Abstract
The outstanding properties of nanocellulose have led to a wide range of applications in packaging, construction, medicine, electronics, cosmetics, environmental solutions, and the food industry. Specifically, cellulose nanocrystals (CNC) have demonstrated excellent biocompatibility, adaptable surface chemistry, low density, optical capabilities, biodegradability, renewability, and good mechanical properties. However, these unique characteristics depend on the raw material, processing, and post-treatment. New opportunities in CNC production are being explored based on unconventional resources and new, environmentally friendly production processes to replace highly polluting and inefficient conventional methods. This review evaluated the current methods for obtaining CNC from green processes, focusing on organic acids, enzymes, mechanical, oxidative, and radiation-based methods.
1. Introduction
The negative environmental impact of plastic materials from fossil sources has demonstrated the need for a paradigm shift and the search for more sustainable solutions. This scenario has led to numerous challenges, e.g., the replacement of these products on the market. A promising option, from renewable resources and biodegradable, is nanocellulose. This novel product presents high potential in packaging, construction, medicine, electronics, cosmetics, environmental solutions, and the food industry. Some key characteristics involve biocompatibility, biodegradability, renewability, optical behavior, high crystallinity, high surface area, and good mechanical properties [1].
The nanocellulose classification involves three main groups according to their morphology, particle size, and insolation method: cellulose nanocrystals (CNC) or nanocrystalline cellulose (NCC), cellulose nanofibers (CNF) or nanofibrillar cellulose (NFC), and bacterial nanocellulose (BNC) [2]. CNC, sometimes called cellulose nanowhiskers, are defined as nano-objects with short and rigid rod-like shapes (3–10 nm diameter), with a pure crystalline structure and aspect ratio between 5 and 50 [3]. Even if CNC characteristics are highly influenced by raw materials and production methods [4], most exhibit high crystallinity, unique optical properties, high mechanical strength (high impact and stiffness, good elongation), biocompatibility, biodegradability, elevated surface area, low density, simple bioconjugation, low thermal expansion, etc. [5].
CNC is obtained via top–down processes from lignocellulosic resources such as bleached cellulose pulps [6,7,8,9], dissolving pulp [10], microcrystalline cellulose [11,12], and agricultural or industrial wastes [13]. Acid hydrolysis is the prevalent and conventional method of producing CNC (Figure 1). It implies the utilization of hydrolysis with strong mineral acids (sulfuric, hydrochloric, phosphoric, nitric, and their combinations) to reach a high crystalline nanostructure and mechanical treatments for CNC separation and homogenization [14]. Nickerson and Habrle [15] pioneered this technique in 1947, successfully synthesizing CNC from cellulose materials by applying sulfuric acid. Then, subsequent research has expanded the range of inorganic acids employed for cellulose hydrolysis.
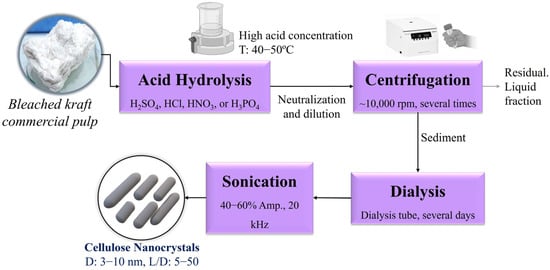
Figure 1.
Conventional methodology to produce cellulose nanocrystals.
Acid hydrolysis to produce CNC involves the cleavage of β-1,4 glycosidic bonds within cellulose chains. Hydronium ions infiltrate the cellulosic structure, preferentially targeting the amorphous regions surrounding and between microfibrils. These disordered amorphous areas are more readily hydrolyzed than the densely packed crystalline regions. The hydrolysis process in amorphous domains exhibits faster kinetics, rapidly reducing the number of glucopyranose units, and the depolymerization of the remaining crystalline regions proceeds at a slower rate due to the hindered accessibility of hydronium ions [16].
Sulfuric acid remains the preferred choice because of its superior hydrolysis efficiency and exceptional CNC dispersibility [17]. During sulfuric acid hydrolysis, negatively charged sulfate groups are introduced by esterification of surface hydroxyl groups [18].
The sulfuric acid hydrolysis conditions reported by different authors are dissimilar. Nevertheless, analogous to using any strong acid, high concentrations of sulfuric acid are always required, along with thorough purification stages [19,20,21]. Consequently, CNC production using this methodology implies material degradation, high operational costs, equipment corrosion, and environmental impacts from the generated effluents [22]. Current research focuses on identifying environmentally friendly CNC production processes to replace conventional methods that are highly polluting and inefficient, contributing to the circular economy.
This review analyzes alternative greener methodologies to produce CNC from lignocellulosic biomass. It presents a general description of the methods and evaluates their advantages and disadvantages, possible limitations, and future perspectives.
2. Materials and Methods
The study was based on the methodological framework of 5 steps suggested by Arkeys & O’Malley [23] and Levac et al. [24]. Figure 2 shows the sequence, which includes the initial question and the main objectives of the review article, as well as its population, concept, and context.
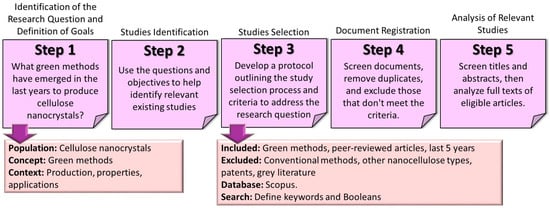
Figure 2.
Applied methodology for the elaboration of this review.
These initial parameters defined the keywords. “AND” was used as a boolean element (cellulose nanocrystals AND oxidative methods, organic solvents, enzymes, organic acids, deep eutectic solvents (DES), ionic liquids (ILs), mechanical treatments, radiation). Publications were restricted to the last 5 years (2019–2024), only in English, and only those included in scientific journals with a final or preprint version. As a result, 350 publications were found in Scopus, selecting 70 for this review. The 70 selected articles consist of original studies that mention nanocrystals, excluding duplicates and grey literature, and focusing exclusively on peer-reviewed scientific journals. Additional articles were incorporated based on suggestions from reviewers. In total, 78 articles were included in the final development.
3. Results
Due to the great interest in obtaining cellulose nano derivatives, researchers continuously seek the most efficient, sustainable, economically viable, and environmentally friendly production technologies to meet their growing demand [22]. Figure 3 summarizes the main green methods investigated in the last years.
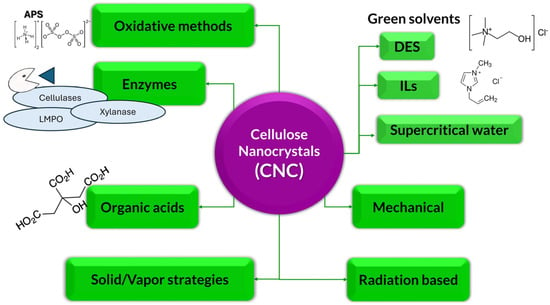
Figure 3.
A general classification of alternative green methods.
Green alternatives for obtaining CNC include organic acids, enzymes, green solvents such as DES or ILs, oxidative reagent-based and radiation-based alternatives, some strategies based on solid or vapor phases, and mechanical processes, as described in the following sections.
3.1. Organic Acids
The production of CNC using organic acids is a promising alternative due to their reduced corrosivity, the possibility of recovery and reuse, and the simultaneous ability to incorporate functional groups during the reaction. Figure 4 exposes a schematic route for fabricating CNC via chemical methods.
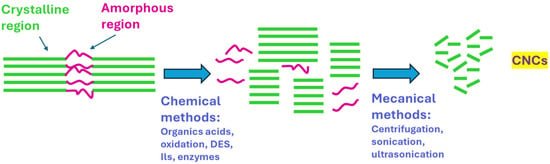
Figure 4.
A schematic route for fabricating CNC via chemical methods.
Mono- and poly-carboxylic organic acids such as citric (CA), acetic (AA), maleic (MA), formic (FA), and oxalic acids (AC) have shown potential for use in obtaining CNC with the mentioned advantages (Table 1). For instance, using FA generates CNC of high thermal stability and crystallinity [8], while OA and CA maximize carboxylation, an advantage for easy dispersion [6,25].

Table 1.
A summary of organic acid treatments for obtaining nanocrystals.
