dregs
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Fermentation of Black Bean Dregs (BBD)
2.2. Effect of CMC and Pectin Coating Concentrations on the Flowability of FBBD
2.3. Effect of Coating Duration on the Physicochemical Properties of FBBD
2.4. Measurement of Particle Size Distribution, Flowability, and Cohesion of Coated FBBD Powder
2.5. Determination of Wettability of Coated FBBD Powder
2.6. Determination of Moisture Content, Water Activity, Appearance, and Color of Coated FBBD Powder
2.7. Analysis of Solubility, Swelling Capacity, and Water-Holding Capacity of Coated FBBD Powder
- Solubility (%) = (m2/m4) × 100;
- Swelling capacity (g/g) = (m3 − m1)/m4;
- Water-holding capacity (g/g) = (m3 − m1)/m0.
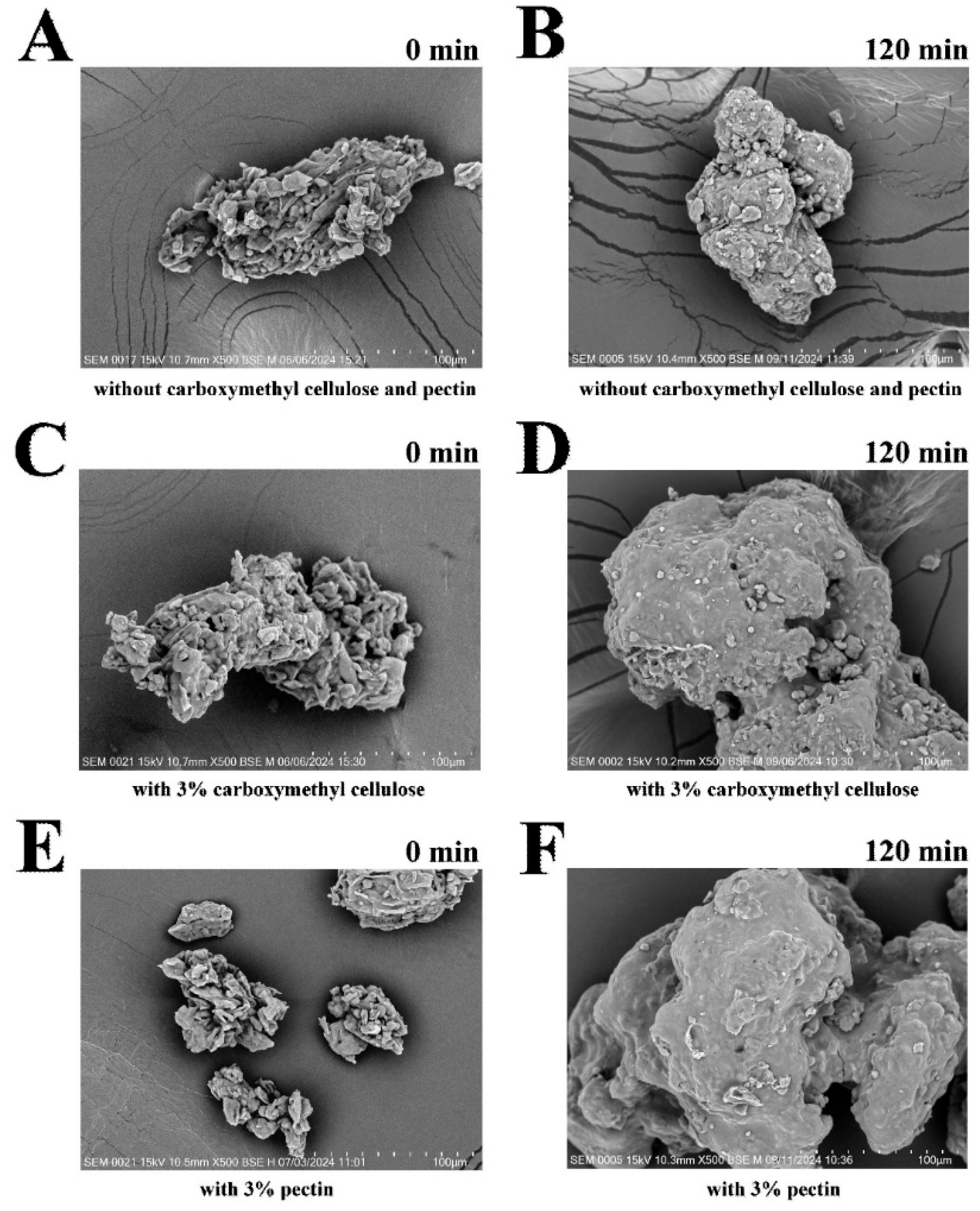
2.8. Microstructural Analysis of Coated FBBD Powder
2.9. Statistics
3. Results and Discussion
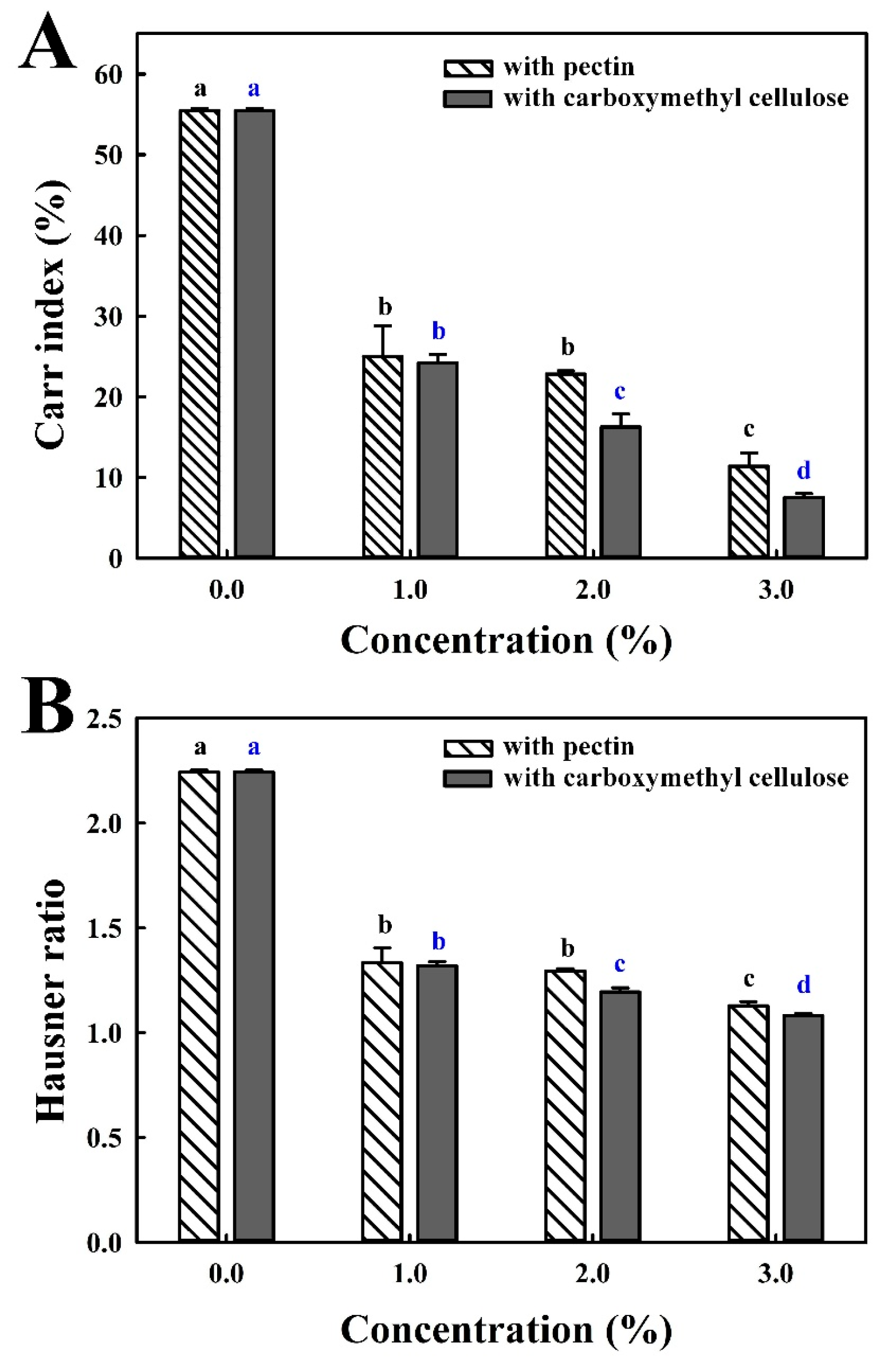
3.1. Effect of CMC and Pectin Coating Exposure on the Flowability of Coated FBBD Powder
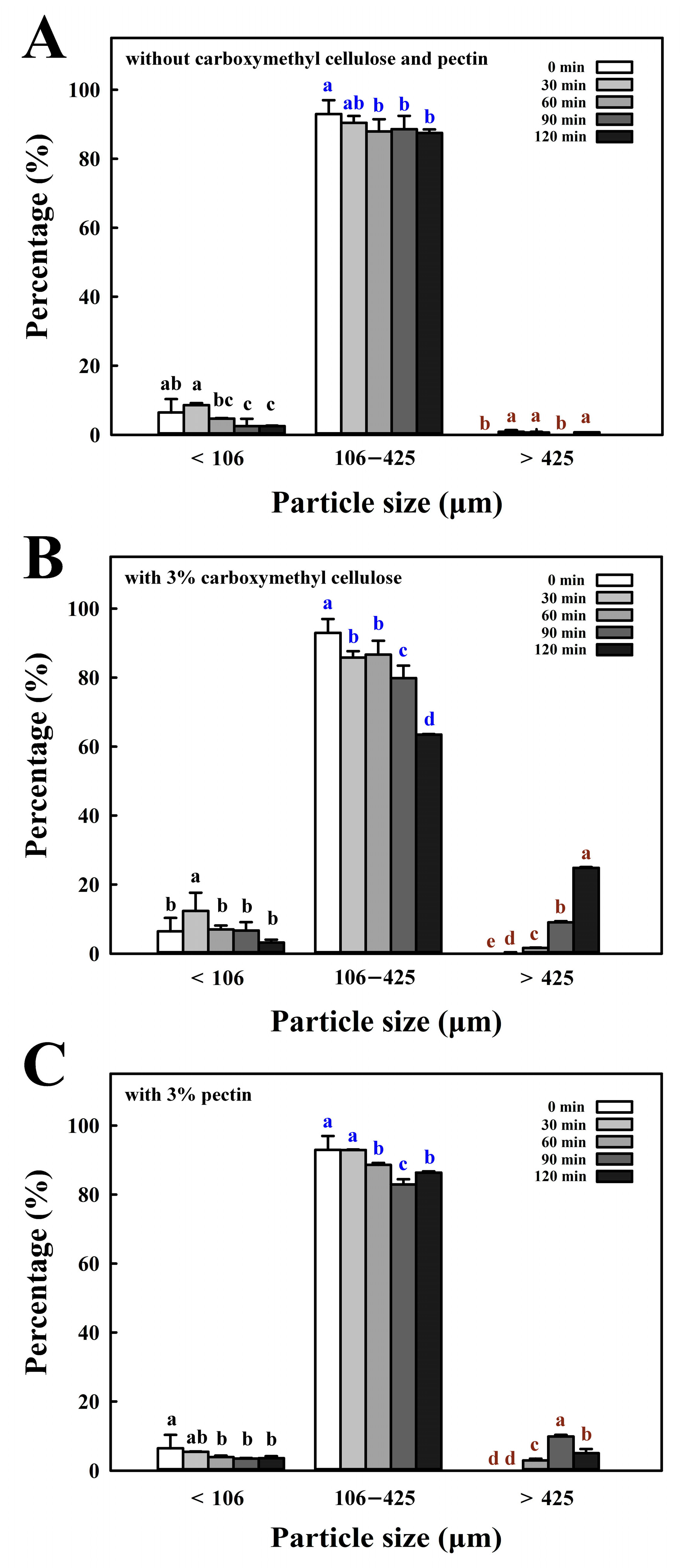
3.2. Effect of Coating with Pectin or Carboxymethyl Cellulose on Particle Size, Microstructure, and Flowability of Coated FBBD
3.3. Effect of Coating Duration on Color, Appearance, Moisture Content, and Water Activity of Coated FBBD Powder
3.4. Effect of Coating Duration on the Wettability, Solubility, and Water-Holding Capacity of Coated FBBD Powder
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.M.; Mat, K.; Ishigaki, G.; Akashi, R. A review of okara (soybean curd residue) utilization as animal feed: Nutritive value and animal performance aspects. Anim. Sci. J. 2021, 92, e13594. [Google Scholar] [PubMed]
- Wang, S.; Swallah, M.S.; Li, J.; Yu, H. Value-added processing and function of okara. In Phytochemicals in Soybeans; Li, Y., Qi, B., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 419–436. [Google Scholar]
