1. Introduction
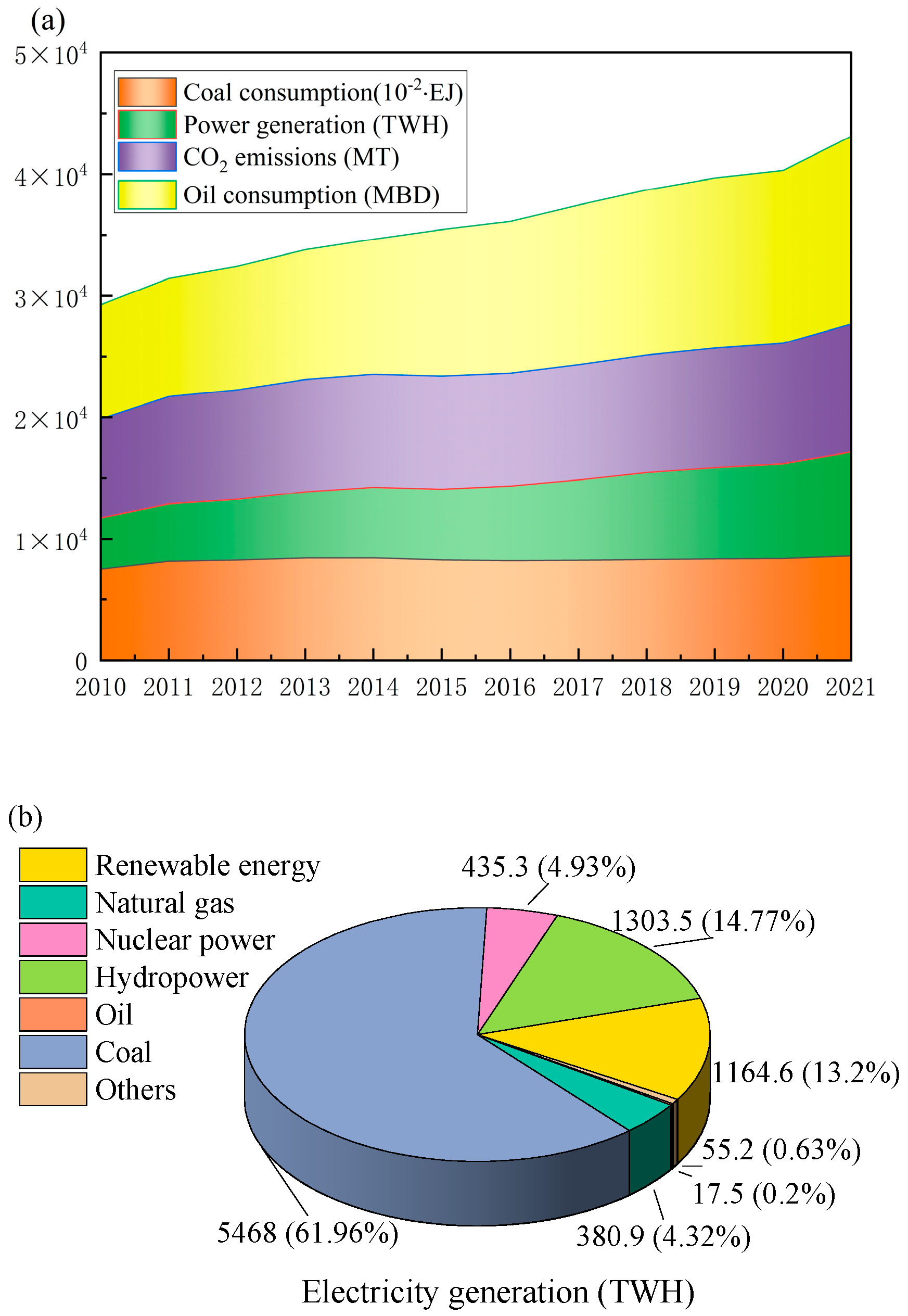
Energy is the cornerstone of contemporary civilization, a pivotal driver for economic development, and an essential material foundation indispensable for the survival and advancement of all entities. With societal development and progress, almost all sectors require increased energy, and the relentless exploitation and utilization of finite energy sources, such as coal and oil, have been ongoing. This unbridled exploitation has led to the depletion of reserves. The global energy issue has emerged as a prominent challenge faced collectively by nations across the globe. According to data from the BP 2022 Statistical Yearbook of World Energy, global primary energy consumption experienced a notable resurgence in 2021, escalating by almost 6%. China’s primary energy consumption showed an annual growth of 7.1%, accounting for approximately 70% of the nation’s total energy consumption. Energy scarcity and environmental pollution become critical bottlenecks constraining the development of ecological civilization and the low-carbon progression of the national economy. As a developing nation, China has proactively implemented measures to meet its energy conservation and carbon reduction targets. Analyzing energy consumption trends from 2010 to 2021 reveals a relatively modest increase in coal consumption (refer to
Figure 1) [
1]. Nevertheless, China’s energy consumption rate remains elevated, with electricity consumption increasing annually. The advancement of high-efficiency, energy-saving, and emission-reducing technologies holds profound strategic significance in propelling national economic development, mitigating environmental pollution, and expediting the transformation of the energy landscape.
Within industrial production, there is inefficient use of low-temperature waste heat, which contributes to energy scarcity. To address this, the proficient recovery and utilization of low-temperature waste heat is a strategic approach for its recovery. In recent years, low-temperature waste heat power generation technology has received considerable attention [
1,
2]. Notably, the ORC is a Rankine cycle employing low-boiling-point organic matter as the working fluid, offering advantages such as a broad range of applicable heat source temperatures, simple equipment, and safety and environmental benefits [
1,
3]. The properties of the organic working fluid have a significant impact on system performance [
4], which highlights the crucial importance of choosing the right organic working fluid for the effective application of ORC technology [
5,
6]. Xie Pan et al. [
7] conducted an assessment of working fluid compatibility at different waste heat temperatures, utilizing thermal efficiency as an evaluation criterion. Guo Hao et al. [
8], employing an exergic analysis methodology, evaluated diverse working fluids based on exergic efficiency and the exergic loss of individual components under specific heat source temperatures. Notably, common research practices indicate a tendency among scholars to employ one or several thermodynamic characteristics, consolidating them into a singular objective function through linear weighting for the optimization of working fluid performance, which can be subject to inherent subjectivity.
DEA is a quantitative method used to assess the relative efficacy of comparable homogeneous units. The establishment of a linear programming model, based solely on input and output indices, enables the derivation of quantitative evaluation results through solution procedures [
9]. The DEA methodology presents distinct advantages, particularly in avoiding subjective biases, simplifying the evaluation process, and enabling quantitative assessments. The methodology offers distinct advantages over traditional evaluation approaches [
10]:
Eliminates Subjectivity: Unlike weighted aggregation methods, DEA does not require predefined weights for evaluation criteria, thus avoiding the subjectivity inherent in subjective weight assignment [
11].
Handles Multi-Dimensionality: DEA effectively manages complex scenarios with multiple inputs and outputs, making it particularly suitable for evaluating systems with interdependent performance indicators.
Data-Driven Objectivity: By directly analyzing raw data without assuming functional relationships, DEA provides a transparent and empirical basis for efficiency assessment [
12].
Benchmarking Capability: DEA identifies efficient DMUs as reference points, enabling underperforming units to benchmark and improve their operations.
These characteristics make DEA an ideal tool for comprehensive performance evaluation in systems where multiple conflicting objectives need to be balanced, such as the organic Rankine cycle (ORC) working fluid selection problem addressed in this study.
