Machine Learning for the Optimization and Performance Prediction of Solid Oxide Electrolysis Cells: A Review
Abstract
1. Introduction
2. Hydrogen Production
3. SOECs Operation
4. Mathematical Modeling of SOECs
4.1. Electrochemical Model
4.2. Mass Balance
4.3. Energy Balance
5. Optimization of SOEC
6. Machine Learning for SOECs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANN | artificial neural network |
| ANFIS | adaptive network-based fuzzy inference system |
| AK | alkaline electrolyzer |
| BHP | biomass hydrogen production |
| BPNN | backpropagation neural network |
| DNN | deep neural network |
| SOECs | solid oxide electrolysis cells |
| ML | machine learning |
| PEM | proton exchange membrane |
| H2 | Hydrogen |
| HP | hydrogen production |
| YSZ | yttria-stabilized zirconia |
| RF | random forest |
| Ni-YSZ | porous nickel–yttria-stabilized zirconia |
| LSM | lanthanum strontium manganite |
| SVR | support vector regression |
| a | transfer coefficient |
| E0 | Standard reversible voltage |
| R | universal gas constant equal (8.314 J/(mole.K)) |
| T | temperature (K) |
| ne | number of electrons transferred per unit mole of steam equal to 2 |
| F | Faraday constant equal to 96,485 (C/mole) |
| total enthalpy on inlet stream of each node along anode (J/s) | |
| Xi | species molar fraction |
| Pan | pressure along the anode stream (pa) |
| Pca | pressure along the cathode stream (pa) |
| Pi | gas partial pressure (pa) |
| J0 | exchange current density (A/m2) |
| activation overpotentials | |
| ohmic overpotentials | |
| concentration overpotentials | |
| LB | lower bound |
| UB | upper bound |
| Gibbs energy change of reaction T and P standard (J/mole) | |
| species molar flow rate | |
| I | current (A) |
| Van | volume fraction of anode stream |
| Vca | volume fraction of cathode stream |
| Vcell | operating voltage (V) |
| total enthalpy | |
| total enthalpy on outlet stream of each node along the cathode (J/s) | |
| average specific heat capacity of species | |
| composed of convection and conduction heat transfer to each node on each of the different layers | |
| nodal activation overpotential at anode stream | |
| nodal activation overpotential at cathode stream | |
| and | weight factors of these two parameters, which are equal to 0.5 |
| f1 | function of the current density of SOEC |
| A | anode |
| C | cathode |
| F | Faraday constant |
| fH1 | function of the hydrogen production rate of SOECs |
References
- Gu, D.; Andreev, K.; Dupre, M.E. Major trends in population growth around the world. China CDC Wkly. 2021, 3, 604. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Xu, X.; Zhang, Y. Transport energy consumption and saving in China. Renew. Sustain. Energy Rev. 2014, 29, 641–655. [Google Scholar] [CrossRef]
