A Comprehensive Review on the Recent Technological Advancements in the Processing, Safety, and Quality Control of Ready-to-Eat Meals
Abstract
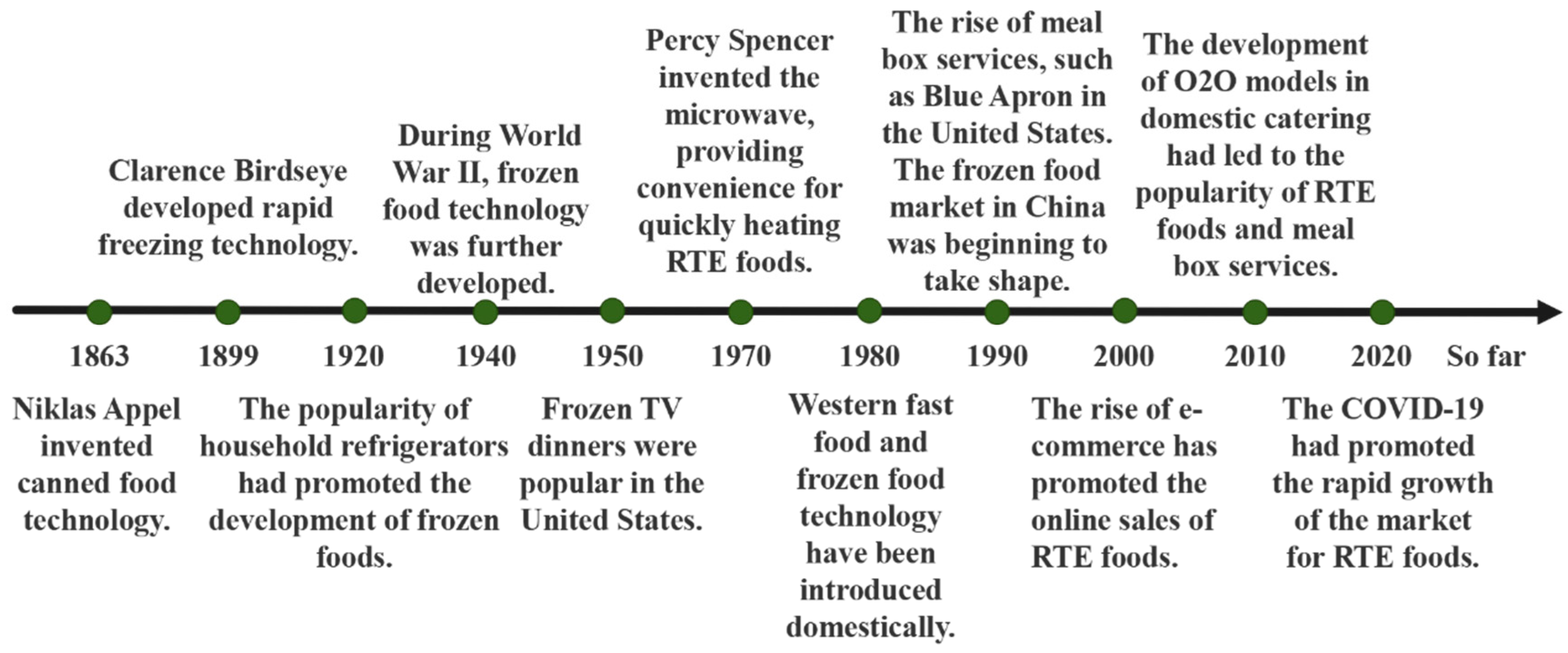
1. Introduction
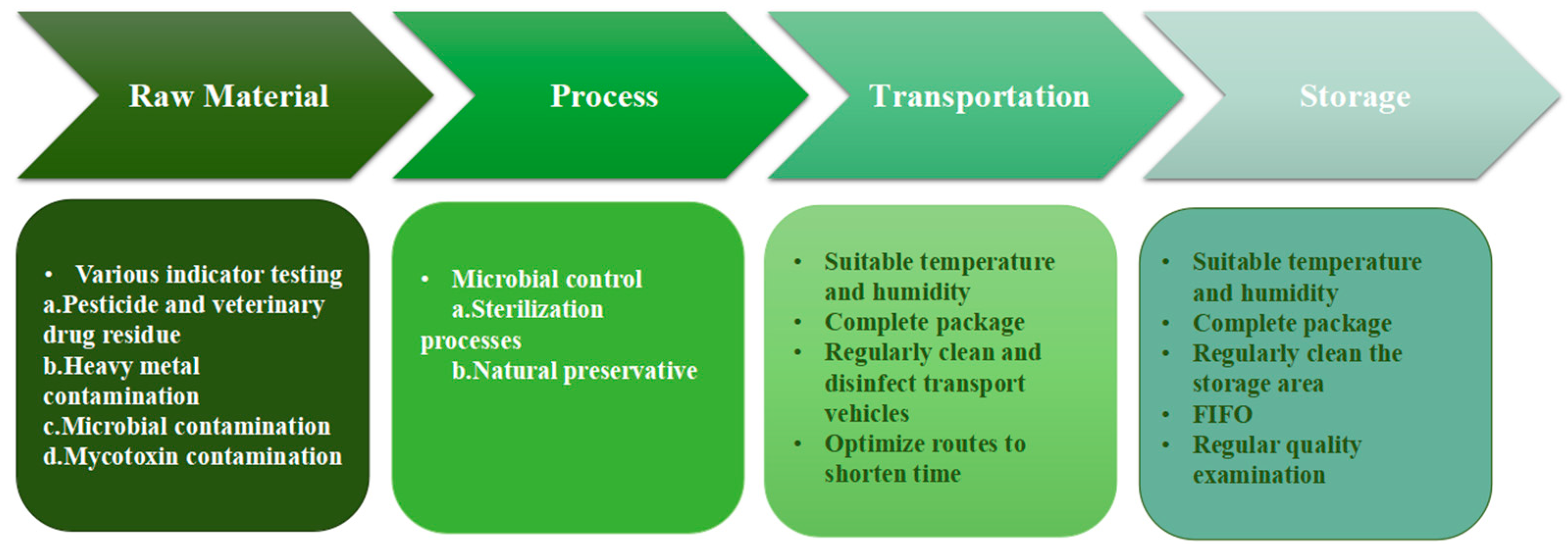
2. Quality and Safety Control of Raw Materials
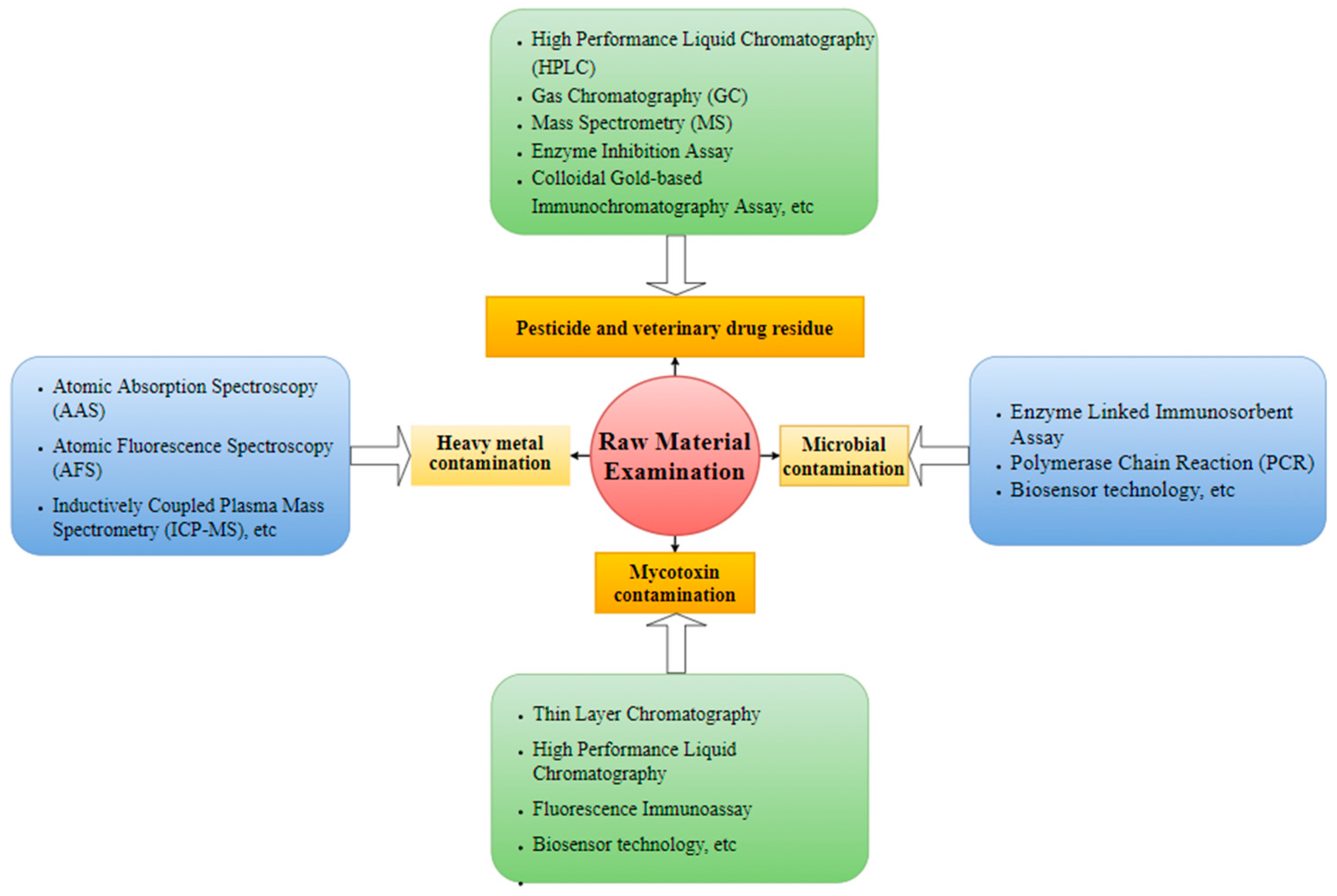
2.1. Contamination Detection in RTEMs
2.2. Adulteration in RTEMs
3. Quality and Safety Control in the Production and Processing Stages
3.1. Control of Microorganisms in RTEMs Through Sterilization Technologies
3.1.1. Conventional Thermal Sterilization
3.1.2. Irradiation Sterilization
Gamma Irradiation
X-Ray Irradiation
Electron Beam
3.1.3. Microwave Sterilization
3.1.4. Radio Frequency
3.2. Microbial Control in RTEMs Using Natural Extracts
3.2.1. Antimicrobial Activity of Natural Extracts
3.2.2. Natural Extracts for Nitrite Inhibition and Replacement
4. Packaging Technologies and Safety Control in RTEMs
4.1. Modified Atmosphere Packaging
4.2. Functional Packaging Materials
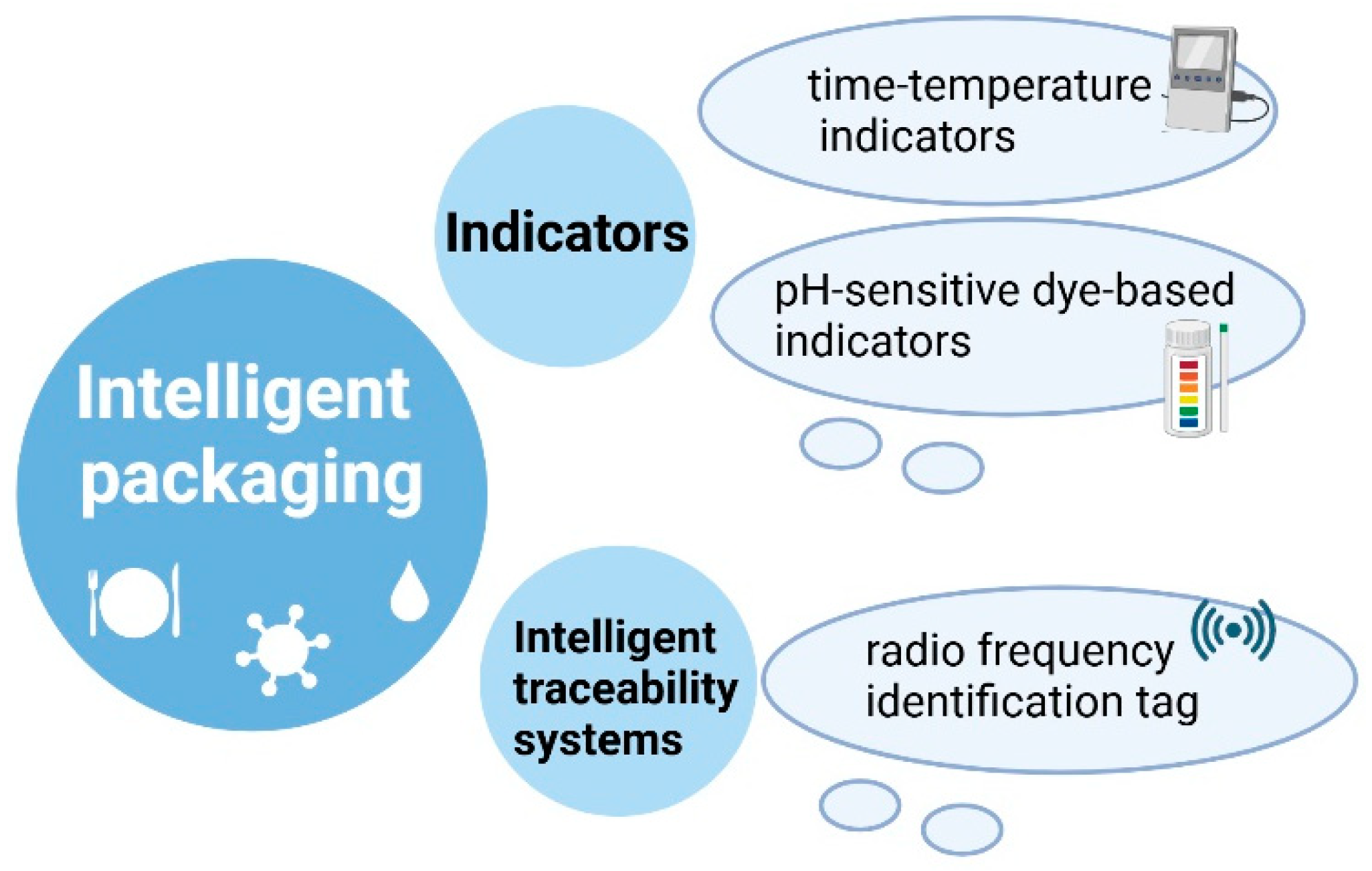
4.3. Intelligent Packaging
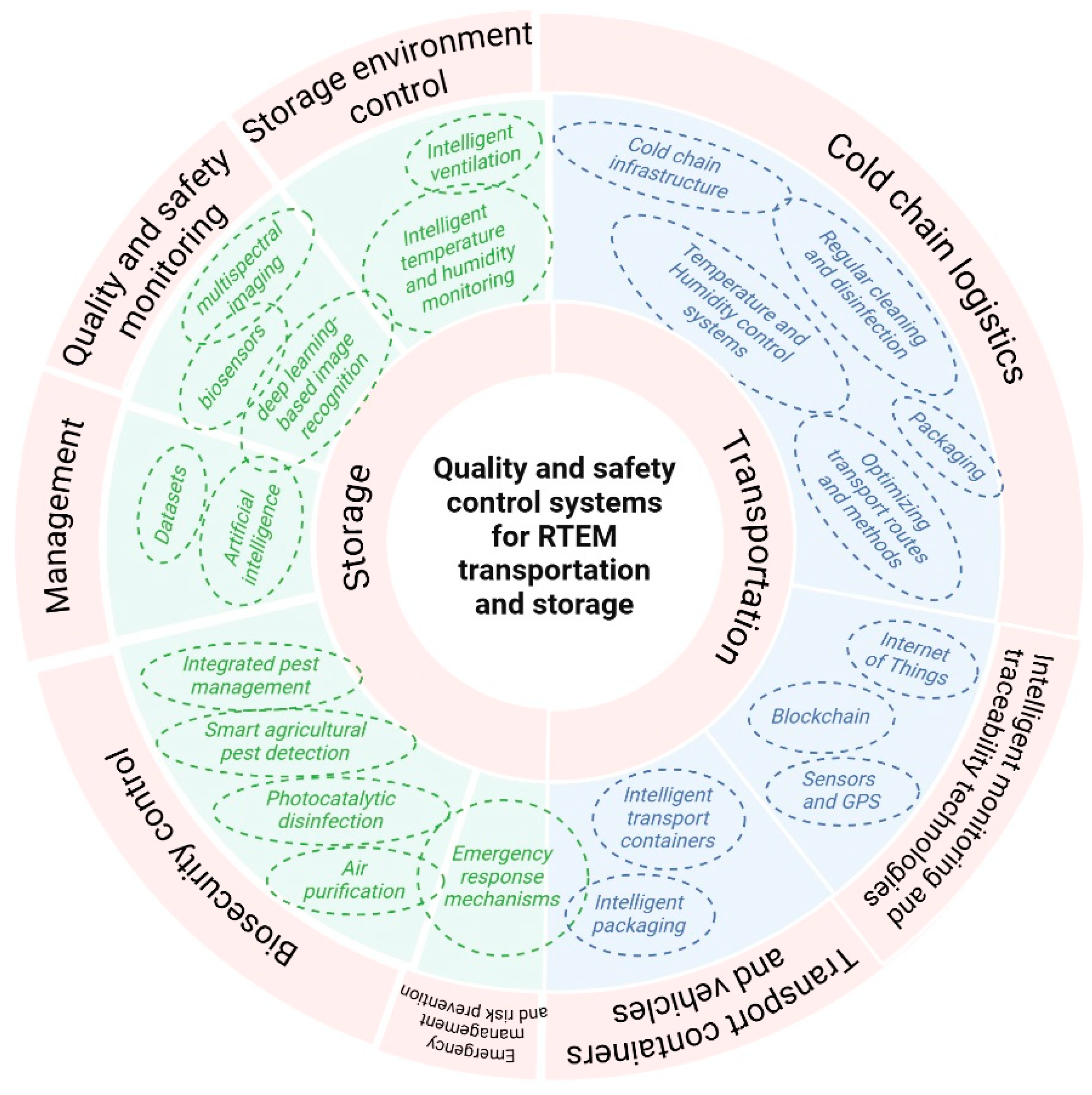
5. Quality and Safety Control Systems
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Torstensson, L.; Johansson, R.; Mark-Herbert, C. Food dishes for sustainable development: A Swedish food retail perspective. Foods 2021, 10, 932. [Google Scholar] [CrossRef]
- Mengistu, D.A.; Belami, D.D.; Tefera, A.A.; Alemeshet Asefa, Y. Bacteriological quality and public health risk of ready-to-eat foods in developing countries: Systematic review and meta-analysis. Microbiol. Insights 2022, 15, 11786361221113916. [Google Scholar] [CrossRef]
- Gizaw, Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019, 24, 68. [Google Scholar] [CrossRef]
- Ivy, R.A.; Wiedmann, M.; Boor, K.J. Listeria monocytogenes grown at 7 °C shows reduced acid survival and an altered transcriptional response to acid shock compared to L. monocytogenes grown at 37 °C. Appl. Environ. Microbiol. 2012, 78, 3824–3836. [Google Scholar] [CrossRef]
- Oliveira, H.; Blocquel, C.; Santos, M.; Fretigny, M.; Correia, T.; Gonçalves, A.; Cabado, A.G.; López, L.B.; Raaholt, B.W.; Ferraris, F.; et al. Semi-industrial development of nutritious and healthy seafood dishes from sustainable species. Food Chem. Toxicol. 2021, 155, 112431. [Google Scholar] [CrossRef]
- Wang, P.; Hu, A.; Fan, X.; Zhao, X.; Ge, Y.; Chen, Y. Bacterial communities in prepared foods available at supermarkets in Beijing, China. Food Res. Int. 2019, 120, 668–678. [Google Scholar] [CrossRef]
- Pires, S.M.; Thomsen, S.T.; Nauta, M.; Poulsen, M.; Jakobsen, L.S. Food safety implications of transitions toward sustainable healthy diets. Food Nutr. Bull. 2020, 41, 104–124. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Technologies and mechanisms for safety control of ready-to-eat muscle foods: An updated review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1886–1901. [Google Scholar] [CrossRef]
- Huang, M.S.; Zhang, M.; Bhandari, B. Recent development in the application of alternative sterilization technologies to prepared dishes: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1188–1196. [Google Scholar] [CrossRef]
- Smyth, A.B.; Song, J.; Cameron, A.C. Modified atmosphere packaged cut iceberg lettuce: Effect of temperature and O2 partial pressure on respiration and quality. J. Agric. Food Chem. 1998, 46, 4556–4562. [Google Scholar] [CrossRef]
- Makinde, O.M.; Ayeni, K.I.; Sulyok, M.; Krska, R.; Adeleke, R.A.; Ezekiel, C.N. Microbiological safety of ready-to-eat foods in low- and middle-income countries: A comprehensive 10-year (2009 to 2018) review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 703–732. [Google Scholar] [CrossRef]
- Yang, S.; Pei, X.; Yang, D.; Zhang, H.; Chen, Q.; Chui, H.; Qiao, X.; Huang, Y.; Liu, Q. Microbial contamination in bulk ready-to-eat meat products of China in 2016. Food Control 2018, 91, 113–122. [Google Scholar] [CrossRef]
- Łepecka, A.; Zielińska, D.; Szymański, P.; Buras, I.; Kołożyn-Krajewska, D. Assessment of the microbiological quality of ready-to-eat salads—Are there any reasons for concern about public health? Int. J. Environ. Res. Public Health 2022, 19, 1582. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Pan, H.; Tie, X.; Ren, Y. Simultaneous determination of 24 sulfonamide residues in meat by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2008, 1200, 144–155. [Google Scholar] [CrossRef]
- Bailac, S.; Ballesteros, O.; Jiménez-Lozano, E.; Barrón, D.; Sanz-Nebot, V.; Navalón, A.; Vílchez, J.L.; Barbosa, J. Determination of quinolones in chicken tissues by liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. A 2004, 1029, 145–151. [Google Scholar] [CrossRef]
- Ricke, S.C.; Kim, S.A.; Shi, Z.; Park, S.H. Molecular-based identification and detection of Salmonella in food production systems: Current perspectives. J. Appl. Microbiol. 2018, 125, 313–327. [Google Scholar] [CrossRef]