This paper proposes a new multi-objective evaluation method for the selection of organic Rankine cycle (ORC) working fluids, and applies this method in the ethylene glycol plant [
13,
14]. First, we conduct ORC process simulations and optimize all candidate working fluids to determine the operating conditions that yield optimal thermal efficiency for each fluid. Under these conditions, we calculate thermodynamic, economic, and environmental indices for each candidate working fluid.
Finally, we perform a multi-objective evaluation to select a working fluid with optimal overall performance. This analytical method establishes a new criterion for screening organic working fluids in ORC applications. The main innovations and contributions include the following:
- (1)
A new methodology based on data envelopment analysis (DEA) for multi-objective assessment of workflows.
- (2)
The evaluation of 62 different working fluids and quantitative assessment of the advantages and disadvantages of these fluids in ORC systems.
- (3)
The evaluation methodology presented in this paper can be extended to the design of hybrid working fluids, thereby saving significant costs related to simulation and experimental studies. It provides a new perspective for the effective screening of working fluids in ORC systems for low-temperature waste heat power generation.
2. Optimization of the Orc System
Low-temperature waste heat power generation technology has the capability to transform the thermal energy within waste gases and liquids generated during industrial production into electrical energy. The ORC system includes an expander, a working fluid pump, and a heat exchanger, as delineated in
Figure 2. The operational principle involves pressurizing a low-temperature and low-pressure working fluid through the pump and sending the medium into the heat exchanger. Within the heat exchanger, the working fluid absorbs heat from the heat source, transitioning into a high-temperature and high-pressure gas. Subsequently, the working fluid enters the expander, whereby a portion of the working fluid’s internal energy is converted into mechanical energy.
In the context of the ORC system for low-temperature waste heat power generation, the cyclic thermal efficiency serves as a pivotal indicator, elucidating the efficacy of converting low-temperature waste heat into electrical energy. Consequently, leveraging the specified low-temperature heat source, the cyclic thermal efficiency is designated as the objective function, with the pump and expander pressures serving as control variables. The Aspen Plus process simulation software is employed for the simulation and optimization of the ORC process. This approach facilitates the derivation of optimal operating conditions for the working fluid, ensuring maximal thermal efficiency.
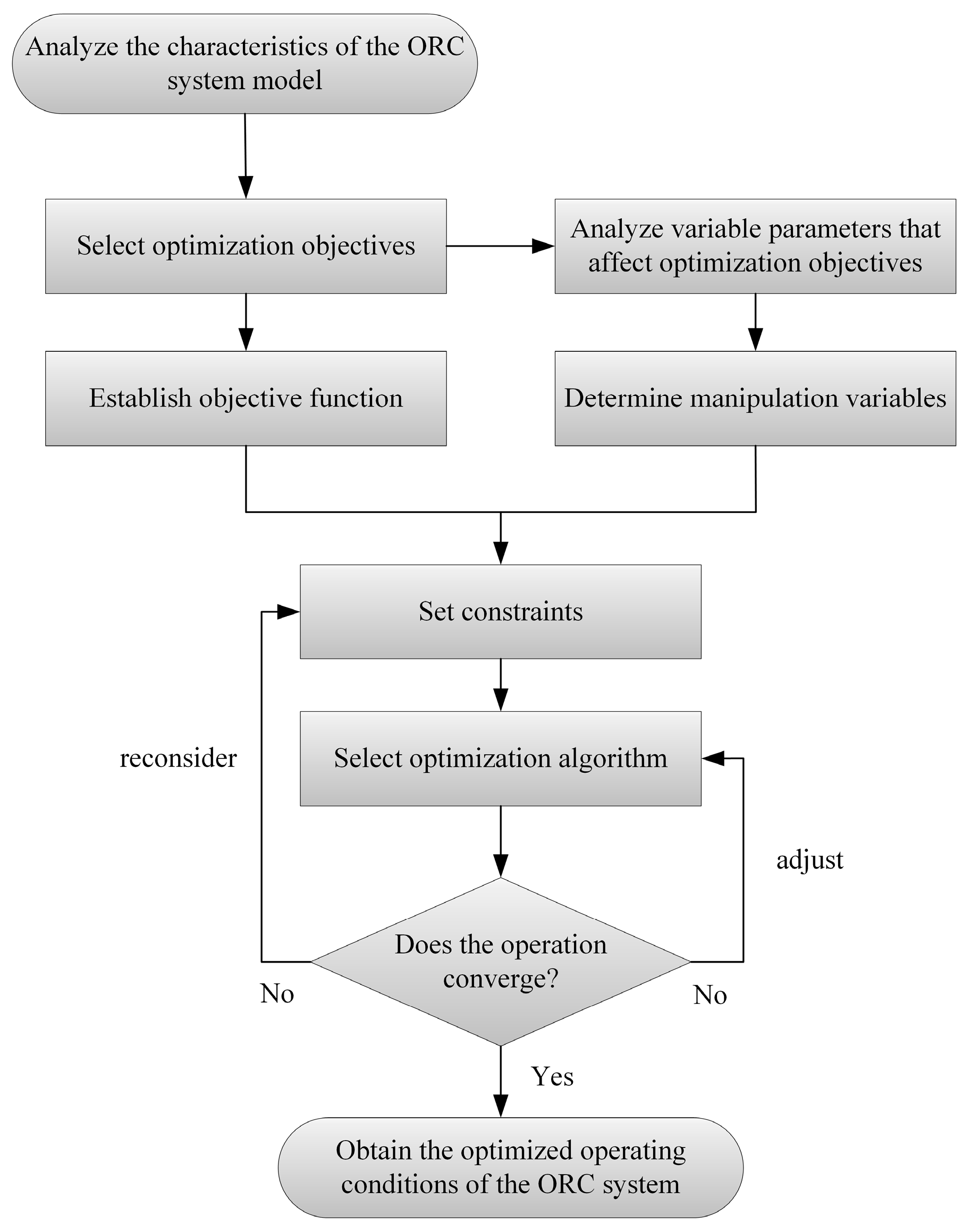
This ORC system operating status optimization diagram starts by analyzing the characteristics of the ORC system model. Then, it progresses through two parallel processes: one is selecting optimization objectives and establishing the objective function, the other is analyzing variable parameters that influence optimization objectives and determining manipulation variables. After setting constraints, an optimization algorithm is chosen. Subsequently, a convergence check is conducted. If the operation does not converge, the algorithm is adjusted or constraints are reconsidered. Once convergence is achieved, the optimized operating conditions of the ORC system are obtained. The optimization procedure is illustrated in
Figure 3.
Optimization is predicated on the following assumptions and conditions:
- (1)
The ORC system maintains a steady-state flow.
- (2)
Friction and pressure losses associated with the flow of organic working fluids in the system are disregarded.
- (3)
Isentropic efficiencies of the expander and pump are set at 0.8.
- (4)
No pressure drops are considered for the evaporator and condenser, with the evaporating temperature fixed at 120 °C.
The specific optimization model is outlined as follows:
Constraint condition:
(1) To ensure that the working fluid steam can be condensed by cooling water, the outlet temperature of the expander is set higher than the cooling water temperature:
(2) According to the cyclic process of the ORC system, the evaporation pressure is higher than the condensation pressure, meaning the outlet pressure of the expander is lower than the inlet pressure of the expander:
(3) The maximum pressure in the system is set below the corresponding subcritical pressure of the working fluid:
(4) To safeguard the expander from damage and extend its lifespan, the outlet flow rate is set to be devoid of liquid phase, and the inlet pressure is lower than the saturated vapor pressure of the working fluid at the evaporation temperature:
(5) To ensure that the expander’s outlet steam is neither superheated nor fails to condense successfully, the outlet pressure is set higher than the saturated vapor pressure of the working fluid at 40 °C:
Following optimization, the operational conditions corresponding to the optimum thermal efficiency of the ORC system are determined for the working fluid, subsequently enabling the calculation of various performance parameters.
