Construction of Novel Nanoflowering MgAl-Double Oxide Konjac Gum for Efficient Enrichment of Uranium (VI) from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of LDO-KGM
2.3. U(VI) Immobilization Experiment
2.4. Characterization Methods
3. Results and Discussion
3.1. Effect of Calcination Temperature and Doping Ratio
3.2. Characterization
3.3. Excellent Performance and Adaptability for U(VI) Removal
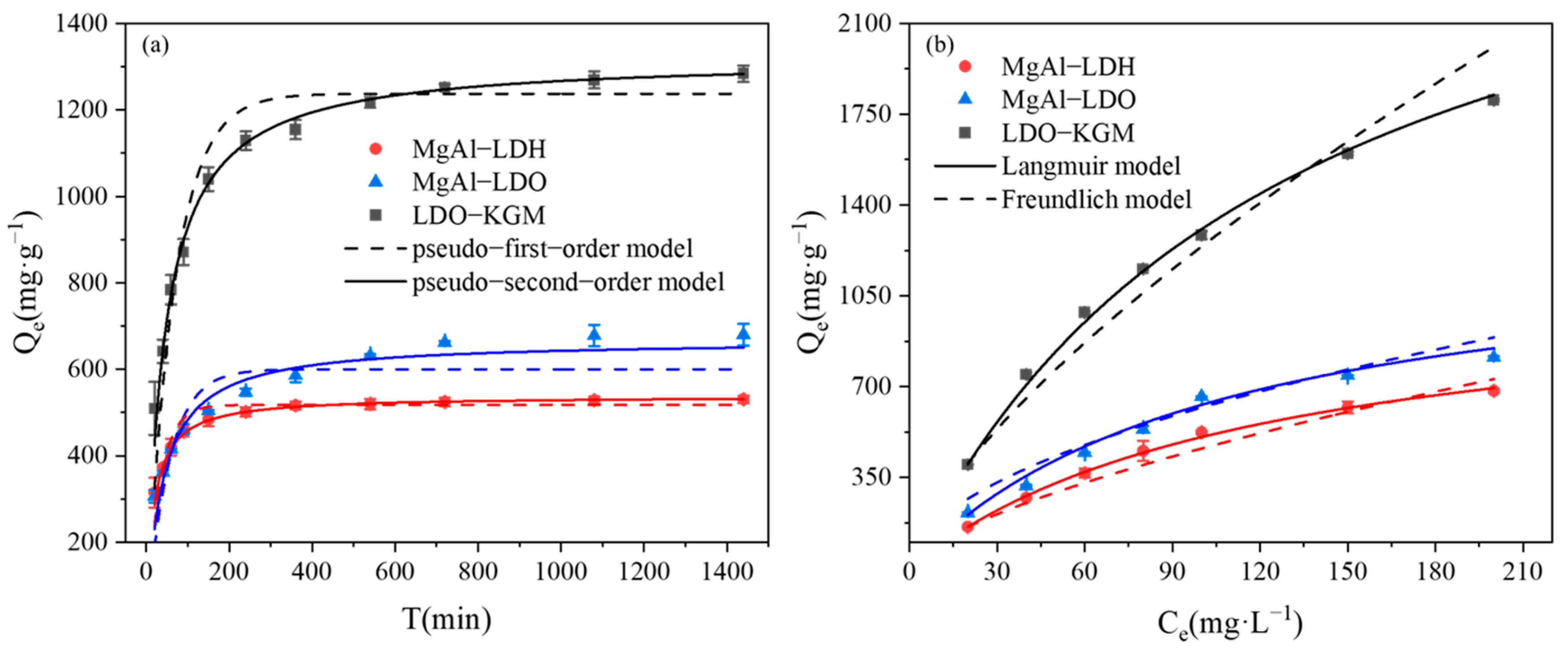
3.3.1. Kinetic Studies and Isotherm Studies
3.3.2. Effect of Coexisting Ions and pH
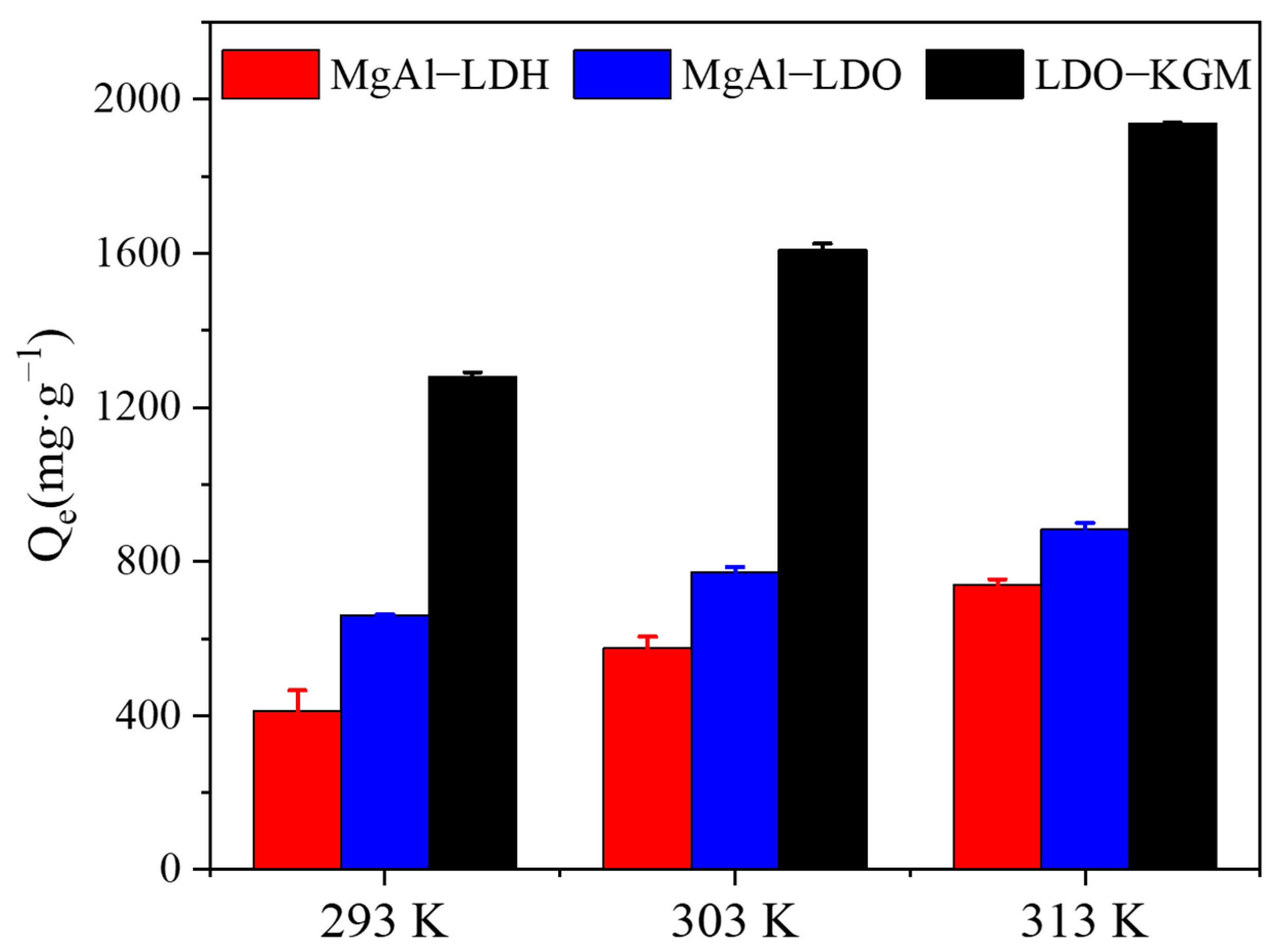
3.3.3. Effect of Temperatures
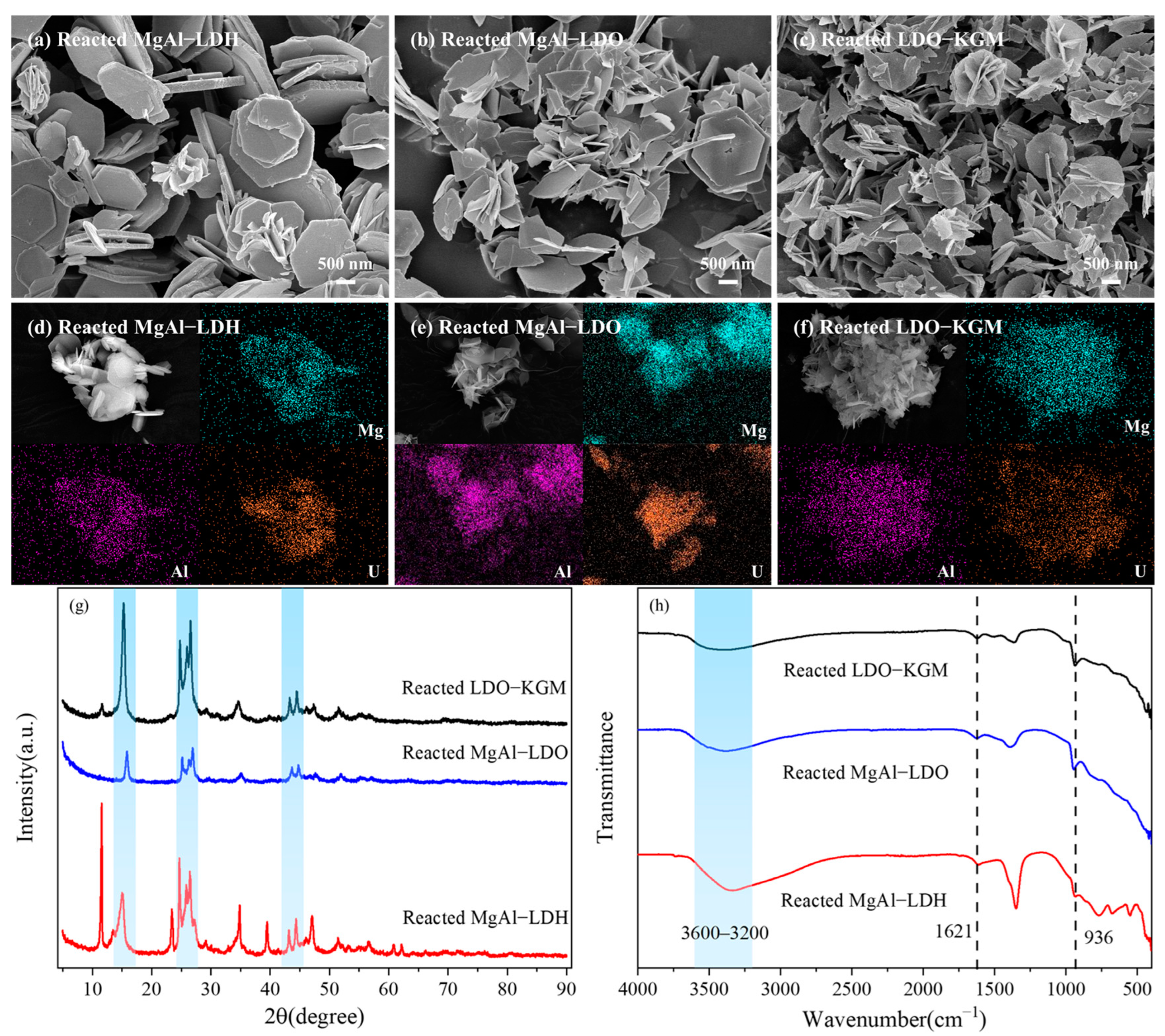
3.4. Adsorption Mechanism
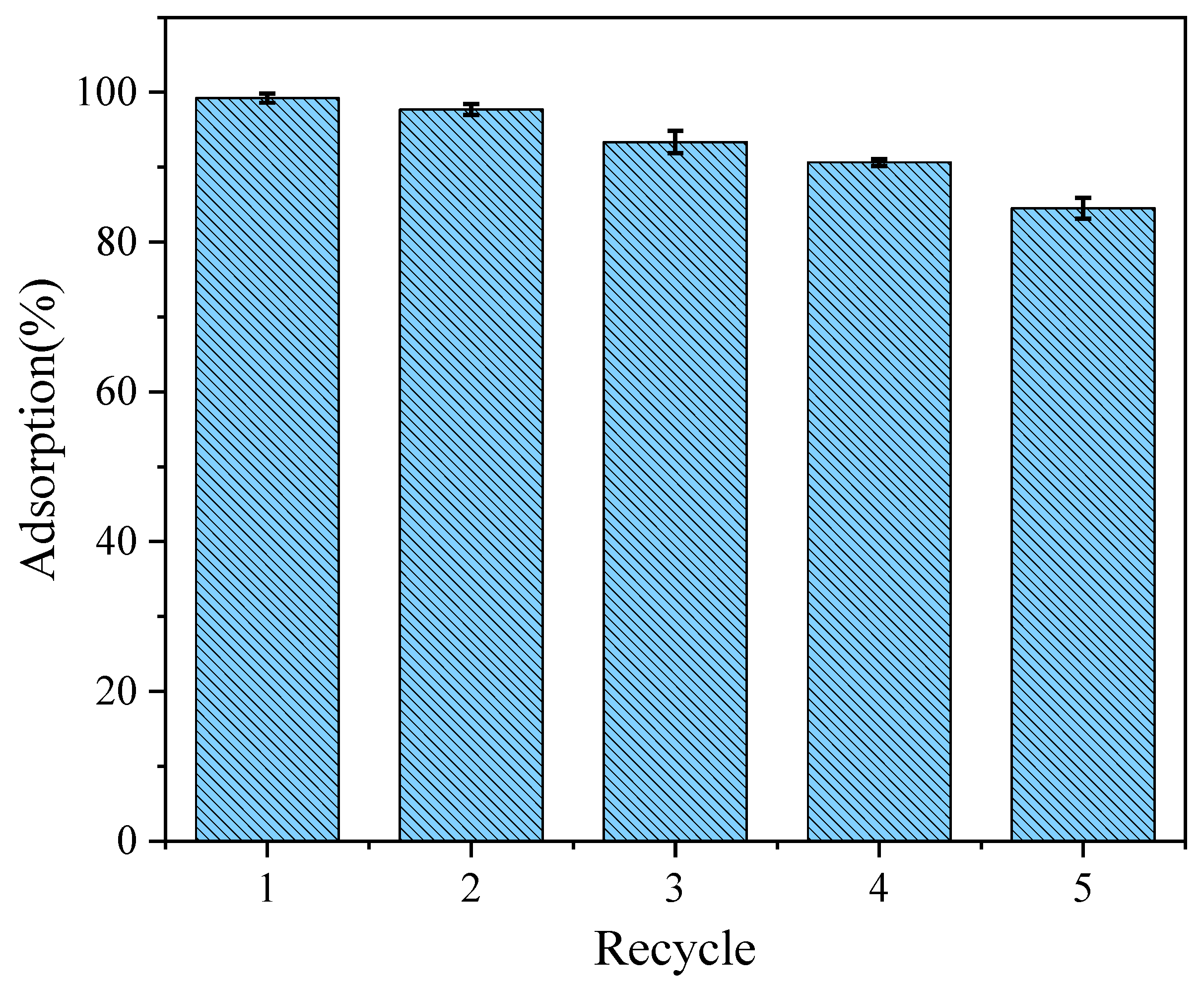
3.5. Reusability and Regeneration
4. Conclusions
5. Insufficient and Prospect
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Li, C.; Chen, X.; Fu, H.; Chen, Y.; Ning, S.; Fujita, T.; Wei, Y.; Wang, X. Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr2+ and Cs+ from contaminated water. J. Colloid Interface Sci. 2022, 615, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.; Sivaprakash, B.; Raja, V.C.V.; Rajamohan, N.; Muthusamy, G. Remediation techniques for uranium removal from polluted environment—Review on methods, mechanism and toxicology. Environ. Pollut. 2022, 302, 119068. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, W.; Chen, Y.; Wang, Y. Uranium (VI) adsorption by copper and copper/iron bimetallic central mofs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124334. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, L.; Xiong, X.; Yuan, L.; Liao, S.; Wang, Y. Removal of uranium (VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheres prepared by a controlled radical polymerization in the presence of dpe. Chem. Eng. J. 2016, 285, 358–367. [Google Scholar] [CrossRef]
- Zheng, N.; Yin, L.; Su, M.; Liu, Z.; Tsang, D.C.W.; Chen, D. Synthesis of shape and structure-dependent hydroxyapatite nanostructures as a superior adsorbent for removal of U(VI). Chem. Eng. J. 2020, 384, 123262. [Google Scholar] [CrossRef]
- Ma, M.; Ye, Z.; Zhang, J.; Wang, Y.; Ning, S.; Yin, X.; Fujita, T.; Chen, Y.; Wu, H.; Wang, X. Synthesis and fabrication of segregative and durable MnO2@chitosan composite aerogel beads for uranium(VI) removal from wastewater. Water Res. 2023, 247, 120819. [Google Scholar] [CrossRef]
