New Molecular Theory and Its Model Applications
Abstract
1. Introduction
2. Calculation Model of Basic Slag Activity
2.1. Theoretical Assumptions
- (1)
- The slag structure is composed of simple molecules of CaO and SiO2 and complex molecules of CaO·SiO2, 2CaO·SiO2, and 3CaO·SiO2, which are collectively referred to as different structural units, and these structural units form an ideal solution.Note: The simple molecules CaO and SiO2 and the complex compound molecules CaO·SiO2, 2CaO·SiO2, and 3CaO·SiO2 considered in the new molecular theory are called structural units rather than molecules per se. There is a debate that CaO may ionize to (Ca2+ + O2−). This complex compound is also called a structural unit here because its existence cannot currently be observed experimentally but is only a hypothesis.
- (2)
- At a given temperature, the formation and decomposition of various oxides and their structural units are balanced, resulting in the formation of complex compounds, and these chemical reactions follow the mass action law.
- (3)
- The various structural units in the slag change continuously within the selected concentration range, exhibiting continuity; that is, no phase change occurs.
- (4)
- Under the above three assumptions, each structural unit in the slag follows the ideal solution model, and the mole fraction of a structural unit representing a simple molecule is equal to the activity of the molecule.
2.2. The Calculation Model of the Mass Action Concentration of Structural Units in the Fundamental Slag System
- 1.
- CaO-SiO2 slag system
- 2.
- CaO-Al2O3 slag system
- 3.
- CaO-Al2O3-SiO2 slag system
2.3. Principle of Standard Free Energy Selection for Complex Molecular Reactions
- (1)
