Mechanical Recycling of Crosslinked High-Density Polyethylene (xHDPE)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Recycling
2.3. Characterization
2.3.1. Crosslink Efficiency
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Tensile Properties
2.3.5. Flexural Properties
2.3.6. Impact Strength
2.3.7. Hardness (Shores A and D)
2.3.8. Density
3. Results
3.1. Crosslink Efficiency
3.2. Morphology
3.3. Differential Scanning Calorimetry
3.4. Thermogravimetric Analysis (TGA)
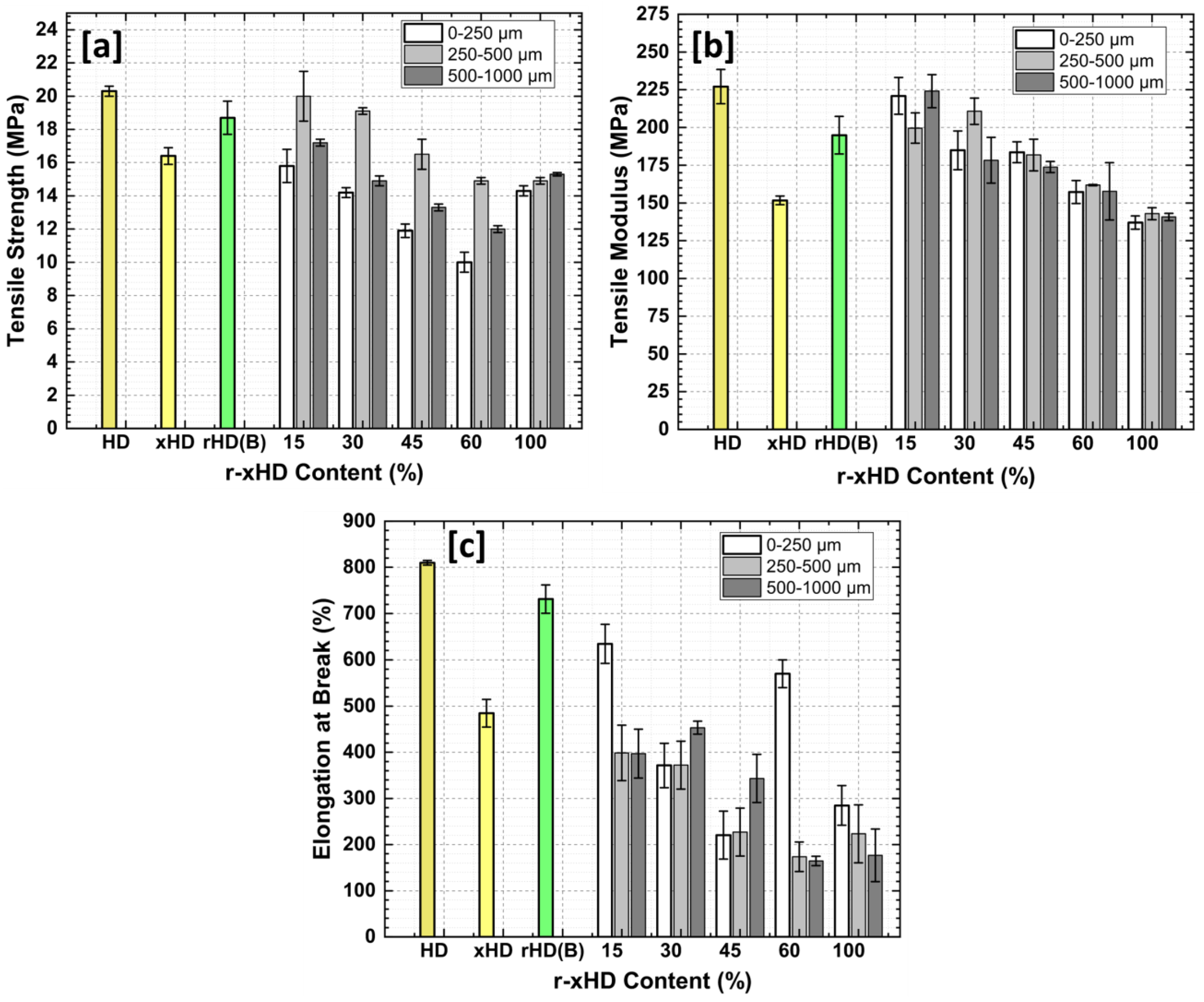
3.5. Tensile Properties
3.6. Flexural Properties
3.7. Impact Strength
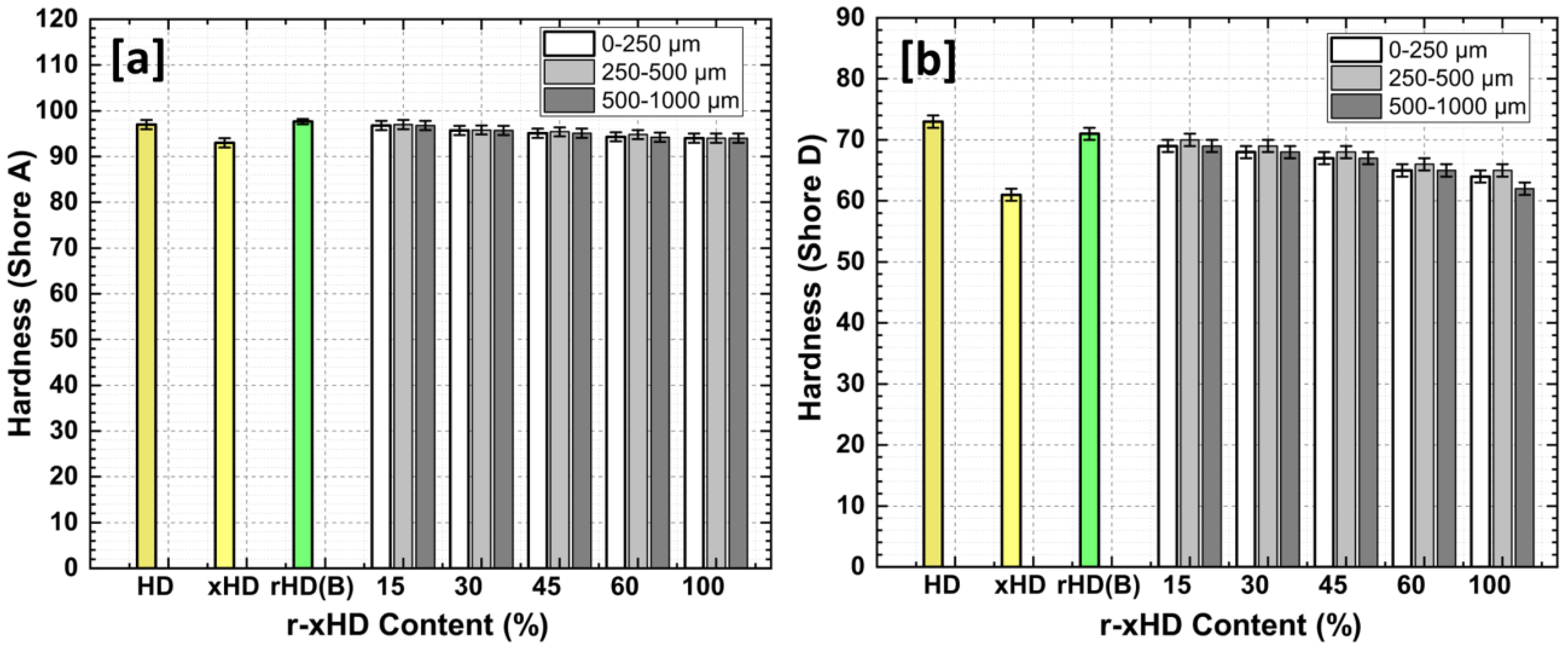
3.8. Hardness (Shore A and Shore D)
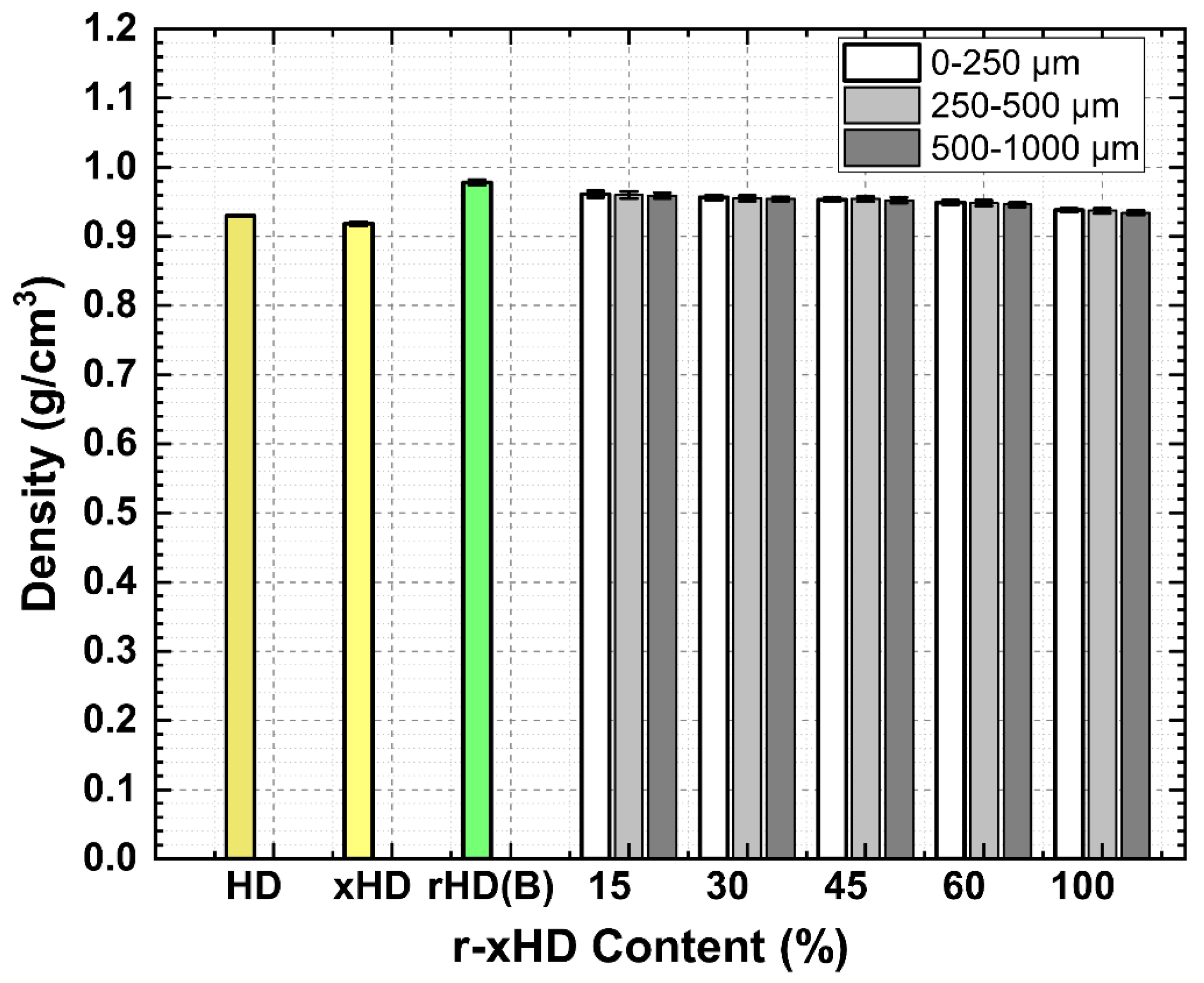
3.9. Density
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ASTM | American Standard for Testing of Materials |
| Cgel | Gel content |
| DCP | Dicumyl peroxide |
| DSC | Differential scanning calorimetry |
| HDPE | High-density polyethylene |
| MDPE | Medium-density polyethylene |
| PE | Polyethylene |
| PP | Polypropylene |
| rHDPE | Recycled high-density polyethylene |
| r-xHDPE | Recycled crosslinked high-density polyethylene |
| rHDPE(B) | Recycled high-density polyethylene from bottles |
| SEM | Scanning electron microscopy |
| Tc | Crystallization temperature |
| TGA | Thermogravimetric analysis |
| Tm | Melting temperature |
| Td10% | Degradation temperature at 10% weight loss |
| Tdmax | Maximum degradation temperature |
| Wf | Final weight of the sample |
| Wi | Initial weight of the sample |
| Xc | Degree of crystallinity |
| xHDPE | Crosslinked high-density polyethylene |
| ΔHm | Melting enthalpy |
References
- Emblem, A. 13-Plastics Properties for Packaging Materials. In Packaging Technology; Emblem, A., Emblem, H., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 287–309. [Google Scholar] [CrossRef]