FA (HCOOH, methanoic acid) is the simplest carboxylic acid used to hydrolyze cellulosic materials to produce CNC. It has a relatively strong acidity (pKa: 3.74), low boiling point (100.8 °C), and easy recovery via distillation. Lv et al. [8] conducted an integrated and sustainable production of CNC and CNF via hydrolysis with FA. Characterization indicated that the prepared CNC exhibited high thermal stability (375 °C) and a high crystallinity index (79%). Another study optimized the non-catalyzed FA-based process for preparing thermally stable spherical CNC from mango seed husk. The yield was approximately 55% more than sulfuric acid-based CNC (Table 1) [26]. Wang et al. [9] developed a mixed system of sulfuric acid and FA, finding that low-concentration sulfuric acid (5–10% w/w) can significantly improve the hydrolysis efficiency of FA (65–80% w/w), reaching a maximum CNC yield of 70.6%. The obtained CNC exhibited a rod-like shape with high crystallinity and good dispersibility in water and some organic phases and high thermal stability, much higher than those traditionally hydrolyzed with sulfuric acid (Table 1) [9]. The post-treatment after obtaining CNC by FA includes different strategies, such as centrifugation (8000 rpm for 4 min), washing with deionized water until neutrality, dispersion in DMAc, and subsequent centrifugation (5000 rpm for 4 min) [8], or suspension centrifugation (8000 rpm for 5 min), sediment washing with deionized water five times by centrifugation, and dialysis with deionized water until the neutral pH. Then, continuous dialyzed suspension sonication using a probe sonicator at 300 W for 10 min and centrifugation at 3000 rpm for 3 min are used to separate the CNC [9].
AA (CH3COOH, ethanoic acid, pKa: 4.75) is another monocarboxylic acid used for CNC production. It is a weak and volatile organic acid. In addition, when AA reacts with cellulose, an acetoxy group can be introduced on the cellulose surface, reducing the cellulose hydrophilicity [20]. An acid hydrolysis system composed of small doses of H2SO4 (5–10%) and large amounts of easily recoverable AA (70–90%) was used to hydrolyze cellulose pulp at 80 °C for several hours with a yield of 81% (Table 1). The obtained suspension was dialyzed, centrifuged, and lyophilized. The resulting CNC showed high thermal and dispersion stability in both aqueous and organic phases [20]. Xu et al. prepared CNC with a one-step reaction via the traditional cellulose acetylation. The isolation and functionalization of CNC simultaneously occurred from acetic anhydride cellulose acetylation, sulfuric acid as the catalyst, and AA as the dispersal agent. Table 1 shows the characteristics of obtained CNC [28].
OA (HOOC-COOH, ethanodioic acid, pKa: 1.25) is a dicarboxylic acid that can be recovered by recrystallization, taking advantage of its low solubility in water at room temperature. Henschen et al. demonstrated that the reaction involves simultaneous esterification and acid hydrolysis, resulting in a highly charged (0.6–1.1 mmol/g) cellulose derivative with an acceptable crystallinity index (Table 1). The authors prepared nanocellulose by a bulk reaction between pulp and OA dihydrate to obtain cellulose oxalate followed by homogenization. Moreover, the H2SO4/OA mixture can effectively improve the shortcomings of single sulfuric acid hydrolysis or single OA hydrolysis [10].
MA (cis-butenedioic acid, HO2CCH = CHCO2H, pKa: 1.90) is another dicarboxylic acid successfully employed for the hydrolysis of cellulose. Optimized ultrasonic-assisted MA hydrolysis of purple sweet potato peels resulted in an 8.17% yield based on the original raw material. The obtained CNC exhibited desirable colloidal and thermal stability and a high degree of crystallinity (Table 1) [29]. Seta et al. [30] introduced a straightforward and environmentally friendly method for producing CNC with high yield and colloidal stability from bamboo fibers. Ball mill pretreatment was utilized to disintegrate and open the structure of bamboo fibers, thereby exposing a higher number of hydroxyl groups on the surface of pulp fibers and facilitating the penetration of acid molecules. This enhanced the accessibility of MA molecules to the cellulose structure, enabling more efficient hydrolysis and the release of crystalline regions. Besides, MA anhydride reacted with hydroxyl groups to introduce additional −COOH groups onto the CNC surface. After the reaction, the obtained suspension was washed, centrifuged at 10,000 rpm for 20 min, and dialyzed. The dialyzed sample was ultrasonicated at 60% power for 4 min and finally centrifuged at different rpm to separate the CNC from the not-completely-hydrolyzed fibers. The obtained CNC presented good characteristics (Table 1) [30].
CA (3-carboxy-3-hydroxypentanodioic acid), a tricarboxylic organic acid commonly found in citrus fruits, has emerged as a promising reagent to produce CNC. Its acidic properties (pKa: 3.13) and ability to form ester linkages with cellulose make it an effective agent for hydrolyzing cellulose fibers, leading to CNC and CNF formation. Previous studies have demonstrated that CA can produce CNC with comparable or superior yields to traditional sulfuric acid methods. The use of CA offers several advantages, including its potential for recovery through simple crystallization processes and its ability to overcome the limitations of weak acidity. With little ultra-sonication at the appropriate preparation stage, CA overcame the difficulties of hydrolyzing cellulose caused by its weak acidity.
The CNC and CNF showed highly stable dispersibility due to their carboxylic acid group content (up to 0.65 mmol/g for CNC and 0.30 mmol/g for CNF) [31]. This property is particularly valuable for various applications, such as composite materials, drug delivery, and filtration. Some studies utilize inorganic acids as catalysts to enhance the weak acidity of organic acids. Worku et al. [21] used CA catalyzed with sulfuric acid (0–15 wt%) to prepare carboxylate CNC, yielding 89.7%. The conductometric titration showed a great carboxylate concentration (Table 1), giving stable dispersibility to the CNC. Since the carboxylic groups are simultaneously introduced to the cellulose surface during hydrolysis, its advantage is the amount and costs of chemical reduction for series and long-step surface functionalizing reactions [21]. In other work, citric/hydrochloric acid mixtures produced carboxylate CNC from commercial microcrystalline cellulose, up to 87.8% yield. The mild acid mixtures demonstrated the potential for facile recovery and recycling for three cycles, exhibiting a negligible impact on the size of CNC, carboxyl content of the citrate CNC surface, zeta potential value, and thermal stability. Similarly, carboxylate CNC with a needle-like shape and good crystallinity was obtained by subjecting CMF to acid hydrolysis with a citric/hydrochloric mixture (Table 1) [12]. The thermal stability was higher than that reported for CNC from other sources extracted by the sulfuric acid hydrolysis process [19].
Technoeconomic analysis revealed that the environmental impact of CA hydrolysis and recovery was substantial, representing approximately 58% of the global warming potential (GWP) for both CA CNC and the combined CA/sulfuric acid CNC. Notably, this GWP was more than double that observed for sulfuric acid CNC. Nonetheless, minimum product selling prices for CA CNC and CA/sulfuric acid CNC were 34.0% and 37.2% higher, respectively, than those for sulfuric acid CNC, primarily due to the elevated cost of CA recovery [32].
3.2. Oxidative Methods
Oxidative methods are treatments based on oxidizing reagents that create carbonyl or carboxylic groups on the surface of hydroxyl groups. These methods introduce surface groups into the CNC that improve dispersion and generate a more chemically reactive surface for functionalization [33]. Green methods of obtaining CNC using oxidizing agents are mainly those based on hydrogen peroxide, ozone, potassium ferrate, and electrochemistry.
Hydrogen peroxide is an environmentally friendly oxidizing agent extensively used in the paper industry for pulp fiber bleaching [34] and dissolving pulps [35]. In addition, metal ions maximize the formation of hydroxyl ions and free radicals, leading to the partial hydrolysis of amorphous cellulose [36]. In general, H2O2 converts the hydroxyl groups on the polymer chains into carbonyl and carboxyl functional groups, often accompanied by macromolecular degradation. The decomposition of H2O2 in the presence of transition metal catalysts, such as copper, iron, or tungstate, can result in the generation of intermediate radical species such as HO• (hydroxyl) and HOO• (hydroperoxyl radical) [37]. These emergent free radicals can ultimately oxidize the alcohol groups and cause the cleavage of glycoside bonds within the polysaccharide chains.
A one-pot method utilizing copper (II) catalyzed oxidation of softwood pulp with H2O2 was employed as a green and efficient way to isolate carboxylate CNC from native cellulose materials, conducting the reaction in an acidic medium under mild conditions. The carboxylate CNC charge content was about 1.0 mmol g–1 with a rod-shaped morphology (diameter 23 nm) and an average length of 263 nm [36]. Later, Koshani, and Van de Ven [38] demonstrated that negatively charged carboxyl groups of carboxylate CNC played a key role in lysozyme immobilization via electrostatic interactions and covalent linkages [38].
In the same direction, the use of ozone to create surface groups to facilitate CNC production was evaluated, considering the impact of lignocellulosic components such as cellulose and lignin [39] and the conditions during ozonation [35].