- Zhu, L.; He, J.; Cao, X.; Huang, K.; Luo, Y.; Xu, W. Development of a double-antibody sandwich ELISA for rapid detection of Bacillus cereus in food. Sci. Rep. 2016, 6, 16092. [Google Scholar]
- Zhu, J.; Yin, H.; Xin, Y.; Wang, L.; Geng, X.; Zhao, H.; Deng, Y. A “turn-on” fluorometric aptasensor for simultaneous detection of Hg(II) and Pb(II) in fruits and vegetables after digestion. J. Food Compos. Anal. 2025, 139, 107102. [Google Scholar] [CrossRef]
- Alieva, R.; Sokolova, S.; Zavyalova, E. A surface-enhanced Raman spectroscopy-based aptasensor for the detection of deoxynivalenol and T-2 mycotoxins. Int. J. Mol. Sci. 2024, 25, 9534. [Google Scholar] [CrossRef]
- Liu, W.G.; Su, Y.Z.; Liu, J.; Zhang, K.; Wang, X.Y.; Chen, Y.G.; Duan, L.C.; Shi, F. Determination of cyflufenamid residues in 12 foodstuffs by QuEChERS-HPLC-MS/MS. Food Chem. 2021, 362, 130148. [Google Scholar] [CrossRef]
- Zhang, L.P.; Zhang, W.Q.; Yang, W.X.; Duan, M.H.; Wei, M.Q. Study on the action mechanism of Euphorbia peplus in the treatment of Alzheimer’s disease based on UPLC-Q-TOF-MS/MS combined with network pharmacology and molecular docking. J. Agric. Biotechnol. 2023, 12, 122–131. [Google Scholar]
- Rocco, K.; Margoum, C.; Richard, L.; Coquery, M. Enhanced database creation with in silico workflows for suspect screening of unknown tebuconazole transformation products in environmental samples by UHPLC-HRMS. J. Hazard. Mater. 2022, 440, 129706. [Google Scholar] [CrossRef]
- Kong, X.H.; Kong, L.Y.; Hu, A.T.; Li, J.J.; Lu, Z.X.; Bie, X.M. Establishment of PCR assay with internal amplification control for rapid detection of Salmonella sp. Appl. Biochem. Microbiol. 2021, 57, 666–674. [Google Scholar] [CrossRef]
- Zheng, Q.; Mikš-Krajnik, M.; Yang, Y.; Xu, W.; Yuk, H.G. Real-time PCR method combined with immunomagnetic separation for detecting healthy and heat-injured Salmonella Typhimurium on raw duck wings. Int. J. Food Microbiol. 2014, 186, 6–13. [Google Scholar] [CrossRef]
- Shin, J.; Miller, M.; Wang, Y.C. Recent advances in CRISPR-based systems for the detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3010–3029. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Yin, J.; Yue, M.; Mu, Y. Microfluidic devices for multiplexed detection of foodborne pathogens. Food Res. Int. 2021, 143, 110246. [Google Scholar] [CrossRef]
- Truchado, P.; Randazzo, W. New challenges for detection and control of foodborne pathogens: From tools to people. Foods 2022, 11, 1788. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, D.; Yu, H.; Yin, H.; Wang, L.; Shen, G.; Geng, X.; Yang, L.; Fei, Y.; Deng, Y. Advances in colorimetric aptasensors for heavy metal ion detection utilizing nanomaterials: A comprehensive review. Anal. Methods 2023, 15, 6320–6343. [Google Scholar] [CrossRef]
- Cui, H.; Wang, S.; Yang, X.; Zhang, W.; Chen, M.; Wu, Y.; Li, S.; Li, L.; Cai, D.; Guo, B.; et al. Predictive models for assessing the risk of Fusarium pseudograminearum mycotoxin contamination in post-harvest wheat with multi-parameter integrated sensors. Food Chem. X 2022, 16, 100472. [Google Scholar] [CrossRef]
- Pezzolato, M.; Baioni, E.; Maurella, C.C.; Varello, K.; Meistro, S.; Balsano, A.; Bozzetta, E. Distinguishing between fresh and frozen-thawed smoked salmon: Histology to detect food adulteration in high-value products. J. Food Prot. 2020, 83, 52–53. [Google Scholar] [CrossRef]
- Sneha, S.; Surjith, S.; Raj, S.M.A. A review on food adulteration detection techniques: Methodologies, applications, and challenges. In Proceedings of the 2023 International Conference on Control, Communication and Computing (ICCC), Thiruvananthapuram, India, 19–21 May 2023; pp. 1–5. [Google Scholar]
- Anagaw, Y.K.; Ayenew, W.; Limenh, L.W.; Geremew, D.T.; Worku, M.C.; Tessema, T.A.; Simegn, W.; Mitku, M.L. Food adulteration: Causes, risks, and detection techniques—Review. SAGE Open Med. 2024, 12, 20503121241250184. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Y.; Fu, K.; Zhang, D.; Wang, Z. Progress in ICP-MS analysis of minerals and heavy metals in traditional medicine. Front. Pharmacol. 2022, 13, 891273. [Google Scholar] [CrossRef]
- Feglo, P.; Sakyi, K. Bacterial contamination of street vending food in Kumasi, Ghana. J. Med. Biomed. Sci. 2012, 1, 1–8. [Google Scholar]
- Chen, Q.; Cao, M.; Chen, H.; Gao, P.; Fu, Y.; Liu, M.; Wang, Y.; Huang, M. Effects of gamma irradiation on microbial safety and quality of stir fry chicken dices with hot chili during storage. Radiat. Phys. Chem. 2016, 127, 122–126. [Google Scholar] [CrossRef]
- Feliciano, C.P.; De Guzman, Z.M.; Tolentino, L.M.M.; Asaad, C.O.; Cobar, M.L.C.; Abrera, G.B.; Baldos, D.T.; Diano, G.T. Microbiological quality of brown rice, ready-to-eat pre-cut fresh fruits, and mixed vegetables irradiated for immuno-compromised patients. Radiat. Phys. Chem. 2017, 130, 397–399. [Google Scholar] [CrossRef]
