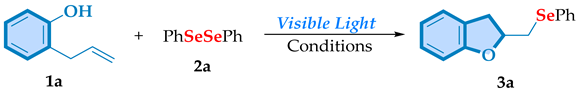
Abstract
This study introduces a visible light-mediated synthesis of 2,3-chalcogenil-dihydrobenzofuran through the oxyselenocyclization of 2-allylphenols in the presence of chalcogenides. Emphasizing sustainability, this method is notably enhanced by proceeding under mild conditions, facilitated by a straightforward I2/SnCl2 as a promoter and blue LED irradiation to activate the process. A variety of functional groups were effectively tolerated under our developed approach, leading to the desired products with yields ranging from good to excellent, demonstrating in this way the versatility of the method. In addition, the synthesized compounds were characterized using 1H and 13C NMR techniques.
1. Introduction
The 2,3-dihydrobenzofuran (2,3-DHB) core is a rigid heterocyclic structure formed by a benzene ring fused with a saturated furan ring at the C-2 and C-3 positions. This chemical structure provides stability and versatility, making it a key scaffold in many bioactive molecules and natural products [1]. Compounds containing the 2,3-DHB core are commonly isolated from plants and fungi, often classified as alkaloids, neolignans, isoflavonoids, and lignans [2,3]. Natural products containing the 2,3-DHB core structure exhibit diverse biological activities, including anti-HIV, antimalarial, anticancer, antinociceptive, anti-inflammatory, antifungal, and antibacterial properties [4]. Notable examples include morphine and codeine (two of the most common opioids), see Refs. [5,6], (+)-conocarpan (secondary metabolite from plants of the Piper species with antifungal activities) [4,7], and (+)-Decursivine (alkaloid isolated from the leaves and stems of Rhaphidophora decursiva Schott with antimalarial activities) [8] (Figure 1, top).
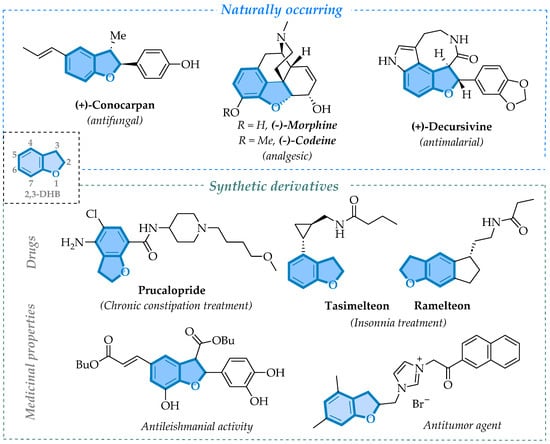
Figure 1.
Bioactive compounds incorporating 2,3-DHB scaffolds.
In addition to the science of natural products, the 2,3-dihydrobenzofuran (2,3-DHB) scaffold has emerged as a valuable framework for creating synthetic derivatives with therapeutic potential and for marketed drugs (Figure 1, bottom) [9,10,11,12,13]. Due to the significant potential of these structures and their remarkable functionalization capability, the combination with organochalcogens, particularly selenium and sulfur-containing compounds, has shown promise in synthetic chemistry and the treatment of neurodegenerative diseases. These derivatives exhibit potent neuroprotective properties, including the inhibition of monoamine oxidase type B (MAO-B), an enzyme associated with oxidative stress and neuronal degeneration in disorders like Parkinson’s and Alzheimer’s diseases [14,15].
This prominent profile led scientists to search for strategies to synthesize 2,3-dihydrobenzofurans functionalized with organochalcogens giving us a variety of interesting approaches. These include selenium-mediated cyclizations with reagents like PhSeCl or diselenides under various oxidative circumstances (e.g., Oxone®, TBHP, or I2O5), as well as ecologically friendly techniques like electrochemical approaches and photoredox catalysis. Many of these techniques have high yields, regioselectivity, and scalability, and they represent significant advances in green chemistry (Scheme 1a) [16,17,18,19,20,21,22,23].
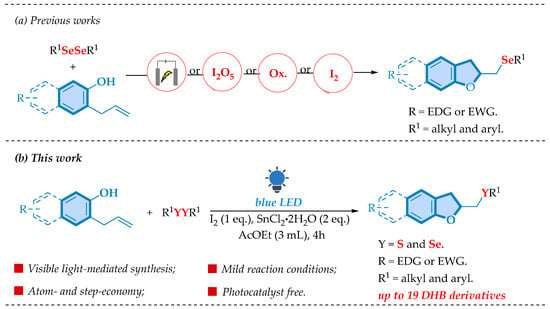
Scheme 1.
Overview.
Despite these advances, contemporary procedures frequently require extra reagents such as oxidants, specialized catalysts, or complex setups, while bringing some limitations of reactional scope. To address these challenges, we propose a visible-light-driven methodology that eliminates the need for photoredox catalysts commonly used [24]. Ethyl acetate is a solvent widely recognized for its low toxicity, biodegradability, and suitability for pharmaceutical applications, recently gaining attention as a green alternative to traditional solvents [25]. Taking advantage of these properties, we present an environmentally friendly and efficient approach to synthesizing chalcogen-containing 2,3-DHB derivatives. In addition to simplifying the reaction conditions, this method aligns with the principles of sustainable chemistry, making it a promising alternative to current strategies (Scheme 1b).
2. Materials and Methods
For the isolation and purification of the compounds using column chromatography, a glass column was used; silica gel was used as the stationary phase with a 0.063–0.2 mesh by Merck (Darmstadt, Germany); and a suitable solvent or solvent mixture was used as the eluent. The fractions and compounds obtained were analyzed by thin-layer chromatography (TLC), using aluminum plates coated with silica gel 60 GF254 provided by Merck (Darmstadt, Germany), 0.25 mm thick, and with particles between 5 and 40 μm in diameter. The substances separated on the chromatographic plates were visualized using several development methods: in an iodine chamber, in an ultraviolet light chamber, or with a vanillin reagent followed by heating at 110 °C. Melting points were obtained on a Fisatom 430D (Sao Paulo, Brazil) apparatus and were uncorrected. All solvents and reagents used in the synthesis, purification, and characterization were purchased from commercial sources Sigma-Aldrich, Merck (Darmstadt, Germany), and Synth (Sao Paulo, Brazil), and used without prior purification. The 13C {1H} NMR spectra were obtained on a Bruker Advance NEO spectrometer (Bruker BioSpin, Rheinstetten, Germany), operating at 500 MHz, employing a direct broadband probe at 125 MHz and 75 MHz.
2.1. General Procedure
In a glass tube, 0.25 mmol of 1a, 1.0 equivalent of the appropriate diaryl dichalcogenide 2a–j and 4a–f, 2.0 equivalents of SnCl2·2H2O, and 1 equivalent of I2 in 3.0 mL of ethyl acetate (EtOAc) were added. The resulting mixture was irradiated with a 50 W blue LED in a room atmosphere for 4 h. The progress of the reaction was monitored by thin-layer chromatography (TLC) and, after verifying the total consumption of the starting material, the mixture was extracted with EtOAc (3 × 25 mL) and water. The obtained product was purified by column chromatography with silica gel, using a hexane/ethyl acetate gradient (9:1) as the eluent. All compounds were fully characterized by 1H and 13C NMR spectroscopy (Supplementary Materials).