- Santamouris, M.; Vasilakopoulou, K. Present and future energy consumption of buildings: Challenges and opportunities towards decarbonisation. e-Prime-Adv. Electr. Eng. Electron. Energy 2021, 1, 100002. [Google Scholar] [CrossRef]
- Mneimneh, F.; Ghazzawi, H.; Abu Hejjeh, M.; Manganelli, M.; Ramakrishna, S. Roadmap to achieving sustainable development via green hydrogen. Energies 2023, 16, 1368. [Google Scholar] [CrossRef]
- Tangato, K.F. The impact of clean technology adoption on carbon emissions: A global perspective. Clean Technol. Environ. Policy 2024, 1–18. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Dickson, R.; Shah, S.F.A.; Niaz, H.; Khan, A.; Liu, J.J.; Lee, M. Availability, versatility, and viability of feedstocks for hydrogen production: Product space perspective. Renew. Sustain. Energy Rev. 2021, 145, 1100843. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and innovative solid oxide electrolysis cell materials: A short review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Sikiru, S.; Oladosu, T.L.; Amosa, T.I.; Olutoki, J.O.; Ansari, M.N.M.; Abioye, K.J.; Soleimani, H. Hydro-gen-powered horizons: Transformative technologies in clean energy generation, distribution, and storage for sustainable innovation. Int. J. Hydrogen Energy 2024, 56, 1152–1182. [Google Scholar] [CrossRef]
- Ni, M. Modeling of a solid oxide electrolysis cell for carbon dioxide electrolysis. Chem. Eng. J. 2010, 164, 246–254. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, S.; Chi, B.; Tu, Z. Technological limitations and recent developments in a solid oxide electrolyzer cell: A review. Int. J. Hydrogen Energy 2024, 50, 548–591. [Google Scholar] [CrossRef]
- Yin, R.; Sun, L.; Khosravi, A.; Malekan, M.; Shi, Y. Control-oriented dynamic modeling and thermodynamic analysis of solid oxide electrolysis system. Energy Convers. Manag. 2022, 271, 116331. [Google Scholar] [CrossRef]
- Zhang, B.; Harun, N.F.; Zhou, N.; Colon-Rodriguez, J.J.; Oryshchyn, D.; Shadle, L.; Tucker, D.; Bayham, S. A real-time multiphysics model of a pressurized solid oxide electrolysis cell (SOEC) for cyber-physical simulation. Energy Convers. Manag. 2023, 298, 117778. [Google Scholar] [CrossRef]
- Babay, M.-A.; Adar, M.; Chebak, A.; Mabrouki, M. Dynamics of gas generation in porous electrode alkaline electrolysis cells: An investigation and optimization using machine learning. Energies 2023, 16, 5365. [Google Scholar] [CrossRef]