- Feliciano, C.P.; De Guzman, Z.M.; Tolentino, L.M.; Cobar, M.L.; Abrera, G.B. Radiation-treated ready-to-eat (RTE) chicken breast adobo for immuno-compromised patients. Food Chem. 2014, 163, 142–146. [Google Scholar] [CrossRef]
- Yun, H.; Lee, K.H.; Lee, H.J.; Lee, J.W.; Ahn, D.U.; Cheorun, J. Effect of high-dose irradiation on quality characteristics of ready-to-eat chicken breast. Radiat. Phys. Chem. 2012, 81, 1107–1110. [Google Scholar] [CrossRef]
- Robertson, C.B.; Andrews, L.S.; Marshall, D.L.; Coggins, P.; Schilling, M.W.; Martin, R.E.; Collette, R. Effect of X-ray irradiation on reducing the risk of listeriosis in ready-to-eat vacuum-packaged smoked mullet. J. Food Prot. 2006, 69, 1561–1564. [Google Scholar] [CrossRef]
- Mahmoud, B.S.M.; Nannapaneni, R.; Chang, S.; Wu, Y.; Coker, R. Improving the safety and quality of raw tuna fillets by X-ray irradiation. Food Control 2016, 60, 569–574. [Google Scholar] [CrossRef]
- Peng, J.; Tang, J.; Luan, D.; Liu, F.; Tang, Z.; Li, F.; Zhang, W. Microwave pasteurization of pre-packaged carrots. J. Food Eng. 2017, 202, 56–64. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; An, Y.; Roknul, A.S.; Adhikari, B. Effects of radio frequency and high-pressure steam sterilization on the color and flavor of prepared Nostoc sphaeroides. J. Sci. Food Agric. 2018, 98, 1719–1724. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Saltaji, S.; Khlifi, M.A.; Salmieri, S.; Dang Vu, K.; Lacroix, M. Active edible coating and γ-irradiation as cold combined treatments to assure the safety of broccoli florets (Brassica oleracea L.). Int. J. Food Microbiol. 2017, 241, 30–38. [Google Scholar] [CrossRef]
- Muriana, P.M.; Quimby, W.; Davidson, C.A.; Grooms, J. Postpackage pasteurization of ready-to-eat deli meats by submersion heating for reduction of Listeria monocytogenes. J. Food Prot. 2002, 65, 963–969. [Google Scholar] [CrossRef]
- McCormick, K.; Han, I.Y.; Acton, J.C.; Sheldon, B.W.; Dawson, P.L. D- and z-values for Listeria monocytogenes and Salmonella typhimurium in packaged low-fat ready-to-eat turkey bologna subjected to a surface pasteurization treatment. Poult. Sci. 2003, 82, 1337–1342. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Isolation of Salmonella from ready-to-eat poultry meat and evaluation of its survival at low temperature, microwaving, and simulated gastric fluids. J. Food Sci. Technol. 2015, 52, 3051–3057. [Google Scholar] [CrossRef]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Radiosensitization of Salmonella spp. and Listeria spp. in ready-to-eat baby spinach leaves. J. Food Sci. 2011, 76, E141–E148. [Google Scholar] [CrossRef]
- Kim, H.J.; Chun, H.H.; Song, H.J.; Song, K.B. Effects of electron beam irradiation on the microbial growth and quality of beef jerky during storage. Radiat. Phys. Chem. 2010, 79, 1165–1168. [Google Scholar] [CrossRef]
- Cambero, M.I.; Cabeza, M.C.; Escudero, R.; Manzano, S.; Garcia-Márquez, I.; Velasco, R.; Ordóñez, J.A. Sanitation of selected ready-to-eat intermediate-moisture foods of animal origin by E-beam irradiation. Foodborne Pathog. Dis. 2012, 9, 594–599. [Google Scholar] [CrossRef]
- Soni, A.; Brightwell, G. Effect of hurdle approaches using conventional and moderate thermal processing technologies for microbial inactivation in fruit and vegetable products. Foods 2022, 11, 1811. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, P.G.; Abhilash, S.; Ravishankar, C.N.; Anandan, R.; Gopal, T.K.S. Heat penetration characteristics and quality changes of Indian mackerel (Rastrelliger kanagurta) canned in brine at different retort temperatures. J. Food Process Eng. 2010, 32, 893–915. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yue, C.Z.; Wang, Z.Q.; Wang, G.Y. Research progress of time-temperature indicators in intelligent food packaging. Food Ferment. Ind. 2023, 49, 328–334. [Google Scholar]
- Norton, T.; Sun, D.W. Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technol. 2008, 1, 2–34. [Google Scholar] [CrossRef]
- Sevenich, R.; Nieder, S.; Rauh, C.; Olivier, S.; Knoerzer, K. High-pressure thermal sterilization (HPTS) and its effect on production of food processing contaminants and quality-related properties in food in comparison to thermal-only processing. In High Pressure Thermal Processing; Knoerzer, K., Sevenich, R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 103–182. [Google Scholar]
- Wang, M.; Kong, B.; Guo, Y.; Yue, C.; Wang, G. Immobilization of laccase on sodium alginate/soluble starch microcapsules to develop a time-temperature indicator for freshness monitoring of Agaricus bisporus. Int. J. Biol. Macromol. 2024, 285, 138166. [Google Scholar]
