Structural, Electrochemical, and Supercapacitor Characterization of Double Metal Oxides Doped Within ZIF-8 Composites
Abstract
1. Introduction
2. Experimental Techniques
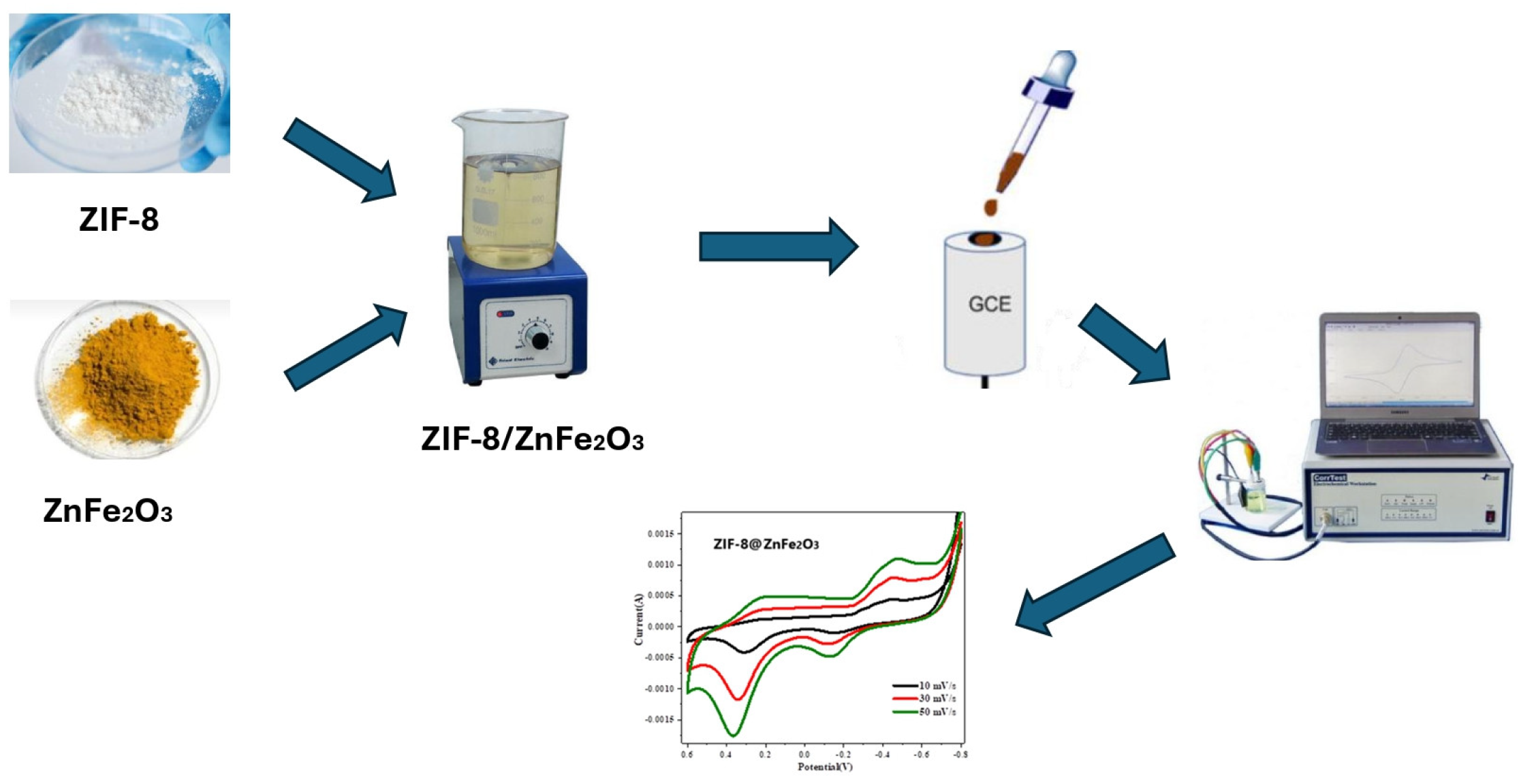
3. Sample Fabrication
4. Results and Discussion
4.1. XRD
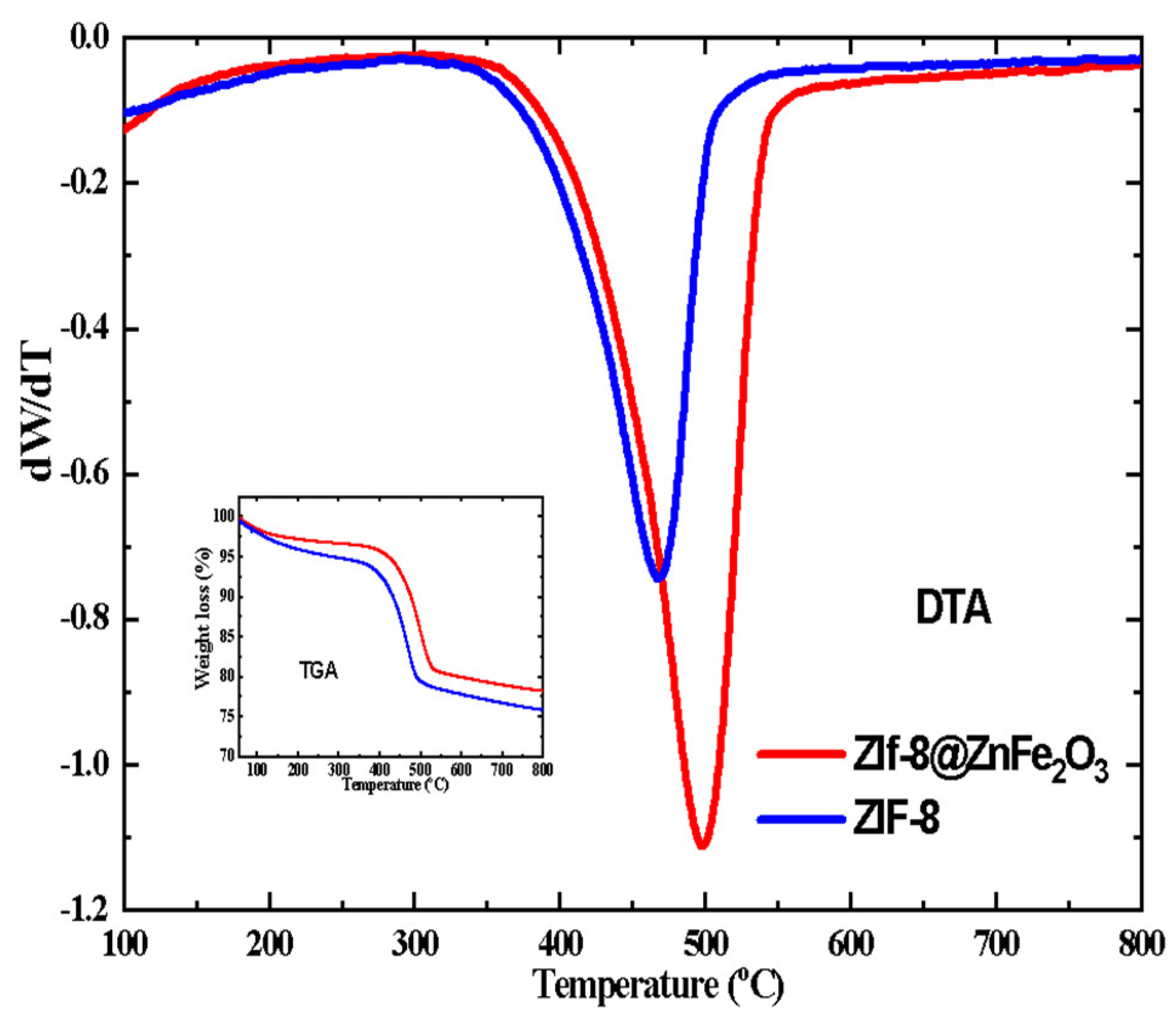
4.2. Thermal Analysis
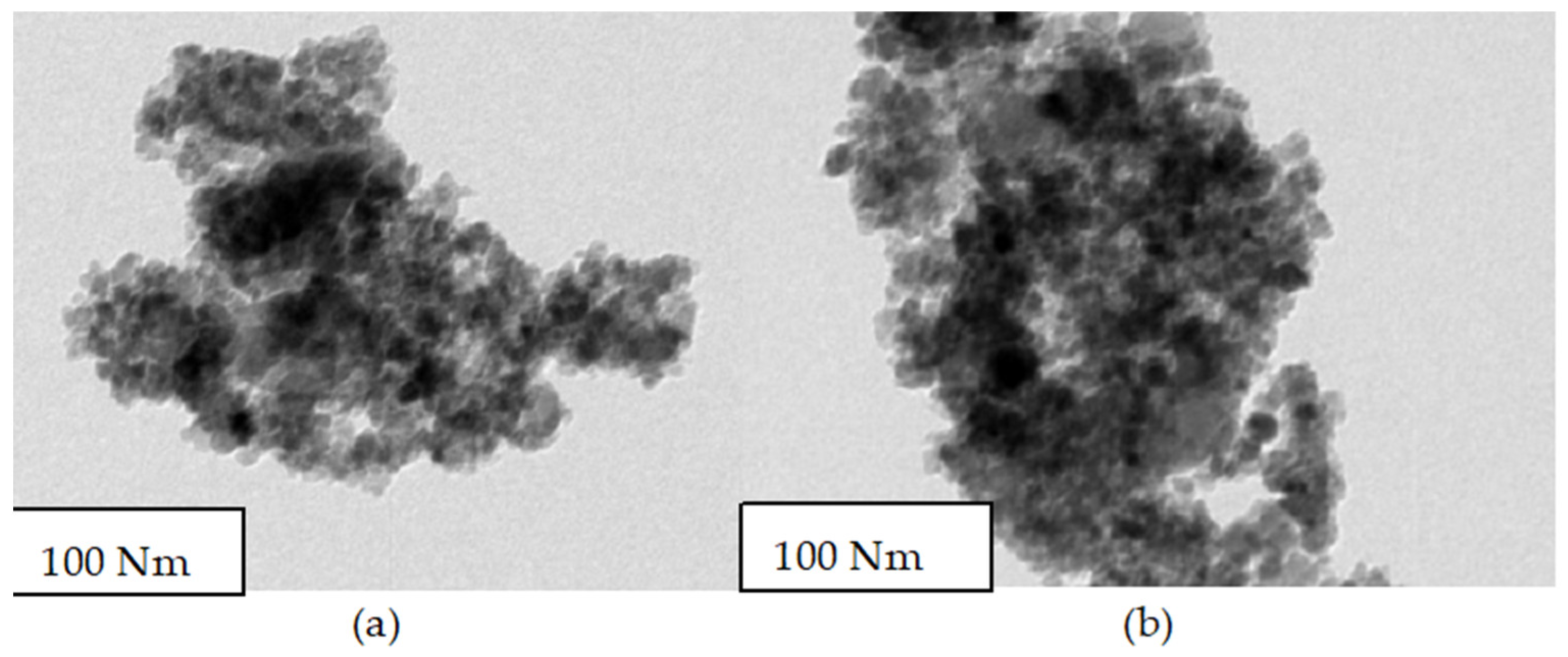
4.3. HRTEM
4.4. FTIR
4.5. Energy Gap
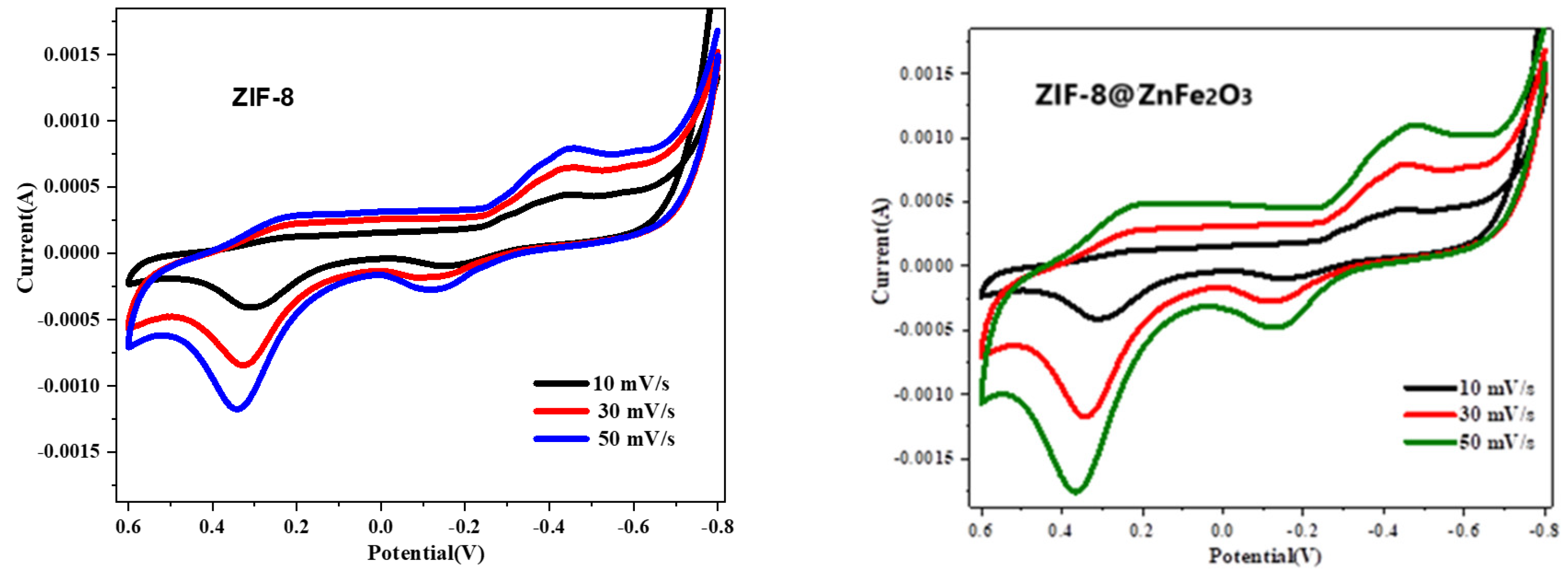
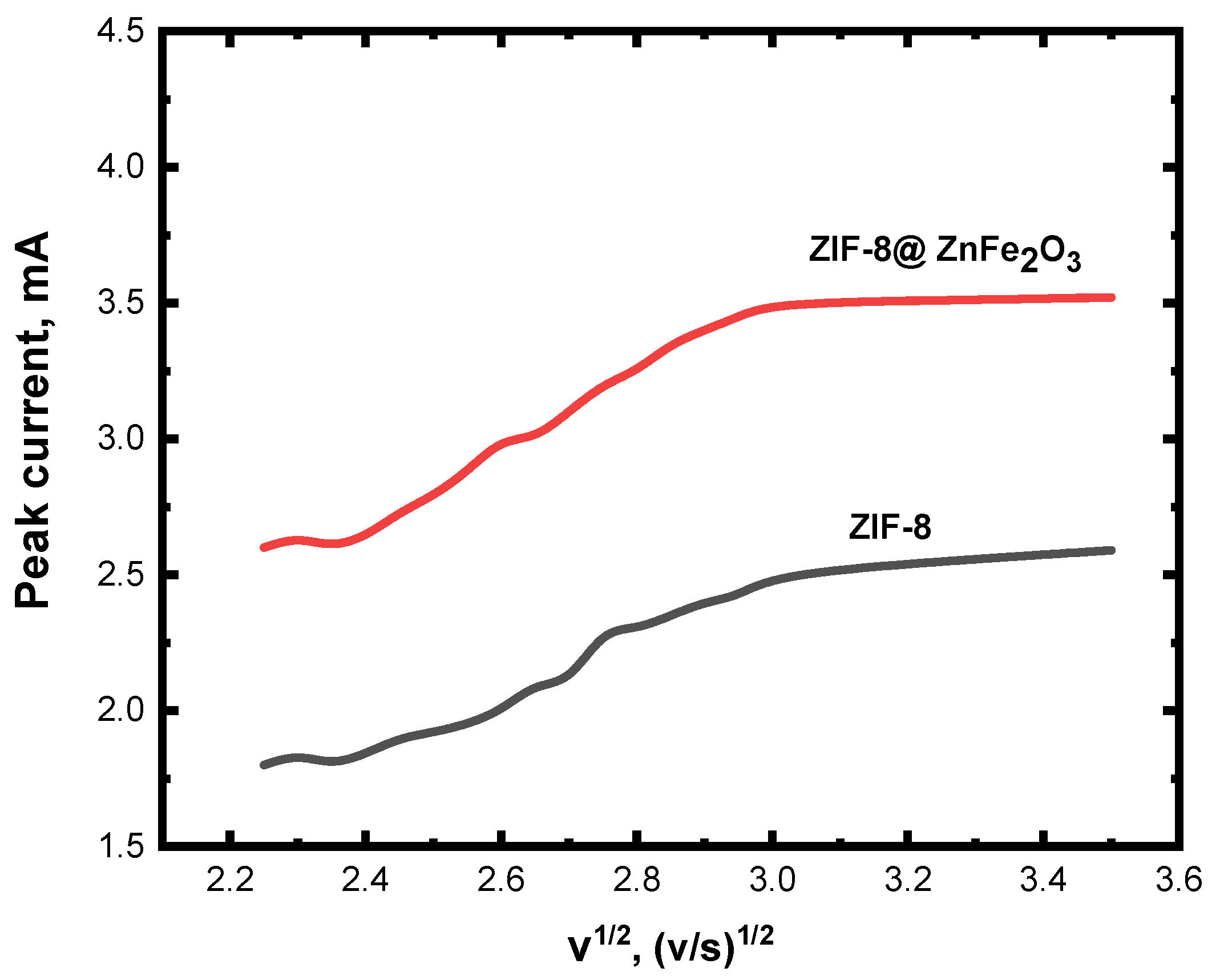
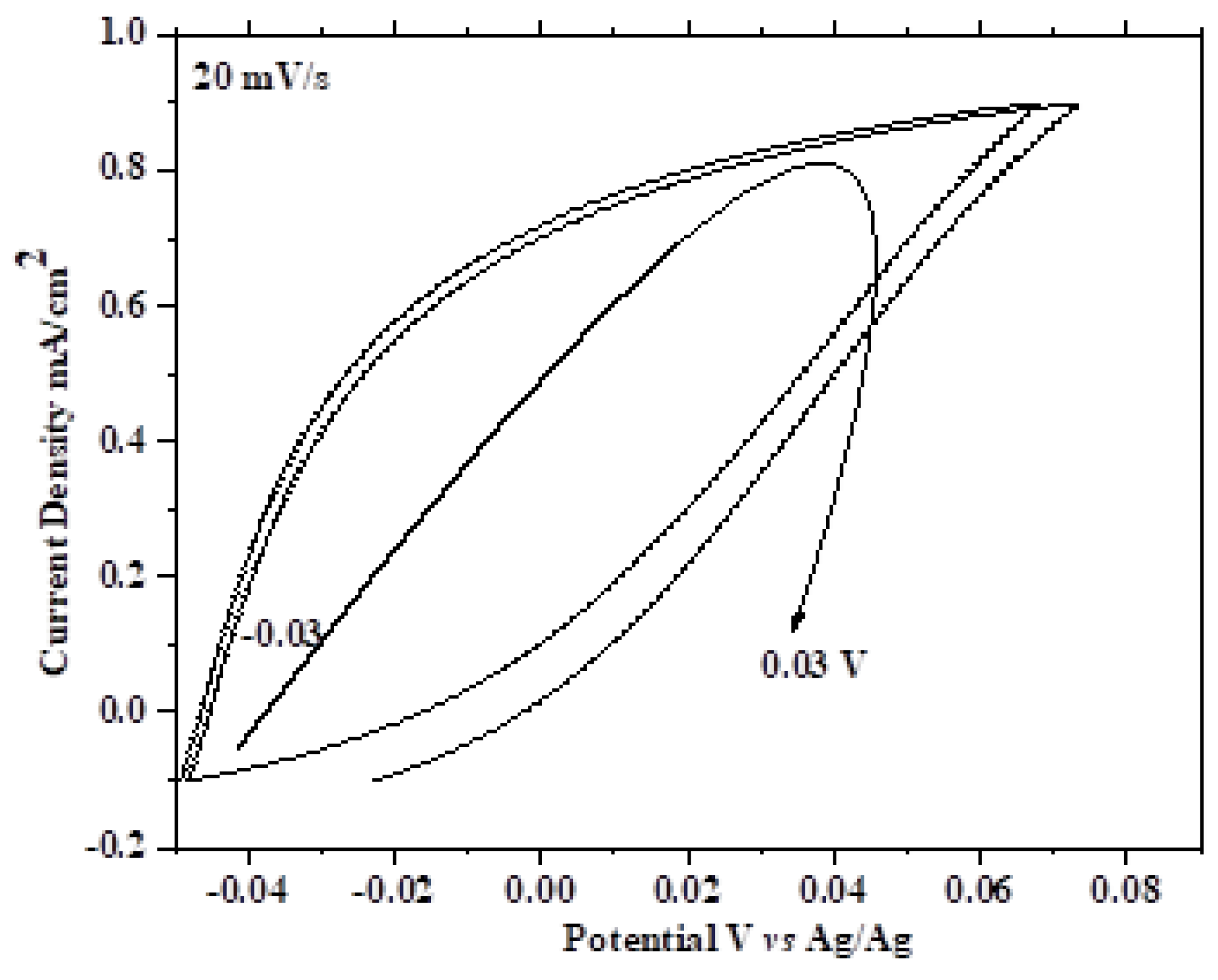
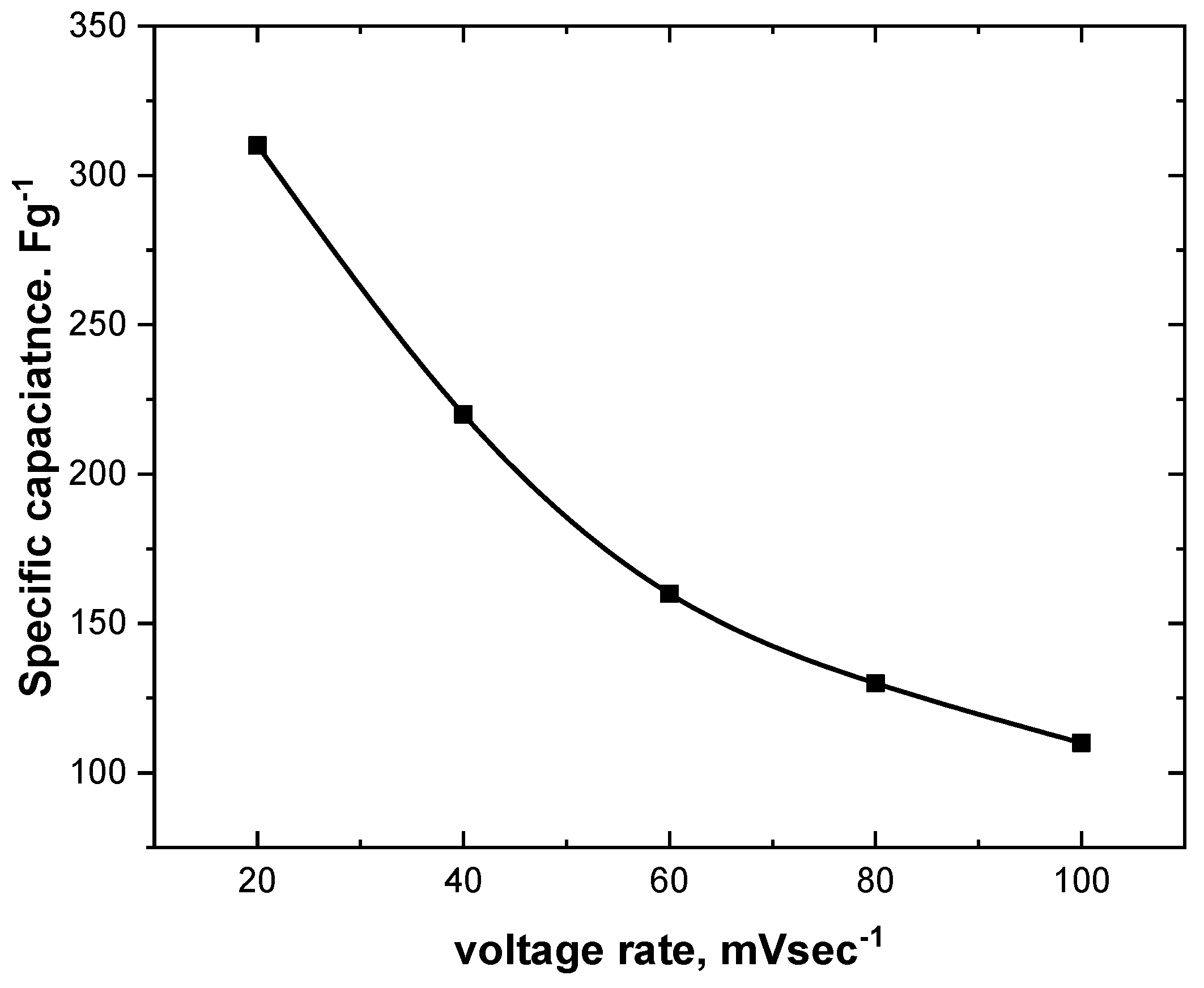
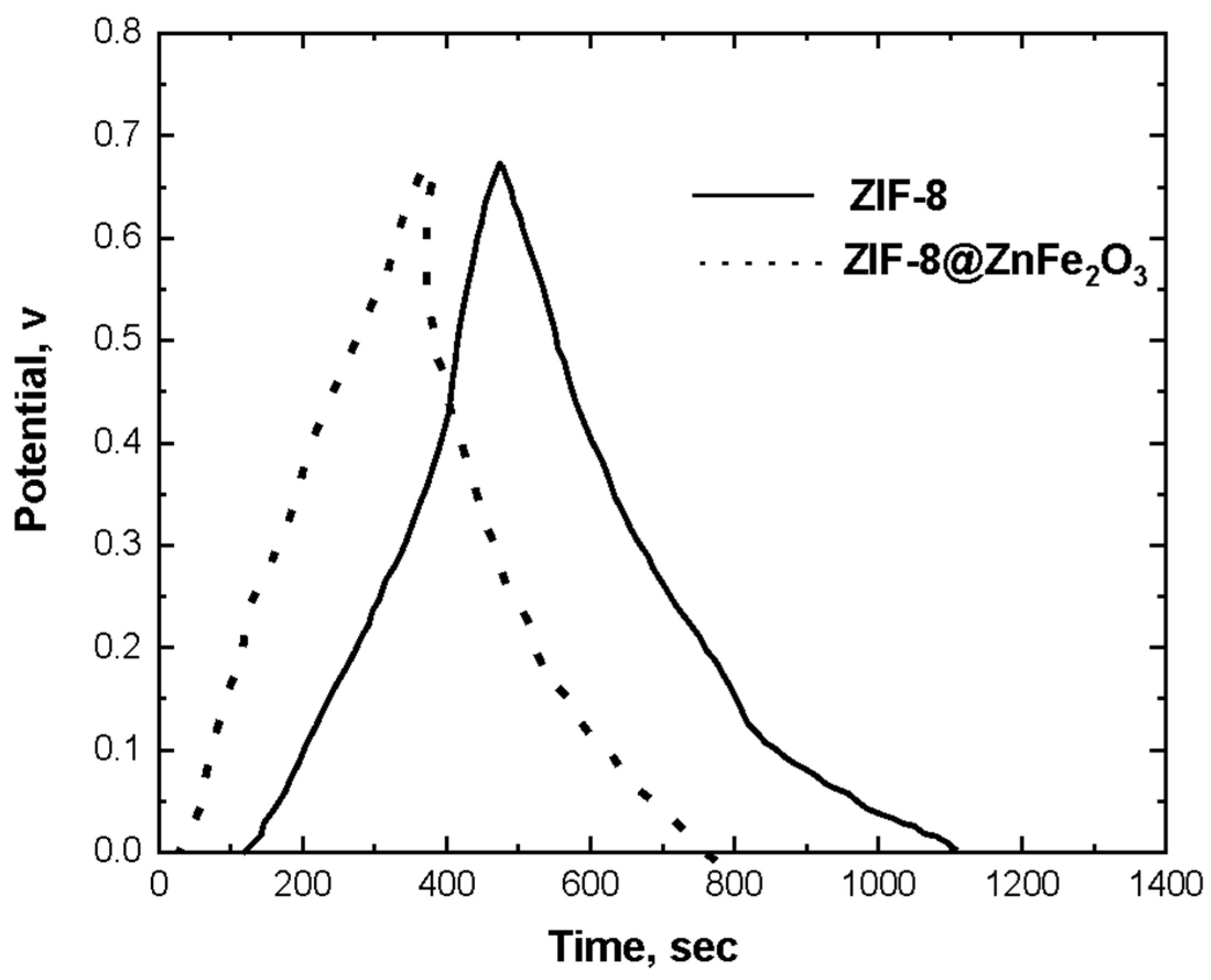
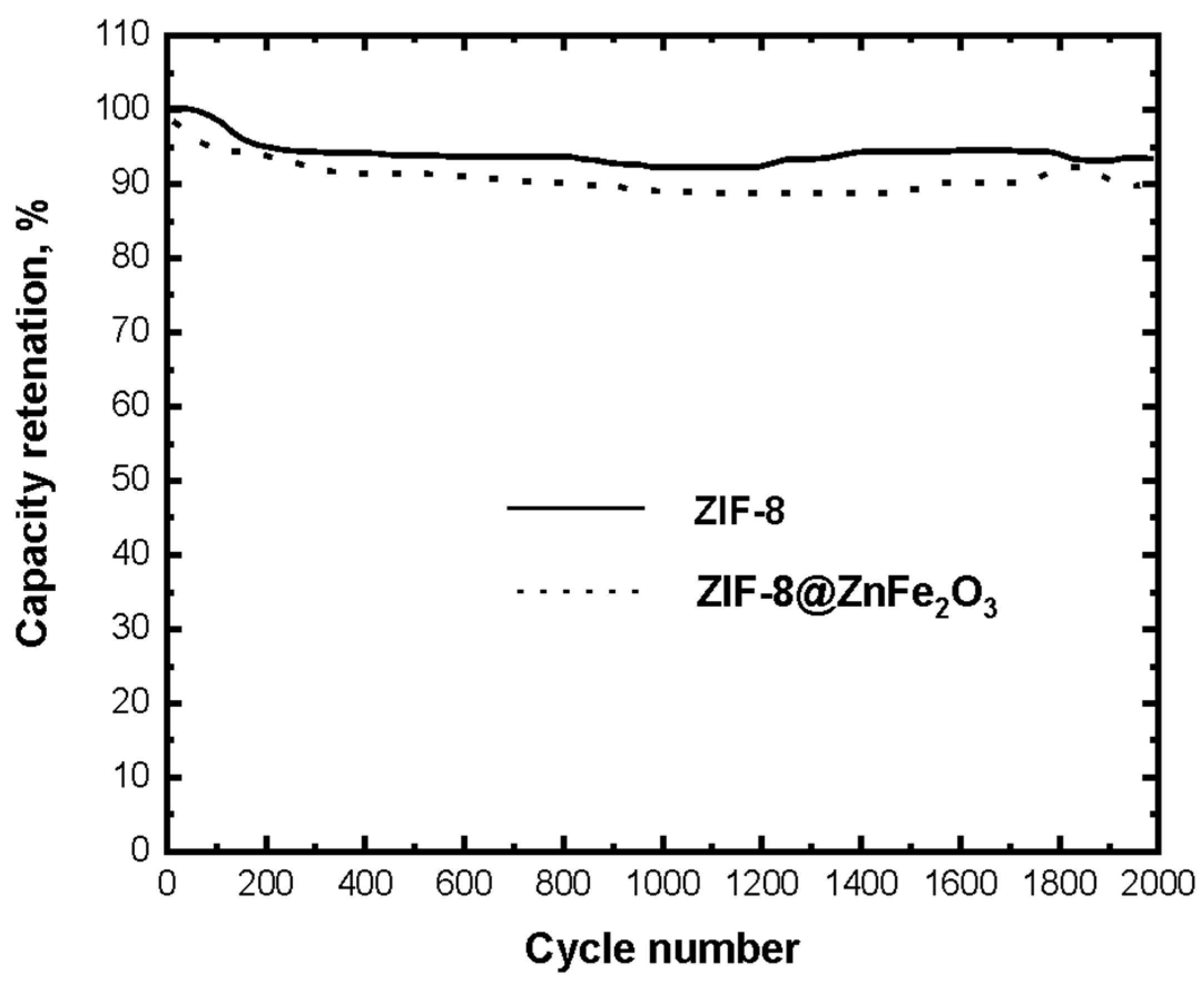
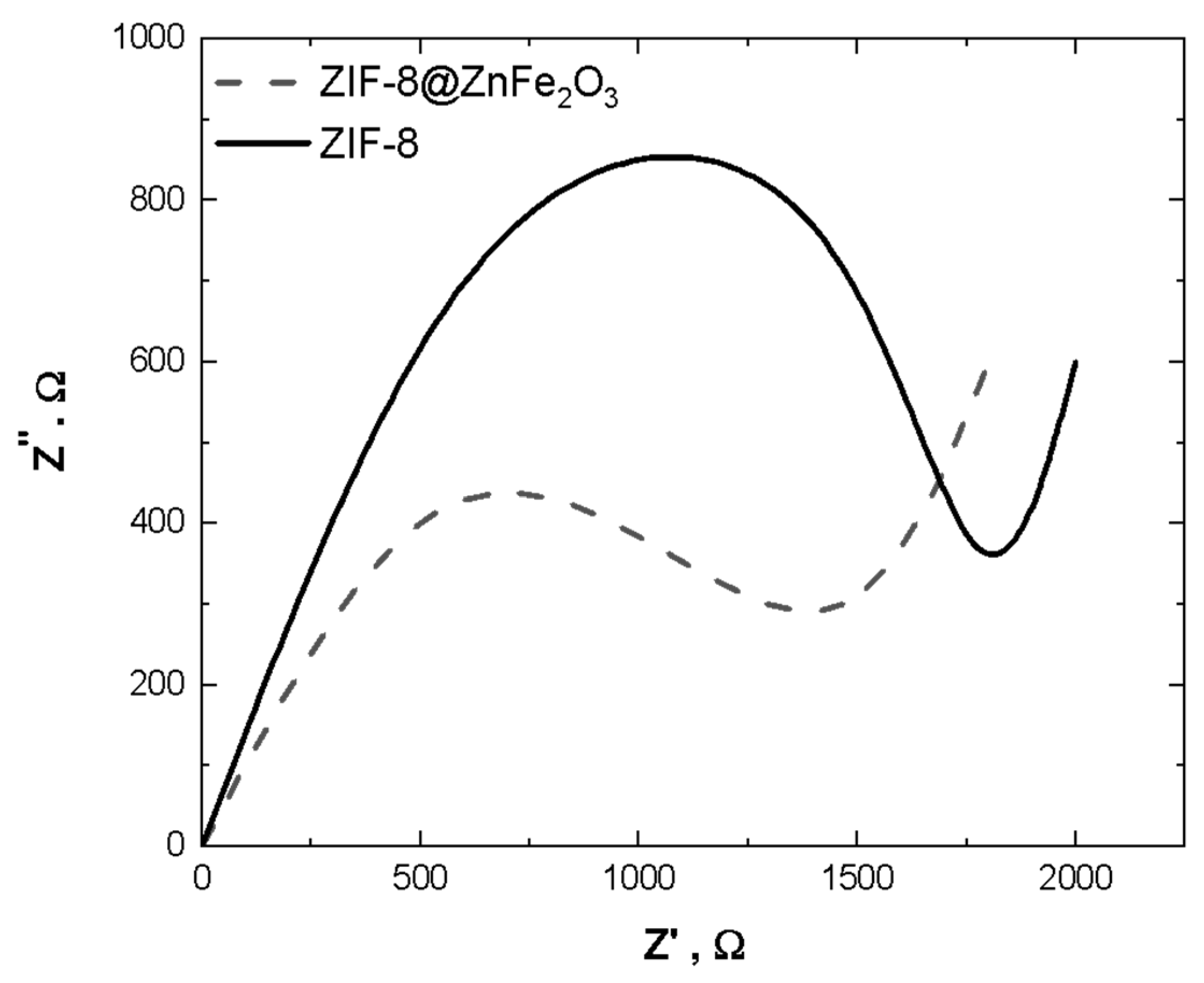
4.6. CV Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging multifunctional metal–organic framework materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Zhang, L.; Lei, J. Multifunctional metal–organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50, 1188–1218. [Google Scholar] [CrossRef]