- The standard state of component i is a pure liquid. Since the melting point of CaO is 2845 K and the temperature of the general slag is approximately 1873 K, which is far lower than the melting point of CaO, CaO is dissolved in the slag in a liquid state. Either pure liquid CaO or pure solid CaO must be chosen as the standard state. If liquid CaO is selected as the standard state for subsequent conversion, CaO, SiO2, and CaO·SiO2 melt from the solid state to a virtual liquid state, and their free energy changes are shown in Equations (13), (15) and (17), respectively.
- (2)
- If the pure solid state is taken as the standard state, reaction (23) becomes Equation (21), where CaO, SiO2, and CaO·SiO2 change only in terms of expression, from a solid pure substance not in the slag to a solid pure substance in the slag, and there is no substantial change.
- (3)
- Zhang divided the CaO-SiO2 slag system into four intervals: below 1523 K, in the range of 1523‒1737 K, in the range of 1737‒2073 K, and above 2073 K. Different standard Gibbs free energies were used to calculate the equilibrium constant K values of the reactions, and the results were slightly different [21].
3. Results and Discussion
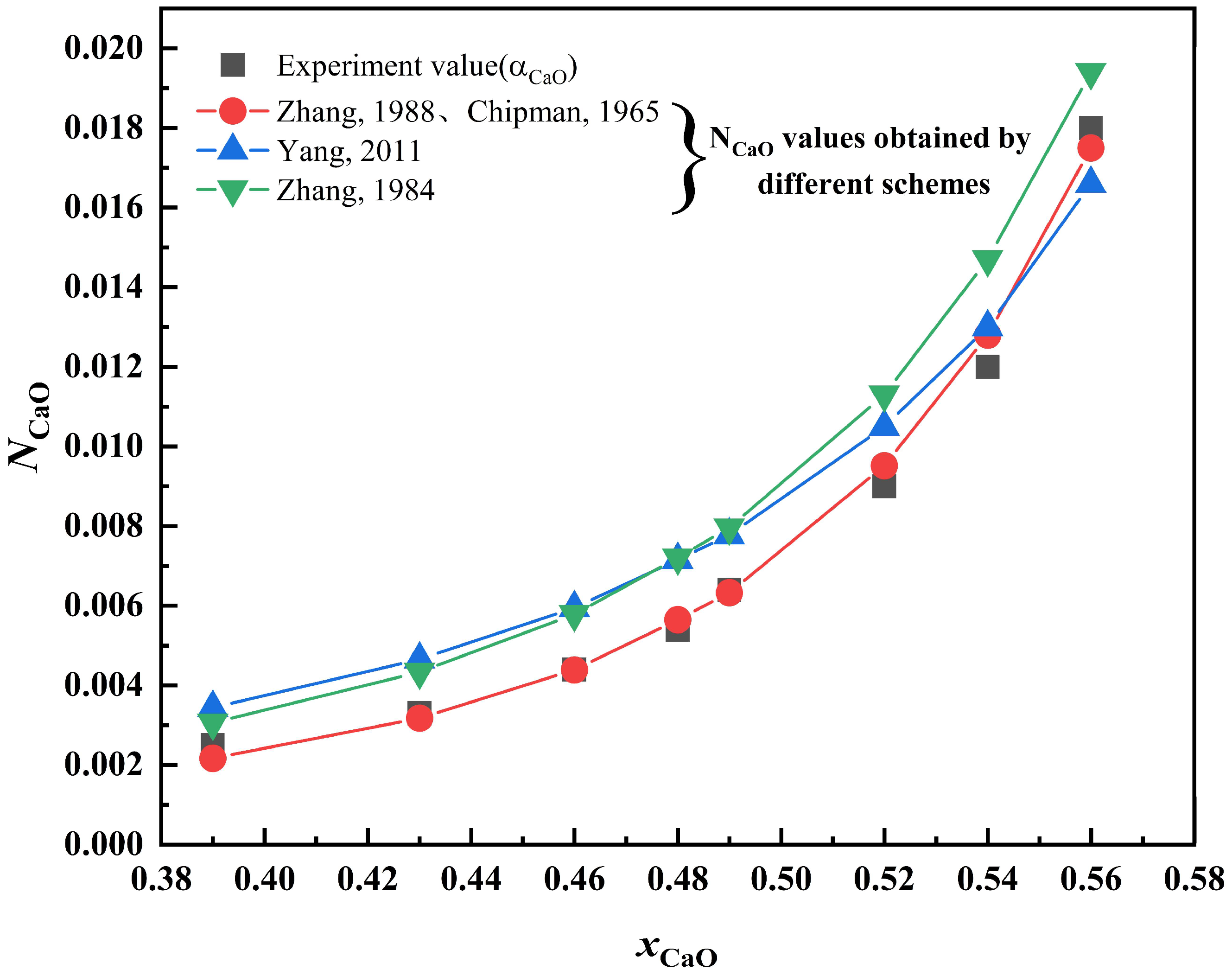
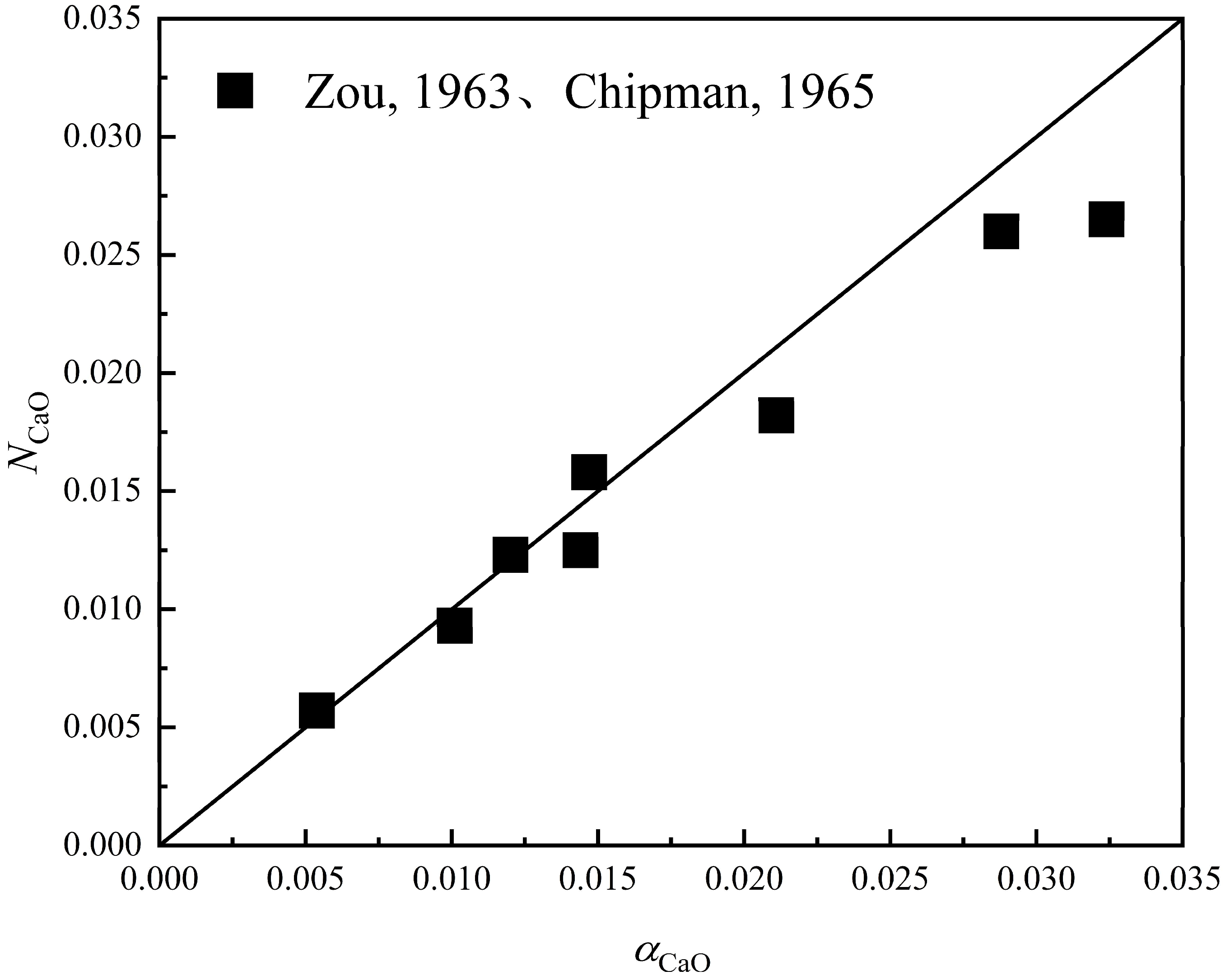
3.1. Comparison of Component Activity with Actual Activity Based on New Molecular Theory
3.2. Limitations of Molecular Theory
3.3. Verification of the New Molecular Theory by Comparison with Different Theories
3.4. Practical Application of New Molecular Theory
- (1)
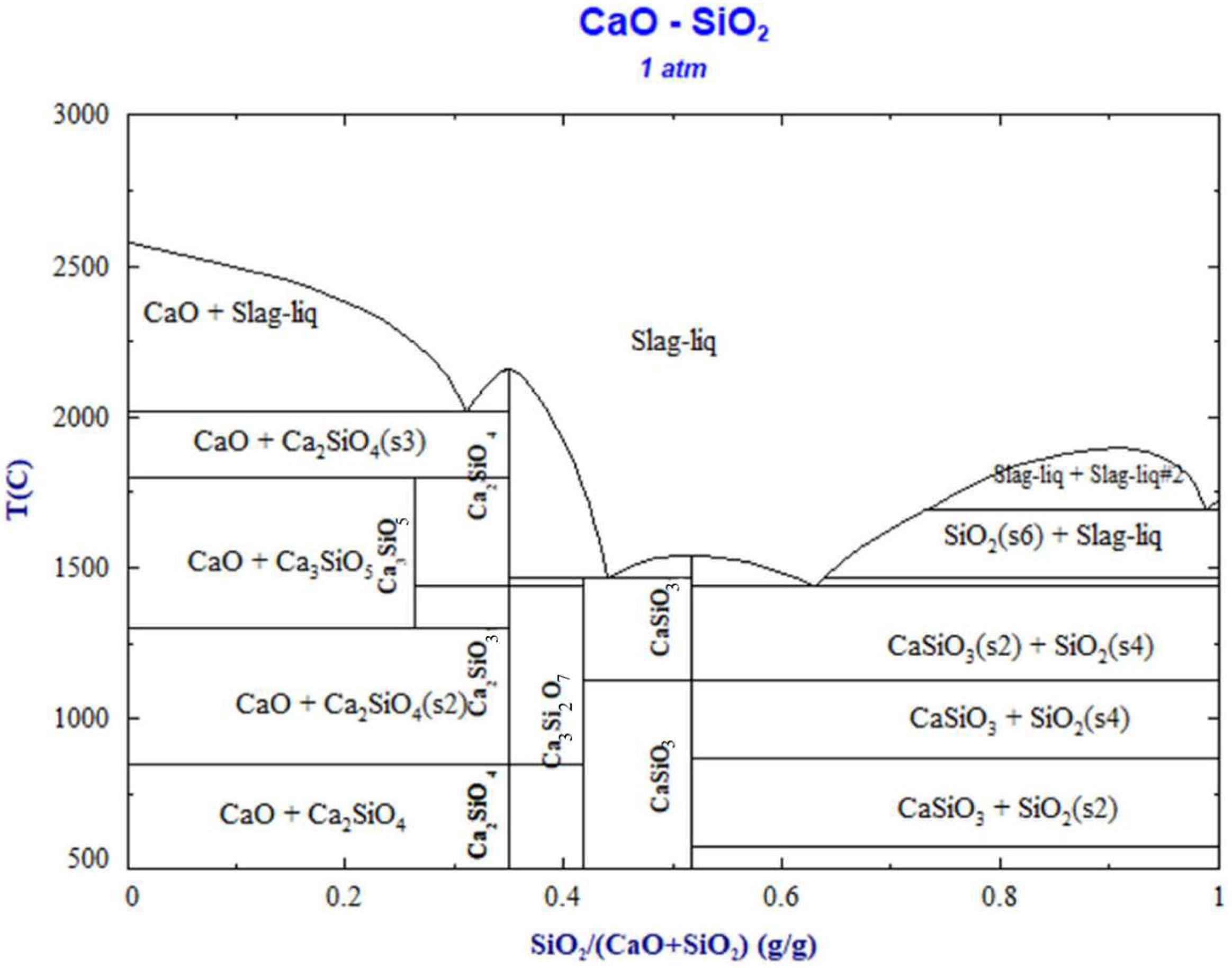
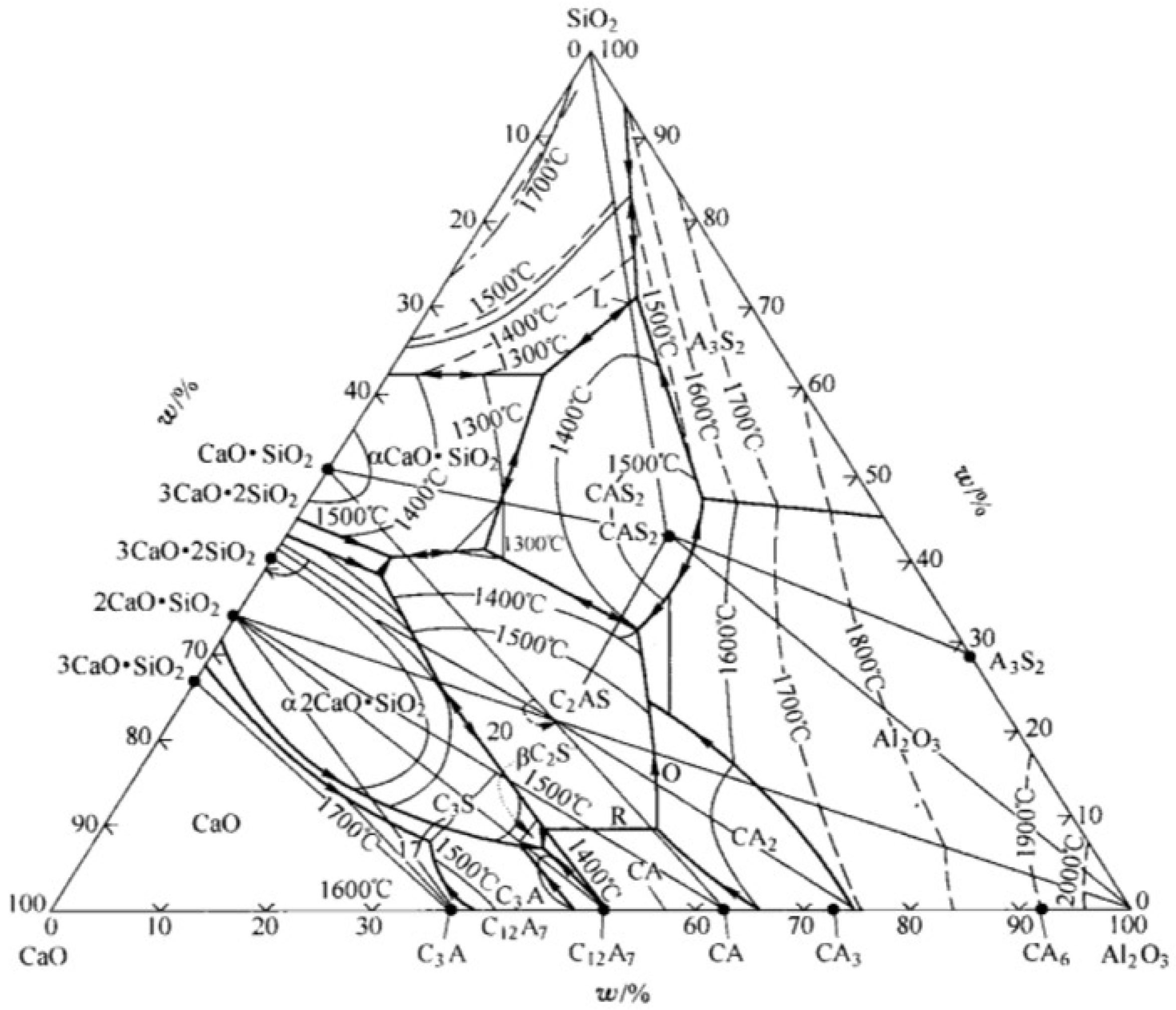
- The relationship between complex molecules and the melting point of slag
- (2)
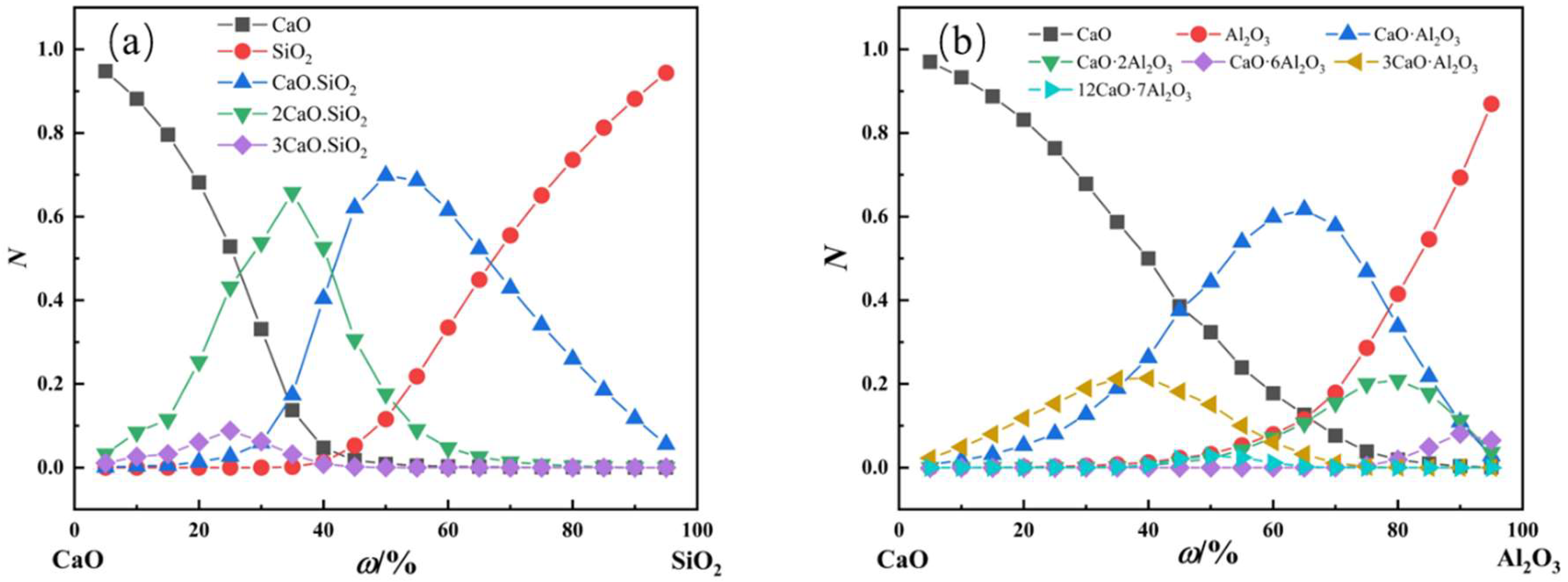
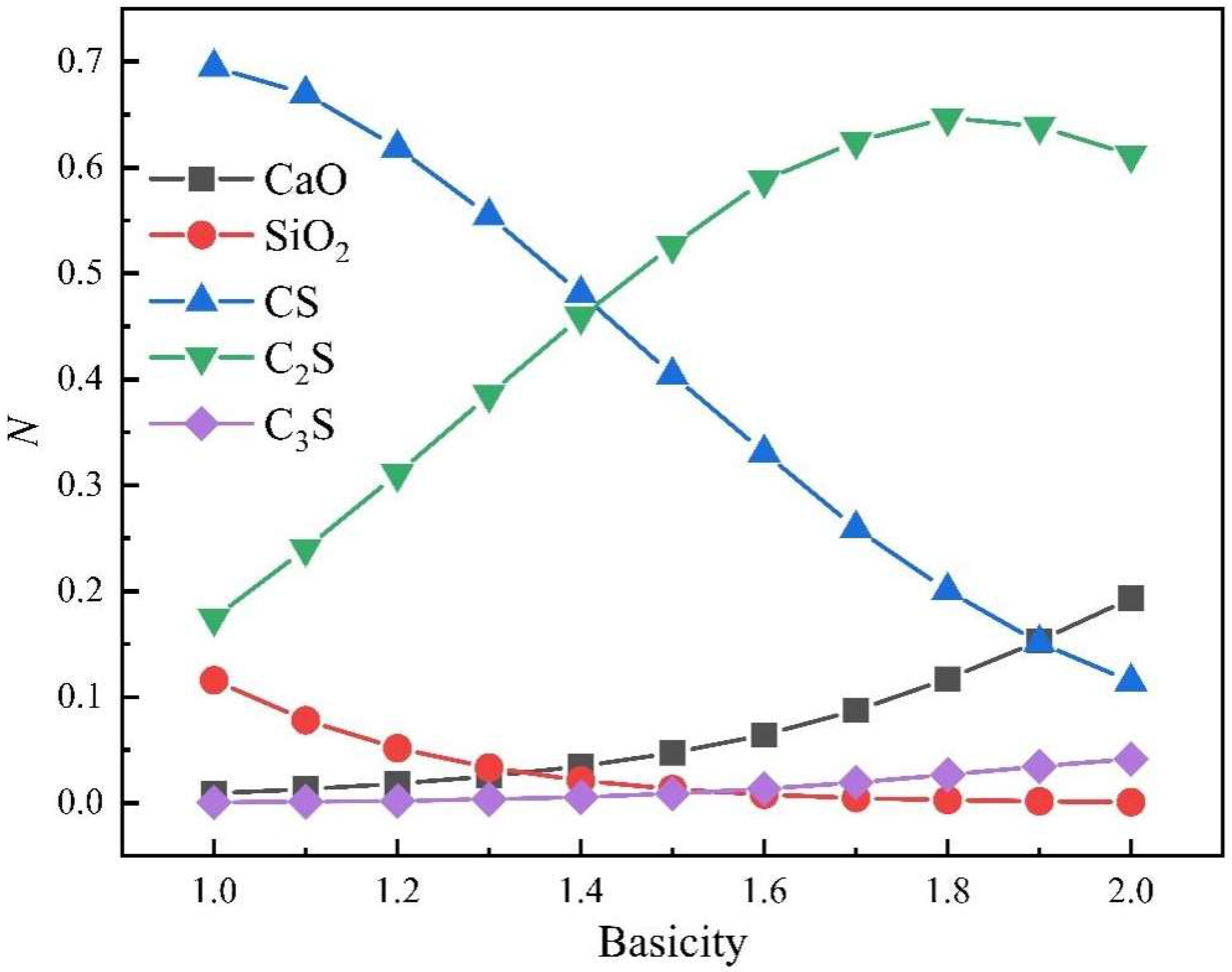