As a green oxidation reagent, potassium ferrate has demonstrated a strong capacity to produce CNC in a one-pot method, granting the potential to produce CNC at an industrial scale [40].
3.3. Enzymes
Enzymatic treatment is a promising green method for isolating CNC from biomass due to its high efficiency, selectivity, and lower energy consumption than other techniques [41,42]. The enzyme processes are neutral and, consequently, have no emissions of harmful chemicals that affect ecology and equipment. Previous studies have demonstrated that various factors influence the effectiveness of cellulose modification, including reaction temperature, pH, substrate type and concentration, cellulose polymerization degree, crystallinity, porosity, and dispersion–morphological properties [41]. While this approach is still under development, further advancements are necessary to scale the process economically [43].
The efficiency of enzymatic processes on an industrial scale can be improved by immobilizing enzymes (deposited on a carefully selected solid support) [44]. The possibility of reusability, working in a continuous process, and the stability gained by the enzymes make them a potential strategy [45]. Cellulase immobilization involves several interactions between support and the enzyme: non-covalent or physical interactions, a more stable covalent system, and binding of the enzyme to affinity tags or ligands (affinity-based system) [46]. Two key factors during cellulase immobilization protocol are the substrate (surface area and chemistry, porosity, chemical composition, shape, and size) and the characteristics of the enzyme characteristics (isoelectric point, surface charge, and structure) [46,47].
Additionally, the lignin content in the lignocellulosic biomass influences the efficiency loss during enzymatic treatment. Lignin not only creates a physical barrier that blocks the access of enzymes to the carbohydrates but also can inhibit their action [48,49]. Lignin can be removed from the cellulose fraction through biomass pretreatments using acidic or alkaline treatments such as organosolv treatments, steam explosion, supercritical CO2, etc. [50]. The influence of pretreatment on the production of CNC by enzymatic methods was observed in sugarcane bagasse biomass [51]. The authors observed that the increase in the alkali load during the pretreatment produced not only dissolved components (lignin, hemicellulose, and extractives) but also favored the enzymatic reaction to produce CNC. As a result, the CNC obtained from pretreated samples presented a more homogeneous distribution and a lower average size [51]
The enzymatic treatment uses a variety of enzyme sources. The cellulase enzyme includes multiple constituents: the endoglucanases, the cellobiohydrolases, and the β-glucosidase. These enzymes work synergistically to hydrolyze cellulose chains: endoglucanases randomly cleave internal β-1,4-glycosidic bonds, cellobiohydrolases progressively cleave chain ends to release cellobiose or glucose, and β-glucosidases hydrolyze cellobiose into glucose. This three-step process initiates with soluble sugars releasing (DP ≤ 6) into the liquid phase. Enzymatic depolymerization by endoglucanases and cellobiohydrolases is the rate-limiting step. Secondary hydrolysis in the liquid phase primarily involves β-glucosidase-mediated conversion of cellobiose to glucose [42]. Controlling this final step is crucial for producing CNC while minimizing glucose formation. Waghmare et al. [52] indicated that endoglucanase is a key enzyme during the enzymatic preparation of CNC.
One of the few studies carried out in recent years is the synthesis of CNC via enzymatic hydrolysis from the lignocellulosic waste of lemongrass after oil extraction. The enzyme did not modify the cellulose structure as it could not penetrate it. Due to the dominance of the 1β structure in the cellulose, hydrolysis takes longer, so the combination of pretreatment, enzymatic hydrolysis, and sonication eliminates the amorphous part of cellulose [42].
Meanwhile, CNC was successfully obtained via enzymatic hydrolysis with the cellulase from engineered strain Penicillium oxalicum using commercial eucalyptus dissolving pulp as the substrate. The total yields of CNC reached 15.7% through three-step enzymatic hydrolysis. The process efficiency decreased in the later stage of enzymatic hydrolysis because of the cellulosic substrate’s high crystallinity, and a simple homogenization treatment can promote enzymatic hydrolysis [53]. Waghmare et al. demonstrated the efficacy of endoglucanases from Myceliophthora thermophila in hydrolyzing eucalyptus dissolving pulp, achieving a maximum yield of 8.8% [52]. Enzymatic hydrolysis also proved promising for producing CNC from pretreated palm oil empty fruit bunch fibers, with a reported yield of 22.53% [54].
Ball miller mechanical pretreatment combined with endoglucanase hydrolysis further enhanced CNC production, achieving a yield of 76% and a crystallinity index of 75% from cellulose Avicel [55]. Yupanqui-Mendoza et al. [43], integrating hydrodynamic cavitation pretreatment with enzymatic hydrolysis, obtained a 60% CNC yield and 81–85% crystallinity index. This approach also significantly reduced energy consumption by 56%, making it more economically feasible [43]. Using a sonication process to eliminate the amorphous part of cellulose produced a similar effect [42], and a combination of defibrillation pretreatment, enzymatic hydrolysis, and sonication substantially improved nanocellulose production yield, reaching up to 83% [56].
3.4. Green Solvents
ILs and DES are solvent methods considered efficient approaches for lignocellulosic component separations, aligning with green chemistry principles.
3.4.1. Deep Eutectic Solvents
DESs emerge as a new generation of green solvents. A DES involves two or three components, primarily a hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) (Figure 5). Choline chloride (ChCl) is the most widespread HBA used, whilst some HBDs are OA, FA, and alkaline glycerol [57].
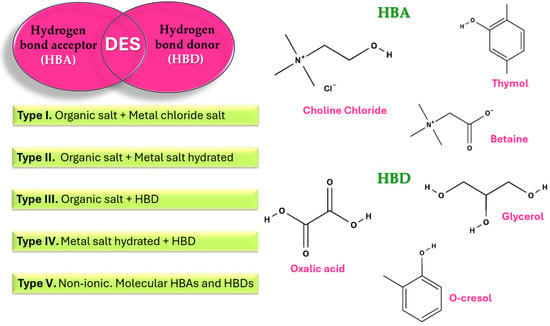
Figure 5.
Types of DES and some commonly applied molecules.
DESs can selectively break ester and hydrogen bonds between lignin and carbohydrates. The strong hydrogen bonding within DESs facilitates their application to a wide range of hydroxyl-rich polymers [58] and can be applied to modify the cellulose surface groups [59]. At the same time, the DESs could enhance the CNC dispersion [59]. For instance, ultrasonicated DES (ChCl + glycerol) produced a stable CNC dispersion before the addition as reinforcement of carrageenan biocomposite [59]. In another study, modified CNCs and films were prepared with two reactive DESs (guanidine sulfamate glycerol and guanidine sulfamate ChCl), resulting in diameters ranging from 3 to 5 nm and lengths up to 204 nm [60]. The CNC presented thermal stability relative to pristine cellulose, and the films showed potential for food packaging applications due to their excellent transparency, high mechanical strength, and good water and gas barriers [60].
DESs offer numerous advantages, including biodegradability, environmental friendliness, and efficient recyclability. Notably, these solvents can be successfully recycled and reused for at least three pretreatment cycles without significant loss of efficacy. However, there are challenges to address, such as high viscosity and production costs [61]. DESs have emerged as promising agents for CNC production. For instance, DESs composed of lactate and ChCl have been employed to obtain CNC from commercial eucalyptus pulp via ultrasonic treatment at a temperature above 120 °C and variable molar ratios. The treatment led to high-yield CNC production. Crystallinity decreased compared to the raw pulp and without changes in thermal stability [61].
In a comparative study, Mariño et al. [62] evaluated three solvent systems for CNC production:
- Recyclable IL dilution ([Hmim] [(HSO4) (H2SO4)]/H2O, 64 wt% IL);
- Recyclable dilution ([Hmim] [(HSO4) (H2SO4)]/H2O, 80 wt% IL);
- Non-recyclable ternary DES (60 wt%/ChCl: OA/30 wt%: PA/10 wt% water).
The first scenario, involving recyclable IL dilution, is the most economically viable and environmentally friendly option. It has a lower raw material cost per gram of CNC produced (US$0.81/g) and reduced environmental impacts, contributing to a more sustainable materials production process. After multiple reuse cycles, the recovered IL maintained its acidity and high CNC production performance. The physicochemical properties of the obtained CNC displayed promising properties [62].
3.4.2. Ionic Liquids
ILs are liquid electrolytes of ions, typically an organic cation and an inorganic or organic anion (Figure 6). These ions form strong, non-covalent interactions, resulting in a melting point below 100 °C. ILs can separate or dissolve the chief components of lignocellulosic biomass, with some capability of extracting all three primary components: cellulose, hemicellulose, and lignin, while others selectively dissolve one or two [63]. Additionally, ILs can facilitate the further degradation of these components into value-added products. Imidazolium-based ILs with chloride or acetate anions are commonly used for the hydrolytic cleavage of glycosidic bonds in cellulose [61]. However, ILs present certain drawbacks, including difficulties in purification, high viscosity, and potential cellulose chain degradation [63].