- Alfadhli, S.; Darwish, A.A.A.; Al-Ghamdi, S.A.; Aljohani, M.M.; Seleim, S.M.; Mahmoud, M.E.; Khasim, S.; Hamdalla, T.A. Synthesis, characterization, optical, and sensing investigations for Fe-BDC doped with 10 wt.% of activated food waste biochar. J. Asian Ceram. Soc. 2024, 12, 23–33. [Google Scholar] [CrossRef]
- Alsharif, M.A.; Alqurashi, R.S.; Hamdalla, T.A. The role of annealing in optimizing the electrical, linear, and nonlinear optical properties ZIf-8@ TiO2 films for possible use in photonic and optoelectronic devices. Phys. Scr. 2024, 99, 065983. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Yang, W.; Shen, W.; Xue, H.; Pang, H. MOF-derived metal oxide composites for advanced electrochemical energy storage. Small 2018, 14, 1704435. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, W.B.; Zhao, L.; Xu, B.Q. MOF-derived binary mixed metal/metal oxide@ carbon nanoporous materials and their novel supercapacitive performances. Phys. Chem. Chem. Phys. 2016, 18, 17941–17948. [Google Scholar] [CrossRef]
- Chen, J.J.; Chen, Y.T.; Senthil Raja, D.; Kang, Y.H.; Tseng, P.C.; Lin, C.H. Metal-organic frameworks to metal/metal oxide embedded carbon matrix: Synthesis, characterization and gas sorption properties. Materials 2015, 8, 5336–5347. [Google Scholar] [CrossRef]
- Hamdalla, T.A.; Al-Ghamdi, S.A.; Alfadhli, S.; Alsharari, A.M.; Chiesa, M.; Khasim, S. PEDOT: PSS Doped Activated Biochar as a Novel Composite Material for Photocatalytic and Efficient Energy Storage Application. Catalysts 2024, 14, 630. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Montemor, M.d.F. Metal oxide and hydroxide–based aqueous supercapacitors: From charge storage mechanisms and functional electrode engineering to need-tailored devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef]