- Zhang, J.; Cheng, J.; Li, Z.; Weng, M.; Zhang, X.; Tang, X.; Pan, Y. Effects of ultra-high pressure, thermal pasteurization, and ultra-high temperature sterilization on color and nutritional components of freshly-squeezed lettuce juice. Food Chem. 2024, 435, 137524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Meenu, M.; Hu, L.; Ren, J.; Ramaswamy, H.S.; Yu, Y. Recent progress in the synergistic bactericidal effect of high pressure and temperature processing in fruits and vegetables and related kinetics. Foods 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.M. Pasteurization of food and beverages by high-pressure processing (HPP) at room temperature: Inactivation of Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Salmonella, and other microbial pathogens. Appl. Sci. 2023, 13, 1193. [Google Scholar] [CrossRef]
- Roberts, P.B. Food irradiation is safe: Half a century of studies. Radiat. Phys. Chem. 2014, 105, 78–82. [Google Scholar] [CrossRef]
- Grandison, A. Food processing technology: Principles and practice. Int. J. Dairy Technol. 2011, 64, 455. [Google Scholar] [CrossRef]
- Smita, M.; Meera, K.; Sundaramoorthy, H.; Jha, D.; Mohan, B.C.; Pavithraa, G.; Reddy, C.K. Influence of γ-irradiation on physicochemical, functional, proximate, and antioxidant characteristics of pigmented rice flours. J. Food Sci. Technol. 2023, 60, 1621–1632. [Google Scholar] [CrossRef]
- Cha, M.Y.; Ha, J.W. Low-energy X-ray irradiation effectively inactivates major foodborne pathogen biofilms on various food contact surfaces. Food Microbiol. 2022, 106, 104054. [Google Scholar] [CrossRef]
- Mahmoud, B.S. Effect of X-ray treatments on inoculated Escherichia coli O157:H7, Salmonella enterica, Shigella flexneri, and Vibrio parahaemolyticus in ready-to-eat shrimp. Food Microbiol. 2009, 26, 860–864. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Moosavi, M.H.; Oliveira, C.A.F.; Vanin, F.; Sant’Ana, A.S. Electron beam irradiation to reduce the mycotoxin and microbial contaminations of cereal-based products: An overview. Food Chem. Toxicol. 2020, 143, 111557. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, L.; Shang, F.; Zhang, Y.; Shuai, L.; Xie, Y.; Duan, Z. Antifungal activity and mechanism of electron beam irradiation against Rhizopus oryzae. J. Food Prot. 2023, 86, 100070. [Google Scholar] [CrossRef]
- Paulina, G.; Piotr, K.; Marzena, Z.; Władysław, M. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2022, 62, 7989–8008. [Google Scholar]
- Resurreccion, F.P.; Tang, J.; Pedrow, P.; Cavalieri, R.; Liu, F.; Tang, Z. Development of a computer simulation model for processing food in a microwave-assisted thermal sterilization (MATS) system. J. Food Eng. 2013, 118, 406–416. [Google Scholar] [CrossRef]
- Hong, Y.K.; Stanley, R.; Tang, J.; Bui, L.; Ghandi, A. Effect of electric field distribution on the heating uniformity of a model ready-to-eat meal in microwave-assisted thermal sterilization using the FDTD method. Foods 2021, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Siddiqui, S.; Slim, S.; İlknur, U.; Arshad, R.N.; Bhat, Z.F.; Bhat, H.F.; Carpena, M.; Prieto, M.A.; Kaddour, A.A.; et al. Emerging technological advances in improving the safety of muscle foods: Framing in the context of the food revolution 4.0. Food Rev. Int. 2022, 40, 37–78. [Google Scholar] [CrossRef]
- Wang, K.; Ran, C.Y.; Cui, B.Z.; Sun, Y.Y.; Fu, H.F.; Chen, X.W.; Wang, Y.Q.; Wang, Y.Y. Sterilizing ready-to-eat poached spicy pork slices using a new device: Combined radio frequency energy and superheated water. Foods 2022, 11, 2841. [Google Scholar] [CrossRef]
- Horita, C.N.; Baptista, R.C.; Caturla, M.Y.R.; Lorenzo, J.M.; Barba, F.J.; Sant’Ana, A.S. Combining reformulation, active packaging, and non-thermal post-packaging decontamination technologies to increase the microbiological quality and safety of cooked ready-to-eat meat products. Trends Food Sci. Technol. 2018, 72, 45–61. [Google Scholar] [CrossRef]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, J.; Zhang, M.; Ke, W.; Shan, K.; Zhao, D.; Li, C. Effect of natural plant extracts on the quality of meat products: A meta-analysis. Food Mater. Res. 2023, 3, 1–15. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Donsì, F.; Barba, F.J.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plant extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Higginbotham, K.L.; Burris, K.P.; Zivanovic, S.; Davidson, P.M.; Stewart, C.N. Aqueous extracts of Hibiscus sabdariffa calyces as an antimicrobial rinse on hot dogs against Listeria monocytogenes and methicillin-resistant Staphylococcus aureus. Food Control 2014, 40, 274–277. [Google Scholar] [CrossRef]
