Abstract
Given the pressing demand for efficient uranium (U(VI)) enrichment and its elimination from wastewater to curtail the risks of radioactive contamination inherent in nuclear energy applications, it is crucial to design materials with high removal efficiency and straightforward separation processes. In the current study, we incorporated konjac gum (KGM) into MgAl-double oxide (MgAl-LDO) and synthesized an innovative, economical, and environmentally friendly LDO-KGM material by using the freeze-drying-calcination (FDC) method, which provided a solution for U(VI) concentration from aqueous solutions. The nanoflower structures LDO-KGM with abundant pore structure and high specific surface area exhibited an optimal U(VI) adsorptive capacity (3019.56 mg·g−1) at pH = 6.0 and 293 K, which was 2.3 times greater than that of MgAl-LDO (1296.39 mg·g−1). LDO-KGM also showed great adaptability for the immobilization of U(VI) over a broad pH range (4.0 to 9.0) and coexisting ions. U(VI) adsorption onto LDO-KGM adhered to the pseudo-second-order kinetic model (R2 ≥ 0.99) and the Langmuir isotherm model (R2 ≥ 0.99). The analysis of thermodynamic parameters derived from isotherms at varying temperatures revealed that U(VI) adsorption onto LDO-KGM was an endothermic and spontaneous process. The mechanism underlying U(VI) adsorption by LDO-KGM was mainly complexation, carbonate co-precipitation, and electrostatic adsorption. Furthermore, the adsorption efficiency of LDO-KGM for U(VI) could still retain more than 84.5% after five cycles. The findings indicate that the synthesized LDO-KGM exhibits potential as an exceptionally potent adsorbent for the purification of wastewater contaminated with U(VI).
1. Introduction
The escalating need for energy resources and the extensive extraction of minerals have caused radionuclides to spread and concentrate within the environment, thereby increasing the threat of radioactive contamination [1]. Uranium-containing wastewater amplifies these dangers, as it is released into the natural environment through nuclear fuel cycles, ore mining, processing, and manufacturing activities at uranium-contaminated sites [2]. When uranium goes (U(VI)) into aquatic ecosystems without proper treatment, it can lead to its accumulation in the food chain [3,4]. U(VI) toxicity poses a significant risk to the proper functioning of vital organs, such as the lungs and kidneys, with severe cases possibly resulting in life-threatening outcomes [5]. Thus, the development of materials that can extract U(VI) from nuclear effluent is critical to reduce environmental pollution and address the energy crisis [6]. Various methodologies have been implemented to eliminate U(VI) from wastewater and nuclear effluents, encompassing precipitation [7], membrane separation [8], solvent extraction [9], phytoremediation [10], and adsorption [11,12]. Among them, adsorption stands out as an exceptionally effective technique for removing U(VI) from wastewater [13,14] and is considered especially attractive due to its cost-effectiveness, robust efficiency, straightforward implementation, and environmentally benign nature [15].
Over the past few years, substantial research efforts have been directed towards exploring diverse sorbent materials for U(VI) elimination from wastewater, including konjac gum (KGM) [16], clays [17], metal–organic frameworks (MOFs) [18], layered double hydroxides (LDHs) [19], activated carbons [20], and several other potential candidates. As a quintessential 2D anionic clay, LDHs possess a distinctive layered architecture, a plethora of reactive sites, and superior stability, making them an ideal candidate for the sequestration of U(VI) [21,22]. Notably, layered double oxides (LDO) exhibit a greater number of active sites, larger specific surface area, and superior chemical and thermal stability compared to the pristine LDHs [23] and, thus, have better U(VI) removal capability. Momin et al. used graphitic carbon nitride (GCN) in LDO to prepare GCN-LDO for U(VI) capture, which showed a substantial adsorption capacity, reaching 158.25 mg·g−1 [24]. Zhu et al. demonstrated that the novel γ-Fe2O3 cross-linked LDO composite (γ-Fe2O3/LDO) exhibited an adsorptive capacity for U(VI) approximately 526.32 mg·g−1 in aqueous media [25]. However, due to the dense surface and low functional group structure of most LDOs, their adsorption performance is limited to a certain degree [26]. Therefore, the development of Mg-Al LDO with a larger specific surface area and a greater number of surface functional groups, such as Mg-O-H, Al-O, and -OH, is highly sought after for the efficient removal of U(VI).
The presence of numerous hydroxyl and carbonyl groups on the saccharide units of KGM enables it to readily form gels with exhibit high porosity, tunable densities, and advantageous porous structures through the action of hydrogen bonding, and its combination with LDO can effectively solve the structure problem of LDO [27,28]. For example, Xiong et al. synthesized hydroxyapatite (HAP) aerogels with high selectivity and capacity by employing a combination of freeze-drying and templating techniques, utilizing KGM as a template [16]. Wang et al. synthesized a 3D porous aerogel, utilizing KGM as a structural template, aimed at U(VI) adsorption [29]. The freeze-drying calcination (FDC) technique has been recognized for its efficacy in preparing ultra low-density porous materials, which have a wide range of applications, such as catalysis and adsorption [30,31].
In the current study, LDO-KGM with a nanoflower structure was prepared by the FDC method using KGM as a template. This material has a larger specific surface, larger pores, and richer functional groups than other composites. By combining KGM with LDO, the structural limitations of LDO can be effectively addressed. This synergy leverages KGM’s advantageous properties to enhance LDO’s performance in terms of capturing U(VI), thereby positioning the composite as a promising material for the selective extraction of U(VI) in wastewater treatment. The morphology and chemical constituents of LDO-KGM were examined using a suite of analytical methods, such as scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), N2 adsorption–desorption analysis, and X-ray photoelectron spectroscopy (XPS). Meanwhile, the impact of several parameters on U(VI) adsorption was explored, including adsorbent concentration, contact time, adsorption isotherms, different ions, pH, and temperature. In addition, a synergistic approach involving XPS and zeta potential analysis was employed to elucidate the adsorption mechanism. The results offer significant understanding for the development and preparation of eco-friendly, high-performance adsorbents tailored for the purification of wastewater containing U(VI).
2. Materials and Methods
2.1. Materials
The laboratory experiments utilized chemical reagents of analytical grade. Supplied included KGM, UO2(NO3)2, Mg(NO3)2·6H2O, Al(NO3)3·9H2O, NaOH, HNO3, NaCl, KCl, MgCl2, CaCl2, NaNO3, Na2CO3, and Na2SO4 were provided by Macklin Biochemical Technology Co., Ltd. (Shanghai, China). The laboratory experiments were conducted using deionized water (DIW), which had a resistivity of 18.2 MΩ·cm.
2.2. Synthesis of LDO-KGM
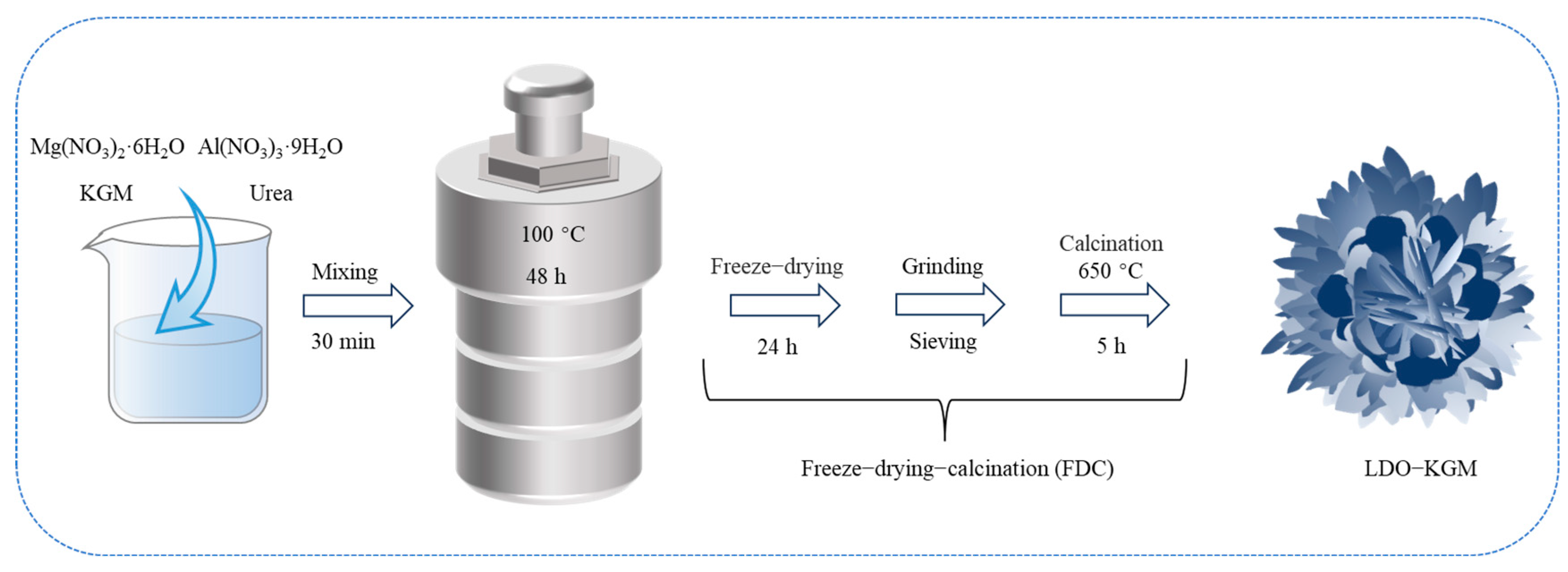
LDH was achieved through a simple one-pot hydrothermal process, utilizing urea as the alkaline agent, and then, LDO-KGM was synthesized according to the FDC method (Figure 1). Research showed that FDC was a widely used way to synthesize products with a porous structure, which could provide more active sites [32]. In detail, 0.008 mol Mg(NO3)2·6H2O, 0.004 mol Al(NO3)3·9H2O, 0.125 mol urea and x g KGM (x = 0.5, 1, 1.5, 2, 2.5) were added to a beaker containing 50 mL DIW, whilst stirring for at least 30 min until completely mixed. Subsequently, the homogeneous solution was poured into a 100 mL Teflon-lined autoclave for hydrothermal processing at a temperature of 100 °C over a period of 48 h. Following this, the resulting material was permitted to gradually cool to ambient temperature. The resulting precipitates were then centrifuged and washed with DIW to eliminate any residual free metals. The washing process was continued until the supernatant became colorless, and the pH of the solution reached approximately neutral, around pH 7. Finally, the precipitates were lyophilized at −60 °C in a freeze-dried machine and named LDH-KGM-x (X was the amount of KGM added), among which LDH-KGM-2 had the best adsorption capacity. The obtained LDH-KGM-2 was calcined at 250, 350, 450, 550, 650, and 750 °C, respectively, and named LDO-KGM-2-Y (Y was the calcination temperature). The calcination process was conducted in a muffle furnace, with the temperature ramped up at a rate of 5 °C/min. The material was then subjected to calcination at the designated target temperature for a period of 5 h. Following the calcination, the furnace was permitted to cool down naturally to ambient temperature. The final obtained material with the best adsorption properties was LDO-KGM-2-650, denoted as LDO-KGM. The MgAl-LDH and MgAl-LDO used for comparison were synthesized without adding KGM, where MgAl-LDO was synthesized through the calcination of MgAl-LDH at 650 °C.
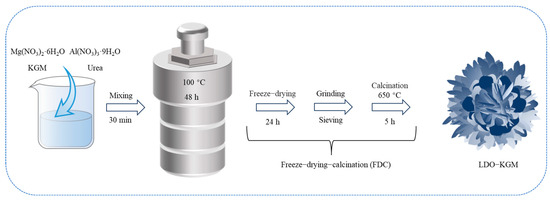
Figure 1.