- Yuan, S.; Duan, X.; Liu, J.; Ye, Y.; Lv, F.; Liu, T.; Wang, Q.; Zhang, X. Recent progress on transition metal oxides as advanced materials for energy conversion and storage. Energy Storage Mater. 2021, 42, 317–369. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Ismail, A.F.; Ismail, N.; Jaafar, J.; Hashim, H. Structural transition from two-dimensional ZIF-L to three-dimensional ZIF-8 nanoparticles in aqueous room temperature synthesis with improved CO2 adsorption. Mater. Charact. 2018, 136, 407–416. [Google Scholar] [CrossRef]
- Yin, R.; Liu, M.; Tang, R.; Yin, L. CdS Nanoparticle-Modified α-Fe2O3/TiO2 Nanorod Array Photoanode for Efficient Photoelectrochemical Water Oxidation. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-atawi, S.; Aljohani, M.M.; Hamdalla, T.A.; Al-Ghamdi, S.A.; Alsharari, A.M.; Khasim, S. Novel Biochar-Modified ZIF-8 Metal–Organic Frameworks as a Potential Material for Optoelectronic and Electrochemical Energy Storage Applications. Catalysts 2024, 14, 705. [Google Scholar] [CrossRef]
- Zhang, Z.; Xian, S.; Xi, H.; Wang, H.; Li, Z. Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Chem. Eng. Sci. 2011, 66, 4878–4888. [Google Scholar] [CrossRef]
- Yu, K.; Lou, L.L.; Liu, S.; Zhou, W. Asymmetric oxygen vacancies: The intrinsic redox active sites in metal oxide catalysts. Adv. Sci. 2020, 7, 1901970. [Google Scholar] [CrossRef]
- Manikandan, V. Efficient Photocatalytic Degradation of Crystal Violet and Rhodamine B Dyes Using RGO@ ZnFe2O3 Nanocomposite. Strength Mater. 2023, 55, 167–178. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, X.Y.; Wu, H.B.; Lou, X.W. Complex nanostructures from materials based on metal–organic frameworks for electrochemical energy storage and conversion. Adv. Mater. 2017, 29, 1703614. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, Y.; Zhang, X.; Zhang, S.; Xue, H.; Pang, H. MOF derived metal oxide composites and their applications in energy storage. Coord. Chem. Rev. 2023, 477, 214949. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Stulp, S.; De Brito, J.F.; Flor, J.B.S.; Frem, R.C.G.; Zanoni, M.V.B. MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B Environ. 2018, 225, 563–573. [Google Scholar] [CrossRef]
- Wang, Z.; Pakoulev, A.; Pang, Y.; Dlott, D.D. Vibrational substructure in the OH stretching band of water. Chem. Phys. Lett. 2003, 378, 281–288. [Google Scholar] [CrossRef]
- Sadem, A.; Aljohani, M.M.; Al-Ghamdi, S.A.; Alsharari, A.M.; Sadque, M.; Hamdalla, T.A. The Optimal Doping Ratio of Fe2O3 for Enhancing the Electrochemical Stability of Zeolitic Imidazolate Framework-8 for Energy Storage Devices. Adv. Condens. Matter Phys. 2024, 2024, 5134666. [Google Scholar]
- Zhang, P.; Sun, F.; Shen, Z.; Cao, D. ZIF-derived porous carbon: A promising supercapacitor electrode material. J. Mater. Chem. A 2014, 2, 12873–12880. [Google Scholar] [CrossRef]
- Li, X.; Hao, C.; Tang, B.; Wang, Y.; Liu, M.; Wang, Y.; Zhu, Y.; Lu, C.; Tang, Z. Supercapacitor electrode materials with hierarchically structured pores from carbonization of MWCNTs and ZIF-8 composites. Nanoscale 2017, 9, 2178–2187. [Google Scholar] [CrossRef]
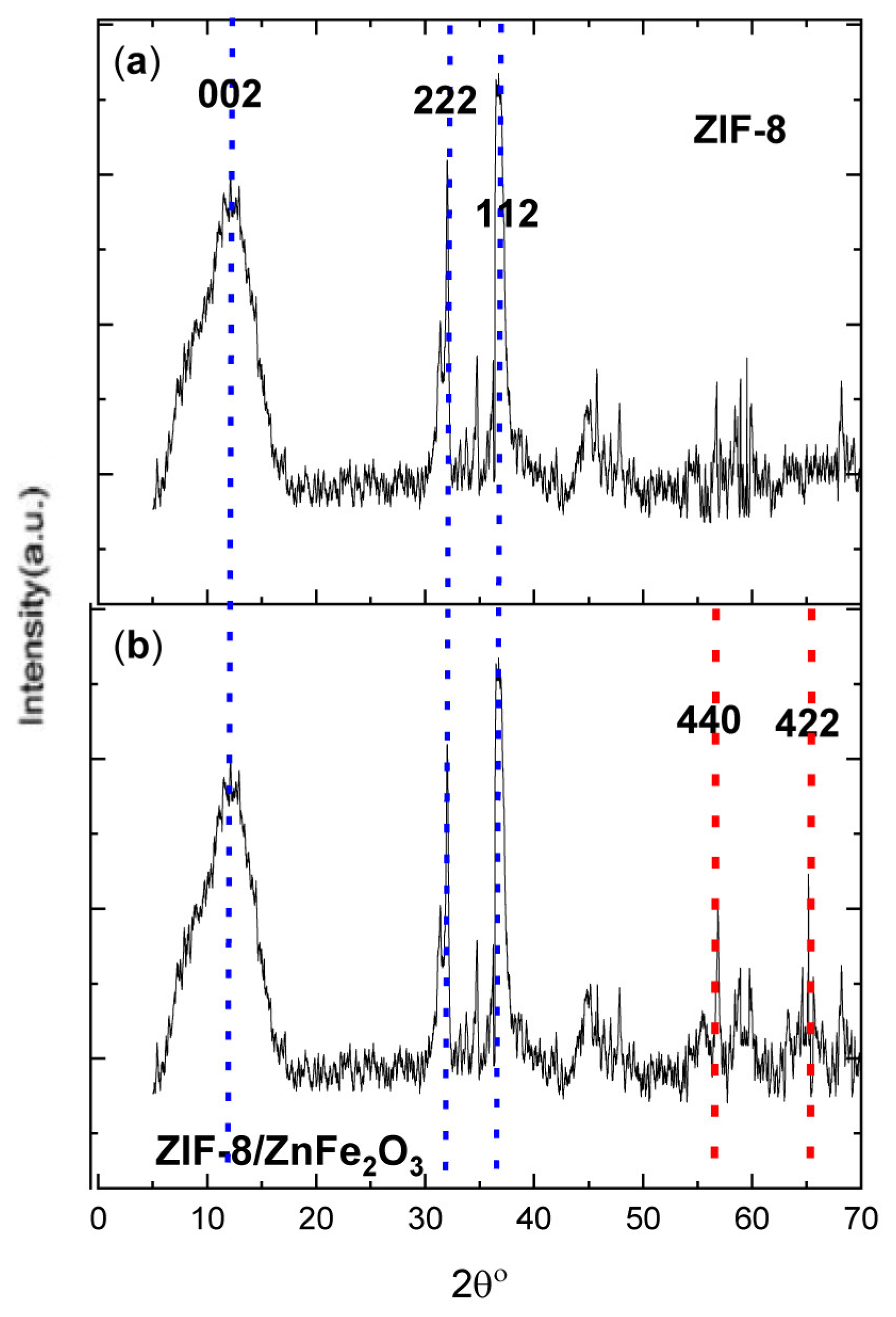


| No. | Compound | 2θ° | D-Spacing [Å] | a, b, c | h, k, l | (Crystallite Size) D, (nm) | Lattice Strain (nm) |
|---|---|---|---|---|---|---|---|
| 1 | ZIF-8/ZnFe2O3 | 12.7° | 3.70 | a = 8.33 b = 7.86 c = 6.91 | (002) | 17.094 | 0.00142 |
| 2 | 32.5° | 3.66 | (222) | 32.065 | 0.00374 | ||
| 3 | 36.8° | 3.95 | (112) | 29.411 | 0.00264 | ||
| 4 | 57.5° | 2.83 | (440) | 21.521 | 0.00612 | ||
| 5 | 64.3° | 3.91 | (442) | 41.341 | 0.00180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saba, S.; Alsharari, A.M.; Aldaleeli, N.Y.; Aljohani, M.M.; Hamdalla, T.A. Structural, Electrochemical, and Supercapacitor Characterization of Double Metal Oxides Doped Within ZIF-8 Composites. Processes 2025, 13, 859. https://doi.org/10.3390/pr13030859
Saba S, Alsharari AM, Aldaleeli NY, Aljohani MM, Hamdalla TA. Structural, Electrochemical, and Supercapacitor Characterization of Double Metal Oxides Doped Within ZIF-8 Composites. Processes. 2025; 13(3):859. https://doi.org/10.3390/pr13030859
Chicago/Turabian StyleSaba, Sadeem, Abdulrhman M. Alsharari, Nadiah Y. Aldaleeli, Meshari M. Aljohani, and Taymour A. Hamdalla. 2025. "Structural, Electrochemical, and Supercapacitor Characterization of Double Metal Oxides Doped Within ZIF-8 Composites" Processes 13, no. 3: 859. https://doi.org/10.3390/pr13030859
APA StyleSaba, S., Alsharari, A. M., Aldaleeli, N. Y., Aljohani, M. M., & Hamdalla, T. A. (2025). Structural, Electrochemical, and Supercapacitor Characterization of Double Metal Oxides Doped Within ZIF-8 Composites. Processes, 13(3), 859. https://doi.org/10.3390/pr13030859