- Li, P.; Hu, Y.; Li, Y.; Bao, Y.; Wang, X.; Piao, C. Co-production of nattokinase and α-amylase from Bacillus natto fermentation using okara. J. Food Process. Preserv. 2022, 46, e17130. [Google Scholar]
- Razavizadeh, S.; Alencikiene, G.; Salaseviciene, A.; Vaiciulyte-Funk, L.; Ertbjerg, P.; Zabulione, A. Impact of fermentation of okara on physicochemical, techno-functional, and sensory properties of meat analogues. Eur. Food Res. Technol. 2021, 247, 2379–2389. [Google Scholar]
- Gupta, S.; Lee, J.J.; Chen, W.N. Analysis of improved nutritional composition of potential functional food (okara) after probiotic solid-state fermentation. J. Agric. Food Chem. 2018, 66, 5373–5381. [Google Scholar]
- Vong, W.C.; Lim, X.Y.; Liu, S.Q. Biotransformation with cellulase, hemicellulase and Yarrowia lipolytica boosts health benefits of okara. Appl. Microbiol. Biotechnol. 2017, 101, 7129–7140. [Google Scholar]
- Vong, W.C.; Hua, X.Y.; Liu, S.Q. Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT-Food Sci. Technol. 2018, 90, 316–322. [Google Scholar]
- Huang, C.; Kuo, M.I.; Chen, B.Y.; Lu, C.P.; Yeh, C.C.; Jao, C.H.; Hsieh, J.F. Effect of radio-frequency drying on the physicochemical properties and isoflavone contents of fermented black bean dregs. Processes 2024, 12, 1294. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, G. Fluid bed technology: Overview and parameters for process selection. Int. J. Pharm. Sci. Drug Res. 2010, 2, 236–246. [Google Scholar]
- Lee, D.; Yoo, B. Cellulose derivatives agglomerated in a fluidized bed: Physical, rheological, and structural properties. Int. J. Biol. Macromol. 2021, 181, 232–240. [Google Scholar]