- Mellah, A.; Chegrouche, S.; Barkat, M. The precipitation of ammonium uranyl carbonate (AUC): Thermodynamic and kinetic investigations. Hydrometallurgy 2007, 85, 163–171. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Mukhopadhyay, S.; Bellary, M.P.; Ghosh, S.K. Studies on membrane stability and recovery of uranium (VI) from aqueous solutions using a liquid emulsion membrane process. Hydrometallurgy 2002, 64, 49–58. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, S.L.; Vijayalakshmi, R. Uranium recovery from phosphoric acid by solvent extraction using a synergistic mixture of di-nonyl phenyl phosphoric acid and tri-n-butyl phosphate. Hydrometallurgy 2004, 73, 63–70. [Google Scholar] [CrossRef]
- Krawczyk-Bärsch, E.; Gerber, U.; Müller, K.; Moll, H.; Rossberg, A.; Steudtner, R.; Merroun, M.L. Multidisciplinary characterization of U(VI) sequestration by acidovorax facilis for bioremediation purposes. J. Hazard. Mater. 2018, 347, 233–241. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Li, X.; Liao, J.; Li, S.; Yang, J.; Yang, Y.; Tang, J.; Liu, N. Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem. Eng. J. 2016, 284, 630–639. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Li, B.; Zhang, M.; Wen, R.; Guo, X.; Li, X.; Zhang, J.; Li, S.; Ma, L. Pore-free matrix with cooperative chelating of hyperbranched ligands for high-performance separation of uranium. ACS Appl. Mater. Interfaces 2016, 8, 28853–28861. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Wang, X.; Dai, Y.; Cao, X.; Wang, Y.; Hua, R.; Feng, H.; Chen, J.; Liu, Y.; et al. Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U(VI): Batch and fixed-bed column studies. Chem. Eng. J. 2019, 370, 1376–1387. [Google Scholar] [CrossRef]
- Zhang, H.; Ruan, Y.; Liang, A.; Shih, K.; Diao, Z.; Su, M.; Hou, L.; Chen, D.; Lu, H.; Kong, L. Carbothermal reduction for preparing nZVI/BC to extract uranium: Insight into the iron species dependent uranium adsorption behavior. J. Clean. Prod. 2019, 239, 117873. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Khan, Z.H.; Ying, H.; Chen, D. Utilization of citrullus lanatus l. Seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: Insights and comparison between modified and raw biochar. Sci. Total Environ. 2021, 771, 144955. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Highly enhanced adsorption performance to uranium(VI) by facile synthesized hydroxyapatite aerogel. J. Hazard. Mater. 2022, 423, 127184. [Google Scholar] [CrossRef]