- Changes in the CaO-SiO2 slag system components with different alkalinity
- (3)
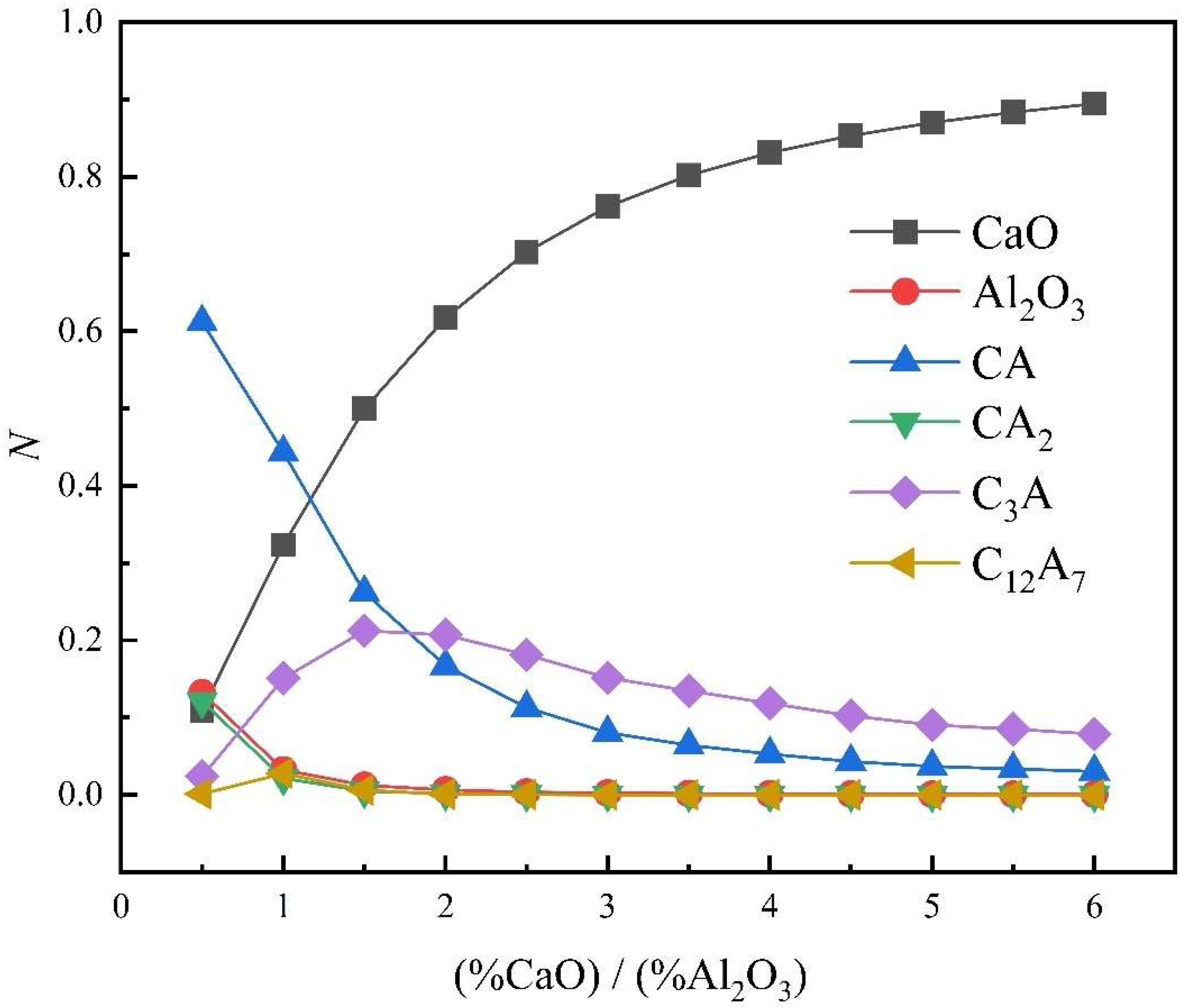
- Variations in CaO-Al2O3 slag system components with different calcium‒aluminum ratios
4. Conclusions
- (1)
- The calculated value based on the new molecular theory is very close to the measured value in different slag systems (CaO-SiO2 and CaO-Al2O3 slag systems). Through the application of the new molecular theory, the limitations of the earlier molecular theory are identified.
- (2)
- In the comparative validation of different theories, the calculated values for the new theory and the coexistence theory are close to the experimental values in the CaO-SiO2 and CaO-SiO2-Al2O3 slag systems, and the results obtained from the new molecular theory are closer to the experimental values in the CaO-Al2O3 slag system than those of other theories.
- (3)
- In the practical application of the new molecular theory, the maximum concentration of each complex molecule is consistent with the position of the melting point of the same solid‒liquid components in the phase diagram. The formation and decomposition of different complex molecules lead to changes in histone activity in the CaO-SiO2 and CaO-Al2O3 slag systems.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X. Chemical Metallurgical Behaviour of Quasi-Steady State Welded Steel-Slag Interface Based on Non-Equilibrium Thermodynamics. Ph.D. Thesis, Tianjin University, Tianjin, China, 2007. [Google Scholar]
- Zhou, X.; Ersson, M.; Zhong, L.; Jönsson, P. Optimization of Combined Blown Converter Process. ISIJ Int. 2014, 54, 2255. [Google Scholar] [CrossRef]
- Tsukasaki, A.; Suzumura, M.; Nishijima, W. Fractionation of Phosphorus in Steelmaking Slags and Aquatic Particulate Materials Using a Sequential Extraction Technique. ISIJ Int. 2015, 55, 183–189. [Google Scholar] [CrossRef][Green Version]