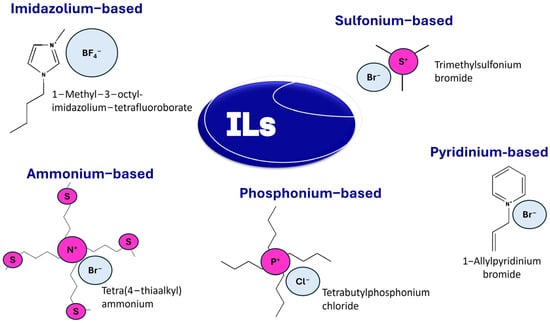
Figure 6.
Some common ILs.
ILs, such as 1-butyl-3-methylimidazolium chloride ([BMIm]Cl), have been employed to prepare CNC from various sources, including cotton gin motes. A promising approach involves minimal cellulose swelling in the IL followed by acid-catalyzed hydrolysis using phosphoric (H3PO4), hydrochloric (HCl), or sulfuric (H2SO4) acid. This method yields CNC with comparable physical properties to sulfate-based CNC, including crystallinity, but with superior thermal stability and reduced surface functionalization, exhibiting excellent colloidal stability for over 90 days [64]. In another study, 1-hexyl-3-methylimidazolium hydrogen sulfate ([Hmim][HSO4]) was used to prepare CNC from commercial microcrystalline cellulose. Optimal conditions identified to produce CNC with desirable characteristics include a temperature of 71 °C and ultrasonication of 69% amplitude for 23 minutes [65]. Ma et al. combined IL pretreatment and solid acid hydrolysis to extract high-purity CNC with excellent thermal stability from microcrystalline cellulose. The resulting CNC exhibited a rod-like shape with an average length of 300 ± 100 nm and a width of 20 ± 10 nm [66]. Likewise, a binary mixture of 1-butyl-3-methylimidazoluim hydrogen sulfate ([Bmim][HSO4]) and dimethyl sulfoxide (DMSO) was compared with pure IL. CNC obtained with IL-cosolvent and pure IL (P-CNC) exhibited an average diameter of 50 nm and 0.77 µm and lengths of 757 nm and 2.11 µm, respectively [67]. Da Silva et al. [68] synthesized CNC through the hydrolysis of microcrystalline cellulose using protic ILs (PILs): 3-diethylamino-propylammonium hexanoate ([DEAPA][Hex]), 3-dimethylamino-1-propylammonium hexanoate ([DMAPA][Hex]), and propylammonium hexanoate ([PA][Hex]) at varying reaction times. All PILs effectively produced CNC, irrespective of the cation size and reaction duration, with a maximum yield of 34%. Crystalline indices consistently decreased, regardless of cation size or reaction time. Thermal stability shifted to higher temperatures, reaching 92 °C, likely due to the influence of PIL cation sizes [67].
3.4.3. Subcritical Water
Subcritical water (SW) is a hydrothermal process in which water is above its vapor pressure, maintaining its liquid form when heated above the boiling point (100 °C) and below the critical point (374 °C). Under these thermodynamic conditions, water exhibits two unique properties: a higher ion product, i.e., higher concentrations of H3O+ species making water slightly acidic, capable of catalyzing chemical reactions, and a relatively low dielectric constant, increasing water’s solubility to dissolve organic materials [69].
SW is an emerging green and efficient hydrothermal technology that reduces harsh process chemicals and could present good extraction performance and easy scalability. Although there is not much new research regarding SW, Osei-Bonsu et al. [70] demonstrated acid-catalyzed digestion of woody-biomass-originated CNC with desirable physical and chemical features dependent on the process parameters (temperature, pressure, and time). Electron microscopy revealed rod-like structures with varying particle size distributions (100–500 nm) dominated by process time. However, colloidal stability was low (versus acid-hydrolyzed CNC) due to the low charges on the surface of CNC [70].
CNC obtained via the SW process offers a high crystallinity index of approximately 79%, having a similar rod-like morphology and aspect ratio to conventionally extracted CNC [71].
3.5. Mechanical Treatments
Purely mechanical treatments allow CNC production without the application of chemical reagents. The ball mill is the most applied equipment. It reduces the size at the expense of reducing the degree of polymerization and crystallinity [72]. The ball mill consists of a rotating hollow cylindrical shell partially filled with steel, ceramic, or rubber-based balls (Figure 7), achieving, in some cases, analogous CNC dimensions to those obtained by acid hydrolysis. As an example, Hernández-Varela et al. [73] treated agave wastes and garlic peels bleached pulps using 72 agate balls (5 mm) per gram of cellulose at a rotation speed of 850 rpm, reaching dimensions less than 100 nm [73].
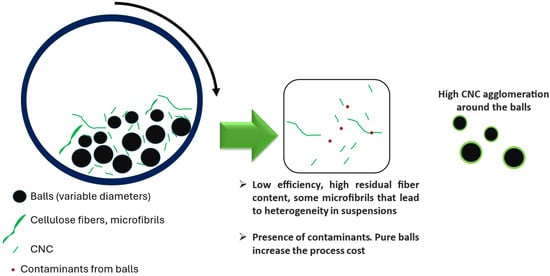
Figure 7.
Ball milling system.
The ball milling process generally leads to a high-size heterogeneity [74], occasionally solved by extra steps such as ultrasound. Another used option is alumina/silica balls (60 balls) instead of agate in different dilution media [75], as well as combined stages of ball milling with alumina/silica balls (balls with variable diameters of 12 and 21 mm) and ultrasound [76]. Ultrasound allows for reducing heterogeneity in sizes and agglomeration of CNC. However, the contamination of CNC appeared in all cases due to the ball milling process.
The studies found that increasing the processing time leads to contamination of the CNC obtained with silica from the balls. Silica or silicon dioxide is a contaminating component of alumina balls (92% purity). Using systems with high-purity alumina or zirconium balls could increase the cost [76]. Studies about using zirconium dioxide (ZrO2) balls for reducing the microcrystalline cellulose size, where 3 g of material was ground with 10 ZrO2 balls (diameter 1 cm) at 500 rpm, for different times (0.5 h up to 24 h), led to a size reduction with amorphization of the material [72].
The effect of ball milling on the fiber was evaluated with cotton powder and stainless-steel balls (6 mm), finding fiber opening, breaking into pieces, reduction in molecular weight, small-scale oxidation, increase in pores, a higher surface area leading to increased water adsorption, decrease in thermal stability, and loss of crystallinity [74]. The treatment with small zirconia balls (0.5 mm) of bamboo-bleached fibers also produced fiber opening. The authors found that a higher ball milling duration and speed led to lower particle size and crystallinity and a re-aggregation phenomenon of tiny fibers, which could also benefit the penetration of chemical components into the fiber structure [30].
As mentioned, ultrasound can assist in ball milling treatment, but high-intensity ultrasonic treatment can also facilitate the isolation of the CNC. High-intensity ultrasound generates the rupture of cellulose fibers through the cavitation generated in the suspension, leading to particle diameter and polydispersity reduction and increased swelling [77].
3.6. Solid/Vapor Strategies
The solid strategy using organic and inorganic solid acids has emerged as a promising alternative to liquid acids, offering benefits such as ease of separation and recyclability [78]. A study evaluated Amberlyst-45 ion-exchange resin as solid acid hydrolysis of previously MCC swollen in [AMIm][Cl]. The authors used 45 wt% of solid acid (relative to cellulose) to extract CNC at 45 °C for 5 h. The obtained CNC had high thermal stability and a rod-like morphology, with an average length and width of 300 and 20 nm, respectively. Furthermore, the solid acid was reusable up to three times without surface purification [66]. Gao et al. [79] extracted CNC from Calotropis gigantea fiber utilizing a nano-solid superacid catalyst of SO42−/TiO2 combined with ball-milling defibrillation. The yield was 55.37% of CNC with a rod-like shape and an average length of 242 nm and width of 8.80 nm. The aspect ratio (length-to-width) of the CNC was 27.5 [79]. The superacid catalyst allows the degradation of the cellulose fibers by Lewis and Bronsted acid sites [78].
Phosphotungstic acid (PTA) is a heteropolyacid with abundant Bronsted acid sites. It is a green and environmentally friendly catalyst [80]. Additionally, PTA has mild reaction conditions and good solubility [80,81]. For example, CNC was obtained under the synergistic effect of ultrasound and PTA, using a concentration of PTA 13.7%, ultrasonication time of 61 min, and reaction time of 6 h. As a result, a short rod-like CNC with a yield of 77.1% was obtained, with excellent stability, and the crystallinity reached 86.9% [80].