The flowchart of preparation of LDO-KGM.
2.3. U(VI) Immobilization Experiment
A series of batch experiments were carried out to determine the adsorptive immobilization characteristics and the intrinsic mechanisms of U(VI) by the synthesized materials. The kinetic studies on U(VI) immobilization were conducted with the following procedure: a 100 mL conical flask contained 100 mL of a 100 mg·L−1 U(VI) solution into which 5.0 mg of the material was introduced, with an initial pH carefully controlled at 6.0 ± 0.1; the mixed solution was then stirred continuously throughout the experiment, and the samples were collected periodically (0, 20, 40, 60, 90, 150, 240, 360, 540, 720, 1080, and 1440 min). In the isotherm experiments, 5.0 mg of material was dispersed to 100 mL of U(VI) solution with concentrations varying between 20 and 200 mg·L−1 at pH of 6.0, followed by agitation for a period of 24 h.
After the reaction, the supernatant liquid was passed through a membrane filter with a pore size of 0.22 μm to isolate the solid phase. The filtrate’s U(VI) concentration was then measured employing a UV–vis spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan). The absorbance was conducted at a wavelength of 651 nm, utilizing arsenazo (III) as the chromogenic reagent at a concentration of 0.05% [33]. Based on these measurements, the U(VI) adsorption capacity (Qe, mg·g−1) (Equation (1)) and the adsorption rate (R, %) (Equation (2)) were determined by employing the subsequent equations [33,34]:
where Qe denotes the adsorption capacity for U(VI) (mg·g−1), C0 denotes the initial concentration of U(VI) solution, Ce denotes the concentration of U(VI) at equilibrium (mg·L−1), V denotes the volume of U(VI) solution (L), M denotes the mass of LDO-KGM (g), and R denotes adsorption rate (%).
Qe = (C0 − Ce) × V/M,
R = (C0 − Ce)/C0 × 100%,
Moreover, the influence of adsorbent concentration, different ions, pH, and temperature were thoroughly investigated. Each data point was obtained from three replicate experiments to ascertain the dependability of the findings, and error bars were supplied.
2.4. Characterization Methods
The specific surface areas and pore size distributions of the materials were evaluated using a Chemisorption Surface Area Analyzer (Micromeritics ASAP 2460, Shanghai, China), employing the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methodologies, respectively. The morphologies of the materials were analyzed using the SEM (Hitachi SU8020, Tokyo, Japan). The physical phases of the materials were analyzed using the XRD (D8 advance Sox-l, Bruker Co., Billerica, MA, USA). The characteristic functional groups of the materials within the wavenumber range of 400–4000 cm−1 were observed using the FTIR (Nicolet 6700, Thermo Fisher Scientific, Waltham, MA, USA). The chemical states of the materials were studied using XPS (Thermo Escalab 250XI, Thermo Fisher Scientific, Waltham, MA, USA). Additionally, the zeta potential of the materials, which indicates their surface charge, was measured before the reaction using a Zeta Potential Analyzer (Zetasizer Nano ZS90, Malvern Panalytical, Malvern, UK).
3. Results and Discussion
3.1. Effect of Calcination Temperature and Doping Ratio
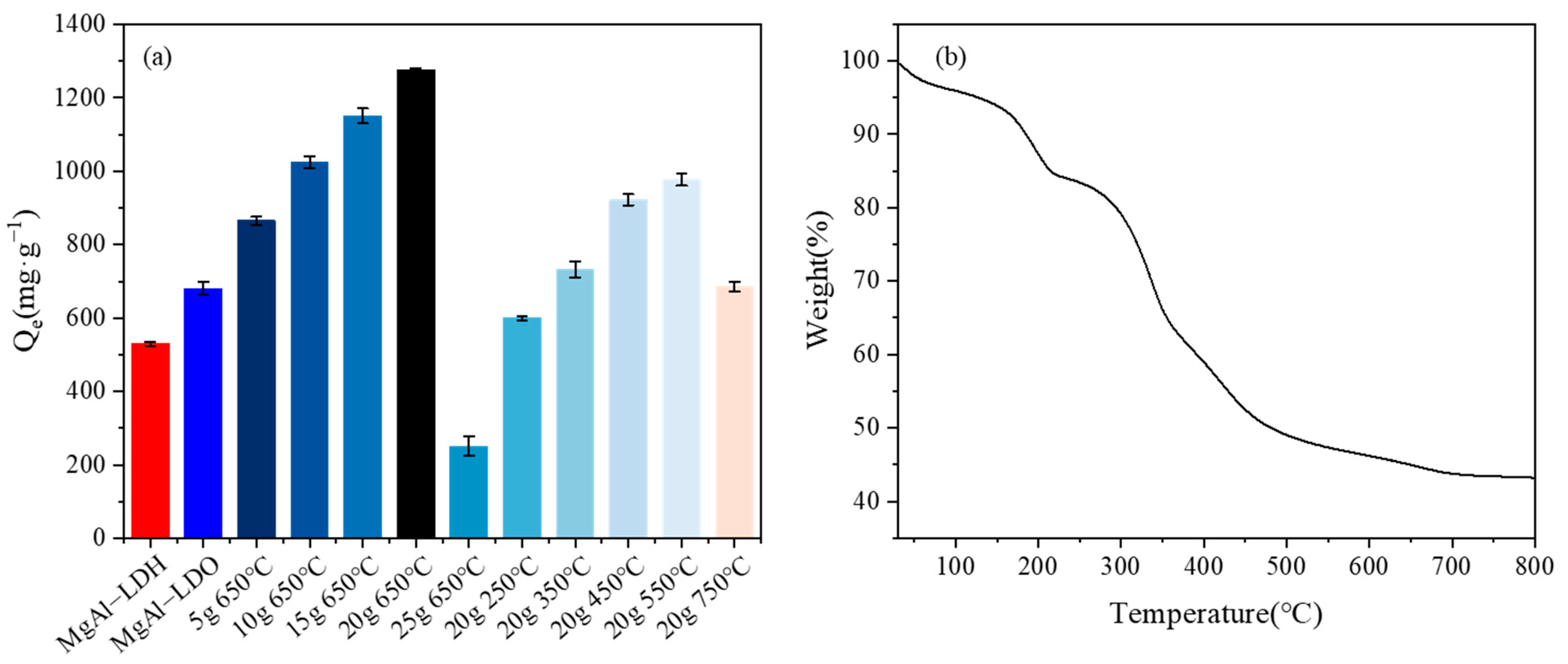
Figure 2a illustrates the differences in adsorptive capacities among different materials. Notably, the enhanced adsorption efficiency for U(VI) by the modified materials was evident, showcasing a significant improvement in their performance compared to their unmodified counterparts. In order to synthesize the materials with the best performance, the impact of the calcination temperature and doping ratio on the adsorption properties were initially examined. This preliminary investigation aimed to determine the optimal conditions for material synthesis, which would result in the best adsorptive behavior. It was observed that the LDO-KGM obtained by calcination at 650 °C with the incorporation of 20 g·L−1 KGM demonstrated optimal U(VI) adsorption capacity; hence, it was used for further investigation. An insufficient amount of KGM made the structure of MgAl-LDH loose, and an excess amount caused the collapse of the MgAl-LDH layered structure [35]. Meanwhile, the KGM in LDH-KGM may occupy the adsorption sites; whereas, during the calcination process, the KGM gradually disappeared and only the fluffy and porous KGM structure remained in the calcined LDO-KGM, and therefore, the adsorption performance was enhanced. The KGM in LDH-KGM could be removed to produce pure LDO at 650 °C [16]. With the ongoing rise in temperature, the metal oxides in LDH sintered and formed stable spinel compounds with the decreasing adsorption performance. The increased temperature affected the structural integrity and adsorptive sites of the LDH, thereby impacting its ability to effectively capture U(VI) [36].
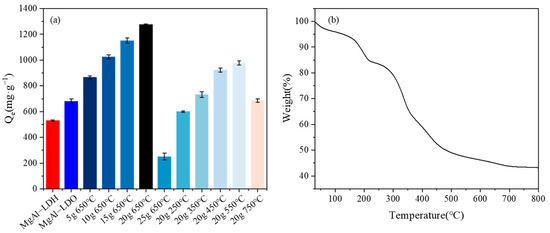
Figure 2.
(a) Adsorption capacity of U(VI) by materials synthesized under different conditions (C0 = 100 mg·L−1, pH = 6.0, T = 293 K); (b) TGA curves of LDO-KGM.
Figure 2b presented the thermogravimetric analysis (TGA) curve of LDO-KGM, revealing a two-stage weight loss process. The initial stage (below 200 °C) corresponded to the evaporation of physically adsorbed water, while the second stage (200–650 °C) was attributed to the thermal decomposition of the KGM template. Above 650 °C, the KGM template was entirely removed, leaving pure LDO, confirming the successful synthesis of LDO-KGM and its robust thermal stability. These TGA results aligned closely with XRD and FTIR data, collectively demonstrating that KGM served as an effective template for LDO-KGM synthesis due to its compatibility with the FDC method and enhanced thermal stability.
3.2. Characterization
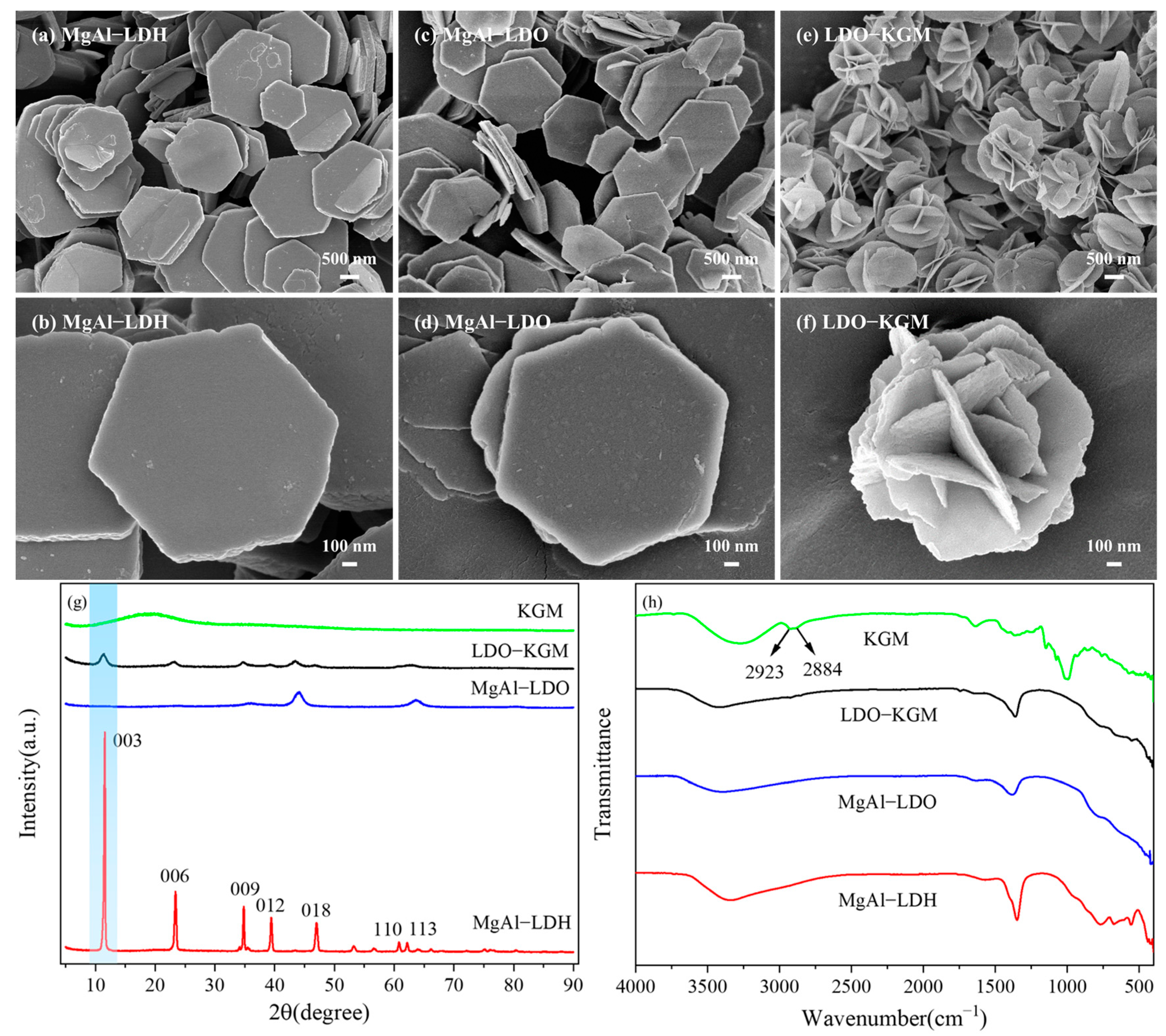
The morphology and porosity of MgAl-LDH, MgAl-LDO, and LDO-KGM were examined through SEM images (Figure 3a–f). MgAl-LDH presented a laminated hexahedron-like morphology, which was a characteristic morphology often observed in LDHs [37]. MgAl-LDO exhibited a structure analogous to that of MgAl-LDH, and both were in a laminated hexahedron-like morphology. Nevertheless, the MgAl-LDO exhibited a rougher surface texture compared to MgAl-LDH, attributed to the removal of interlayer water molecules and the breakdown of carbonate ions during calcination. The as-obtained LDO-KGM consisted primarily of nanoflower structures. Due to the addition of KGM, the layered structure of MgAl-LDO underwent a transformation, adopting a flower-like morphology accompanied by an increased specific surface area.