- Redhwi, H.H.; Siddiqui, M.N.; Andrady, A.L.; Furquan, S.A.; Hussain, S. Durability of High-Density Polyethylene (HDPE)- and Polypropylene (PP)-Based Wood-Plastic Composites—Part 1: Mechanical Properties of the Composite Materials. J. Compos. Science. 2023, 7, 163. [Google Scholar] [CrossRef]
- Aocharoen, Y.; Chotickai, P. Compressive Mechanical and Durability Properties of Concrete with Polyethylene Terephthalate and High-Density Polyethylene Aggregates. Clean. Eng. Technol. 2023, 12, 100600. [Google Scholar] [CrossRef]
- Arrakhiz, F.Z.; El Achaby, M.; Kakou, A.C.; Vaudreuil, S.; Benmoussa, K.; Bouhfid, R.; Fassi-Fehri, O.; Qaiss, A. Mechanical Properties of High Density Polyethylene Reinforced with Chemically Modified Coir Fibers: Impact of Chemical Treatments. Mater. Des. 2012, 37, 379–383. [Google Scholar] [CrossRef]
- Seven, K.M.; Cogen, J.M.; Gilchrist, J.F. Nucleating Agents for High-Density Polyethylene—A Review. Polym. Eng. Sci. 2016, 56, 541–554. [Google Scholar] [CrossRef]
- Khalaf, M.N. Mechanical Properties of Filled High Density Polyethylene. J. Saudi Chem. Soc. 2015, 19, 88–91. [Google Scholar] [CrossRef]
- Boldt, R.; Gohs, U.; Wagenknecht, U.; Stamm, M. Process-Induced Morphology and Mechanical Properties of High-Density Polyethylene. Polymer 2018, 136, 179–186. [Google Scholar] [CrossRef]
- Ritums, J.E.; Mattozzi, A.; Gedde, U.W.; Hedenqvist, M.S.; Bergman, G.; Palmlöf, M. Mechanical Properties of High-Density Polyethylene and Crosslinked High-Density Polyethylene in Crude Oil and Its Components. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 641–648. [Google Scholar] [CrossRef]