- Bachmaf, S.; Merkel, B.J. Sorption of uranium(VI) at the clay mineral–water interface. Environ. Earth Sci. 2011, 63, 925–934. [Google Scholar] [CrossRef]
- Liu, S.; Luo, M.; Li, J.; Luo, F.; Ke, L.; Ma, J. Adsorption equilibrium and kinetics of uranium onto porous azo-metal–organic frameworks. J. Radioanal. Nucl. Chem. 2016, 310, 353–362. [Google Scholar] [CrossRef]
- Yang, P.; Li, S.; Liu, C.; Liu, X. Interface-constrained layered double hydroxides for stable uranium capture in highly acidic industrial wastewater. ACS Appl. Mater. Interfaces 2021, 13, 17988–17997. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Xie, L.; Zhong, Y.; Xiang, R.; Fu, G.; Xu, Y.; Cheng, Y.; Liu, Z.; Wen, T.; Zhao, Y.; Liu, X. Sono-assisted preparation of Fe(II)-Al(III) layered double hydroxides and their application for removing uranium (VI). Chem. Eng. J. 2017, 328, 574–584. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Wang, X.; Sheng, G.; Wang, S.; Ai, Y.; Ji, Y.; Liu, Y.; Hayat, T.; Wang, X. Glycerol-modified binary layered double hydroxide nanocomposites for uranium immobilization via extended x-ray absorption fine structure technique and density functional theory calculation. ACS Sustain. Chem. Eng. 2017, 5, 3583–3595. [Google Scholar] [CrossRef]
- Yao, W.; Yu, S.; Wang, J.; Zou, Y.; Lu, S.; Ai, Y.; Alharbi, N.S.; Alsaedi, A.; Hayat, T.; Wang, X. Enhanced removal of methyl orange on calcined glycerol-modified nanocrystallined Mg/Al layered double hydroxides. Chem. Eng. J. 2017, 307, 476–486. [Google Scholar] [CrossRef]
- Momin, Z.H.; Lingamdinne, L.P.; Kulkarni, R.; Pal, C.A.; Choi, Y.; Chang, Y.; Koduru, J.R. Exploring recyclable alginate-enhanced gcn-ldo sponge for U(VI) and Cd(II) removal: Insights from batch and column studies. J. Hazard. Mater. 2024, 469, 134015. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Wang, H.; Xie, Y.; Wakeel, M.; Wahid, A.; Zhang, X. Gamma-ferric oxide nanoparticles decoration onto porous layered double oxide belts for efficient removal of uranyl. J. Colloid Interface Sci. 2019, 535, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, X.; Liang, Y.; Yu, S.; Gu, P.; Sun, Y.; Xu, C.; Chen, J.; Hayat, T.; Alsaedi, A.; et al. Synthesis of novel flower-like layered double oxides/carbon dots nanocomposites for U(VI) and 241Am(III) efficient removal: Batch and exafs studies. Chem. Eng. J. 2018, 332, 775–786. [Google Scholar] [CrossRef]
