Synthesis of γ-AlOOH Nanosheets and Their Adsorption Properties for Heavy Metal Ions
Abstract
:1. Introduction
2. Experiment
2.1. Physical and Chemical Characterization
2.2. Synthesis of AlOOH Nanosheets
2.3. Cu2+ Adsorption Experiments
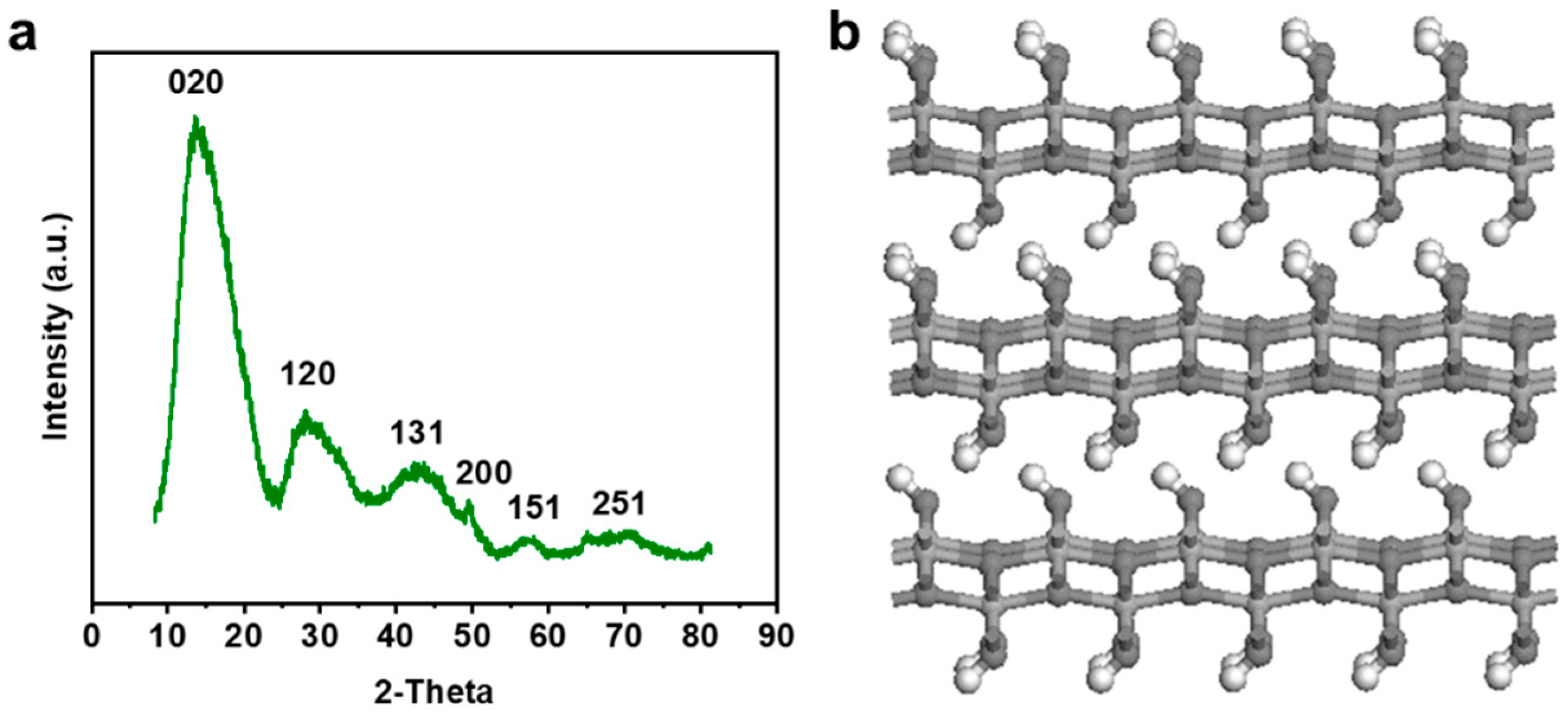
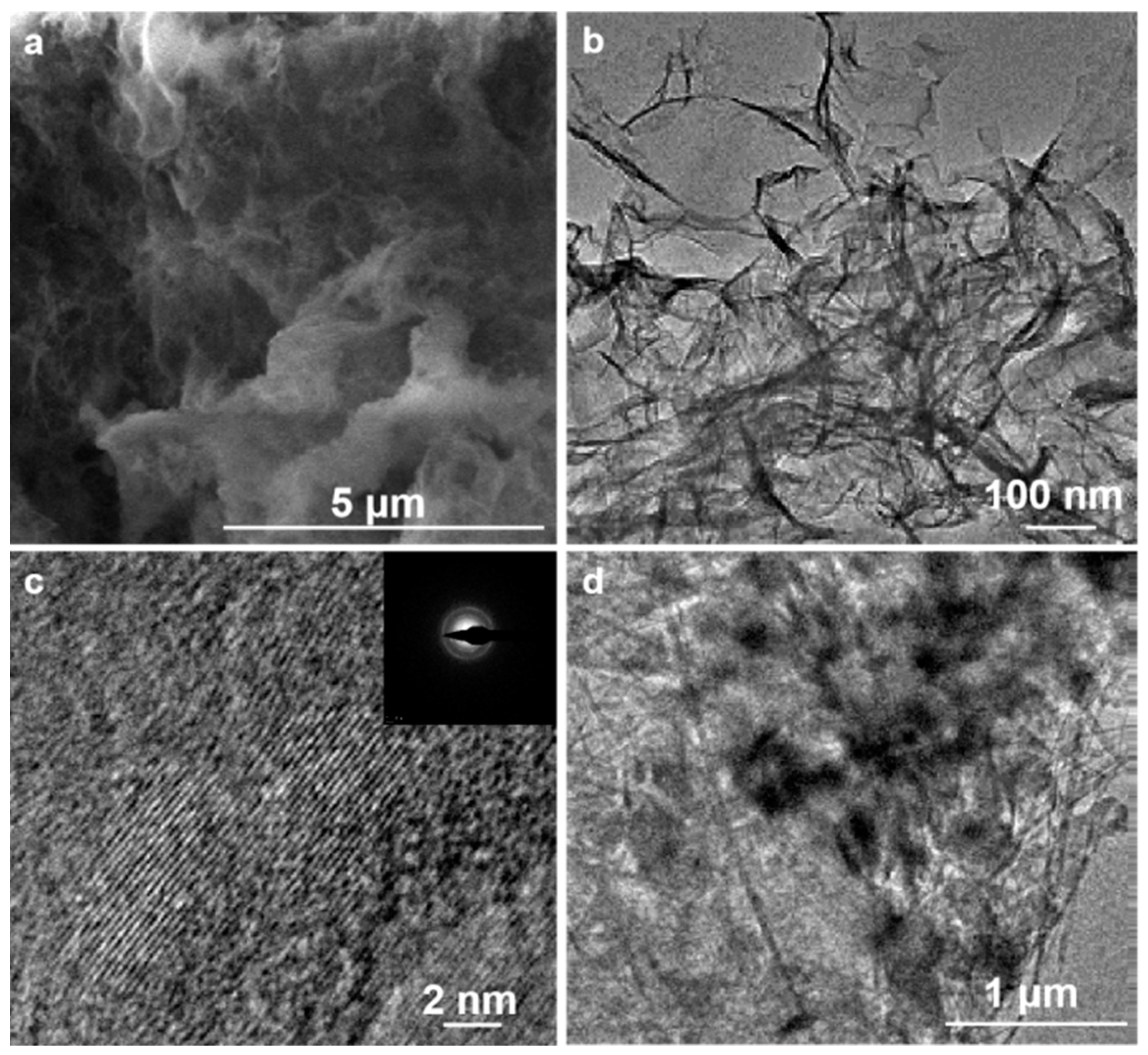
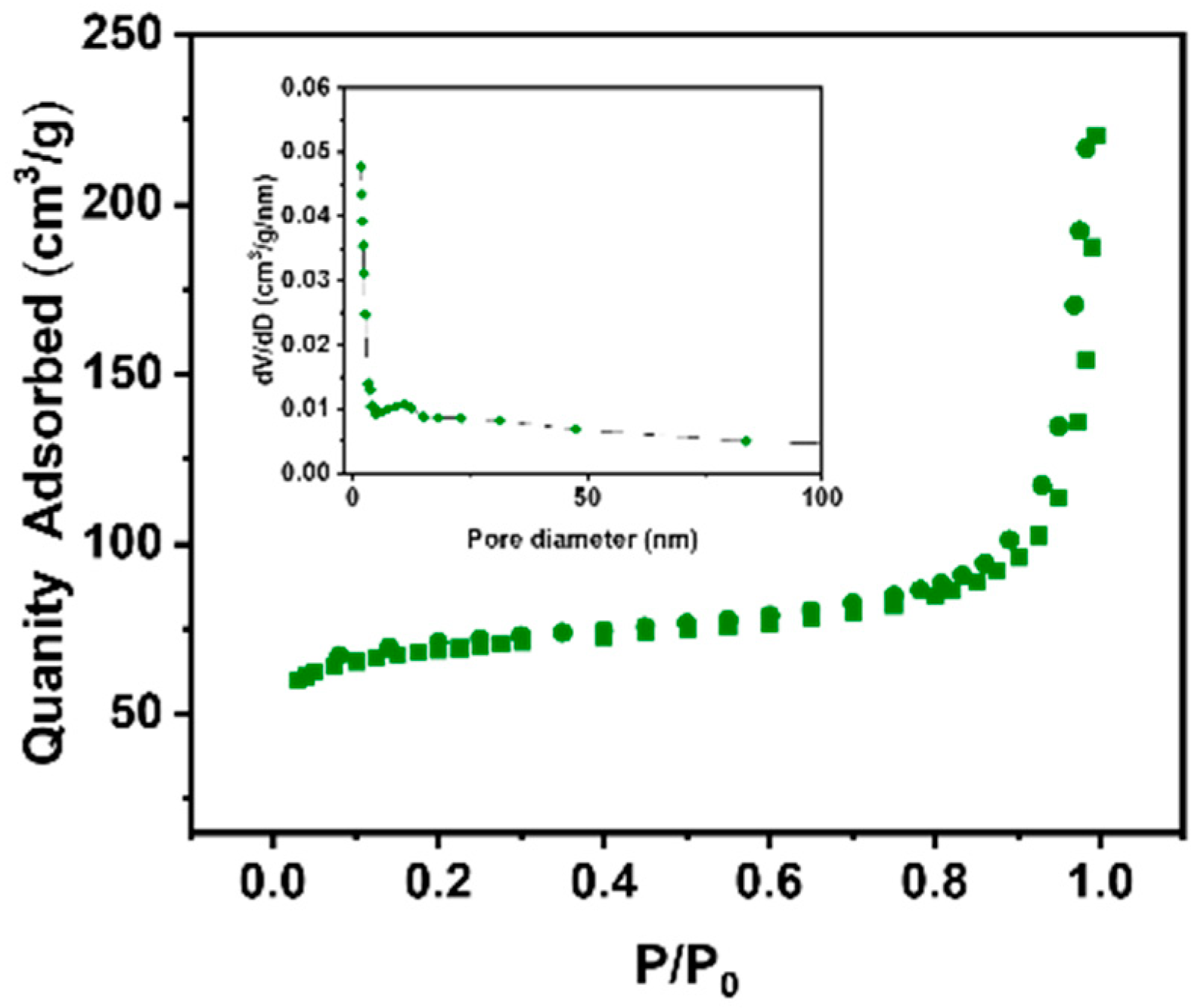
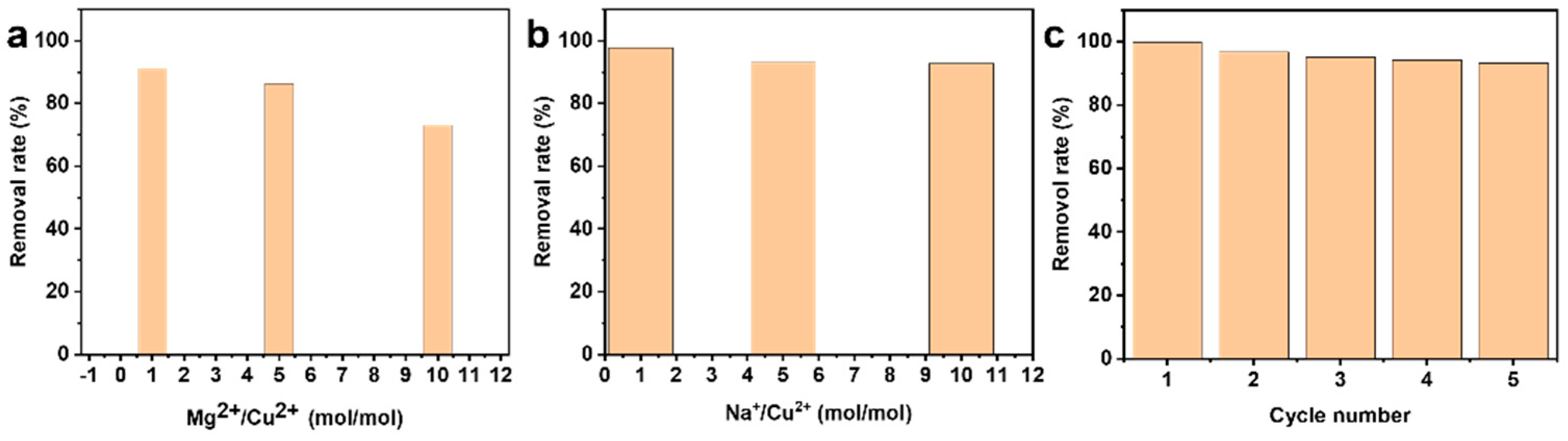
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Lin, G.; Zeng, B.; Li, J.; Wang, Z.; Wang, S.; Hu, T.; Zhang, L. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem. Eng. J. 2023, 460, 141710. [Google Scholar]
- Dhokpande, S.R.; Deshmukh, S.M.; Khandekar, A.; Sankhe, A. A review outlook on methods for removal of heavy metal ions from wastewater. Sep. Purif. Technol. 2024, 350, 127868. [Google Scholar]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar]
- Wang, S.; Song, W.; Liu, E.; Zhao, P.; Ng, H.Y.; Wang, X. Efficient, facile and recyclable coating strategy to improve heavy metals removal by UF membrane in drinking water purification. Sep. Purif. Technol. 2025, 363, 131995. [Google Scholar]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [PubMed]