- Price, A.; Díaz, P.; Bañón, S.; Garrido, M.D. Natural extracts versus sodium ascorbate to extend the shelf life of meat-based ready-to-eat meals. Food Sci. Technol. Int. 2013, 19, 427–438. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S.; Over, K.; Ricke, S.C.; Slavik, M.F.; Gbur, E.; Davis, B.; Acosta, S. Effect of partial replacement of potassium lactate and sodium diacetate by natural green tea and grape seed extracts and postpackaging thermal treatment on the growth of Listeria monocytogenes in hotdog model system. Int. J. Food Sci. Technol. 2012, 48, 918–926. [Google Scholar] [CrossRef]
- Cadet, M. Antimicrobial efficacy of flower extract from Alpinia galanga (Linn.) Swartz against Listeria monocytogenes and Staphylococcus aureus in a ready-to-eat turkey ham product. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2012. [Google Scholar]
- Odedina, G.F.; Vongkamjan, K.; Voravuthikunchai, S.P. Use of Rhodomyrtus tomentosa ethanolic leaf extract for the bio-control of Listeria monocytogenes post-cooking contamination in cooked chicken meat. J. Food Sci. Technol. 2016, 53, 4234–4243. [Google Scholar] [CrossRef]
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Ch’ng, S.E.; Younas, U.; Babji, A.S. Cinnamon bark deodorized aqueous extract as potential natural antioxidant in meat emulsion system: A comparative study with synthetic and natural food antioxidants. J. Food Sci. Technol. 2012, 51, 3269–3276. [Google Scholar] [CrossRef]
- Moarefian, M.; Barzegar, M.; Sattari, M. Cinnamomum zeylanicum essential oil as a natural antioxidant and antibacterial agent in cooked sausage. J. Food Biochem. 2013, 37, 62–69. [Google Scholar] [CrossRef]
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; de Godoy, S.H.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; de Lima, C.G.; et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016, 118, 15–21. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant extracts obtained with green solvents as natural antioxidants in fresh meat products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.X.; Zhang, M.; Adhikari, B.; Liu, K. Garlic essential oil emulsions stabilized by microwave dry-heating induced protein-pectin conjugates and their application in controlling nitrite content in prepared vegetable dishes. Food Hydrocoll. 2023, 136, 108267. [Google Scholar] [CrossRef]
- Magdalena, E.S.; Agnieszka, N.; Agata, C. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.X.; Zhang, M.; Mujumdar, A.S.; Wang, H.Q. Inhibition of nitrite in prepared dish of Brassica chinensis L. during storage via non-extractable phenols in hawthorn pomace: A comparison of different extraction methods. Food Chem. 2022, 393, 133344. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Tomović, V.; Kocić-Tanackov, S.; Đurović, S.; Zeković, Z.; Belović, M.; Torbica, A.; Jokanović, M.; Urumović, N. Tomato pomace extract and organic peppermint essential oil as effective sodium nitrite replacement in cooked pork sausages. Food Chem. 2020, 330, 127202. [Google Scholar] [CrossRef]
- Mi, K.Y.; Hwa, P.J.; Sun, Y.K. Nitrite formation from vegetable sources and its use as a preservative in cooked sausage. J. Sci. Food Agric. 2017, 97, 1774–1783. [Google Scholar]
- Erceg, T.; Vukić, N.; Šovljanski, O.; Teofilović, V.; Porobić, S.; Baloš, S.; Kojić, S.; Terek, P.; Banjanin, B.; Rakić, S. Preparation and characterization of biodegradable cellulose acetate-based films with novel plasticizer obtained by polyethylene terephthalate glycolysis intended for active packaging. Cellulose 2023, 30, 5825–5844. [Google Scholar] [CrossRef]
- Albaridi, N.A.; Badr, A.N.; Ali, H.S.; Shehata, M.G. Outstanding approach to enhance the safety of ready-to-eat rice and extend the refrigerated preservation. Foods 2022, 11, 1928. [Google Scholar] [CrossRef]
- Siriskar, D.A.; Khedkar, G.D.; Lior, D. Production of salted and pressed anchovies (Stolephorus sp.) and its quality evaluation during storage. J. Food Sci. Technol. 2013, 50, 1172–1178. [Google Scholar] [CrossRef]
- Domínguez, R.; Bohrer, B.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Sustainable production technology in food. In Packaging Systems; Lorenzo, J.M., Munekata, P.E.S., Barba, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 49–69. [Google Scholar]
- Katarzyna, M.; Patrycja, L.; Dorota, Z.; Michal, S.; Katarzyna, Z.; Piotr, L.; Anna, Z. The influence of chestnut flour on the quality of gluten-free bread. Appl. Sci. 2022, 12, 8340. [Google Scholar] [CrossRef]
- Nichols, B.W.; Morales, G.M.B.; Douglas, S.L.; Johnson, G.F.; Cordero, R.J.B.; Belk, A.D.; Ball, J.J.; Sawyer, J.T. Thermoforming vacuum packaging influences fresh pork loin chop characteristics. Foods 2024, 13, 2701. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, C.; Zhou, F.; Liu, C.; Tian, M.; Zeng, X.; Jiang, A. Vacuum packaging and ascorbic acid synergistically maintain the quality and flavor of fresh-cut potatoes. LWT 2022, 162, 113356. [Google Scholar] [CrossRef]
- Stamatis, N.; Arkoudelos, J. Quality assessment of Scomber colias japonicus under modified atmosphere and vacuum packaging. Food Control 2005, 18, 292–300. [Google Scholar] [CrossRef]