- Chen, T.; Shi, P.; Zhang, J.; Li, Y.; Duan, T.; Dai, L.; Wang, L.; Yu, X.; Zhu, W. Natural polymer konjac glucomannan mediated assembly of graphene oxide as versatile sponges for water pollution control. Carbohydr. Polym. 2018, 202, 425–433. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, J.; Jiang, C.; Liu, S.; Li, Y. Ultralight, hydrophobic, monolithic konjac glucomannan-silica composite aerogel with thermal insulation and mechanical properties. Carbohydr. Polym. 2019, 207, 246–255. [Google Scholar] [CrossRef]
- Wang, R.; Dong, H.; Li, X.; Zhou, L.; Zhu, W.; Chen, T. Biomass encapsulated zif-8-derived ZnO carbon aerogels for efficient uranium extraction by synergistic adsorption-photoreduction. Chem. Eng. J. 2023, 478, 147331. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Zhang, L. Improved catalytic activity on the thermal decomposition of ammonium perchlorate and efficient adsorption of uranium using a novel ultra-low density Al2O3-based aerogels. J. Hazard. Mater. 2020, 387, 122015. [Google Scholar] [CrossRef]
- Hu, W.; Sun, T.; Liu, C.; Yu, L.; Ahamad, T.; Ma, Z. Refined microstructure and enhanced mechanical properties in mo-Y2O3 alloys prepared by freeze-drying method and subsequent low temperature sintering. J. Mater. Sci. Technol. 2021, 88, 36–44. [Google Scholar] [CrossRef]
- Hu, W.; Kong, X.; Du, Z.; Khan, A.; Ma, Z. Synthesis and characterization of nano tic dispersed strengthening w alloys via freeze-drying. J. Alloy. Compd. 2021, 859, 157774. [Google Scholar] [CrossRef]
- Tang, M.; Chen, J.; Wang, P.; Wang, C.; Ao, Y. Highly efficient adsorption of uranium(VI) from aqueous solution by a novel adsorbent: Titanium phosphate nanotubes. Environ. Sci. Nano 2018, 5, 2304–2314. [Google Scholar] [CrossRef]