- Pellenz, L.; de Oliveira, C.R.S.; Júnior, A.H.d.S.; da Silva, L.J.S.; de Souza, A.A.U.; Souza, S.M.d.A.G.U.d.; Borba, F.H.; da Silva, A. A comprehensive guide for characterization of adsorbent materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar]
- Liu, P.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes: Via capacitive deionization. J. Mater. Chem. A 2017, 5, 14748–14757. [Google Scholar]
- Björklund, K.; Li, L.Y. Adsorption of organic stormwater pollutants onto activated carbon from sewage sludge. J. Environ. Manag. 2017, 197, 490–497. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Yang, C.; Niu, D.; Zhang, F.; Zhong, Y.; Cao, T.; Li, L.; Liu, Y. Absorption mechanisms of lead in aqueous solutions with hydrous manganese dioxide. Water Sci. Technol. 2018, 77, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, R.; Roy, P.; Periasamy, A.P.; Chen, Y.W.; Liang, C.T.; Chang, H.T. Fe2O3/Al2O3 microboxes for efficient removal of heavy metal ions. New J. Chem. 2017, 41, 7751–7757. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, P.P.; Yadav, V.; Gupta, H.; Kulshrestha, V. Synthesis and characterization of different metal oxide and GO composites for removal of toxic metal ions. Sep. Sci. Technol. 