2.2. Characterization
2-((Phenylselanyl)methyl)-2,3-dihydrobenzofuran (3a) [15] 0.072 g. Yield: 95%, white solid, mp 58–60 °C (literature 60–61 °C). 1H NMR (500 MHz, CDCl3) δ (ppm) = 7.56–7.52 (m, 2H), 7.39–7.37 (m, 3H), 7.12 (d, J = 7.4, 1H), 7.08 (t, J = 7.9, 1H), 6.82 (t, J = 7.3, 1H), 6.73 (d, J = 7.9 Hz, 1H), 4.92 (dddd, J = 9.1, 7.7, 6.8 and 5.5 Hz, 1H), 3.36–3.28 (m, 2H), 3.08 (dd, J = 12.5 and 7.7 Hz, 1H), 3.00 (dd, J = 15.7 and 6.8 Hz, 1H). 13C-APT NMR (CDCl3, 125 MHz) δ (ppm) = 158.9, 133.1, 129.3, 129.2, 128.1, 127.3, 126.2, 125.7, 120.6, 109.5, 81.9, 35.7, 32.5.
2-(((4-Chlorophenyl)selanyl)methyl)-2,3-dihydrobenzofuran (3b) [15] 0.077 g. Yield: 95%, yellow oil. 1H NMR (500 MHz, CDCl3) δ (ppm) = 7.46 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 8.5 Hz, 2H), 7.12 (d, J = 7.3 Hz, 2H), 7.09 (t, J = 7.9 Hz, 1H), 6.83 (t, J = 7.3 Hz, 1H), 6.73 (d, J = 7.9 Hz, 1H), 4.94–4.88 (m, 1H), 3.34 (dd, J = 15.7 and 9.0 Hz, 1H), 3.27 (dd, J = 12.7 and 5.8 Hz, 1H), 3.08 (dd, J = 12.7 Hz and 7.4 Hz, 1H), 3.01 (dd, J = 15.7 and 6.8 Hz, 1H). 13C-APT NMR (CDCl3, 125 MHz) δ (ppm) = 159.1, 134.4, 133.6, 129.3, 128.1, 127.6, 126.0, 125.9, 120.6, 109.5, 81.7, 35.5, 33.1.
2-(((4-Fluorophenyl)selanyl)methyl)-2,3-dihydrobenzofuran (3c) [15] 0.075 g. Yield: 96%, white solid, mp 57–60 °C (literature 61–62 °C). 1H NMR (500 MHz, CDCl3) δ (ppm) = 7.54 (dd, J = 8.8 and 5.4 Hz, 2H), 7.13 (d, J = 7.4 Hz, 1H), 7.10 (t, J = 7.8 Hz, 1H), 6.97 (t, J = 8.8 Hz, 2H), 6.83 (t, J = 7.4 Hz, 1H), 6.73 (d, J = 8.8 Hz, 1H), 4.94–4.88 (m, 1H), 3.35 (dd, J = 15.8 and 9.1 Hz, 1H), 3.26 (dd, J = 12.6 and 5.6 Hz, 1H), 3.07 (dd, J = 12.6 and 7.4 Hz; 1H); 3.01 (dd, J = 15.8 and 5.6 Hz, 1H). 13C-APT NMR (CDCl3, 75 MHz) δ (ppm) = 162.5 (d, JC-F = 246.0 Hz), 159.1, 135.8 (d, JC-F = 7.7 Hz), 128.1, 126.1, 125.0, 123.8 (d, JC-F = 3.4 Hz), 120.6, 116.3 (d, JC-F = 21.3 Hz), 109.5, 81.8, 35.5, 33.6.
2-(((4-Methoxyphenyl)selanyl)methyl)-2,3-dihydrobenzofuran (3e) [15] 0.069 g. Yield: 85%, orange oil. 1H NMR (500 MHz, CDCl3) δ (ppm) = 7.52 (d, J = 8.9 Hz, 2H), 7.13 (d, J = 7.4 Hz, 1H); 7.09 (t, J = 8.0 Hz, 1H), 6.84–6.81 (m, 3H), 6.74 (d, J = 8.0 Hz, 1H), 4.89 (dddd, J = 9.0, 7.8, 7.0, 5.5 Hz, 1H), 3.80 (s, 3H), 3.33 (dd, J = 15.7 and 9.0 Hz, 1H), 3.23 (dd, J = 12.4 and 5.5 Hz, 1H), 3.02–2.98 (m, 2H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 159.6, 159.2, 136.0, 128.0, 126.2, 125.0, 120.5, 119.0, 114.9, 109.5, 82.0, 77.3, 77.0, 76.7, 55.3, 35.5, 33.7.
2-((p-Tolylselanyl)methyl)-2,3-dihydrobenzofuran (3f) [15] 0.055 g. Yield: 71%, white solid, mp 45–47 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) = 7.45 (d, J = 8.1 Hz, 2H), 7.13 (dd, J = 7.4 and 0.8 Hz, 1H), 7.11–7.07 (m, 3H), 6.82 (td, J = 7.4 and 0.9 Hz, 1H), 6.74 (d, J = 8.0 Hz; 1H); 4.90 (dddd, J = 9.0, 7.8, 6.8 and 5.5 Hz, 1H), 3.34 (dd, J = 15.7 and 9.0 Hz, 1H), 3.28 (dd, J = 12.4 and 5.4 Hz, 1H), 3.06–2.98 (m, 2H), 2.32 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ (ppm) = 159.2, 137.5, 133.6, 130.0, 128.0, 126.2, 125.4, 125.0, 120.5, 109.5, 81,9, 35.5, 33.0, 21.1.
2-((Naphthalen-1-ylselanyl)methyl)-2,3-dihydrobenzofuran (3g) [15] 0.078 g. Yield: 91%, white solid, mp 82–85 °C. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 8.43 (d, J = 8.3 Hz, 1H), 7.87 (dd, J = 7.1 and 0.9 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.82 (d, J = 8.3 Hz, 1H), 7.57 (ddd, J = 8.3, 6.8 and 1.3 Hz; 1H), 7.54–7.49 (m,1H), 7.37 (t, J = 7.6 Hz; 1H), 7.11 (d, J = 7.3 Hz; 1H), 7.08 (t, J = 7.4 Hz; 1H), 6.72 (d, J = 8.0 Hz; 1H), 4.91–4.85 (m, 1H), 3.38–3.30 (m, 2H), 3.10 (dd, J = 12.4 Hz and 7.8 Hz; 1H), 3.03 (dd, J = 15.8 Hz and 6.8 Hz, 1H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 159.1, 134.4, 134.0, 133.3, 128.9, 128.7, 128.4, 128.0, 127.7, 126.8, 126.3, 126.2, 125.8, 125.0, 120.5, 109.5, 81.9, 35.5, 32.9.
2-((Mesitylselanyl)methyl)-2,3-dihydrobenzofuran (3h) [15] 0.055 g. Yield: 65%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.05 (d, J = 7.4 Hz, 1H), 7.01 (t, J = 7.8 Hz, 1H), 6.85 (s, 2H), 6.74 (t, J = 7.4 Hz, 1H), 6.65 (d, J = 7.9 Hz, 1H), 4.74–4.68 (m, 1H), 3.25 (dd, J = 15.7, 9.0 Hz, 1H), 2.98 (dd, J = 12.1, 5.5 Hz, 1H), 2.92 (dd, J = 15.8, 6.8 Hz, 1H), 2.75 (dd, J = 12.1, 8.0 Hz, 1H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 159.2, 143.1, 138.4, 128.6, 128.0, 127.0, 126.2, 125.0, 120.4, 109.4, 82.3, 32.4, 35.6, 24.4, 20.9.