- Yoon, S.J.; Bak, J.; Yoo, B. Rheological and tribological properties of native potato starch agglomerated by fluidized bed granulator. Int. J. Biol. Macromol. 2024, 264, 130600. [Google Scholar]
- Guignon, B.; Duquenoy, A.; Dumoulin, E.D. Fluid bed encapsulation of particles: Principles and practice. Dry. Technol. 2002, 20, 419–447. [Google Scholar] [CrossRef]
- Palamanit, A.; Soponronnarit, S.; Prachayawarakorn, S.; Tungtrakul, P. Effects of inlet air temperature and spray rate of coating solution on quality attributes of turmeric extract coated rice using top-spray fluidized bed coating technique. J. Food Eng. 2013, 114, 132–138. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Das Purkayastha, M.; Mukherjee, A. Recent progress in pectin extraction and their applications in developing films and coatings for sustainable food packaging: A review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef]
- Toǧrul, H.; Arslan, N. Carboxymethyl cellulose from sugar beet pulp cellulose as a hydrophilic polymer in coating of mandarin. J. Food Eng. 2004, 62, 271–279. [Google Scholar] [CrossRef]
- Moraga, S.V.; Villa, M.P.; Bertín, D.E.; Cotabarren, I.M.; Piña, J.; Pedernera, M.; Bucalá, V. Fluidized-bed melt granulation: The effect of operating variables on process performance and granule properties. Powder Technol. 2015, 286, 654–667. [Google Scholar] [CrossRef]
- Geldart, D.; Abdullah, E.C.; Hassanpour, A.; Nwoke, L.C.; Wouters, I.J.C.P. Characterization of powder flowability using measurement of angle of repose. China Particuology 2006, 4, 104–107. [Google Scholar] [CrossRef]
- Ji, J.; Fitzpatrick, J.; Cronin, K.; Maguire, P.; Zhang, H.; Miao, S. Rehydration behaviours of high protein dairy powders: The influence of agglomeration on wettability, dispersibility and solubility. Food Hydrocoll. 2016, 58, 194–203. [Google Scholar] [CrossRef]