2019, 54, 426–433. [Google Scholar] [CrossRef]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpää, M.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci. Total Environ. 2024, 912, 169160. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Mohamed, A.A. A systematic review of layered double hydroxide-based materials for environmental remediation of heavy metals and dye pollutants. Inorg. Chem. Commun. 2023, 148, 110325. [Google Scholar] [CrossRef]
- Alnasrawi, F.A.; Mohammed, A.A. Mohammed, Enhancement of Cd2+ removal on CuMgAl-layered double hydroxide/montmorillonite nanocomposite: Kinetic, isotherm, and thermodynamic studies. Arab. J. Chem. 2023, 16, 104471. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, L.; Shi, D.; Cai, W.; Guo, L. Al-Li Alloy Chemical Milling Waste Solution Synthesis of γ-AlOOH Bundles for Cr(VI) Rapid Adsorption. ChemistrySelect 2020, 5, 5732–5741. [Google Scholar] [CrossRef]
- Vo, T.K.; Park, H.-K.; Nam, C.-W.; Kim, S.-D.; Kim, J. Facile synthesis and characterization of γ-AlOOH/PVA composite granules for Cr(VI) adsorption. J. Ind. Eng. Chem. 2018, 60, 485–492. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jia, Y.; Jin, Z.; Yu, X.Y.; Xu, W.H.; Luo, T.; Zhu, B.J.; Liu, J.H.; Huang, X.J. Self-assembled, monodispersed, flower-like γ-AlOOH hierarchical superstructures for efficient and fast removal of heavy metal ions from water. CrystEngComm 2012, 14, 3005. [Google Scholar] [CrossRef]
- Granados-Correa, F.; Corral-Capulin, N.G.; Olguín, M.T.; Acosta-León, C.E. Comparison of the Cd(II) adsorption processes between boehmite (-AlOOH) and goethite (-FeOOH). Chem. Eng. J. 2011, 171, 1027–1034. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, Y.; Du, J.; Zhang, W.; Huang, Y.; Zhao, Q.; Duan, L.; Liu, Y.; Naidu, R.; Pan, X. Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions. Processes 2021, 9, 1829. [Google Scholar] [CrossRef]
- Zhang, Z.; He, S.; Zhang, Y.; Zhang, K.; Wang, J.; Jing, R.; Yang, X.; Hu, Z.; Lin, X.; Li, Y. Spectroscopic investigation of Cu2+, Pb2+ and Cd2+ adsorption behaviors by chitosan-coated argillaceous limestone: Competition and mechanisms. Environ. Pollut. 2019, 254, 112938. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, F.; Li, X.Y.; Shih, K.; Zeng, E.Y. Adsorption and Thermal Stabilization of Pb2+ and Cu2+ by Zeolite. Ind. Eng. Chem. Res. 2016, 55, 8767–8773. [Google Scholar] [CrossRef]