2-((Thiophen-2-ylselanyl)methyl)-2,3-dihydrobenzofuran (3i) [15] 0.062 g. Yield: 83%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.31 (dd, J = 5.3, 1.2 Hz, 1H), 7.17 (dd, J = 3.5, 1.3 Hz, 1H), 7.07 (d, J = 7.4 Hz, 1H), 7.02 (t, J = 8.0 Hz, 1H), 6.90 (dd, J = 5.3, 3.5 Hz, 1H), 6.76 (td, J = 7.4, 1.0 Hz, 1H), 6.68 (d, J = 8.0 Hz, 1H), 4.88–4.81 (m, 1H), 3.30 (dd, J = 15.8, 9.0 Hz, 1H), 3.13 (dd, J = 12.5, 5.9 Hz, 1H), 2.96–2.88 (m, 2H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 159.1, 136.1, 131.1, 128.08, 128.06, 126.1, 125.0, 122.7, 120.5, 109.5, 81.5, 36.0, 35.4.
2-((Butylselanyl)methyl)-2,3-dihydrobenzofuran (3j) [23] 0.058 g. Yield: 85%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.08 (d, J = 7.4 Hz, 3H), 7.03 (t, J = 7.7 Hz, 3H), 6.76 (t, J = 7.4 Hz, 2H), 6.69 (d, J = 8.0 Hz, 3H), 4.89 (ddd, J = 9.0, 7.4, 7.2, 5.6 Hz, 2H), 3.30 (dd, J = 15.6, 9.0 Hz, 3H), 2.95 (dd, J = 15.8, 7.2 Hz, 3H), 2.87 (dd, J = 12.6, 5.6 Hz, 2H), 2.73 (dd, J = 12.5, 7.4 Hz, 2H), 2.59 (t, J = 7.5 Hz, 4H), 1.59 (qt, J = 7.5 Hz, 2H), 1.34 (sext, J = 7.5 Hz, 3H), 0.85 (t, J = 7.4 Hz, 7H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 159.2, 128.0, 126.3, 125.0, 120.4, 109.4, 82.7, 35.7, 32.7, 28.6, 24.6, 22.9, 13.6.
2-((Phenylthio)methyl)-2,3-dihydrobenzofuran (5a) [23] 0.032 g. Yield: 53%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.42 (d, J = 7.2 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.22 (t, J = 7.4 Hz, 1H), 7.16 (d, J = 7.3 Hz, 1H), 7.11 (t, J = 7.9 Hz, 1H), 6.85 (t, J = 7.3 Hz, 1H), 6.76 (d, J = 7.9 Hz, 1H) 4.94–4.88 (m, 1H), 3.40–3.33 (m, 2H), 3.12–3.05 (m, 2H). 13C NMR (CDCl3, 75 MHz) δ (ppm) = 159.1, 135.4, 129.9, 129.0, 128.0, 126.5, 126.0, 125.0, 120.6, 109.5, 81.0, 38.9, 34.8.
2-(((4-Chlorophenyl)thio)methyl)-2,3-dihydrobenzofuran (5b) [23] 0.030 g. Yield: 43%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.34 (d, J = 8.6 Hz, 2H), 7.27 (d, J = 8.6 Hz, 2H), 7.16 (d, J = 7.4 Hz, 1H), 7.11 (t, J = 8.0 Hz, 1H), 6.86 (t, J = 7.4 Hz, 1H), 6.77 (d, J = 8.0 Hz, 1H), 4.93–4.87 (m, 1H), 3.38–3.31 (m, 2H), 3.13–3.04 (m, 2H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 159.0, 134.0, 132.7, 131.3, 129.2, 128.2, 125.9, 125.0, 120.7, 109.6, 81.0, 39.3, 34.9.
2-(((4-Fluorophenyl)thio)methyl)-2,3-dihydrobenzofuran (5c) [23] 0.025 g. Yield: 38%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.42 (dd, J = 8.8, 5.2 Hz, 2H), 7.15 (d, J = 7.4 Hz, 1H), 7.10 (t, J = 7.9 Hz, 1H), 7.00 (t, J = 8.8 Hz, 2H), 6.84 (td, J = 7.4, 0.8 Hz, 1H), 6.74 (d, J = 7.9 Hz, 1H), 4.89–4.84 (m, 1H), 3.34 (dd, J = 15.8, 9.1 Hz, 1H), 3.29 (dd, J = 13.5, 5.7 Hz, 1H), 3.09–3.03 (m, 1H). 13C NMR (CDCl3, 100 MHz) δ (ppm) = 162.1 (d, JC-F = 243.0 Hz), 159.07, 133.1 (d, J = 8.0 Hz), 130.3 (d, J = 3.0 Hz), 128.1, 126.0, 125.0, 120.3, 116.2 (d, JC-F = 22.0 Hz), 109.5, 81.0, 40.3, 34.8.
2-((p-Tolylthio)methyl)-2,3-dihydrobenzofuran (5d) [23] 0.046 g. Yield: 70%, colorless oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.32 (d, J = 8.3 Hz, 2H), 7.16–7.08 (m, 4H), 6.83 (t, J = 7.4 Hz, 1H), 6.75 (d, J = 8.0 Hz, 1H), 4.90–4.83 (m, 1H), 3.35–3.30 (m, 2H), 3.07–3.00 (m, 2H), 2.32 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ (ppm) = 159.1, 136.8, 131.5, 130.8, 129.8, 128.0, 126.1, 125.0, 120.6, 109.5, 81.1, 39.6, 34.8, 21.0.
2-((o-Tolylthio)methyl)-2,3-dihydrobenzofuran (5e) [23] 0.058 g. Yield: 89%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.37 (dd, J = 7.6 and 1.7 Hz, 1H), 7.19–7.08 (m, 5H), 6.88–6.82 (m, 1H), 6.78–6.75 (m, 1H), 4.92–4.86 (m, 1H), 3.38–3.30 (m, 2H), 3.11–3.03 (m, 2H), 2.40 (s, 3H). 13C-APT NMR (CDCl3, 75 MHz) δ (ppm) = 159.1, 138.3, 134.5, 130.2, 129.1, 128.0, 126.5, 126.4, 126.0, 125.0, 120.6, 109.5, 81.0, 38.2, 34.9, 20.4.
2-((Phenyltellanyl)methyl)-2,3-dihydrobenzofuran (5f) [15] 0.022 g. Yield: 26%, white solid, mp 40–43 °C (literature 46–47 °C). 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.78 (d, J = 7.5 Hz, 2H), 7.30 (t, J = 7.5 Hz, 1H), 7.21 (t, J = 7.5 Hz, 1H), 7.12–7.08 (m, 2H), 6.84 (t, J = 7.4 Hz, 1H), 6.72 (d, J = 8.1 Hz, 1H), 5.04–4.98 (m, 1H), 3.41–3.32 (m, 2H), 3.14 (dd, J = 12.1 and 8.1 Hz, 1H), 2.97 (dd, J = 15.7 and 7.0 Hz, 1H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 159.2, 138.7, 129.3, 128.0, 126.3, 124.9, 120.5, 110.9, 109.5, 83.3, 36.8, 14.6.
2,7-Bis((phenylselanyl)methyl)-1,2,6,7-tetrahydronaphtho [1,2-b:5,6-b’]difuran (6b) [14] 0.056 g. Yield: 40%, yellow oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.57 (m, 4H), 7.38 (d, J = 8.2 Hz, 2H), 7.27–7.25 (m, 6H), 7.22 (d, J = 8.2 Hz, 2H), 5.14 (quint, J = 7.6, 2H), 3.52 (dd, J = 15.3 and 9.2 Hz, 2H), 3.43 (dd, J = 12.5 and 5.3 Hz, 2H), 3.20–3.13 (m, 4H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 154.6, 133.1, 129.4, 129.2, 127.3, 122.4, 121.0, 118.9, 113.9, 82.6, 36.4, 32.9.