- Xiong, J.; Fan, Y.; Luo, F. Grafting functional groups in metal–organic frameworks for U(VI) sorption from aqueous solutions. Dalton Trans. 2020, 49, 12536–12545. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, J.; Kang, Y.; Zhang, H.; Ge, Y.; Yuan, N.; Xing, Y.; Ma, W.; Yang, Z.; Zou, L.; et al. Ultralight carbon aerogel composites derived from MOFs and cross-linked chitosan: Synthesis, characterization, and U(VI) adsorption. Chem. Eng. J. 2023, 455, 140749. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Li, L.; Jiang, L.; Ahmed, Z.; Dang, Z.; Wu, P. Memory effect induced the enhancement of uranium (VI) immobilization on low-cost MgAl-double oxide: Mechanism insight and resources recovery. J. Hazard. Mater. 2021, 401, 123447. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Gong, B.; Fang, Y.; Zhu, N. Fabrication and photocatalytic properties of a visible-light responsive nanohybrid based on self-assembly of carboxyl graphene and ZnAl layered double hydroxides. J. Mater. Chem. A 2014, 2, 5534–5540. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Zhang, Y.; Guo, Z.; Luo, Y.; Mao, C. Engineering ultrafine nis cocatalysts as active sites to boost photocatalytic hydrogen production of MgAl layered double hydroxide. Appl. Surf. Sci. 2020, 506, 144999. [Google Scholar] [CrossRef]
- Lv, X.; Qin, X.; Wang, K.; Peng, Y.; Wang, P.; Jiang, G. Nanoscale zero valent iron supported on MgAl-LDH-decorated reduced graphene oxide: Enhanced performance in Cr(VI) removal, mechanism and regeneration. J. Hazard. Mater. 2019, 373, 176–186. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Zhu, Y.; Tran, L. Simultaneous adsorption of Cd2+ and bpa on amphoteric surfactant activated montmorillonite. Chemosphere 2016, 144, 1026–1032. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Xu, M.; Chen, K.; Tan, X.; Wakeel, M.; Alharbi, N.S. In situ carbothermal reduction synthesis of Fe nanocrystals embedded into n-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction. Chem. Eng. J. 2018, 331, 395–405. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Wang, X.; Xie, Z.; Deng, N. Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J. Hazard. Mater. 2009, 168, 1053–1058. [Google Scholar] [CrossRef]
- Wu, P.; Dai, Y.; Long, H.; Zhu, N.; Li, P.; Wu, J.; Dang, Z. Characterization of organo-montmorillonites and comparison for Sr(II) removal: Equilibrium and kinetic studies. Chem. Eng. J. 2012, 191, 288–296. [Google Scholar] [CrossRef]
- Yin, L.; Hu, Y.; Ma, R.; Wen, T.; Wang, X.; Hu, B.; Yu, Z.; Hayat, T.; Alsaedi, A.; Wang, X. Smart construction of mesoporous carbon templated hierarchical Mg-Al and Ni-Al layered double hydroxides for remarkably enhanced U(VI) management. Chem. Eng. J. 2019, 359, 1550–1562. [Google Scholar] [CrossRef]