- Bassey, A.P.; Chen, Y.; Zhu, Z.; Odeyemi, O.A.; Frimpong, E.B.; Ye, K.; Li, C.; Zhou, G. Assessment of quality characteristics and bacterial community of modified atmosphere packaged chilled pork loins using 16S rRNA amplicon sequencing analysis. Food Res. Int. 2021, 145, 110412. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.; Zhu, L.; Luo, X.; Mao, Y.; Hopkins, D.L.; Zhang, Y.; Dong, P. Effect of modified atmosphere packaging on shelf life and bacterial community of roast duck meat. Food Res. Int. 2020, 137, 109645. [Google Scholar] [CrossRef]
- Cortellino, G.; Gobbi, S.; Bianchi, G.; Rizzolo, A. Modified atmosphere packaging for shelf life extension of fresh-cut apples. Trends Food Sci. Technol. 2015, 46, 320–330. [Google Scholar] [CrossRef]
- Ping, K.; Sun, J.; Yang, Y.I.; Hou, W.F.; Min, T.; Wang, H.X. Optimization of gas mixture ratio of modified atmosphere packaging for chilled meat by simplex-centroid design. Sci. Technol. Food Ind. 2016, 7, 310–315. [Google Scholar]
- Yam, K.L.; Lee, D.S. Emerging Food Packaging Technologies: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Sandhya, S. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, X.; Bhandari, B.; Fang, Z. Recent developments in film and gas research in modified atmosphere packaging of fresh foods. Crit. Rev. Food Sci. Nutr. 2016, 56, 2174–2182. [Google Scholar] [CrossRef]
- Irkin, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of cheese whey bioactive proteins and peptides in the development of antimicrobial edible film composite: A review of recent trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Iseppi, R.; Camellini, S.; Sabia, C.; Messi, P. Combined antimicrobial use of essential oils and bacteriocin bacLP17 as seafood biopreservative to control Listeria monocytogenes both in planktonic and in sessile forms. Res. Microbiol. 2020, 171, 351–356. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of titanium dioxide and zinc oxide nanoparticles supported in 4A zeolite and evaluation of the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Gupta, P.C.; Upadhyay, S.; Rai, S.; Mishra, P. Metal and metal-oxide based nanomaterials: Synthesis, agricultural, biomedical and environmental interventions. In Antimicrobial Applications of Zinc Oxide Nanoparticles in Food Packaging Industry; Bachheti, R.K., Bachheti, A., Husen, A., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 67–93. [Google Scholar]
- Lotfi, S.; Ahari, H.; Sahraeyan, R. The effect of silver nanocomposite packaging based on melt mixing and sol–gel methods on shelf life extension of fresh chicken stored at 4 °C. J. Food Process. Preserv. 2019, 39, e12625. [Google Scholar] [CrossRef]
- Deng, J.; Ding, Q.M.; Li, W.; Zhang, Y.; Chen, S. Preparation of Nano-Silver-Containing Polyethylene Composite Film and Ag Ion Migration into Food-Simulants. J. Nanosci. Nanotechnol. 2020, 20, 1613–1621. [Google Scholar] [CrossRef]
- Velásquez, E.; López de Dicastillo, C.; Patiño Vidal, C.; Rodríguez, A. Feasibility of valorization of post-consumer recycled flexible polypropylene by adding fumed nanosilica for its potential use in food packaging toward sustainability. Polymers 2023, 15, 1081. [Google Scholar] [CrossRef]
- Huang, Y.; Huo, S.; Mo, J. Highly effective and broad-spectrum antimicrobial quaternary ammonium salts containing camphene structure: Preparation, surface-active properties, and bioassay. ACS Omega 2023, 8, 34687–34697. [Google Scholar] [CrossRef]
- Hauser, C.; Thielmann, J.; Muranyi, P. Organic acids: Usage and potential in antimicrobial packaging. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 563–580. [Google Scholar]
- Sung, S.Y.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Tsironi, T.; Dermesonlouoglou, E.; Giannoglou, M.; Gogou, E.; Katsaros, G.; Taoukis, P. Shelf-life prediction models for ready-to-eat fresh cut salads: Testing in real cold chain. Int. J. Food Microbiol. 2017, 240, 131–140. [Google Scholar] [CrossRef]
- Lim, S.; Gunasekaran, S.; Imm, J.Y. Gelatin-templated gold nanoparticles as novel time–temperature indicator. J. Food Sci. 2012, 77, N45–N49. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.F.M.; Picciani, P.H.S.; Calado, V.M.A.; Tonon, R.V. Gelatin-based nanobiocomposite films as sensitive layers for monitoring relative humidity in food packaging. Food Bioprocess Technol. 2020, 13, 1063–1073. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Yousefi, H.; Ali, M.M.; Su, H.M.; Filipe, C.D.M.; Didar, T.F. Sentinel wraps: Real-time monitoring of food contamination by printing DNAzyme probes on food packaging. ACS Nano 2018, 12, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Kerry, J.P.; Butler, P. Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley & Sons: West Sussex, UK, 2008. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global food losses and food waste: Extent, causes and prevention. Food Agric. Organ. United Nations 2011, 38, 29. [Google Scholar]