2-((Phenylselanyl)methyl)-2,3-dihydronaphtho [1,2-b]furan (6c) [15] 0.043 g. Yield: 50%, colorless oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.87–7.85 (m, 1H), 7.78–7.76 (m, 1H), 7.59–7.55 (m, 2H), 7.40–7.35 (m, 3H), 7.28–7.25 (m, 4H), 5.19–5.1 (m, 1H), 3.52 (dd, J = 15.5 and 9.3 Hz, 2H), 3.42 (dd, J = 12.5 and 5.3 Hz, 2H), 3.22–3.14 (m, 2H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 156.7, 133.2, 130.8, 129.3, 129.1, 128.7, 127.4, 126.7, 123.0, 122.7, 117.8, 112.1 82.7, 34.5, 33.0.
1-(2-((Phenylselanyl)methyl)-2,3-dihydrobenzofuran-5-yl)ethan-1-one (6e) [14] 0.073 g. Yield: 87%, colorless oil. 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.80–7.78 (m, 2H), 7.57–7.54 (m, 2H), 7.30–7.26 (m, 3H), 6.75 (d, J = 8.2 Hz, 1H), 5.05 (dddd, J = 9.0, 7.6, 6.7 and 5.4 Hz, 1H), 3.39 (dd, J = 15.9 and 9.0 Hz, 1H), 3.33 (dd, J = 12.7 and 5.4 Hz, 1H), 3.12 (dd, J = 12.7 and 7.6 Hz, 1H), 3.05 (dd, J = 15.9 and 6.7 Hz, 1H), 2.53 (s, 3H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 196.6, 163.4, 133.2, 130.9, 130.4, 129.2, 129.0, 127.5, 127.0, 125.6, 109.0, 83.3, 34.8, 32.6, 26.4.
5-Allyl-7-methoxy-2-((phenylselanyl)methyl)-2,3-dihydrobenzofuran (6f) [15] 0.064 g. Yield: 70%, yellow solid, mp 43–46 °C (literature: 46–49 °C). 1H NMR (CDCl3, 500 MHz) δ (ppm) = 7.55–7.53 (m, 2H), 7.27–7.25 (m, 3H), 6.60 (s, 1H), 6.55 (s, 1H), 5.94 (ddt, J = 16.8, 10.1, 6.7 Hz, 1H), 5.08 (d, = 16.8, 1.8 Hz, 2H), 5.00–4.93 (m, 1H), 3.84 (s, 3H), 3.43 (dd, J = 12.5, 4.3 Hz, 1H), 3.40–3.30 (m, 3H), 3.09 (dd, J = 12.5, 9.1 Hz, 1H), 3.03 (dd, J = 15.6, 7.1 Hz, 1H). 13C NMR (CDCl3, 125 MHz) δ (ppm) = 145.9, 144.0, 137.9, 133.3, 132.8, 129.2, 127.2, 122.0, 117.1, 115.5, 111.5, 109.0, 82.7, 55.9, 40.0, 35.9, 32.2.
3. Results and Discussion
Our investigation commenced with the selection of 2-allylphenol 1a and diphenyl diselenide 2a as model substrates for the synthesis of 2-[(phenylselanyl)methyl]-2,3-dihydrobenzofuran 3a. The initial reaction (Table 1, entry 1) was performed with 0.25 mmol of 1a and 2a, Eosin Y (5 mol%) as a photocatalyst, and blue LED light (50 W) in dichloromethane (DCM). This configuration yielded only 41% of the desired product after 24 h, emphasizing that the use of light could be a way to obtain these molecules; however, prior optimization is necessary to increase the efficiency of the reaction. Based on previous works [26,27], it was decided to use InCl3 as an additive, considering that this modification was essential for better yields. An amount of 2 equiv. of the InCl3 was added to the reaction mixture, offering the targetwith a 56% yield (Table 1, entry 2). Afterward, other additives were investigated and SnCl2·2H2O was found to be the most effective, yielding a product with a 63% yield, while with the use of SnCl4 no product was detected (Table 1, entries 3–6). Furthermore, we increased the amount of additive, but no significative impact was observed (Table 1, entry 5).

Table 1.
Optimization of the reaction a.
Iodine is well established as an effective promoter in chalcogen cyclization reactions [28,29,30]. Therefore, to enhance the yield of product 3a, 1 equivalent of iodine was introduced into the reaction mixture, resulting in the target product being obtained with a 72% yield after 4 h (Table 1, entry 7). Notably, the reaction proceeded even in the absence of a photocatalyst, affording the desired product with an 83% yield (Table 1, entry 8). To our delight, the yield was advanced to 98%, when 0.1875 mmol of the 2a was used for the model reaction (Table 1, entry 9). Furthermore, we found that the reaction efficiency was dependent on the loading of iodine. The addition of 2 equiv. to the reaction mixture had no noticeable effect on the reaction, while with the use of 0.1 equiv. only traces of the product 3a were detected (Table 1, entries 10–11). Next, we explored the use of different light sources, such as green LED and white LED; however, no formation of the target product was observed under these conditions (Table 1, entries 12–13). Similar results have been documented in the literature, highlighting the effectiveness of blue LED as an excellent light source for transformations involving organochalcogen compounds [31,32,33,34,35]. Various solvents, including MeCN, acetone, toluene, hexane, AcOEt, THF, and EtOH, were evaluated (Table 1, entries 14–20). Among them, AcOEt yielded results comparable to DCM, affording product 3a in 95% yield (Table 1, entry 18). Therefore, to achieve a more environmentally friendly synthesis by avoiding the use of organochlorine solvents, AcOEt was selected as the optimal solvent for this transformation. Additionally, a notable observation was that conducting the reaction under an inert atmosphere resulted in a decreased yield of 56%, suggesting that ambient air conditions are more favorable for this procedure (Table 1, entry 21). Subsequently, when the reaction was conducted in the absence of light irradiation (in the dark), the target product was obtained with only a 21% yield, highlighting the significance of the visible light-initiated process (Table 1, entry 22).
To further assess the generality of this protocol, first, we examined the substrate scope of diselenide derivatives. In this way, a set of diselenides with electron-withdrawing (EWG) and electron-donating groups (EDGs), as well as alkyl and heteroaromatic different substituents, were synthesized to assess the scope of the optimized reaction (Scheme 2). To our delight, the methodology showed excellent compatibility with some electron-withdrawing groups, for example, the para-chloro derivative (3b) with a 95% yield and the para-fluorine derivative (3c) with a 96% yield, both displaying excellent efficiency. However, the para-nitro derivative (3d) did not produce the target molecule. Similar phenomena have been reported in the literature, where the use of para-nitro diselenide resulted in the corresponding products with lower yields [36,37]. Significantly, electron-donating substituents, such as para-methoxy derivative (3e), showed a good yield of 85%, as well as the para-methyl derivative (3f), where it was possible to achieve a yield of 71%. Furthermore, a high yield of 91% was obtained for sterically bulkier diorganyl diselenides, such as the naphthyl-substituted diselenide (3g). These results suggested that bigger aromatic groups enhanced the reaction’s efficiency. To our surprise, going in another direction, the mesityl-substituted derivative (3h) yielded 65%. We next continued to study the range of diselenide derivatives applying aliphatic substituents with the butyl-substituted diselenide (3j) achieving a good yield of 85%. These results are quite impressive since, generally, reactions using this type of substituent tend to be less reactive, which further highlights the methodology developed by us.