- Wang, Y.; Seo, B.; Wang, B.; Zamel, N.; Jiao, K.; Adroher, X.C. Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy AI 2020, 1, 100014. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, C.; Ju, B.; Xu, H.; Sun, D.; Dang, Y. Estimation of in-situ biogas upgrading in microbial electrolysis cells via direct electron transfer: Two-stage machine learning modeling based on a NARX-BP hybrid neural network. Bioresour. Technol. 2021, 330, 124965. [Google Scholar] [CrossRef] [PubMed]
- Abdelkareem, M.A.; Soudan, B.; Mahmoud, M.S.; Sayed, E.T.; AlMallahi, M.N.; Inayat, A.; Al Radi, M.; Olabi, A.G. Progress of artificial neural networks applications in hydrogen production. Chem. Eng. Res. Des. 2022, 182, 66–86. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelghafar, A.A.; Maghrabie, H.M.; Sayed, E.T.; Rezk, H.; Al Radi, M.; Abdelkareem, M.A. Application of artificial intelligence for prediction, optimization, and control of thermal energy storage systems. Therm. Sci. Eng. Prog. 2023, 39, 101730. [Google Scholar] [CrossRef]
- Mohamed, A.; Ibrahem, H.; Yang, R.; Kim, K. Optimization of proton exchange membrane electrolyzer cell de-sign using machine learning. Energies 2022, 15, 6657. [Google Scholar] [CrossRef]
- Raeesi, M.; Moradzadeh, A.; Ardejani, F.D.; Rahimi, M. Classification and identification of hydrocarbon reservoir lithofacies and their heterogeneity using seismic attributes, logs data and artificial neural networks. J. Pet. Sci. Eng. 2012, 82, 151–165. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, S.; Chen, Y.; Rui, Z.; Hua, K.; Wu, Y.; Li, X.; Duan, X.; Wang, X.; Li, J.; et al. Application of machine learning in optimizing proton exchange membrane fuel cells: A review. Energy AI 2022, 9, 100170. [Google Scholar] [CrossRef]
- Salehi, Z.; Tofigh, M.; Vafaeenezhad, S.; Kharazmi, A.; Smith, D.J.; Koch, C.R.; Shahbakhti, M. Performance Prediction of a Range of Diverse Solid Oxide Fuel Cells using Deep Learning and Principal Component Analysis. IFAC-Papers OnLine 2024, 58, 935–940. [Google Scholar] [CrossRef]
- Szemer, M.; Buchaniec, S.; Prokop, T.; Brus, G. Topology-Informed Machine Learning for Efficient Prediction of Solid Oxide Fuel Cell Electrode Polarization. arXiv 2024, arXiv:2410.05307. [Google Scholar]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Lahrichi, A.; El Issmaeli, Y.; Kalanur, S.S.; Pollet, B.G. Advancements, strategies, and prospects of solid oxide electrolysis cells (SOECs): Towards enhanced performance and large-scale sustainable hydrogen production. J. Energy Chem. 2024, 94, 688–715. [Google Scholar] [CrossRef]