The strategy involving vapor/gas phase acid hydrolysis for CNC production involves wet cellulose fibers hydrolysis in the presence of HCl vapor. The hydrolysis mechanism is as follows: HCl gas is first adsorbed onto the surface water layer of the cellulose fibers, followed by HCl dissociation to generate a high local acid concentration, and finally, the acid hydrolyzes the cellulose to obtain CNC. This gaseous acid hydrolysis strategy offers advantages over traditional liquid acid hydrolysis since it eliminates the need for repeated centrifugation and dialysis, producing significant time and water consumption savings. However, it presents certain drawbacks, such as the relatively high vapor pressure of the reaction, which poses some safety risks, and the need to address vapor recycling [78,82,83].
The moisture content in cellulose resources before HCl gas treatment plays a key role in hydrolysis, rather than the studied range of exposure time to acidic gas and the well-preserved cellular structure of wood [84]. Through XRD analysis, Leboucher et al. [83] demonstrated that vapor-phase HCl hydrolysis opens the cellulose structure without affecting crystallinity and with minimal monomer production compared to the popular sulfuric acid hydrolysis. XRD showed no significant crystallinity changes, while lateral size variations were substantial, on the order of 22% on hydrophilic surfaces. Moreover, based on their results, they assume that HCl hydrolysis does not proceed through complete hydrolysis of the less-ordered regions, unlike H2SO4 hydrolysis, which explains the high yield of the method (>90%) [83].
Furthermore, combinations of HCl acid hydrolysis and other methods have been explored to prepare charged CNC, e.g., a methodology integrating TEMPO-mediated electrochemical oxidation and HCl gas pre-hydrolysis. HCl hydrolyzes the amorphous regions of cellulose, yielding microfibrils, while TEMPO oxidation introduces carboxyl groups onto the crystallite surface, enhancing the dispersion of CNC in aqueous media [85]. Li et al. [86] developed an optimized methodology combining dilute acid steam hydrolysis and enzymatic hydrolysis to produce a novel cellulose mesh with properties distinct from conventional nanocellulose. This material demonstrated a significantly enhanced specific surface area, high yield and crystallinity, and excellent thermal stability [86].
3.7. Radiation-Based Treatments
More recent studies have evaluated the application of radiation to avoid large amounts of acid or high reaction times [87]. For example, accelerated electron irradiation (electron beam irradiation, EBI) promoted higher efficiency during CNC production through size reduction, less reagent consumption, and lower reaction times [87,88]. Wu et al. [89] evaluated the application of EBI before a high-pressure homogenizer. The authors reached a rod-shaped structural nanocellulose with diameters between 12 and 20 nm and lengths up to 300 nm [89].
Gamma radiation (γ-radiation) is another option to reduce the sizes while maintaining uniformity [90]. Previous studies have shown that gamma radiation of cellulosic components in the presence of humidity increases carboxyl groups due to radical species that occur because of water radiolysis [91].
Microwave irradiation has been applied to assist a chemical–mechanical (alkaline treatment and high-speed blender) treatment to obtain an average particle size (DLS) of 75 nm [92].
4. Discussion
The previous section reviewed various sustainable methods to replace traditional CNC production processes, examining the key studies and their characteristics. The following section assesses their advantages and disadvantages, the potential limitations associated with these new strategies, and the future challenges they may pose. Table 2 presents the advantages and disadvantages of each method analyzed in the preceding paragraphs. Additionally, it includes numerical values for yields, reaction times, and temperatures, expressed as ranges based on the data extracted from each cited reference throughout this document.

Table 2.
Comparison between conventional and green methods.
Three key characteristics of the CNC were selected for analysis: functionalization due to its importance in industrial applications, improved thermal degradation compared to the raw material, and the crystallinity index indicative of crystal purity (Table 2).
Table 2 shows that chemical methods (such as acids, ILs, and DES) can produce cellulose nanocrystals (CNC) and functionalize them in a single step, which is crucial for reducing production costs. Functionalization can affect thermal degradation. For example, in the case of carboxylation or the addition of sulfate groups, it could decrease the maximum degradation temperature. On the other hand, esterification with organic acids, such as OA or MA, improves thermal stability. In the case of methods that do not functionalize the CNC by themselves (mechanical, enzymatic, hydrothermal treatments), the improvement in thermal stability is due to the selectivity of the hydrolysis without generating other degradation products or the presence of chemical contaminants. On the other hand, the increase or improvement in the crystallinity index indicates the elimination or reduction of the amorphous regions of the cellulose and the release of the crystalline regions, whose value depends on the starting material and the efficiency of the CNC production treatment.
In brief, although most processes present environmental, economic, or process-related drawbacks, green processes are susceptible to optimization and could be a valid option for obtaining CNC.
4.1. Advantages of the Green Methods
CNCs have garnered significant attention due to their diverse applications across various fields. Consequently, researchers actively seek the most efficient, sustainable, economically viable, and environmentally friendly production technologies to meet the growing demand for these materials. Conventional production methods often exhibit limitations such as environmental damage, corrosivity, and low yields. To overcome these shortcomings, scientists have explored the potential of emerging processing technologies, as discussed in this review.
The utilization of mild organic acids presents a promising alternative due to their reduced corrosivity, potential for recovery and reuse, and the simultaneous ability to incorporate functional groups during the reaction. For instance, CA, a non-toxic and relatively weak acid, exhibits minimal environmental impact and can be readily recycled. Furthermore, the resulting CNC demonstrates excellent thermal stability, and the organic acid can be introduced as a functional group onto the CNC surface via ester bonds, enabling further functionalization of the material.
Solid acid hydrolysis offers the advantages of milder reaction conditions and easier recovery from the reaction mixture, facilitating acid recycling.
Enzymatic treatments emerge as a promising approach for isolating CNC from biomass. They present high efficiency, selectivity, and lower energy consumption than other techniques. Enzymatic processes operate under neutral conditions, thereby eliminating the emission of harmful chemicals that can adversely affect both the environment and equipment.
ILs and DESs have emerged as efficient solvent systems for lignocellulosic component separations, aligning with green chemistry principles. DESs have excellent recovery performance and high yields, making them a promising green solvent. Because DESs have low vapor pressure, they are less volatile, reducing the risk of evaporation and air pollution. Moreover, in many cases, they are biodegradable, can be reused, and exhibit lower toxicity compared to conventional solvents.
Mechanical methods do not need chemical reagents, eliminating the solvent or catalyst recovery requirement. They also function as auxiliary techniques for other methods.
Radiation-based techniques are efficient in reducing time and chemical reagents, leading to CNC with larger sizes and increasing the generation of hydroxyl groups in the presence of water.
4.2. Current Limitations
Green methods for CNC production exhibit some limitations that require further overcoming but are straightforward to improve.
Table 2 outlines the drawbacks associated with each method. While some can be readily addressed, others present more significant obstacles to production scale-up. For instance, some organic acids can be noxious or unsafe, requiring careful recovery and reuse procedures. Moreover, acid hydrolysis often requires prolonged reaction times and elevated temperatures due to the inherent weakness of these acids. Solid acid hydrolysis exhibits limitations such as reduced contact between the solid acid and cellulose, prolonged reaction times, and a broader particle size distribution. Also, the high cost of solid acids can hinder large-scale CNC production despite the minimal equipment corrosion. Enzymatic hydrolysis also suffers from extended reaction times. Additionally, the high cost of enzymes can significantly limit their application in large-scale CNC production. While both mechanical and enzymatic methods are environmentally friendly and can be employed to produce CNC, the associated high production costs hinder their large-scale implementation. Mechanical methods for producing nanometric cellulose require the application of high shear forces, which results in increased energy consumption.
Moreover, these processes can adversely affect the morphology of cellulose fibers, potentially reducing crystallinity and resulting in materials with low particle uniformity. The use of ILs presents some drawbacks, including purification challenges, high viscosity, and the potential for cellulose chain degradation. Similarly, DESs exhibit high viscosity and production costs. Furthermore, the recovery of DESs can be complex and expensive, limiting their widespread application. Finally, radiation-based methods’ limitations involve high equipment investments, high energy consumption, and higher risks associated with exposure during the process.
5. Conclusions and Future Perspectives
The green techniques evaluated in the analyzed literature for CNC production have demonstrated efficiency in achieving similar properties to traditional acid processes but with lower environmental impacts, in some cases even with less degradation of materials.