- Han, S.O.; Lee, D.W.; Han, O.H. Thermal Degradation of Crosslinked High Density Polyethylene. Polym. Degrad. Stab. 1999, 63, 237–243. [Google Scholar] [CrossRef]
- Sirisinha, K.; Chuaythong, P. Reprocessable Silane-Crosslinked Polyethylene: Property and Utilization as Toughness Enhancer for High-Density Polyethylene. J. Mater. Sci. 2014, 49, 5182–5189. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Morshedian, J.; Wagenknecht, U.; Jafari, S.H. An Investigation of Chemical Crosslinking Effect on Properties of High-Density Polyethylene. Polymer 2003, 44, 4301–4309. [Google Scholar] [CrossRef]
- Ahmad, H.; Rodrigue, D. Crosslinked Polyethylene: A Review on the Crosslinking Techniques, Manufacturing Methods, Applications, and Recycling. Polym. Eng. Sci. 2022, 62, 2376–2401. [Google Scholar] [CrossRef]
- Ahmad, H.; Rostami-Tapeh-Esmaeil, E.; Rodrigue, D. The Effect of Chemical Crosslinking on the Properties of Rotomolded High Density Polyethylene. J. Appl. Polym. Sci. 2024, 141, e54744. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Chen, L.; Li, Y.; Sun, M.; Duan, X.; Liang, W. Structures and Impact Strength Variation of Chemically Crosslinked High-Density Polyethylene: Effect of Crosslinking Density. Rsc Adv. 2021, 11, 6791–6797. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Maniadi, A. Sustainable Additive Manufacturing: Mechanical Response of High-Density Polyethylene over Multiple Recycling Processes. Recycling 2021, 6, 4. [Google Scholar] [CrossRef]
- Abeysinghe, S.; Gunasekara, C.; Bandara, C.; Nguyen, K.; Dissanayake, R.; Mendis, P. Engineering Performance of Concrete Incorporated with Recycled High-Density Polyethylene (HDPE)—A Systematic Review. Polymers 2021, 13, 1885. [Google Scholar] [CrossRef]
- Huang, K.; Isayev, A.I.; Zhong, J. Ultrasonic Decrosslinking of Crosslinked High-Density Polyethylene: Effect of Screw Design. J. Appl. Polym. Sci. 2014, 131, 40680. [Google Scholar] [CrossRef]