3. Calculation of Evaluation Indicators
3.1. Thermodynamic Model
The system’s thermal efficiency, exergic efficiency, and net output work are outcomes derived from the principles of the first and second laws of thermodynamics. These metrics serve to elucidate the efficacy of waste heat recovery within the system. Specifically, the system’s thermal efficiency is articulated as follows:
QEva (kW) signifies the heat absorbed by the working fluid at intermediate pressure within the evaporator, while
Wnet (kW) denotes the system’s net output work, computed as follows:
where
mwf represents the mass flow rate of the organic working fluid (kg·s
−1) and hi signifies the enthalpy value at each point measured (J·kg
−1).
WExp (kW) quantifies the work accomplished through the entropy expansion of the working fluid within the expander, while
WPum (kW) denotes the work expended due to the entropy compression of the working fluid within the pump.
The exergic efficiency of the system is as follows:
is the exergic loss of the system and is the total exergic loss of all components in the system. The exergic loss of all components can be calculated as follows:
Exergic loss of evaporator
IEva (kW):
Exergic loss of expander
IExp (kW):
Exergic loss of condenser
ICon (kW):
Exergic loss of pump
IPum (kW):
where the subscripts delineate distinct parameters: “hot” denotes the heat flow from the heat exchanger, “
cold” designates the cold flow, “out” signifies the output of the unit component, and “
in” corresponds to the input. “
EXQ” symbolizes the heat from a constant-temperature heat source
Q, kW. “
Ei” represents the exergy of component
i, kW. “
T0” denotes the ambient temperature, K, while “Tm” signifies the temperature of the heat source, K.
3.2. Economic Model
Investment costs predominantly encompass both equipment costs and annual operating costs. The equipment cost, specifically pertaining to the expander, evaporator, condenser, and pump, can be determined through the application of the subsequent formula [
15]:
Y signifies the cost of each component (USD). For pumps and expanders,
Q represents the work (kW), while for evaporators and condensers
Q denotes the heat transfer area (m
2).
QB represents the basic size of the unit component,
M corresponds to the cost index of the unit component, and
ZB represents the benchmark cost of the unit component. The associated cost calculation coefficient for each unit component [
16,
17] is outlined in
Table 1.
Annual operating expenditures comprise both annual electricity consumption and annual water consumption. The annual cost attributed to electricity consumption is denoted as
V (USD) and determined by the subsequent formula:
Cele represents the unit price of electricity, set at 0.11 USD/kWh, and
t denotes the annual operating time of the system device, calculated as 8000 h [
18].
Annual water charges are recorded as
U (USD) and are calculated as follows:
Ccw is the unit price of water charge, set at 0.35 USD/GJ, QCon is the condenser heat load (GJ/h), and t is the system operating time in a year, calculated as 8000 h.
The ORC system will have exergic loss during operation, and the economic waste caused by exergic loss is called the exergic loss cost, which is calculated as follows:
represents the overall exergic loss cost of the system, while
,
,
, and
represent the exergic loss cost of the expander, evaporator, condenser, and pump, respectively. The formula is as follows:
indicates the exergic cost of a unit part k, Ik indicates the exergic cost of a unit part K, and indicates the average cost of fuel used per exergic unit.
For the convenience of explanation, based on the fact that the investment cost dominates compared with the operation and maintenance cost [
18], this study has employed a simplified model that ignores the operation and maintenance cost. If it is necessary to further establish a more accurate and rigorous model, the operation and maintenance cost should be taken into account.
3.3. Environmental Model
The organic Rankine cycle (ORC) system stands as a means to repurpose industrial waste heat, geothermal energy, and other energy sources, concurrently diminishing CO2 emissions in accordance with energy conservation and emission reduction imperatives. Consequently, the reduction in CO2 emissions assumes significance as a pivotal metric for gauging the operational efficacy of the system. Exergic environmental factors, including CO2 emission reduction, are chosen to appraise the merits and drawbacks of the organic working medium within the ORC system. The calculation model for these indices is articulated as follows:
System CO
2 emission reduction [
19]:
where
is the CO
2 emission coefficient: that is, the amount of CO
2 emitted during the production of unit electric energy, usually 0.872 kg/(kWh) [
20].
The exergic environmental factors of the system are as follows:
The exergy environmental factor serves as an indicator of the potential impact on the environment. A smaller exergy value implies a reduced environmental impact.
4. Multi-Objective Evaluation Strategy
The DEA method is an outcome of interdisciplinary research involving operations research, management science, and mathematical economics. Primarily applied for assessing the relative efficiency of homogeneous decision-making units (DMUs) with multiple inputs and outputs, DEA stands out as a non-parametric method.
As a non-parametric methodology that does not require predefined weights [
21], DEA automatically optimizes the combined weight of the input and output through linear programming. It is completely data-driven and avoids subjective assignment biases. Its advantages lie in not requiring preset weights. It is suitable for multi-input and multi-output scenarios and objectively reflects the relative efficiency of decision-making units.
Its principal advantage over other methods lies in its exemption from assuming the production function and estimating pertinent parameters in advance. Instead, it yields relatively objective and practical quantitative evaluation results based directly on the original input and output data. The fundamental models of DEA are categorized into the Charnes–Cooper–Rhodes (CCR) model, based on constant returns to scale [
22], and the Bankers–Charnes–Cooper (BCC) model, based on variable returns to scale [
23]. The efficiency calculated by the CCR model typically represents overall efficiency, while that derived from the BCC model signifies pure technical efficiency. In this study, we mainly focused on overall efficiency and chose the CCR model to comprehensively evaluate the working fluid of the ORC system.
Consider a scenario where there exists
n decision units (DMUs), each possessing
m inputs and
s outputs. Denoted as input
Xj and output
Yj, respectively, we express these as
Xj = (
x1j,
x2j, …,
xmj)
T,
Yj = (
y1j,
y2j, …,
ysj)
T, and
j = 1, 2, …,
n. For a designated DMU
k, with k = 1, 2, … n, the dual programming of the CCR model, essential for assessing its validity, is formulated as follows [
13]:
where
ε denotes a non-Archimedean infinitesimally small quantity (typically an arbitrarily small positive number, often set at 10
−6);
θ represents the effective value of DMUk, signifying the efficient utilization degree of output relative to input;
λ is the combination ratio of the jth decision unit within an effectively reconstructed DMU combination relative to DMUk; and
S+ denotes the slack variable, while
S− represents the residual variable, indicating potential input redundancy and output deficiency of the DMU. A DMU is deemed optimal when
θ equals 1, and both
S+ and
S− are zero. In cases where
θ equals 1 but
S+ or
S− is non-zero, the DMU is considered a weak DEA efficient. In such instances, within a system comprising DMUs, the input index can reduce
S− while maintaining the original output or increase the output
S+ without altering the original input. When
θ is less than 1, the DMU is categorized as non-DEA efficient, with smaller values indicating a greater distance from the optimal frontier.