Future perspectives regarding green methods to produce CNC involve process optimization, technology improvement, energy cost reduction, solvent reduction or recovery, and advances in innovative systems. For example, methods based on green solvents still require research to achieve efficient reagent recovery systems, avoiding waste of reagents and increasing their use cycles. In the case of energy consumption, one of the alternatives is the use of hybrid treatments, such as combining chemical treatments with mechanical ones. Pretreatment of the raw material improves enzymatic systems.
Author Contributions
E.P.D.: conceptualization, investigation, writing—original draft, and formal analysis. N.E.: methodology, data curation, and writing—review and editing. M.C.A.: writing—review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Universidad Tecnológica Nacional (UTN) and Universidad Nacional de Misiones (UNAM), for their support.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Acetic Acid |
| CA | Citric Acid |
| CNC | Cellulose nanocrystals |
| CNF | Cellulose nanofibers |
| ChCl | Choline Chloride |
| DES | Deep eutectic solvent |
| EBI | Electron beam irradiation |
| FA | Formic Acid |
| GWP | Global warming potential |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| ILs | Ionic liquids |
| MA | Maleic Acid |
| OA | Oxalic Acid |
| SW | Subcritical water |
References
- Arockiasamy, F.S.; Manoharan, B.; Santhi, V.M.; Prakalathan, K.; Periasamy, D.; Dhandapani, A.; Natarajan, V.; Senthilkumar, K.; Muthu Kumar, T.S.; Ilyas, R.A. Navigating the Nano-World Future: Harnessing Cellulose Nanocrystals from Green Sources for Sustainable Innovation. Heliyon 2024, 11, e41188. [Google Scholar] [CrossRef]
- ISO/TS 20477; Standard Terms and Their Definition for Cellulose Nanomaterial. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent Advancement in Isolation, Processing, Characterization and Applications of Emerging Nanocellulose: A Review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Zhang, X.; Haq, F.; Ullah, A.; Ullah, R.; Khan, F.U.; Iqbal, M. Advance Study of Cellulose Nanocrystals Properties and Applications. J. Polym. Environ. 2020, 28, 1117–1128. [Google Scholar] [CrossRef]
- Bondancia, T.J.; de Aguiar, J.; Batista, G.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Production of Nanocellulose Using Citric Acid in a Biorefinery Concept: Effect of the Hydrolysis Reaction Time and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 11505–11516. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly Efficient and Sustainable Preparation of Carboxylic and Thermostable Cellulose Nanocrystals via FeCl3 -Catalyzed Innocuous Citric Acid Hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Lv, D.; Du, H.; Che, X.; Wu, M.; Zhang, Y.; Liu, C.; Nie, S.; Zhang, X.; Li, B. Tailored and Integrated Production of Functional Cellulose Nanocrystals and Cellulose Nanofibrils via Sustainable Formic Acid Hydrolysis: Kinetic Study and Characterization. ACS Sustain. Chem. Eng. 2019, 7, 9449–9463. [Google Scholar] [CrossRef]
- Wang, H.; Du, H.; Liu, K.; Liu, H.; Xu, T.; Zhang, S.; Chen, X.; Zhang, R.; Li, H.; Xie, H.; et al. Sustainable Preparation of Bifunctional Cellulose Nanocrystals via Mixed H2SO4/Formic Acid Hydrolysis. Carbohydr. Polym. 2021, 266, 118107. [Google Scholar] [CrossRef]
- Henschen, J.; Li, D.; Ek, M. Preparation of Cellulose Nanomaterials via Cellulose Oxalates. Carbohydr. Polym. 2019, 213, 208–216. [Google Scholar] [CrossRef]
- Tang, F.; Li, Y.; Huang, J.; Tang, J.; Chen, X.; Yu, H.-Y.; Zhou, Y.; Tang, D. An Environmentally Friendly and Economical Strategy to Cyclically Produce Cellulose Nanocrystals with High Thermal Stability and High Yield. Green Chem. 2021, 23, 4866–4872. [Google Scholar] [CrossRef]
- Yu, H.; Abdalkarim, S.Y.H.; Zhang, H.; Wang, C.; Tam, K.C. Simple Process to Produce High-Yield Cellulose Nanocrystals Using Recyclable Citric/Hydrochloric Acids. ACS Sustain. Chem. Eng. 2019, 7, 4912–4923. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lin, Y.; Qin, Y.; He, R.; Wang, M.; Sun, Q.; Peng, Y. Nanocellulose from Agro-Industrial Wastes: A Review on Sources, Production, Applications, and Current Challenges. Food Res. Int. 2024, 192, 114741. [Google Scholar] [CrossRef] [PubMed]
- Magagula, L.P.; Masemola, C.M.; Ballim, M.A.; Tetana, Z.N.; Moloto, N.; Linganiso, E.C. Lignocellulosic Biomass Waste-Derived Cellulose Nanocrystals and Carbon Nanomaterials: A Review. Int. J. Mol. Sci. 2022, 23, 4310. [Google Scholar] [CrossRef]
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.-Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline Cellulose Isolation via Acid Hydrolysis from Non-Woody Biomass: Importance of Hydrolysis Parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Shang, Y.; Li, B.; Du, H. Sustainable Preparation of Cellulose Nanocrystals: State of the Art and Perspectives. Green Chem. 2022, 24, 9346–9372. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M.; Kaiser, E.Q.; Archibald, D.D. Quantification of Cellulose Nanowhiskers Sulfate Esterification Levels. Carbohydr. Polym. 2013, 92, 1809–1816. [Google Scholar] [CrossRef]
- Kassab, Z.; Syafri, E.; Tamraoui, Y.; Hannache, H.; Qaiss, A.E.K.; El Achaby, M. Characteristics of Sulfated and Carboxylated Cellulose Nanocrystals Extracted from Juncus Plant Stems. Int. J. Biol. Macromol. 2020, 154, 1419–1425. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Du, H.; Wang, X.; Liu, W.; Duan, Y.; Zhang, X.; Sun, L.; Zhang, X.; Si, C. Highly Efficient Preparation of Functional and Thermostable Cellulose Nanocrystals via H2SO4 Intensified Acetic Acid Hydrolysis. Carbohydr. Polym. 2020, 239, 116233. [Google Scholar] [CrossRef]
- Worku, L.A.; Bachheti, R.K.; Tadesse, M.G. Preparation and Characterization of Carboxylated Cellulose Nanocrystals from Oxytenanthera abyssinica (Ethiopian Lowland Bamboo) Cellulose via Citric Acid Anhydrous Hydrolysis Catalyzed by Sulfuric Acid. Biomass Biorefin. 2024, 14, 28807–28823. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging Technologies for the Production of Nanocellulose from Lignocellulosic Biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Lin, C.; Wang, P.; Liu, Y.; Lv, Y.; Ye, X.; Liu, M.; Zhu, J.Y. Chiral Self-Assembly Behavior of Carboxylated Cellulose Nanocrystals Isolated by Recyclable Oxalic Acid from Degreasing Cotton. ACS Sustain. Chem. Eng. 2023, 11, 8035–8043. [Google Scholar] [CrossRef]
- Bello, F.; Chimphango, A. Non-Catalyzed Formic Acid-Based Process for Preparing Thermally Stable Spherical Cellulose Nanocrystals from Mango Seed Husk. Biomass Convers. Biorefin. 2024, 14, 1133–1148. [Google Scholar] [CrossRef]
- Jia, W.; Liu, Y. Two Characteristic Cellulose Nanocrystals (CNCs) Obtained from Oxalic Acid and Sulfuric Acid Processing. Cellulose 2019, 26, 8351–8365. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Wu, Q.; Kuang, Y. Acetylated Cellulose Nanocrystals with High-Crystallinity Obtained by One-Step Reaction from the Traditional Acetylation of Cellulose. Carbohydr. Polym. 2020, 229, 115553. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Mu, T.; Li, Q.; Richel, A. Preparation of Cellulose Nanocrystals from Purple Sweet Potato Peels by Ultrasound-Assisted Maleic Acid Hydrolysis. Food Chem. 2023, 403, 134496. [Google Scholar] [CrossRef]
- Seta, F.T.; An, X.; Liu, L.; Zhang, H.; Yang, J.; Zhang, W.; Nie, S.; Yao, S.; Cao, H.; Xu, Q.; et al. Preparation and Characterization of High Yield Cellulose Nanocrystals (CNC) Derived from Ball Mill Pretreatment and Maleic Acid Hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef]