- Kamaroddin, M.F.A.; Sabli, N.; Abdullah, T.A.T.; Siajam, S.I.; Abdullah, L.C.; Jalil, A.A.; Ahmad, A. Membrane-based electrolysis for hydrogen production: A review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of renewable energy sources in tandem with electrolysis: A technology review for green hydrogen production. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Wei, X.; Sharma, S.; Waeber, A.; Wen, D.; Sampathkumar, S.N.; Margni, M.; Maréchal, F.; Van Herle, J. Comparative life cycle analysis of electrolyzer technologies for hydrogen production: Manufacturing and operations. Joule 2024, 8, 3347–3372. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief review of hydrogen production methods and their challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Darko, C.K.; Obiako, P.C.; Kuang, B.; Sun, X.; Jenkins, K. A comparative analysis of different hydrogen production methods and their environmental impact. Clean Technol. 2023, 5, 1344–1380. [Google Scholar] [CrossRef]
- Shu, K.; Guan, B.; Zhuang, Z.; Chen, J.; Zhu, L.; Ma, Z.; Huang, Z. Reshaping the energy landscape: Explorations and strategic perspectives on hydrogen energy preparation, efficient storage, safe transportation and wide applications. Int. J. Hydrogen Energy 2025, 97, 160–213. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A. (Eds.) Advances in Hydrogen Production, Storage and Distribution; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Posdziech, O.; Schwarze, K.; Brabandt, J. Efficient hydrogen production for industry and electricity storage via high-temperature electrolysis. Int. J. Hydrogen Energy 2019, 44, 19089–19101. [Google Scholar] [CrossRef]
- Norman, E.; Maestre, V.; Ortiz, A.; Ortiz, I. Steam electrolysis for green hydrogen generation. State of the art and research perspective. Renew. Sustain. Energy Rev. 2024, 202, 114725. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, G.Y.; Yun, J.W. Characteristics of LaCo1-x NixO3-δ Coated on Ni/YSZ Anode using CH 4 Fuel in Solid Oxide Fuel Cells. J. Electrochem. Sci. Technol. 2020, 11, 336–345. [Google Scholar]
- Vafaeenezhad, S.; Hanifi, A.R.; A Laguna-Bercero, M.; Etsell, T.H.; Sarkar, P. Microstructure and long-term stability of Ni–YSZ anode supported fuel cells: A review. Mater. Futur. 2022, 1, 042101. [Google Scholar] [CrossRef]
- Yefsah, L. Study of the reaction mechanisms and durability of innovative oxygen electrode materials for high temperature electrolysis. Ph.D. Dissertation, Université Grenoble Alpes, Grenoble, France, 2023. [Google Scholar]
- Khalili, M.; Bahnamiri, F.K.; Mehrpooya, M. An integrated process configuration of solid oxide fuel/electrolyzer cells (SOFC-SOEC) and solar organic Rankine cycle (ORC) for cogeneration applications. Int. J. Energy Res. 2021, 45, 11018–11040. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Bello, I.T.; Ojelade, O.A.; Yousuf, A.; Duan, C.; Kazempoor, P. Operational and scaling-up barriers of SOEC and mitigation strategies to boost H2 production-a comprehensive review. Int. J. Hydrogen Energy 2023, 48, 33017–33041. [Google Scholar] [CrossRef]