- Pang, H.; Wu, Y.; Huang, S.; Ding, C.; Li, S.; Wang, X.; Yu, S.; Chen, Z.; Song, G.; Wang, X. Macroscopic and microscopic investigation of uranium elimination by Ca–Mg–Al-layered double hydroxide supported nanoscale zero valent iron. Inorg. Chem. Front. 2018, 5, 2657–2665. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Liu, Q.; Wang, J.; Jing, X.; Liu, L.; Liu, J.; Song, D. Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Q.; Liu, J.; Chen, R.; Zhang, H.; Li, R.; Wang, J. Ni–Mn LDH-decorated 3d Fe-inserted and n-doped carbon framework composites for efficient uranium(VI) removal. Environ. Sci. Nano 2018, 5, 467–475. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Li, K.; Zhang, Y.; Zhu, W. Construction of novel magnesium oxide aerogel for highly efficient separation of uranium(VI) from wastewater. Sep. Purif. Technol. 2022, 295, 121296. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Wang, J.; Xu, C.; Liang, Y.; Yao, W.; Wang, X.; Yu, S.; Chen, J.; Sun, Y.; et al. Superior immobilization of U(VI) and 243Am(III) on polyethyleneimine modified lamellar carbon nitride composite from water environment. Chem. Eng. J. 2017, 326, 863–874. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ou, X.; Li, J.; Zhou, Y.; Zhang, Z.; Dong, S.; Lin, Z. Interfacial engineering improved the selective extraction of uranyl from saline water by nano-Mg(OH)2 and the underlying mechanism. ACS Sustain. Chem. Eng. 2016, 4, 801–809. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y. Effective removal of uranium from aqueous solution by using novel sustainable porous Al2O3 materials derived from different precursors of aluminum. Inorg. Chem. Front. 2020, 7, 765–776. [Google Scholar] [CrossRef]
- Liu, J.; Shi, S.; Li, C.; Hong, X.; Gu, Z.; Li, F.; Zhai, J.; Zhang, Q.; Liao, J.; Liu, N.; et al. U(VI) adsorption by one-step hydrothermally synthesized cetyltrimethylammonium bromide modified hydroxyapatite-bentonite composites from phosphate-carbonate coexisted solution. Appl. Clay Sci. 2021, 203, 106027. [Google Scholar] [CrossRef]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Pang, H.; Li, L.; Jiang, S.; Wen, T.; Zhuang, L.; Hu, B.; Wang, X. Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from mil-53. Chem. Eng. J. 2018, 353, 157–166. [Google Scholar] [CrossRef]