Utilizing the DEA method, a multi-objective evaluation of the organic working fluid within the ORC system is conducted, considering performance, economy, and environmental aspects. The evaluation framework is delineated in
Figure 4. The objective of this assessment is to identify the optimal working fluid from a myriad of options for the system. As depicted in the figure, the evaluation strategy encompasses three assessment systems: thermodynamic and economic performance, thermodynamic and environmental performance, and thermodynamic, economic, and environmental performance. Through a cross-combination approach, working fluid evaluation outcomes catering to diverse user requirements are ascertained.
The working fluid is a crucial working fluid for realizing waste heat power conversion. It can transfer heat from a lower temperature to a higher temperature. The existing literature reports that there are more than 140 kinds of working fluids suitable for ORC systems. Based on the concept of low-carbon environmental protection, in order to better achieve the goal of low-carbon and near-zero emissions, according to the content of the Kigali Amendment to the Montreal Protocol, the application of hydrofluorocarbon CFCs, HCFCs, PFCs, and other working fluids has been gradually canceled, which can meet the ORC system. The working fluid is gradually reduced to more than 60 kinds. The 62 working fluids selected in this paper take into account environmental factors (global warming potential ozone depletion potential (ODP), global warming potential (GWP)), basic physical properties of working fluids, and other factors, and study the comprehensive performance of these working fluids in ORC systems, as detailed in
Table 2.
This DEA-based multi-objective evaluation strategy for ORC working fluids starts by selecting a working fluid set, obtaining its basic physical properties, and building both the ORC system and its optimization model, iterating the optimization process until convergence to acquire optimal operating parameters. It then incorporates economic performance indicators (investment cost, effective energy loss cost), thermodynamic performance indicators (thermal efficiency, exergy efficiency, net output power), and environmental performance indicators (effective energy environmental factors, CO
2 reduction emissions). Finally, through three DEA evaluations (DEA evaluation 1, DEA evaluation 2, DEA evaluation 3), the strategy integrates these multi-objective indicators and outputs comprehensive evaluation results. The procedure is illustrated in
Figure 4.
5. Results
5.1. Single-Objective Evaluation Results
Leveraging the optimization and computational outcomes (normalization) of the ORC system, a univariate evaluation of the working fluid is undertaken, employing thermodynamic performance metrics such as thermal efficiency, net output work, and exergic efficiency. Furthermore, economic performance is gauged using investment cost and exergic loss cost, while environmental considerations encompass CO2 emission reduction and exergic environmental factors. These parameters collectively serve as the evaluation indices for this comprehensive assessment of the working fluid.
5.1.1. Thermodynamic Index Evaluation Results
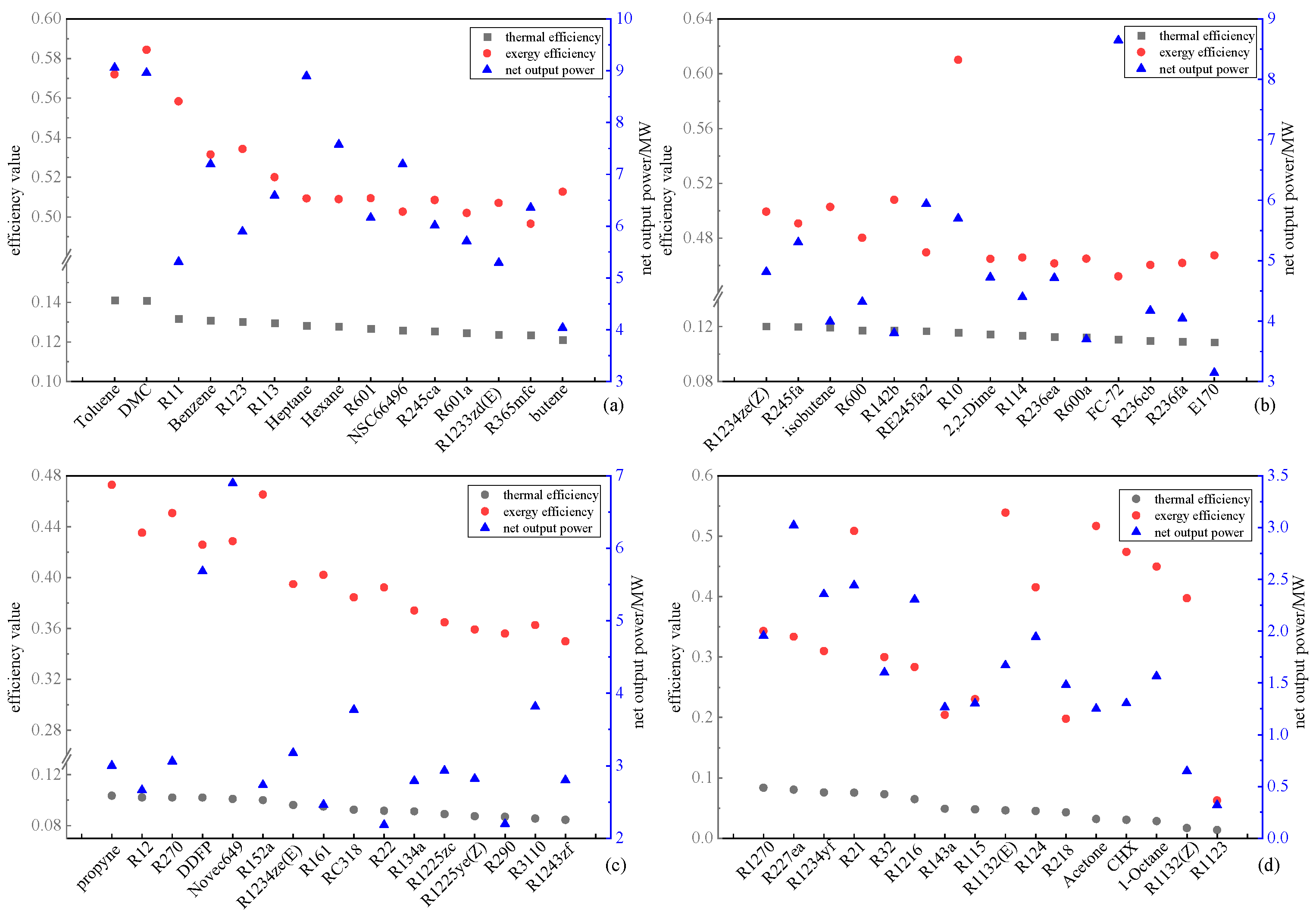
The results of the single-objective evaluation, utilizing thermodynamic performance as the primary index, are presented in
Figure 5. Thermodynamic performance serves as a metric reflecting the extent of the ORC system’s recovery and utilization of waste heat. Taking thermal efficiency as an illustrative example, a higher thermal efficiency signifies an improved waste heat recovery effect, consequently elevating the priority of the corresponding working fluid. The working fluids are categorized into four grades—A, B, C, and D—based on the thermal efficiency value, ranging from high to low, as depicted in
Figure 5 (a), (b), (c), and (d), respectively. Grade A denotes the superior working fluid, followed by grades B and C, with Grade D representing the least favorable. It is evident that substantial disparities exist in the single-objective evaluation outcomes across the three indices. For instance, working fluid R1132(E) holds the 55th and 53rd positions in the thermal efficiency and net work output evaluations, designating it as a Grade D working fluid. However, it secures the fifth position in the exergic efficiency evaluation, classifying it as a Grade A working fluid. Notably, the figure illustrates a counteractive trend between net output work and thermal efficiency for certain working fluids like Heptane, R10, DDFP, Novec649, etc. This phenomenon is linked to the working fluid’s capacity to absorb heat in the evaporator, where the rate of heat absorption surpasses the increase in net output work, resulting in a scenario where thermal efficiency decreases with the rise in net power generation.