- Wendel, C.H.; Braun, R.J. Design and techno-economic analysis of high efficiency reversible solid oxide cell systems for distributed energy storage. Appl. Energy 2016, 172, 118–131. [Google Scholar] [CrossRef]
- Daneshpour, R.; Mehrpooya, M. Design and optimization of a combined solar thermophotovoltaic power generation and solid oxide electrolyser for hydrogen production. Energy Convers. Manag. 2018, 176, 274–286. [Google Scholar] [CrossRef]
- Sohal, M.S.; O’Brien, J.E.; Stoots, C.M.; McKellar, M.G.; Herring, J.S.; Harvego, E.A. Challenges in Generating Hydrogen by high Temperature Electrolysis Using Solid Oxide Cells (No. INL/CON-08-14038); Idaho National Lab.(INL): Idaho Falls, ID, USA, 2008. [Google Scholar]
- Jin, X.; Xue, X. Mathematical modeling analysis of regenerative solid oxide fuel cells in switching mode conditions. J. Power Sources 2010, 195, 6652–6658. [Google Scholar] [CrossRef]
- del Pozo Gonzalez, H.; Torrell, M.; Bernadet, L.; Bianchi, F.D.; Trilla, L.; Tarancón, A.; Domínguez-García, J.L. Mathematical modeling and thermal control of a 1.5 kW reversible solid oxide stack for 24/7 hydrogen plants. Mathematics 2023, 11, 366. [Google Scholar] [CrossRef]
- Boyer, H.; Shimpalee, S.; Weidner, J.W.; Ma, Z. Mathematical Modeling of a Proton-Conducting Solid Oxide Electrolyzer Cell Using Computational Fluid Dynamics Simulation. ECS Meet. Abstr. 2019, MA2019-02, 2359. [Google Scholar] [CrossRef]
- Sharma, S.; Stanley, R.; Kumari, N. Electrochemical modeling and simulation of CO2 reduction in solid oxide electrolysis cells. J. Hazardous Toxic Radioact. Waste 2022, 26, 04022005. [Google Scholar] [CrossRef]
- Udagawa, J. Hydrogen production through steam electrolysis: Model-based evaluation of an intermediate temperature solid oxide electrolysis cell. Ph.D. Dissertation, Department of Earth Science and Engineering, Imperial College London, London, UK, 2008. [Google Scholar]
- Xie, Y.; Xue, X. Modeling of solid oxide electrolysis cell for syngas generation with detailed surface chemistry. Solid State Ionics 2012, 224, 64–73. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, Y.; Li, W.; Ni, M.; Cai, N. Elementary reaction modeling and experimental characterization of solid oxide fuel-assisted steam electrolysis cells. Int. J. Hydrogen Energy 2014, 39, 10359–10373. [Google Scholar] [CrossRef]
- Kazempoor, P.; Braun, R.J. Model validation and performance analysis of regenerative solid oxide cells: Electrolytic operation. Int. J. Hydrogen Energy 2014, 39, 2669–2684. [Google Scholar] [CrossRef]