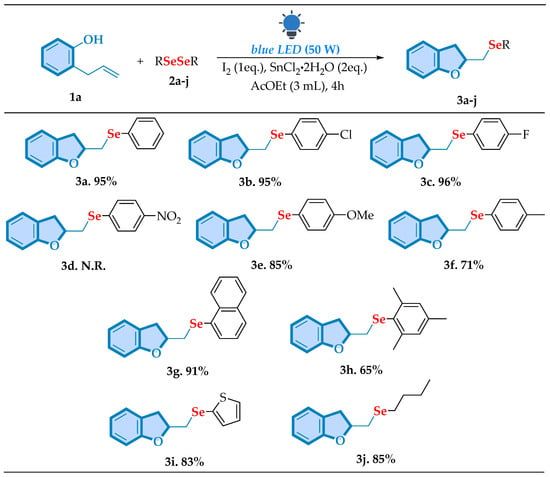
Scheme 2.
Scope of cyclization of 1a with different diselenides a,b. a Reaction conditions: a mixture of 2-allylphenol 1a (0.25 mmol), diorganyl dichalcogenide 2a–j (0.1875 mmol), I2 (0.25 mmol), and SnCl2·2H2O (0.5 mmol) in 3 mL of the AcOEt (3.0 mmol) mixture was stirred under visible light irradiation (LED chip, 50 W) at room temperature under open-air conditions for a period of 4 h. b Isolated yield.
Encouraged by the excellent results achieved in the formation of selenium-functionalized products, we further explored the adaptability of the visible light-mediated chalcogen cyclization reaction by investigating diorganyl disulfides and diorganyl ditellurides as substrates under the previously optimized reaction conditions (Scheme 3).
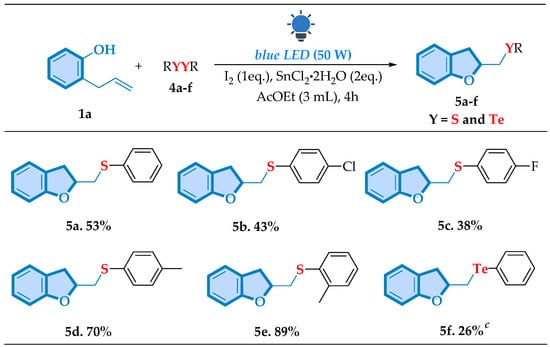
Scheme 3.
Scope of cyclization of 1a with different dichalcogenides a,b,c. a Reaction conditions: a mixture of 2-allylphenol 1a (0.25 mmol), diorganyl dichalcogenide 4a–f (0.1875 mmol), I2 (0.25 mmol), and SnCl2·2H2O (0.5 mmol) in 3 mL of the AcOEt (3.0 mmol) was stirred under visible light irradiation (LED chip, 50 W) at room temperature under open-air conditions for a period of 4 h. b Isolated yield; c reaction using SnCl4 instead of SnCl2·2H2O.
Thus, it was gratifying to demonstrate that our method proved to be efficient in obtaining sulfur and tellurium compounds, thereby expanding our compound library to other chalcogen derivatives. For sulfur-containing derivatives, the phenyl disulfide (5a) provided a moderate yield of 53%, indicating that sulfur is compatible with the reaction but less efficient compared to selenium-based systems. The para-chloro derivative (5b) and the para-fluor derivative (5c) gave lower yields of 43% and 38%, respectively, suggesting that the electron-withdrawing nature of the chlorine and fluorine atoms may slightly hinder the reaction. Notably, the para-methyl derivative (5d) produced a higher yield of 70% and in the same behavior with the ortho-methyl compound (5e), we were able to achieve the product with an excellent yield of 89%, which might be attributed to both the electron-donating nature of the methyl group and a favorable steric environment that facilitates the reaction. However, the methodology required modifications for tellurium-containing compounds since the phenyl ditelluride (5f) produced the target molecule only when SnCl4 was employed instead of SnCl2·2H2O, resulting in a 26% yield.
Building on these promising results, we further investigated the scope of allylphenol derivatives (Scheme 4). Thereby, five different substrates were tested and gave us the products 6b–c and 6e–f with yields performing between moderate to excellent (40–87%). Regrettably, the desired compounds 6d and 6f, with pyridine and aldehyde derivatives, were not obtained. This result suggests that these functional groups may introduce electronic hindrances that prevent the reaction from occurring efficiently.
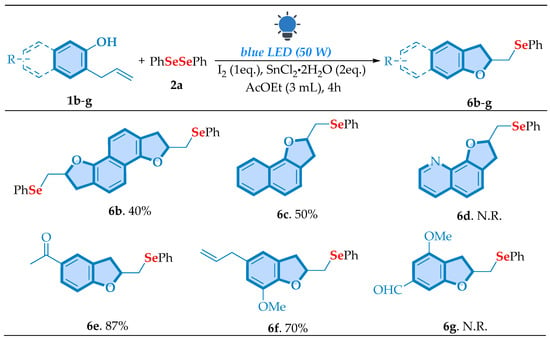
Scheme 4.
Scope of cyclization of different phenols with diphenyl diselenide 2a a,b. a Reaction conditions: a mixture of 2-allylphenol 1b–g (0.25 mmol), diorganyl diselenide 2a (0.1875 mmol), I2 (0.25 mmol), and SnCl2·2H2O (0.5 mmol) in 3 mL of the AcOEt (3.0 mmol) was stirred under visible light irradiation (LED chip, 50 W) at room temperature under open-air conditions for a period of 4 h. b Isolated yield.
To assess the scalability and synthetic applicability of this methodology, a gram-scale synthesis of 3a was conducted, yielding 86% after 4 h (Scheme 5).
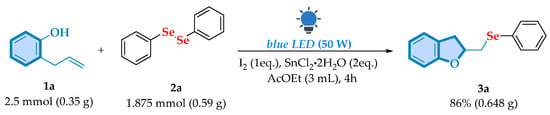
Scheme 5.
Gram-scale experiment.
Initially, radical trapping experiments were performed. The addition of common radical scavengers, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and 2,6-di-tert-butyl-4-methylphenol (BHT), under standard reaction conditions, resulted in the formation of the target product with yields of 85% and 93%, respectively (Scheme 6, top). These findings indicate that a free radical pathway is not involved in the reaction mechanism. Three sets of control experiments were further carried out to address certain concerns (Scheme 6, bottom). The absence of iodine led to complete inhibition of the reaction, demonstrating its critical role in this transformation. Additionally, a marked reduction in the yield of product 3a was observed when SnCl2·2H2O was excluded, confirming its key contribution to the reaction process.
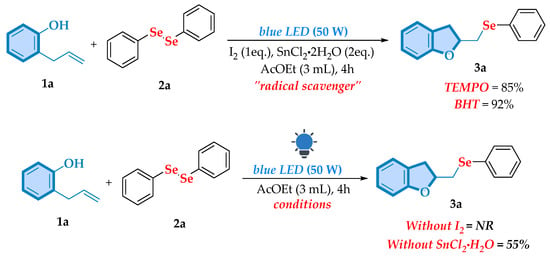
Scheme 6.
Control experiments around the reaction mechanism.
Based on the mechanistic studies outlined above, along with the relevant literature precedents [38,39,40], a tentative reaction mechanism is proposed, as depicted in Scheme 7. In this sense, we believe that the predominant step of the reaction is an anionic pathway since with the use of radical inhibitors the reaction had only a small decrease in yield of reaction. Thus, one possibility is that the reaction occurs in a classical protocol via the formation of the electrophilic species PhSeI, in the presence of iodine [41,42,43,44,45,46,47,48]. However, since we used SnCl2·2H2O, we believe that, acting as a Lewis acid [49,50,51,52,53,54,55,56], it increases the electrophilicity of selenyl iodide forming an intermediate A. Thus, this intermediate subsequently reacts with 2-allylphenols 1a to produce the selenium ion B, accompanied by the elimination of −SnICl2. Intramolecular cyclization of intermediate B then leads to the final product C after the loss of protons. Furthermore, as indicated by the control reaction performed without SnCl2·2H2O, the selenocyclization of 1a still proceeds but results in a minor conversion (see Scheme 7, bottom). However, it is not possible to completely rule out a radical pathway in the reaction, which can lead to the production of PhSeI via homolytic cleavage of molecular iodine (I2) under blue LED irradiation to give iodine radical species (I·) [57,58,59,60]. Furthermore, as an alternative non-SnCl2·2H2O pathway, the photogenerated PhSe· radical reacts with I· to also form PhSeI. This species can then interact with the electron-rich C–C double bond of 1a to form intermediate B. Nucleophilic cyclization of cation B leads to the target product.