- Pompe, R.; Briesen, H.; Datta, A.K. Understanding puffing in a domestic microwave oven. J. Food Process Eng. 2020, 43, e13429. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Yuan, Y.; Peng, X.; Zhang, Q.; Zhang, S.; Xie, C.; Zhang, X.; Yan, S.; Xu, J.; et al. Effect of spray-drying and freeze-drying on the properties of soybean hydrolysates. J. Chem. 2020, 2020, 9201457. [Google Scholar] [CrossRef]
- Dias, I.; Laranjo, M.; Potes, M.E.; Agulheiro-Santos, A.C.; Ricardo-Rodrigues, S.; Fraqueza, M.J.; Oliveira, M.; Elias, M. Staphylococcus spp. and Lactobacillus sakei starters with high level of inoculation and an extended fermentation step improve safety of fermented sausages. Fermentation 2022, 8, 49. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lin, J.H.; Chang, S.Y. Physicochemical properties of waxy and normal corn starches treated in different anhydrous alcohols with hydrochloric acid. Food Hydrocoll. 2006, 20, 332–339. [Google Scholar]
- Jeong, G.Y.; Bak, J.H.; Yoo, B. Physical and rheological properties of xanthan gum agglomerated in fluidized bed: Effect of HPMC as a binder. Int. J. Biol. Macromol. 2019, 121, 424–428. [Google Scholar] [PubMed]
- Lee, D.; Yoo, B. Agglomerate growth of xanthan gum powder during fluidized-bed agglomeration process. Polymers 2022, 14, 4018. [Google Scholar] [CrossRef]
- Sakai, E.; Masuda, K.; Kakinuma, Y.; Aikawa, Y. Effects of shape and packing density of powder particles on the fluidity of cement pastes with limestone powder. J. Adv. Concr. Technol. 2009, 7, 347–354. [Google Scholar]
- Ferreira, L.S.; Chaves, M.A.; Dacanal, G.C.; Pinho, S.C. Wet agglomeration by high shear of binary mixtures of curcumin-loaded lyophilized liposomes and cornstarch: Powder characterization and incorporation in cakes. Food Biosci. 2018, 25, 74–82. [Google Scholar]