5.1.2. Evaluation Results of Economic Indicators
As depicted in
Figure 6, both exergic cost and exergic loss cost represent expenses incurred during enterprise operations. A lower cost is advantageous for enterprise functionality, signifying a higher priority for the corresponding working fluid. Hence, the working fluids are categorized into four grades—A, B, C, and D—based on the order of investment cost, ranging from low to high. Reflecting
Figure 6 (a), (b), (c), and (d), respectively, Grade A designates the superior working fluid, followed by Grades B and C, with Grade D representing the least favorable. Notably, substantial differences emerge in the ranking of working chemicals concerning investment cost and exergic loss cost, including instances of inverse rankings. For instance, R1123 is classified as Grade A and optimal in the assessment of investment cost, while earning a Grade C in the evaluation of exergic loss cost.
5.1.3. Environmental Index Evaluation Results
Exergic environmental factors and CO
2 reduction serve as measures to assess whether an enterprise’s operations align with the principles of green and low-carbon development, as illustrated in
Figure 7. A lower exergic environmental factor implies reduced environmental impact, elevating the priority of the corresponding working fluid. Exergic environmental factors associated with working fluids are categorized into four grades—A, B, C, and D—aligning with
Figure 7 (a), (b), (c), and (d), respectively. Grade A denotes the superior working fluid, followed by Grades B and C, with Grade D representing the least favorable. Notably, working fluids such as R1132(Z), Acetone, 1-Octane, CHX, and R1132(E) exhibit minimal exergic factors, indicating optimal performance. Conversely, when CO
2 emission reduction is utilized as an evaluation index, Toluene, DMC, Heptane, and FC-72 emerge with the highest CO
2 emission reduction and superior performance. The two evaluation outcomes display inconsistencies, with some working fluids even securing opposing rankings. For instance, working fluid R1132(Z) claims the top position in the former evaluation, marking it as the best, while it ranks second to last in the latter assessment.
In conclusion, it becomes evident that the single-objective evaluation method, when applied to assess working fluids, tends to be one-sided and lacks objectivity. The adoption of multiple single-objective evaluation combinations may result in disparities or even contradictions in the categorization of working quality across different evaluation indexes, preventing the effective screening of working fluids. Consequently, we propose the subsequent multi-objective evaluation method based on the DEA method.
5.2. Results of Multi-Objective Evaluation