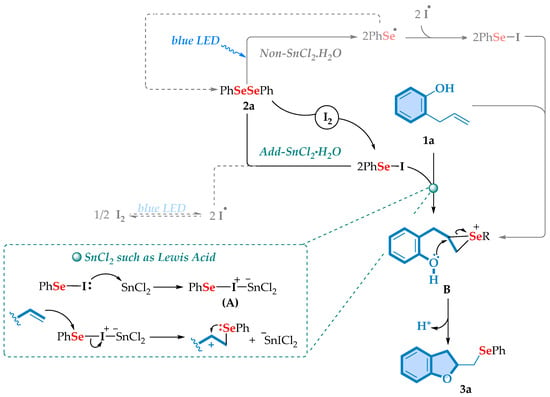
Scheme 7.
A plausible mechanism for the formation of chalcogenyl-2,3-dihydrobenzofurans under visible light irradiation.
Compared to previously published methods [16,17,18,19,20,21,22,23], the methodology presented in this work offers several advantages for the synthesis of chalcogen-functionalized 2,3-dihydrobenzofurans. While prior approaches reported a maximum of 83% for selenide derivatives and yields ranging from 40% to 70% for sulfur compounds, our investigation consistently showed better yields, surpassing 90% for numerous compounds. Furthermore, our method made it possible to access more structurally complex compounds that had only been briefly examined in one previous study, like the synthesis of compound 6b [16]. Utilizing light as a driving force instead of the harsh oxidants or high temperatures that are frequently needed in other protocols is another significant benefit of our technology. This improves efficiency and is consistent with more energy-efficient and sustainable synthetic techniques.
4. Conclusions
In summary, we developed a mild, sustainable, and operationally simple method of visible light-mediated intramolecular oxychalcogen cyclization of 2-allylphenol derivatives with different dichalcogenides. This approach aligns with green chemistry strategies, employing environmentally friendly conditions without the need for photocatalysts or other more robust conditions. Additionally, the reaction is scalable, highlighting its practicality and utility. The reaction demonstrated excellent compatibility with a range of substituents on both substrates, efficiently yielding 19 derivatives in good to excellent yields, thereby offering a practical approach for the synthesis of chalcogenyl-2,3-DHB.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr13041038/s1. Supplementary Figures: Spectroscopic characterization for the Synthesized Compounds.
Author Contributions
Conceptualization, L.S.G., P.C.N. and T.J.P.; Methodology, L.S.G. and M.C.S.; Resources, V.N.; Writing—original draft, L.S.G.; Writing—review & editing, L.S.G., P.C.N., T.J.P. and V.N.; Supervision, V.N.; Project administration, V.N.; Funding acquisition, V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ (E-26/202.911/2019, E-26/200.414/2020, E-26/210.325/2022, and E-26/200.235/2023), Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (310656/2021-4 and 152903/2022-4), and INCT-Catalise.
Data Availability Statement
All data used to support the findings of this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cordeiro, P.; Rebelo, A.; Menezes, V.; Reis, J.S.; Nascimento, V. Fontes Naturais e Sintéticas de derivados de 2,3-Dihidrobenzofuranos: Uma Abordagem Recente. Rev. Virtual Quím. 2023, 15, 374–401. [Google Scholar] [CrossRef]
- Chen, Z.; Pitchakuntla, M.; Jia, Y. Synthetic approaches to natural products containing 2,3-dihydrobenzofuran skeleton. Nat. Prod. Rep. 2019, 36, 666–690. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-W.; Chai, H.-B.; Keller, W.J.; Kinghorn, D.A. Lignans and Other Constituents of the Fruits of Euterpe oleracea (Açai) with Antioxidant and Cytoprotective Activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Dapkekar, A.B.; Sreenivasulu, C.; Kishore, D.R.; Satyanarayana, G. Recent Advances Towards the Synthesis of Dihydrobenzofurans and Dihydroisobenzofurans. Asian J. Org. Chem. 2022, 11, e202200012. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.-M.; Zhang, C.-S.; Liu, S.-Z.; Tian, J.-M.; Wang, S.-H.; Zhang, X.-M.; Tu, Y.-Q. Enantioselective synthesis of cis-hydrobenzofurans bearing all-carbon quaternary stereocenters and application to total synthesis of (−)-morphine. Nature 2019, 10, 2507. [Google Scholar] [CrossRef]
- Zarin, M.K.Z.; Dehaen, W.; Salehi, P.; Asl, A.A.B. Synthesis and Modification of Morphine and Codeine, Leading to Diverse Libraries with Improved Pain Relief Properties. Pharmaceutics 2023, 15, 1779. [Google Scholar] [CrossRef]
- Da Fonseca, A.C.C.; De Queiroz, L.N.; Felisberto, J.S.; Ramos, Y.J.; Marques, A.M.; Wermelinger, G.F.; Pontes, B.; Moreira, D.L.; Robbs, B.K. Cytotoxic effect of pure compounds from Piper rivinoides Kunth against oral squamous cell carcinoma. Nat. Prod. Res. 2021, 35, 6163–6167. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, Q.; Li, C. Total Synthesis of (+)-Decursivine. Org. Lett. 2011, 13, 5302–5305. [Google Scholar] [CrossRef]
- Ashraf, R.; Zahoor, A.F.; Ali, K.G.; Nazeer, U.; Saif, M.J.; Mansha, A.; Chaudhry, A.R.; Irfan, A. Development of novel transition metal-catalyzed synthetic approaches for the synthesis of a dihydrobenzofuran nucleus: A review. RSC Adv. 2024, 14, 14539–14581. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.-D.; Li, Y.; Yang, L.-J.; Wang, X.-Q.; Zhang, G.-L.; Zhang, H.-B. Design, synthesis and cytotoxic activities of novel hybrid compounds between dihydrobenzofuran and imidazole. Org. Biomol. Chem. 2011, 9, 4250–4255. [Google Scholar] [CrossRef]
- Wang, D.-H.; Yu, J.-Q. Highly Convergent Total Synthesis of (+)-Lithospermic Acid via a Late-Stage Intermolecular C−H Olefination. J. Am. Chem. Soc. 2011, 133, 5767–5769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-A.; Yue, L.; Zhu, J.; Ren, H.; Zhang, H.; Hu, D.-Y.; Han, G.; Feng, J.; Nan, Z.-D. Total synthesis of Tasimelteon. Tetrahedron Lett. 2019, 60, 1986–1988. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Drug Insight: The use of melatonergic agonists for the treatment of insomnia—Focus on ramelteon. Nat. Clin. Pract. Neurol. 2007, 3, 221–228. [Google Scholar] [CrossRef]
- Scheide, M.R.; Schneider, A.R.; Jardim, G.A.M.; Martins, G.M.; Durigon, D.C.; Saba, S.; Rafique, J.; Braga, A.L. Electrochemical synthesis of selenyl-dihydrofurans via anodic selenofunctionalization of allyl-naphthol/phenol derivatives and their anti-Alzheimer activity. Org. Biomol. Chem. 2020, 18, 4916–4921. [Google Scholar] [CrossRef]