- Huang, K.; Isayev, A.I. Ultrasonic Decrosslinking of Crosslinked High-Density Polyethylene: Effect of Degree of Crosslinking. RSC Adv. 2014, 4, 38877–38892. [Google Scholar] [CrossRef]
- Huang, K.; Isayev, A. Comparison between Decrosslinking of Crosslinked High and Low Density Polyethylenes via Ultrasonically Aided Extrusion. Polymer 2015, 70, 290–306. [Google Scholar] [CrossRef]
- Zack, S.J.; Herrold, N.T.; Wakabayashi, K. Mechanochemical Modification of Crosslinked Low-Density Polyethylene: Effect of Solid-State Shear Pulverization on Crosslinks, Branches, and Chain Lengths. SPE Polym. 2022, 3, 152–162. [Google Scholar] [CrossRef]
- Wu, H.; Liang, M.; Lu, C. Morphological and Structural Development of Recycled Crosslinked Polyethylene During Solid-State Mechanochemical Milling. J. Appl. Polym. Sci. 2011, 122, 257–264. [Google Scholar] [CrossRef]
- Wu, H.; Liang, M.; Lu, C. Non-Isothermal Crystallization Kinetics of Peroxide-Crosslinked Polyethylene: Effect of Solid State Mechanochemical Milling. Thermochim. Acta 2012, 545, 148–156. [Google Scholar] [CrossRef]
- Roumeli, E.; Paraskevopoulos, K.M.; Bikiaris, D.; Chrissafis, K. Effect of High Energy Ball Milling on the Structure and Mechanical Properties of Cross-Linked High Density Polyethylene. J. Mater. Sci. 2013, 48, 6753–6761. [Google Scholar] [CrossRef]
- Sun, F.; Yang, S.; Wang, Q. Selective Decomposition Process and Mechanism of Si–O–Si Cross-Linking Bonds in Silane Cross-Linked Polyethylene by Solid-State Shear Milling. Ind. Eng. Chem. Res. 2020, 59, 12896–12905. [Google Scholar] [CrossRef]