- Tan, P.; Sun, J.; Hu, Y.; Fang, Z.; Bi, Q.; Chen, Y.; Cheng, J. Adsorption of Cu2+, Cd2+ and Ni2+ from aqueous single metal solutions on graphene oxide membranes. J. Hazard. Mater. 2015, 297, 251–260. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, L.; Peng, R.; Tang, H. Synthesis of γ-AlOOH Nanosheets and Their Adsorption Properties for Heavy Metal Ions. Processes 2025, 13, 1037. https://doi.org/10.3390/pr13041037
Wang S, Wang L, Peng R, Tang H. Synthesis of γ-AlOOH Nanosheets and Their Adsorption Properties for Heavy Metal Ions. Processes. 2025; 13(4):1037. https://doi.org/10.3390/pr13041037
Chicago/Turabian StyleWang, Shile, Lili Wang, Ruichao Peng, and Hongding Tang. 2025. "Synthesis of γ-AlOOH Nanosheets and Their Adsorption Properties for Heavy Metal Ions" Processes 13, no. 4: 1037. https://doi.org/10.3390/pr13041037
APA StyleWang, S., Wang, L., Peng, R., & Tang, H. (2025). Synthesis of γ-AlOOH Nanosheets and Their Adsorption Properties for Heavy Metal Ions. Processes, 13(4), 1037. https://doi.org/10.3390/pr13041037