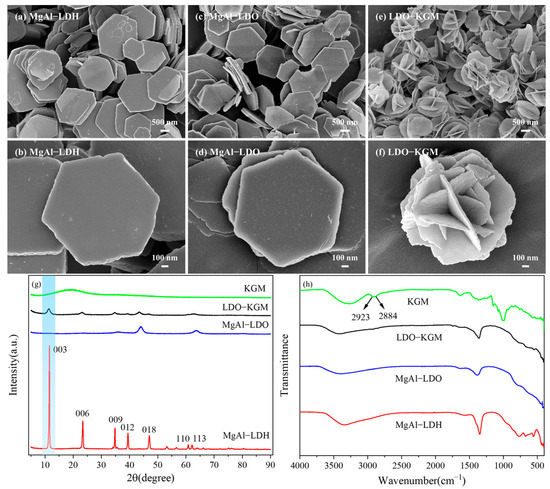
Figure 3.
SEM images of (a,b) MgAl-LDH; (c,d) MgAl-LDO; (e,f) LDO-KGM; (g) XRD patterns; and (h) FTIR spectra of MgAl-LDH, MgAl-LDO, LDO-KGM, and KGM.
The XRD analysis (Figure 3g) provided additional clarity on the crystallographic and chemical configurations of MgAl-LDH, MgAl-LDO, and LDO-KGM. The distinctive peaks indicative of (003), (006), (009), (012), (018), (110), and (113) of MgAl-LDH were identified at 11.55°, 23.38°, 34.80°, 39.41°, 46.97°, 60.78°, and 62.19°, respectively [38], which was consistent with its typical patterns (JCPDS No. 70-2151) [39]. However, in comparison to MgAl-LDH, the MgAl-LDO peaks became weaker and broader, indicating the lower crystallinity and lattice defects of the MgAl-LDO. Interestingly, for MgAl-LDO, carbonate decomposition during calcination led to the disappearance of the 003 reflection [36], which reappeared in LDO-KGM after the addition of KGM (Figure 3g). Besides, many peaks that disappeared in MgAl-LDO reappeared in LDO-KGM. In contrast to pure KGM, the LDO-KGM showed no characteristic peak associated with KGM at 2θ = 20.68°. This observation further confirmed the KGM in LDH-KGM, which was removed to yield pure LDO-KGM at 650 °C. The functional groups of three materials and KGM were illustrated in the FTIR spectrum (Figure 3h). As for KGM, adsorption bands at 2923 cm−1 and 2884 cm−1 were ascribed to the stretching vibration of C-H bonds that were inherent to KGM [16]. Notably, the absence of peaks at 2923 cm−1 and 2884 cm−1 in LDO-KGM suggested that the KGM had become completely decomposed following the heat treatment process at 650 °C. Drawing from the findings, it can be confidently stated that the pure LDO-KGM has been successfully synthesized.
It had been demonstrated that the specific surface area, electrostatic forces, and structural characteristics of materials have a profound influence on their adsorptive capacity [40]. In an effort to pinpoint the key determinant governing the adsorptive interaction of MgAl-LDH, MgAl-LDO, and LDO-KGM with respect to U(VI), the impact of the specific surface area, as calculated using the BET method, was evaluated. As shown in Table 1, the specific surface area of LDO-KGM (231.22 m2·g−1) was 30.26 times greater than that of MgAl-LDH (7.64 m2·g−1) and 1.38 times higher than that of MgAl-LDO (167.17 m2·g−1), respectively. This marked increase in surface area implied a higher probability of effective collisions between LDO-KGM and U(VI), thereby enhancing the adsorption capacity of LDO-KGM for U(VI) [30]. As shown in Figure S1, the adsorption isotherm of MgAl-LDO revealed a type H2 hysteresis loop, demonstrating a relatively uniform mesoporous structure. In contrast, MgAl-LDH and LDO-KGM exhibited type H3 hysteresis loops, indicative of lamellar or layered materials with non-uniform pore size distributions. While all three materials possessed mesoporous characteristics, their pore size distributions exhibited notable differences. Upon calcination, the transformation of MgAl-LDH into MgAl-LDO resulted in a reduction in the average pore size, likely due to structural reorganization and densification. Notably, the incorporation of a KGM matrix into LDO-KGM led to an increase in the average pore size, attributed to the fluffy and porous structure of KGM, which effectively altered the material’s porosity and pore architecture.

Table 1.
Structural information of adsorbents.
3.3. Excellent Performance and Adaptability for U(VI) Removal
3.3.1. Kinetic Studies and Isotherm Studies
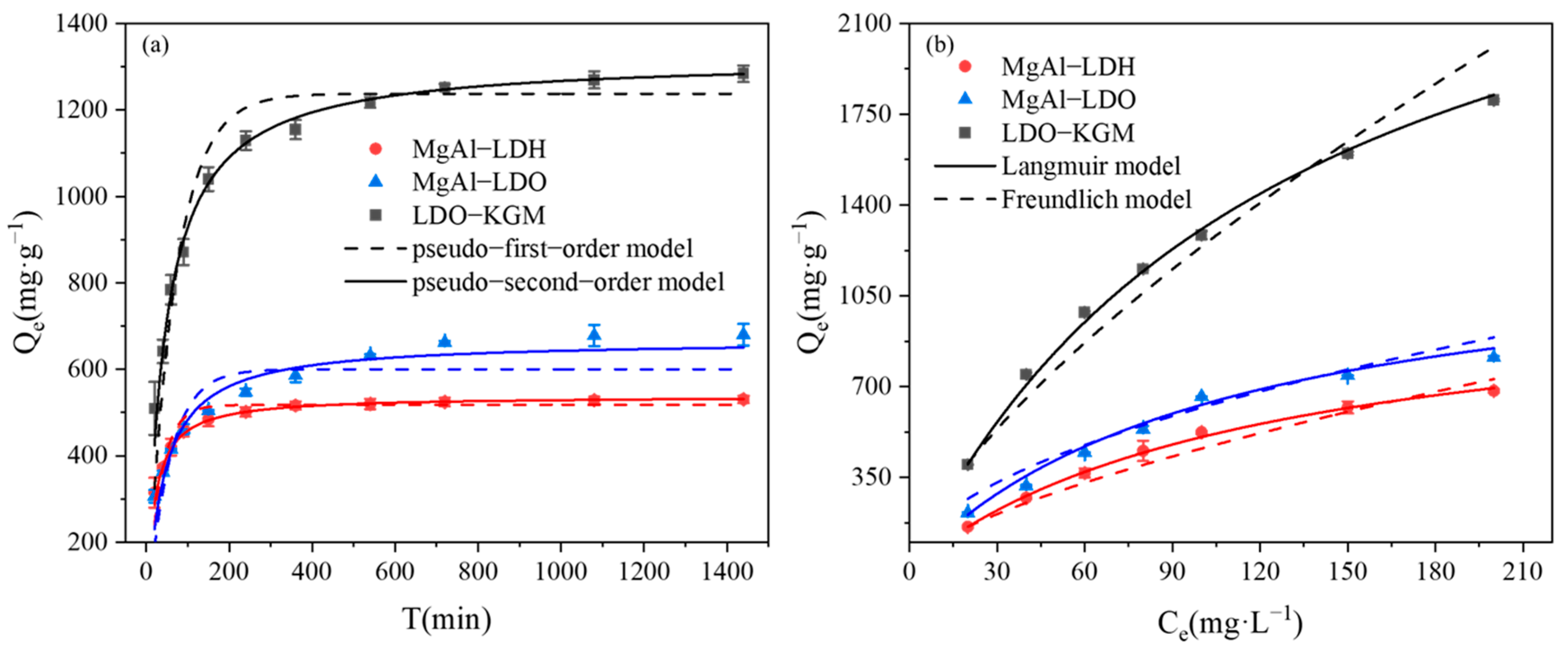
The excellent physicochemical properties of the successfully modified material with the greatly improved adsorption capacity for U(VI) were further demonstrated. Next, we continued to investigate the influence of reaction conditions on the immobilization capacity for U(VI). The efficiency of U(VI) immobilization was positively correlated with the dosage of the materials (Figure S2). Among the various materials, the dosage of 0.05 g·L−1 was more effective in immobilizing U(VI), and thus, this concentration was chosen for further study. As depicted in Figure 4a, each material exhibited a swift adsorption rate for U(VI) during the initial 180 min. The ranking of U(VI) immobilization capacities was as follows: LDO-KGM > MgAl-LDO > MgAl-LDH. To clarify the mechanism behind the immobilization of U(VI), we fitted the experimental data to both pseudo-first-order and pseudo-second-order kinetic models. The parameters obtained from the fitting are detailed in Table S1. The analysis revealed that for all materials, the pseudo-second-order model demonstrated superior correlation coefficients (R2 = 0.99) when compared to the pseudo-first-order model (R2 = 0.92), implying that the process of U(VI) immobilization was largely governed by chemical reactions [41,42]. Consequently, LDO-KGM demonstrates the capability to serve as a highly efficient adsorbent for the mitigation of U(VI) contamination events.
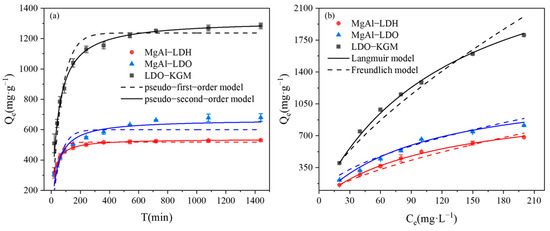
Figure 4.
(a) Adsorption kinetic of U(VI) on materials (C0 = 100 mg·L−1, pH = 6.0, T = 293 K); (b) adsorption isotherm of U(VI) on materials (C0 = 20–200 mg·L−1, pH = 6.0, T = 293 K).