- Azevedo, A.R.; Cordeiro, P.S.; Strelow, D.N.; Andrade, K.N.; Neto, M.R.S.; Fiorot, R.G.; Brüning, C.A.; Braga, A.L.; Lião, L.M.; Bortolatto, C.F.; et al. Green Approach for the Synthesis of Chalcogenyl-2,3-dihydrobenzofuran Derivatives Through Allyl-phenols/Naphthols and Their Potential as MAO-B Inhibitors. Chem. Asian J. 2023, 18, e202300586. [Google Scholar] [CrossRef]
- Hellwig, P.S.; Barcellos, A.M.; Furst, C.G.; Alberto, E.E.; Perin, G. Oxyselenocyclization of 2-Allylphenols for the Synthesis of 2,3-Dihydrobenzofuran Selenides. ChemistrySelect 2021, 6, 13884–13889. [Google Scholar] [CrossRef]
- Bartz, R.H.; Souz, P.S.; Iarocz, L.E.B.; Hellwig, P.S.; Jacob, R.G.; Silva, M.S.; Lenardão, E.J.; Perin, G. Greening the Synthesis of 2,3-Dihydrobenzofuran Selenides: I2/TBHP-Promoted Selenocyclization of 2-Allylphenols. Eur. J. Org. Chem. 2025, 28, e202401243. [Google Scholar] [CrossRef]
- Zhou, C.F.; Zhang, Y.-Q.; Ling, Y.; Ming, L.; Xi, X.; Liu, G.-Q.; Zhang, Y. Time-economical synthesis of selenofunctionalized heterocycles via I2O5-mediated selenylative heterocyclization. Org. Biomol. Chem. 2022, 20, 420–426. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Magolda, R.L.; Sipio, W.J.; Barnette, W.E.; Lysenko, Z.; Joullie, M.M. Phenylselenoetherification. A highly efficient cyclization process for the synthesis of oxygen- and sulfur-heterocycles. J. Am. Chem. Soc. 1980, 102, 3784–3793. [Google Scholar] [CrossRef]
- Kostić, M.; Verdía, P.; Fernández-Stefanuto, V.; Puchta, R.; Tojo, E. A mild and efficient procedure for alkenols oxyselenocyclization by using ionic liquids. J. Phys. Org. Chem. 2019, 32, e3928. [Google Scholar] [CrossRef]
- Okuma, K.; Seto, J.-I. Synthesis of Indoles, 3,1-Benzoxazines, and Quinolines from 2-Alkenylanilides and Active Seleniums. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1014–1020. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, T.; Jaing, X.; Yeung, Y.-Y. Cyclopropenium Sulfide as Lewis Base Catalyst for Chemoselective and Regioselective Electrophilic Selenylation of Phenols. ACS Catal. 2024, 14, 9198–9206. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Tingoli, M.; Bartoli, D.; Balducci, R. Ring-closure reactions initiated by the peroxydisulfate ion oxidation of diphenyl diselenide. J. Org. Chem. 1990, 55, 429–434. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef]
- Vergaelen, M.; Verbraeken, B.; Van Guyse, J.F.R.; Podevyn, A.; Tigrine, A.; De la Rosa, V.R.; Monnery, B.D.; Hoogenboom, R. Ethyl acetate as solvent for the synthesis of poly(2-ethyl-2- oxazoline). Green Chem. 2020, 22, 1747–1753. [Google Scholar] [CrossRef]
- Peglow, T.J.; Nobre, P.C.; Thomaz, J.P.S.S.C.; Vieira, M.M.; Junior, H.C.S.; Dalberto, B.T.; Schneider, P.H.; Rodembusch, F.S.; Nascimento, V. Synthesis and Photophysical Evaluation of Dialkynyl-N-(het)arylpyrroles: Promising Key Compounds in Fluorescence Chemistry. Asian J. Org. Chem. 2024, 13, e202300655. [Google Scholar] [CrossRef]
- Peglow, T.J.; Martins, C.C.; Da Motta, K.P.; Luchese, C.; Wilhelm, E.A.; Stieler, R.; Schneider, P.H. Synthesis and biological evaluation of 5-chalcogenyl-benzo[h]quinolines via photocyclization of arylethynylpyridine derivatives. New J. Chem. 2022, 46, 23030–23038. [Google Scholar] [CrossRef]
- Li, Y.-N.; Chen, F.; Zhang, X.-G.; Tu, H.-Y. Iodine-Mediated Regioselective Radical Cyclization of o-Vinylaryl Isocyanides with Disulfides/Diselenides Leading to 2-Chalcogenated Quinolines. Adv. Synth. Catal. 2023, 365, 3814–3818. [Google Scholar] [CrossRef]
- Meng, Z.; Shi, M.; Wei, Y. Iodine radical mediated cascade [3 + 2] carbocyclization of ene-vinylidenecyclopropanes with thiols and selenols via photoredox catalysis. Org. Chem. Front. 2024, 11, 1395–1403. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, G.; Jiang, L.; Dong, Y.; Zhan, X.; Sun, F.; Du, Y. Construction of C-S and C-Se Bonds Mediated by Hypervalent Iodine Reagents Under Metal-Free Conditions. Curr. Org. Chem. 2022, 26, 1935–1953. [Google Scholar] [CrossRef]
- Tian, S.-Y.; Ai, J.-J.; Han, J.-H.; Rao, W.; Shen, S.-S.; Sheng, D.; Wang, S.-Y. Photoinduced Construction of Thieno [3,4-c]quinolin-4(5H)-ones/Selenopheno [3,4-c]quinolin-4(5H)-ones Using Diphenyl Disulfide or Diphenyl Diselenide as Sulfur or Selenium Sources. J. Org. Chem. 2023, 88, 828–837. [Google Scholar] [CrossRef]
- Jiang, S.; Leng, Y.; Wang, P.; Sui, K.; Ma, N.; Wu, Y. Visible-Light-Induced Regioselective Selenohydroxylation of Enamine Amides with Diaryl Diselenides. Eur. J. Org. Chem. 2024, 27, e202400600. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Wang, Y.; Zhu, H.; Le, Z.; Xie, Z. Photo-induced organoselenium-catalyzed synthesis of 2-substituted quinazoline derivatives. J. Mol. Struct. 2024, 1297, 136940. [Google Scholar] [CrossRef]
- Chillal, A.S.; Bhawale, R.T.; Kshirsagar, U.A. Photoinduced Regioselective Chalcogenation and Thiocyanation of 4H-Pyrido [1,2-a] pyrimidin-4-ones Under Benign Conditions. Eur. J. Org. Chem. 2023, 26, e202300665. [Google Scholar] [CrossRef]
- Dalberto, B.T.; Vieira, M.M.; Padilha, N.B.; Stieler, R.; Schneider, P.H. Visible-Light-Mediated Cyclization of 1,3-Diones and Chalcogenoalkynes: An Eco-Friendly Protocol for the Regioselective Formation of Polysubstituted Chalcogenofurans. ChemCatChem 2024, 16, e202400869. [Google Scholar] [CrossRef]
- Rodrigues, I.; Barcellos, A.M.; Belladona, A.L.; Roehrs, J.A.; Cargnelutti, R.; Alves, D.; Perin, G.; Schumacher, R.F. Oxone®-mediated direct arylselenylation of imidazo [2,1-b]thiazoles, imidazo [1,2-a]pyridines and 1H-pyrazoles. Tetrahedron 2018, 74, 4242–4246. [Google Scholar] [CrossRef]