Exergic loss cost, investment cost, and exergic environmental factors are designated as input indices, whereas exergic efficiency, net output work, exergic efficiency, and CO2 emission reduction are designated as output indices. This consideration is based on the thermodynamic, economic, and environmental performance of the ORC system during operation, stemming from the optimization and computation results of various indices. The evaluation and screening of the working fluid for the ORC system are undertaken using the DEA multi-objective evaluation method.
5.2.1. Evaluation of Thermodynamic and Economic Performance
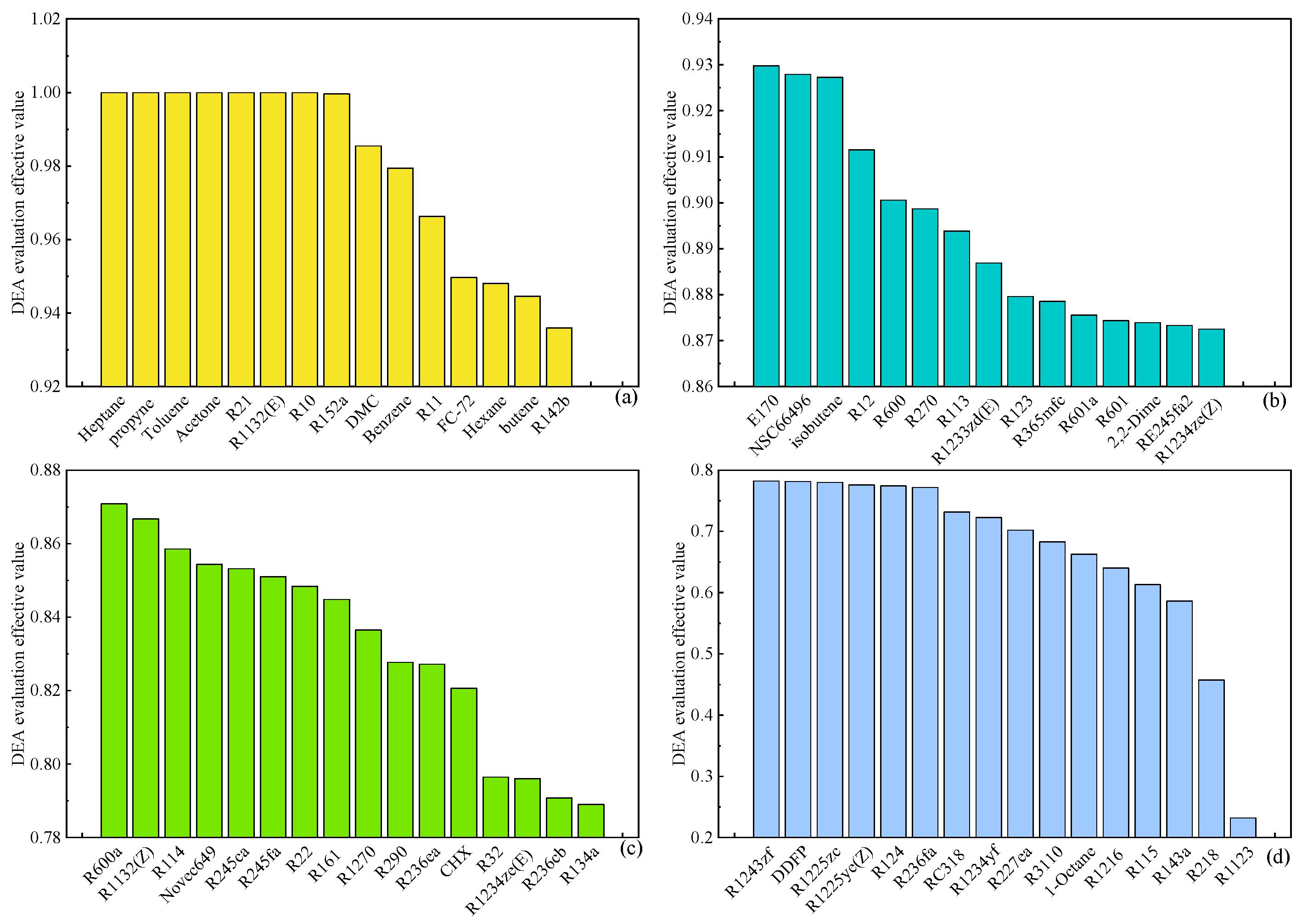
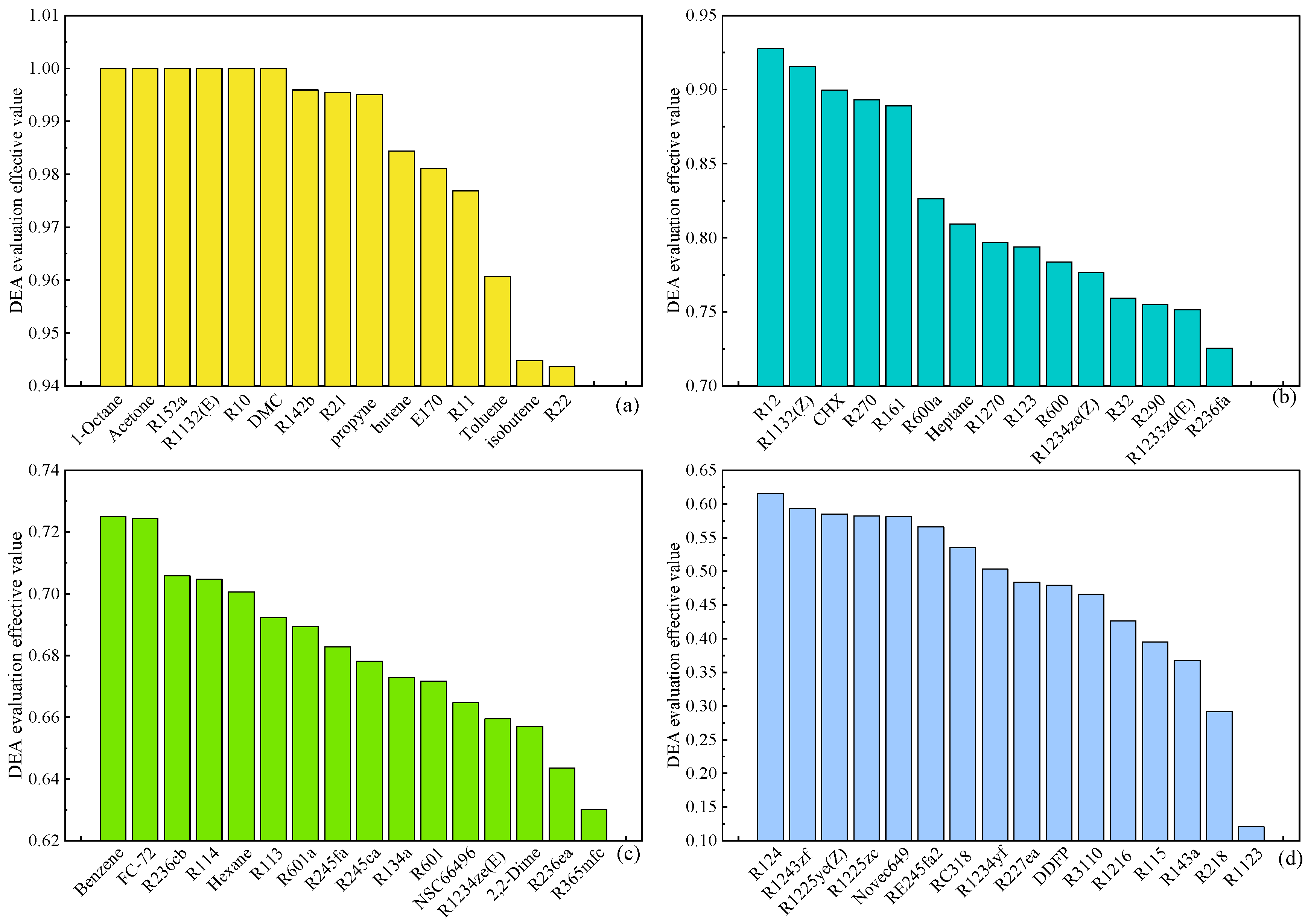
Figure 8 illustrates the outcomes of the DEA multi-objective evaluation of organic working fluids in the ORC system, utilizing thermodynamic and economic performance parameters as indices at an evaporation temperature of 120 °C. The working fluids are categorized into four grades—A, B, C, and D—based on the effective values of the DEA evaluation, corresponding to
Figure 8 (a), (b), (c), and (d), respectively, with Grade D representing the least favorable category. Working substances such as Heptane, Propyne, Toluene, Acetone, R21, R1132(E), and R10 achieved Grade A status, each with an evaluation effective value of 1. This denotes that these working substances exhibit the most comprehensive performance among all evaluated options and should be prioritized when selecting working substances for the ORC system. Conversely, working fluids like R1123, R218, R143a, and others falling under Grade D demonstrate a poor overall performance, making them unsuitable for the ORC system.
5.2.2. Evaluation Based on Thermodynamic and Environmental Performance
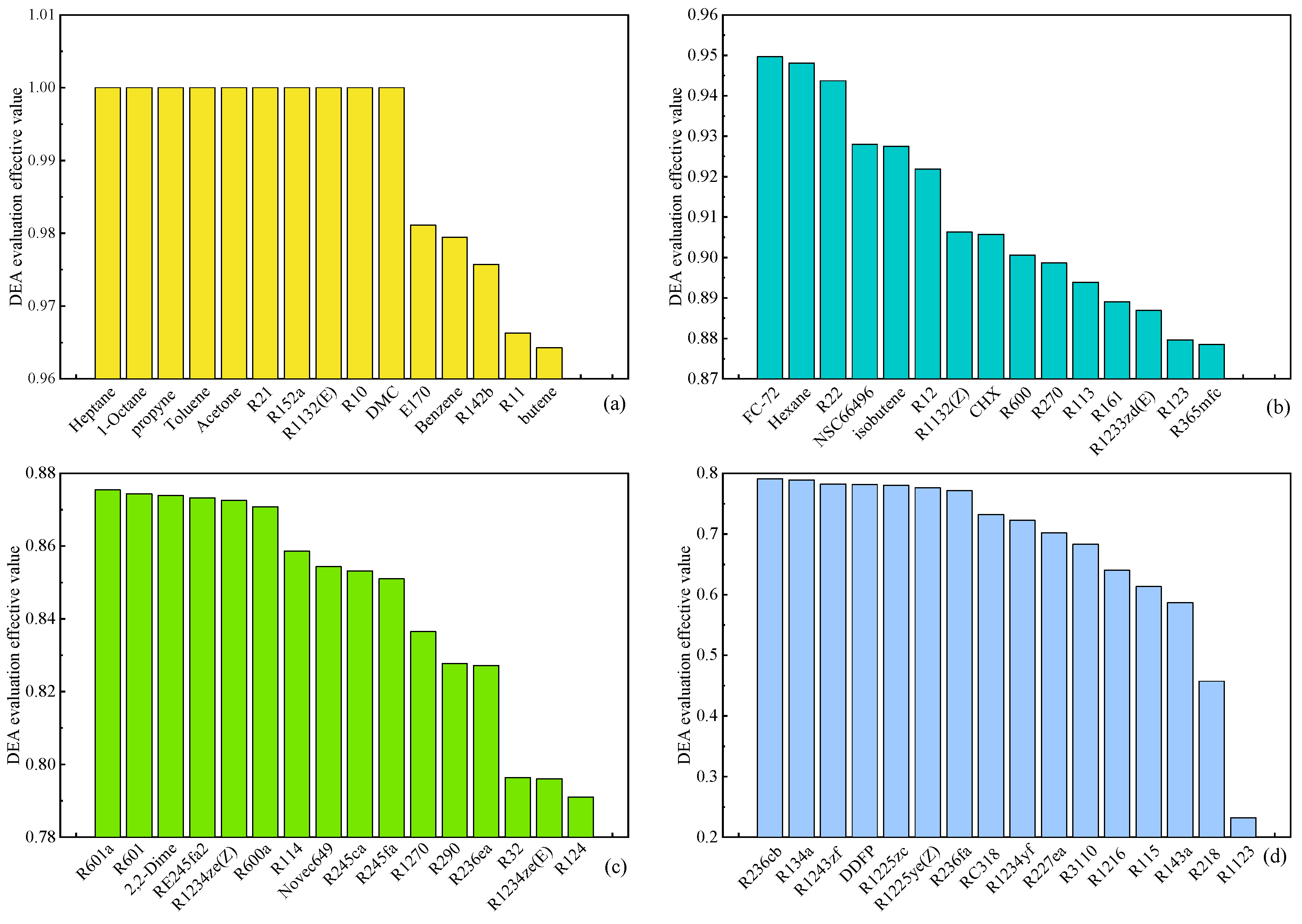
Figure 9 depicts the outcomes of the multi-objective DEA evaluation for organic working fluids in the ORC system, utilizing thermodynamic and environmental performance parameters as indices at an evaporation temperature of 120 °C. The working fluids are classified into four grades—A, B, C, and D—based on the effective values of the DEA evaluation, corresponding to
Figure 9 (a), (b), (c), and (d), respectively, with Grade D being the least favorable. Notably, working fluids such as 1-Octane, Acetone, R152a, R1132(E), R10, and DMC achieved an effective value of 1, showcasing the most optimal comprehensive performance when applied in the ORC system. On the contrary, working fluids like R1123, R218, R143a, and others falling under Grade D exhibited lower effective values and an inferior comprehensive performance compared to other options, rendering them unsuitable for use as circulating working fluids in the ORC system.