In the current study, the Langmuir and Freundlich adsorption isotherm models were utilized to assess the maximum immobilization capacity (Qm) of the synthesized materials. As shown in Figure 4b, the Qe of the three materials exhibited a steep rise with an increase in the initial U(VI) concentration until all accessible reactive sites were saturated. The relevant parameters for the model are presented in Table S2. An examination of the correlation coefficient (R2) indicated that the Langmuir model offered a greater description of the U(VI) immobilization than the Freundlich model, suggesting that U(VI) molecules were homogeneously adsorbed as a monolayer on the reactive sites [43]. The calculated Qm for LDO-KGM (3019.56 mg·g−1) significantly exceeded those of both MgAl-LDH (1107.74 mg·g−1) and MgAl-LDO (1296.39 mg·g−1). Furthermore, in comparison to the adsorption performance of various studied materials, LDO-KGM is a highly effective adsorbent for U(VI) with good application prospects (Table 2) [30,36,44,45,46,47,48].

Table 2.
Comparison of the maximum adsorption amounts of the LDO-KGM and those of other adsorbents towards U(VI).
3.3.2. Effect of Coexisting Ions and pH
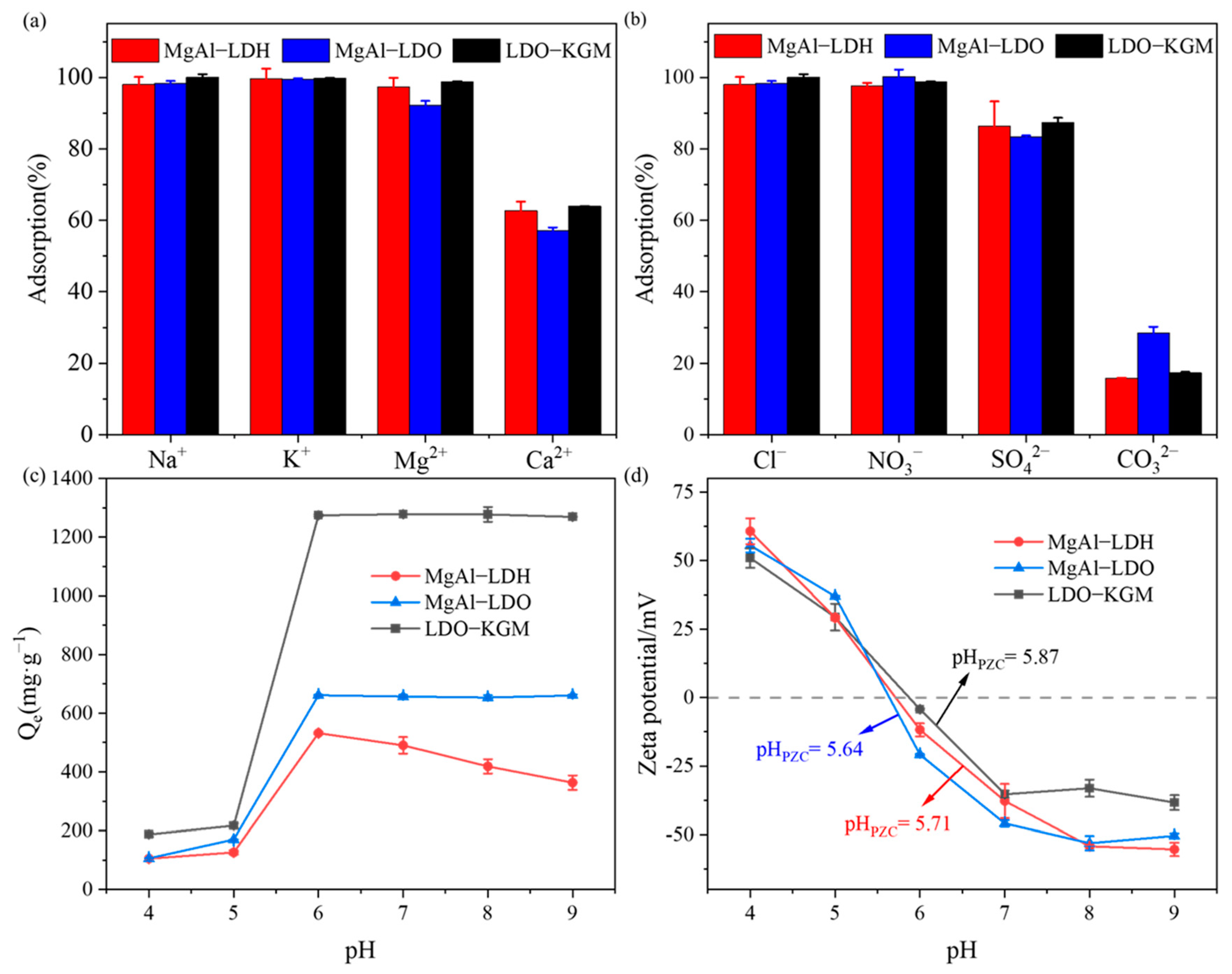
Considering the omnipresent nature of coexisting ions in natural water systems, the impact of diverse coexisting cations and anions on U(VI) adsorption by the prepared samples was explored (Figure 5a,b). As illustrated in Figure 5a, K+, Na+, and Mg2+ exhibited little influence on the adsorptive capacity of the MgAl-LDH, MgAl-LDO, and LDO-KGM. However, the adsorptive capacity of MgAl-LDH, MgAl-LDO, and LDO-KGM for U(VI) decreased by more than 40% in the presence of Ca2+. The decrease was likely because Ca2+ with the high valence was more prone to occupy the adsorption sites on the surfaces of materials [6], and the formation of CaUO2(CO3)32− complexes impeded the movement of positively charged uranyl ions (UO22+) to the adsorbent surface [49].
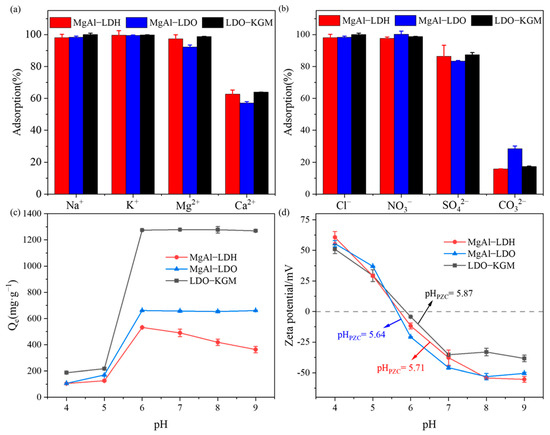
Figure 5.
Effect of coexisting cation (a) and anion (b) on U(VI) immobilization by materials (C0 = 100 mg·L−1 for (a,b), pH = 6.0, T = 293 K, ionic strength = 0.01 mol·L−1); (c) effect of pH on U(VI) immobilization (C0 = 100 mg·L−1, pH = 4–9, T = 293 K); (d) zeta potential of materials before reaction with U(VI).
As shown in Figure 5b, the inhibitory influence of coexisting anions on adsorption followed the order: CO32− > SO42− >NO3− ≈ Cl−. In the presence of Cl− and NO3−, the U(VI) immobilization capacity decreased negligibly. Nevertheless, the adsorptive capacity of MgAl-LDH, MgAl-LDO, and LDO-KGM for U(VI) was markedly reduced in the presence of CO32−, which can likely be ascribed to complex formation (such as UO2CO3 and UO2(CO3)22−) between UO22+ and CO32− [50,51]. Similarly, UO2(SO4)22− complexes were formed between SO42− and UO22+, which resulted in a reduction in U(VI) adsorption capacities by MgAl-LDH, MgAl-LDO, and LDO-KGM [6]. Therefore, implementing an appropriate desalination step prior to treatment can enhance the removal of U(VI) from saline wastewater containing this ion.
The pH of the solution is recognized as a pivotal parameter that significantly impacts the adsorption of U(VI) onto materials, as it significantly impacts the chemical form of U(VI), the charge on the surface, and the reactivity of the adsorbent’s active sites in aqueous media. As depicted in Figure 5c, the adsorptive capacity of MgAl-LDH, MgAl-LDO, and LDO-KGM for U(VI) was considerably enhanced within the range of 4.0–6.0. For MgAl-LDH, a significant decline in the rate of U(VI) removal was detected at a pH greater than 6.0. Therefore, MgAl-LDO and LDO-KGM demonstrated applicability across a broad spectrum of pH values.
3.3.3. Effect of Temperatures
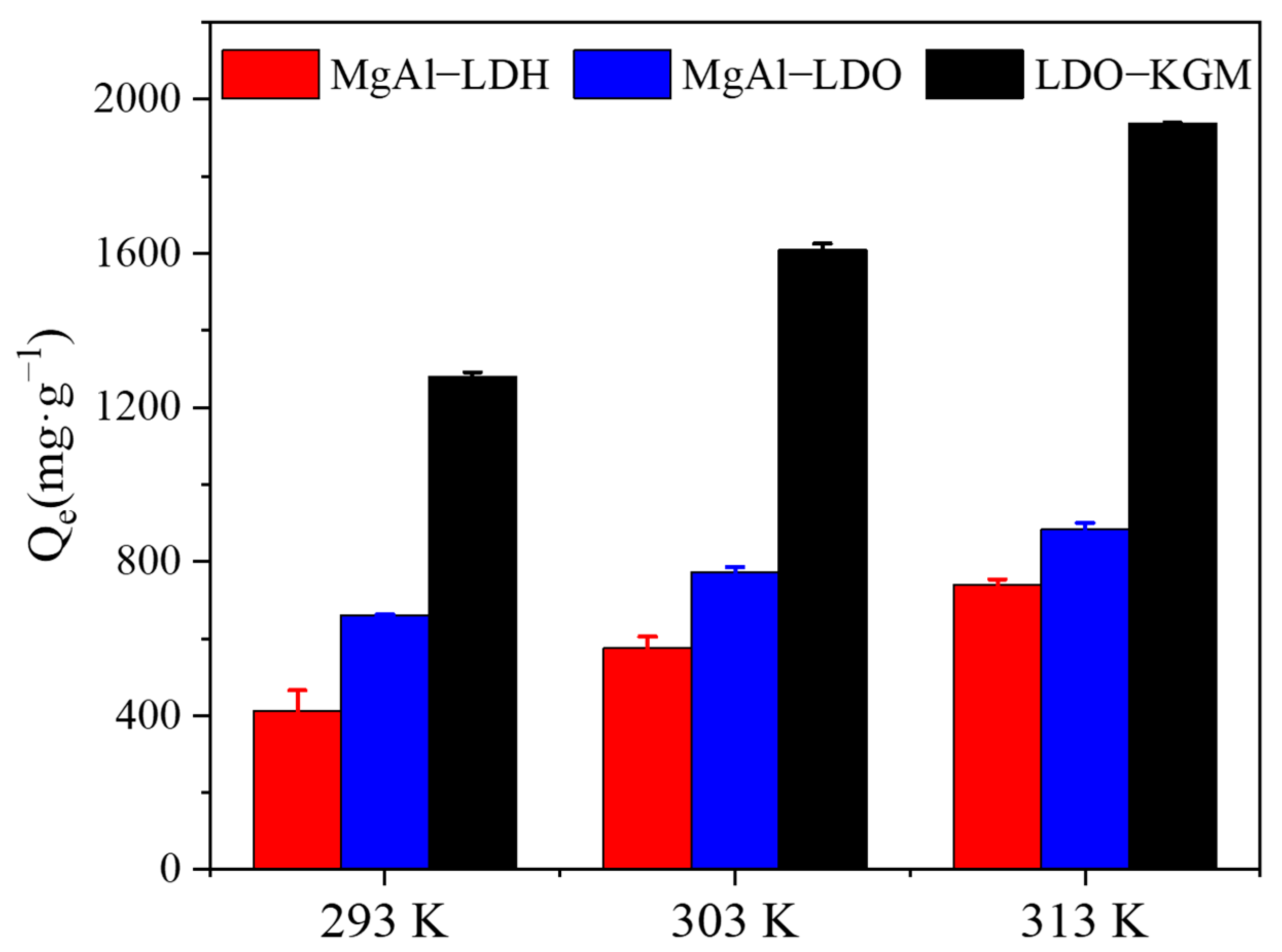
As demonstrated in Figure 6, temperature fluctuations significantly affected the adsorption performance of the adsorbent towards U(VI). The quantity of U(VI) adsorbed escalated with rising temperatures. To assess the thermodynamic nature of U(VI) adsorption, the Gibbs free energy change (Equation (3)) and Van’t Hoff equation (Equation (4)), which included entropy change (ΔS), enthalpy change (ΔH), and Gibbs energy (ΔG), were introduced. The thermodynamic fitting outcomes were presented in Figure 6 and Table S3 [52,53]:
where R represents the universal gas constant (8.314 J·mol−1·K−1), T represents the absolute temperature (K), and KL represents the thermodynamic equilibrium coefficient (L·g−1), which is calculated from a plot of lnKd, and the intercept is lnKL.