- Ji, H.; Xiang, Z.; Qi, H.; Han, T.; Pranovich, A.; Song, T. Strategy towards One-Step Preparation of Carboxylic Cellulose Nanocrystals and Nanofibrils with High Yield, Carboxylation and Highly Stable Dispersibility Using Innocuous Citric Acid. Green Chem. 2019, 21, 1956–1964. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Batista, G.; de Aguiar, J.; Lorevice, M.V.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Cellulose Nanocrystals from Sugar Cane Bagasse Using Organic and/or Inorganic Acids: Techno-Economic Analysis and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2022, 10, 4660–4676. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Martinsson, A.; Hasani, M.; Potthast, A.; Theliander, H. Modification of Softwood Kraft Pulp Fibres Using Hydrogen Peroxide at Acidic Conditions. Cellulose 2020, 27, 7191–7202. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Olmos, G.V.; Taleb, M.C.; Felissia, F.E.; Ehman, N.V.; Peresin, M.S.; Area, M.C.; Maximino, M.G. Dissolving Pulp from Eucalyptus Sawdust for Regenerated Cellulose Products. Cellulose 2022, 29, 4645–4659. [Google Scholar] [CrossRef]
- Koshani, R.; van de Ven, T.G.M.; Madadlou, A. Characterization of Carboxylated Cellulose Nanocrytals Isolated through Catalyst-Assisted H2O2 Oxidation in a One-Step Procedure. J. Agric. Food Chem. 2018, 66, 7692–7700. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. Advanced Oxidation Process: A Remediation Technique for Organic and Non-Biodegradable Pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Koshani, R.; van de Ven, T.G.M. Carboxylated Cellulose Nanocrystals Developed by Cu-Assisted H2O2 Oxidation as Green Nanocarriers for Efficient Lysozyme Immobilization. J. Agric. Food Chem. 2020, 68, 5938–5950. [Google Scholar] [CrossRef]
- Valls, C.; Cusola, O.; Roncero, M.B. Evaluating the Potential of Ozone in Creating Functional Groups on Cellulose. Cellulose 2022, 29, 6595–6610. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Li, Y.; Xu, H.; Liu, R.; Zhang, Y.; Zhang, Z.; Yuan, Y.; Zong, L.; Zhou, L.; et al. Oxidation with Potassium Ferrate for the One-Pot Preparation of Carboxylated Cellulose II Nanocrystals. Carbohydr. Polym. 2024, 329, 121796. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Kumari, P.; Seth, R.; Meena, A.; Sharma, D. Enzymatic Synthesis of Cellulose Nanocrystals from Lemongrass and Its Application in Improving Anti-Cancer Drug Release, Uptake and Efficacy. Ind. Crops. Prod. 2023, 192, 115933. [Google Scholar] [CrossRef]
- Yupanqui-Mendoza, S.L.; Prado, C.A.; dos Santos, J.C.; Arantes, V. Hydrodynamic Cavitation as a Promising Pretreatment Technology to Enhance the Efficiency of Cellulose Nanocrystal Production via Enzymatic Hydrolysis. Chem. Eng. J. 2023, 472, 144821. [Google Scholar] [CrossRef]
- Pota, G.; Gallucci, N.; Cavasso, D.; Krauss, I.R.; Vitiello, G.; López-Gallego, F.; Costantini, A.; Paduano, L.; Califano, V. Controlling the Adsorption of β-Glucosidase onto Wrinkled SiO2 Nanoparticles To Boost the Yield of Immobilization of an Efficient Biocatalyst. Langmuir 2023, 39, 1482–1494. [Google Scholar] [CrossRef]
- Pota, G.; Sapienza Salerno, A.; Costantini, A.; Silvestri, B.; Passaro, J.; Califano, V. Co-Immobilization of Cellulase and β-Glucosidase into Mesoporous Silica Nanoparticles for the Hydrolysis of Cellulose Extracted from Eriobotrya Japonica Leaves. Langmuir 2022, 38, 5481–5493. [Google Scholar] [CrossRef]
- Xu, C.; Tong, S.; Sun, L.; Gu, X. Cellulase Immobilization to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass: An All-Inclusive Review. Carbohydr. Polym. 2023, 321, 121319. [Google Scholar] [CrossRef]
- De Souza Lima, J.; Boemo, A.P.S.I.; de Araújo, P.H.H.; de Oliveira, D. Immobilization of Endoglucanase on Kaolin by Adsorption and Covalent Bonding. Bioprocess Biosyst. Eng. 2021, 44, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Meng, X.; Ragauskas, A.J.; Lai, C.; Ling, Z.; Huang, C.; Yong, Q. Unlocking the Secret of Lignin-Enzyme Interactions: Recent Advances in Developing State-of-the-Art Analytical Techniques. Biotechnol. Adv. 2022, 54, 107830. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin–Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 2019, 37, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kermanshahi-pour, A.; Brar, S.K.; Xu, C.C.; He, Q.S.; Evans, S.; Rainey, J.K. Enzymatic Digestibility of Lignocellulosic Wood Biomass: Effect of Enzyme Treatment in Supercritical Carbon Dioxide and Biomass Pretreatment. Heliyon 2023, 9, e21811. [Google Scholar] [CrossRef]
- De Oliveira Júnior, S.D.; Asevedo, E.A.; de Araújo, J.S.; Brito, P.B.; dos Santos Cruz Costa, C.L.; de Macedo, G.R.; dos Santos, E.S. Enzymatic Extract of Aspergillus Fumigatus CCT 7873 for Hydrolysis of Sugarcane Bagasse and Generation of Cellulose Nanocrystals (CNC). Biomass Convers. Biorefin. 2022, 12, 5515–5526. [Google Scholar] [CrossRef]
- Waghmare, P.; Xu, N.; Waghmare, P.; Liu, G.; Qu, Y.; Li, X.; Zhao, J. Production and Characterization of Cellulose Nanocrystals from Eucalyptus Dissolving Pulp Using Endoglucanases from Myceliophthora thermophila. Int. J. Mol. Sci. 2023, 24, 10676. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Xu, N.; Guo, Y.; Liu, G.; Zhao, J. Preparation of Cellulose Nanocrystals from Commercial Dissolving Pulp Using an Engineered Cellulase System. Bioresour. Bioprocess. 2023, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Sikder, B.H.; Rashid, S.S.; Ab Rahim, M.H.; Ramli, A.N.M.; Roslan, R.; Sasi, A.A.; Mustafa, A.H. Enzymatic Cellulose Nanocrystal Production from Pretreated Palm Oil Empty Fruit Bunch Fibers. Mater. Today Proc. 2023, 107, 249–253. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Beneventi, D.; Dufresne, A.; Operamolla, A. High Yield Synthesis of Cellulose Nanocrystals from Avicel by Mechano-Enzymatic Approach. ChemistrySelect 2024, 9, e202401511. [Google Scholar] [CrossRef]
- Dias, I.K.R.; Lacerda, B.K.; Arantes, V. High-Yield Production of Rod-like and Spherical Nanocellulose by Controlled Enzymatic Hydrolysis of Mechanically Pretreated Cellulose. Int. J. Biol. Macromol. 2023, 242, 125053. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of Hydrogen Bond Donor on the Choline Chloride-Based Deep Eutectic Solvent-Mediated Extraction of Lignin from Pine Wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, K.; Liu, M.; Xu, M.; Zhao, T. Deep Eutectic Solvents with Multiple Hydroxyl Sites for Efficient and Reversible Absorption of SF6. J. Mol. Liq. 2022, 356, 119052. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Extraction of Cellulose Nanocrystals Using a Recyclable Deep Eutectic Solvent. Cellulose 2020, 27, 1301–1314. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Dong, C.; Li, R.; Zhang, X.; Wang, T.; Zhang, K. Transparent, Thermal Stable, Water Resistant and High Gas Barrier Films from Cellulose Nanocrystals Prepared by Reactive Deep Eutectic Solvents. Int. J. Biol. Macromol. 2024, 276, 134107. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, H.; Xu, X.; Li, L.; Kang, H.; Li, D. Recent Advancements in the Synthesis, Functionalization, and Utilization of Cellulose Nanocrystals. Resour. Chem. Mater. 2024, 10073. [Google Scholar] [CrossRef]