- Palmer, D.; Levina, M.; Nokhodchi, A.; Douroumis, D.; Farrell, T.; Rajabi-Siahboomi, A. The influence of sodium carboxymethylcellulose on drug release from polyethylene oxide extended release matrices. AAPS PharmSciTech 2011, 12, 862–871. [Google Scholar]
- Singh, J.; Kaur, K.; Kumar, P. Optimizing microencapsulation of α-tocopherol with pectin and sodium alginate. J. Food Sci. Technol. 2018, 55, 3625–3631. [Google Scholar]
- Bozkurt, S.; Altay, Ö.; Alemdar, F.; Türker, M.; Koç, M.; Kaymak-Ertekin, F. The effect of different materials on the coating of baker’s yeast (Saccharomyces cerevisiae) with the fluidized bed method. J. Coat. Technol. Res. 2023, 20, 1661–1676. [Google Scholar]
- Bhavya, S.N.; Prakash, J. Nutritional and sensory quality of buns enriched with soy fiber (Okara). J. Eng. Process. Manag. 2018, 10, 23–31. [Google Scholar]
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Kong, F.; Chang, S.K. Changes in protein characteristics during soybean storage under adverse conditions as related to tofu making. J. Agric. Food Chem. 2013, 61, 387–393. [Google Scholar] [PubMed]
- López-Malo, A.; Alzamora, S.M. Water activity and microorganism control: Past and future. In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; Gutiérrez-López, G., Alamilla-Beltrán, L., del Pilar Buera, M., Welti-Chanes, J., Parada-Arias, E., Barbosa-Cánovas, G., Eds.; Springer Publishing: New York, NY, USA, 2015; pp. 245–262. [Google Scholar]
- Peleg, M. A new look at models of the combined effect of temperature, pH, water activity, or other factors on microbial growth rate. Food Eng. Rev. 2022, 14, 31–44. [Google Scholar]