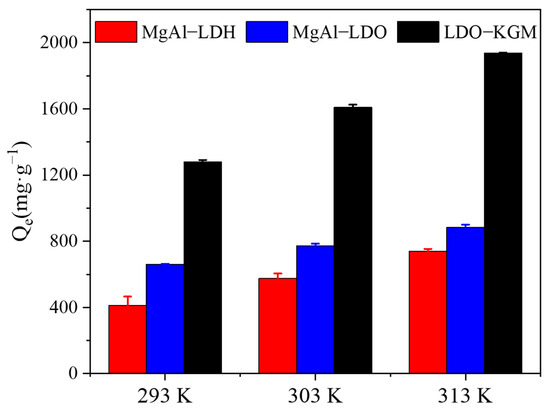
Figure 6.
Effect of temperatures on U(VI) immobilization (C0 = 100 mg·L−1, pH = 6, T = 293–313 K).
As shown in Table S3, the computed values for both ΔH and ΔG were found to be positive, and the decrease in ΔG values with rising temperature, indicating that the adsorption of U(VI) onto MgAl-LDH, MgAl-LDO, and LDO-KGM were endothermic, with increasing entropy and spontaneous process. Furthermore, the sorption of U(VI) was found to be favored at higher temperatures [54]. The ΔS demonstrated a significantly positive value, indicating that the adsorption of U(VI) by MgAl-LDH, MgAl-LDO, and LDO-KGM was characterized by an irreversible and spontaneous reaction process [55].
3.4. Adsorption Mechanism
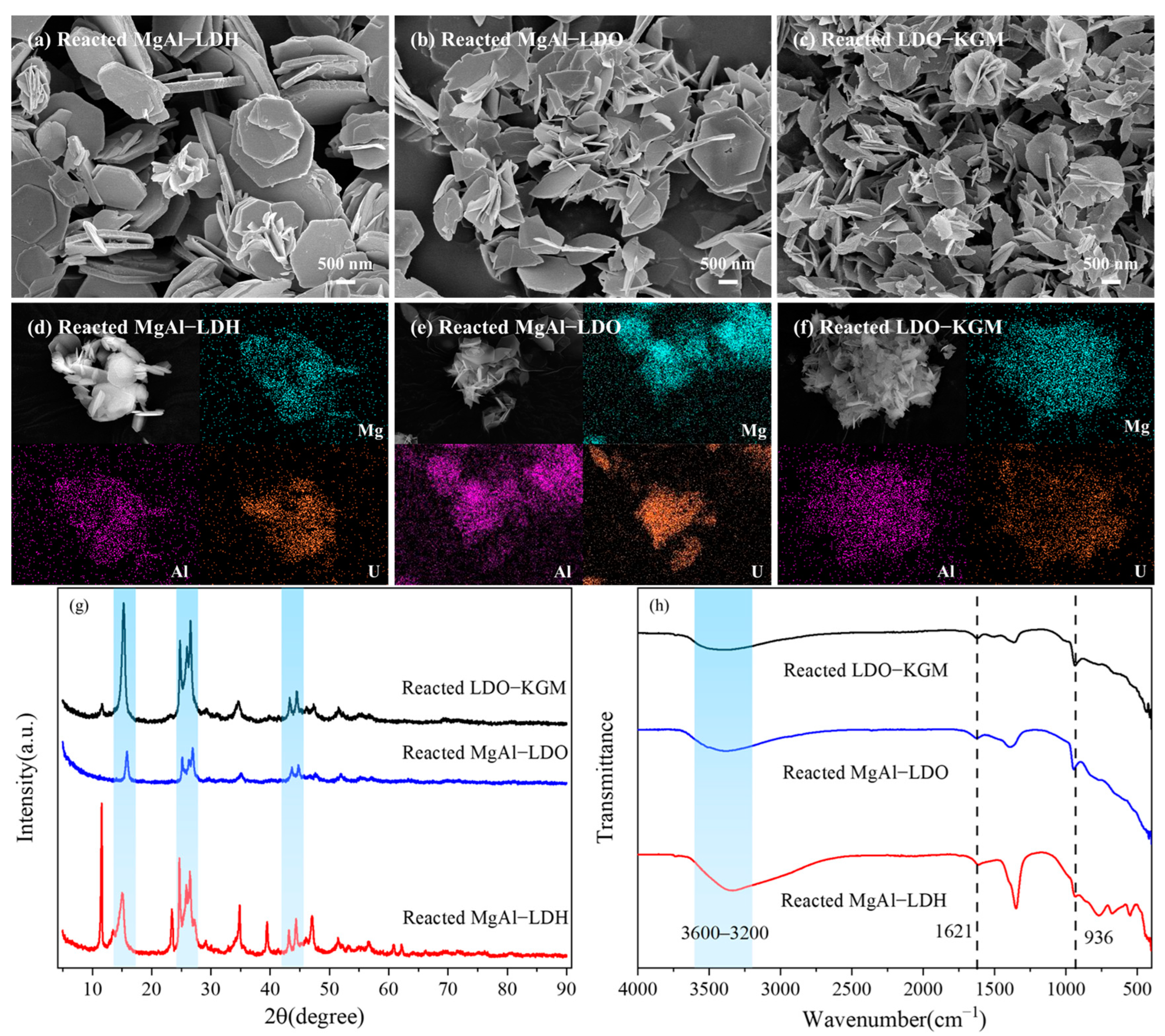
Figure 7a–f presented SEM images along with the corresponding elemental distribution maps of MgAl-LDH, MgAl-LDO, and LDO-KGM following the adsorption process, demonstrating a homogeneous distribution of U(VI) on the surfaces of materials. The interaction between MgAl-LDH, MgAl-LDO, and LDO-KGM with U(VI) resulted in the generation of numerous small surface fragments, implying that U(VI) was strongly complexed and immobilized on the surfaces [36]. Furthermore, the uniform distribution of U(VI) on the adsorbent surfaces was closely correlated with the presence of magnesium (Mg) and aluminum (Al), indicating an immobilization process that was not only homogeneous but also intimately linked to the oxygen-containing functional groups present on the materials (Figure 7d–f) [56].
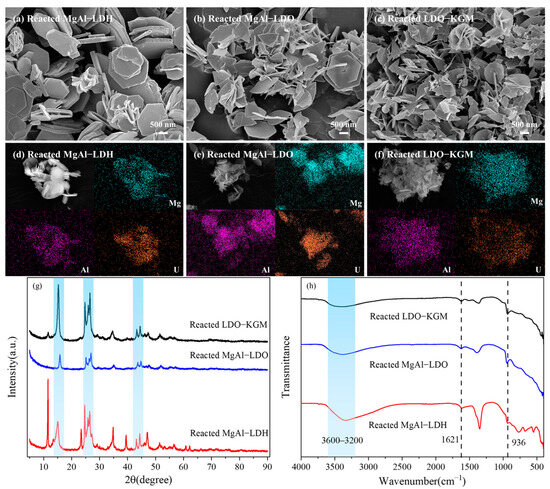
Figure 7.
SEM and the corresponding EDS mapping of MgAl-LDH (a,d), MgAl-LDO (b,e), and LDO-KGM (c,f) after reacting with U(VI), Mg (cyan zone), Al (rose zone), U(VI) (orange zone); (g) XRD patterns and (h) FTIR spectra of MgAl-LDH, MgAl-LDO, and LDO-KGM after reacting with U(VI).
To confirm the mechanism, the influence of adsorbent structural features for U(VI) immobilization was explored by XRD (Figure 7g). The XRD patterns of MgAl-LDH, MgAl-LDO, and LDO-KGM following U(VI) adsorption exhibited a little change, indicating that the crystal structures of these materials were largely preserved without significant destruction [57]. However, the intensities of some MgAl-LDH peaks were reduced gradually, indicating that the adsorption of U(VI) affects the original layered structure of MgAl-LDH. Following the adsorption of U(VI), the XRD pattern of MgAl-LDH, MgAl-LDO, and LDO-KGM exhibited similar additional diffraction peaks within the 2θ range of 15° to 48° (Figure 7g). It was observed that the clear and sharp new peaks appeared at 15.26° and 26.45° in MgAl-LDH, MgAl-LDO, and LDO-KGM. This indicated that U(VI) formed complexes with the hydroxyl groups present on the metal layer of MgAl-LDH, MgAl-LDO, and LDO-KGM. After the immobilization of U(VI), a noticeable weakening of the (003) plane was observed, implying that U(VI) had bound to the carbonate present on the material surfaces [36]. Moreover, the observed diffraction peaks at 24.68°, 25.88°, and 44.39° may be contributed to the uranyl carbonate hydrate species (PDF 34-0578), indicating that the uranyl carbonate hydrate complexes were entrapped in the reconstructed structure [36]. In summary, the sorption mechanism of U(VI) onto the materials may be surface complexation and precipitation with carbonate.
The FTIR spectrum provided functional groups present in the three materials following their interaction with U(VI) (Figure 7h). The prominent and broad adsorption band observed in the range of 3600–3200 cm−1 on MgAl-LDH, MgAl-LDO, and LDO-KGM was attributed to the -OH stretching vibrations of the physically adsorbed water, while the distinctive peak at 1621 cm−1 was attributed to the bending vibration of the interlayer water molecules [58]. Subsequent to U(VI) adsorption, the peaks corresponding to -OH in MgAl-LDH, MgAl-LDO, and LDO-KGM were red-shifted, which was a stretching vibration of -OH, implying that the H of -OH on the materials was more reactive during the reaction and could be easily replaced by UO22+. A novel peak emerged at 936 cm−1 on reacted materials, which can be associated with the asymmetric stretching vibration of UO22+, further indicating that U(VI) has been effectively incorporated into the structures of MgAl-LDH, MgAl-LDO, and LDO-KGM through the adsorption process [59]. The results were in agreement with the XRD analysis.