- Dippenaar, R. Industrial uses of slag (the use and re-use of iron and steelmaking slags). Ironmak. Steelmak. 2013, 32, 35–46. [Google Scholar] [CrossRef]
- Stovpchenko, G.; Togobitskaya, D.; Lisova, L.; Stepanenko, D.; Medovar, L. Predictive models for molten slags viscosity and electrical conductivity based on directed chemical bonds concept. Ironmak. Steelmak. 2022, 49, 572–580. [Google Scholar] [CrossRef]
- Min, D.J.; Tsukihashi, F. Recent advances in understanding physical properties of metallurgical slags. Met. Mater. Int. 2017, 23, 1–19. [Google Scholar] [CrossRef]
- Zhao, L. Study on Thermodynamic Properties of CaO-SiO2-Al2O3-MgO-FetO slag System. Ph.D. Thesis, Northeastern University, Shenyang, China, 2013. [Google Scholar]
- Rein, R.H.; Chipman, J. Activities in the liquid solution SiO2-CaO-MgO-Al2O3 at 1600 °C. Trans. Metall. Soc. AIME 1965, 233, 415–425. [Google Scholar]
- Zhang, Z.Q.; Zhou, J.-C.; Zou, Y.-X. Activity of CaO in SiO2-CaO-MgO-Al2O3 slag. J. Met. 1986, 22, 76–84. [Google Scholar]
- Mikimoto, G.; Omori, Y. Activity of CaO in CaO-SiO2-Al2O3 slag system. J. Jpn. Soc. Met. 1961, 25, 139–143. [Google Scholar]
- Uchida, S.; Tsukihashi, F.; Sano, N. The Phase Relations of the CaO-MnO-SiO2 System in Connection with the Smelting Reduction of Manganese Ore. Tetsu-to-Hagane 2009, 77, 490–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, W.; Olsen, S.E. Reaction equilibria in the production of manganese ferroalloys. Metall. Mater. Trans. B 1996, 27, 5–17. [Google Scholar] [CrossRef]
- Deo, J.B.; Gaye, H. Investigation on the properties of components in liquid slags by EMF method. Ber. Bunsen-Ges. Phys. Chem. 1952, 32, 54–60. [Google Scholar]