- Menon, V.; Fu, Q.; Janardhanan, V.M.; Deutschmann, O. A model-based understanding of solid-oxide electrolysis cells (SOECs) for syngas production by H2O/CO2 co-electrolysis. J. Power Sources 2015, 274, 768–781. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Tu, Z. Numerical simulation of flow channel geometries optimization for the planar solid oxide electrolysis cell. Int. J. Hydrogen Energy 2023, 52, 288–301. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mastropasqua, L.; Saeedmanesh, A.; Brouwer, J. Investigating electric heater failure scenarios in solid oxide electrolysis cell systems with a novel three-dimensional dynamic SOEC stack model. Energy Convers. Manag. X 2024, 24, 100805. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mastropasqua, L.; Saeedmanesh, A.; Brouwer, J. Sustained long-term efficiency in solid oxide electrolysis systems through innovative reversible electrochemical heat management. Energy Convers. Manag. 2024, 308, 118405. [Google Scholar] [CrossRef]
- Mansilla, C.; Sigurvinsson, J.; Bontemps, A.; Maréchal, A.; Werkoff, F. Heat management for hydrogen production by high temperature steam electrolysis. Energy 2007, 32, 423–430. [Google Scholar] [CrossRef]
- Mogensen, M.B.; Chen, M.; Frandsen, H.L.; Graves, C.; Hansen, J.B.; Hansen, K.V.; Hauch, A.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; et al. Reversible solid-oxide cells for clean and sustainable energy. Clean Energy 2019, 3, 175–201. [Google Scholar] [CrossRef]
- Gopalan, S.; Mosleh, M.; Hartvigsen, J.J.; McConnell, R.D. Analysis of self-sustaining recuperative solid oxide electrolysis systems. J. Power Sources 2008, 185, 1328–1333. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, B.; Chung, C.Y.; Bu, S.; Wang, Q.; Voropai, N. A coordinated multi-energy trading framework for strategic hydrogen provider in electricity and hydrogen markets. IEEE Trans. Smart Grid 2022, 14, 1403–1417. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; de Haart, L.G.J.; Guillon, O.; Eichel, R.-A.; Menzler, N.H. Solid oxide electrolysis cells—Current material development and industrial application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- Martsinchyk, K.; Martsinchyk, A.; Łazor, M.; Shuhayeu, P.; Kupecki, J.; Niemczyk, A.; Błesznowski, M.; Milewski, J. Feasibility study and techno-economic assessment of power-to-gas (P2G) technology based on solid oxide electrolysis (SOE). J. Environ. Manag. 2024, 354, 120425. [Google Scholar] [CrossRef]
- Kabir, M.M.; Roy, S.K.; Alam, F.; Nam, S.Y.; Im, K.S.; Tijing, L.; Shon, H.K. Machine learning-based prediction and optimization of green hydrogen production technologies from water industries for a circular economy. Desalination 2023, 567, 116992. [Google Scholar] [CrossRef]