- Sun, F.; Bai, S.; Wang, Q. Structures and Properties of Waste Silicone Cross-Linked Polyethylene de-Cross-Linked Selectively by Solid-State Shear Mechanochemical Technology. J. Vinyl Addit. Technol. 2018, 25, 149–158. [Google Scholar] [CrossRef]
- Gao, P.; Krantz, J.; Ferki, O.; Nieduzak, Z.; Perry, S.; Sobkowicz, M.J.; Masato, D. Thermo-Mechanical Recycling via Ultrahigh-Speed Extrusion of Film-Grade Recycled LDPE and Injection Molding. Sustain. Mater. Technol. 2023, 38, e00719. [Google Scholar] [CrossRef]
- Manas, D.; Manas, M.; Mizera, A.; Stoklasek, P.; Navratil, J.; Sehnalek, S.; Drabek, P. The High Density Polyethylene Composite with Recycled Radiation Cross-Linked Filler of RHDPEx. Polymers 2018, 10, 1361. [Google Scholar] [CrossRef]
- Freitas, R.S.; Bonse, B.C. Cross-Linked Polyethylene (XLPE) as Filler in High-Density Polyethylene: Effect of Content and Particle Size. AIP Conf. Proc. 2019, 2055, 20009. [Google Scholar] [CrossRef]
- Bawareth, M.; Xu, W.; Ravichandran, D.; Zhu, Y.; Jambhulkar, S.; Fonseca, N.; Miquelard-Garnier, G.; Camille, V.; Matthew, L.; Campbell, W.; et al. Crosslinked Polyethylene (XLPE) Recycling via Foams. Polymers 2022, 14, 2589. [Google Scholar] [CrossRef]
- Lindqvist, K.; Andersson, M.; Boss, A.; Oxfall, H. Thermal and Mechanical Properties of Blends Containing PP and Recycled XLPE Cable Waste. J. Polym. Environ. 2019, 27, 386–394. [Google Scholar] [CrossRef]
- Ahmad, H.; Rodrigue, D. High-Performance Wood-Reinforced Crosslinked High-Density Polyethylene Composites. Polym. Eng. Sci. 2024, 64, 2459–2475. [Google Scholar] [CrossRef]
- Díaz, S.; Ortega, Z.; McCourt, M.; Kearns, M.P.; Benítez, A.N. Recycling of Polymeric Fraction of Cable Waste by Rotational Moulding. Waste Manag. 2018, 76, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Selvin, M.; Shah, S.; Maria, H.J.; Thomas, S.; Tuladhar, R.; Jacob, M. Review on Recycling of Cross-Linked Polyethylene. Ind. Eng. Chem. Res. 2024, 63, 1200–1214. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Brigandi, P.J.; Moubarak, M.; Sengupta, S.S.; Epps, T.H. Cross-Linked Polyolefins: Opportunities for Fostering Circularity Throughout the Materials Lifecycle. ACS Appl. Polym. Mater. 2024, 6, 11859–11876. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Rodrigue, D. Mechanical Decrosslinking and Reprocessing of Crosslinked Rotomolded Polypropylene Using Cryogenic-Assisted Shear Pulverization and Compression Molding. Recycling 2024, 9, 129. [Google Scholar] [CrossRef]
- Onffroy, P.R.; Herrold, N.T.; Goehrig, H.G.; Yuen, K.; Wakabayashi, K. Polylactic Acid Chemical Foaming Assisted by Solid-State Processing: Solid-State Shear Pulverization and Cryogenic Milling. Polymers 2022, 14, 4480. [Google Scholar] [CrossRef]
- Lee, A.; Ko, Y.; Ju, S.; Shin, Y.; Jeon, J.; Kim, S.; Oh, Y.; Yi, J.-W.; Um, M.-K.; Park, T. Cryogenic Impact-Induced Pulverization Process for Fast, Highly Crystallized, and Nucleating Agent-Free PLA with High Heat Resistance. ACS Appl. Polym. Mater. 2024, 6, 1234. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Haponiuk, J.T.; Colom, X.; Saeb, M.R. Green Approaches in Rubber Recycling Technologies: Present Status and Future Perspective. ACS Sustain. Chem. Eng. 2023, 11, 8706–8726. [Google Scholar] [CrossRef]
- Ren, M.; Wu, W.; Shi, Q.; Wu, L.; Zhang, C. Study on the Surface Properties of the Regenerated Polyurethane Foam Micropowder via Cryogenic Pulverization and Its Application. J. Mater. Res. Technol. 2023, 23, 808–818. [Google Scholar] [CrossRef]
- Lee, K.; Jo, Y.; Seok Nam, J.; Yu, H.; Kim, Y.-J. Dry-Film Technology Employing Cryo-Pulverized Polytetrafluoroethylene Binder for All-Solid-State Batteries. Chem. Eng. J. 2024, 487, 150221. [Google Scholar] [CrossRef]