5.2.3. Evaluation Based on Thermodynamic, Economic, and Environmental Performance
The evaluation outcomes of ORC system working fluids utilizing the DEA multi-objective evaluation method are illustrated in
Figure 10. The effective value derived from the DEA evaluation serves as an indicator of the comprehensive performance of a given working fluid under the current evaluation criteria. A higher effective value correlates with a more superior comprehensive performance of the working fluid. As denoted in
Figure 10 (a), (b), (c), and (d), respectively, Grade A represents the optimal working fluid, Grade B follows, then Grade C, and finally Grade D is deemed the least favorable. Notably, Heptane, 1-Octane, Propyne, Toluene, Acetone, R21, R152a, R1132(E), R10, and DMC are classified as Grade A working substances, each achieving an effective value of 1 in the DEA evaluation. This signifies that the comprehensive performance of these working substances surpasses that of others, recommending them as the preferred circulating working fluids for the ORC system. Conversely, Class D working fluids exhibit an inferior performance compared to others, warranting their exclusion from the recommended circulating working fluids for the ORC system.
The findings reveal that the count of optimal working fluid rises as the evaporation temperature declines, aligning with the characteristic of system performance fluctuations in response to evaporation temperature variations. Discrepancies in the optimal working fluid across the three scenarios signify that the DEA method comprehensively assesses and ranks the performance of organic working fluids, taking into account variations in evaluation indices. The overall trend observed in the majority of working fluids remains consistent, indicating that the assessment ranking of working fluids adheres to the performance patterns of these fluids under each evaluation index.
In conclusion, upon contrasting the outcomes of multi-objective evaluation with those of single-objective evaluation, it becomes apparent that the evaluation and ranking outcomes of working fluid quality by the DEA method generally exhibit greater conformity with the single-objective evaluation criteria for working fluid. This alignment indicates consistency with the corresponding system performance characteristics of the working fluid. Nevertheless, within a limited scope, the ranking of working fluid deviates from the single-objective result, suggesting a comprehensive evaluation outcome achieved through the reconciliation of multiple evaluation indicators.
5.3. Application Cases of Multi-Objective Evaluation
To validate the precision of the multi-objective evaluation outcomes, we conducted applied research on the working fluid Heptane, chosen due to its commendable performance in the multi-objective evaluation results. A comparative analysis of the application effects between Heptane and the working fluid R245fa commonly used in the existing literature was undertaken [
24,
25]. The results, depicted in
Figure 11, reveal notable advantages for Heptane. Specifically, in contrast to R245fa, Heptane demonstrates a 5.02% reduction in exergy damage, a 0.24% increase in thermal efficiency, and a substantial 2.04% augmentation in net work output. These findings underscore the superior performance of Heptane, affirming the efficacy of the multi-objective evaluation approach in selecting the optimal working fluid.
6. Conclusions
In this study, single-objective and multi-objective evaluations were conducted to study the screening of working fluids in the ORC system. The limitations inherent in the single-objective evaluation approach were duly addressed. A multi-objective evaluation method, employing data envelopment analysis (DEA), was introduced to comprehensively assess and rank organic working fluids within the ORC system, facilitating the selection of the optimal working fluid.
Although some traditional refrigerants have acceptable performance in terms of CO2 emission reduction, some may have high global warming potential (GWP) values that could offset the benefits brought about by the emission reduction. Some are also accompanied by potential hazards due to their toxicity. Therefore, they need to be selected with caution. We suggest giving priority to working fluids with a low GWP and complying with safety standards to meet international environmental protection regulations and safety requirements, and to enhance the practical guiding value of the research.
The key conclusions derived from this study are as follows:
(1) Single-objective evaluations were performed for 62 working fluids, resulting in disparate screening outcomes due to the singular nature of the evaluations and subjective influences. The results indicated that Toluene and DMC have a better advantage in thermodynamic performance. The investment cost of R1123 is the lowest, while the exergy loss of R1132(Z) is the lowest.
(2) The DEA multi-objective evaluation method emerged as a robust approach capable of harmonizing diverse evaluation indicators. It swiftly and accurately gauged the comprehensive performance of various working fluids within the ORC system, delineating their prioritized applications. This method effectively overcame the limitations associated with single-objective evaluations.
(3) The working fluid Heptane obtained by DEA multi-objective evaluation showed better advantages than R245fa, the working fluid commonly used in the literature. The application results of multi-objective evaluation indicated that Heptane demonstrates a 5.02% reduction in exergy damage, a 0.24% increase in thermal efficiency, and a substantial 2.04% augmentation in net work output.
The proposed multi-objective evaluation method, grounded in DEA, offers an innovative approach for assessing and ranking ORC working fluids under distinct evaporation temperatures and varied combination indexes. This method circumvents issues related to a limited working fluid dataset and reliance on singular evaluation indices. The strategy provides precise, concise data, offering practical guidance for the intricate process of selecting working fluids, especially when confronted with variations in low-temperature heat source temperatures and diverse operational requisites in industrial production. Hence, this screening strategy holds substantial practical application value.