- Cardona, L.M.; Cortés-Rodríguez, M.; Galeano, F.J.C. Optimization of fluidized bed agglomeration process of a pineapple powder mixture using a binder solution of ginger extract and vitamin C. LWT-Food Sci. Technol. 2022, 171, 114075. [Google Scholar]
- Bhattachar, S.N.; Wesley, J.A.; Seadeek, C. Evaluation of the chemiluminescent nitrogen detector for solubility determinations to support drug discovery. J. Pharm. Biomed. Anal. 2006, 41, 152–157. [Google Scholar]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar]


| Duration of Coating (min) | L* | a* | b* | ΔE | |
|---|---|---|---|---|---|
| Without CMC and pectin | 0 | 45.5 ± 0.1 a | 7.6 ± 0.1 a | 17.2 ± 0.1 a | 0 a |
| 60 | 39.9 ± 0.3 b | 8.0 ± 0.1 b | 15.8 ± 0.1 b | 5.7 ± 0.35 b | |
| 120 | 41.1 ± 0.2 b | 7.9 ± 0.1 b | 15.9 ± 0.1 b | 4.5 ± 0.30 c | |
| With 3% CMC | 0 | 45.5 ± 0.1 a | 7.6 ± 0.1 a | 17.2 ± 0.1 a | 0 a |
| 60 | 34.7 ± 0.4 b | 8.7 ± 0.1 b | 15.7 ± 0.1 b | 10.9 ± 0.4 b | |
| 120 | 32.9 ± 0.2 b | 8.9 ± 0.1 b | 15.3 ± 0.1 c | 12.7 ± 0.2 c | |
| With 3% pectin | 0 | 45.5 ± 0.1 a | 7.6 ± 0.1 a | 17.2 ± 0.1 a | 0 a |
| 60 | 33.9 ± 0.2 b | 9.2 ± 0.1 b | 16.3 ± 0.1 b | 11.6 ± 0.2 b | |
| 120 | 32.6 ± 0.2 c | 9.2 ± 0.1 b | 16.1 ± 0.1 b | 13.0 ± 0.1 c |
| Duration of Coating (min) | Water Content (%) | Water Activity | |
|---|---|---|---|
| Without CMC and pectin | 0 | 5.0 ± 0.1 a | 0.39 ± 0.01 a |
| 60 | 4.6 ± 0.1 b | 0.33 ± 0.01 b | |
| 120 | 4.6 ± 0.1 b | 0.32 ± 0.01 b | |
| With 3% CMC | 0 | 5.0 ± 0.1 a | 0.39 ± 0.01 a |
| 60 | 5.4 ± 0.1 b | 0.37 ± 0.01 b | |
| 120 | 6.6 ± 0.1 c | 0.46 ± 0.01 c | |
| With 3% pectin | 0 | 5.0 ± 0.1 a | 0.39 ± 0.01 a |
| 60 | 5.9 ± 0.1 b | 0.40 ± 0.01 a | |
| 120 | 6.5 ± 0.1 c | 0.42 ± 0.01 b |
| Duration of Coating (min) | Solubility (%) | Water-Holding Capacity (g/g) | |