- Huang, K.; Isayev, A.I. Ultrasonic Decrosslinking of Peroxide Crosslinked HDPE in Twin Screw Extrusion. II. Simulation and Experiment. Polym. Eng. Sci. 2017, 57, 1047. [Google Scholar] [CrossRef]
- Baek, B.K.; La, Y.H.; Lee, A.S.; Han, H.; Kim, S.H.; Hong, S.M.; Koo, C.M. Decrosslinking Reaction Kinetics of Silane-Crosslinked Polyethylene in Sub- and Supercritical Fluids. Polym. Degrad. Stab. 2016, 130, 103–108. [Google Scholar] [CrossRef]
- Ahmad, H.; Rodrigue, D. Upcycling of Recycled Polyethylene for Rotomolding Applications via Dicumyl Peroxide Crosslinking. J. Appl. Polym. Sci. 2024, 141, e56236. [Google Scholar] [CrossRef]
- Krupa, I.; Luyt, A.S. Mechanical Properties of Uncrosslinked and Crosslinked Linear Low-density Polyethylene/Wax Blends. J. Appl. Polym. Sci. 2001, 81, 973–980. [Google Scholar] [CrossRef]
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. Nanomaterials. ASTM Standard: West Conshohocken, PA, USA, 2022; pp. 1–7.
- ASTM D790-17; Standard Test Method for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM Standard: West Conshohocken, PA, USA, 2017; pp. 1–12.
- ASTM D6110-18; Standard Test Method for Determining the Charpy Impact Resistance of Notched Specimens of Plastics. ASTM Standard: West Conshohocken, PA, USA, 2017; pp. 1–17.
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM Standard: West Conshohocken, PA, USA, 2021; pp. 1–13.
- Das, P.; Tiwari, P. Thermal Degradation Kinetics of Plastics and Model Selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
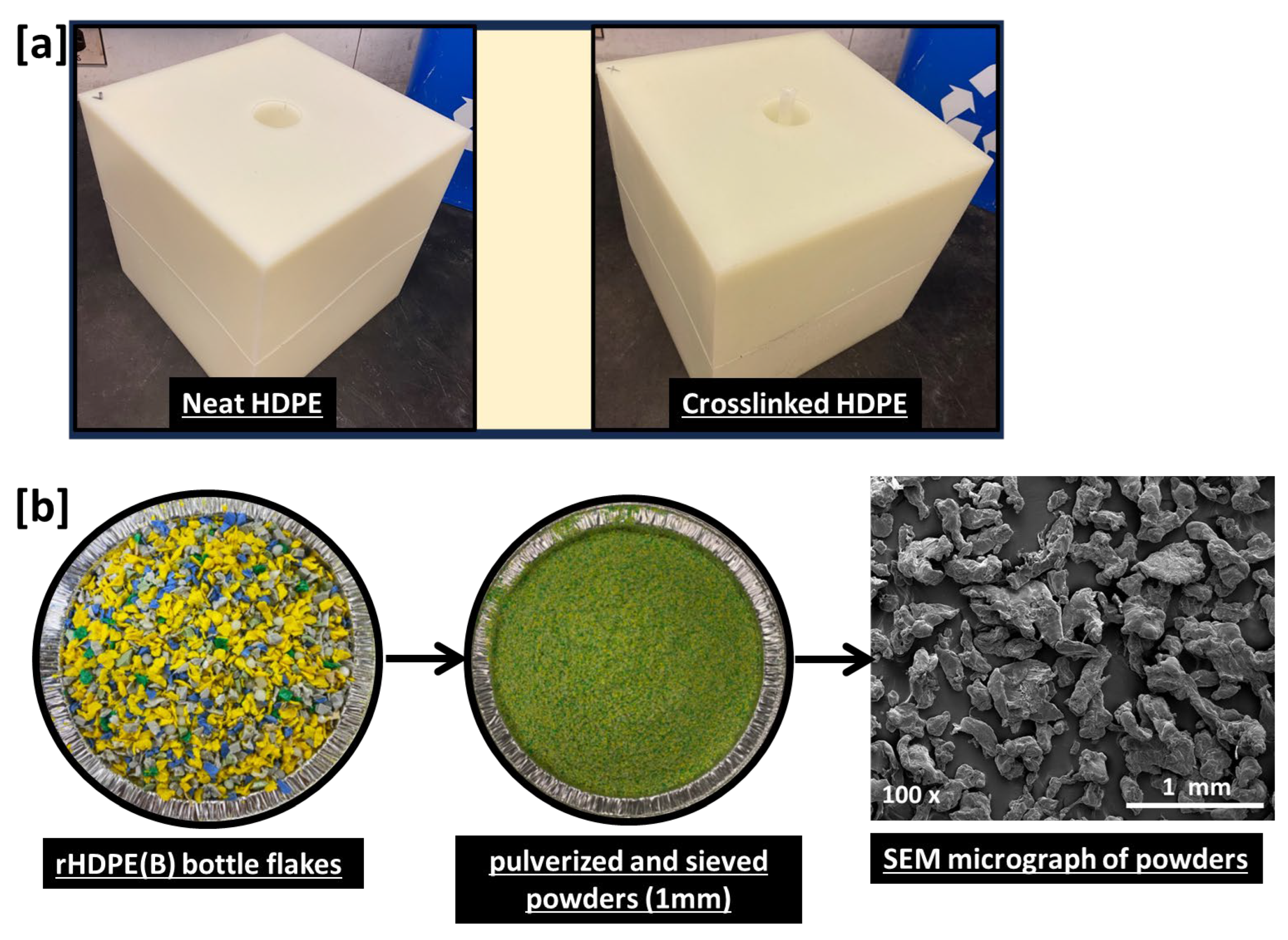

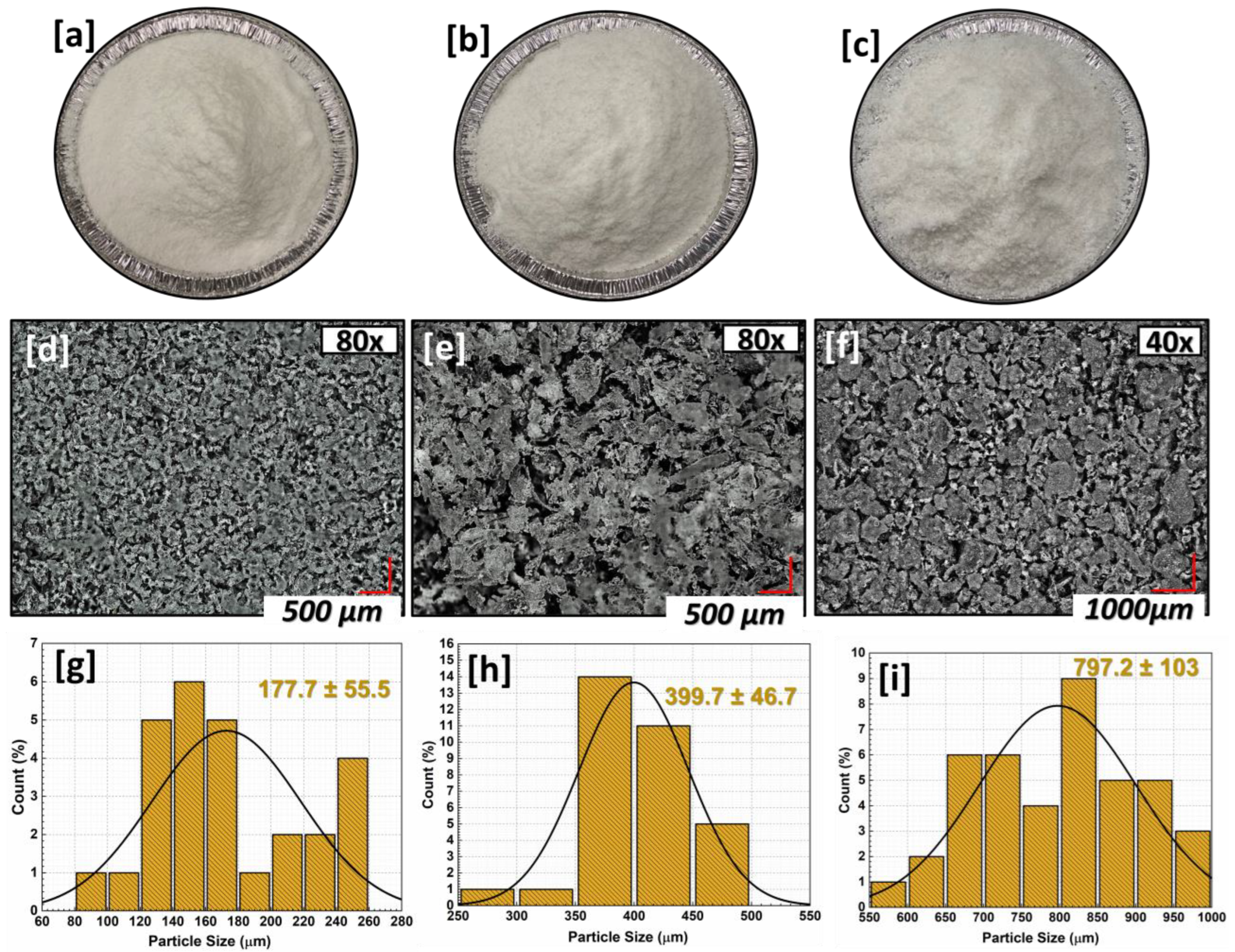


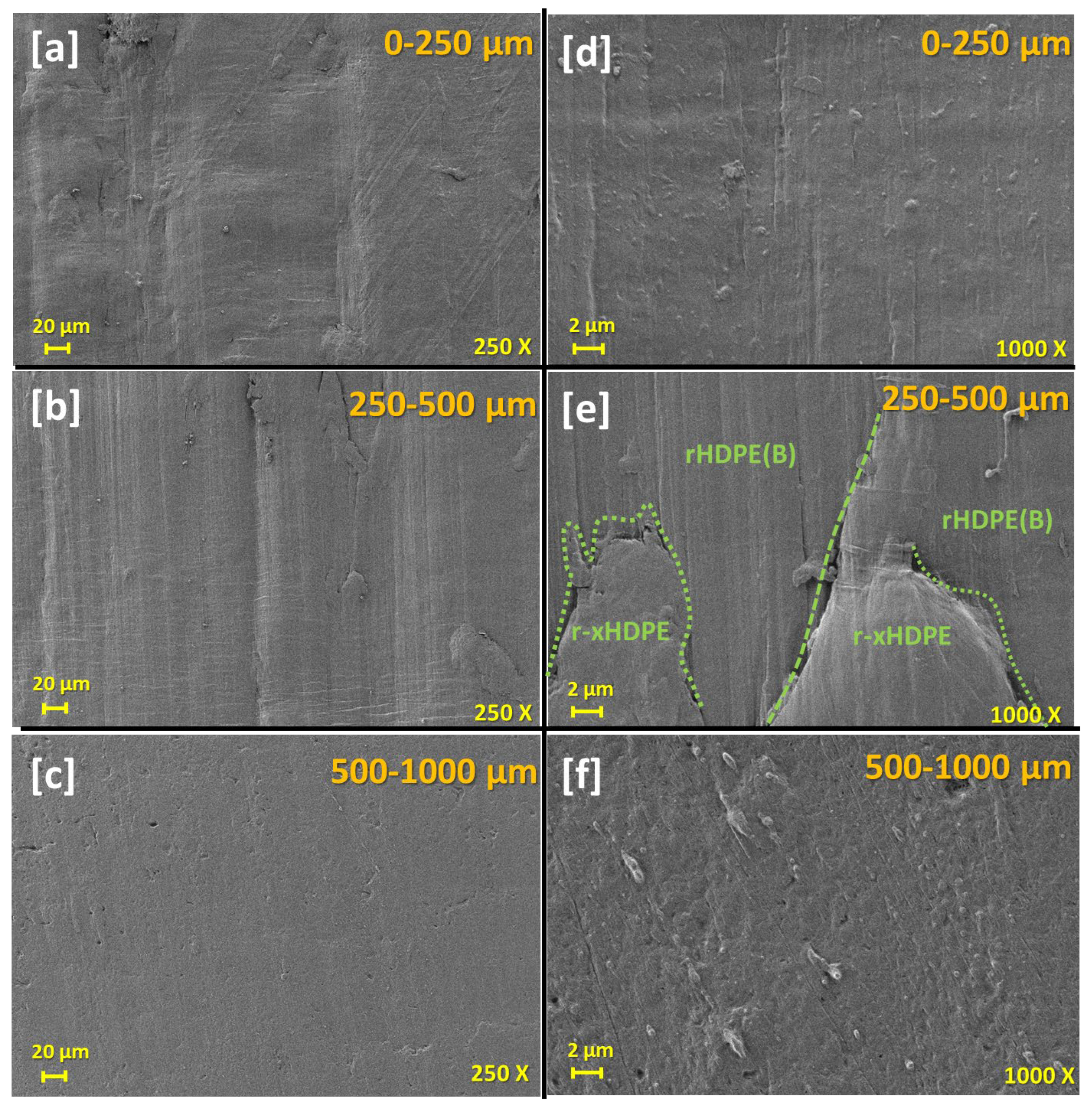
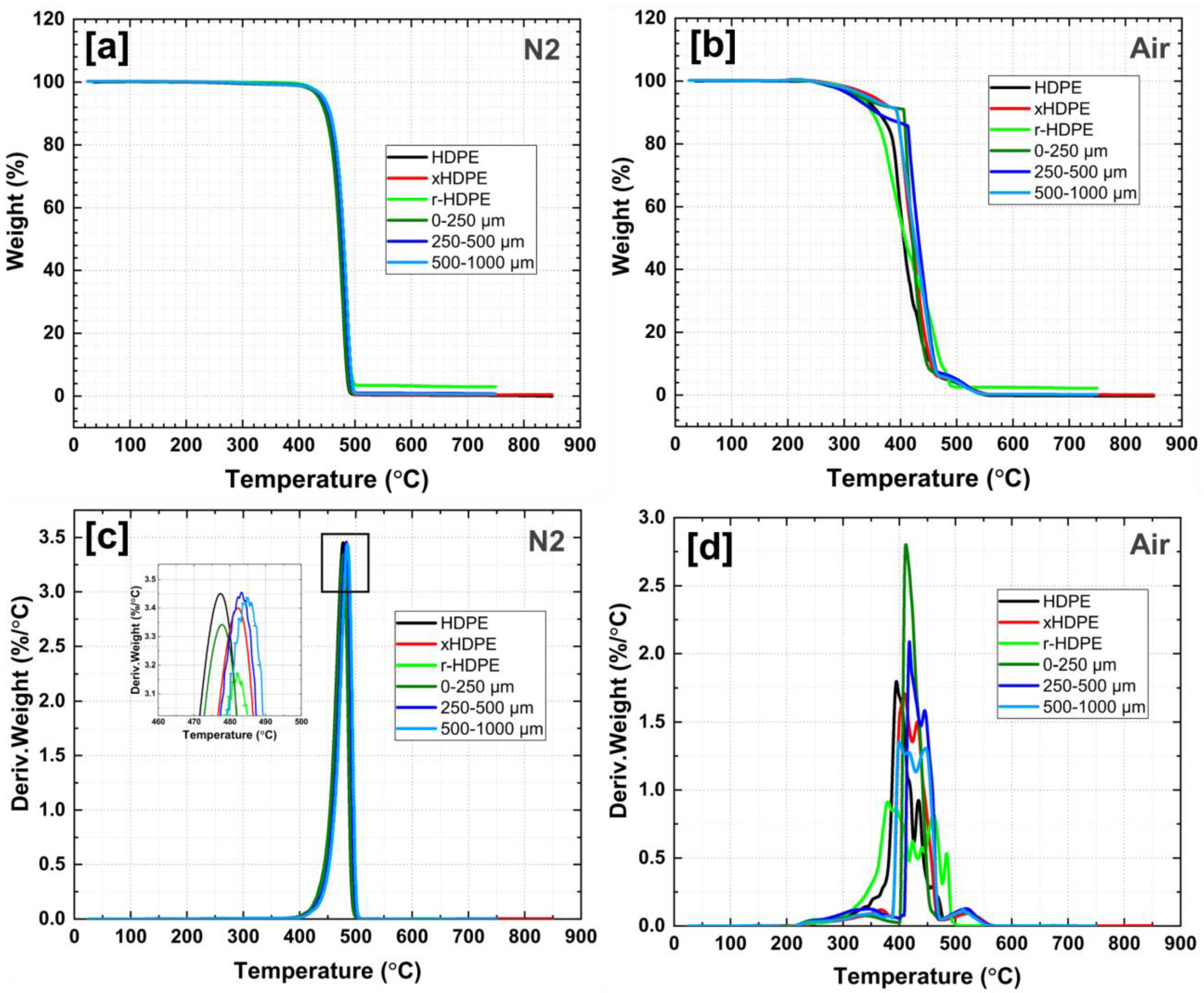