- Bilgiç, G.; Öztürk, B.; Atasever, S.; Şahin, M.; Kaplan, H. Prediction of hydrogen production by magnetic field effect water electrolysis using artificial neural network predictive models. Int. J. Hydrogen Energy 2023, 48, 20164–20175. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-economic and environmental impact assessment of hydrogen production processes using bio-waste as renewable energy resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Gu, C.; Wang, X.; Song, Q.; Li, H.; Qiao, Y. Prediction of gas-liquid-solid product distribution after solid waste pyrolysis process based on artificial neural network model. Int. J. Energy Res. 2021, 45, 13786–13800. [Google Scholar] [CrossRef]
- Aydinli, B.; Caglar, A.; Pekol, S.; Karaci, A. The prediction of potential energy and matter production from biomass pyrolysis with artificial neural network. Energy Explor. Exploit. 2017, 35, 698–712. [Google Scholar] [CrossRef]
- Zbay, G.; Kökten, E.S. Modeling of bio-oil production by pyrolysis of woody biomass: Artificial neural network approach. Politek. Derg. 2020, 23, 1255–1264. [Google Scholar]
- Nasr, N.; Hafez, H.; El Naggar, M.H.; Nakhla, G. Application of artificial neural networks for modeling of biohydrogen production. Int. J. Hydrogen Energy 2013, 38, 3189–3195. [Google Scholar] [CrossRef]
- Kargbo, H.O.; Zhang, J.; Phan, A.N. Robust modelling development for optimisation of hydrogen production from biomass gasification process using bootstrap aggregated neural network. Int. J. Hydrogen Energy 2022, 48, 10812–10828. [Google Scholar] [CrossRef]
- Iqbal, S.; Aftab, K.; tul Jannat, F.; Baig, M.A.; Kalsoom, U. A bibliographic analysis of optimization of hydrogen production via electrochemical method using machine learning. Fuel 2024, 372, 132126. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, L.; Xiao, J.; Wen, R.; Zhang, F.; Zhang, D. Machine learning-assisted prediction and optimization of solid oxide electrolysis cell for green hydrogen production. Green Chem. Eng. 2024, 2, 154–168. [Google Scholar] [CrossRef]
- Prakasham, R.; Sathish, T.; Brahmaiah, P. Imperative role of neural networks coupled genetic algorithm on optimization of biohydrogen yield. Int. J. Hydrogen Energy 2011, 36, 4332–4339. [Google Scholar] [CrossRef]
- Yang, K.; Liu, J.; Wang, Y.; Shi, X.; Wang, J.; Lu, Q.; Ciucci, F.; Yang, Z. Machine-learning-assisted prediction of long-term performance degradation on solid oxide fuel cell cathodes induced by chromium poisoning. J. Mater. Chem. A 2022, 10, 23683–23690. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Q.; Wu, Q.; Zheng, Y.; Zhou, J.; Tu, Z.; Chan, S.H. Modelling of solid oxide electrolyser cell using extreme learning machine. Electrochim. Acta 2017, 251, 137–144. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Chen, J.; Li, M.; Song, Q.; Huang, Y.; Wu, X.; Xu, Y.; Li, X. Prediction of Hydrogen Production from Solid Oxide Electrolytic Cells Based on ANN and SVM Machine Learning Methods. Atmosphere 2024, 15, 1344. [Google Scholar] [CrossRef]