- Kay, D.A.R.; Taylor, J. Activities of silica in the lime-alumina-silica system. Trans. Faraday Soc. 1960, 56, 1372–1386. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, P.; Cao, Z. Activity of CaO in liquid CaO-SiO2 and CaO-SiO2-Al2O3 slags. J. Met. 1963, 6, 121–130. [Google Scholar]
- Huang, X. Principles of Iron and Steel Metallurgy; Metallurgical Industry Press: Beijing, China, 1990. [Google Scholar]
- Guo, Y. Influence of Desulfurization Capacity and a(Al2O3) of CaO-SiO2-MgO-Al2O3 Quaternary Slag System. Master’s Thesis, Northeastern University, Shenyang, China, 2019. [Google Scholar]
- Wei, Q.C. Metallurgical Thermodynamics; Chongqing University Press: Chongqing, China, 1996. [Google Scholar]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts; Metallurgical Industry Press: Beijing, China, 1998. [Google Scholar]
- Tan, J. Theoretical Activity Calculation and Viscosity Determination of Ionic Molecular Coexistence in Converter Slag. Ph.D. Thesis, Liaoning University of Science and Technology, Anshan, China, 2019. [Google Scholar]
- Zhang, J. Calculation model for the concentration of CaO-SiO2 slag system. J. Beijing Iron Steel Inst. 1988, 10, 412–421. [Google Scholar]
- Chen, J. Handbook of Commonly Used Steelmaking Charts and Data; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Turkdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: New York, NY, USA, 1980; pp. 5–24. [Google Scholar]
- Liang, Y.; Che, Y. Inorganic Thermodynamics Data Book; Northeastern University Press: Shenyang, China, 1993; p. 86. [Google Scholar]
- Zhang, J. Thermodynamic Calculations of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Zhang, J.; Yuan, W.X. Calculation model for the action concentration of CaO-Al2O3-SiO2 slag. J. Beijing Iron Steel Inst. 1995, 17, 7. [Google Scholar] [CrossRef]