|---|---|---|---|
| Without CMC and pectin | 0 | 29.4 ± 0.3 a | 4.9 ± 0.2 a |
| 60 | 33.1 ± 0.4 b | 4.8 ± 0.3 a | |
| 120 | 34.8 ± 0.1 c | 4.4 ± 0.1 a | |
| With 3% CMC | 0 | 29.4 ± 0.3 a | 4.9 ± 0.3 a |
| 60 | 34.2 ± 1.9 b | 4.2 ± 0.1 b | |
| 120 | 35.5 ± 0.9 c | 4.3 ± 0.1 b | |
| With 3% pectin | 0 | 29.4 ± 0.3 a | 4.9 ± 0.3 a |
| 60 | 34.8 ± 0.7 b | 4.1 ± 0.1 b | |
| 120 | 31.6 ± 0.8 c | 4.0 ± 0.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Kuo, M.-I.; Lu, C.-P.; Chen, B.-Y.; Yeh, C.-C.; Chang, C.-I.; Jao, C.-H.; Lai, Y.-C.; Hsieh, J.-F. Effects of Fluidized Bed Coating with Carboxymethyl Cellulose and Pectin on the Physicochemical Properties of Fermented Black Bean Dregs. Processes 2025, 13, 1066. https://doi.org/10.3390/pr13041066
Huang C, Kuo M-I, Lu C-P, Chen B-Y, Yeh C-C, Chang C-I, Jao C-H, Lai Y-C, Hsieh J-F. Effects of Fluidized Bed Coating with Carboxymethyl Cellulose and Pectin on the Physicochemical Properties of Fermented Black Bean Dregs. Processes. 2025; 13(4):1066. https://doi.org/10.3390/pr13041066
Chicago/Turabian StyleHuang, Cheng, Meng-I Kuo, Chun-Ping Lu, Bang-Yuan Chen, Chien-Cheng Yeh, Chia-I Chang, Cheng-Hsun Jao, Yi-Chung Lai, and Jung-Feng Hsieh. 2025. "Effects of Fluidized Bed Coating with Carboxymethyl Cellulose and Pectin on the Physicochemical Properties of Fermented Black Bean Dregs" Processes 13, no. 4: 1066. https://doi.org/10.3390/pr13041066
APA StyleHuang, C., Kuo, M.-I., Lu, C.-P., Chen, B.-Y., Yeh, C.-C., Chang, C.-I., Jao, C.-H., Lai, Y.-C., & Hsieh, J.-F. (2025). Effects of Fluidized Bed Coating with Carboxymethyl Cellulose and Pectin on the Physicochemical Properties of Fermented Black Bean Dregs. Processes, 13(4), 1066. https://doi.org/10.3390/pr13041066