- Golbabaei, M.H.; Varnoosfaderani, M.S.; Zare, A.; Salari, H.; Hemmati, F.; Abdoli, H.; Hamawandi, B. Performance Analysis of Anode-Supported Solid Oxide Fuel Cells: A Machine Learning Approach. Materials 2022, 15, 7760. [Google Scholar] [CrossRef] [PubMed]



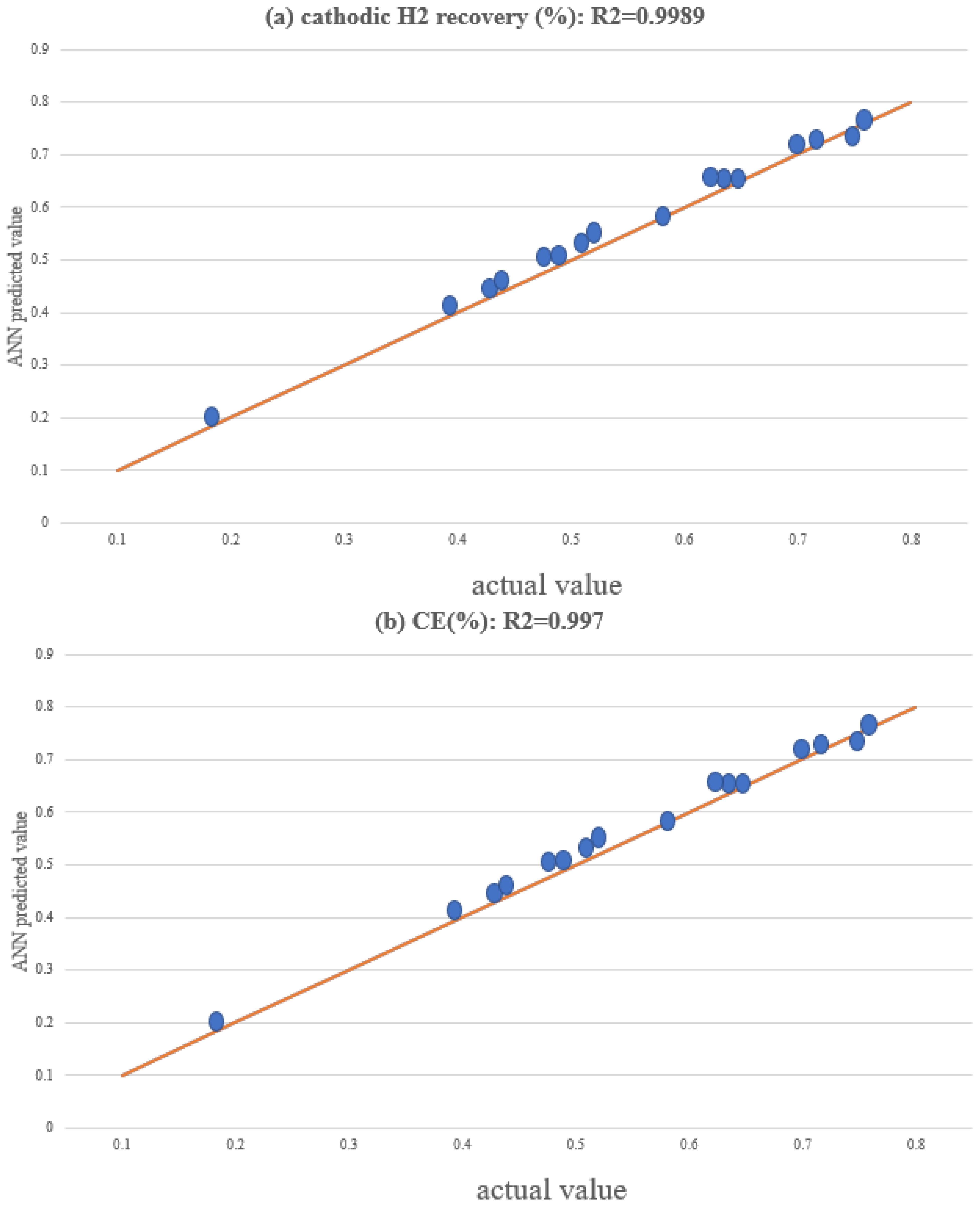
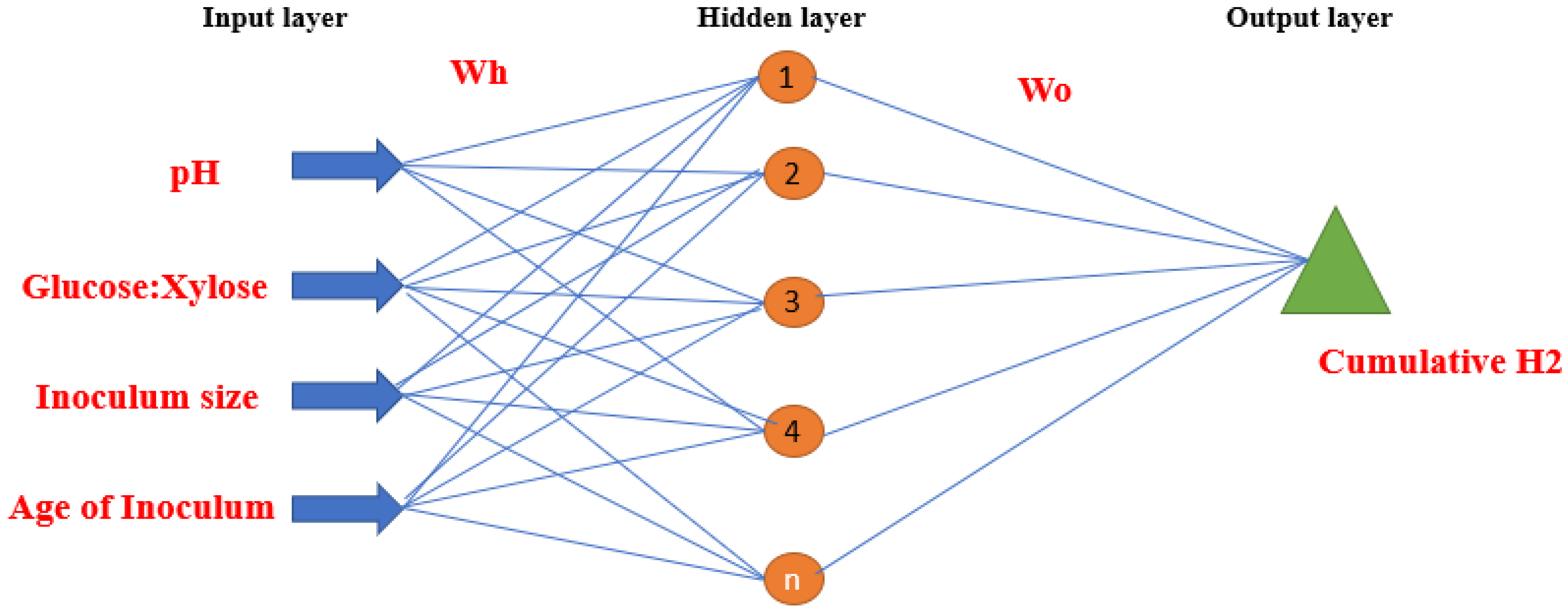
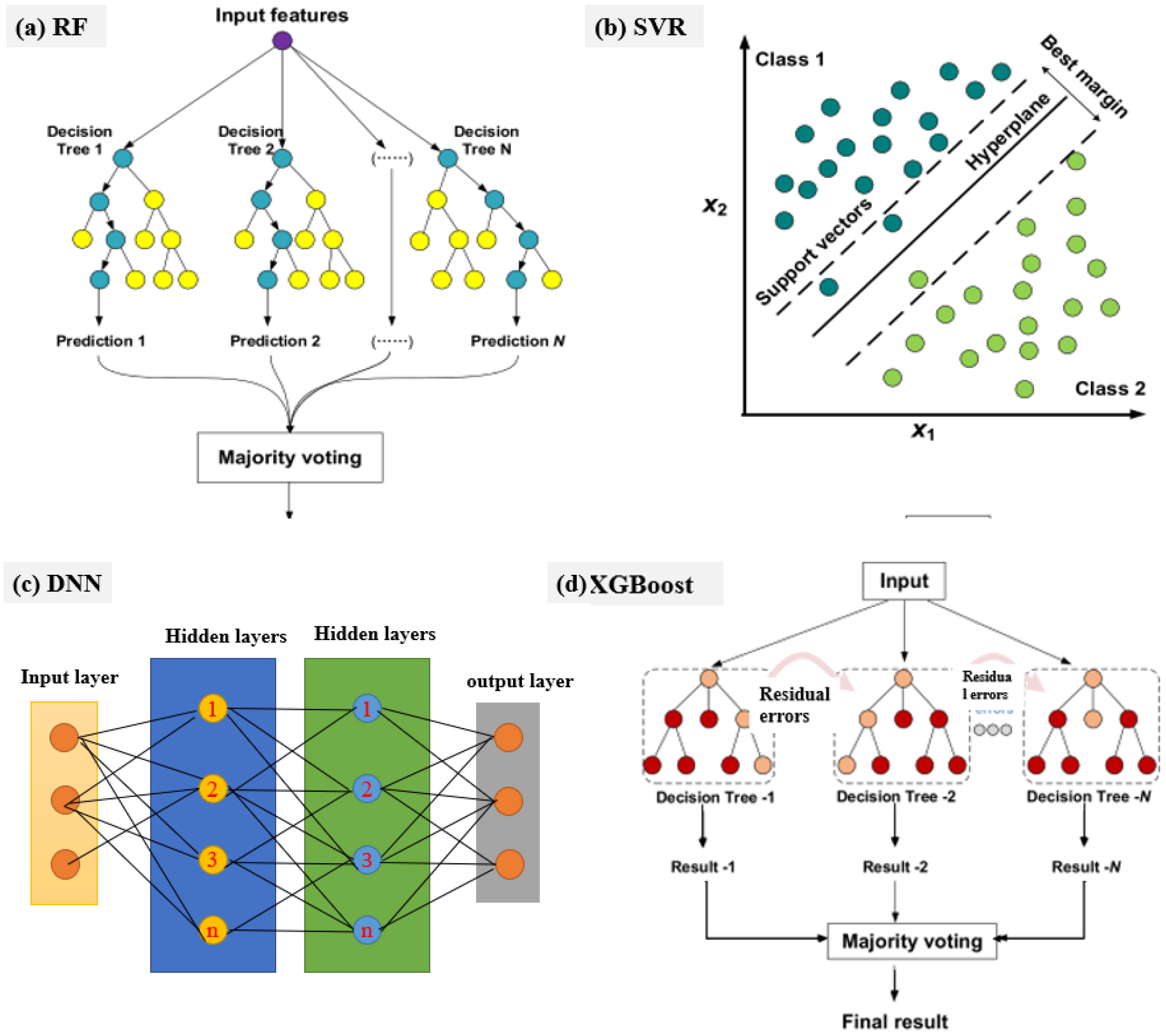

| Parameter | Alkaline Electrolyzers (AWE) | PEM Electrolyzers | Solid Oxide Electrolyzers (SOE) |
|---|---|---|---|
| Cell Temperature (°C) | 60–100 | 50–90 | 500–850 |
| Cell Pressure (bar) | 1–30 | 20–50 | 1–15 |
| Current Density (A/cm2) | 0.2–0.5 | 1.0–2.0 | 0.3–1.0 |
| Cell Voltage (V) | 1.8–2.4 | 1.6–2.0 | 1.0–1.5 |
| Power Density (W/cm2) | 0.4–1.2 | 1.6–4.0 | 0.3–1.5 |
| Voltage Efficiency (%) | 60–70 | 65–80 | 85–95 |
| Specific System Energy Consumption (kWh/Nm3) | 4.5–5.5 | 4.2–5.0 | 3.5–4.0 |
| Hydrogen Production (Nm3/hr) | 10–1000 (system-dependent) | 10–500 (system-dependent) | 5–200 (system-dependent) |
| Hydrogen Purity (%) | 99.5–99.9 | 99.99–99.999 | 99.9–99.99 |
| CAPEX Cost (USD/kW) | 800–1200 | 1400–2000 | 2000–3000 (pre-commercial) |
| OPEX Cost (USD/kg H2) | 0.5–1.0 | 0.8–1.5 | 0.7–1.2 (estimated) |
| Type of Membrane | Diaphragm (e.g., asbestos-free polysulfone) | Solid polymer (e.g., Nafion) | Solid ceramic (e.g., yttria-stabilized zirconia, YSZ) |
| Type of Anode | Nickel (Ni) or Ni-based alloys | Platinum (Pt) or Iridium oxide (IrO2) | Perovskite (e.g., LSM: La0.8Sr0.2MnO3) |
| Type of Cathode | Nickel (Ni) or Ni-Mo alloys | Platinum (Pt) or Pt/C | Ni-YSZ cermet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makki Abadi, M.; Rashidi, M.M. Machine Learning for the Optimization and Performance Prediction of Solid Oxide Electrolysis Cells: A Review. Processes 2025, 13, 875. https://doi.org/10.3390/pr13030875
Makki Abadi M, Rashidi MM. Machine Learning for the Optimization and Performance Prediction of Solid Oxide Electrolysis Cells: A Review. Processes. 2025; 13(3):875. https://doi.org/10.3390/pr13030875
Chicago/Turabian StyleMakki Abadi, Mahmoud, and Mohammad Mehdi Rashidi. 2025. "Machine Learning for the Optimization and Performance Prediction of Solid Oxide Electrolysis Cells: A Review" Processes 13, no. 3: 875. https://doi.org/10.3390/pr13030875
APA StyleMakki Abadi, M., & Rashidi, M. M. (2025). Machine Learning for the Optimization and Performance Prediction of Solid Oxide Electrolysis Cells: A Review. Processes, 13(3), 875. https://doi.org/10.3390/pr13030875