- Hu, W.; Lu, S.; Song, W.; Chen, T.; Hayat, T.; Alsaedi, N.S.; Chen, C.; Liu, H. Competitive adsorption of U(VI) and Co(II) on montmorillonite: A batch and spectroscopic approach. Appl. Clay Sci. 2018, 157, 121–129. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.; Baltrus, J.P.; Stevens, R.W.; Khatri, R.; Chuang, S.S.C.; Filburn, T. Improved immobilized carbon dioxide capture sorbents. Fuel Process. Technol. 2005, 86, 1449–1455. [Google Scholar] [CrossRef]
- Tu, J.; Peng, X.; Wang, S.; Tian, C.; Deng, H.; Dang, Z.; Lu, G.; Shi, Z.; Lin, Z. Effective capture of aqueous uranium from saline lake with magnesium-based binary and ternary layered double hydroxides. Sci. Total Environ. 2019, 677, 556–563. [Google Scholar] [CrossRef]
- Chernobay, G.B.; Chesalov, Y.A.; Baltakhinov, V.P.; Popova, G.Y.; Andrushkevich, T.V. In situ ftir study of β-picoline transformations on V–Ti–O catalysts. Catal. Today 2011, 164, 58–61. [Google Scholar] [CrossRef]
- Lefèvre, G.; Noinville, S.; Fédoroff, M. Study of uranyl sorption onto hematite by in situ attenuated total reflection–infrared spectroscopy. J. Colloid Interface Sci. 2006, 296, 608–613. [Google Scholar] [CrossRef]
- Lei, Y.; Li, K.; Liao, J.; Zhang, Y.; Zhang, L.; Zhu, W. Design of 3d alumina-doped magnesium oxide aerogels with a high efficiency removal of uranium(VI) from wastewater. Inorg. Chem. Front. 2021, 8, 2561–2574. [Google Scholar] [CrossRef]
- Mei, H.; Tan, X.; Tan, L.; Meng, Y.; Chen, C.; Fang, M.; Wang, X. Retention of U(VI) by the formation of Fe precipitates from oxidation of Fe(II). ACS Earth Space Chem. 2018, 2, 968–976. [Google Scholar] [CrossRef]
- Wu, C.; Tu, J.; Tian, C.; Geng, J.; Lin, Z.; Dang, Z. Defective magnesium ferrite nano-platelets for the adsorption of As(V): The role of surface hydroxyl groups. Environ. Pollut. 2018, 235, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y. Application of surface complexation modeling on adsorption of uranium at water-solid interface: A review. Environ. Pollut. 2021, 278, 116861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, C.; Li, J.; Wang, X. Preparation of montmorillonite@carbon composite and its application for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2015, 349, 129–137. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hosseini, S.S.; Akbari, A.; Ramavandi, B. Hydroxyapatite biomaterial production from chicken (femur and beak) and fishbone waste through a chemical less method for Cd2+ removal from shipbuilding wastewater. J. Hazard. Mater. 2021, 413, 125428. [Google Scholar] [CrossRef]





| Adsorbents | SBET/(m2·g−1) | Da/nm | Vt/(cm3·g−1) |
|---|---|---|---|
| MgAl-LDH | 7.95 | 10.73 | 0.02 |
| MgAl-LDO | 167.17 | 2.74 | 0.11 |
| LDO-KGM | 231.22 | 5.62 | 0.32 |
| Adsorbent | Experimental Conditions | Qm (mg·g−1) | Equilibrium Time (h) | References |
|---|---|---|---|---|
| CMK-3@MgAl LDH | pH = 5.0, T = 298 K | 171.00 | 0.5 | [44] |
| CaMgAl-LDH/nZVI | pH = 5.0, T = 298 K | 216.10 | 4 | [45] |
| rGO/LDH | pH = 4.0, T = 298 K | 277.80 | 2 | [46] |
| Fe-NCNF-LDH | pH = 6.0, T = 298 K | 532.25 | 4 | [47] |
| Al2O3 aerogel | pH = 7.0, T = 298 K | 746.30 | 24 | [30] |
| MgO-N aerogel | pH = 4.0, T = 298 K | 1061.80 | 1.67 | [48] |
| MgAl-LDO-500 | pH = 6.0, T = 303 K | 1098.90 | 0.25 | [36] |
| LDO-KGM | pH = 6.0, T = 293 K | 3019.56 | 24 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Wu, J.; Shang, Z.; Chen, M.; Li, B.; Wang, T.; Sun, L.; Dang, Z.; Zhu, N.; Wu, P. Construction of Novel Nanoflowering MgAl-Double Oxide Konjac Gum for Efficient Enrichment of Uranium (VI) from Wastewater. Processes 2025, 13, 876. https://doi.org/10.3390/pr13030876
Gong P, Wu J, Shang Z, Chen M, Li B, Wang T, Sun L, Dang Z, Zhu N, Wu P. Construction of Novel Nanoflowering MgAl-Double Oxide Konjac Gum for Efficient Enrichment of Uranium (VI) from Wastewater. Processes. 2025; 13(3):876. https://doi.org/10.3390/pr13030876
Chicago/Turabian StyleGong, Ping, Jiayan Wu, Zhongbo Shang, Meiqing Chen, Bo Li, Tianming Wang, Leiye Sun, Zhi Dang, Nengwu Zhu, and Pingxiao Wu. 2025. "Construction of Novel Nanoflowering MgAl-Double Oxide Konjac Gum for Efficient Enrichment of Uranium (VI) from Wastewater" Processes 13, no. 3: 876. https://doi.org/10.3390/pr13030876
APA StyleGong, P., Wu, J., Shang, Z., Chen, M., Li, B., Wang, T., Sun, L., Dang, Z., Zhu, N., & Wu, P. (2025). Construction of Novel Nanoflowering MgAl-Double Oxide Konjac Gum for Efficient Enrichment of Uranium (VI) from Wastewater. Processes, 13(3), 876. https://doi.org/10.3390/pr13030876