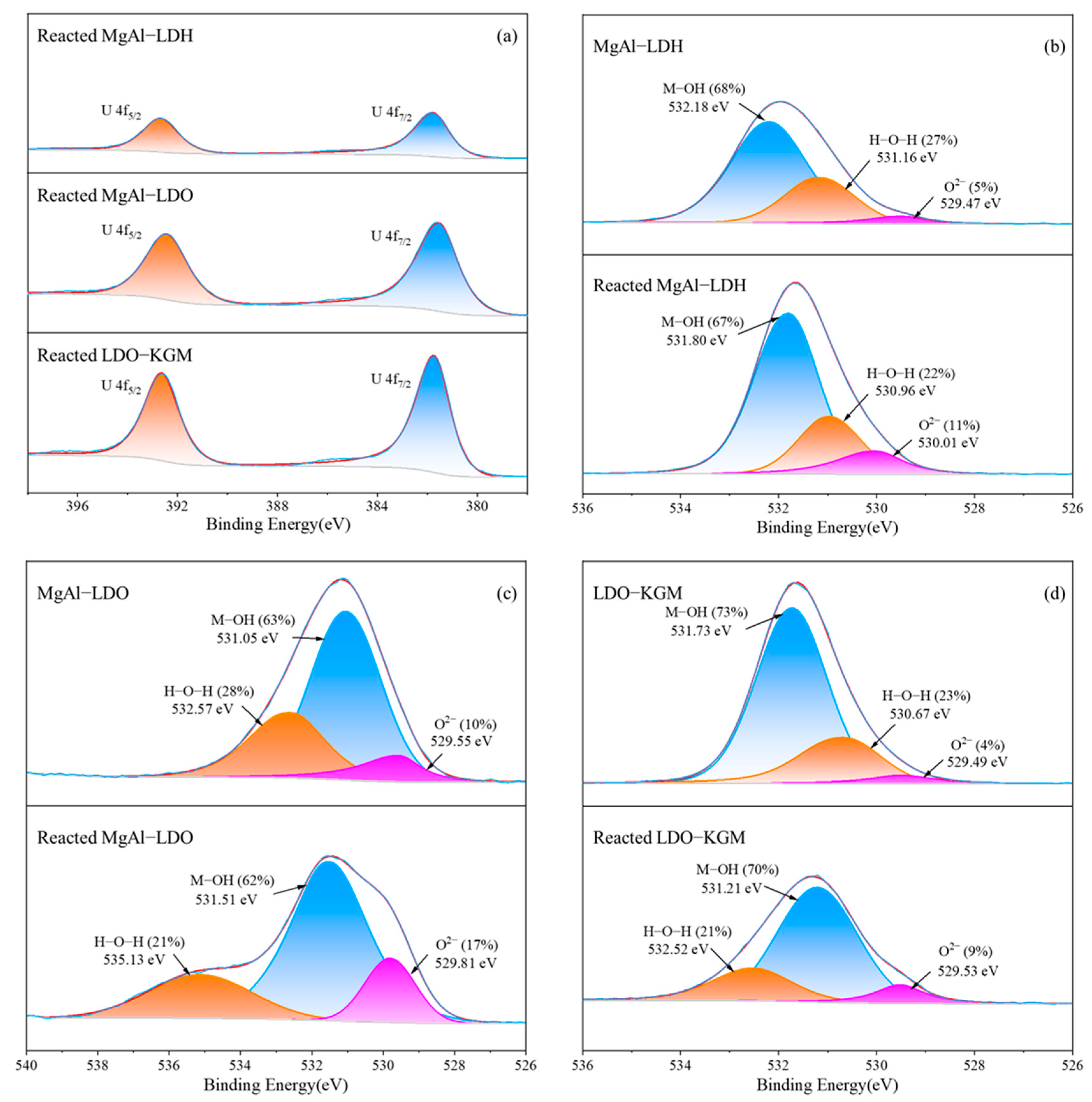
The XPS was utilized to analyze the surface chemistry of the materials, both prior to and following the reaction, as depicted in Figure 8. Analysis revealed that the intense peaks of U 4f were introduced by the adsorption process and could be resolved into the following two distinct peaks: U 4f7/2 at a binding energy of 381.74 eV and U 4f5/2 at 392.64 eV. This result indicated that no redox reaction between U(VI) and other materials occurs [57]. Instead, U(VI) formed chemical bonds with the reactive surface sites of the material [60]. In comparison with MgAl-LDH and MgAl-LDO, the reacted LDO-KGM displayed enhanced peak intensity at the corresponding binding energies, indicating a more robust binding affinity between U(VI) and LDO-KGM. This stronger interaction aligns with LDO-KGM’s superior capacity for immobilizing U(VI).
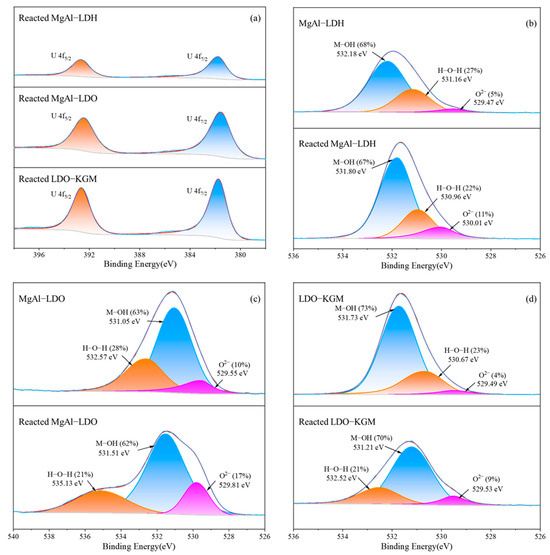
Figure 8.
High-resolution XPS spectrum of (a) U 4f; (b–d) O 1s before and after U(VI) adsorption.
Figure 8b–d presented the O 1s spectra for three materials, both prior to and following the U(VI) immobilization. As illustrated in Figure 8d, LDO-KGM had three O 1s overlapping peaks at 529.49, 530.67, and 531.73 eV, which were ascribed to the vibration of O2−, H-O-H, and M-OH (where M represented Mg or Al), respectively [61,62]. During the reaction, M-O on the LDO-KGM surface was hydrolyzed to yield M-OH, which subsequently participated in surface complexation with U(VI), leading to the formation of M-O-U bonds. Therefore, after the reaction, the proportion of M-OH in the LDO-KGM decreased from 73% to 70%, and the relative abundance of O2− escalated from 4% to 9%. Following the adsorption of U(VI), alterations in the relative concentrations and positions of these O-donor functional groups were observed, with the binding energy of M-OH bonds shifting to lower values and that of H-O-H and O2− shifting to higher values. The reaction processes of MgAl-LDH and MgAl-LDO were similar to those of LDO-KGM. The findings substantiated that the oxygen-containing functional groups on the materials’ surface played a crucial role as enrichment and active sites for coordination with U(VI) during adsorption.
The presence of coexisting cations exerted a significant competitive influence on the adsorption of U(VI) onto the materials, in which the stretching vibration of -OH, and the formation of uranyl carbonate hydrate complexes suggested that the uptake of U(VI) by materials was predominantly influenced by surface complexation and carbonate co-precipitation. To obtain a more comprehensive understanding of the fundamental mechanisms underlying the adsorption of U(VI) by three materials, the point of zero charge (pHpzc) of the materials was investigated (Figure 5c,d).
At pH values below 6.0, the removal efficiency for U(VI) was relatively low, as both materials and UO22+ were positively charged below pHpzc (Figure 5d). This led to significant electrostatic repulsion between UO22+ and adsorbents, which inhibit the U(VI) removal behavior of the materials. Furthermore, the solution contained a high concentration of H+ (pH < 6.0), which were more likely to be adsorbed onto the adsorbent surfaces compared to UO22+ [63]. This preferential adsorption of H+ contributed to the reduced efficiency in removing U(VI) from the solution. However, the adsorbent still had some adsorption capacity, indicating the occurrence of surface complexes between U(VI) species and the oxygen-containing functional groups present on the adsorbent [64]. At a pH value of approximately 6.0, the removal efficiency for U(VI) was at its peak, attributed to the strong electrostatic attraction between negatively charged materials and positively charged UO22+. When the pH surpassed 6.0, the three materials exhibited disparate trends. In alkaline environments, the solution’s OH− concentration increased, and U(VI) could form stable U(VI)-containing complexes (Figure S3), such as (UO2)3(OH)7− [65], which in turn, underwent electrostatic repulsion with negatively charged materials or complexed with OH− on the surface of materials. For MgAl-LDH, the adsorption capacity decreased, because the complexation on the surface was weaker than electrostatic repulsion. In contrast, MgAl-LDO and LDO-KGM demonstrated exceptional efficiency in removing U(VI), attributed to their robust surface complexation capabilities.
3.5. Reusability and Regeneration
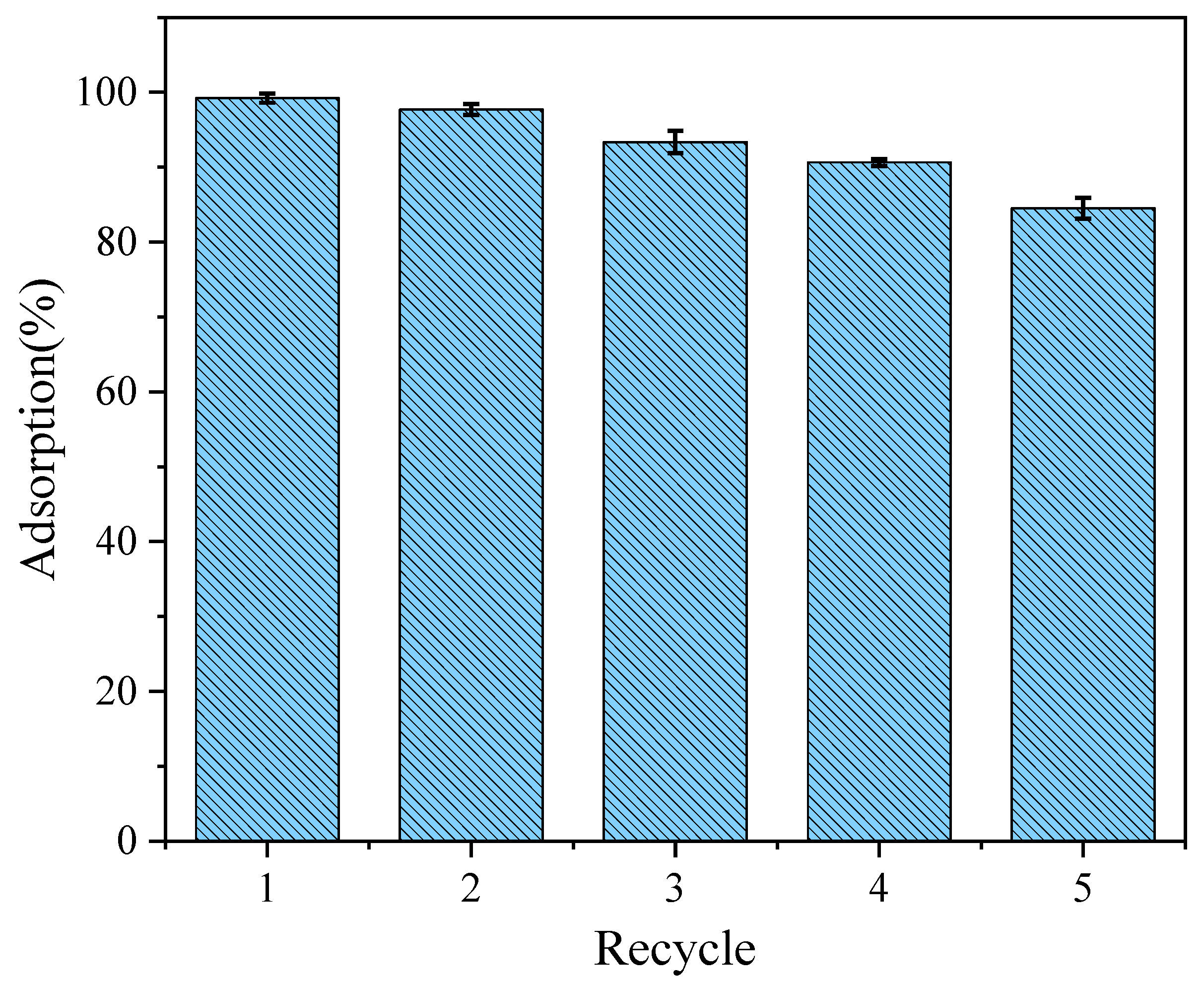
The reusability of adsorbents is an important factor for their practical application. Simultaneously, recycling adsorbents can save cost and reduce the waste of resources; therefore, it was also necessary to study the recycling performance of the adsorbents. On this basis, the reusability of the LDO-KGM was studied in five cycles of regeneration (Figure 9). During this process, the LDO-KGM were filtered out in the process of collecting the supernatants for the recycling experiments. Then, the collected LDO-KGM were eluted with hydrochloric acid of pH 3 and stirred for 24 h to desorb. After desorption, the LDO-KGM, which were restored to the state before adsorption, were repeatedly washed with DI water and then dried for the next adsorption experiment. After five cycles, the removal rates of LDO-KGM were 84.5%, which showed that LDO-KGM had good regeneration characteristics for the removal of U(VI).