| Sample Code | HDPE (% wt.) | xHDPE (% wt.) | rHDPE (% wt.) | r-xHDPE (% wt.) |
|---|---|---|---|---|
| HD | 100 | 0 | 0 | 0 |
| xHD | 0 | 100 | 0 | 0 |
| rHD (B) | 0 | 0 | 100 | 0 |
| 15 | 0 | 0 | 85 | 15 |
| 30 | 0 | 0 | 70 | 30 |
| 45 | 0 | 0 | 55 | 45 |
| 60 | 0 | 0 | 40 | 60 |
| 100 | 0 | 0 | 0 | 100 |
| Sample | Tm (°C) | Tc (°C) | ∆Hm (J/g) | Xc (%) |
|---|---|---|---|---|
| HDPE | 131.9 | 114.5 | 181.1 | 62.7 |
| rHDPE(B) | 129.1 | 109.3 | 155.5 | 53.8 |
| xHDPE | 128.6 | 109.2 | 132.2 | 45.8 |
| r-xHDPE (0–250 µm) | 128.9 | 109.6 | 128.1 | 44.4 |
| r-xHDPE (250–500 µm) | 128.4 | 103.8 | 127.1 | 44.0 |
| r-xHDPE (500–1000 µm) | 128.3 | 103.1 | 123.7 | 42.8 |
| Sample | Td (N2) | Td (Air) | ||
|---|---|---|---|---|
| Td10% (°C) | Tdmax (°C) | Td10% (°C) | Tdmax (°C) | |
| HDPE | 447.9 | 477.2 | 360.6 | 396.2 |
| rHDPE(B) | 451.4 | 481.7 | 349.2 | 379.8 |
| xHDPE | 451.6 | 483.2 | 393.5 | 410.3 |
| r-xHDPE (0–250 µm) | 447.2 | 477.7 | 406.1 | 410.8 |
| r-xHDPE (250–500 µm) | 453.7 | 483.4 | 356.1 | 419.2 |
| r-xHDPE (500–1000 µm) | 455.8 | 485.3 | 393.8 | 399.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, H.; Rodrigue, D. Mechanical Recycling of Crosslinked High-Density Polyethylene (xHDPE). Processes 2025, 13, 809. https://doi.org/10.3390/pr13030809
Ahmad H, Rodrigue D. Mechanical Recycling of Crosslinked High-Density Polyethylene (xHDPE). Processes. 2025; 13(3):809. https://doi.org/10.3390/pr13030809
Chicago/Turabian StyleAhmad, Hibal, and Denis Rodrigue. 2025. "Mechanical Recycling of Crosslinked High-Density Polyethylene (xHDPE)" Processes 13, no. 3: 809. https://doi.org/10.3390/pr13030809
APA StyleAhmad, H., & Rodrigue, D. (2025). Mechanical Recycling of Crosslinked High-Density Polyethylene (xHDPE). Processes, 13(3), 809. https://doi.org/10.3390/pr13030809