- Bian, M.; Hua, J.; Ma, T.; Xu, J.; Cai, C.; Yang, Z.; Liu, C.; He, W.; Fang, Z.; Guo, K. Continuous-flow electrosynthesis of selenium-substituted iminoisobenzofuran via oxidative cyclization of olefinic amides and diselenides. Org. Biomol. Chem. 2021, 19, 3207–3212. [Google Scholar] [CrossRef]
- Beukeaw, D.; Noikham, M.; Yotphan, S. Iodine/persulfate-promoted site-selective direct thiolation of quinolones and uracils. Tetrahedron 2019, 75, 130537. [Google Scholar] [CrossRef]
- Kumar, P.; Bhalla, A. Reaction Pattern and Mechanistic Aspects of Iodine and Iodine-Based Reagents in Selenylation of Aliphatic, Aromatic, and (Hetero)Cyclic Systems. Top Curr. Chem. 2024, 382, 12. [Google Scholar] [CrossRef]
- Kosso, A.R.O.; Kabri, Y.; Broggi, J.; Redon, S.; Vanelle, P. Sequential Regioselective Diorganochalcogenations of Imidazo [1,2-a]pyrimidines Using I2/H3PO4 in Dimethylsulfoxide. J. Org. Chem. 2020, 85, 3071–3081. [Google Scholar] [CrossRef]
- Win, K.M.N.; Sonawane, A.D.; Koketsu, M. Synthesis of selenated tetracyclic indoloazulenes via iodine and diorganyl diselenides. Org. Biomol. Chem. 2021, 19, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Palomba, M.; Angeli, A.; Galdini, R.; Hughineata, A.J.; Pering, G.; Lenardão, E.J.; Marini, F.; Santi, C.; Supuran, C.T.; Bagnoli, L. Iodine/Oxone® oxidative system for the synthesis of selenylindoles bearing a benzenesulfonamide moiety as carbonic anhydrase I, II, IX, and XII inhibitors. Org. Biomol. Chem. 2024, 22, 6532–6542. [Google Scholar] [CrossRef] [PubMed]
- Dhurey, A.; Mandal, S.; Pramanik, A. I2/DMSO-Promoted Synthesis of Diaryl Sulfide- and Selenide-Embedded Arylhydrazones. J. Org. Chem. 2023, 88, 5377–5390. [Google Scholar] [CrossRef] [PubMed]
- Thedy, M.E.C.; Gularte, M.M.; Azeredo, J.B.; Braga, A.L. I2/DMSO Mediated Direct Selenylation of Uracils with Diorganoyl Diselenides—A Simple Protocol to Access 5-Selanyl-Uracils. ChemistrySelect 2024, 9, e202402057. [Google Scholar] [CrossRef]
- Jiang, H.; Schen, H.; Li, C.; Jin, Z.; Shang, Y.; Chen, Y.; Yi, M.; Du, J.; Gui, Q.-W. Synthesis of Seleno Oxindoles via Iodine-Induced Radical Cyclization of N-Arylacrylamides with Diorganyl Diselenides. Synthesis 2022, 54, 2669–2676. [Google Scholar] [CrossRef]
- Yi, R.; Liu, S.; Gao, H.; Liang, Z.; Xu, X.; Li, N. Iodine-promoted direct thiolation (selenylation) of imidazole with disulfides (diselenide): A convenient and metal-free protocol for the synthesis of 2-arylthio(seleno)imidazole. Tetrahedron 2020, 76, 130951. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Yu, L.; Xu, Q.; Jiang, X. Visible light-promoted, iodine-catalyzed selenoalkoxylation of olefins with diselenides and alcohols in the presence of hydrogen peroxide/air oxidant: An efficient access to α-alkoxyl selenides. Sci. China Chem. 2018, 61, 294–299. [Google Scholar] [CrossRef]
- Du, H.-A.; Tang, R.-Y.; Deng, C.-L.; Liu, Y.; Li, J.-H.; Zhang, X.-G. Iron-Facilitated Iodine-Mediated Electrophilic Annulation of N,N-Dimethyl-2-alkynylanilines with Disulfides or Diselenides. Adv. Synth. Catal 2011, 353, 2739–2748. [Google Scholar] [CrossRef]
- Tran, V.H.; Nguyen, A.T.; Kim, H.-K. Tin(II) Chloride-Catalyzed Direct Esterification and Amidation of tert-Butyl Esters Using α,α-Dichlorodiphenylmethane Under Mild Conditions. J. Org. Chem. 2023, 88, 13291–13302. [Google Scholar] [CrossRef]
- Masuyama, Y.; Hayashi, M.; Suzuki, N. SnCl2-Catalyzed Propargylic Substitution of Propargylic Alcohols with Carbon and Nitrogen Nucleophiles. Eur. J. Org. Chem. 2013, 2013, 2914–2921. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Agrawal, N.; Mali, S.M.; Jadhav, S.V.; Gopi, H.N. Tin(ii) chloride assisted synthesis of N-protected γ-amino β-keto esters through semipinacol rearrangement. Org. Biomol. Chem. 2010, 8, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Perumal, P.T. SnCl2·2H2O—An Alternative to Lewis Acidic Ionic Liquids. Chem. Lett. 2006, 35, 632–633. [Google Scholar] [CrossRef]
- Akiyama, T.; Onuma, Y. Tin(ii) chloride mediated allylation of aldimines generated in situ with allylstannane in water. J. Chem. Soc. 2002, 1, 1157–1158. [Google Scholar] [CrossRef]
- Aichhorn, S.; Himmelsbach, M.; Schöfberger, W. Synthesis of quinoxalines or quinolin-8-amines from N-propargyl aniline derivatives employing tin and indium chlorides. Org. Biomol. Chem. 2015, 13, 9373–9380. [Google Scholar] [CrossRef]
- Marjani, A.P.; Khalafy, J.; Salami, F.; Mohammadlou, M. Tin(II) Chloride Catalyzed Synthesis of New Pyrazolo [5,4-b]quinolines under Solvent-Free Conditions. Synthesis 2015, 47, 1656–1660. [Google Scholar] [CrossRef]
- Menezes, F.D.L.; Guimarães, M.D.O.; Silva, M.J. Highly Selective SnCl2-Catalyzed Solketal Synthesis at Room Temperature. Ind. Eng. Chem. Res. 2013, 52, 16709–16713. [Google Scholar] [CrossRef]
- Schendera, E.; Unkel, L.-N.; Quyen, P.P.H.; Salkewitz, G.; Hoffmann, F.; Villinger, A.; Brasholz, M. Visible-Light-Mediated Aerobic Tandem Dehydrogenative Povarov/Aromatization Reaction: Synthesis of Isocryptolepines. Chem. Eur. J. 2020, 26, 269–274. [Google Scholar] [CrossRef]
- Maejima, S.; Yamaguchi, E.; Itoh, A. Intermolecular Tandem Addition/Esterification Reaction of Alkenes with Malonates Leading to γ-Lactones Mediated by Molecular Iodine under Visible Light Irradiation. Adv. Synth. Catal. 2017, 359, 3883–3887. [Google Scholar] [CrossRef]
- Maejima, S.; Yamaguchi, E.; Itoh, A. trans-Diastereoselective Syntheses of γ-Lactones by Visible Light-Iodine-Mediated Carboesterification of Alkenes. ACS Omega 2019, 4, 4856–4870. [Google Scholar] [CrossRef]
- Maejima, S.; Yamaguchi, E.; Itoh, A. Three-Component Iminolactonization Reaction via Bifunctionalization of Olefins Using Molecular Iodine and Visible Light. J. Org. Chem. 2020, 85, 10709–10718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).