- Yu, J. Modeling and Application of CaO-SiO2-MgO-Al2O3 Activity in Quaternary Slag System. Ph.D. Thesis, Northeastern University, Shenyang, China, 2016. [Google Scholar]
- Li, Z.; Ma, G.; Liu, M.; Zou, J. Calculation Model for Activity of FeO in Quaternary Slag System SiO2-CaO-Al2O3-FeO. Metals 2018, 8, 714. [Google Scholar] [CrossRef]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A Thermodynamic Model of Phosphorus Distribution Ratio between CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 Slags and Molten Steel during a Top–Bottom Combined Blown Converter Steelmaking Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Duan, S.C.; Li, C.; Guo, X.L.; Guo, H.J.; Guo, J.; Yang, W.S. A thermodynamic model for calculating manganese distribution ratio between CaO-SiO2-MgO-FeO-MnO-Al2O3-TiO2-CaF2 ironmaking slags and carbon saturated hot metal based on the IMCT. Ironmak. Steelmak. 2018, 45, 655–664. [Google Scholar] [CrossRef]
- Li, P. Characterization of Slag Desulfurization and Dephosphorization Capacity Based on Slag Ion and Molecular Coexistence Theory. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2016. [Google Scholar]
- Zhang, J. On the coexistence theory of slag structure. J. Beijing Iron Steel Inst. 1984, 18, 21–29. [Google Scholar]
- Zou, Y.X.; Zhou, J.C.; Xu, Y.S. Some studies on the thermodynamics of metallurgical melts. J. Met. 1982, 18, 127–139. [Google Scholar]
- Meta Techx Engineers. Association of Iron and Steel Engineers of the Federal Republic of Germany. In Slag Atlas; Metallurgical Industry Press: Beijing, China, 1989; pp. 147–148. [Google Scholar]



| Slag Theory | Founder | Theoretical Assumption | Advantage | Limitation |
|---|---|---|---|---|