- Mariño, M.A.; Rueda-Ordonez, D.; Paredes, M.G.; Tapia, R.A.; Pita, R.; Pavez, P. Recycled Ionic Liquid vs. Deep Eutectic Solvent in Cellulose Nanocrystals Production: Characterization, Techno-Economic Analysis, and Life Cycle Assessment. J. Clean. Prod. 2024, 472, 143461. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Condon, B.D. Cellulose Hydrolysis Using Ionic Liquids and Inorganic Acids under Dilute Conditions: Morphological Comparison of Nanocellulose. RSC Adv. 2020, 10, 39413–39424. [Google Scholar] [CrossRef] [PubMed]
- Haron, G.A.S.; Mahmood, H.; Bin Noh, H.; Moniruzzaman, M. Fabrication and Characterization of 3D Printable Nanocomposite Filament Based on Cellulose Nanocrystals and Polylactic Acid Using Ionic Liquids. J. Appl. Polym. Sci. 2024, 141, e54780. [Google Scholar] [CrossRef]
- Ma, L.; Xu, Y.; Chen, J.; Dong, C.; Pang, Z. Preparation of Cellulose Nanocrystals by Synergistic Action of Ionic Liquid and Recyclable Solid Acid under Mild Conditions. Molecules 2023, 28, 3070. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Bin Noh, H.; Goto, M.; Moniruzzaman, M. Cellulose Nanocrystals Preparation from Microcrystalline Cellulose Using Ionic Liquid-DMSO Binary Mixture as a Processing Medium. J. Mol. Liq. 2022, 346, 118208. [Google Scholar] [CrossRef]
- Da Silva, J.B.A.; Vieira, S.R.; Pessôa, L.C.; Santana, J.S.; Lemos, P.V.F.; de Souza, C.O.; Cardoso, L.G.; de Assis, D.J.; Mussagy, C.U.; Santos Ebinuma, V.C.; et al. Impact of Ionic Liquid’s Cation Alkyl Chain Length and Reaction Time on Cellulose Nanocrystals Preparation. Carbohydr. Polym. Technol. Appl. 2023, 6, 100390. [Google Scholar] [CrossRef]
- Kulkarni, S.P. Supercritical Water Hydrolysis of Cellulose: State-of-the-Art of Green Depolymerisation Technique. Biomass Bioenergy 2024, 184, 107182. [Google Scholar] [CrossRef]
- Osei-Bonsu, R.; Hoque, M.; McMichael, P.S.; Foster, E.J. Subcritical Water Digestion of Woody Biomass: Extraction of Cellulose Nanomaterials under Acid-Lean Condition. Nanoscale Adv. 2024, 6, 3923–3933. [Google Scholar] [CrossRef]
- Singh, S.; Bhardwaj, S.; Tiwari, P.; Dev, K.; Ghosh, K.; Maji, P.K. Recent Advances in Cellulose Nanocrystals-Based Sensors: A Review. Mater. Adv. 2024, 5, 2622–2654. [Google Scholar] [CrossRef]
- Lan, L.; Chen, H.; Lee, D.; Xu, S.; Skillen, N.; Tedstone, A.; Robertson, P.; Garforth, A.; Daly, H.; Hardacre, C.; et al. Effect of Ball-Milling Pretreatment of Cellulose on Its Photoreforming for H2 Production. ACS Sustain. Chem. Eng. 2022, 10, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Varela, J.D.; Chanona-Pérez, J.J.; Calderón Benavides, H.A.; Cervantes Sodi, F.; Vicente-Flores, M. Effect of Ball Milling on Cellulose Nanoparticles Structure Obtained from Garlic and Agave Waste. Carbohydr. Polym. 2021, 255, 117347. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Wang, T.; Makarem, M.; Santiago Cintrón, M.; Cheng, H.N.; Kang, X.; Bacher, M.; Potthast, A.; Rosenau, T.; King, H.; et al. Effects of Ball Milling on the Structure of Cotton Cellulose. Cellulose 2019, 26, 305–328. [Google Scholar] [CrossRef]
- Kano, F.S.; de Souza, A.G.; Rosa, D.d.S. Variation of the Milling Conditions in the Obtaining of Nanocellulose from the Paper Sludge. Matéria 2019, 24, e12406. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Souza, A.G.; Nunes, L.L.; Shahi, N.; Rangari, V.K.; Rosa, D.d.S. Use of Ball Mill to Prepare Nanocellulose from Eucalyptus Biomass: Challenges and Process Optimization by Combined Method. Mater. Today Commun. 2020, 22, 100755. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Zheng, L.; Tian, T.; Teng, F.; Li, Y. Preparation and Characterization of Okara Nanocellulose Fabricated Using Sonication or High-Pressure Homogenization Treatments. Carbohydr. Polym. 2021, 255, 117364. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustain. Syst. 2022, 6, 2100100. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient Extraction of Cellulose Nanocrystals from Waste Calotropis Gigantea Fiber by SO42−/TiO2 Nano-Solid Superacid Catalyst Combined with Ball Milling Exfoliation. Ind. Crops. Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Gui, X.; Wan, Z.; Zhang, H.; Niu, M.; Guo, Y.; Li, H. Preparation of Cellulose Nanocrystals by Ultrasonication-Assisted Phosphotungstic Acid Method: An Effective Method of High Yield and Friendly Environment. Ind. Crops. Prod. 2024, 222, 119780. [Google Scholar] [CrossRef]
- Chen, B.-H.; Wang, Z.-Q.; Jin, Z.-C.; Gou, Z.-C.; Tang, S.-S.; Yu, X.-X.; Chen, H.; Chen, G.; Su, Y.-J. Optimized Phosphotungstic Acid Pretreatment for Enhancing Cellulase Adsorption and Biomass Saccharification in Corn Stover. Biomass Convers. Biorefin. 2023, 13, 9249–9264. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, Q.; An, X.; Ji, X.; Tian, Z.; Liu, S.; Yang, G. Recent Advances in Sustainable Preparation of Cellulose Nanocrystals via Solid Acid Hydrolysis: A Mini-Review. Int. J. Biol. Macromol. 2023, 253, 127353. [Google Scholar] [CrossRef] [PubMed]
- Leboucher, J.; Bazin, P.; Goux, D.; El Siblani, H.; Travert, A.; Barbulée, A.; Bréard, J.; Duchemin, B. High-Yield Cellulose Hydrolysis by HCl Vapor: Co-Crystallization, Deuterium Accessibility and High-Temperature Thermal Stability. Cellulose 2020, 27, 3085–3105. [Google Scholar] [CrossRef]
- Lourençon, T.; Altgen, M.; Pääkkönen, T.; Guccini, V.; Penttilä, P.; Kontturi, E.; Rautkari, L. Effect of Moisture on Polymer Deconstruction in HCl Gas Hydrolysis of Wood. ACS Omega 2022, 7, 7074–7083. [Google Scholar] [CrossRef]
- Yousefi, N.; Hannonen, J.; Fliri, L.; Peljo, P.; Kontturi, E. Highly Charged Cellulose Nanocrystals via Electrochemical Oxidation. Nano Lett. 2024, 24, 14610–14614. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Z.; Dang, W.; Lin, Z.; Wang, H.; Wang, H.; Ye, D.; Yao, R. High-Yield and Scalable Cellulose Nanomesh Preparation via Dilute Acid Vapor and Enzymatic Hydrolysis-Mediated Nanofabrication. Carbohydr. Polym. 2024, 323, 121370. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Kim, J.-H.; Lee, J.-S. Efficient Isolation of Cellulose Nanocrystals from Seaweed Waste via a Radiation Process and Their Conversion to Porous Nanocarbon for Energy Storage System. Molecules 2024, 29, 4844. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Lei, H.; Villota, E.; Zhao, Y.; Wang, C.; Huo, E.; Zhang, Q.; Mateo, W.; Lin, X. High Yield Production of Nanocrystalline Cellulose by Microwave-Assisted Dilute-Acid Pretreatment Combined with Enzymatic Hydrolysis. Chem. Eng. Process. 2021, 160, 108292. [Google Scholar] [CrossRef]
- Wu, Q.; Ding, C.; Wang, B.; Rong, L.; Mao, Z.; Feng, X. Green, Chemical-Free, and High-Yielding Extraction of Nanocellulose from Waste Cotton Fabric Enabled by Electron Beam Irradiation. Int. J. Biol. Macromol. 2024, 267, 131461. [Google Scholar] [CrossRef]
- Whba, F.; Mohamed, F.; Whba, R.; Idris, M.I.; Noor, N.M.; Bin Mahmood, M.K. Synthesis and Characterization of Cellulose Nanocrystals/Gd2O3 Nanocomposite as a Dual-Mode Contrast Agent for MRI via Gamma-Ray Irradiation. Radiat. Phys. Chem. 2024, 221, 111727. [Google Scholar] [CrossRef]
- Muscolino, E.; Sabatino, M.A.; Jonsson, M.; Dispenza, C. The Role of Water in Radiation-Induced Fragmentation of Cellulosic Backbone Polysaccharides. Cellulose 2024, 31, 841–856. [Google Scholar] [CrossRef]
- Varshney, S.; Mulpuru, V.; Mishra, N.; Gupta, M.K. Microwave-Irradiated Novel Isolation of Nanocellulose from Waste Rice Husk via Modified Chemo-Mechanical Route: Characterization, in-Silico Prediction, and Its Antibacterial Activity. Mater. Technol. 2022, 37, 2608–2622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).