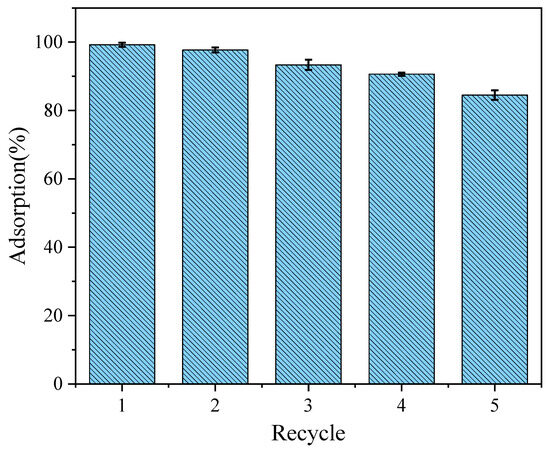
Figure 9.
Recycling of LDO-KGM in the adsorption of U(VI) (C0 = 100 mg·L−1, pH = 6.0, T = 293 K).
4. Conclusions
LDO-KGM, synthesized by the FDC method using KGM as a template, has demonstrated its effectiveness as an adsorbent for removing U(VI) from wastewater. The high specific surface area and plentiful active adsorption sites of LDO-KGM stem from its porous architecture and oxygen-containing functional groups. The U(VI) adsorption behavior followed the pseudo-second-order kinetic model (R2 ≥ 0.99) and the Langmuir isotherm model (R2 ≥ 0.99), which indicated that the primary adsorption mechanism at the molecular level was chemisorption. The Langmuir isotherm model determined a maximum adsorption capacity of 3019.56 mg·g−1, outperforming other adsorbents derived from LDHs or KGM, as reported in the literature. Moreover, LDO-KGM retained over 84.5% U(VI) adsorption efficiency after five consecutive cycles, underscoring its excellent recyclability. Complexation, carbonate co-precipitation, and electrostatic adsorption were the predominant mechanisms for U(VI) immobilization. Consequently, our findings not only elucidate the adsorption mechanism of U(VI) by LDO-KGM but also advance the design of high-performance LDO-based materials for environmental applications. Importantly, the synthesis method, combined with the material’s high capacity and recyclability, suggests strong potential for cost-effective, large-scale implementation in water purification and related fields, such as heavy-metal remediation. This work bridges fundamental research with practical engineering solutions, offering a sustainable pathway for next-generation adsorbent development.
5. Insufficient and Prospect
The current investigation was predominantly confined to idealized laboratory conditions involving single-component U(VI) solutions, while critical challenges inherent to authentic radioactive effluents—particularly synergistic interference from dissolved organic colloids, hypersaline matrices, and coexisting actinides—remained insufficiently characterized. This methodological simplification risks overestimating the material’s ability in nuclear wastewater treatment scenarios. To bridge this technology readiness gap, the following two strategic directions are proposed: (1) the systematic evaluation of chemical stability under authentic multi-pollutant systems containing competitive radionuclides (such as Th4+, Pu4+); and (2) optimization through different templating agents (such as cellulose, chitosan) or doping strategies (the introduction of magnetic components, multi-metal synergism) to enhance the adsorption selectivity, cyclic stability, and functional versatility of the materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13030876/s1, Figure S1: (a) N2 adsorption–desorption isotherms and (b) the average pore size distributions of materials; Figure S2: Effect of materials dosages on U(VI) immobilization (C0 = 100 mg·L−1, pH = 6.0, T = 293 K); Figure S3: Species distribution of U(VI) as a function of pH (C0 = 100 mg·L−1); Figure S4: XPS spectra of materials before (a) and after (b) reacted with U(VI); Table S1: The kinetic model parameters for the immobilization of U(VI) on materials at pH 6.0; Table S2: Langmuir and Freundlich isotherm constants for U(VI) immobilization on adsorbents; Table S3: Thermodynamic parameters of adsorbents.
Author Contributions
Conceptualization, N.Z. and Z.D.; methodology, P.G., J.W., Z.S., M.C. and B.L.; software, P.G.; validation, P.G.; formal analysis, P.G.; investigation, P.G.; resources, P.G.; data curation, P.G.; writing—original draft preparation, P.G.; writing—review and editing, J.W., Z.S., M.C., B.L., T.W. and L.S.; visualization, P.G.; supervision, P.W.; project administration, P.W., Z.D. and N.Z.; funding acquisition, P.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant numbers 42207254 and 42172042.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, H.; Li, C.; Chen, X.; Fu, H.; Chen, Y.; Ning, S.; Fujita, T.; Wei, Y.; Wang, X. Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr2+ and Cs+ from contaminated water. J. Colloid Interface Sci. 2022, 615, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.; Sivaprakash, B.; Raja, V.C.V.; Rajamohan, N.; Muthusamy, G. Remediation techniques for uranium removal from polluted environment—Review on methods, mechanism and toxicology. Environ. Pollut. 2022, 302, 119068. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, W.; Chen, Y.; Wang, Y. Uranium (VI) adsorption by copper and copper/iron bimetallic central mofs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124334. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, L.; Xiong, X.; Yuan, L.; Liao, S.; Wang, Y. Removal of uranium (VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheres prepared by a controlled radical polymerization in the presence of dpe. Chem. Eng. J. 2016, 285, 358–367. [Google Scholar] [CrossRef]
- Zheng, N.; Yin, L.; Su, M.; Liu, Z.; Tsang, D.C.W.; Chen, D. Synthesis of shape and structure-dependent hydroxyapatite nanostructures as a superior adsorbent for removal of U(VI). Chem. Eng. J. 2020, 384, 123262. [Google Scholar] [CrossRef]
- Ma, M.; Ye, Z.; Zhang, J.; Wang, Y.; Ning, S.; Yin, X.; Fujita, T.; Chen, Y.; Wu, H.; Wang, X. Synthesis and fabrication of segregative and durable MnO2@chitosan composite aerogel beads for uranium(VI) removal from wastewater. Water Res. 2023, 247, 120819. [Google Scholar] [CrossRef]
- Mellah, A.; Chegrouche, S.; Barkat, M. The precipitation of ammonium uranyl carbonate (AUC): Thermodynamic and kinetic investigations. Hydrometallurgy 2007, 85, 163–171. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Mukhopadhyay, S.; Bellary, M.P.; Ghosh, S.K. Studies on membrane stability and recovery of uranium (VI) from aqueous solutions using a liquid emulsion membrane process. Hydrometallurgy 2002, 64, 49–58. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, S.L.; Vijayalakshmi, R. Uranium recovery from phosphoric acid by solvent extraction using a synergistic mixture of di-nonyl phenyl phosphoric acid and tri-n-butyl phosphate. Hydrometallurgy 2004, 73, 63–70. [Google Scholar] [CrossRef]
- Krawczyk-Bärsch, E.; Gerber, U.; Müller, K.; Moll, H.; Rossberg, A.; Steudtner, R.; Merroun, M.L. Multidisciplinary characterization of U(VI) sequestration by acidovorax facilis for bioremediation purposes. J. Hazard. Mater. 2018, 347, 233–241. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Li, X.; Liao, J.; Li, S.; Yang, J.; Yang, Y.; Tang, J.; Liu, N. Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem. Eng. J. 2016, 284, 630–639. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Li, B.; Zhang, M.; Wen, R.; Guo, X.; Li, X.; Zhang, J.; Li, S.; Ma, L. Pore-free matrix with cooperative chelating of hyperbranched ligands for high-performance separation of uranium. ACS Appl. Mater. Interfaces 2016, 8, 28853–28861. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Wang, X.; Dai, Y.; Cao, X.; Wang, Y.; Hua, R.; Feng, H.; Chen, J.; Liu, Y.; et al. Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U(VI): Batch and fixed-bed column studies. Chem. Eng. J. 2019, 370, 1376–1387. [Google Scholar] [CrossRef]
- Zhang, H.; Ruan, Y.; Liang, A.; Shih, K.; Diao, Z.; Su, M.; Hou, L.; Chen, D.; Lu, H.; Kong, L. Carbothermal reduction for preparing nZVI/BC to extract uranium: Insight into the iron species dependent uranium adsorption behavior. J. Clean. Prod. 2019, 239, 117873. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Khan, Z.H.; Ying, H.; Chen, D. Utilization of citrullus lanatus l. Seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: Insights and comparison between modified and raw biochar. Sci. Total Environ. 2021, 771, 144955. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Li, Q.; Liao, J.; Zhang, Y.; Zhu, W. Highly enhanced adsorption performance to uranium(VI) by facile synthesized hydroxyapatite aerogel. J. Hazard. Mater. 2022, 423, 127184. [Google Scholar] [CrossRef]
- Bachmaf, S.; Merkel, B.J. Sorption of uranium(VI) at the clay mineral–water interface. Environ. Earth Sci. 2011, 63, 925–934. [Google Scholar] [CrossRef]
- Liu, S.; Luo, M.; Li, J.; Luo, F.; Ke, L.; Ma, J. Adsorption equilibrium and kinetics of uranium onto porous azo-metal–organic frameworks. J. Radioanal. Nucl. Chem. 2016, 310, 353–362. [Google Scholar] [CrossRef]
- Yang, P.; Li, S.; Liu, C.; Liu, X. Interface-constrained layered double hydroxides for stable uranium capture in highly acidic industrial wastewater. ACS Appl. Mater. Interfaces 2021, 13, 17988–17997. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Xie, L.; Zhong, Y.; Xiang, R.; Fu, G.; Xu, Y.; Cheng, Y.; Liu, Z.; Wen, T.; Zhao, Y.; Liu, X. Sono-assisted preparation of Fe(II)-Al(III) layered double hydroxides and their application for removing uranium (VI). Chem. Eng. J. 2017, 328, 574–584. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Wang, X.; Sheng, G.; Wang, S.; Ai, Y.; Ji, Y.; Liu, Y.; Hayat, T.; Wang, X. Glycerol-modified binary layered double hydroxide nanocomposites for uranium immobilization via extended x-ray absorption fine structure technique and density functional theory calculation. ACS Sustain. Chem. Eng. 2017, 5, 3583–3595. [Google Scholar] [CrossRef]
- Yao, W.; Yu, S.; Wang, J.; Zou, Y.; Lu, S.; Ai, Y.; Alharbi, N.S.; Alsaedi, A.; Hayat, T.; Wang, X. Enhanced removal of methyl orange on calcined glycerol-modified nanocrystallined Mg/Al layered double hydroxides. Chem. Eng. J. 2017, 307, 476–486. [Google Scholar] [CrossRef]