| Ion theory | Herasymenko |
| It provides a better description of interslag reaction. |
|
| Molecular theory | H. Schenck and J. Chipman |
|
|
|
| Coexistence theory | Chuyikau and Zhang et al. |
|
| Consideration of the ionization of basic oxides is needed when calculating activity. |
| Species | Structural Units of Simple and Complex Molecules | Structural Unit Number | Number of Moles of the Structure/mol | Structural Unit Mass Action Concentration |
|---|---|---|---|---|
| Simple molecules | CaO | 1 | ||
| SiO2 | 2 | |||
| Complex molecules | CaO·SiO2 | 3 | ||
| 2CaO·SiO2 | 4 | |||
| 3CaO·SiO2 | 5 |
| Reactions | Reference | ||
|---|---|---|---|
| [21] | |||
| [21] | |||
| [8] |
| Species | Structural Units of Simple and Complex Molecules | Structural Unit Number | Number of Moles of the Structure/mol | Structural Unit Mass Action Concentration |
|---|---|---|---|---|
| Simple molecules | CaO | 1 | ||
| Al2O3 | 2 | |||
| Complex molecules | CaO·Al2O3 | 3 | ||
| CaO·2Al2O3 | 4 | |||
| CaO·6Al2O3 | 5 | |||
| 3CaO·Al2O3 | 6 | |||
| 12CaO·7Al2O3 | 7 |
| Reactions | Reference | ||
|---|---|---|---|
| [23] | |||
| [24] | |||
| [24] | |||
| [8] | |||
| [8] |
| Species | Structural Units of Simple and Complex Molecules | Structural Unit Number | Number of Moles of the Structure/mol | Structural Unit Mass Action Concentration |
|---|---|---|---|---|
| Simple molecules | CaO | 1 | ||
| SiO2 | 2 | |||
| Al2O3 | 3 | |||
| Complex molecules | CaO·SiO2 | 4 | ||
| 2CaO·SiO2 | 5 | |||
| 3CaO·SiO2 | 6 | |||
| CaO·Al2O3 | 7 | |||
| 12CaO·7Al2O3 | 8 | |||
| 3CaO·Al2O3 | 9 | |||
| CaO·2Al2O3 | 10 | |||
| CaO·6Al2O3 | 11 | |||
| 2CaO·Al2O3·SiO2 | 12 | |||
| CaO·Al2O3·2SiO2 | 13 | |||
| 3Al2O3·2SiO2 | 14 |
| Reactions | Reference | ||
|---|---|---|---|