- Pan, S. Research on Quality Control of Fresh Food Supply Chain. Acad. J. Bus. Manag. 2024, 6, 278–283. [Google Scholar]
- Bai, L.; Liu, M.; Sun, Y. Overview of Food Preservation and Traceability Technology in the Smart Cold Chain System. Foods 2023, 12, 2881. [Google Scholar] [CrossRef]
- Roos, Y.H. Glass transition and re-crystallization phenomena of frozen materials and their effect on frozen food quality. Foods 2021, 10, 447. [Google Scholar] [CrossRef]
- Chu, J.O.; Jeong, H.S.; Park, J.P.; Park, K.; Kim, S.K.; Yi, H.; Choi, C.H. Capsule-based colorimetric temperature monitoring system for customizable cold chain management. Chem. Eng. J. 2023, 455, 140753. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J. Design of Intelligent Temperature and Humidity Monitoring System Based on STM32. J. Phys. Conf. Ser. 2023, 2493, 012002. [Google Scholar] [CrossRef]
- Zatsu, V.; Shine, A.E.; Tharakan, J.M.; Peter, D.; Ranganathan, T.V.; Alotaibi, S.S.; Mugabi, R.; Muhsinah, A.B.; Waseem, M.; Nayik, G.A. Revolutionizing the food industry: The transformative power of Artificial Intelligence—A review. Food Chem. X 2024, 24, 101867. [Google Scholar] [CrossRef] [PubMed]
- Khade, H.D.; Saxena, S.; Hajare, S.N.; Gautam, S. Gamma radiation processing for extending shelf-life and ensuring quality of minimally processed ready-to-eat onions. J. Food Sci. Technol. 2023, 60, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Trebar, M.; Liu, X.; Li, Z.; Wu, Y.; Zhang, X. Developing an advanced multi-parameter monitoring system for wireless real-time monitoring and traceability of chilled chicken in cold chain. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019; Volume 1. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, G.; Zheng, S.; Gao, X.; Xiang, Z.; Gao, M.; Wang, C.; Liu, M.; Zhong, J. Advanced photocatalytic disinfection mechanisms and their challenges. J. Environ. Manag. 2024, 366, 121875. [Google Scholar] [CrossRef]
- Brincat, J.P.; Sardella, D.; Muscat, A.; Decelis, S.; Grima, J.N.; Valdramidis, V.; Gatt, R. A review of the state-of-the-art in air filtration technologies as may be applied to cold storage warehouses. Trends Food Sci. Technol. 2016, 50, 175–185. [Google Scholar] [CrossRef]
- Geng, Z.; Liu, F.; Shang, D.; Han, Y.; Shang, Y.; Chu, C. Early warning and control of food safety risk using an improved AHC-RBF neural network integrating AHP-EW. J. Food Eng. 2021, 292, 110239. [Google Scholar] [CrossRef]
- Torky, M.; Hassanein, A.E. Integrating blockchain and the internet of things in precision agriculture: Analysis, opportunities, and challenges. Comput. Electron. Agric. 2020, 178, 105476. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, X.; Huang, J. Adaptive multi-temperature control for transport and storage containers enabled by phase-change materials. Nat. Commun. 2023, 14, 5449. [Google Scholar] [CrossRef]
- Nnachi, R.C.; Sui, N.; Ke, B.; Luo, Z.; Bhalla, N.; He, D.; Yang, Z. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef]
- Zhu, L.; Spachos, P.; Pensini, E.; Plataniotis, K.N. Deep learning and machine vision for food processing: A survey. Curr. Res. Food Sci. 2021, 4, 233–249. [Google Scholar] [CrossRef] [PubMed]




| Food Name | Detection Index | Detection Method | Refs |
|---|---|---|---|
| Meat | Pesticide residue | LC-MS/MS | [14] |
| Chicken | Veterinary drug residue | GC-MS/MS | [15] |
| Seafood | Salmonella | Real-time PCR | [16] |
| Vegetables | Escherichia coli O157:H7 | ELISA | [17] |
| Fruit | Heavy metal | Inductively coupled plasma mass spectrometry (ICP-MS) | [18] |
| Wheat grains | T-2 mycotoxins | Surface-enhanced Raman spectroscopy (SERS) | [19] |
| Product | Sterilization Technology | Factor | Effect | Refs |
|---|---|---|---|---|
| Pre-made stir-fried chicken with chili peppers | γ-ray | 10/20 kGy | Ensure shelf stability, microbiological safety, organoleptic quality, and nutritional value of pre-made dishes | [35] |
| Pre-made mixed vegetables | γ-ray | 1 kGy | Reduced microbial contamination levels in pre-made mixed vegetables | [36] |
| Pre-made chicken breasts | γ-ray combined refrigerated and vacuum-packed processing | 25 kGy | Extend shelf life to 60 days | [37] |
| Pre-made chicken breasts | γ-ray | 40/5 kGy | After 10 days of irradiation treatment, the 40 kGy irradiated samples showed a more significant improvement in microbiological quality than the 5 kGy but were also accompanied by off-flavors that could affect sensory properties | [38] |
| Pre-made smoked osprey | X-ray | 2.0 kGy | Highly effective in killing bacteria without altering their flavor | [39] |
| Pre-made shrimp | X-ray | 0.75 kGy | Significantly reduced the initial flora on the surface of prepared shrimps below the detectable limit | [40] |