- Momin, Z.H.; Lingamdinne, L.P.; Kulkarni, R.; Pal, C.A.; Choi, Y.; Chang, Y.; Koduru, J.R. Exploring recyclable alginate-enhanced gcn-ldo sponge for U(VI) and Cd(II) removal: Insights from batch and column studies. J. Hazard. Mater. 2024, 469, 134015. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Wang, H.; Xie, Y.; Wakeel, M.; Wahid, A.; Zhang, X. Gamma-ferric oxide nanoparticles decoration onto porous layered double oxide belts for efficient removal of uranyl. J. Colloid Interface Sci. 2019, 535, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, X.; Liang, Y.; Yu, S.; Gu, P.; Sun, Y.; Xu, C.; Chen, J.; Hayat, T.; Alsaedi, A.; et al. Synthesis of novel flower-like layered double oxides/carbon dots nanocomposites for U(VI) and 241Am(III) efficient removal: Batch and exafs studies. Chem. Eng. J. 2018, 332, 775–786. [Google Scholar] [CrossRef]
- Chen, T.; Shi, P.; Zhang, J.; Li, Y.; Duan, T.; Dai, L.; Wang, L.; Yu, X.; Zhu, W. Natural polymer konjac glucomannan mediated assembly of graphene oxide as versatile sponges for water pollution control. Carbohydr. Polym. 2018, 202, 425–433. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, J.; Jiang, C.; Liu, S.; Li, Y. Ultralight, hydrophobic, monolithic konjac glucomannan-silica composite aerogel with thermal insulation and mechanical properties. Carbohydr. Polym. 2019, 207, 246–255. [Google Scholar] [CrossRef]
- Wang, R.; Dong, H.; Li, X.; Zhou, L.; Zhu, W.; Chen, T. Biomass encapsulated zif-8-derived ZnO carbon aerogels for efficient uranium extraction by synergistic adsorption-photoreduction. Chem. Eng. J. 2023, 478, 147331. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Zhang, L. Improved catalytic activity on the thermal decomposition of ammonium perchlorate and efficient adsorption of uranium using a novel ultra-low density Al2O3-based aerogels. J. Hazard. Mater. 2020, 387, 122015. [Google Scholar] [CrossRef]
- Hu, W.; Sun, T.; Liu, C.; Yu, L.; Ahamad, T.; Ma, Z. Refined microstructure and enhanced mechanical properties in mo-Y2O3 alloys prepared by freeze-drying method and subsequent low temperature sintering. J. Mater. Sci. Technol. 2021, 88, 36–44. [Google Scholar] [CrossRef]
- Hu, W.; Kong, X.; Du, Z.; Khan, A.; Ma, Z. Synthesis and characterization of nano tic dispersed strengthening w alloys via freeze-drying. J. Alloy. Compd. 2021, 859, 157774. [Google Scholar] [CrossRef]
- Tang, M.; Chen, J.; Wang, P.; Wang, C.; Ao, Y. Highly efficient adsorption of uranium(VI) from aqueous solution by a novel adsorbent: Titanium phosphate nanotubes. Environ. Sci. Nano 2018, 5, 2304–2314. [Google Scholar] [CrossRef]
- Xiong, J.; Fan, Y.; Luo, F. Grafting functional groups in metal–organic frameworks for U(VI) sorption from aqueous solutions. Dalton Trans. 2020, 49, 12536–12545. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, J.; Kang, Y.; Zhang, H.; Ge, Y.; Yuan, N.; Xing, Y.; Ma, W.; Yang, Z.; Zou, L.; et al. Ultralight carbon aerogel composites derived from MOFs and cross-linked chitosan: Synthesis, characterization, and U(VI) adsorption. Chem. Eng. J. 2023, 455, 140749. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Li, L.; Jiang, L.; Ahmed, Z.; Dang, Z.; Wu, P. Memory effect induced the enhancement of uranium (VI) immobilization on low-cost MgAl-double oxide: Mechanism insight and resources recovery. J. Hazard. Mater. 2021, 401, 123447. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Gong, B.; Fang, Y.; Zhu, N. Fabrication and photocatalytic properties of a visible-light responsive nanohybrid based on self-assembly of carboxyl graphene and ZnAl layered double hydroxides. J. Mater. Chem. A 2014, 2, 5534–5540. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Zhang, Y.; Guo, Z.; Luo, Y.; Mao, C. Engineering ultrafine nis cocatalysts as active sites to boost photocatalytic hydrogen production of MgAl layered double hydroxide. Appl. Surf. Sci. 2020, 506, 144999. [Google Scholar] [CrossRef]
- Lv, X.; Qin, X.; Wang, K.; Peng, Y.; Wang, P.; Jiang, G. Nanoscale zero valent iron supported on MgAl-LDH-decorated reduced graphene oxide: Enhanced performance in Cr(VI) removal, mechanism and regeneration. J. Hazard. Mater. 2019, 373, 176–186. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Zhu, Y.; Tran, L. Simultaneous adsorption of Cd2+ and bpa on amphoteric surfactant activated montmorillonite. Chemosphere 2016, 144, 1026–1032. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Xu, M.; Chen, K.; Tan, X.; Wakeel, M.; Alharbi, N.S. In situ carbothermal reduction synthesis of Fe nanocrystals embedded into n-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction. Chem. Eng. J. 2018, 331, 395–405. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Wang, X.; Xie, Z.; Deng, N. Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J. Hazard. Mater. 2009, 168, 1053–1058. [Google Scholar] [CrossRef]
- Wu, P.; Dai, Y.; Long, H.; Zhu, N.; Li, P.; Wu, J.; Dang, Z. Characterization of organo-montmorillonites and comparison for Sr(II) removal: Equilibrium and kinetic studies. Chem. Eng. J. 2012, 191, 288–296. [Google Scholar] [CrossRef]
- Yin, L.; Hu, Y.; Ma, R.; Wen, T.; Wang, X.; Hu, B.; Yu, Z.; Hayat, T.; Alsaedi, A.; Wang, X. Smart construction of mesoporous carbon templated hierarchical Mg-Al and Ni-Al layered double hydroxides for remarkably enhanced U(VI) management. Chem. Eng. J. 2019, 359, 1550–1562. [Google Scholar] [CrossRef]
- Pang, H.; Wu, Y.; Huang, S.; Ding, C.; Li, S.; Wang, X.; Yu, S.; Chen, Z.; Song, G.; Wang, X. Macroscopic and microscopic investigation of uranium elimination by Ca–Mg–Al-layered double hydroxide supported nanoscale zero valent iron. Inorg. Chem. Front. 2018, 5, 2657–2665. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Liu, Q.; Wang, J.; Jing, X.; Liu, L.; Liu, J.; Song, D. Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Q.; Liu, J.; Chen, R.; Zhang, H.; Li, R.; Wang, J. Ni–Mn LDH-decorated 3d Fe-inserted and n-doped carbon framework composites for efficient uranium(VI) removal. Environ. Sci. Nano 2018, 5, 467–475. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Li, K.; Zhang, Y.; Zhu, W. Construction of novel magnesium oxide aerogel for highly efficient separation of uranium(VI) from wastewater. Sep. Purif. Technol. 2022, 295, 121296. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Wang, J.; Xu, C.; Liang, Y.; Yao, W.; Wang, X.; Yu, S.; Chen, J.; Sun, Y.; et al. Superior immobilization of U(VI) and 243Am(III) on polyethyleneimine modified lamellar carbon nitride composite from water environment. Chem. Eng. J. 2017, 326, 863–874. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ou, X.; Li, J.; Zhou, Y.; Zhang, Z.; Dong, S.; Lin, Z. Interfacial engineering improved the selective extraction of uranyl from saline water by nano-Mg(OH)2 and the underlying mechanism. ACS Sustain. Chem. Eng. 2016, 4, 801–809. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y. Effective removal of uranium from aqueous solution by using novel sustainable porous Al2O3 materials derived from different precursors of aluminum. Inorg. Chem. Front. 2020, 7, 765–776. [Google Scholar] [CrossRef]
- Liu, J.; Shi, S.; Li, C.; Hong, X.; Gu, Z.; Li, F.; Zhai, J.; Zhang, Q.; Liao, J.; Liu, N.; et al. U(VI) adsorption by one-step hydrothermally synthesized cetyltrimethylammonium bromide modified hydroxyapatite-bentonite composites from phosphate-carbonate coexisted solution. Appl. Clay Sci. 2021, 203, 106027. [Google Scholar] [CrossRef]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Pang, H.; Li, L.; Jiang, S.; Wen, T.; Zhuang, L.; Hu, B.; Wang, X. Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from mil-53. Chem. Eng. J. 2018, 353, 157–166. [Google Scholar] [CrossRef]
- Hu, W.; Lu, S.; Song, W.; Chen, T.; Hayat, T.; Alsaedi, N.S.; Chen, C.; Liu, H. Competitive adsorption of U(VI) and Co(II) on montmorillonite: A batch and spectroscopic approach. Appl. Clay Sci. 2018, 157, 121–129. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.; Baltrus, J.P.; Stevens, R.W.; Khatri, R.; Chuang, S.S.C.; Filburn, T. Improved immobilized carbon dioxide capture sorbents. Fuel Process. Technol. 2005, 86, 1449–1455. [Google Scholar] [CrossRef]
- Tu, J.; Peng, X.; Wang, S.; Tian, C.; Deng, H.; Dang, Z.; Lu, G.; Shi, Z.; Lin, Z. Effective capture of aqueous uranium from saline lake with magnesium-based binary and ternary layered double hydroxides. Sci. Total Environ. 2019, 677, 556–563. [Google Scholar] [CrossRef]
- Chernobay, G.B.; Chesalov, Y.A.; Baltakhinov, V.P.; Popova, G.Y.; Andrushkevich, T.V. In situ ftir study of β-picoline transformations on V–Ti–O catalysts. Catal. Today 2011, 164, 58–61. [Google Scholar] [CrossRef]
- Lefèvre, G.; Noinville, S.; Fédoroff, M. Study of uranyl sorption onto hematite by in situ attenuated total reflection–infrared spectroscopy. J. Colloid Interface Sci. 2006, 296, 608–613. [Google Scholar] [CrossRef]
- Lei, Y.; Li, K.; Liao, J.; Zhang, Y.; Zhang, L.; Zhu, W. Design of 3d alumina-doped magnesium oxide aerogels with a high efficiency removal of uranium(VI) from wastewater. Inorg. Chem. Front. 2021, 8, 2561–2574. [Google Scholar] [CrossRef]
- Mei, H.; Tan, X.; Tan, L.; Meng, Y.; Chen, C.; Fang, M.; Wang, X. Retention of U(VI) by the formation of Fe precipitates from oxidation of Fe(II). ACS Earth Space Chem. 2018, 2, 968–976. [Google Scholar] [CrossRef]
- Wu, C.; Tu, J.; Tian, C.; Geng, J.; Lin, Z.; Dang, Z. Defective magnesium ferrite nano-platelets for the adsorption of As(V): The role of surface hydroxyl groups. Environ. Pollut. 2018, 235, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y. Application of surface complexation modeling on adsorption of uranium at water-solid interface: A review. Environ. Pollut. 2021, 278, 116861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, C.; Li, J.; Wang, X. Preparation of montmorillonite@carbon composite and its application for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2015, 349, 129–137. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hosseini, S.S.; Akbari, A.; Ramavandi, B. Hydroxyapatite biomaterial production from chicken (femur and beak) and fishbone waste through a chemical less method for Cd2+ removal from shipbuilding wastewater. J. Hazard. Mater. 2021, 413, 125428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).