| [21] | |||
| [21] | |||
| [8] | |||
| [23] | |||
| [8] | |||
| [8] | |||
| [24] | |||
| [24] | |||
| [8] | |||
| [8] | |||
| [24] |
| Slag Composition | [5,12] | for Different Complex Molecules | ||||||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | ||||||
| 0.481 | 0.519 | 0.00539 | 0.0155 | 0.0068 | 0.0403 | 0.698 | 0.109 | 0.000217 |
| 0.518 | 0.482 | 0.0101 | 0.0845 | 0.0079 | 0.0434 | 0.698 | 0.178 | 0.000581 |
| 0.537 | 0.463 | 0.012 | 0.145 | 0.0086 | 0.0451 | 0.675 | 0.227 | 0.000976 |
| 0.538 | 0.462 | 0.0144 | 0.149 | 0.0087 | 0.0452 | 0.6735 | 0.230 | 0.00101 |
| 0.553 | 0.447 | 0.0147 | 0.199 | 0.0094 | 0.0468 | 0.6415 | 0.276 | 0.00152 |
| 0.562 | 0.438 | 0.0211 | 0.225 | 0.0098 | 0.0478 | 0.6195 | 0.308 | 0.00195 |
| 0.583 | 0.417 | 0.0288 | 0.287 | 0.0112 | 0.0502 | 0.552 | 0.390 | 0.00352 |
| 0.584 | 0.416 | 0.0324 | 0.290 | 0.0112 | 0.0504 | 0.547 | 0.392 | 0.00359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Duan, S.; Huang, J.; Peng, X.; Yang, W.; Zheng, X.; Luo, Y.; Guo, H. New Molecular Theory and Its Model Applications. Processes 2025, 13, 828. https://doi.org/10.3390/pr13030828
Wang Q, Duan S, Huang J, Peng X, Yang W, Zheng X, Luo Y, Guo H. New Molecular Theory and Its Model Applications. Processes. 2025; 13(3):828. https://doi.org/10.3390/pr13030828
Chicago/Turabian StyleWang, Qixin, Shengchao Duan, Junhan Huang, Xuecheng Peng, Wensheng Yang, Xiaodan Zheng, Yiwa Luo, and Hanjie Guo. 2025. "New Molecular Theory and Its Model Applications" Processes 13, no. 3: 828. https://doi.org/10.3390/pr13030828
APA StyleWang, Q., Duan, S., Huang, J., Peng, X., Yang, W., Zheng, X., Luo, Y., & Guo, H. (2025). New Molecular Theory and Its Model Applications. Processes, 13(3), 828. https://doi.org/10.3390/pr13030828