| Simulated pre-made carrots | Microwave/hot water | Retained the color of carrots better treated by microwave | [41] | |
| Pre-made carrots | Nano-zinc oxide composite radio frequency heating | 6 kW, 27 MHz, 20 min | Significantly reduced colony counts during storage and extended shelf life up to 60 days compared to carrots treated only with radio frequency heating | [42] |
| Pre-made broccoli | γ-ray with active coating | 0.4 kGy | Active coating and irradiation treatment had a synergistic sterilizing effect on prepared broccoli, extending shelf life | [43] |
| Raw tuna fillets | X-ray | 0.6 kGy | Significant (p < 0.05) decrease in Salmonella enterica counts | [40] |
| Pre-cooked ham | Thermal sterilization | 90.6–96.1 °C for 2 min | Reduced mixture of four Listeria monocytogenes strains significantly | [44] |
| Pre-made low-fat turkey jumbo salami | Thermal sterilization | 85 °C water bath heating for 10 s | Inactivated all Listeria monocytogenes on the surface | [45] |
| Pre-made poultry | Microwave | 900 W for 90 s | Effective removal targets bacteria | [46] |
| Pre-made spinach | Electron beam | Aerosol packaging (100% O2 and N2:O2 [2:1]), low dose (less than 1 kGy) | Reduced Salmonella spp. and Listeria spp. | [47] |
| Pre-made beef jerky | Electron beam | 10 kGy | Significantly reduced the total number of aerobic bacteria, increasing microbiological safety without altering the jerky quality | [48] |
| Pre-made Iberian dry-cured ham, dried beef, and smoked tuna | Electron beam | 1.5 kGy | Increased the shelf life of the product | [49] |
| Sterilization Technology | Advantages | Disadvantages |
|---|---|---|
| Thermal sterilization | Reducing/eliminating pathogens that cause foodborne illness; extend shelf life | Impact on food sensory and quality; long processing time |
| Irradiation | Reducing/eliminating pathogens that cause foodborne illness; extending shelf life; no residual hazardous substances or additional nutritional changes; high sanitation and permeability; package products; no heat or wastewater generation | Require equipment to prevent radiation leakage; produce off-flavors after irradiation; exist concerns about irradiation technology |
| Microwave | Shorten heat treatment time; reduce the impact of heat treatment on food quality; sterilization | Uneven heating |
| Radiofrequency | Rapid heating; sterilization; stronger penetration ability | Uneven heating |
| Product | Natural Additive | Effect | Refs |
|---|---|---|---|
| All-beef hot dog | Freeze-dried Hibiscus sabdariffa flower extract | 240 mg/mL was most effective in preventing or reducing Listeria monocytogenes and methicillin-resistant Staphylococcus aureus | [75] |
| Cooked pork balls | Grape seed and green tea extract | Samples containing green tea and grape seed extracts had lower thiobarbituric acid reactive substances, major volatile compounds, and microbial counts than sodium ascorbate samples and inhibited the formation of cholesterol oxidation products | [76] |
| Hot dog | Green tea (0.35%) and grape seed (0.22%) | Significant inhibited Listeria monocytogenes | [77] |
| Pre-made turkey ham | Galangal flower extract | Significantly inhibited Staphylococcus aureus and Listeria monocytogenes, with no adverse effects on sample color or pH | [78] |
| Cooked chicken | Follicular red yeast leaf ethanol extract | Inhibited Listeria monocytogenes | [79] |
| Chicken meatballs | Cinnamon extract | Decreased peroxide value without any effect on sensory properties | [80] |
| Sausages | Cinnamon essential oil | Reduced peroxide value, no effect on sensory properties | [81] |
| Pork sausage | Microencapsulated cornus officinalis extract | Reduced lipid oxidation in fresh pork sausage | [82] |
| Meat | Fruit extracts | Extend shelf life and health-promoting attributes | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xu, G.; Hu, S. A Comprehensive Review on the Recent Technological Advancements in the Processing, Safety, and Quality Control of Ready-to-Eat Meals. Processes 2025, 13, 901. https://doi.org/10.3390/pr13030901
Zhang Z, Xu G, Hu S. A Comprehensive Review on the Recent Technological Advancements in the Processing, Safety, and Quality Control of Ready-to-Eat Meals. Processes. 2025; 13(3):901. https://doi.org/10.3390/pr13030901
Chicago/Turabian StyleZhang, Zhi, Guangzhi Xu, and Shengqun Hu. 2025. "A Comprehensive Review on the Recent Technological Advancements in the Processing, Safety, and Quality Control of Ready-to-Eat Meals" Processes 13, no. 3: 901. https://doi.org/10.3390/pr13030901
APA StyleZhang, Z., Xu, G., & Hu, S. (2025). A Comprehensive Review on the Recent Technological Advancements in the Processing, Safety, and Quality Control of Ready-to-Eat Meals. Processes, 13(3), 901. https://doi.org/10.3390/pr13030901