Author Contributions
L.Z.: Writing—original draft, Visualization, Software, Investigation, Formal analysis. L.W.: Writing—review and editing, Software, Conceptualization. X.S.: Writing—review and editing, Supervision, Conceptualization. L.X.: Supervision, Funding acquisition, Conceptualization. S.T.: Writing—review and editing, Supervision, Funding acquisition, Conceptualization. S.X.: Supervision, Funding acquisition, Conceptualization. S.J.: Supervision, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (No. 22178190), the National Youth Natural Science Foundation of China (No. 22108138, 22408195), the Key Research and Development Program of Shandong Province (Major Scientific and Technological Innovation Project) (No. 2024CXGC010405), and Shandong Provincial Key Laboratory of Chemical Process Simulation and Optimization Industrial Software (PKL2024F23).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| mass flow rate |
| power |
| heat duty |
| overall exergic loss of all components in the system |
| exergic loss of the system |
| IEva | exergy loss of the evaporator |
| IExp | exergy loss of the turbine |
| ICon | exergy loss of the condenser |
| Tm | temperature of the reservoir |
| EXQ | exergy of constant-temperature heat source Q |
| Y | cost of each component |
| specific enthalpy, (J·kg−1) |
| ambient temperature, (25 °C) |
| M | unit component cost index |
| ZB | base cost of the unit component |
| QB | basic capacity |
| V | annual cost of electricity consumption |
| Cele | unit price of electricity |
| t | operating time of the system device in a year |
| U | annual water cost |
| correction factor for materials of construction |
| correction factor for design pressure |
| correction factor for design temperature |
| overall exergic loss cost of the system |
| Ik | exergic cost of a unit part k |
| average cost of fuel per exergic unit |
| CO2 emission coefficient |
| ε | non-Archimedean infinitesimally small quantity |
| θ | effective value of DMUk |
| λ | combination proportion of the jth decision-making unit |
| exergic environmental factor |
| environmental damage effect factor |
| coefficient of environmental impact |
| exergy stability coefficient |
| Abbreviations |
| ORC | organic Rankine cycle |
| CCR | Charnes–Cooper–Rhodes |
| BCC | Banker–Charnes–Cooper |
| DEA | data envelopment analysis |
| CON | condenser |
| EVA | evaporator |
| TUR | turbine |
References
- BP. BP Statistical Review of World Energy; BP: London, UK, 2021. [Google Scholar]
- Xu, Z.Y.; Wang, R.Z.; Yang, C. Perspectives for Low-Temperature Waste Heat Recovery. Energy 2019, 176, 1037–1043. [Google Scholar] [CrossRef]
- Liu, Z.; Preston, D.J. Enhanced Condensation for Improved Energy Efficiency. Joule 2019, 3, 1182–1184. [Google Scholar] [CrossRef]
- Yang, S.; Yang, S.; Wang, Y.; Qian, Y. Low Grade Waste Heat Recovery with a Novel Cascade Absorption Heat Transformer. Energy 2017, 130, 461–472. [Google Scholar] [CrossRef]
- Emadi, M.A.; Chitgar, N.; Oyewunmi, O.A.; Markides, C.N. Working-Fluid Selection and Thermoeconomic Optimisation of a Combined Cycle Cogeneration Dual-Loop Organic Rankine Cycle (ORC) System for Solid Oxide Fuel Cell (SOFC) Waste-Heat Recovery. Appl. Energy 2020, 261, 114384. [Google Scholar] [CrossRef]
- Roumpedakis, T.C.; Loumpardis, G.; Monokrousou, E.; Braimakis, K.; Charalampidis, A.; Karellas, S. Exergetic and Economic Analysis of a Solar Driven Small Scale ORC. Renew. Energy 2020, 157, 1008–1024. [Google Scholar] [CrossRef]
- Desai, N.B.; Bandyopadhyay, S. Thermo-Economic Comparisons between Solar Steam Rankine and Organic Rankine Cycles. Appl. Therm. Eng. 2016, 105, 862–875. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Liu, Q.; Duan, Y.; Yang, Z. Thermo-Economic Performance Analyses and Comparison of Two Turbine Layouts for Organic Rankine Cycles with Dual-Pressure Evaporation. Energy Convers. Manag. 2018, 164, 603–614. [Google Scholar] [CrossRef]
- Heberle, F.; Preißinger, M.; Brüggemann, D. Zeotropic Mixtures as Working Fluids in Organic Rankine Cycles for Low-Enthalpy Geothermal Resources. Renew. Energy 2012, 37, 364–370. [Google Scholar] [CrossRef]
- Fathi, B.; Ashena, M.; Bahari, A.R. Energy, Environmental, and Economic Efficiency in Fossil Fuel Exporting Countries: A Modified Data Envelopment Analysis Approach. Sustain. Prod. Consum. 2021, 26, 588–596. [Google Scholar] [CrossRef]
- Mohd Chachuli, F.S.; Ahmad Ludin, N.; Md Jedi, M.A.; Hamid, N.H. Transition of Renewable Energy Policies in Malaysia: Benchmarking with Data Envelopment Analysis. Renew. Sustain. Energy Rev. 2021, 150, 111456. [Google Scholar] [CrossRef]
- Wang, S.; Xing, L.; Chen, X.; Song, M. Evaluating and Enhancing Natural Resource Asset Management Efficiency in China: A Data Envelopment Analysis Study. Resour. Policy 2024, 92, 105000. [Google Scholar] [CrossRef]
- Xiang, S.; Wang, L.; Wang, Y.; Bi, R.; Xia, L.; Sun, X. Exploration of Gradient Energy-Saving Separation Processes for Ethylene Glycol Mixtures Based on Energy, Exergy, Environment, and Economic Analyses. Sep. Purif. Technol. 2021, 279, 119787. [Google Scholar] [CrossRef]
- Wang, L.; Niu, B.; Xia, L.; Zhao, W.; Sun, X.; Xiang, S. Towards on Energy-Saving Separation of Ethylene Glycol Mixture from Production Plants Based on Energy, Exergy, Economic, Environmental, Exergoeconomic, and Exergoenvironmental Analyses. J. Clean. Prod. 2022, 377, 134388. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Li, X.; Zhu, Q. Sustainability Performance Analysis of Environment Innovation Systems Using a Two-Stage Network DEA Model with Shared Resources. Front. Eng. Manag. 2022, 9, 425–438. [Google Scholar] [CrossRef]
- Henriques, C.O.; Gouveia, C.M.; Tenente, M.; Da Silva, P.P. Employing Value-Based DEA in the Eco-Efficiency Assessment of the Electricity Sector. Econ. Anal. Policy 2022, 73, 826–844. [Google Scholar] [CrossRef]
- Peng, C.; Feng, D.; Guo, S. Material Selection in Green Design: A Method Combining DEA and TOPSIS. Sustainability 2021, 13, 5497. [Google Scholar] [CrossRef]
- Bi, G.; Shao, Y.; Song, W.; Yang, F.; Luo, Y. A Performance Evaluation of China’s Coal-Fired Power Generation with Pollutant Mitigation Options. J. Clean. Prod. 2018, 171, 867–876. [Google Scholar] [CrossRef]
- Song, M.-L.; Zhang, L.-L.; Liu, W.; Fisher, R. Bootstrap-DEA Analysis of BRICS’ Energy Efficiency Based on Small Sample Data. Appl. Energy 2013, 112, 1049–1055. [Google Scholar] [CrossRef]
- Jradi, S.; Chameeva, T.B.; Delhomme, B.; Jaegler, A. Tracking Carbon Footprint in French Vineyards: A DEA Performance Assessment. J. Clean. Prod. 2018, 192, 43–54. [Google Scholar] [CrossRef]
- Banker, R.D.; Charnes, A.; Cooper, W.W. Some Models for Estimating Technical and Scale Inefficiencies in Data Envelopment Analysis. Manag. Sci. 1984, 30, 1078–1092. [Google Scholar] [CrossRef]
- Lukawski, M.Z.; Tester, J.W.; DiPippo, R. Impact of Molecular Structure of Working Fluids on Performance of Organic Rankine Cycles (ORCs). Sustain. Energy Fuels 2017, 1, 1098–1111. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Teng, J.; Dong, S.; Wang, Y.; Xiang, S.; Sun, X. Study on an Energy-Saving Process for Separation Ethylene Elycol Mixture through Heat-Pump, Heat-Integration and ORC Driven by Waste-Heat. Energy 2022, 243, 122985. [Google Scholar] [CrossRef]
- Wang, E.; Zhang, H.; Fan, B.; Wu, Y. Optimized Performances Comparison of Organic Rankine Cycles for Low Grade Waste Heat Recovery. J. Mech. Sci. Technol. 2012, 26, 2301–2312. [Google Scholar] [CrossRef]
- Hu, B.; Guo, J.; Yang, Y.; Shao, Y. Selection of Working Fluid for Organic Rankine Cycle Used in Low Temperature Geothermal Power Plant. Energy Rep. 2022, 8, 179–186. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).